Abstract
Microphysiological systems replicate human organ function and are promising technologies for discovery of translatable biomarkers, pharmaceuticals, and regenerative therapies. Because microphysiological systems require complex microscale anatomical structures and heterogeneous cell populations, a major challenge remains to manufacture and operate these products with reproducible and standardized function. In this Perspective, three stages of microphysiological system monitoring, including process, development, and function, are assessed. The unique features and remaining technical challenges for the required sensors are discussed. Monitoring of microphysiological systems requires nondestructive, continuous biosensors and imaging techniques. With such tools, the extent of cellular and tissue development, as well as function, can be autonomously determined and optimized by correlating physical and chemical sensor outputs with markers of physiological performance. Ultimately, data fusion and analyses across process, development, and function monitors can be implemented to adopt microphysiological systems for broad research and commercial applications.
Keywords: microphysiological system (MPS), organ-on-chip, biosensor, real-time analysis, data, cyber-physical system
Graphical Abstract
Microphysiological systems (MPSs) (i.e., “organ-on-chip”) that replicate human organ function are promising technologies for drug discovery, disease modeling, therapeutic efficacy testing, toxicology, and personalized medicine.1–13 Nevertheless, incomplete characterization, inefficient data collection, and inconsistent analyses significantly impede cost-effective translation of cell and tissue engineering strategies for MPSs. The state-of-the-art methods for manufacturing and operating MPSs are labor intensive, expensive, and lack standardization. For analyses, researchers rely on downstream and post facto methods of data collection, including histology, fluorescence imaging after sample fixation, and biomolecular assays. To monitor temporal changes in MPSs, replicates are fabricated, and a portion are sacrificed at specific time points throughout the duration of a study. Underlying this method are flawed assumptions: during data collection, every MPS represents a biological replicate, and all MPSs in an experimental group have negligible variability to make statistically significant comparisons. These methods and assumptions are often not amenable to scale-up or high-power evaluation, as variability is often compounded during downstream analysis.
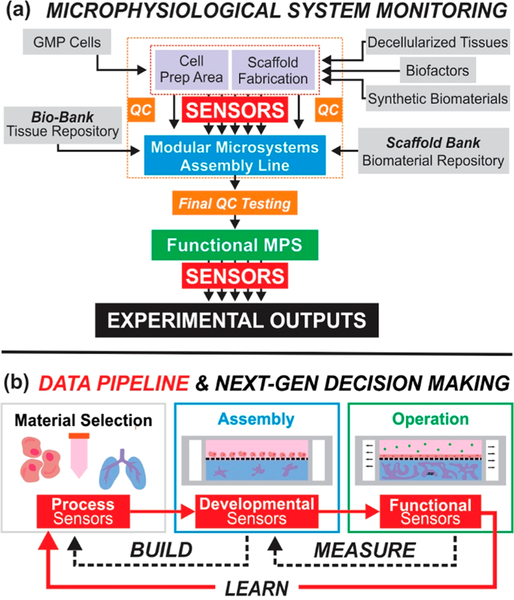
An idealized model of the MPS manufacturing and operating pipeline is summarized in Figure 1a. This figure illustrates the proposed, central role of nondestructive, in-line sensors. Viewed collectively, the ideal MPS pipeline will contain inline sensors that continuously assess development and function without the need for off-line assays or batch testing. These sensors will be integrated into an “on-chip” network for monitoring intraconstruct environmental parameters such as oxygen, pH, fluid perfusion, electrophysiology, and metabolite concentration. Furthermore, on-chip sensor data would be fused with label-free methods for assessing tissue morphology, quality, and function, such as optical microscopy, microcomputed tomography (μ-CT), ultrasound, and photoacoustic imaging. Such automated, self-contained platforms for monitoring MPSs will provide both scalability and autonomous decision-making potential. The fusion of process, development, and function monitoring via advanced data harnessing and artificial intelligence are necessary to “close-the-loop” and generate a complete cyber-physical system.
Figure 1.
(a) Schematic illustrating the significance and central role of sensors in next-generation MPSs. (b) For improved translational potential and impact, data fusion and feedback must be continuous between all steps in the life of an MPS, from manufacture to operation.
Although one may argue if he or she is provided with the material or supply purchased, then the metadata is unnecessary, an ideal manufacturing setting requires synergistic data sharing between all steps of the MPS manufacturing process. For example, a research MPS workflow including (1) a biological resource company manufactures biologics, validated with necessary process sensors, and sends them to (2) the research scientist developing and assembling the MPS. The research scientist introduces the raw materials to the device, monitors via developmental sensors to ensure proper maturation, and then (3) runs experimental protocols with incorporated functional sensors or sends to a collaborator to test developed technologies. This workflow ideally would generate large data sets from all steps of MPS fabrication and operation, although this data is rarely communicated back to adjust preceding protocols or materials. Lack of upstream data movement results in the absence of beneficial feedback loops in all areas of MPS development and function, therefore limiting MPS reproducibility while increasing variability.
With this vision in mind, the primary technological questions of this perspective are as follows: “What controllable, extrinsic parameters can noninvasively measure MPS manufacturing and operation? What developmental and functional sensors currently exist, and which novel sensors will achieve continuous monitoring that will lead to next-generation MPSs?”
TAXONOMY OF SENSING FOR MICROPHYSIOLOGICAL SYSTEMS
The central pivot in developing such cyber-physical systems is the integration of sensors (see Figure 1b) (i.e., noninvasive, nondestructive, and connected sensors) that will collect phenotypic and genotypic data in both cell cultures and engineered tissues. To achieve comprehensive monitoring of MPSs, technologies must be exploited that span the gamut of sensing modalities, including thermal, acoustic, optical, chemical, and electrical sensors. For a comprehensive overview of sensing techniques, we refer you to the following reviews.14–16 The target analytes for a comprehensive monitoring system for MPSs span the physiochemical gamut, from small molecules and proteins to microanatomical imaging and force mapping.17–19 Therefore, identifying and characterizing types of sensors for monitoring MPSs requires classifying the stages of MPS manufacturing.
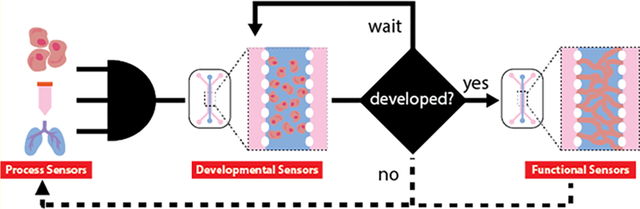
A recommended sensing taxonomy for MPSs is presented in Table 1. Sensors, which analyze the entire MPS from manufacture through operation, can be categorized into (1) process, (2) development, and (3) function monitors. We suggest the use of the term “monitors”, instead of sensors, as the physical embodiment of a sensor may be used to gather quite different information at stages in the lifespan of an MPS. Process monitors include all sensors employed to evaluate the “raw materials” of the MPS, such as cells, culture media, scaffolds, and growth factors. Development monitors quantify the growth and maturity of the integral cell population from a milieu of heterogeneous cells and biomaterials inside the MPS. Lastly, function monitors record physiological performance to ensure proper operation or identify and record changes—pathological, experimentally induced, or otherwise. MPS characterization can be classified based on position of the sensors in the operation pipeline (i.e., in-line, at-line, on-line, or off-line). In-line and on-line analysis provide data collection in real-time; in-line sensors are engineered directly into the MPS, whereas on-line sensors analyze automatically collected samples from the MPS. In-line and on-line sensors are integrated sensors. At-line and off-line analysis are not realtime and require manual sample collection, which is tested on-site or at an off-site laboratory, respectively. Of these characterization modalities, in-line and on-line sensors provide a means to initiate feedback control of MPS operation, whereas information generated from at-line or off-line sensors can only be integrated to pre and postoperational analyses. Although the in-line characterization of cell and biomaterial products for MPSs is actively being explored, robust design criteria and nondestructive monitoring of the development and function have not yet been fully realized.20–24
Table 1.
Taxonomy of Sensors for Monitoring Microphysiological Systems (MPSs)
| target | sensor | sampling rate | operating time | physiological relevance | ref. | |
|---|---|---|---|---|---|---|
| development | cellular organization | TEER | daily | 62 days | epithelial barrier formation, function | 56 |
| cellular organization | TEER | daily | 10 days | barrier integrity | 58 | |
| contractile stress | strain gauge | seconds | 28 days | cardiomyocyte maturation | 38 | |
| DNA | impedance | 10 min | 2 days | cell mitotic division | 95 | |
| glycerol | phosphorescence | 90 s | 2+ hrs | adipocyte secretion | 43 | |
| oxygen | optical | 440 ns | 5 h | culture environment | 96 | |
| oxygen | phosphorescence | 2s | 24 h | culture environment | 97 | |
| oxygen, pH, cell adhesion | electrochemical | 20 min | 11 days | control of culture conditions; cell proliferation | 94 | |
| scaffold stiffness | strain gauge | daily | 20 days | matrix remodeling; tissue maturation | 68 | |
| strain | Wheatstone bridge | daily | 20 days | endothelial barrier forming to create perfusable lumen | 68 | |
| function | acidic metabolites | electrochemical | seconds | 400 s | cellular metabolism; dose-response | 17 |
| albumin, GSTα, CK-MB | electrochemical | seconds | 120 days | nephrotoxicity; cardiotoxicity | 88 | |
| albumin, GSTα | electrochemical | 4 h | 7 days | metabolite monitoring | 98 | |
| cell growth | impedance | 2s | 24 h | cardiotoxicity; drug efficacy | 99 | |
| cell migration | impedance | real-time | 200 min | cancer cell migration; metastasis | 100 | |
| cell viability | impedance | 6 h | 2 days | chemosensitivity | 55 | |
| differentiation | impedance | 180 s | 17 days | stem cell differentiation | 101 | |
| γ-glutamyl transpeptidase | smartphone-based fluorescence microscopy | n/a | drug-induced nephrotoxicity | 102 | ||
| glucose, lactate, oxygen | optical | 1h | 24 h | glucose metabolism; mitochondrial function/dysfunction | 45 | |
| glucose, lactate, oxygen | optical, electrochemical | real-time | 21 days | metabolite monitoring | 103 | |
| glucose, lactate | electrochemical | real-time | 16 h | cellular metabolism; toxicity | 104 | |
| HGF, TGF-β1 | optical | daily | 7 days | cell secreting growth factors | 75 | |
| lactate, oxygen | electrochemical | 10 min | 3 days | metabolite monitoring | 105 | |
| TGF-β | electrochemical | near realtime | 10+ hrs | cellular response to alcohol injury | 86 |
PROCESS MONITORING
Comprehensive characterization of all biological and synthetic materials introduced to an MPS is vital to ensure the system operates as designed (i.e., a basic tenet of quality control demands that all material inputs should be defined such that the product outputs are both predictable and within specification). For MPSs, well-defined inputs provide support to realize more physiologically relevant outputs. Defining material inputs is often challenging when considering cell-based and bioderived materials. Variability in raw biomaterials arises in both sourcing and processing. As a system comparable to biomanufacturing, process and quality control would be beneficial for ensuring reproducibility across other bio-products. Passing data collected during the upstream production of cells or biomaterials directly to the MPS developer can ensure consistent biological materials (Figure 1b). To date, the role of cell and material validation falls on the MPS developer; this is an untenable model for the future scale-up of MPS fabrication and use. Manufacturer characterization of cells and raw biomaterials assures MPS developers receive the starting materials, thereby standardizing initial MPS conditions and decreasing MPS variability. When the material validation is the responsibility of the MPS developers, raw materials may be well-defined but not standardized across MPS development sites. Furthermore, standard equipment used for materials characterization, such as high-performance chromatography, requires both expertise and equipment and may not be readily available to MPS developers.
Process sensors have been utilized in large-scale biomanufacturing for centuries, often to streamline production of biologics, such as in the food, cosmetic, and pharmaceutical industries.25–29 Biological components, such as hydrogels, media, and cells, are tested stringently before they are sent to MPS research laboratories.30–34 For cell culture, media components are manufactured with high yield and low variability to reduce researchers’ concerns of confounding variables during experimentation. Although process sensing is highly optimized to guarantee low variability in biological materials, the data gap between manufacturers and researchers may limit MPS functionality. Researchers are provided simplistic validation data sheets ensuring cell types are as advertised and media or scaffold material is per specifications, but quantitative processing data beyond general ranges are seldom provided. Furthermore, many materials that come from biological sources are poorly defined because of variability in source and overall sample complexity (i.e., too many growth factors or proteins to reasonably define). For example, batch-to-batch variability in tumor-derived cellular scaffolds, such as Matrigel, is a reoccurring hurdle in tissue engineering.35 As biological processing techniques improve, well-characterized materials should become standard in the fields of regenerative medicine and, therefore, in MPSs.
Characterization issues that arise in biomaterial inputs are exponentially more detrimental when considering cell line, primary human cell, and stem cell inputs. As cell sources have become more commercialized, there is extensive quality control in production, but quality assurance concerns remain. Cells are sold with minimal validation, and there are often doubts that the cells are as identified. Commonly misidentified cell lines are compiled within a database (http://iclac.org/databases/cross-contaminations). Stem cells would allow all biological materials to come from the same germline, providing seamless interactions, with limited immunological responses, within a multicellular-engineered tissue structure. For application in MPSs, preprocessing facilities could differentiate pluripotent stem cells before introduction to a system. From a biomanufacturing point of view, inventories of pluripotent stem cells and in-house differentiation could allow research laboratories to order multiple cell types from the same germline. Alternatively, pluripotent stem cells could be introduced to an MPS, exposed to the relevant mechanical and chemical stimuli, and differentiation could be monitored on-chip. Current technologies are being developed that would allow in-line and off-line sensing of stem cell differentiation; these sensors will be discussed in the following section.36–38
DEVELOPMENTAL MONITORING
Biosynthetic tissues, composed of cells and hydrogels, which are inherently variable, are difficult to create in isolation and even more difficult to create in replicate; thus, developmental monitoring ensures that an MPS organ is consistently achieved. During MPS fabrication, there are many variables that will affect the ultimate output, including but not limited to media formulations and cell secretions, geometry, and function of incorporated structures (i.e., 3D scaffold macro and micro-geometry and chemistry), and cell phenotype, genotype, and epigenetic lineage. Sensing modalities include biochemical sensing, fluorescent tagging and subsequent imaging, impedance monitoring, and mechanical testing. Many of these sensors have been incorporated into biological assays and cell cultures, but the future for developmental monitoring is vast when considering integration into MPSs. Multiplexed sensing, recording, and processing of real-time MPS data could provide novel insights into the optimal nutrients and culture conditions needed to maintain cell viability and growth for weeks. Beyond optimization, we can use data analytics in real-time to respond to changes in culture conditions, adjusting inputs to obtain desired results. For example, media composition can be tracked as a stem cell differentiates to determine how differentiation is progressing and allow growth factors to be removed or added to encourage further differentiation or even quiescence.
Furthermore, by creating a system with all integrated sensing and pumping mechanisms needed to function, a device typically limited for laboratory use may have off-the-shelf applications. This could range from cell culture identification, stem cell differentiation, or even full tissue and MPS functional monitoring. Herein, we describe specific classes of sensors that need to be integrated into a viable MPS. Achievement of development monitoring could improve throughput limitations that restrict translational potential of MPSs, as a major problem with MPSs is the lack of scalability.14,15
Measurement of Nutrients and Metabolites.
Quantifying concentrations of media components and metabolites is key to define how an MPS is operating, ensuring a cell population is receiving proper nutrients to operate. Aseptic sampling techniques are available that allow at-line monitoring of such molecules with high-performance liquid chromatography (HPLC), though HPLC requires separate sample preparation steps and does not offer real-time data.39 Integrated biosensors show potential for providing real-time information regarding media composition.40,41 Furthermore, biosensors currently being explored provide in-line, real-time quantification of oxygen levels in an MPS, offering information on the MPS environment as well as cell proliferation and death.42 At-line monitoring systems can also be used to investigate cellular secretions; for example, Clark et al. demonstrated the use of an on-chip analysis to look at glycerol secretion from adipocytes.43 An enzyme assay kit was incorporated in the MPS, which was able to sample supernatant media via capillary connection and mix with agents from a commercial enzyme assay for glycerol. With only a 5 min mixing and incubation time, glycerol concentration could be determined via fluorescent microscopy. Other sensing mechanisms are used to monitor nutrients and metabolites in MPMs, though these mechanisms are often limited by lifetime. As shown in Table 1, electrochemical sensing modalities offer the chance to sample more often, as compared to electrical or optical measurements, but often with shorter total run times for experiments. For example, Misun et al. developed a lactose and glucose enzyme-based electrochemical sensor for monitoring hanging-drop culture with extreme sensitivity, although only for 1 day.44 Sensing lifetimes can be extended by designing chambers with interchangeable sensors or sampling small volumes of liquids at single time points, though these methods may not be realistic to achieve continuous monitoring.45,46
Discussed in more detail concerning function sensors, these types of sensing techniques could offer valuable real-time information on cell health based on concentrations of gaseous species to understand what is happening within the MPS. Ideally, we would like to input known media, gas, and temperature into our system while reading resultant oxidative species, nutrients metabolized, and temperature fluctuations to gauge not only the reliability of the MPS setup but also cellular health and function.
Measurement of Proteins and Growth Factors.
Along with sampling nutrients, monitoring MPS development includes sampling media for in-line or at-line measurement of introduced or secreted proteins and growth factors. Standard measurement of proteins or nucleic acids, such as enzyme-linked immunosorbent assays (ELISA), nucleic acid amplification (NAA), and lateral flow assays (LFA), require intensive sample preparation and back-end analysis while only providing information at a single time point. Furthermore, without specialized equipment, these analyses must be sent to collaborating laboratories or companies for off-line monitoring, eliminating instantaneous feedback control. Many at-line microfluidic measurements of cell products exist, though these technologies are more commonly used in point-of-care diagnostics.47–49 To use these techniques for MPS sensing, perfused culture media can be sampled and analyzed for cell secretomes. Riahi et al. designed an automated microfluidic electrochemical immunosensor for on-line testing of media perfused through a microfluidic bioreactor.36 Magnetic beads were functionalized with an antibody to test the liver biomarker transferrin secreted by hepatocytes. Media was sampled from the bioreactor via connected tubing, and automated valves were used to move the sample through the reaction chamber into the detection chamber. Electrochemical immunosensing was performed with an electrode setup to measure a current differential dependent on the concentration of transferrin. This platform could be slightly modified with different antibodies to detect various biomarkers. Furthermore, incorporating parallel microchannels would allow the monitoring of a variety of cell secretomes for multiplexed detection of media composition.
Electrical Measurements.
In-line impedimetric methods can monitor cell proliferation and barrier selectivity, similar to technologies available for static well-plate cultures.50,51 To quantify proliferation, a low-frequency current is driven across two electrodes, and the measured voltage is used to determine impedance within the system.52–54 Higher impedances correlate with a higher number of cells or full coverage of closely oriented cells within the well. Lei et al. demonstrated similar technology in an MPS of human oral cancer within an agarose scaffold.55 Higher cell-seeding densities resulted in higher impedance measurements, and impedance decreased with increasing cancer drug concentration and incubation time.
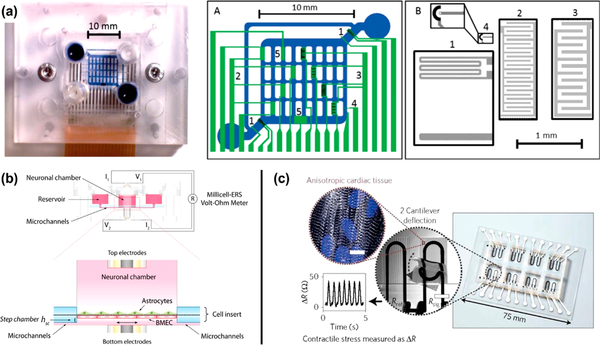
As an alternative, similar technology is used to measure the impedance across an MPS barrier, resulting in measurements that can be directly compared to those of in vivo systems. Electrode-based sensing mechanisms have facilitated in-line measurement of epithelial MPS barrier permeability with physiologically relevant air–liquid or liquid–liquid interfaces as well as flow rates.56 To assess barrier permeability in static cell culture systems, measurements of transepithelial electrical resistance (TEER) are performed on monolayers of epithelial cells cultured on permeable polycarbonate membranes inside Transwell systems.57 TEER is measured across the epithelial monolayer, driving a low-frequency square wave across the barrier and reading the resultant voltage to determine the system resistance. When the barrier is tightened, as cells are connected via tight junctions, the TEER goes up. Low TEER values mean that the barrier is compromised or not fully formed. When the TEER values indicate that an MPS is underdeveloped, supplemental factors can be introduced to induce proliferation, or the barrier can simply be given more time to develop. Specifically, such techniques have been used in an lung and intestinal MPS over months, validating cell proliferation and barrier formation over time. Similar technology is also used to monitor vasculature tissue MPSs, referred to as transendothelial electrical resistance. Wang et al. built an MPS of the blood–brain barrier (BBB) to study endothelial barrier permeability (Figure 2b).58 Pellet-based silver electrodes inserted inside the MPS measured TEER with and without the treatment of the drug doxorubicin, indicating barrier damage and disruption. TEER measurements were coupled with fluorescence imaging to calculate values of endothelial barrier permeability. At the state-of-the-art currently, it is necessary to pair electrical measurements with fluorescent imaging, such as with fluorescein-tagged dextran molecules with varying molecular weights, to confirm conclusions made by TEER. Unfortunately, low-frequency TEER is limited to resistive values, only providing information on gaps in cell growth caused by antagonist-induced cell death. To learn more dynamic characteristics of cell cultures in an MPS, impedances at higher frequencies can be obtained to determine capacitive characteristics of the system.59 Therefore, electrical measurements can be used to identify specific biological phenomena instead of relying only on overall cell viability.
Figure 2.
Microphysiological systems with developmental sensors. (a) On-chip electrothermal micropumps (1), interdigitated electrodes (2, 3), oxygen sensors (4), and pH sensors (5) are all integrated into a single MPS to observe cell proliferation and viability. Reprinted with permission from Bonk 2015.94 Copyright 2015 MDPI AG. (b) A blood–brain barrier MPS includes neural and endothelial cell growth chambers with applied shear as well as embedded electrodes connected to a volt–ohm meter to measure transendothelial electrical resistance as a metric of BBB development. Reprinted with permission from Wang 2017.58 Copyright 2016 Wiley Periodicals, Inc. (c) 3D printed strain sensors are integrated into an MPS, using cantilever deflection to determine cardiomyocyte self-assembly in engineered tissue. Reprinted with permission from Lind 2017.38 Copyright 2017 Macmillan Publishers Limited.
On-Chip Microscopy.
Optical techniques characterize biological phenomena, typically via immunofluorometry and fluorescence microscopy. Standard microscopes are composed of a single optical objective, limiting users to a single field of view at a time. Automated mechanical stages are often integrated with microscopes to collect images from different fields of view and fluorescent wavelengths.64 With many pictures, machine learning can be used to automate data analysis, identifying morphological features of cell populations to determine biological results without user bias. Matsuoka et al. has developed this technology to track mesenchymal stem cell differentiation in a dish.60 Although microscopy partnered with computational machine learning allows for real-time optical sensing of parallel devices while eliminating the user interface, such set ups are large, stationary, and expensive.
For image-based quantification to be practical as a sensing technique for MPSs, microscopes must be integrated into the chip design. This on-chip microscopy offers the possibility of a fully integrated, ready-to-use MPS, expanding on technology that is typically limited to a laboratory setting. Some groups have made headway in this area.61,62 An on-chip microscope was developed by Zhang et al. by simply repurposing a webcam CMOS chip and lens to create a fully integrated “mini-microscope”.62 Similar CMOS-based technology has been used to integrate contact fluorescence microscopy and microfluidic chips containing cell culture on thin-film glass, though resolution is limited for most microscopes incorporated on MPSs.63 Higher-resolution objectives are possible from a technical standpoint, but even with such a high data density, images are commonly only presented as representative, failing to provide quality quantitative data. Furthermore, mechanical automation of on-chip microscopes has never been demonstrated, limiting high-resolution objectives to a single field of view and wavelength. On-chip microscopes must also be adaptable to culture conditions and incorporate mechanisms to collect images in different focal planes for 3D culture. Adapting hardware for incubator conditions and including mechanical components may not be a cost-effective solution for collecting real-time data on MPSs. Sensor integration for parallel monitoring has a much lower data density, but the information obtained can be maximized with design or postprocessing techniques.
Supplemental Sensing.
MPSs incorporate microfluidic controls, especially in MPSs that seek to recapitulate microvasculature. Angiogenesis has been demonstrated in a myriad of MPSs, though quantification is limited to immunohistochemistry in fixed tissue structures or fluorescently labeled molecule tracking through formed lumens and endothelial barriers.65,66 Temiz et al. created an on-line flow monitor for very low flow rates that has a sub-nanoliter dead volume.67 As the interest in connected, multiorgan MPSs arise, there will be more demand to maintain stable perfusion throughout the system using on-line flow monitors. For example, Liu et al. introduced a method for sensing pressure within a microfluidic device with embedded electrofluidic pressure sensors.17 Wheatstone bridge resistor configurations are arranged within the sensing portion of the device to determine hydrostatic pressure at different locations because of deformed PDMS. Measured values were compared to predicted values, and flow rates were adjusted to achieve desired pressure. This system provides a well-controlled mechanical environment for shear sensitive cell populations and has possible applications in determining pressure differentials in more complex tissue MPSs.
In addition to flow sensing, the mechanics of incorporated tissue scaffolds influence cellular proliferation and architecture as MPSs develop. Cells respond to matrix mechanics, reorganizing and degrading hydrogel scaffolds while synthesizing and secreting native collagen. Scaffold reorganization is difficult to measure without sacrificing the MPS, though strain sensors have been integrated for real-time quantification of scaffold properties. Recently, Liu et al. integrated carbon nanotube (CNT) strain sensors into a skin MPS to measure hydrogel stiffness continually over time.68 Using this platform, it was found that degradable hydrogels with embedded human mesenchymal stromal cells (MSCs) softened over time when statically cultured, whereas hydrogels containing MSCs cultured under cyclic tensile stimulation for 15 days initially softened but recovered stiffness over time. The results, along with end point histological analysis, revealed that the MSCs produced more collagen when under cyclic stimulation. Strain sensors have also been used to detect contractile stresses of developing cardiac tissues (Figure 2c).38 The contractile development of human induced plupotent stem cells (hiPS)-cardiomyocytes was measured via continuous electronic readout from a cardiac MPS. The longitudinal contractile stress was measured daily to reveal a gradual increase, while the spontaneous beating rate decreased, supporting increased cardiomyocyte maturity. Twitch stress also increased with time to show the structural development of the laminar cardiac tissue with increasing sarcomere packing density, sarcomere length, sarcomerogenesis, and myofibrillogenesis. The integration of soft strain gauges into the cardiac MPS allowed for streamlined data acquisition and knowledge concerning functionality to be realized in real-time. Another common strategy for monitoring the mechanics of cardiac tissue is by culturing tissue between micropillars. Engineered tissue is cultured between or on top of flexible micropillars, and contractile forces are measured by determining pillar displacement.69–71 Although this displacement is most commonly determined by optically tracking micropillars, electrodes can be integrated into micropillars to track beating rate as well as pace cardiac tissue.72 Metrics of cardiac development are variable in both amplitude and frequency; therefore, real-time assessment and optimization of development require a continuous data stream for adaptive feedback.
FUNCTIONAL MONITORING
Although it may take many days, if not multiple weeks, to complete the fabrication of an MPS, the experimental run for a single MPS may be less than 10 h (Table 1). Once an MPS has been fabricated, and the desired cell structures or 3D tissue constructs have been formed, developmental sensors take on a new role—monitoring the function of the MPS. For example, it has been posited that an ideal MPS for testing toxicology or pharmacodynamics/pharmacokinetics would include a network of connected organ systems that “monitors epithelial and endothelial barrier functions, exposure to environmental and infectious agents, and absorption and metabolism of drugs in real-time and at high-resolution inflammatory responses.”73 Functional sensors can be used to suggest therapeutic windows in injury models, such as drug toxicity and efficacy, streamline discovery of key cellular players in disease progression, and even elucidate fundamental biological pathways. To date however, there have been few examples of such a comprehensive MPS. Below we describe the few reported examples.
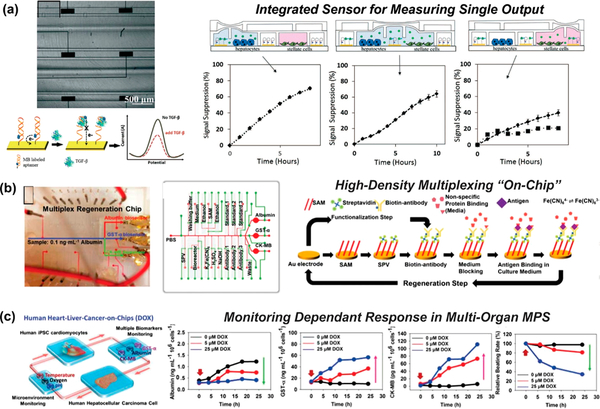
Cell secretomes, or protein secretions by cells into the extracellular space, are important functional readouts. Researchers have integrated biosensors into MPSs to monitor changes in cell secretomes and other cell byproducts.19,36,45,46,74,75 Because the liver has been a popular MPS to build for nearly two decades76–85 and was the first MPS commercialized for drug development studies (LiverChip, CN Bio Innovations), it is not surprising that many examples of functional sensors integrated into MPSs are for the liver. Zhou et al. fabricated a device with integrated biochemical sensors for detecting liver cell signaling from both hepatocytes and stellate cells following alcohol injury (Figure 3a).86 They fabricated a multicompartmented device that allows for initial separation of different liver cell types and sensing chambers. Each sensing chamber has patterned gold electrodes and immobilized aptamers for electrochemical sensing of secreted cytokine transforming growth factor-beta (TGF-β). By manipulating the exposure of certain chambers to each other at different times, they detected changes in TGF-β concentrations because of different cell–cell interactions after injury via ethanol. Because TGF-β1 concentrations were significantly higher in the MPS, as compared to conditioned media and transwell experiments, the MPS showed that the close proximity of two native liver cells in culture promoted paracrine crosstalk and potentially activated the epithelial-to mesenchymal transition (EMT) of hepatocytes. By integrating the functional sensors for TGF-β, they measured electrical changes (current) over time for 24 h. Although the results are informative, aptasensor saturation remains a limiting factor in monitoring secretion rates for longer experiments. Further developing the aptasensors to be regenerative on the liver MPS would alleviate this limitation. Recently, the commercialized liver MPS, LiverChip, has been used to identify secreted inflammatory cytokines that promote cancer growth and proliferation.87 By coculturing hepatic stellate cells with breast cancer cells and comparing this to the LiverChip stellate cell secretome, key cytokines, interleukin 8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1), were identified to promote metastatic outgrowth in otherwise dormant breast cancer tissue.
Figure 3.
Microphysiological systems with integrated functional sensors. (a) (left) Bright field image showing gold electrodes (black rectangles) integrated in a liver injury MPS to monitor transforming growth factor (TGF)-β and the principle of TGF-β detection. (right) Monitoring TGF-β release in an MPS while (1) hepatocytes were exposed to culture media containing alcohol, (2) intercellular communication occurred between injured hepatocytes and stellate cells, and (3) stellate cells were sequestered from hepatocytes. Squares curve (□) represents control experiment where neutralizing antibody was used at the hepatocyte–stellate cell communication stage. Reprinted with permission from Zhou 2015.86 Copyright 2015 The Royal Society of Chemistry. (b) (left) Photograph of automated multiplexed regeneration microfluidic chip. (middle) Schematic showing the design of the multiplexed microfluidic chip for precisely timed injections of the chemicals for electrochemical detection. (right) Schematic diagram showing the functionalization and regeneration process for measuring soluble antigens. Reprinted with permission from Zhang 2017.88 Copyright 2017 National Academy of Sciences. (c) (left) Schematic diagram of biomimetic human heart-liver-cancer-on-chips. (right) Graphs of in-line automated electrochemical measurements of albumin and GST-α secreted from the liver cancer organoids, electrochemical measurements of creatine kinase MB (CK-MB) from the cardiac organoids, and beating analysis of the cardiac organoids. Reprinted with permission from Zhang 2017.88 Copyright 2017 National Academy of Sciences.
Ambitious connections between MPSs of different organ systems have been realized that integrate multiple developmental and functional sensors. One such multisensor-integrated device was previously discussed, and it includes a modular system capable of perfusing a liver MPS and a heart MPS while detecting a biomarker along with physical and chemical parameters (Figure 3b,c).88 Zhang et al. use in-line microfluidic sensors to achieve automated monitoring. The first sensor is an immunosensor that is based on antibody, or aptamer, immobilization on gold electrodes via streptavidin/biotin bonding. Multiple biomarkers can be detected, including albumin, glutathione S-transferase α, and creatine kinase MB (CK-MB), and multiplexed on the same sensor. A second set of developmental sensors detected pH changes, via optical measurements, oxygen, via fluorescence of ruthenium dye, and temperature, via a thermistor probe. Mini-microscopes were added under the two connected MPSs to monitor cell behavior via real-time imaging, as previously described. The combined liver-chip and heart-chip modular system was challenged with the addition of the drug acetaminophen. Results revealed stable pH, oxygen, and temperature throughout the experiment and detection of significant differences in secreted biomarkers at different doses of the drug as well as cell morphological and viability changes. Zhang et al. demonstrate the value of integrated sensors, as a two-organ drug toxicity study was performed in real-time, without sacrificing either MPS. Although their system used PDMS, which can have problems with absorbing molecules, it is an incredible step forward in the space of instrumented MPSs.
These findings using data provided by sensors on an MPS show that complex cell signaling can be investigated on ex vivo platforms. Whether an injury, such as from alcohol, promotes upregulation of protein production or a drug induces toxicity across multiple organs, functional measurements can reveal important human responses. Often, MPSs are designed to provide information concerning how two organs interact or affect one another; furthermore, as disease research focuses more on the mechanisms of action between and within cells, functional MPS sensors can provide significant insight into specific pathways that promote or mitigate disease progression.
Unfortunately, much of the sensing techniques required to provide a multiplexed output beyond pH and oxygen require single use antibody-based sensors. In some applications, this is reasonable, as an array of sensors can provide data at multiple time points, still maintaining at-line operation though with no real-time output. To expand the use of this technology and the possible data output that can be obtained through multiplexing, an extended lifetime or regeneration of biological recognition interfaces would be necessary.89
FUTURE MONITORING: MULTIPLEXED SENSING, LEARNING, AND DECISION-MAKING
Challenges facing MPS research are myriad. Creating sufficient biological complexity while maintaining control to gain valuable insights is paramount, but reproducibility is a major hurdle. To predict systemic response, an MPS must contain multiple, connected engineered organs, but synergistic interactions between engineered organs cannot be elucidated without real-time recording and analyses. Continuous, noninvasive sensing of development and function is the vital next step to prepare MPSs for adoption across research laboratories and translation to clinical applications.90
In 2014, John Wikswo posed the question, “how does one diagnose the “health” of an individual organ construct or an entire microphysiological system?”90 This is the current crux of validating and using any MPS. MPS post facto measurements are detrimental, because they are used to draw broader conclusions about an evolving system. This low data density is a major issue and limits the potential for next-generation analysis and decision-making. Including more sensors or multiplexing sensors will increase resolution and allow for continuous monitoring. Sparse data can also be improved by including many points of measurement that are then fused via algorithmic methodologies to resolve multiform problems that arise in complex biological systems.
We believe that the answer will be enabled by the fusion of sensors systems and data science technologies (i.e., cyber-physical systems). Cyber-physical techniques and technologies will enable (1) better understanding and predictive labels for identifying cell viability, performance, and function to be used in engineered tissues and (2) the development of an automated methodology to identify and confirm the optimal conditions for manufacturing, which then subsequently responds with knowledge-based selection of cells and supporting cells to optimize the function and reduce variability of engineered tissues. Once powerful data sets are accumulated from nondestructive and destructive analyses, next-generation learning and pattern-recognition analytics can be deployed to close-the-loop between selection of raw materials, engineering of human tissue, and optimized function of MPSs, and such capability will directly translate into a means for studying complex physiologic and pathophysiologic phenomena.
For any such cyber-physical system to be realized, we will have to overcome multiple barriers across hardware and software platforms. Integrated sensors will need to store extended data sets across developmental and functional stages of MPS fabrication. Electrochemical sensors capable of longer lifetimes and optical sensors with higher resolution and sensitivity could help close the gap in integrated MPS sensor technologies. Multiplexed sensing schemes with diverse data are paramount, though data fusion is a roadblock in realizing autonomous decision-making for MPSs. Comparing and integrating multiple data sources from heterogeneous conditions, with varying spatial and temporal resolutions, requires complex analytical tools. Even further, acquiring and labeling input data to create the ground truth for autonomous decision-making systems is a tedious and user intensive task that is subject to bias. As MPS sensing techniques become commonplace and many laboratories output data sets, the required ground truth data may be available to develop and support decision-making systems. Machine learning for automation of single-sensor measurements or identification of key candidates in a drug screen have been implemented, though use in a multiplexed MPS has yet to be realized.91,92 This technology has enormous potential in both single-organ systems and the emerging efforts toward body-on-a-chip and is key in achieving technology transfer from the research laboratory to clinical settings and personalized medicine.93
ACKNOWLEDGMENTS
This work was done in collaboration with the National Science Foundation (EEC1160483) through the NSF Nanosystems Engineering Research Center (NERC) for Advanced Self-Powered Systems of Integrated Sensors and Technologies (ASSIST). A.T.Y. is supported through the NIH Integrative Vascular Biology Traineeship (NIH T32HL069768). K.R.R. is supported by the National Institutes of Health under award number F31DK118859-02.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Abaci HE; Shuler ML Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr Biol-Uk 2015, 7 (4), 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Takebe T; Zhang BY; Radisic M Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell 2017, 21 (3), 297–300. [DOI] [PubMed] [Google Scholar]
- (3).Skardal A; Murphy SV; Devarasetty M; Mead I; Kang HW; Seol YJ; Shrike Zhang Y; Shin SR; Zhao L; Aleman J; Hall AR; Shupe TD; Kleensang A; Dokmeci MR; Jin Lee S; Jackson JD; Yoo JJ; Hartung T; Khademhosseini A; Soker S; Bishop CE; Atala A Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep 2017, 7, 8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zhang BY; Radisic M Organ-on-a-chip devices advance to market. Lab Chip 2017, 17 (14), 2395–2420. [DOI] [PubMed] [Google Scholar]
- (5).Cho S; Yoon JY Organ-on-a-chip for assessing environmental toxicants. Curr. Opin. Biotechnol 2017, 45, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zheng FY; Fu FF; Cheng Y; Wang CY; Zhao YJ; Gu ZZ Organ-on-a-Chip Systems: Microengineering to Biomimic Living Systems. Small 2016, 12 (17), 2253–2282. [DOI] [PubMed] [Google Scholar]
- (7).Lee LP Microphysiological Analytic Platforms (MAPs): Precision Organs on Chip. Adv. Healthcare Mater. 2018, 7 (2), 1701488. [DOI] [PubMed] [Google Scholar]
- (8).Geraili A; Jafari P; Hassani MS; Araghi BH; Mohammadi MH; Ghafari AM; Tamrin SH; Modarres HP; Kolahchi AR; Ahadian S; Sanati-Nezhad A Controlling Differentiation of Stem Cells for Developing Personalized Organ-on-Chip Platforms. Adv. Healthcare Mater. 2018, 7 (2), 1700426. [DOI] [PubMed] [Google Scholar]
- (9).Prantil-Baun R; Novak R; Das D; Somayaji MR; Przekwas A; Ingber DE Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu. Rev. Pharmacol. Toxicol 2018, 58, 37–64. [DOI] [PubMed] [Google Scholar]
- (10).Caballero D; Kaushik S; Correlo VM; Oliveira JM; Reis RL; Kundu SC Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials 2017, 149, 98–115. [DOI] [PubMed] [Google Scholar]
- (11).Biselli E; Agliari E; Barra A; Bertani FR; Gerardino A; De Ninno A; Mencattini A; Di Giuseppe D; Mattei F; Schiavoni G; Lucarini V; Vacchelli E; Kroemer G; Di Natale C; Martinelli E; Businaro L Organs on chip approach: a tool to evaluate cancer-immune cells interactions. Sci. Rep 2017, 7, 12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kodzius R; Schulze F; Gao XH; Schneider MR Organ-on-Chip Technology: Current State and Future Developments. Genes 2017, 8 (10), 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Low LA; Tagle DA Organs-on-chips: Progress, challenges, and future directions. Exp. Biol. Med 2017, 242 (16), 1573–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kilic T; Navaee F; Stradolini F; Renaud P; Carrara S Organs-on-chip monitoring: sensors and other strategies. Micro-physiological Systems 2018, 2, 5. [Google Scholar]
- (15).Zhang B; Korolj A; Lai BFL; Radisic M Advances in organ-on-a-chip engineering. Nature Reviews Materials 2018, 3 (8), 257–278. [Google Scholar]
- (16).Modena MM; Chawla K; Misun PM; Hierlemann A Smart Cell Culture Systems: Integration of Sensors and Actuators into Microphysiological Systems. ACS Chem. Biol 2018, 13 (7), 1767–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hu N; Wu C; Ha D; Wang T; Liu Q; Wang P A novel microphysiometer based on high sensitivity LAPS and microfluidic system for cellular metabolism study and rapid drug screening. Biosens. Bioelectron 2013, 40 (1), 167–73. [DOI] [PubMed] [Google Scholar]
- (18).Joeris K; Frerichs JG; Konstantinov K; Scheper T In-situ microscopy: Online process monitoring of mammalian cell cultures. Cytotechnology 2002, 38 (1–3), 129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Shin SR; Zhang YS; Kim DJ; Manbohi A; Avci H; Silvestri A; Aleman J; Hu N; Kilic T; Keung W; Righi M; Assawes P; Alhadrami HA; Li RA; Dokmeci MR; Khademhosseini A Aptamer-Based Microfluidic Electrochemical Biosensor for Monitoring Cell-Secreted Trace Cardiac Biomarkers. Anal. Chem 2016, 88 (20), 10019–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Banaeiyan AA; Theobald J; Paukstyte J; Wolfl S; Adiels CB; Goksor M Design and fabrication of a scalable liver-lobule-ona-chip microphysiological platform. Biofabrication 2017, 9 (1), 015014. [DOI] [PubMed] [Google Scholar]
- (21).Doryab A; Amoabediny G; Salehi-Najafabadi A Advances in pulmonary therapy and drug development: Lung tissue engineering to lung-on-a-chip. Biotechnol. Adv 2016, 34 (5), 588–596. [DOI] [PubMed] [Google Scholar]
- (22).Marsano A; Conficconi C; Lemme M; Occhetta P; Gaudiello E; Votta E; Cerino G; Redaelli A; Rasponi M Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016, 16 (3), 599–610. [DOI] [PubMed] [Google Scholar]
- (23).Ware BR; Khetani SR Engineered Liver Platforms for Different Phases of Drug Development. Trends Biotechnol. 2017, 35(2), 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Xiao S; Coppeta JR; Rogers HB; Isenberg BC; Zhu J; Olalekan SA; McKinnon KE; Dokic D; Rashedi AS; Haisenleder DJ; Malpani SS; Arnold-Murray CA; Chen K; Jiang M; Bai L; Nguyen CT; Zhang J; Laronda MM; Hope TJ; Maniar KP; Pavone ME; Avram MJ; Sefton EC; Getsios S; Burdette JE; Kim JJ; Borenstein JT; Woodruff TK A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun 2017, 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Beutel S; Henkel S In situ sensor techniques in modern bioprocess monitoring. Appl. Microbiol. Biotechnol 2011, 91 (6), 1493–505. [DOI] [PubMed] [Google Scholar]
- (26).Bluma A; Hopfner T; Lindner P; Rehbock C; Beutel S; Riechers D; Hitzmann B; Scheper T In-situ imaging sensors for bioprocess monitoring: state of the art. Anal. Bioanal. Chem 2010, 398 (6), 2429–2438. [DOI] [PubMed] [Google Scholar]
- (27).Biechele P; Busse C; Solle D; Scheper T; Reardon K Sensor systems for bioprocess monitoring. Eng. Life Sci. 2015, 15 (5), 469–488. [Google Scholar]
- (28).Scheper T; Hitzmann B; Stark E; Ulber R; Faurie R; Sosnitza P; Reardon KF Bioanalytics: detailed insight into bioprocesses. Anal. Chim. Acta 1999, 400, 121–134. [Google Scholar]
- (29).Zydney AL Perspectives on integrated continuous bioprocessing - opportunities and challenges. Curr. Opin. Chem. Eng 2015, 10, 8–13. [Google Scholar]
- (30).Le H; Vishwanathan N; Jacob NM; Gadgil M; Hu WS Cell line development for biomanufacturing processes: recent advances and an outlook. Biotechnol. Lett 2015, 37 (8), 1553–64. [DOI] [PubMed] [Google Scholar]
- (31).Lewis AM; Abu-Absi NR; Borys MC; Li ZJ The use of ″Omics technology to rationally improve industrial mammalian cell line performance. Biotechnol. Bioeng 2016, 113 (1), 26–38. [DOI] [PubMed] [Google Scholar]
- (32).Sandor M; Rudinger F; Bienert R; Grimm C; Solle D; Scheper T Comparative study of non-invasive monitoring via infrared spectroscopy for mammalian cell cultivations. J. Biotechnol 2013, 168 (4), 636–645. [DOI] [PubMed] [Google Scholar]
- (33).Ozturk SS Equipment for Large-Scale Mammalian Cell Culture. Adv. Biochem. Eng./Biotechnol. 2013, 139, 69–92. [DOI] [PubMed] [Google Scholar]
- (34).Arnold SA; Crowley J; Woods N; Harvey LM; McNeil B In-situ near infrared spectroscopy to monitor key analytes in mammalian cell cultivation. Biotechnol. Bioeng 2003, 84 (1), 13–19. [DOI] [PubMed] [Google Scholar]
- (35).Nguyen EH; Daly WT; Le NNT; Farnoodian M; Belair DG; Schwartz MP; Lebakken CS; Ananiev GE; Saghiri MA; Knudsen TB; Sheibani N; Murphy WL Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed Eng. 2017, 1 (7), 0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Riahi R; Shaegh SA; Ghaderi M; Zhang YS; Shin SR; Aleman J; Massa S; Kim D; Dokmeci MR; Khademhosseini A Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep 2016, 6, 24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Faley SL; Copland M; Wlodkowic D; Kolch W; Seale KT; Wikswo JP; Cooper JM Microfluidic single cell arrays to interrogate signalling dynamics of individual, patient-derived hematopoietic stem cells. Lab Chip 2009, 9 (18), 2659–64. [DOI] [PubMed] [Google Scholar]
- (38).Lind JU; Busbee TA; Valentine AD; Pasqualini FS; Yuan H; Yadid M; Park SJ; Kotikian A; Nesmith AP; Campbell PH; Vlassak JJ; Lewis JA; Parker KK Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater 2017, 16 (3), 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Stoll T; Pugeaud P; von Stockar U; Marison IW A simple HPLC technique for accurate monitoring of mammalian cell metabolism. Cytotechnology 1994, 14 (2), 123–8. [DOI] [PubMed] [Google Scholar]
- (40).Pereira Rodrigues NP; Sakai Y; Fujii T Cell-based microfluidic biochip for the electrochemical real-time monitoring of glucose and oxygen. Sens. Actuators, B 2008, 132 (2), 608–613. [Google Scholar]
- (41).Ge X; Hanson M; Shen H; Kostov Y; Brorson KA; Frey DD; Moreira AR; Rao G Validation of an optical sensor-based high-throughput bioreactor system for mammalian cell culture. J. Biotechnol 2006, 122 (3), 293–306. [DOI] [PubMed] [Google Scholar]
- (42).Rivera KR; Pozdin VA; Young AT; Erb PD; Wisniewski NA; Magness ST; Daniele M Integrated phosphorescence-based photonic biosensor (iPOB) for monitoring oxygen levels in 3D cell culture systems. Biosens. Bioelectron 2019, 123, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Clark AM; Sousa KM; Jennings C; MacDougald OA; Kennedy RT Continuous-flow enzyme assay on a microfluidic chip for monitoring glycerol secretion from cultured adipocytes. Anal. Chem 2009, 81 (6), 2350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Misun PMRJ; Rothe J; Schmid YRF; Hierlemann A; Frey O Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst Nanoeng 2016, 2, 16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Bavli D; Prill S; Ezra E; Levy G; Cohen M; Vinken M; Vanfleteren J; Jaeger M; Nahmias Y Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. U. S. A 2016, 113(16), E2231–E2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Prill S; Jaeger MS; Duschl C Long-term microfluidic glucose and lactate monitoring in hepatic cell culture. Biomicrofluidics 2014, 8 (3), 034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Mirasoli M; Guardigli M; Michelini E; Roda A Recent advancements in chemical luminescence-based lab-on-chip and microfluidic platforms for bioanalysis. J. Pharm. Biomed. Anal 2014, 87, 36–52. [DOI] [PubMed] [Google Scholar]
- (48).Rodriguez NM; Wong WS; Liu LN; Dewar R; Klapperich CM A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip 2016, 16 (4), 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lafleur LK; Bishop JD; Heiniger EK; Gallagher RP; Wheeler MD; Kauffman P; Zhang XH; Kline EC; Buser JR; Kumar S; Byrnes SA; Vermeulen NMJ; Scarr NK; Belousov Y; Mahoney W; Toley BJ; Ladd PD; Lutz BR; Yager P A rapid, instrument-free, sample-to-result nucleic acid amplification test. Lab Chip 2016, 16 (19), 3777–3787. [DOI] [PubMed] [Google Scholar]
- (50).Canali C; Heiskanen A; Muhammad HB; Hoyum P; Pettersen FJ; Hemmingsen M; Wolff A; Dufva M; Martinsen OG; Emneus J Bioimpedance monitoring of 3D cell culturing–complementary electrode configurations for enhanced spatial sensitivity. Biosens. Bioelectron 2015, 63, 72–79. [DOI] [PubMed] [Google Scholar]
- (51).Lei KF; Liu TK; Tsang NM Towards a high throughput impedimetric screening of chemosensitivity of cancer cells suspended in hydrogel and cultured in a paper substrate. Biosens. Bioelectron 2018, 100, 355–360. [DOI] [PubMed] [Google Scholar]
- (52).Giaever I; Keese CR Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. U. S.A. 1984, 81 (12), 3761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Liu Q; Yu J; Xiao L; Tang JC; Zhang Y; Wang P; Yang M Impedance studies of bio-behavior and chemosensitivity of cancer cells by micro-electrode arrays. Biosens. Bioelectron 2009, 24 (5), 1305–10. [DOI] [PubMed] [Google Scholar]
- (54).Hong J; Kandasamy K; Marimuthu M; Choi CS; Kim S Electrical cell-substrate impedance sensing as a non-invasive tool for cancer cell study. Analyst 2011, 136 (2), 237–245. [DOI] [PubMed] [Google Scholar]
- (55).Lei KF; Wu MH; Hsu CW; Chen YD Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens. Bioelectron 2014, 51, 16–21. [DOI] [PubMed] [Google Scholar]
- (56).Henry OYF; Villenave R; Cronce MJ; Leineweber WD; Benz MA; Ingber DE Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 2017, 17 (13), 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Domenech M; Yu H; Warrick J; Badders NM; Meyvantsson I; Alexander CM; Beebe DJ Cellular observations enabled by microculture: paracrine signaling and population demographics. Integr Biol. (Camb) 2009, 1 (3), 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Wang YI; Abaci HE; Shuler ML Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng 2017, 114 (1), 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Benson K; Cramer S; Galla HJ Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS 2013, 10 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Matsuoka F; Takeuchi I; Agata H; Kagami H; Shiono H; Kiyota Y; Honda H; Kato R Morphology-based prediction of osteogenic differentiation potential of human mesenchymal stem cells. PLoS One 2013, 8 (2), No. e55082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Kim SB; Koo KI; Bae H; Dokmeci MR; Hamilton GA; Bahinski A; Kim SM; Ingber DE; Khademhosseini A A mini-microscope for in situ monitoring of cells. Lab Chip 2012, 12(20), 3976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Zhang YS; Ribas J; Nadhman A; Aleman J; Selimovic S; Lesher-Perez SC; Wang T; Manoharan V; Shin SR; Damilano A; Annabi N; Dokmeci MR; Takayama S; Khademhosseini A A cost-effective fluorescence mini-microscope for biomedical applications. Lab Chip 2015, 15 (18), 3661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Takehara HKO; Kazutaka O; Haruta M; Noda T; Sasagawa K; Tokuda T; Ohta J On-chip cell analysis platform: Implementation of contact fluorescence microscopy in microfluidic chips. AIP Adv. 2017, 7 (9), 095213. [Google Scholar]
- (64).Lanz HL; Saleh A; Kramer B; Cairns J; Ng CP; Yu J; Trietsch SJ; Hankemeier T; Joore J; Vulto P; Weinshilboum R; Wang L Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform. BMC Cancer 2017, 17 (1), 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Kim C; Kasuya J; Jeon J; Chung S; Kamm RD A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 2015, 15 (1), 301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Kim S; Kim W; Lim S; Jeon JS Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering 2017, 4 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Temiz Y; Delamarche E Sub-nanoliter, real-time flow monitoring in microfluidic chips using a portable device and smartphone. Sci. Rep 2018, 8 (1), 10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Liu H; MacQueen LA; Usprech JF; Maleki H; Sider KL; Doyle MG; Sun Y; Simmons CA Microdevice arrays with strain sensors for 3D mechanical stimulation and monitoring of engineered tissues. Biomaterials 2018, 172, 30–40. [DOI] [PubMed] [Google Scholar]
- (69).Boudou T; Legant WR; Mu AB; Borochin MA; Thavandiran N; Radisic M; Zandstra PW; Epstein JA; Margulies KB; Chen CS A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Eng., Part A 2012, 18 (9–10), 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Oyunbaatar NE; Lee DH; Patil SJ; Kim ES; Lee DW Biomechanical Characterization of Cardiomyocyte Using PDMS Pillar with Microgrooves. Sensors 2016, 16 (8), 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Kalman B; Monge C; Bigot A; Mouly V; Picart C; Boudou T Engineering human 3D micromuscles with co-culture of fibroblasts and myoblasts. Comput. Method Biomec 2015, 18, 1960–1961. [DOI] [PubMed] [Google Scholar]
- (72).Zhang N; Stauffer F; Simona BR; Zhang F; Zhang ZM; Huang NP; Voros J Multifunctional 3D electrode platform for real-time in situ monitoring and stimulation of cardiac tissues. Biosens. Bioelectron 2018, 112, 149–155. [DOI] [PubMed] [Google Scholar]
- (73).Marx U; Andersson TB; Bahinski A; Beilmann M; Beken S; Cassee FR; Cirit M; Daneshian M; Fitzpatrick S; Frey O; Gaertner C; Giese C; Griffith L; Hartung T; Heringa MB; Hoeng J; de Jong WH; Kojima H; Kuehnl J; Leist M; Luch A; Maschmeyer I; Sakharov D; Sips AJ; Steger-Hartmann T; Tagle DA; Tonevitsky A; Tralau T; Tsyb S; van de Stolpe A; Vandebriel R; Vulto P; Wang J; Wiest J; Rodenburg M; Roth A Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. ALTEX 2016, 33 (3), 272–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Prill S; Bavli D; Levy G; Ezra E; Schmalzlin E; Jaeger MS; Schwarz M; Duschl C; Cohen M; Nahmias Y Real-time monitoring of oxygen uptake in hepatic bioreactor shows CYP450-independent mitochondrial toxicity of acetaminophen and amiodarone. Arch. Toxicol 2016, 90 (5), 1181–1191. [DOI] [PubMed] [Google Scholar]
- (75).Son KJ; Gheibi P; Stybayeva G; Rahimian A; Revzin A Detecting cell-secreted growth factors in microfluidic devices using bead-based biosensors. Microsyst Nanoeng 2017, 3, 17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Allen JW; Bhatia SN Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol. Bioeng 2003, 82 (3), 253–62. [DOI] [PubMed] [Google Scholar]
- (77).Allen JW; Khetani SR; Bhatia SN In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol. Sci 2005, 84 (1), 110–9. [DOI] [PubMed] [Google Scholar]
- (78).Dash A; Inman W; Hoffmaster K; Sevidal S; Kelly J; Obach RS; Griffith LG; Tannenbaum SR Liver tissue engineering in the evaluation of drug safety. Expert Opin. Drug Metab. Toxicol 2009, 5 (10), 1159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Domansky K; Inman W; Serdy J; Dash A; Lim MHM; Griffith LG Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 2010, 10 (1), 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Du Y; Li N; Yang H; Luo CH; Gong YX; Tong CF; Gao YX; Lu SQ; Long M Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip 2017, 17 (5), 782–794. [DOI] [PubMed] [Google Scholar]
- (81).Khazali AS; Clark AM; Wells A A Pathway to Personalizing Therapy for Metastases Using Liver-on-a-Chip Platforms. Stem Cell Rev. 2017, 13 (3), 364–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Lee PJ; Hung PJ; Lee LP An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng 2007, 97 (5), 1340–1346. [DOI] [PubMed] [Google Scholar]
- (83).Tilles AW; Baskaran H; Roy P; Yarmush ML; Toner M Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol. Bioeng 2001, 73 (5), 379–89. [DOI] [PubMed] [Google Scholar]
- (84).Tsamandouras N; Kostrzewski T; Stokes CL; Griffith LG; Hughes DJ; Cirit M Quantitative Assessment of Population Variability in Hepatic Drug Metabolism Using a Perfused Three-Dimensional Human Liver Microphysiological System. J. Pharmacol. Exp. Ther 2017, 360 (1), 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Xiao W; Perry G; Komori K; Sakai Y New physiologically-relevant liver tissue model based on hierarchically cocultured primary rat hepatocytes with liver endothelial cells. Integr Biol. (Camb) 2015, 7(11), 1412–22. [DOI] [PubMed] [Google Scholar]
- (86).Zhou Q; Patel D; Kwa T; Haque A; Matharu Z; Stybayeva G; Gao Y; Diehl AM; Revzin A Liver injury-on-a-chip: microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 2015, 15(23), 4467–78. [DOI] [PubMed] [Google Scholar]
- (87).Khazali AS; Clark AM; Wells A Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. Br. J. Cancer 2018, 118 (4), 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Zhang YS; Aleman J; Shin SR; Kilic T; Kim D; Mousavi Shaegh SA; Massa S; Riahi R; Chae S; Hu N; Avci H; Zhang W; Silvestri A; Sanati Nezhad A; Manbohi A; De Ferrari F; Polini A; Calzone G; Shaikh N; Alerasool P; Budina E; Kang J; Bhise N; Ribas J; Pourmand A; Skardal A; Shupe T; Bishop CE; Dokmeci MR; Atala A; Khademhosseini A Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (12), E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Goode JA; Rushworth JV; Millner PA Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31 (23), 6267–76. [DOI] [PubMed] [Google Scholar]
- (90).Wikswo JP The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. (London, U. K.) 2014, 239 (9), 1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Peel S; Corrigan AM; Ehrhardt B; Jang KJ; Caetano-Pinto P; Boeckeler M; Rubins JE; Kodella K; Petropolis DB; Ronxhi J; Kulkarni G; Foster AJ; Williams D; Hamilton GA; Ewart L Introducing an automated high content confocal imaging approach for Organs-on-Chips. Lab Chip 2019, 19 (3), 410–421. [DOI] [PubMed] [Google Scholar]
- (92).Urban G; Bache KM; Phan D; Sobrino A; Shmakov AK; Hachey SJ; Hughes C; Baldi P Deep Learning for Drug Discovery and Cancer Research: Automated Analysis of Vascularization Images. IEEE/ACM Trans. Comput. Biol. Bioinf 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Riordon J; Sovilj D; Sanner S; Sinton D; Young EWK Deep Learning with Microfluidics for Biotechnology. Trends Biotechnol. 2019, 37 (3), 310–324. [DOI] [PubMed] [Google Scholar]
- (94).Bonk SM; Stubbe M; Buehler SM; Tautorat C; Baumann W; Klinkenberg ED; Gimsa J Design and Character ization of a Sensorized Microfluidic Cell-Culture System with Electro-Thermal Micro-Pumps and Sensors for Cell Adhesion, Oxygen, and pH on a Glass Chip. Biosensors 2015, 5 (3), 513–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Ghenim L; Kaji H; Hoshino Y; Ishibashi T; Haguet V; Gidrol X; Nishizawa M Monitoring impedance changes associated with motility and mitosis of a single cell. Lab Chip 2010, 10 (19), 2546–50. [DOI] [PubMed] [Google Scholar]
- (96).Mehta G; Mehta K; Sud D; Song JW; Bersano-Begey T; Futai N; Heo YS; Mycek MA; Linderman JJ; Takayama S Quantitative measurement and control of oxygen levels in micro-fluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed. Microdevices 2007, 9 (2), 123–134. [DOI] [PubMed] [Google Scholar]
- (97).Rivera KR; Pozdin VA; Young AT; Erb PD; Wisniewski NA; Magness ST; Daniele M Integrated phosphorescence-based photonic biosensor (iPOB) for monitoring oxygen levels in 3D cell culture systems. Biosens. Bioelectron 2019, 123, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Shin SR; Kilic T; Zhang YS; Avci H; Hu N; Kim D; Branco C; Aleman J; Massa S; Silvestri A; Kang J; Desalvo A; Hussaini MA; Chae SK; Polini A; Bhise N; Hussain MA; Lee H; Dokmeci MR; Khademhosseini A Label-Free and Regenerative Electrochemical Microfluidic Biosensors for Continual Monitoring of Cell Secretomes. Adv. Sci. (Weinh) 2017, 4 (5), 1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Zhang X; Wang T; Wang P; Hu N High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip. Micromachines (Basel) 2016, 7 (7), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Nguyen TA; Yin TI; Reyes D; Urban GA Microfluidic chip with integrated electrical cell-impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal. Chem 2013, 85 (22), 11068–76. [DOI] [PubMed] [Google Scholar]
- (101).Bagnaninchi PO; Drummond N Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (16), 6462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Cho S; Islas-Robles A; Nicolini AM; Monks TJ; Yoon JY In situ, dual-mode monitoring of organ-on-a-chip with smartphone-based fluorescence microscope. Biosens. Bioelectron 2016, 86, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Simmons AD; Williams C 3rd; Degoix A; Sikavitsas VI Sensing metabolites for the monitoring of tissue engineered construct cellularity in perfusion bioreactors. Biosens. Bioelectron 2017, 90, 443–449. [DOI] [PubMed] [Google Scholar]
- (104).Pemberton RM; Cox T; Tuffin R; Drago GA; Griffiths J; Pittson R; Johnson G; Xu J; Sage IC; Davies R; Jackson SK; Kenna G; Luxton R; Hart JP Fabrication and evaluation of a micro(bio)sensor array chip for multiple parallel measurements of important cell biomarkers. Sensors 2014, 14 (11), 20519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Weltin A; Hammer S; Noor F; Kaminski Y; Kieninger J; Urban GA Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens. Bioelectron 2017, 87, 941–948. [DOI] [PubMed] [Google Scholar]