SUMMARY
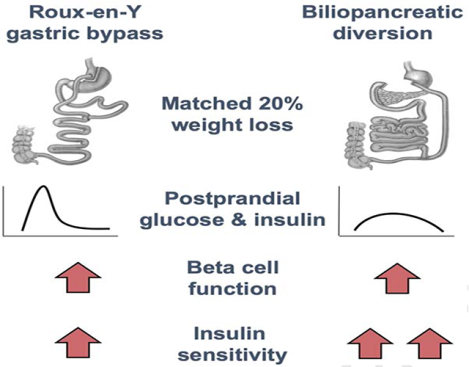
Diabetes remission is greater after biliopancreatic diversion (BPD) than Roux-en-Y gastric bypass (RYGB) surgery. We used a mixed-meal test with ingested and infused glucose tracers and the hyperinsulinemic-euglycemic clamp procedure with glucose tracer infusion to assess the effect of 20% weight loss induced by either RYGB or BPD on glucoregulation in people with obesity (ClinicalTrials.gov number: ). The rate of appearance of ingested glucose into the circulation was much slower and the postprandial increases in plasma glucose and insulin concentrations were markedly blunted after BPD than after RYGB. Insulin sensitivity, assessed as glucose disposal rate during insulin infusion, was ~45% greater after BPD than RYGB, whereas β-cell function was not different between groups. These results demonstrate that, compared with matched percentage-weight loss induced by RYGB, BPD has unique beneficial effects on glycemic control, manifested by slower postprandial glucose absorption, blunted postprandial plasma glucose and insulin excursions, and greater improvement in insulin sensitivity.
Keywords: insulin sensitivity, bariatric surgery, obesity
Graphical Abstract
Diabetes remission is greater after biliopancreatic diversion (BPD) than Roux-en-Y gastric bypass (RYGB) surgery. Harris and Kayser et al. report that in individuals matched for percentage weight loss after surgery, BPD induces the same improvement in β-cell function but a greater improvement in insulin sensitivity than RYGB.
INTRODUCTION
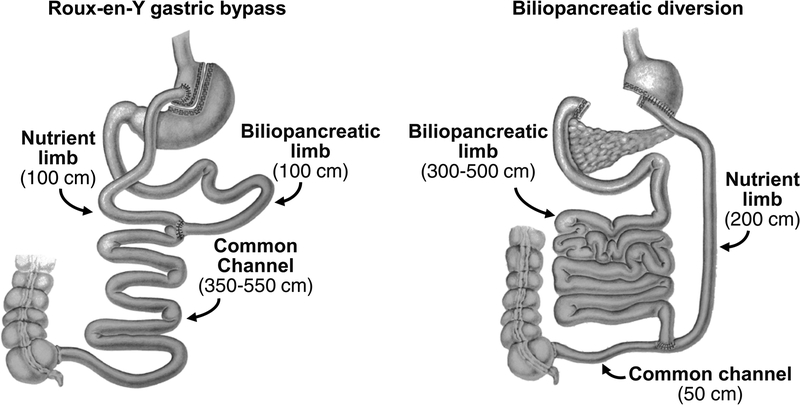
Type 2 diabetes (T2D) is a major public health problem in the United States and many other countries because of its high prevalence, serious comorbidities, adverse effects on quality of life, and considerable economic impact related to the cost of medical therapy and lost work productivity (Egede and Zheng, 2003; Manuel and Schultz, 2004; Seuring et al., 2015). Although many medications are available to treat T2D, less than 50% of patients with T2D achieve adequate glycemic control with medical therapy (Liebl et al., 2002; Resnick et al., 2006). Data from several randomized controlled trials have demonstrated that bariatric surgery is more effective than intensive medical therapy for managing T2D, and many patients with T2D achieve normal glycemia after bariatric surgery without the need for diabetes medications (Dixon et al., 2008; Mingrone et al., 2012; Mingrone et al., 2015; Schauer et al., 2017). Bariatric surgical procedures that bypass the upper gastrointestinal tract, such as biliopancreatic diversion (BPD) and Roux-en-Y gastric bypass (RYGB) (Figure 1), result in greater T2D remission rates than those that maintain intestinal continuity (Rubino et al., 2010). Moreover, the rate of T2D remission is greater after BPD than after RYGB surgery, even when weight loss after both procedures is the same (Mingrone et al., 2012; Panunzi et al., 2016), suggesting that weight loss-independent effects of BPD contribute to its therapeutic efficacy.
Figure 1.
Diagram of Roux-en-Y gastric bypass and biliopancreatic diversion surgeries. Roux-en-Y gastric bypass involves the creation of a small gastric pouch (<30 ml) that is anastomosed to a segment of jejunum, which was transected at 75 cm from the Ligament of Treitz. Intestinal continuity is restored via an anastomosis between the nutrient limb and the excluded biliopancreatic limb 100 cm distal to the gastro-jejunostomy to form a 100-cm nutrient limb, a 100-cm biliopancreatic limb, and a variable length (350–550 cm) common channel. Biliopancreatic diversion involves a horizontal gastrectomy, and anastomosis of the remaining stomach (~300 ml) to the small intestine, 250 cm from the ileocecal valve. The excluded biliopancreatic limb is anastomosed to the distal ileum, 50 cm from the ileocecal valve to form a 200-cm nutrient limb, a variable length (300–500 cm) biliopancreatic limb, and a 50-cm common channel.
The mechanism(s) responsible for the superior effect of BPD relative to RYGB in treating T2D is not known, but likely involves one or more of the key factors involved in the pathogenesis of T2D; namely, insulin sensitivity, β-cell function, and the metabolic response to meal ingestion. Accordingly, the purpose of the present study was to determine the effect of matched percentage-weight loss (20%) induced by BPD and RYGB on insulin sensitivity, β-cell function and the metabolic response to mixed meal ingestion in people with class III obesity (body mass index [BMI] ≥40.0 kg/m2). We hypothesized that the weight loss-induced improvements in metabolic function would be greater after BPD than RYGB surgery. We also hypothesized that postprandial plasma concentrations of metabolites and hormones involved in regulating glucose homeostasis, particularly bile acids and fibroblast growth factor 19 (FGF19), which inhibits bile acid synthesis, would differ between the two surgery groups. The hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotopically labeled glucose tracer infusion was used to assess whole-body insulin sensitivity and mixed meal ingestion in conjunction with ingested and infused stable isotopically-labeled glucose tracers was used to assess β-cell function and the metabolic response to food consumption.
RESULTS AND DISCUSSION
At baseline, mean BMI was ~15% lower in the RYGB group than in the BPD group (P < 0.05). Subjects in both the RYGB and BPD groups lost ~20% (19.7 ± 0.6% and 20.3 ± 0.4%, respectively) of their body weight at the time of post weight loss metabolic testing (16.0 ± 1.8 and 26.5 ± 3.1 weeks after surgery in the RYGB and BPD groups, respectively). Changes in fat mass and fat-free mass were also not different between groups (Table 1).
Table 1.
Body composition and metabolic response to mixed meal ingestion before and after 20% weight loss induced by Roux-en-Y gastric bypass and biliopancreatic diversion surgeries
| Roux-en-Y gastric bypass | Biliopancreatic diversion | PANCOVA | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Body mass index (kg/m2) | 47.6 ± 1.7 | 38.3 ± 1.4** | 56.1 ± 2.3# | 44.7 ± 1.8#** | NA |
| Body weight (kg) | 148.3 ± 6.3 | 119.0 ± 5.1** | 163.1 ± 7.8 | 130 ± 6.2** | NA |
| Fat-free mass (kg) | 79.7 ± 3.3 | 70.2 ± 3.4** | 78.0 ± 5.0 | 66.3 ± 3.9** | 0.12 |
| Body fat (%) | 45.7 ± 2.3 | 40.8 ± 2.3** | 52.5 ± 1.3 | 49.2 ± 1.1** | 0.12 |
| Basal plasma glucose (mg/dl) | 98.2 ± 2.0 | 89.1 ± 1.9* | 101.3 ± 2.3 | 93.9 ± 1.6* | 0.82 |
| HbA1c(%) | 5.8 ± 0.1 | 5.2 ± 0.2* | 6 ± 0.2 | 5.5 ±0.2* | 0.76 |
| Basal plasma insulin (μU/ml) | 28.7 ± 4.6 | 7.3 ± 1.6** | 26.6 ± 2.8 | 11.9 ± 1.7** | 0.14 |
| Plasma glucose AUC (g/dl × 5 h) | 31.8 ± 0.8 | 31.4 ± 0.8 | 34.3 ± 0.9 | 31.5 ± 0.8* | 0.47 |
| Plasma insulin AUC (mU/ml × 5 h) | 18.7 ± 1.9 | 13.5 ± 2.2** | 19.2 ± 2.6 | 8.4 ± 1.1** | 0.04 |
| Peak plasma glucose (mg/dl) | 144.7 ± 6.2 | 173.0 ± 5.9** | 160.9 ± 6.8 | 129.5 ± 7.2** | ≤0.01 |
| Peak plasma insulin (μU/ml) | 168.0 ± 18.5 | 197.8 ± 35.0 | 162.4 ± 23.9 | 66.7 ± 11.4** | ≤0.01 |
| ISR AUC (μmol/min × 5 h) | 214.8 ± 18.3 | 178.3 ± 16.3** | 235.7 ± 15.9 | 154.9 ± 12.1** | 0.03 |
| Insulin MCR (l/min) | 1.7 ± 0.1 | 2.2 ± 0.2** | 2.0 ± 0.1 | 3.0 ± 0.3** | 0.02 |
| Suppression of endogenous glucose Ra (5h-AUC % below basal) | 50.3 ± 2.9 | 36.9 ± 2.4* | 52.1 ± 2.6 | 55.3 ± 2.9 | ≤0.01 |
Data are means ± SEM. Value significantly different from the corresponding Roux-en-Y gastric bypass value,
P<0.01. Value significantly different from the corresponding Before value,
P<0.05 and
P<0.01. Pancova, between group differences after weight loss adjusted for the baseline value and BMI. Abbreviations: AUC, area under the curve; ISR, insulin secretion rate; MCR, metabolic clearance rate; NA, not applicable; Ra, rate of appearance.
Weight loss induced by RYGB and BPD causes marked differences in the metabolic response to mixed meal ingestion
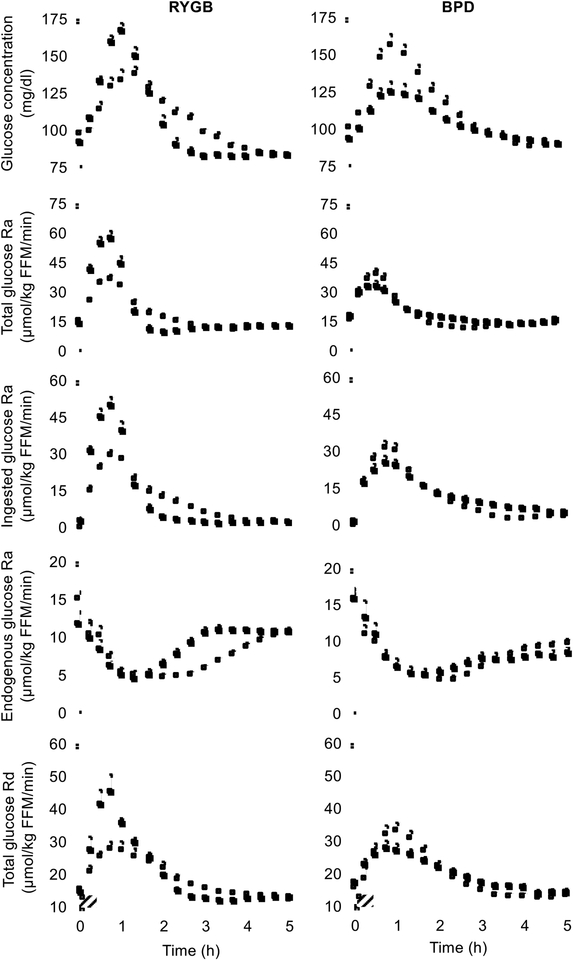
To determine how each surgical procedure affected postprandial plasma glucose and insulin kinetics, we used a combination of ingested and infused stable isotopically labeled glucose tracers to assess the metabolic response to mixed meal ingestion. Basal plasma glucose and insulin concentrations decreased after weight loss in both groups, without significant differences between groups (Table 1). The 5-h postprandial plasma glucose concentration area under the curve (AUC) decreased by ~10% (P < 0.05) after weight loss in the BPD cohort, but remained unchanged in the RYGB group; however, this difference between groups was not statistically significant (Table 1). Moreover, the shape of the postprandial plasma glucose concentration curve after weight loss was different in the two surgery groups; peak postprandial plasma glucose concentration was much higher after than before weight loss in the RYGB group but much lower after than before weight loss in the BPD group (Table 1 and Figure 2). Peak total glucose rate of appearance (Ra), ingested glucose Ra and total glucose disposal rate (Rd) were higher after than before weight loss in the RYGB group, whereas these values were lower after than before weight loss in the BPD group (Figure 2). The blunted increase in plasma glucose concentration and ingested glucose Ra after BPD surgery was not due to a decrease in the rate of gastric emptying because the rate of gastric emptying, assessed by scintigraphy after ingestion of a technetium-99m labeled mixed meal (Farrell, 2019) in selected subjects, was markedly accelerated (Supplemental Figure 1). Both RYGB and BPD caused maximal meal-induced suppression of endogenous glucose production during the first 60 minutes after meal ingestion with a more rapid return toward baseline in the RYGB than the BPD group (Figure 2). Therefore, total 5-h suppression of endogenous glucose production rate was less after than before RYGB surgery, but did not change after BPD surgery (Table 1). The percent of ingested glucose that appeared in the systemic circulation during the 5-h postprandial period after weight loss was not different from values obtained before weight loss in either the RYGB (89 ± 6% and 95 ± 6%, respectively) or the BPD (96 ± 5% and 98 ± 5%, respectively) groups, demonstrating any differences in plasma glucose concentrations or kinetics between surgery groups was not caused by differences in total glucose absorption.
Figure 2. Weight loss induced by RYGB and BPD causes marked differences in glucose dynamics during mixed meal ingestion.
Plasma glucose concentrations, total glucose Ra into the systemic circulation, ingested glucose Ra, endogenous glucose Ra, and total glucose Rd from the systemic circulation after ingesting a mixed-meal over 30 min (striped box) before (white squares) and after (black squares) 20% weight loss induced by RYGB or BPD surgery. Abbreviations: BPD, biliopancreatic diversion; FFM, fat-free mass. Ra, rate of appearance; Rd, disposal rate; RYGB, Roux-en-Y gastric bypass. Data are means ± SEM. See also Figure S1.
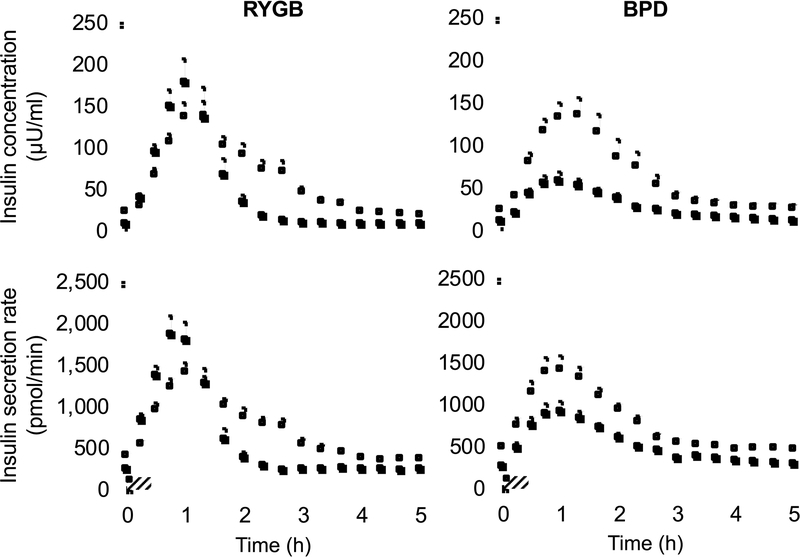
Total 5-h postprandial plasma insulin concentration AUC decreased after weight loss in both surgical groups, but the decline was greater in those who had BPD than those who had RYGB surgery (Table 1). The shape of the postprandial plasma insulin concentration curves closely followed the glucose curves and was also different after RYGB and BPD surgeries, manifested by an early increase above pre-surgery values followed by a rapid decline to below pre-surgery values in the RYGB group and a blunted plasma insulin response throughout the 5-h postprandial period after than before surgery in the BPD group (Figure 3). The difference in postprandial plasma insulin concentration after surgery in the two groups was due to both a greater decrease in insulin secretion rate and a greater increase in insulin metabolic clearance rate after BPD than after RYGB surgery (Figure 3, Table 1).
Figure 3. Weight loss induced by RYGB and BPD causes marked differences in the insulin response to mixed meal ingestion.
Plasma insulin concentrations and insulin secretion rate after ingesting a mixed-meal over 30 min (striped box) before (white squares) and after (black squares) 20% weight loss induced by Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion (BPD) surgery. Data are means ± SEM.
These data demonstrate marked differences in the metabolic response to mixed meal ingestion after BPD and RYGB. Although both BPD and RYGB involve bypass of the upper gastrointestinal tract, bile acids and pancreatic enzymes are delivered much more distally in the small intestine with the BPD than the RYGB procedure, which limits the length of intestine for optimal digestion and absorption of ingested food to 50 cm in patients who have BPD compared with 350–550 cm in those who have RYGB. Although the same total amount of ingested glucose was delivered into the systemic circulation after RYGB and BPD in our subjects, the rate of appearance of ingested glucose was much slower and the peak in plasma glucose was much lower after BPD than RYGB. The slower rate of appearance was likely caused by a slower rate of upper intestinal glucose absorption, rather than delayed gastric emptying, even though simple glucose, which does not require intraluminal digestion, was consumed. It is likely that the diversion of sodium-rich bile to the distal small intestine after BPD contributed to the decreased rate of glucose absorption by preventing the normal postprandial increase in intraluminal sodium needed for sodium-glucose cotransport, which is the major mechanism for glucose absorption by the small intestine (Ferraris and Diamond, 1997). This explanation is supported by data from a study conducted in a minipig model that demonstrated biliary diversion impaired intestinal glucose absorption, but was restored by increasing the intraluminal sodium content (Baud et al., 2016). The delayed rate of appearance of ingested glucose into the systemic circulation was the primary factor responsible for minimizing the postprandial increase in plasma glucose concentration and insulin secretion after BPD, because the suppression of endogenous glucose production was not different before and after surgery and glucose Rd was lower after than before surgery. These results demonstrate completely divergent changes in glucose and insulin kinetics in response to mixed-meal ingestion after RYGB and BPD, even though both procedures cause rapid gastric emptying and bypass the upper gastrointestinal tract.
Plasma metabolomics show distinct effects of BPD on fasting and postprandial fatty acid and bile acid metabolism
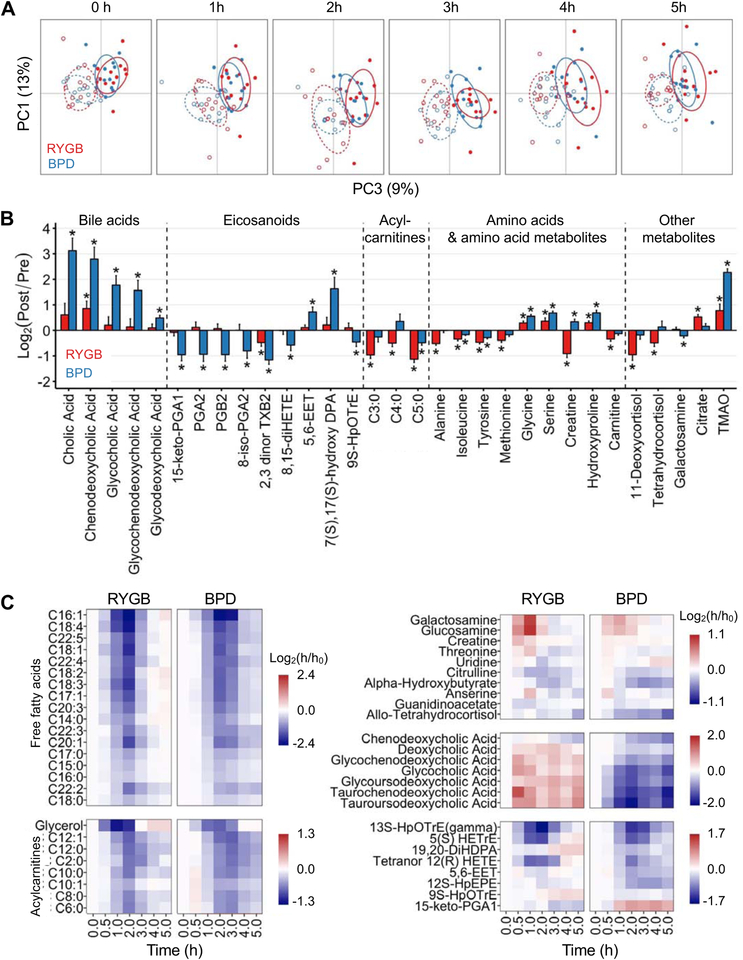
By using an LC-MS-based metabolomics platform, we identified and monitored 216 circulating metabolites in plasma before and after weight loss in the two surgery groups. Principal components analysis demonstrated a global shift in circulating metabolite abundances that was primarily caused by weight loss, rather than the specific type of surgical procedure (Figure 4A and Supplemental Figure 2). The change in only 14% (31 of 216) of the metabolites after weight loss was significantly different between surgical groups, manifested by: i) greater increases in bile acids, specific anti-inflammatory eicosanoids (5,6-EET and 7S, 17S-OH-DPA), certain amino acids (glycine, serine, hydroxyproline and creatine), and trimethylamine N-oxide (TMAO) after BPD than RYGB; ii) greater decreases in prostaglandins A and B after BPD than RYGB; and iii) greater decreases in other amino acids, short-chain acyl carnitines and cortisol metabolites after RYGB than BPD (Figure 4B).
Figure 4. Weight loss after BPD causes distinct changes in the fasting and postprandial metabolome than weight loss after RYGB.
Principal components analysis of plasma metabolite abundances during basal postabsorptive conditions (0 h) and throughout the postprandial period (1 h-5 h) before (solid lines) and after (broken lines) weight loss induced by RYGB (red) and BPD (blue) surgeries (A). Changes in basal plasma metabolite abundances that were different (2-way interaction FDR <0.3) after 20% weight loss induced by RYGB (red bars) and BPD (blue bars) surgery; * post-hoc within-group paired (pre-post weight loss) t-test FDR <0.1 (B). Heatmaps showing the meal-induced changes in plasma metabolite abundances that were different after 20% weight loss induced by RYGB and BPD (3-way interaction FDR <0.1 and post-hoc 2-way interaction FDR <0.1 in at least one of the two groups) (C). Abbreviations: BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass. See also Figure S2 and Table S1.
Weight-loss induced by biliopancreatic diversion with duodenal switch and by RYGB, but not vertical banded gastroplasty, have also been shown to increase plasma TMAO concentrations (Tremaroli et al., 2015; Troseid et al., 2016); we are not aware of any studies that evaluated plasma TMAO after BPD surgery. It is possible that changes in the gut microbiome induced by upper gastrointestinal bypass are responsible for the increase in plasma TMAO because TMAO is produced by intestinal microbiota or by hepatic oxidation of TMA, which is generated by gut microbial metabolism of dietary phosphatidylcholine and carnitine (Falony et al., 2015). The marked difference in plasma TMAO between RYGB and BPD surgeries in our subjects suggests an increased production of TMA, presumably caused by the greater delivery of unabsorbed phosphatidylcholine and carnitine into the colon and metabolism by colonic bacteria, in conjunction with increased intrahepatic oxidation of TMA to TMAO (Bennett et al., 2013). High plasma TMAO concentrations are associated with an increased risk of myocardial infarction and stroke (Heianza et al., 2017; Tang et al., 2013; Wang et al., 2011). However, the incidence of myocardial infarction and stroke in people with obesity and T2D who had BPD surgery is lower than in those treated with medical therapy alone (Iaconelli et al., 2011). Additional studies are needed to determine whether the large increase in plasma TMAO after BPD diminishes the cardiovascular benefits of weight loss after this procedure. Given the effect of BPD on plasma TMAO concentrations, which likely reflects alterations in gut microbial metabolism, we also measured basal plasma concentrations of short-chain fatty acids (acetate, propionate, and butyrate), but these microbial fermentation products did not change after surgery in either the RYGB or BPD group (Supplemental Table 1).
The effect of mixed meal ingestion on postprandial plasma metabolite abundances was not different in the RYGB and BPD groups before surgery (Figure 4A). However, after weight loss, the postprandial changes in the abundances of 50 of the 216 monitored metabolites were significantly different between the two surgery groups (Figure 4C). Surgery-induced weight loss caused: i) a greater rebound, after initial suppression, of free fatty acids, medium-chain acylcarnitines and several eicosanoids after RYGB than BPD; ii) a greater early increase in the amino sugars glucosamine and galactosamine after RYGB than BPD; and iii) an increase in bile acids after RYGB, but a marked decrease in bile acids after BPD.
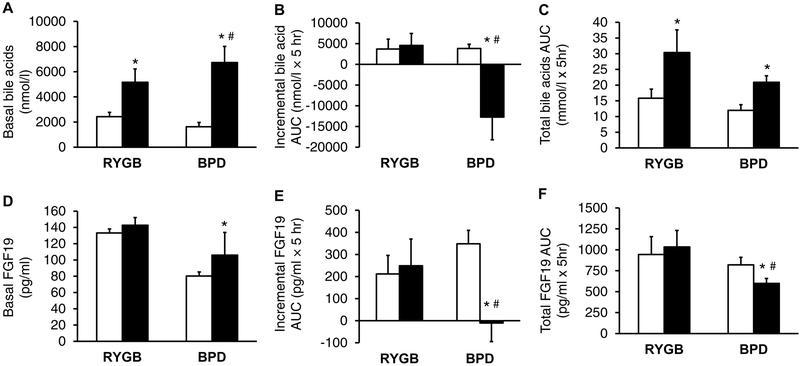
Because of the differences in the effect of BPD and RYGB on basal and postprandial bile acids after BPD than after RYGB, we also performed a quantitative analysis of plasma bile acid and FGF19 concentrations to provide a more comprehensive characterization of the effects of each surgery on bile acid metabolism. Basal plasma bile acid concentrations were much greater after than before surgery-induced weight loss in both surgery groups, but the increase was greater in the BPD than the RYGB group (Figure 5A). The postprandial change in plasma bile acid concentrations, assessed as the incremental 5-h AUC above or below the basal value after mixed meal ingestion, did not change after RYGB surgery but markedly decreased in the BPD group (Figure 5B), whereas the total postprandial bile acid AUC above zero increased after both RYGB and BPD, without a significant difference between the two groups (Figure 5C). Basal plasma FGF19 concentration did not change after RYGB but increased after BPD (Figure 5D). The incremental postprandial 5-h AUC and total postprandial AUC for plasma FGF19 did not change after RYGB, but decreased after BPD (Figures 5E and 5F). The postprandial increase in plasma bile acids after RYGB, which has also been observed by others (Ahmad et al., 2013; Kohli et al., 2013; Sachdev et al., 2016; Simonen et al., 2012), is likely caused by a decrease in hepatic bile acid clearance (Chavez-Talavera et al., 2017). The mechanism responsible for the decline in postprandial plasma bile acids after BPD is not known, but it is likely that the large bile acid load delivered through the excluded biliopancreatic limb to the distal ileum overwhelmed the capacity of intestinal bile acid transporters, causing a large loss of bile acids through the colon and a subsequent decrease in enterohepatic bile acid recycling and plasma concentrations. In addition, our findings suggest the decrease in ileal bile acid uptake was responsible for the postprandial decline in plasma FGF19, because bile acid activation of ileal farnesoid X receptors stimulate FGF19 production (Inagaki et al., 2005). The marked increase in basal (postabsorptive) plasma bile acid concentrations observed in our subjects after BPD surgery are consistent with the findings previously reported by Ferrannini and colleagues (Ferrannini et al., 2015), and support their conclusion that BPD causes an increase in basal hepatic bile acid synthesis. That BPD alters FGF19 suggested that FGF21 could also be involved.
Figure 5. Weight loss after BPD causes a greater increase in fasting plasma bile acids than weight loss after RYGB.
Basal concentrations, incremental 5-h postprandial concentration areas under the curve (AUC) above basal values, and total 5-h postprandial concentration AUC above zero for bile acids (A–C) and FGF19 (D–F) before (white bars) and after (black bars) 20% weight loss induced by Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion (BPD) surgery. * Value significantly different from the corresponding before weight loss value, p<0.05. #Value significantly different from the RYGB value, p<0.05. Abbreviations: BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass. Data are means ± SEM.
We next evaluated plasma FGF21 concentrations in a subset of 5 participants who had RYGB and 5 who had BPD. Mean basal plasma FGF21 concentration, the incremental increase in postprandial FGF21 5-h AUC above the basal value, and the total increase in postprandial FGF21 5-h AUC above zero tended to be greater after than before weight loss in both surgery groups (Supplemental Figure 3). However, only the total increase in postprandial FGF21 5-h AUC above zero after RYGB surgery was significantly different than the value obtained before surgery (P < 0.05) and there were no significant differences in any FGF21 outcomes between surgical groups, presumably because of the small number of study subjects.
Matched percentage weight loss correlated with greater improvement in insulin sensitivity after BPD than RYGB, but improvement in β-cell function was not different between surgery groups
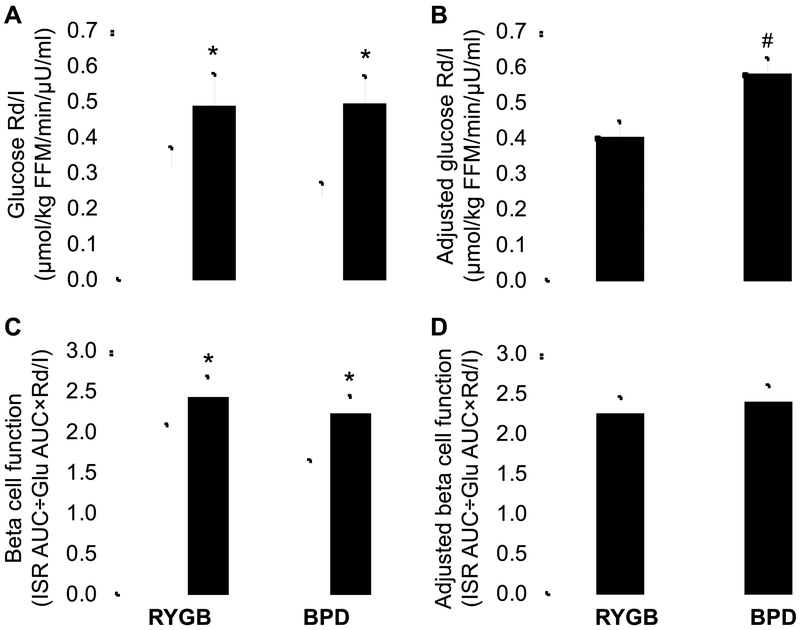
We evaluated insulin sensitivity before and after weight loss by conducting a hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically labeled glucose tracer infusion that allowed the assessment of glucose kinetics. Insulin sensitivity, assessed as glucose Rd divided by plasma insulin (I) concentration, was greater after than before weight loss in both surgery groups (Figure 6A), but the weight loss-induced increase was significantly greater in the BPD than the RYGB group after adjusting for differences in baseline insulin sensitivity and BMI between groups (Figure 6B). Our finding of greater insulin sensitivity after matched weight loss induced by BPD than RYGB surgery is consistent with the results from previous studies that evaluated the effect of BPD and RYGB on insulin sensitivity within the first 6 months after surgery (Ferrannini et al., 2015; Muscelli et al., 2005). However, it is difficult to make definitive conclusions from those studies because weight loss was greater in the BPD than the RYGB groups, which could have enhanced insulin action. The mechanism responsible for the greater improvement in insulin sensitivity after BPD than RYGB observed in our subjects is not clear, but our data suggest that a decrease in daily postprandial plasma glucose, glucosamine and insulin excursions has therapeutic effects on insulin action. Increased plasma glucose, glucosamine and insulin can cause insulin resistance by stimulating the hexosamine biosynthetic pathway, which produces intracellular metabolites that inhibit GLUT-4 and the insulin signaling cascade (Giaccari et al., 2009), and by downregulating insulin receptor binding affinity and insulin receptor number (Buren et al., 2003). Even short-term (24-h to 72-h) increases in plasma glucose and insulin in healthy subjects cause both hepatic and skeletal muscle insulin resistance and impair non-oxidative glucose disposal (Iozzo et al., 2001; Shannon et al., 2018), whereas normalizing fasting and postprandial plasma glucose and insulin concentrations by blocking SGLT glucose transport in people with T2D improves insulin sensitivity (Kahn et al., 1991; Rossetti et al., 1987). Furthermore, it is likely that the blunted postprandial glucose and insulin concentrations observed in our subjects who had BPD surgery would be even more pronounced when ingesting their usual meals at home, because of differences in carbohydrate composition. The carbohydrate in our test meal was composed entirely of simple glucose, whereas meals consumed at home likely contain complex carbohydrates, which are more difficult to absorb after BPD surgery because the diversion of secreted pancreatic enzymes to the terminal ileum prevents proximal intestinal carbohydrate digestion. It is also possible that the alterations in circulating anti-inflammatory and pro-inflammatory eicosanoids observed in the subjects who had BPD surgery could have contributed to an increase in insulin sensitivity, but the precise metabolic pathways for these potential links require further study.
Figure 6. Matched weight loss causes a greater improvement in insulin sensitivity, but not β-cell function, after BPD than RYGB.
Glucose Rd/I during the hyperinsulinemic-euglycemic clamp procedure (A) and β-cell function assessed after mixed meal ingestion (C) before (white bars) and after (black bars) 20% weight loss induced by RYGB or BPD surgery and glucose Rd/I after weight loss adjusted for baseline Rd/I and body mass index (B) and β-cell function after weight loss adjusted for baseline β-cell function and body mass index (D). * Value significantly different from the corresponding before weight loss value, p<0.05. #Value significantly different from the RYGB value, p<0.05. Abbreviations: BPD, biliopancreatic diversion; FFM, fat-free mass; Glu, glucose; I, plasma insulin concentration; ISR, insulin secretion rate; Rd, disposal rate; RYGB, Roux-en-Y gastric bypass. Data are means ± SEM.
We also evaluated β-cell function by determining insulin secretion in response to postprandial changes in plasma glucose in relationship to insulin sensitivity (Rd/I during the hyperinsulinemic-euglycemic clamp procedure). This index of β-cell function improved in both surgery groups after matched percentage weight loss (Figure 6C), without a significant difference between groups when adjusted for baseline β-cell function and BMI (Figure 6D). Assessing the β-cell response in relationship to the degree of insulin sensitivity is particularly important in our study because weight loss improved insulin sensitivity, which decreases the amount of insulin needed for glycemic control. Even though total postprandial insulin secretion rate decreased after weight loss in both surgical groups, β-cell function still increased because the increase in insulin sensitivity was greater than the decline in insulin secretion. Data from previous studies also found RYGB and BPD surgeries improve β-cell function (Bradley et al., 2012b; Ferrannini and Mingrone, 2009), but the two procedures were not directly compared in the same study.
In conclusion, our data demonstrate marked differences in the effect of matched weight loss induced by BPD and RYGB on several key factors that regulate glucose homeostasis. Compared with RYGB surgery, BPD causes a greater improvement in insulin-mediated glucose disposal and profound differences in the metabolic response to mixed-meal ingestion, manifested by a slow rate of intestinal glucose absorption and appearance of ingested glucose into the systemic circulation. These factors minimize the postprandial increase in plasma glucose and insulin concentrations and likely contribute to the superiority of BPD over RYGB in achieving remission of T2D.
Limitations of Study
Our study has several limitations. First, BPD is only done in a limited number of medical centers and represents a very small percentage of all bariatric surgery procedures performed annually. Therefore, the major aim of our study was to elucidate the metabolic mechanisms that might help explain why the rate of remission of T2D is greater after BPD than RYGB surgery, even when weight loss is the same in both groups of patients (Mingrone et al., 2012), and was not designed to inform clinical guidelines or make surgical recommendations. Second, our study was not a randomized trial, and mean BMI was greater in the BPD group than in the RYGB group, which is typical of clinical practice. Although we adjusted for the differences in baseline BMI in our outcome measures and matched percentage weight loss in both surgery groups, the possible influence of other unmeasured confounders on the results cannot be excluded. Third, the rate of percent weight loss was slower in the participants who had BPD than in those who had RYGB; consequently, the timing of the follow-up studies conducted after 20% weight loss was different between the two groups. Finally, our study was conducted in people with obesity who did not have T2D in order to eliminate the potential confounding effects of diabetes medications on our outcome measures. Therefore, we cannot exclude the possibility that the results would be different in people with T2D.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Samuel Klein (sklein@wustl.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Study subjects
Patients with class III obesity (BMI ≥40.0 kg/m2) who were scheduled to undergo RYGB (n = 12, 11 men) or BPD (n = 12, 6 men) surgeries at Catholic University in Rome, Italy participated in this study. All subjects completed a medical evaluation, including a history and physical examination and standard blood tests. Many participants in both the RYGB and BPD groups had cardiometabolic diseases: hypercholesterolemia (total cholesterol ≥ 240 mg/dL or LDL-C ≥ 160 mg/dL, or treatment with atorvastatin) in 8 RYGB and 9 BPD participants, hypertension (systolic or diastolic blood pressure > 140/90 mmHg, or treatment with antihypertensive medication) in 7 RYGB and 7 BPD participants, and impaired fasting glucose (plasma glucose ≥100 mg/dl) in 7 RYGB and 5 BPD participants. People with diabetes were excluded to avoid the potential confounding effect of diabetes medications and postoperative changes in medications on the outcome measures. In addition, those with previous intestinal surgery or a history of inflammatory intestinal disease, and those who were taking medications that could influence the study outcome measures were excluded. A CONSORT flow diagram for the study is provided in Supplemental Figure 4. All subjects gave written informed consent before participating in this study, which was approved by the Institutional Human Research Review Committee of Catholic University in Rome, Italy (ClinicalTrials.gov ).
METHOD DETAILS
Study visits
The mixed meal metabolic study and the hyperinsulinemic-euglycemic clamp procedure were performed on two consecutive days in the Clinical Research Unit at Catholic University in Rome, Italy. Body fat mass (FM) and fat-free mass (FFM) were determined by using dual energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Chicago IL) in the afternoon after the mixed meal metabolic study.
Mixed meal metabolic study
Subjects were admitted to the Clinical Research Unit in the morning after they fasted for ~10 h overnight at home before completing an 8.5 h mixed meal metabolic study, as we have previously described (Bradley et al., 2012a). A catheter was inserted into a forearm vein for stable isotopically-labeled glucose tracer infusion and a second catheter was placed into a contralateral hand vein, which was heated to 55 °C by using a thermostatically controlled box, to obtain arterialized venous blood samples. At 0700 h, a primed, continuous infusion of [6,6-2H2]glucose (priming dose: 22 μmol/kg; infusion rate: 0.22 μmol/kg/min) was started and maintained until the end of the study. At 1030 h, subjects ingested a liquid meal (210 ml, containing 46 g glucose mixed with 0.9 g [U-13C]glucose, 9 g fat, and 9 g protein), which was provided in 7 equal aliquots every 5 min over 30 min. Blood samples were obtained before starting the tracer infusion (time 0 min), every 10 min for 30 min before consuming the meal (time 180, 190, 200 and 210 min of tracer infusion), and every 15 min for the first hour and then every 20 min for the next 4 hours after starting the meal to determine plasma glucose, insulin, c-peptide, bile acid and FGF19 concentrations, and plasma glucose tracer-to-tracee ratios (TTRs).
Hyperinsulinemic-euglycemic clamp procedure
Subjects were admitted to the Clinical Research Unit in the morning after they fasted for ~10 h overnight at home before completing a 7-h hyperinsulinemic-euglycemic clamp procedure, as we have previously described (Bradley et al., 2012a). One catheter was inserted into a forearm vein to infuse insulin, dextrose and a stable isotopically-labeled glucose tracer, and a second catheter was inserted into a contralateral hand vein, which was heated to 55 °C by using a thermostatically controlled box, to obtain arterialized venous blood samples. At 0700 h, a primed, continuous infusion of [6,6-2H2]glucose (priming dose: 22 μmol/kg; infusion rate: 0.22 μmol/kg/min) was started and maintained for 3.5 h. At 1030 h, insulin was infused at a rate of 50 mU·m−2 body surface area· min−1 (initiated with a priming dose of 200 mU·m−2·min−1 for 5 min and then 100 mU·m−2·min−1 for 5 min) for ~3.5 h. Euglycemia (plasma glucose ~100 mg/dl) was maintained by variable rate infusion of a 20% dextrose solution enriched to 2.5% with [6,6-2H2]glucose. Blood samples were obtained immediately before starting the tracer infusion and every 10 min during the final 30 min of the basal period and the clamp procedure to determine plasma glucose and insulin concentrations, and glucose kinetics.
Surgical procedures
After baseline testing was completed, RYGB and BPD surgeries were performed by using standard techniques, as previously described (Bradley et al., 2012a; Mingrone et al., 2012) (Figure 1). The RYGB procedure involves the use of a surgical stapler to create a small (~30 ml) vertically-oriented gastric pouch, which is completely divided from the gastric remnant. The pouch was anastomosed to a segment of jejunum, which was transected at 75 cm from the Ligament of Treitz. Bowel continuity was restored via an entero-entero anastomosis between the excluded biliopancreatic limb and nutrient limb, 100 cm distal to the gastro-jejunostomy to form a 100-cm nutrient limb, a 100-cm biliopancreatic limb, and a variable length (350–550 cm) common channel, where biliopancreatic secretions can come into contact with ingested food. The BPD procedure involves a distal horizontal gastrectomy, which removes about 60% of the stomach leaving a residual stomach volume of ~300 mL, and a stapled closure of the duodenal stump. The small intestine is then transected at 250 cm from the ileocecal valve, and its distal end is anastomosed to the remaining stomach. The excluded biliopancreatic limb is anastomosed to the distal ileum, 50 cm from the ileocecal valve to form a 200-cm nutrient limb, a variable length (300–500 cm) biliopancreatic limb and a 50-cm common channel where biliopancreatic secretions can come into contact with ingested food.
Repeat studies after weight loss
After surgery, all subjects participated in standard medical follow-up evaluations by the surgery team. Both the RYGB and BPD participants consumed the same 600 kcal/day diet immediately after surgery for 3 days before discharge from the hospital (fruit purees and two servings of Fortimel® Protein [240 kcal each]) and were prescribed the same 850 kcal/day diet for the first 4 weeks after surgery (Fortimel® Protein 3 x/day and purees of fruits and vegetables). Participants were instructed to consume a 1200–1400 kcal/day diet consisting of rice, fish, minced meat, vegetables, and fruit from 1–3 months after surgery. After 3 months, participants were instructed to avoid high-sugar beverages, but otherwise consume an ad libitum diet. Subjects were contacted weekly by the research team to monitor changes in body weight. After 20 ± 2% (grand mean) weight loss was achieved, body composition analysis, the mixed meal metabolic study and the hyperinsulinemic-euglycemic clamp procedure were repeated.
Sample analyses
Plasma glucose concentration was measured by using a Roche Cobas C501 (Roche Diagnostics, Indianapolis, IN), insulin by using electrochemiluminescence technology (Elecsys 2010, Roche Diagnostics), c-peptide by using an enzyme-linked immunosorbent assay (Millipore, Billerica, MA), and FGF19 and FGF21 by using enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN). Plasma bile acid concentrations were measured by using ultrahigh performance liquid chromatography/multiple reactions monitoring mass spectrometry (UPLC-MRM MS) (Han et al., 2015). Plasma glucose TTRs were determined by using gas chromatography-mass spectrometry, as previously described (Fabbrini et al., 2009).
Metabolomics
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) based metabolomics was performed on deproteinated plasma collected immediately before and for 5 hours after mixed meal ingestion. Polar metabolites were analyzed by using a Thermo QExactive orbitrap mass spectrometer coupled to a Thermo Vanquish UPLC system, as previously described (Garratt et al., 2018). Bioactive lipids (e.g. free fatty acids, eicosanoids, and bile acids) were profiled on the same LC-MS/MS system, as previously described (Lagerborg et al., 2019; Watrous et al., 2019). A total of 216 metabolites were annotated by using an in-house library of commercially available standards or MS/MS fragmentation patterns. Metabolite abundances were log2 transformed for presentation and statistical analysis. Gas chromatography mass spectrometry with isotopically-labeled internal standards was used to measure plasma short-chain fatty acids (Rey et al., 2013).
Calculations
Metabolic response to mixed meal ingestion
The 5-h postprandial AUCs for plasma glucose, insulin, c-peptide, bile acid and FGF19 concentrations were calculated by using the trapezoid method (Ong et al., 1994). The postprandial insulin secretion rate (ISR) was calculated by using stochastic deconvolution of c-peptide concentrations (Sparacino et al., 2002; Van Cauter et al., 1992). The postprandial insulin metabolic clearance rate was calculated as the ratio of ISR AUC (pmol/min x 5h) ÷ plasma insulin concentration AUC (pmol/l x 5h). Total (endogenous and meal-derived) glucose Ra into the systemic circulation, ingested glucose Ra, endogenous glucose Ra, and total glucose Rd from plasma were calculated as previously described (Bradley et al., 2012a). The percent of ingested glucose that appeared in the systemic circulation was calculated from the plasma and meal glucose TTR and Ra values using Steele’s equation as described previously (Bradley et al., 2012a; Gastaldelli et al., 2007). The suppression of endgenous glucose Ra during the meal was calculated as the area above the endogenous glucose Ra time course bounded by basal Ra divided by total area below basal Ra.
Hyperinsulinemic-euglycemic clamp procedure
Glucose Ra was calculated by dividing the tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal and insulin infusion periods (Bradley et al., 2014). Glucose Rd is equal to endogenous glucose Ra plus the rates of exogenously infused dextrose and the glucose tracer. Insulin-stimulated glucose Rd divided by steady state plasma insulin concentration (glucose Rd/I) was used as a measure of whole-body (primarily skeletal muscle) insulin sensitivity.
β-cell function
β-cell function was calculated as the product of the β-cell response to the mixed meal ingestion (assessed as the ISR AUC to glucose concentration AUC ratio) and insulin sensitivity (glucose Rd/I).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed by using SPSS Version 25 (IBM, Armonk, NY) and R (The R Foundation for Statistical Computing, Vienna, Austria). Within-subject effects of weight loss on plasma glucose and hormone concentrations and glucose kinetics were assessed by using Student’s t-test for paired samples. Group differences in BMI and body weight were assessed by using Student’s t-test for independent samples. Differences in body composition and metabolic outcomes after weight loss between the BPD and RYGB groups were assessed by using analysis of covariance (ANCOVA) with adjustments for the baseline outcome value and BMI. A P value < 0.05 was considered statistically significant. Data are presented as mean ± SEM. Based on data from our previous study conducted in participants with obesity before and after 20% weight loss induced by RYGB surgery (Bradley et al., 2012a), we estimated that 12 subjects would be required in each group to detect at least a 40% mean difference in insulin-stimulated glucose Rd between groups with 80% power at the 0.05 alpha level.
Principal components analysis was used to explore global patterns in the metabolomics data and a scree plot was used to choose the number of PCs. The effects of RYGB and BPD surgeries on basal metabolite abundances were tested by using 2-way (group and time, i.e., before/after surgery) mixed effects ANOVA and paired Student’s t-test for post-hoc testing. The effects of meal ingestion before and after weight loss in the two groups were compared by using 3-way (with group, meal time, and time, i.e., before/after surgery) ANOVA; significant group x time interactions were further evaluated by using 2-way (stratification by surgery) mixed effects ANOVA post hoc. Benjamini-Hochberg false discovery rate (FDR)-adjusted p-values were used to determine statistical significance. For basal postabsorptive values, an FDR < 30% for the interaction and < 10% for the post-hoc paired t-test in at least one of the surgery groups was used to identify a statistically significant different response in the two groups. For meal kinetics, a more conservative threshold was used to limit metabolites to those with temporal patterns distinctly different between surgeries: an FDR < 10% for both the 3-way interaction and a 2-way interaction in at least one of the surgeries was considered statistically significant.
Supplementary Material
Highlights
Glucose metabolism was studied before and after 20% weight loss from RYGB or BPD
RYGB accelerates, whereas BPD slows, the absorption of glucose from a mixed meal
BPD causes greater improvement in insulin sensitivity than RYGB
β-cell function is equally improved after weight loss induced by RYGB or BPD
Context and Significance
Bariatric surgery is currently the most effective therapy for type 2 diabetes (T2D). Diabetes remission (i.e., blood glucose and %HbA1c in the normal range without diabetes medications) occurs more commonly after biliopancreatic diversion (BPD) than other bariatric procedures, including the more common Roux-en-Y gastric bypass (RYGB). Sam Klein and his colleagues found that in individuals who underwent either BPD or RYGB and were matched for postoperative weight loss, BPD resulted in slower glucose absorption and a blunted increase in blood insulin concentrations after a meal, and a greater improvement in whole-body insulin sensitivity. These results help explain the unique therapeutic effects of BPD and provide a framework for developing new therapies that mimic the physiology of BPD for preventing and treating T2D.
ACKNOWLEDGEMENTS
The authors thank Jennifer Shew, Freida Custodio, and Dr. Adewole L. Okunade for their technical assistance in processing and analyzing study samples, Drs. Jiye Cheng and Jeff Gordon for analysis of plasma short-chain fatty acid concentrations, Prof. Bart Staels for insightful discussions regarding bile acid physiology, and the study subjects for their participation.
This study was supported by National Institutes of Health grants DK101578, DK56341 (Nutrition Obesity Research Center), DK20579 (Diabetes Research Center), UL1 TR000448 (Clinical Translational Science Award, including KL2 sub-award TR 000450), T32HL130357, K01DK116917, S10OD020025, R01ES027595, and HL20948 and support from the Pershing Square Foundation.
Footnotes
DECLARATION OF INTERESTS
S.K. is a shareholder of Aspire Bariatrics, receives research funding from Merck Research Laboratories and Janssen Pharmaceuticals, and serves as a member of the Merck Global Diabetes and Metabolism Scientific Advisory Board. The other authors have nothing to disclose.
REFERENCES
- Ahmad NN, Pfalzer A, and Kaplan LM (2013). Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int. J. Obes. (Lond) 37, 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, Devienne M, Ghunaim M, Bonner C, Quenon A, et al. (2016). Bile Diversion in Roux-en-Y Gastric Bypass Modulates Sodium-Dependent Glucose Intestinal Uptake. Cell Metab 23, 547–553. [DOI] [PubMed] [Google Scholar]
- Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 17, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, et al. (2012a). Gastric bypass and banding equally improve insulin sensitivity and β cell function. J. Clin. Invest 122, 4667–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Magkos F, Eagon JC, Varela JE, Gastaldelli A, Okunade AL, Patterson BW, and Klein S (2014). Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity 22, 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Magkos F, and Klein S (2012b). Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology 143, 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buren J, Liu HX, Lauritz J, and Eriksson JW (2003). High glucose and insulin in combination cause insulin receptor substrate-1 and −2 depletion and protein kinase B desensitisation in primary cultured rat adipocytes: possible implications for insulin resistance in type 2 diabetes. Eur. J. Endocrinol 148, 157–167. [DOI] [PubMed] [Google Scholar]
- Chavez-Talavera O, Baud G, Spinelli V, Daoudi M, Kouach M, Goossens JF, Vallez E, Caiazzo R, Ghunaim M, Hubert T, et al. (2017). Roux-en-Y gastric bypass increases systemic but not portal bile acid concentrations by decreasing hepatic bile acid uptake in minipigs. Int. J. Obes. (Lond) 41, 664–668. [DOI] [PubMed] [Google Scholar]
- Dixon JB, O’Brien PE, Playfair J, Chapman L, Schächter LM, Skinner S, Proietto J, Bailey M, and Anderson M (2008). Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299, 316–323. [DOI] [PubMed] [Google Scholar]
- Egede LE, and Zheng D (2003). Independent Factors Associated With Major Depressive Disorder in a National Sample of Individuals With Diabetes. Diabetes Care 26, 104–111. [DOI] [PubMed] [Google Scholar]
- Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, and Klein S (2009). Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 106, 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G, Vieira-Silva S, and Raes J (2015). Microbiology Meets Big Data: The Case of Gut Microbiota-Derived Trimethylamine. Annu. Rev. Microbiol 69, 305–321. [DOI] [PubMed] [Google Scholar]
- Farrell MB (2019). Gastric Emptying Scintigraphy. J. Nucl. Med. Technol 47, 111–119. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Camastra S, Astiarraga B, Nannipieri M, Castro-Perez J, Xie D, Wang L, Chakravarthy M, and Haeusler RA (2015). Increased Bile Acid Synthesis and Deconjugation After Biliopancreatic Diversion. Diabetes 64, 3377–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, and Mingrone G (2009). Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care 32, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris RP, and Diamond J (1997). Regulation of intestinal sugar transport. Physiol. Rev 77, 257–302. [DOI] [PubMed] [Google Scholar]
- Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, and Miller RA (2018). Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell, e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A, Casolaro A, Pettiti M, Nannipieri M, Ciociaro D, Frascerra S, Buzzigoli E, Baldi S, Mari A, and Ferrannini E (2007). Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin. Pharmacol. Ther 81, 205–212. [DOI] [PubMed] [Google Scholar]
- Giaccari A, Sorice G, and Muscogiuri G (2009). Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutrition, metabolism, and cardiovascular diseases: Nutr. Metab. Cardiovasc. Dis 19, 365–377. [DOI] [PubMed] [Google Scholar]
- Han J, Liu Y, Wang R, Yang J, Ling V, and Borchers CH (2015). Metabolic profiling of bile acids in human and mouse blood by LC-MS/MS in combination with phospholipid-depletion solid-phase extraction. Anal. Chem 87, 1127–1136. [DOI] [PubMed] [Google Scholar]
- Heianza Y, Ma W, Manson JE, Rexrode KM, and Qi L (2017). Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli A, Panunzi S, De Gaetano A, Manco M, Guidone C, Leccesi L, Gniuli D, Nanni G, Castagneto M, Ghirlanda G, et al. (2011). Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care 34, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. (2005). Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2, 217–225. [DOI] [PubMed] [Google Scholar]
- Iozzo P, Pratipanawatr T, Pijl H, Vogt C, Kumar V, Pipek R, Matsuda M, Mandarino LJ, Cusi KJ, and DeFronzo RA (2001). Physiological hyperinsulinemia impairs insulin-stimulated glycogen synthase activity and glycogen synthesis. Am. J. Physiol. Endocrinol. Metab 280, E712–719. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Shulman GI, DeFronzo RA, Cushman SW, and Rossetti L (1991). Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J. Clin. Invest 87, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, and Klein S (2013). Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J. Clin. Endocrinol. Metab 98, E708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerborg KA, Watrous JD, Cheng S, and Jain M (2019). High-Throughput Measure of Bioactive Lipids Using Non-targeted Mass Spectrometry. Methods Mol. Biol 1862, 17–35. [DOI] [PubMed] [Google Scholar]
- Liebl A, Mata M, and Eschwege E (2002). Evaluation of risk factors for development of complications in Type II diabetes in Europe. Diabetologia 45, S23–28. [DOI] [PubMed] [Google Scholar]
- Manuel DG, and Schultz SE (2004). Health-Related Quality of Life and Health-Adjusted Life Expectancy of People With Diabetes in Ontario, Canada, 1996–1997. Diabetes Care 27, 407-. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, et al. (2012). Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med 366, 1577–1585. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, and Rubino F (2015). Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386, 964–973. [DOI] [PubMed] [Google Scholar]
- Muscelli E, Mingrone G, Camastra S, Manco M, Pereira JA, Pareja JC, and Ferrannini E (2005). Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am. J. Med 118, 51–57. [DOI] [PubMed] [Google Scholar]
- Ong JM, Simsolo RB, Saghizadeh M, Pauer A, and Kern PA (1994). Expression of lipoprotein lipase in rat muscle: regulation by feeding and hypothyroidism. J. Lipid Res 35, 1542–1551. [PubMed] [Google Scholar]
- Panunzi S, Carlsson L, De Gaetano A, Peltonen M, Rice T, Sjostrom L, Mingrone G, and Dixon JB (2016). Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care 39, 166–174. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Foster GL, Bardsley J, and Ratner RE (2006). Achievement of American Diabetes Association Clinical Practice Recommendations Among U.S. Adults With Diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care 29, 531–537. [DOI] [PubMed] [Google Scholar]
- Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, and Gordon JI (2013). Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci. USA 110, 13582–13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L, Smith D, Shulman GI, Papachristou D, and DeFronzo RA (1987). Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Invest 79, 1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino F, Schauer PR, Kaplan LM, and Cummings DE (2010). Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu. Rev. Med 61, 393–411. [DOI] [PubMed] [Google Scholar]
- Sachdev S, Wang Q, Billington C, Connett J, Ahmed L, Inabnet W, Chua S, Ikramuddin S, and Korner J (2016). FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes. Surg 26, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, et al. (2017). Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N. Engl. J. Med 376, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuring T, Archangelidi O, and Suhrcke M (2015). The Economic Costs of Type 2 Diabetes: A Global Systematic Review. PharmacoEconomics 33, 811–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Merovci A, Xiong J, Tripathy D, Lorenzo F, McClain D, Abdul-Ghani M, Norton L, and DeFronzo RA (2018). Effect of Chronic Hyperglycemia on Glucose Metabolism in Subjects With Normal Glucose Tolerance. Diabetes 67, 2507–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, et al. (2012). Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes. Surg 22, 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparacino G, Pillonetto G, Capello M, De Nicolao G, and Cobelli C (2002). WINSTODEC: a stochastic deconvolution interactive program for physiological and pharmacokinetic systems. Comput. Methods Programs Biomed 67, 67–77. [DOI] [PubMed] [Google Scholar]
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, and Hazen SL (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med 368, 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V, Karlsson F, Werling M, Stahlman M, Kovatcheva-Datchary P, Olbers T, Fandriks L, le Roux CW, Nielsen J, and Backhed F (2015). Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab 22, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, and Lappegard KT (2016). Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery. Metab. Syndr. Relat. Disord 14, 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Mestrez F, Sturis J, and Polonsky KS (1992). Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41, 368–377. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous JD, Niiranen TJ, Lagerborg KA, Henglin M, Xu YJ, Rong J, Sharma S, Vasan RS, Larson MG, Armando A, et al. (2019). Directed Non-targeted Mass Spectrometry and Chemical Networking for Discovery of Eicosanoids and Related Oxylipins. Cell Chem. Biol 26, 433–442 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.