Abstract
Objective:
To describe an unusual presentation of a follicle-stimulating hormone-secreting pituitary adenoma leading to ovarian hyperstimulation syndrome. We also discuss the pathophysiology and subsequent management of these tumors.
Methods:
This is a case report and review of the literature. A 37-year-old female with menorrhagia was found to have bilateral multilocular adnexal cysts with concern for ovarian hyperstimulation syndrome.
Results:
Labs revealed elevated follicle-stimulating hormone, and an eminent estradiol level. Pituitary magnetic resonance imaging revealed a macroadenoma with cavernous sinus invasion. The patient went on to have a successful transsphenoidal resection with normalization of hormones and subsequent resumption of menstrual cycles.
Conclusion:
With the help of this case report, we illustrate the pathogenesis of functioning gonadotroph adenomas as well as the management challenges associated with these tumors. Our case is unique in its presentation with severe hyperestrogenemia and cavernous sinus invasion pointing towards a clinically aggressive adenoma. It is important to increase awareness of these tumors to ensure timely and effective management of their complications.
INTRODUCTION
Gonadotroph cell adenomas are the most common histological subtype of pituitary adenomas, accounting for 15 to 40% of all pituitary adenomas and 80% of nonfunctioning pituitary adenomas (1,2). The majority of immunohistochemically confirmed gonadotroph adenomas are hormonally silent, or nonfunctioning. Functioning gonadotroph adenomas (FGAs) are much rarer pituitary tumors leading to gonadal stimulation and ovarian hyperstimulation syndrome (OHSS) in premenopausal women. Their pathogenesis remains ambiguous. We report a case of a female patient with an FGA leading to hyperestrogenemia and OHSS along with a review of the medical literature.
CASE REPORT
A 37-year-old female with a history of Graves disease presented to her gynecologist with an outside diagnosis of anemia and menorrhagia. A pelvic magnetic resonance imaging (MRI) scan showed multiple fibroids, including multilocular cystic lesions measuring 5.1 cm on the left and 6.3 cm on the right in the adnexa. Labs were significant for elevated prolactin at 112 ng/mL (reference range is 4 to 26 ng/mL), diluted prolactin was not available, elevated estradiol at 576 pg/mL (reference ranges are 21 to 251 pg/mL [follicular phase], 38 to 649 pg/mL [midcycle peak], and 21 to 312 pg/mL [luteal phase]), follicle-stimulating hormone (FSH) was 10.5 mIU/mL, luteinizing hormone (LH) was <0.2 mIU/mL, elevated inhibin A at 294 pg/mL (reference range for premenopausal women is <98 pg/mL), and elevated inhibin B at 479 pg/mL (reference range for premenopausal women is <153 pg/mL). Pituitary MRI scan revealed an 18.0 × 18.5-mm adenoma with mild mass effect on the optic chiasm (Fig. 1). The patient was started on cabergoline by an outside physician. However, the prolactin elevation was likely due to stalk effect and cabergoline was subsequently stopped.
Fig. 1.
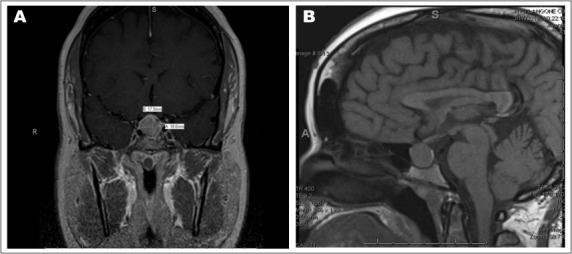
Pituitary magnetic resonance images post-contrast showing coronal (A) and sagittal (B) views of T1. They show a pituitary adenoma extending into suprasellar cistern.
The patient continued to experience severe menorrhagia and irregular cycles necessitating blood transfusions and emergency surgery to remove a large submucosal fibroid. Transvaginal ultrasound revealed bilateral ovaries replaced by large multiloculated septated cystic masses, characteristic for ovarian hyperstimulation (Fig. 2). The clinical presentation, labs, and pelvic imaging with ovarian hyperstimulation increased the suspicion for an FSH-secreting adenoma. Repeat labs revealed an estradiol of 8,372 pg/mL, elevated FSH of 41.7 mIU/mL, and low LH of 0.9 mIU/mL (Table 1).
Fig. 2.
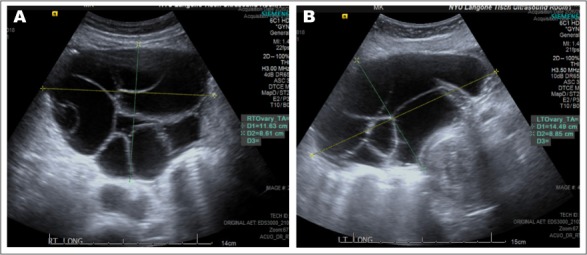
Transvaginal ultrasound showing right (A) and left (B) large multiloculated septated cystic ovaries.
Table 1.
Laboratory Testing Values Pre- and Post-Surgery
| Reference values | 04/13/2018 (9 weeks pre-surgery) | 04/23/2018 (8 weeks pre-surgery) | 06/28/2018 (10 days post-surgery) | 09/19/2018 (13 weeks post-surgery) | |
|---|---|---|---|---|---|
| FSH (mIU/mL) | Follicular phase 3.0–8.0, Midcycle peak 3.0–17.0, Luteal phase 1.4–5.5, Postmenopausal 27.0–133 | 41.7 | 47.9 | 2.5 | 4.9 |
| LH (mIU/mL) | Follicular phase 1.8–12.0, Midcycle peak 8.0–89.0, Luteal phase 0.6–14.0, Postmenopausal 5.0–62.0 | 0.9 | 1.4 | 0.3 | 8.8 |
| Estradiol (pg/mL) | Follicular phase 21–251, Midcycle peak 38–649, Luteal phase 21–312, Postmenopausal without HRT <28, Postmenopausal with HRT <144 | 8,372 | 8,310 | 14 | 311 |
| Progesterone (ng/mL) | Follicular phase <1.0, Luteal phase 2.6–21.5, Postmenopausal <0.5 | 6.3 | - | - | - |
| Prolactin (ng/mL) | 4–26 | 8.5 | 19.7 | 2.5 | 10.1 |
| Prolactin dilution study (ng/mL) | 2.9–26.0 | - | 17.3 | - | - |
| β-hCG (mIU/mL) | <2 | - | - | - | |
| Testosterone (ng/dL) | Premenopausal female 9–55 | - | 144 | - | - |
| DHEA sulfate (μg/dL) | 45–270 | - | 73 | - | - |
| Androstenedione (ng/mL) | 0.260–2.140 | - | 3.120 | - | - |
| Insulin-like growth factor-1 (ng/mL) | 80–277 | - | 150 | - | - |
| Cortisol @ 09:56 am (μg/dL) | Before 10:00 am 3.7–19.4 | - | 15.8 | - | - |
| ACTH (pg/mL) | 6–58 | - | 21 | - | - |
She was referred to neurosurgery and had an endoscopic transsphenoidal resection of the tumor. The tumor was noted to be necrotic and soft. The tumor was invasive into the right medial wall of the cavernous sinus. There were no major complications during the procedure and the patient's FSH levels dropped from 41.7 to 1.8 mIU/mL 24 hours after surgery. The surgical pathology revealed tumor cells positive for chromogranin, Cam5.2, and FSH, variable for LH, and negative for adrenocorticotropic hormone, growth hormone, prolactin, and thyroid-stimulating hormone. The Ki-67 proliferation index was <2% and no obvious mitotic figures were found. The medial wall of the cavernous sinus was invaded by the pituitary adenoma (Fig. 3).
Fig. 3.
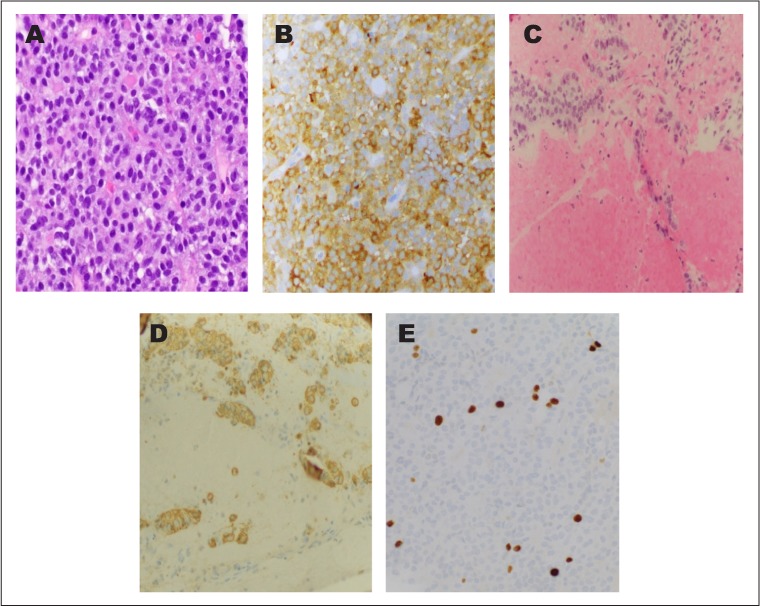
Photomicrographs of the follicle-stimulating hormone-producing adenoma. Monotonous population of tumor cells on hematoxylin and eosin staining (A, ×40) and strong follicle-stimulating hormone immunoreactivity (B, ×20). Pituitary adenoma invading the medial wall of the cavernous sinus (C, ×20) highlighted by an immunostain for chromogranin (D, ×20). A Ki-67 proliferation marker highlights <2% of tumor nuclei (E, ×40).
Postoperatively, the patient remained stable with minimal headache or visual symptoms. Hormone levels initially decreased to low levels showing 2.5 mIU/mL FSH, 14 pg/mL estradiol, and 2.5 ng/mL prolactin (Table 1) and the patient was amenorrheic, indicating possible postoperative hypopituitarism. However, 10 weeks after surgery, her menstrual cycles resumed with an FSH of 4.9 mIU/mL and estradiol of 311 pg/mL (Table 1). Follow-up MRI scan of the brain 12 weeks after surgery revealed no evidence of any residual adenoma.
DISCUSSION
Gonadotroph adenomas can be divided into functioning and nonfunctioning adenomas. FGAs express and secrete one or both of the gonadotropins FSH and LH, in biologically active forms, and usually manifest with hormone excess and varied clinical presentations. Menstrual irregularities including oligoamenorrhea, menorrhagia, menometrorrhagia, and rarely, FSH-driven OHSS are described in premenopausal females. Precocious puberty and possible subsequent OHSS may be seen in younger children as well (3–5). In a rare and fascinating case, Vargas et al (6) describe a 19-year-old patient with a history of precocious puberty at age 7 and subsequent OHSS leading to bilateral oophorectomy secondary to a TSH- and FSH-cosecreting adenoma.
A diagnosis of FGA is suspected if FSH is elevated or within the reference range, LH is low with a consequent increase in the FSH/LH ratio, and relevant clinical symptoms are present (4). Clinical symptoms by themselves are not pathognomonic (4). Nonfunctioning pituitary adenomas are characterized by presence of mass effect symptoms including visual disturbances, headaches, and hypopituitarism, and the absence of hormone hypersecretion symptoms (3). They can be “totally silent,” where the adenomas do not secrete sufficient amounts of hormonal products to cause an elevation of the serum concentration, or “clinically silent,” where the hormonal products do not result in clinical signs or symptoms typical of that hormone (7).
FGAs produce intact and biologically active FSH with an increased bioactivity-to-immunoreactivity ratio (5). Increased bioactivity of the hormones can explain why patients with FGAs with “physiological” FSH levels can still cause symptoms of hormone excess (5). One possible explanation for this is that basic FSH isoforms are more active than acidic ones; this was shown in a case report of a male with an FGA where chromatofocusing analysis of FSH isoforms revealed more basic isoforms of FSH in the adenoma, as opposed to more acidic FSH isoforms with pH <5.5 in normal pituitaries and clinically nonfunctioning gonadotroph adenomas (8). The degree of glycosylation of the gonadotropins also affects their bioactivity, so secretion of glycosylated variants with increased biological activity may also play a role. Despite these hypotheses, the underlying mechanism driving the production and secretion of gonadotropins in FGAs still remains unclear (5).
As mentioned, in premenopausal women functioning gonadotroph adenomas may be associated with menorrhagia and OHSS. OHSS includes enlarged bilateral ovaries with multiseptated cysts of variable size, typically >5 cm in diameter, which is much larger than the ≤3 cm cysts found in polycystic ovaries (5,9). This diagnosis will only be seen in premenopausal females because the response of the ovary to FSH stimuli is important for the onset of the syndrome and in postmenopausal women the ovaries have been depleted of their preantral follicles, and are no longer sensitive to FSH stimulation (2,5).
Our patient presented with menorrhagia and severe anemia with ovarian cysts measuring between 5 to 10 cm. The cysts can have characteristic “soap-bubble” or “wheel-spoke” appearance as the enlarged follicles are arranged peripherally around ovarian parenchyma with normal stroma compressed between the follicles forming pseudosepta (5) (Fig. 2). In a 2015 review (10), 32 cases of FSH-secreting adenomas with OHSS were reported. Clinically, gonadotroph adenomas are macroadenomas, but have an invasion rate as low as 20% (11). In our case, there was some invasion into the right medial wall of the cavernous sinus, which is relatively rare, raising concern for a clinically aggressive pituitary adenoma (12–14). A 2017 paper (13) reviewing the World Health Organization classification of pituitary adenomas discusses how tumor subtyping, assessment of tumor proliferation potentials through mitotic count and Ki-67 index, as well as clinical parameters such as tumor invasion should be considered for detecting clinically aggressive adenomas.
Fig. 4.
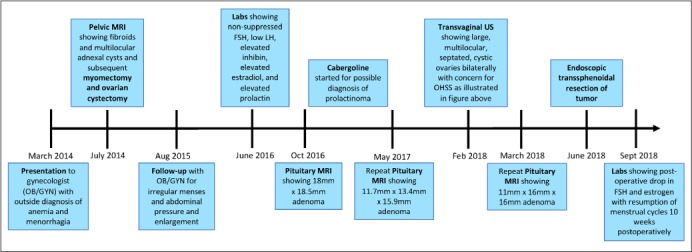
Timeline of events and interventions.
The characteristic hormonal profile in reported cases of gonadotropin-secreting OHSS, including in our case, shows normal-to-elevated FSH levels, suppressed LH levels, elevated estradiol levels, and supranormal concentrations of prolactin, mostly due to pituitary stalk compression (10). Cases with normal FSH concentrations capable of inducing OHSS could likely be due to enhanced FSH bioactivity (10). Low LH levels typically seen are thought to be primarily due to estrogen-induced negative feedback or compression of normal pituitary tissue. While the majority of cases of OHSS involve FSH-secreting tumors with the hormonal profile described above, several of these have some positivity for LH on histology. There has also been a report of an LH-secreting pituitary adenoma causing OHSS, where FSH and LH levels were normal, but estradiol was elevated (15).
Elevated inhibin levels can also be seen in patients with FSH-secreting adenomas (5), including our patient. This is because inhibin A is produced by luteinized granulosa cells under the stimulation of FSH and will therefore be elevated when there is increased secretion of FSH. Inhibin A typically acts to decrease FSH secretion via negative feedback, but decreased inhibin responsiveness of the pituitary adenomas could possibly contribute to the persistence of autonomous FSH secretion (16). In many of the case reports, the patients also had elevated testosterone levels, another feature seen in our patient. This is likely due to LH-driven stimulation of the thecal cells resulting in increased androgen production. Under the control of FSH, these androgens can be aromatized to estradiol in the granulosa cells, further increasing the estradiol levels (17). The maximum estradiol levels in reported cases of OHSS caused by gonadotroph pituitary adenomas ranged from 29 to 18,617 pg/mL with an average of 2,554 pg/mL (10). Only 3 cases had levels similar to or above our patient's estradiol level of 8,372 pg/mL. Estrogen is associated with prothrombotic alterations in coagulation proteins and has been commonly associated with hypercoagulation (18). Despite the very high levels of estrogen, OHSS caused by gonadotropin-secreting adenomas does not typically present with ascites or hypercoagulability for unclear reasons (15). Only 1 case report describes a patient with an FGA and estradiol levels reaching 18,617 pg/mL who presented with thromboembolism (19). However, the patient presented with the thromboembolic event during gestation, which confounded the presentation (19). In other case reports, similar hypercoagulability has not been mentioned, as is the case with our patient (5,10).
OHSS secondary to an FSH-secreting pituitary adenoma is seen to resolve with successful transsphenoidal tumor resection of the pituitary adenoma. This leads to regression of the enlarged multicystic ovaries, restoration of regular menstrual cycles, and normalization of hormone levels, as was seen in our patient during early postoperative follow up (10). In cases with persistence, or recurrence of these tumors, ovarian hyperstimulation can recur, making the subsequent management challenging. In many of these reported cases, recurrent or residual tumors were treated with repeat transsphenoidal surgery, radiation therapy, or both (16,17,20–23).
Dopamine agonists such as bromocriptine and cabergoline have been shown to reduce estradiol levels and decrease ovarian size, improving OHSS, but have not been shown to be effective in tumor reduction. Similarly, somatostatin analogs, such as octreotide, have been shown to resolve OHSS due to tumor recurrence, but again had no effect on adenoma size (5,21). Gonadotropin-releasing hormone analogs have not been shown to be effective, and in some cases they increased gonadotropin secretion and exacerbated OHSS (5). Surgical excision of the gonadotroph adenoma can lead to restoration of normal gonadotropin secretion, ovarian function, menstrual cycles, and possibly fertility.
CONCLUSION
We describe the difference between functioning and nonfunctioning gonadotropin adenomas with the help of an interesting case. We also describe the pathogenesis of these tumors leading to the clinical presentation of FGAs, with emphasis on the underlying mechanisms behind the hormonal findings characteristically seen. We additionally illustrate the management challenges associated with FGAs given high incidence of menstrual irregularities, OHSS, and infertility. Increased awareness is important for early diagnosis, amelioration of hormonal hypersecretion sequelae, restoration of fertility, and minimization of pituitary mass effect consequences to ensure optimal management of these tumors.
Abbreviations:
- FGA
functioning gonadotroph adenoma
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- OHSS
ovarian hyperstimulation syndrome
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Cooper O, Geller JL, Melmed S. Ovarian hyperstimulation syndrome caused by an FSH-secreting pituitary adenoma. Nat Clin Pract Endocrinol Metab. 2008;4:234–238. doi: 10.1038/ncpendmet0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawaguchi T, Ogawa Y, Ito K, Watanabe M, Tominaga T. Follicle-stimulating hormone-secreting pituitary adenoma manifesting as recurrent ovarian cysts in a young woman--latent risk of unidentified ovarian hyperstimulation: a case report. BMC Res Notes. 2013;6:408. doi: 10.1186/1756-0500-6-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caretto A, Lanzi R, Piani C, Molgora M, Mortini P, Losa M. Ovarian hyperstimulation syndrome due to follicle-stimulating hormone-secreting pituitary adenomas. Pituitary. 2017;20:553–560. doi: 10.1007/s11102-017-0817-7. [DOI] [PubMed] [Google Scholar]
- 4.Cote DJ, Smith TR, Sandler CN et al. Functional gonadotroph adenomas: case series and report of literature. Neurosurgery. 2016;79:823–831. doi: 10.1227/NEU.0000000000001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntali G, Capatina C, Grossman A, Karavitaki N. Clinical review: functioning gonadotroph adenomas. J Clin Endocrinol Metab. 2014;99:4423–4433. doi: 10.1210/jc.2014-2362. [DOI] [PubMed] [Google Scholar]
- 6.Vargas G, Balcazar-Hernandez LJ, Melgar V et al. An FSH and TSH pituitary adenoma, presenting with precocious puberty and central hyperthyroidism. Endocrinol Diabetes Metab Case Rep. 2017;2017 doi: 10.1530/EDM-17-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayson SE, Snyder PJ. Silent (clinically nonfunctioning) pituitary adenomas. J Neurooncol. 2014;117:429–436. doi: 10.1007/s11060-014-1425-2. [DOI] [PubMed] [Google Scholar]
- 8.Pigny P, Henric B, Lahlou N et al. A gonadotroph adenoma with a high proportion of basic FSH isohormones by chromatofocusing. J Clin Endocrinol Metab. 1996;81:2407–2408. doi: 10.1210/jcem.81.6.8964889. [DOI] [PubMed] [Google Scholar]
- 9.Macchia E, Simoncini T, Raffaelli V, Lombardi M, Iannelli A, Martino E. A functioning FSH-secreting pituitary macroadenoma causing an ovarian hyperstimulation syndrome with multiple cysts resected and relapsed after leuprolide in a reproductive-aged woman. Gynecol Endocrinol. 2012;28:56–59. doi: 10.3109/09513590.2011.588758. [DOI] [PubMed] [Google Scholar]
- 10.Halupczok J, Kluba-Szyszka A, Bidzińska-Speichert B, Knychalski B. Ovarian hyperstimulation caused by gonadotroph pituitary adenoma--review. Adv Clin Exp Med. 2015;24:695–703. doi: 10.17219/acem/25212. [DOI] [PubMed] [Google Scholar]
- 11.Young WF, Jr, Scheithauer BW, Kovacs KT, Horvath E, Davis DH, Randall RV. Gonadotroph adenoma of the pituitary gland: a clinicopathologic analysis of 100 cases. Mayo Clin Proc. 1996;71:649–656. doi: 10.1016/S0025-6196(11)63002-4. [DOI] [PubMed] [Google Scholar]
- 12.Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;39:758–766. doi: 10.1016/j.humpath.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Mete O, Lopes MB. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr Pathol. 2017;28:228–243. doi: 10.1007/s12022-017-9498-z. [DOI] [PubMed] [Google Scholar]
- 14.Trouillas J, Roy P, Sturm N et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126:123–135. doi: 10.1007/s00401-013-1084-y. [DOI] [PubMed] [Google Scholar]
- 15.Castelo-Branco C, del Pino M, Valladares E. Ovarian hyperstimulation, hyperprolactinaemia and LH gonadotroph adenoma. Reprod Biomed Online. 2009;19:153–155. doi: 10.1016/s1472-6483(10)60065-x. [DOI] [PubMed] [Google Scholar]
- 16.Garmes HM, Grassiotto OR, Fernandes YB et al. A pituitary adenoma secreting follicle-stimulating hormone with ovarian hyperstimulation: treatment using a gonadotropin-releasing hormone antagonist. Fertil Steril. 2012;97:231–234. doi: 10.1016/j.fertnstert.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Christin-Maitre S, Rongières-Bertrand C, Kottler ML et al. A spontaneous and severe hyperstimulation of the ovaries revealing a gonadotroph adenoma. J Clin Endocrinol Metab. 1998;83:3450–3453. doi: 10.1210/jcem.83.10.5182. [DOI] [PubMed] [Google Scholar]
- 18.Trenor CC, III, Chung RJ, Michelson AD et al. Hormonal contraception and thrombotic risk: a multidisciplinary approach. Pediatrics. 2011;127:347–357. doi: 10.1542/peds.2010-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanaya M, Baba T, Kitajima Y et al. Continuous follicle-stimulating hormone exposure from pituitary adenoma causes periodic follicle recruitment and atresia, which mimics ovarian hyperstimulation syndrome. Int J Womens Health. 2012;4:427–431. doi: 10.2147/IJWH.S33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djerassi A, Coutifaris C, West VA et al. Gonadotroph adenoma in a premenopausal woman secreting follicle-stimulating hormone and causing ovarian hyperstimulation. J Clin Endocrinol Metab. 1995;80:591–594. doi: 10.1210/jcem.80.2.7852525. [DOI] [PubMed] [Google Scholar]
- 21.Karapanou O, Tzanela M, Tamouridis N, Tsagarakis S. Gonadotroph pituitary macroadenoma inducing ovarian hyperstimulation syndrome: successful response to octreotide therapy. Hormones (Athens) 2012;11:199–202. doi: 10.14310/horm.2002.1347. [DOI] [PubMed] [Google Scholar]
- 22.Pentz-Vidovíc I, Skorić T, Grubisić G et al. Evolution of clinical symptoms in a young woman with a recurrent gonadotroph adenoma causing ovarian hyperstimulation. Eur J Endocrinol. 2000;143:607–614. doi: 10.1530/eje.0.1430607. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JE, Spandorfer S, Fasouliotis SJ, Lin K, Rosenwaks Z. Spontaneous ovarian hyperstimulation caused by a follicle-stimulating hormone-secreting pituitary adenoma. Fertil Steril. 2005;83:208–210. doi: 10.1016/j.fertnstert.2004.06.061. [DOI] [PubMed] [Google Scholar]


