Abstract
Objective:
To report 2 interesting cases of pituitary metastasis (PM) with differing presentations, and briefly review the literature regarding the incidence, presentation, and natural history of PMs.
Methods:
Case report and literature review.
Results:
Patient 1 had known widely metastatic papillary thyroid cancer and presented with signs and symptoms of anterior pituitary dysfunction. He was found to have an undetectable AM cortisol. Further lab evaluation showed complete anterior panhypopituitarism. Pituitary magnetic resonance imaging (MRI) revealed an infiltrative pituitary lesion, which was presumed to be a meta-static lesion from his thyroid cancer. Patient 2 presented with an acute increase in urinary frequency and polydipsia. Water deprivation testing confirmed central diabetes insipidus (DI). Brain MRI showed an infiltrative pituitary lesion, which was the first sign of recurrent metastatic colon cancer. Subsequently, he developed the rapid onset of complete anterior panhypopituitarism. Review of the literature shows that when PMs produce symptoms, the most common presentation has traditionally been central DI. However, this is shifting as the incidence of anterior dysfunction and cranial nerve abnormalities is rising in more recent reviews.
Conclusion:
Central DI has traditionally been the most common presenting symptom of PM, however symptoms reflective of anterior pituitary dysfunction and cranial nerve abnormalities are being reported with increasing frequency. PM should remain in the differential in any form of pituitary dysfunction.
INTRODUCTION
Pituitary metastases (PMs) are rare and often overlooked in the differential diagnosis of pituitary lesions. Classically, symptomatic PMs present with diabetes insipidus (DI), but more recent reporting suggests a rising prevalence of other presentations, particularly anterior pituitary dysfunction (1–3). While PMs often come from breast and lung malignancies, they are reported in over 30 types of cancers and should be considered in any cancer patient with a pituitary lesion (3). Here we report 2 cases of PMs related to uncommon primary cancer types that presented in contrasting ways. We review the most recent literature to highlight the newest trends in presentation, primary cancer, and prognosis.
CASE REPORT
Case 1
An 86-year-old male was admitted with 2 months of progressive weakness, fatigue, and decreased appetite; he denied increased urinary frequency. He had a history of poorly differentiated papillary thyroid carcinoma (PTC) diagnosed 15 years prior, treated with a thyroidectomy, multiple radioactive iodine treatments, and repeat neck surgeries to treat persistent disease. He had known pulmonary, abdominal, and skeletal metastases. His most recent thyroglobulin was 27,405 ng/mL with negative thyroglobulin antibodies. Initially, he was found to have anemia and mild hyponatremia. Further evaluation showed a fully suppressed thyroid stimulating hormone (TSH) despite a normal free thyroxine (FT4), an extremely low 8:00 am cortisol, undetectable adrenocorticotropic hormone, low luteinizing hormone, low to normal follicle-stimulating hormone, and mildly elevated prolactin (Table 1). A pituitary magnetic resonance image (MRI) showed an infiltrative process enlarging the pituitary gland, extending through the stalk and into the hypothalamus as well as other cerebral lesions, concerning for metastatic disease (Fig. 1).
Table 1.
Case 1 Laboratory Values
| Lab test | Result | Normal range |
|---|---|---|
| Hgb | 9.4 g/dL | 13.5–17.9 g/dL |
| Na | 135 mEq/L | 136–145 mEq/L |
| 8:00 am cortisol | 0.8 mcg/dL | 4.3–22.4 mcg/dL |
| ACTH | <5 pg/mL | 6–50 pg/mL |
| TSH | <0.01 uIU/mL | 0.3–5.0 uIU/mL |
| Free T4 | 1.43 ng/dL | 0.76–1.46 ng/dL |
| LH | 0.12 mIU/mL | 3.1–34.6 mIU/mL |
| FSH | 1.53 mIU/mL | 1.4–18.1 mIU/mL |
| Prolactin | 38.94 ng/mL | 2.1–17.7 ng/mL |
Abbreviations: ACTH = adrenocorticotropic hormone; FSH = follicular stimulating hormone; Hgb = hemoglobin; LH = luteinizing hormone; Na = sodium; T4 = thyroxine; TSH = thyroid stimulating hormone.
Fig. 1.
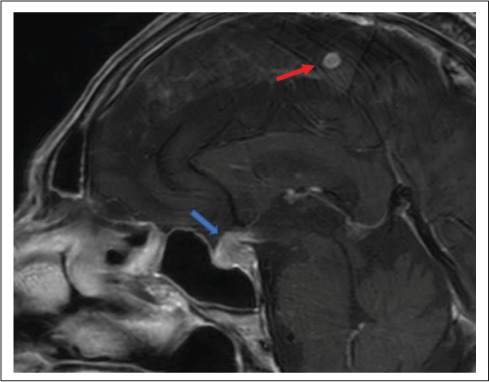
Case 1 pituitary MRI, sagittal view. Blue arrow = enlarged pituitary gland with enhancing, thickened stalk; red arrow = cerebral metastasis. MRI = magnetic resonance imaging.
Given these findings, he was diagnosed with anterior panhypopituitarism due to presumed metastatic PTC. His symptoms and hyponatremia improved with hydrocortisone replacement. He never developed DI, visual disturbance, or cranial neuropathies. He completed 3000 centigray (cGy) of palliative radiotherapy to his pituitary mass. Subsequent MRI showed diminished size of his pituitary lesion. He was started on lenvatinib and had a biochemical response with reduction in his thyroglobulin to 17,219 ng/mL within 2 months. Unfortunately, he could not tolerate the therapy and it was discontinued. Over the next 2 months his thyroglobulin rose to 71,000 ng/mL. Due to progression of his disease he enrolled in hospice care and passed away 7 months after presenting with panhypopituitarism.
Case 2
A 56-year-old male with a history of melanoma (resected), heavy tobacco-use, and remote stage IIIb colorectal cancer was referred for a 3-week history of self-reported excessive thirst, increased urinary frequency, and nocturia. Initial labs showed normal glucose, sodium, and serum osmolality with low urine osmolality (Table 2). An immediate inpatient water deprivation test confirmed central DI. Further labs revealed intact anterior pituitary function (Table 2). A pituitary MRI was obtained and showed an infiltrative process within the pituitary gland, stalk, and hypothalamus concerning for metastatic disease (Fig. 2). Positron emission tomography scan confirmed multiple sites concerning for metastasis including lesions in the lung, kidney, lymph node, and thoracic spine. Given his heavy smoking and history of multiple malignancies, mediastinal lymph node and renal mass biopsies were done and confirmed recurrent colorectal adenocarcinoma.
Table 2.
Case 2 Lab Results
| Lab test | Week 1 result | Week 4 result | Normal range |
|---|---|---|---|
| Glucose | 96 mg/dL | 70–100 mg/dL | |
| Sodium | 145 mEq/L | 136–145 mEq/L | |
| Serum Osm | 299 mOsm/kg | 276–305 mOsm/kg | |
| Urine Osm | 92 mOsm/kg | 500–800 mOsm/kg | |
| 8:00 am Cortisol | 19.3 mcg/dL | 2.3 mcg/dL | 4.3–22.4 mcg/dL |
| Total testosterone | 201 ng/dL | 21 ng/dL | 241–827 ng/dL |
| LH | 3.3 mIU/mL | 1.5–9.3 mIU/mL | |
| TSH | 0.75 uIU/mL | 0.11 uIU/mL | 0.3–5 uIU/mL |
| Free T4 | 0.81 ng/dL | 0.67 ng/dL | 0.76–1.46 ng/dL |
Abbreviations: LH = luteinizing hormone; Osm = osmolality; T4 = thyroxine; TSH = thyroid stimulating hormone.
Fig. 2.
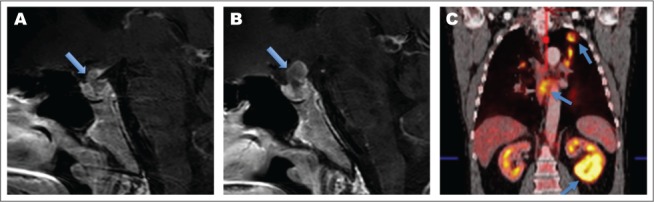
Case 2 imaging. A, Initial pituitary MRI, sagittal view, time 0. Arrow showing thickened stalk. B, Follow-up MRI, sagittal view, time 4 weeks. Arrow showing continued stalk enlargement, highlighting rapid growth. C, Initial PET scan. Arrows showing left lung mass, mediastinal lymphadenopathy, and a renal mass. MRI = magnetic resonance imaging; PET = positron emission tomography.
He was treated with desmopressin and referred to radiation oncology for palliative treatment of his PM. However, 4 weeks later, prior to initiating radiation, he developed severe fatigue, orthostatic lightheadedness, and hot flashes; he denied any visual disturbance. Labs confirmed the development of anterior pituitary dysfunction with low 8:00 am cortisol, total testosterone, TSH, and FT4 (Table 2). Repeat MRI confirmed growth of his PM (Fig. 2). He was started on hydrocortisone, levothyroxine, and testosterone, and was referred for urgent visual field testing which returned normal.
He symptomatically improved with hormone replacement and completed radiation to his pituitary gland (3,000 cGy). He was started on chemotherapy with folinic acid, 5-fluorouracil and oxaliplatin, then irinotecan and oxaliplatin, but was intolerant due to side effects so therapy was discontinued. He had disease progression and developed visual field loss. Despite treatment he passed away 9 months after his initial presentation.
DISCUSSION
PMs are rare, comprising less than 1% of pituitary lesions found on transsphenoidal surgeries, and less than 1% of all intracranial metastases (2,3). However, autopsy studies have shown incidental PM rates of 2 to 13% in patients with known cancer, so they may be more common than we realize (2,3). Pituitary lesions in cancer patients are most often metastases, however 1 to 16% of pituitary lesions found in cancer patients are benign adenomas, so differentiation is important (2). Although particularly rare, symptomatic PMs are important as disruption of the hormonal cascade can cause serious downstream effects, as seen in both of our cases.
While many cancers have been reported to metastasize to the pituitary gland, breast and lung are the most common and represent over 60% of reported PM cases (3). In our first case, PTC was the presumed cause. Thyroid cancer is a rare cause of PM, with an incidence of 2 to 3% of all reported PMs (2,3). While PTC is the most common type of thyroid cancer, it accounts for less than 50% of reported PMs of thyroid origin, likely due to its typically less aggressive nature (3–7). In case 2, while the PM was clearly from metastatic colorectal cancer as evidenced by the biopsies, this patient was a heavy smoker with a history of melanoma, and had lesions found in the kidney, lung, spine, and lymph nodes with no lesions in the colon. This made other primary sites an initial consideration. Interestingly, PMs from lung, renal, and melanoma origin are all more commonly reported than PMs from colorectal origin, with rates of 24.2%, 4.9%, 3.1% and 2.8%, respectively (3).
In addition to differing sites of primary malignancy, these cases differed in clinical presentation. A previous review on this subject has reported that only 2.5 to 18.2% of PMs are symptomatic (2). Three larger reviews have investigated the symptoms associated with PMs and, interestingly, the most commonly reported presenting symptom has changed over time. In a 1989 review, 70% of symptomatic PMs presented with central DI, while only 15% presented with anterior pituitary deficiencies (1). A review in 2004 reported a decrease in DI rates to 42% with increasing rates of anterior dysfunction to 23.6% (2). Lastly, the most recent review in 2015 showed a further decrease in the rate of DI to 32.8% and the rate of anterior dysfunction higher at 39.7% (3). The reason for this changing trend in PM symptomatology is unclear, but it may be related to publication bias or differences in reported primary cancer sites which may have differing predilection towards certain presenting symptoms. Nevertheless, given the increasing prevalence of anterior dysfunction in PMs, it is important to take vague symptoms like nausea, abdominal pain, fatigue, weight loss, and lightheadedness seriously in cancer patients, and consider evaluation for pituitary hormonal disruption and malignant infiltration of the pituitary.
Another important aspect in the diagnosis of PM is imaging characteristics. Unfortunately, imaging alone is often inadequate to correctly diagnose PMs, as findings can overlap with other pituitary pathology. In patients with widely metastatic cancers, it can often be presumed that a pituitary lesion is a metastasis, but occasionally pituitary symptoms and lesions are the first sign of a malignancy, so imaging findings may become more important in building the differential. The most common MRI findings in reported PM cases are sellar- and suprasellar-enhancing masses, stalk enhancement with thickening, dumbbell shaped lesions due to indentation by the diaphragma sellae, bony erosion without sellar enlargement, and rapid tumor growth over time (2,3). Both of our cases had thickening and enhancement of the infundibulum, and the second case also demonstrated rapid tumor enlargement. These imaging findings paired with each patient's clinical history made PM the most likely diagnosis.
Unfortunately, PMs hold a poor prognosis with a mean survival of 6 to 7 months after diagnosis, and 10 to 36% 1-year-survival rate (2,8). Our patients displayed the rapid mortality often seen in patients with PMs, both passing within 1 year of diagnosis. The rapid progression to death is not related to pituitary dysfunction but rather to the aggressiveness of the underlying cancer (2). Findings associated with shorter survival are in patients over 65 years, metastasis from small cell lung cancer, and short time-frame between cancer diagnosis and PM detection (2). Interventions such as surgery, chemotherapy and radiation, including stereotactic radiosurgery, do not improve survival but may help preserve visual fields and pituitary function (2,3,9).
CONCLUSION
PMs are rare but have been reported with increasing frequency in the literature. In the past, the most common symptomatic presentation of PM was DI; however, over time the most common presentation has shifted away from DI and toward anterior dysfunction. Prognosis remains poor for patients with PM, with a mean survival time after diagnosis of 6 to 7 months. Unfortunately, no therapies have been shown to improve survival in these patients. Overall, although rare, PM should remain in the differential of symptomatic pituitary dysfunction so proper diagnosis and treatment can be performed.
Abbreviations
- DI
diabetes insipidus
- MRI
magnetic resonance imaging
- PM
pituitary metastasis
- PTC
papillary thyroid carcinoma
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Mccormick PC, Post KD, Kandji AD, Hays AP. Metastatic carcinoma to the pituitary gland. Br J Neurosurg. 1989;3:71–79. doi: 10.3109/02688698909001028. [DOI] [PubMed] [Google Scholar]
- 2.Komninos J, Vlassopoulou V, Protopapa D et al. Tumors meta-static to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89:574–580. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 3.He W, Chen F, Dalm B, Kirby PA, Greenlee JDW. Metastatic involvement of the pituitary gland: a systematic review with pooled individual patient data analysis. Pituitary. 2015;18:159–168. doi: 10.1007/s11102-014-0552-2. [DOI] [PubMed] [Google Scholar]
- 4.Bhatoe HS, Badwal S, Dutta V, Kannan N. Pituitary metastasis from medullary carcinoma of the thyroid: case report and review of literature. J Neurooncol. 2008;89:63–67. doi: 10.1007/s11060-008-9586-5. [DOI] [PubMed] [Google Scholar]
- 5.Prodam F, Pagano L, Belcastro S et al. Pituitary metastases from follicular thyroid carcinoma. Thyroid. 2010;20:823–830. doi: 10.1089/thy.2009.0256. [DOI] [PubMed] [Google Scholar]
- 6.Santarpia L, Gagel RF, Sherman SI, Sarlis NJ, Evans DB, Hoff AO. Diabetes insipidus and panhypopituitarism due to intrasellar metastasis from medullary thyroid cancer. Head Neck. 2009;31:419–423. doi: 10.1002/hed.20911. [DOI] [PubMed] [Google Scholar]
- 7.Williams MD, Asa SL, Fuller GN. Medullary thyroid carcinoma metastatic to the pituitary gland: an unusual site of metastasis. Ann Diagn Pathol. 2008;12:199–203. doi: 10.1016/j.anndiagpath.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Morita A, Meyer FB, Laws ER., Jr. Symptomatic pituitary metastases. J Neurosurg. 1998;89:69–73. doi: 10.3171/jns.1998.89.1.0069. [DOI] [PubMed] [Google Scholar]
- 9.Kano H, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for pituitary metastases. Surg Neurol. 2009;72:248–255. doi: 10.1016/j.surneu.2008.06.003. [DOI] [PubMed] [Google Scholar]


