Abstract
The consumption of alcohol during gestation is detrimental to the developing central nervous system. One functional outcome of this exposure is impaired spatial processing, defined as sensing and integrating information pertaining to spatial navigation and spatial memory. The hippocampus, entorhinal cortex, and anterior thalamus are brain regions implicated in spatial processing and are highly susceptible to the effects of developmental alcohol exposure. Some of the observed effects of alcohol on spatial processing may be attributed to changes at the synaptic to circuit level. In this review, we first describe the impact of developmental alcohol exposure on spatial behavior followed by a summary of the development of brain areas involved in spatial processing. We then provide an examination of the consequences of prenatal and early postnatal alcohol exposure in rodents on hippocampal, anterior thalamus, and entorhinal cortex-dependent spatial processing from the cellular to behavioral level and highlight several unanswered questions that provide a framework for future investigation.
Keywords: fetal alcohol, navigation, spatial behavior, hippocampus, thalamus, entorhinal
1. Introduction
Alcohol is a known teratogen, that can freely cross the placenta, resulting in widespread damage and alterations to many systems in the developing embryo, including the nervous system (Guerri and Sanchis, 1985). Alcohol use during pregnancy has been directly linked to many adverse outcomes: spontaneous abortions (Henriksen et al., 2004), stillbirth (Kesmodel et al., 2002), premature birth (Albertsen et al., 2004; Kesmodel et al., 2000; Patra et al., 2011), intrauterine growth retardation (Patra et al., 2011; Yang et al., 2001), low birthweight (O’Callaghan et al., 2003; Patra et al., 2011), and cognitive impairments (discussed in Marquardt and Brigman, 2016). Fetal alcohol spectrum disorder (FASD) is the general term that encompasses all adverse effects associated with prenatal alcohol exposure. While there is an increasing societal awareness that alcohol consumption during pregnancy can lead to numerous adverse outcomes, prenatal alcohol exposure remains one of the most common developmental insults (Day et al., 2002; Green et al., 2009; Thomas et al., 1998). Using data collected from the National Birth Defects Prevention Study, Ethen et al., (2009) estimated that during 1997 to 2002 one-third of women in the US consumed alcohol during pregnancy and 5–10% reported incidents of binge drinking defined as 4 or more standard drinks on one occasion. More recent evidence estimates that globally 9.8% of women consume alcohol while pregnant, and one out of every 67 women who consumed alcohol during pregnancy delivers a child with fetal alcohol syndrome (FAS) (Popova et al., 2017), which is the most severe and debilitating form of FASD (Chudley, 2005; Cook et al., 2016; Riley et al., 2011). Further, there is a growing body of evidence that suggests even moderate alcohol consumption during pregnancy, which is the most prevalent and underestimated alcohol consumption during pregnancy, can also lead to lifelong cognitive impairments such as working memory, behavioral flexibility, and deficits in episodic memory (Green et al., 2009; Mattson et al., 1999; Streissguth et al., 1991).
One of the most consistent behavioral and cognitive manifestations of developmental alcohol exposure in humans and in rodent models, even when the exposure is in moderate amounts, is deficits in spatial processing (see Fig. 1) (Hamilton et al., 2003; Marquardt and Brigman, 2016; Sutherland et al., 1997, 2000; Uecker and Nadel, 1996). For the purposes of this review, spatial processing is defined as sensing and integrating information pertaining to spatial navigation, or guided locomotion to and from locations within familiar or unfamiliar environments, as well as the ability to recognize previously learned spatial locations. The neurobiological basis of spatial processing has been linked to the hippocampal formation as well as the limbic thalamus (Fig. 2) (Bliss and Collingridge, 1993; Cullen and Taube, 2017; Epstein et al., 2017; Hardcastle et al., 2017; Lisman et al., 2017; Morris et al., 1982; Moser et al., 2017; O’Keefe and Nadel, 1978; Olton et al., 1979; Schmidt-Hieber and Nolan, 2017; Squire, 1992). This linkage is due in large part to the discovery of hippocampal-limbic neurons that fire action potentials correlated with the animal’s spatial behavior (Fig. 3). The hippocampus, which has received the most considerable attention with respect to the developmental impact of alcohol on cognition, contains neurons that discharge when animals are within particular locations of the environment and are called “place” cells (Fig. 3A). Neurons that fire as a function of the animal’s “head direction” have been identified in several cortical and thalamic limbic regions, but most prominently in the anterior thalamus (Fig. 3B). In addition, neurons that fire in several regions of the environment arranged in a hexagonal “grid” have been identified one synapse upstream in the medial entorhinal cortex (Fig. 3C). Together, these neural populations are thought to provide an animal with critical spatial information regarding their location and orientation in the environment and play a role in an animal’s self-localization in an environment and accurate navigation.
Figure 1.
The effects of developmental alcohol exposure on spatial navigation and memory tasks. Results of studies which utilized behavioral spatial navigation and memory tasks after exposing animals to alcohol prenatally or postnatally. Each row represents a different study or a different component of a study in the case of multiple groups or experiments. Rows are organized by exposure period throughout the three trimesters (color bars indicate exposure duration and dose) and the dose of alcohol in terms of blood alcohol content (each group of identical exposure periods is organized by dose). The color map illustrates the alcohol dose (dark = low; bright = high). Dose indicates the concentration of alcohol given with blood alcohol concentration (BAC) in parentheses in mg/dl when reported; (na) when not reported. In the “Results” columns, “Impaired” represents if the animals were impaired at completing the task. Experiments showing impairments are also indicated by gray shading. It’s important to note that, in some studies, behavior may have been different between the groups (different MWT probe behavior) yet performance (latency, errors, etc.) may have been identical. In those situations, the “Impaired” column would read “N” as each group was able to perform the task at similar levels. This table was adapted from and expanded on from (Marquardt and Brigman, 2016).
Figure 2.
Anatomy of rat brain. A. 3D Animation of a mouse brain. Locations of major regions involved with spatial navigation and memory are represented; Hippocampus, Anterior Thalamus, and Entorhinal Cortex. © 2015 Allen Institute for Brain Science. Allen Brain Atlas API.
Available from: brain-map.org/api/index.html. (B) Schematic showing the anatomical relationship between the hippocampus (HPC), anterior thalamic nuclei (ATN), and entorhinal cortex (EC). Note that the anterior thalamic nuclei project to the hippocampus indirectly via the postsubiculum (PoS), medial entorhinal cortex (mEC), and retrosplenial cortex (RSC). (C) Coronal section of a rat brain at anterior/posterior (AP) −1.78 with the anterior thalamic nuclei labeled. AD: anterodorsal nucleus thalamus; AV: anteroventral nucleus thalamus; AMd: anteromedial nucleus thalamic, dorsal part; IAM: interanteromedial nucleus thalamus; LD: laterodorsal nucleus thalamus. (D) Coronal section of a rat brain at AP −3.7 with the hippocampus labeled. DGlb: dentate gyrus, lateral blade; DGmb: dentate gyrus, medial blade. (E) Coronal section of a rat brain at AP −1.78 with the entorhinal cortex labeled. ENTm 1–6: entorhinal area, medial part, dorsal zone, layers 1–6; ENTL 1–6: entorhinal area, lateral part, layers 1–6. (B-D) A mid-sagittal section is represented above each coronal section with a red line indicating the AP coordinate for each coronal section. Atlas images obtained and adapted from (Swanson, 2004). Available from: http://larrywswanson.com/.
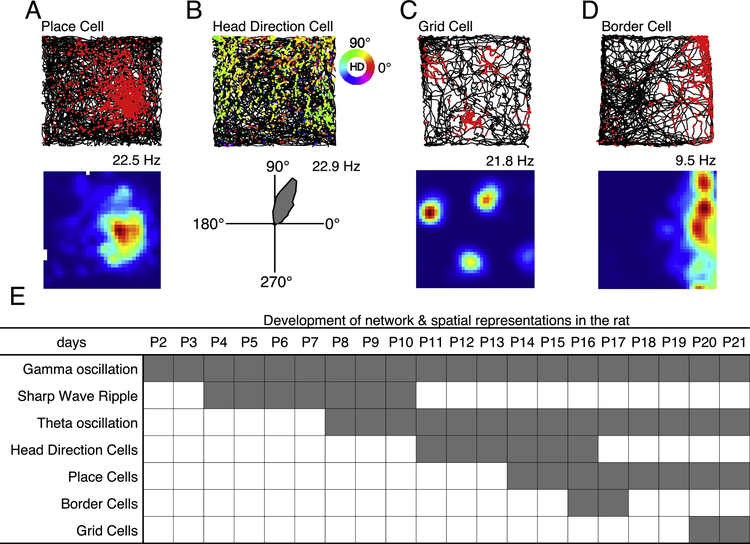
Figure 3.
Firing patterns of major cell types that may functionally constitute the cognitive map. (A) Place cell. Top: action potentials, or spikes, (red) on path (black). Note that the spikes cluster in one physical location. Bottom: Occupancy normalized heat map of the above place cell. Note that the warmer colors cluster in the same physical location as the above plot indicating that this cell is a place cell. (B) A head direction (HD) cell with a preferred firing direction at 72 degrees. Top: spikes on rat’s path with each spike color-coded by the rat’s head direction at the time of each spike. Note that most of the spikes in this plot are a mixture between yellow and green which indicates that this HD cell might have a preferred firing direction around 70 degrees when compared with the circular color bar to the right. Bottom: circular tuning curve representing the HD cell’s preferred firing direction and firing rate in spikes per second with a peak rate of 22.9Hz. (C) A grid cell with four firing fields. (D) Border cell that prefers the east most border. (E) Rodent postnatal developmental timeline, from postnatal day 2 through postnatal day 21, of hippocampal network activity (rows 1–3) and spatial cell emergence (rows 4–7). The start of each gray bar indicates the earliest reported emergence of the signal and the end of the bar indicates when each signal is mature or adult-like. (A-D) all example cells were compiled from ongoing unpublished projects. The numbers to the upper right of each lower plot indicate the peak firing rate of each cell in number of spikes per second. Top: spikes from an individual cell (colored dots) on rat’s path throughout the entire recording session (black).
A central aim of the present review is to provide a comprehensive summary of the prenatal and postnatal developmental impact of alcohol exposure on the neurobiology of spatial processing. First, we present a large body of evidence demonstrating that developmental alcohol exposure has a significant impact on spatial learning and memory. Our aim in this first section is to evaluate the range of behavior assessments used to investigate the integrity of spatial processing but to also highlight the specificity in behavioral deficits that follow developmental alcohol exposure. Secondly, we summarize research suggesting a linkage between spatial behavior deficits and underlying neurobiological changes. Although the neural systems involved in spatial processing likely involve a broad network of hippocampal and cortical-limbic and cortical-thalamic regions (Clark et al., 2018; Hinman et al., 2018), our review focuses on three central “hubs” within this network: the hippocampus proper, entorhinal cortex, and anterior thalamus. These three interconnected regions (Fig. 2B) have been shown to play a central role in the expression of place, grid, and head direction signals as well as accurate spatial navigation (Moser et al., 2017). In the sections below, we summarize evidence suggesting that these neural systems are significantly impacted by developmental alcohol exposure, which we argue may mediate deficits in spatial problem solving reported after prenatal and postnatal alcohol exposure. Lastly, our review summarizes a number of unanswered questions that could potentially provide a framework for future investigation. Because most memory tasks in humans are largely verbal in nature, the assessment of spatial processing may serve as an avenue for comparative research between humans and animal models of FASD. Further, although memory deficits associated with FASD have also been reported in non-spatial categories (object memory, working memory, etc.), we suggest that a thorough understanding of the neurobiological changes associated with spatial memory deficits should provide a foundation for extension to these other memory domains (Eichenbaum et al., 2007; Martin and O’Keefe, 1998; Squire, 1992).
2. Developmental Alcohol Exposure and Spatial Behavior
First, it must be clarified that gestation and development in rodents differ from that of humans. The human gestation period is characterized by three trimesters, all of which occur prenatally or before birth. In humans, a period of rapid neural growth occurs between weeks 25 and 38 of gestation in the third-trimester, peaks at birth, and then tapers off in early years (Dobbing and Sands, 1978). Compared to humans, the rat and mouse gestational period is much shorter (rats: 21–22 days; mice: 18–21 days), and rodent offspring continue to undergo neural development postnatally or after birth (Cronise et al., 2001; Tran, 2000; Wigal and Amsel, 1990). Because of this, the first two rodent trimesters occur prenatally, and the third-trimester occurs postnatally. Broken down, the first-trimester equivalent occurs on gestational days (G) 1–10, the second-trimester equivalent corresponds to G11–G18/22 and the third-trimester equivalent corresponds to postnatal (P) days P1–P10.
2.1. Morris water task
A large proportion of behavioral studies assessing spatial processing following alcohol exposure have largely made use of the Morris water task (MWT). The MWT is traditionally used to assess place learning, the ability to navigate to the precise location of the hidden platform within the pool (Fig. 4A) (Morris, 1984; Morris et al., 1982). Animals can also navigate by learning to swim in the direction of the platform; sometimes termed directional responding (Hamilton et al., 2007, 2008; Knierim and Hamilton, 2011). The basic procedure of the MWT involves placing an animal at a semi-random location near the border of a large circular pool filled with opaque water. In most applications of this task, the animal can escape the water by finding a hidden escape platform which is located a few centimeters below the surface of the water; other applications can use a cued platform. Over several trials, the animal learns to effectively locate the hidden platform, resulting in a decrease in the latency and cumulative swim distance to the platform location. Several studies indicate that animals can use a combination of cues to localize the platform including distal cues located along the testing room walls (Hamilton et al., 2007), the pool walls (Hamilton et al., 2008), and self-motion cues (e.g., optic flow, proprioceptive, and vestibular) generated from the animals transport to the pool (Clark et al., 2015; Stackman et al., 2012). To assess retention of place learning, probe tests can be performed at the end of training by removing the hidden platform and allowing the animals to swim for 60 sec. Impairments in the accuracy of spatial learning and memory is well documented following damage of the hippocampal formation (Morris et al., 1982), including damage of the entorhinal cortex, and the limbic thalamus (Clark and Harvey, 2016; Hales et al., 2014; Steffenach et al., 2005).
Figure 4.
Overhead schematic of spatial navigation tasks. (A) Morris water maze. (B) Radial arm maze. (C) T-Maze. (D) Y-Maze. (E) Object-place paired-associate task. Black shapes represent different objects. (B-D) Black circles represent reward locations.
As summarized in Figure 1, a general trend exists in which moderate developmental alcohol (blood alcohol content (BAC) ~ 7–120mg/dl) spares, but high dose developmental alcohol (>BAC 120mg/dl) impairs rodent spatial learning in the MWT. Several studies indicate that high dose prenatal alcohol exposure produces a clear detrimental effect on MWT performance as measured by increased escape latency during task acquisition and less focused search behavior at the platform location during probe trials (An and Zhang, 2013; Blanchard et al., 1987; Christie et al., 2005; Gianoulakis, 1990; Iqbal et al., 2004; Kim et al., 1997; Matthews and Simson, 1998). In addition, high dose postnatal alcohol exposure reportedly creates detrimental effects in this task (Girard and Wainwright, 2002; Goodlett and Johnson, 1997; Goodlett et al., 1987; Kelly et al., 1988; Thomas et al., 2007; Wagner et al., 2014). With respect to task acquisition, deficits in MWT performance following developmental alcohol exposure are dose-dependent. Specifically, task acquisition in the MWT is unaffected following moderate levels (BAC: 7–100mg/dl) of prenatal (Cullen et al., 2014; Hamilton et al., 2014; Rodriguez et al., 2016a; Savage et al., 2002; Sutherland et al., 2000) or postnatal alcohol exposure (Zink et al., 2011). In contrast, high dose alcohol exposure either prenatally or postnatally can significantly impair MWT acquisition (see Fig. 1). However, it is important to note that two studies assessing high dose (BAC: 265,350mg/dl) prenatal (Dursun et al., 2006) and postnatal (Goodlett and Johnson, 1997) alcohol exposure found unaffected MWT performance.
While task acquisition in the MWT is largely unaffected by moderate prenatal alcohol exposure, some studies demonstrate impaired flexibility or impaired spatial working memory for locations in the MWT (Hamilton et al., 2014; Rodriguez et al., 2016b; Sutherland et al., 2000). Assessments for behavioral flexibility or spatial working memory have been typically conducted by moving the platform to a new location in the pool during the probe test. In general, rats exposed to moderate prenatal alcohol exposure tend to perseverate in the previous platform location rather than acquire the new location (see Fig. 1) (Hamilton et al., 2014; Rodriguez et al., 2016b). In addition, deficits after moderate prenatal alcohol exposure have been reported during post-acquisition retention tests. For instance in Savage et al., (2010), animals exposed to a moderate dose of prenatal alcohol were given 12 training trials in a single session in the MWT followed by another 12 trials of training 4 days later. On the first day of training, rats exposed to moderate alcohol prenatally reached asymptotic performance similar to control animals. However, on the first trial of retention testing, the alcohol exposed animals performed significantly worse compared to control animals. In contrast, other studies have reported intact post-acquisition retention for the hidden platform (Cullen et al., 2014). For instance, Cullen et al., (2014) found that low dose alcohol exposure fails to impair spatial memory by rats 24hrs after MWT acquisition. However, Cullen et al., (2014) used a relatively smaller pool (110cm in diameter) during task acquisition and retention testing, perhaps limiting the demands on spatial learning and memory. Matthews and Simson, (1998) provided evidence of retention deficits in the MWT following a high dose exposure of alcohol prenatally in rats. This deficit was particularly apparent when the training-probe test interval was set at 3 days, but not after a 1-day interval. Together, these observations suggest that prenatal ethanol exposure can produce retention deficits after MWT training, provided that the training-testing delay is extended to at least 3 days.
2.2. Radial arm maze
The radial arm maze typically consists of eight equally spaced arms that radiate from a small circular central platform with the end of each arm containing a food reward (Fig. 4B) (Olton et al., 1979). The animal is trained to collect each food reward. In the spatial working memory variant of the task, all of the arms are baited with food and the animal is required to retrieve each of the food rewards. Entries into arms already visited are counted as spatial working memory errors. In the spatial reference memory variant of the task, a subset of the arms is baited, and the same arms are rewarded between testing sessions. Entries into unbaited arms are counted as spatial reference memory errors (Harvey et al., 2017). Although some studies have employed the radial arm maze task in order to examine developmental alcohol exposure (Fig. 1), these studies have preferentially used the spatial working memory variant of the task. In general, most studies report deficits in this task variant (Hall et al., 1994; Reyes et al., 1989). However, Sluyter et al., (2005) found no effect on radial arm maze performance after prenatal alcohol exposure. One explanation for this difference in results may be related to the fact that Sluyter et al., (2005) used adult mice (P90), while Hall et al., (1994) & Reyes et al., (1989) used adolescent rats (P26, P60). Thus, it is possible that maturation eliminated the deficit caused by prenatal alcohol. In contrast to these findings, Wozniak et al., (2004) found that radial arm maze performance was impaired in adult mice exposed to alcohol postnatally.
While the studies above evaluated the impact of high dose development exposure to alcohol, a recent study assessed spatial working memory in the radial arm maze after moderate prenatal alcohol exposure (Brady et al., 2012). The study measured performance by adult mice (P90–150) in a delayed non-match-to-place variant of the task. Briefly, the task required that animals discriminate between a recently visited maze arm and a second previously unavailable maze arm. Importantly, the authors varied the angular distance between the sample and choice arms with some tests involving a separation of two arms and in other tests the 4 arms between the sample and choice arms. Overall, the authors reported that alcohol exposed animals made a greater number of errors when the choice and sample arms were separated by two arms, but not when they were separated by four arms. One explanation for this finding is that the nearby or adjacent maze arms share similarities in the stimuli that define their spatial locations (i.e., distal cues that are visible from both maze arms). Thus, discrimination between the shared spatial features of these locations might be more challenging after developmental alcohol exposure.
2.3. Spatial alternation tasks
The second most used task to examine developmental alcohol exposure’s effects on spatial processing is the Y or T-maze alternation task (Fig. 1). Briefly, alternation tasks involve first placing an animal on the base of the ‘T’ or ‘Y’ and allowing them to choose one of the two goal arms (Fig. 4C–D) (Deacon and Rawlins, 2006; Lalonde, 2002). When two trials are given, the animal will tend to choose the arm not visited before. This tendency is then reinforced with selective rewards when the animal correctly alternates between the two arms. Thus, entries into previously visited arms are counted as spatial working memory errors. Importantly, this task is hippocampal and anterior thalamic-dependent as rats with hippocampal or anterior thalamic lesions are unable to correctly alternate between the two arms (Clark and Harvey, 2016; Lalonde, 2002).
Developmental alcohol exposure negatively affects the ability to accurately alternate on the Y and T-maze with prenatal (Lee and Rabe, 1999; Riley et al., 1979; Wainwright et al., 1990; Zimmerberg et al., 1989, 1991) and postnatal exposures (O’Leary-Moore et al., 2006; Thomas, 2004; Thomas et al., 1996, 1997). In addition, similar to the performance on the MWT, alternation task performance seems to be dose-dependent as rats with moderate prenatal (Cullen et al., 2014; Riley et al., 1979) and moderate postnatal alcohol exposure (Zink et al., 2011) are unaffected. Further, there is evidence that male rats are more affected than female rats following prenatal exposure (Zimmerberg et al., 1989, 1991). Zimmerberg et al., (1991) used a variant of the task to investigate the impact of prenatal alcohol on spatial working versus spatial reference memory performance. Spatial reference memory errors were measured by counting the number of visits down a “blind alley” that occupied a fixed location in the stem of the T maze. Overall, the authors reported that males and females with prenatal alcohol exposure made a greater number of spatial reference memory errors (blind alley entries) compared with controls, but only males made a greater number of spatial working memory errors (entries into previously visited arms).
2.4. Object-place paired-associate task
While moderate prenatal alcohol exposure seems to leave task acquisition in the MWT unaffected, performance deficits have been detected on dry land tasks in which animals are required to associate specific items with spatial locations. For example, Sanchez et al., (2019) exposed pregnant rats to a moderate amount of alcohol (BAC: 60mg/dl) throughout gestation and tested their adult offspring on an object-place paired associate task (Fig. 4E). In this task, rats are trained to discriminate between an identical pair of objects presented at two spatial locations. Rats are reinforced with a food reward only after selecting a specific object when it appears in a specific location, but not when it is presented in a second location. Sanchez et al., (2019) found that moderate prenatal alcohol exposure significantly disrupted the acquisition of this paired-associate task. Importantly, Sanchez et al., (2019) reported that rats exposed to moderate alcohol prenatally could accurately discriminate between objects. In addition, some recent preliminary findings indicate that performance is intact even when the two objects have significant overlap in their perceptual similarity (Sanchez et al., 2018), suggesting that deficits in this task are likely not related to the overall complexity of the task (e.g., Clausing et al., 1995). These observations also lend support for the conclusion that exposure to moderate alcohol prenatally appears to impair performance in tasks requiring the discrimination between two or more spatial locations (Brady et al., 2012).
2.5. Human spatial behavior
Several studies have reported impairments by human subjects with FAS and FASD in visual-spatial tasks (Aronson et al., 1985; Kaemingk and Halverson, 2000; Streissguth et al., 1989; Uecker and Nadel, 1996). In general, testing required that subjects accurately identify spatial relationships between features of an object, such as texture, color, size, or orientation on a paper or a 2D computer screen. Note that these tasks do not involve navigation, nor do they require the subject’s awareness of their own location with respect to the environment. Further, visual-spatial ability may rely on different or partially overlapping neural systems as spatial navigation (Broadbent et al., 2004; Eichenbaum et al., 2007; Kolb et al., 1994; Winters et al., 2004). Experiments involving an assessment of a subject’s awareness of the space around them, their location within this space, and how to navigate to and from different locations, has received less attention. To address this gap in the literature, Hamilton et al., (2003) assessed FAS’s effect on spatial processing by examining adolescent (9.5–16.5 years old) male’s performance on a virtual Morris water maze task. Participants were trained to virtually navigate to a hidden platform with several environmental cues available. This was followed with a probe trial where the platform was removed and a final set of trials in which the platform was visible. Hamilton et al., (2003) found that individuals with FAS traveled further to find the hidden platform during training and made indirect trajectories to the platform area during the probe session. Importantly, performance by subjects with FAS was similar to control subjects when the platform was visible. In addition, subjects with FAS and control subjects navigated at a similar speed. The latter similarities suggest that differences in visual acuity, motor control, and motivation are not likely contributing factors to the spatial navigation impairments detected in hidden platform testing. These findings are consistent with many rodent studies which found deficits in the MWT following developmental alcohol exposure, as discussed above.
Mattson et al., (2010) used an identical virtual Morris water maze task and found that children with FAS spent a greater amount of time in the target quadrant during the probe trial. While this finding does not suggest navigation or spatial memory impairment, it might suggest reduced behavioral flexibility in searching other spatial locations for the platform. Woods et al., (2018) examined children (9.4 years old) with FASD in a virtual environment visually similar to the virtual Morris water maze task used in Hamilton et al., (2003) during fMRI scanning. However, the task only involved passively viewing of a recording of another person navigating the environment to locate a hidden platform and did not require participant input. After the scan, the participants were then able to navigate the virtual environment on their own using a joystick to find a hidden platform location. Results revealed that while females with FASD were unaffected in their ability to navigate the virtual environment, FASD in males was associated with poorer performance as well as decreased activation of parahippocampal gyrus and temporal lobes. Similarly, sex differences have also been discovered in rodents. Specifically, rodent studies that have found DG LTP impairments in males but not females following developmental alcohol exposure (Patten et al., 2013a, 2013b; Sickmann et al., 2014). The mechanism behind this effect is currently unknown, however, some work suggests that sex difference maybe be due in-part to altered gonadal hormones or gonadal-adrenal interactions following prenatal alcohol exposure (Weinberg et al., 2008). Lastly, human navigation tasks following prenatal alcohol exposure have only been conducted in children with the main goal of finding better diagnostic criteria. Thus, it is unknown whether adult humans also show spatial processing impairments following prenatal alcohol exposure.
2.6. Summary and considerations for future investigation
A general aim of this first section was to summarize evidence suggesting a strong linkage between developmental alcohol exposure and deficits in spatial processing. As expected, spatial impairments are expressed across a broad range of behavior tasks following high dose alcohol exposure (MWT, radial arm maze, and spatial alternation). However, more subtle but specific deficits are observed after moderate alcohol exposure. Specifically, moderate prenatal alcohol exposure impairs the retention of a hidden platform location after MWT learning, but not task acquisition. In contrast to the MWT, moderate prenatal alcohol exposure can result in performance deficits in dry land maze tasks, particularly when rats are required to associate a specific object with a particular maze arm (object-place paired associate task), or when rats are required to discriminate between nearby maze arms.
The specific impairments described above could be mediated by a common neurobiological substrate. For instance, the radial arm maze and object-place tasks require that animals determine their spatial location and guide their actions toward objects or maze arms. Similarly, retention of prior MWT learning requires that animals initially self-localize in the environment to guide subsequent behavior in the pool. The capacity to discriminate between spatial locations and recall previously learning spatial relationships is thought to be a fundamental computational operation of the hippocampal formation. Indeed, it is well documented that performance in the MWT, radial arm maze, spatial alternation, and object-place paired associate tasks are hippocampal-dependent (Hunsaker et al., 2013; Lalonde, 2002; Lee and Kim, 2010; Morris et al., 1982). We have also noted that damage to extra-hippocampal regions such as the entorhinal cortex and anterior thalamus can produce a similar pattern of spatial deficits (Clark and Harvey, 2016; Hales et al., 2014; Steffenach et al., 2005). In the sections below we motivate the hypothesis that developmental alcohol exposure may involve modification to circuitry involving the hippocampus, anterior thalamus, and entorhinal cortex.
It is important to consider some caveats to the behavioral work summarized above. First, developmental alcohol exposure can produce deficits across a range of visual and sensory-motor domains, especially following high dose exposure (e.g., Hamilton et al., 2014; Lantz et al., 2014; Vernescu et al., 2012). Although disruption to the acquisition of these non-spatial variables could influence outcomes in spatial behavior tasks, some studies have controlled for this possibility. For instance, as described above, human subjects with FASD are capable of accurately performing tasks such as the cued platform variant of the virtual MWT wherein the goal location is visually marked thus controlling for visual acuity differences. Accurate performance in this task also suggests that FASD subjects are sufficiently motivated to complete the virtual MWT procedures. Further, moderate prenatal alcohol exposure does not disrupt the acquisition of hidden platform variants of the MWT(Cullen et al., 2014; Hamilton et al., 2014; Savage et al., 2010; Sutherland et al., 2000), nor does it impair discrimination between distinct objects or distinct maze arms (Brady et al., 2012; Sanchez et al., 2018). Again, accurate performance in these tasks suggest that differences in visual acuity or motivation are not central to the spatial deficits described after moderate alcohol exposure. In contrast to the findings above, some studies have reported visual discrimination deficits in rats after alcohol pre- or postnatal alcohol exposure at higher doses (Girard and Wainwright, 2002; Popović et al., 2006). However, other studies have reported intact object discrimination by rats and mice following high dose exposures of pre/postnatal alcohol (Kim et al., 1997; Wozniak et al., 2004).These latter studies highlight the importance of including assessments of non-spatial and motivational characteristics especially in models of high dose alcohol exposure.
Locomotor or exploratory activity, which is generally measured in short duration tests in large open fields, has been linked to the accurate processing of spatial information (O’Keefe and Nadel, 1978; Poulter et al., 2018; Thompson et al., 2018), and is commonly used to provide a general assessment of movement characteristics and motivation. Although measures of locomotor behavior have generally failed to detect activity differences after moderate alcohol exposure (Brady et al., 2012; Patten et al., 2016), high dose exposure rodent models have produced mixed results with some studies reporting elevated locomotor activity and others reporting no change (reviewed in Marquardt and Brigman, 2016). The latter discrepancy warrants further investigation. Because most experiment have used general measures of locomotor activity (distance traveled, movement speed, etc.) in a single test session, future studies could benefit from a more detailed characterization of locomotor activity coupled with daily testing of open field behaviors (e.g., Lehmann et al., 2007).
An additional consideration is that although previous studies have failed to detect spatial deficits in some tasks (e.g., MWT and spatial alternation), it is possible that different strategies are used to solve the spatial problem. In previous work using the MWT, detailed analysis of the swim path has revealed subtle differences not detectable with standard MWT measures (Berkowitz et al., 2018; Faraji et al., 2018; Garthe et al., 2009; Gehring et al., 2015). For instance, swim paths can be qualitatively or quantitatively categorized into non-spatial or spatial movements (Berkowitz et al., 2018; Garthe et al., 2009; Gehring et al., 2015; Graziano et al., 2003; Wolfer and Lipp, 2000). Escape latency may be increased if the use of non-spatial movements (e.g., thigmotaxis) are employed more often than spatial movements (e.g., direct swims to platform). Furthermore, animals may be able to use non-spatial movements more efficiently over time corresponding to improved escape latency over acquisition trials (e.g., Berkowitz et al., 2018; Garthe et al., 2009). Garthe et al., (2009) found that mice with suppressed hippocampal neurogenesis became more efficient at using swim movements that were not spatially directed, indicating the improvement in their performance was not solely attributable to spatial learning. Finally, path analysis has been shown to dissociate functional contributions from different hippocampal sub-regions in MWT performance (Ruediger et al., 2012). Overall, the use of path analysis methods during MWT acquisition may be able to reveal impairments that general MWT measures cannot completely capture.
3. Developmental Alcohol Exposure and the Neurobiology of Spatial Processing
The studies discussed above establish that deficits in spatial processing represent a consistent behavioral manifestation of developmental alcohol exposure. In the sections below, we turn our attention to the neurobiological bases of spatial processing impairments after developmental alcohol exposure, with particular emphasis on the hippocampus, entorhinal cortex, and anterior thalamus (Fig. 2). These three regions play a critical role in the establishment of neural representations of spatial location and directional orientation (Fig. 3) and the integrity of these regions have been linked with performance in the behavioral tasks summarized in the section above. In considering the impact of developmental alcohol exposure on these neural systems, we focus our attention on the precise timing of their development (Bayer, 1980a, 1980b; Bayer et al., 1993). Examining the timing of exposure is crucial because brain structures can be affected to a greater or lesser extent depending on the developmental timing of alcohol exposure (Adams-Chapman, 2009; Gressens et al., 1992; Guerri et al., 2009; Rubert et al., 2006). Thus, to better understand functional impairments following alcohol exposure, the following sections will review the development of brain areas involved in spatial processing, as well as the structural and functional outcomes after developmental alcohol exposure.
4. Influence of Alcohol Exposure on Hippocampal Development
The hippocampal formation has a crucial role in encoding spatial, (Morris et al., 1982) and episodic memories (Eichenbaum et al., 2007; Scoville and Milner, 1957). Briefly, the hippocampal formation is composed of multiple structures: hippocampus proper (dentate gyrus (DG), CA1, CA2, CA3), subicular complex (subiculum, presubiculum, postsubiculum, and parasubiculum), and entorhinal cortex (Fig. 2) (Amaral and Witter, 1989). Studies using mammalian subjects, including humans, nonhuman primates, and rodents are in agreement that selective lesions of the hippocampal formation are sufficient to produce severe impairments in certain forms of spatial learning, memory, and navigation (Squire, 1992). The hippocampus proper contains place cells which consistently increase in firing rate when an animal visits a particular location (O’Keefe, 1976) (Fig. 3A), with each hippocampal place cell firing in a unique environmental location thereby covering the spatial layout of each environment encountered by the animal. Together, the population activity is thought to form a “cognitive map” of space giving an animal a sense of location (O’Keefe and Nadel, 1978). Finally, it is important to note that while most place cell findings have emerged from rodent studies, place cells and place-like signals are found in multiple other species such as bats, non-human primates, and in humans (Ekstrom et al., 2003; Hori et al., 2005; Yartsev and Ulanovsky, 2013).
4.1. General overview of hippocampal development
In rats, hippocampal development starts at the latter half of the second-trimester and continues into adulthood. Hippocampal CA3 pyramidal cells arise between G16-G20 while reaching peak growth rates on G17, and CA1 pyramidal cells emerge between G17-G20 (Bayer et al., 1993) (Fig. 5). DG granule cells originate on G20 and continue to form postnatally (Bayer et al., 1993). While around 15% of DG cells are formed prenatally, approximately 85% of DG cells emerge after birth during the first postnatal week. This neurogenesis reduces during the second and third postnatal weeks so that the DG appears mature around weaning around P21 (Bayer et al., 1993), however DG neurogenesis continues to occur across the lifespan (Christie and Cameron, 2006). As for hippocampal interneurons, studies in mice have found that CA1 and CA3 GABAergic interneurons are generated between G12-G13 and between G13-G14 in the DG (Soriano et al., 1989a, 1989b). Concerning the development of incoming connections, synaptic fibers from the entorhinal cortex appear in CA1-CA3 around G17 and in DG around G18 (Ceranik et al., 1999; Supèr and Soriano, 1994). In humans, it is estimated that pyramidal cells in the hippocampus emerge from the 7th through the 14th week of development, and DG granule cells emerge on the 12th week then continue to emerge throughout the lifespan (Boldrini et al., 2018). Together, depending on the timing and duration of alcohol exposure, different hippocampal regions may be more susceptible to the teratogenic effects of alcohol.
Figure 5.
Estimated timelines of regional neurogenesis in rats and humans. The scale for rats is represented in days and the scale for humans is represented in weeks. Adapted from Bayer, Altman, Russo, & Zhang (1993). See figure 2 for anatomical locations of regions. Abbreviations: LD: lateral dorsal, AV: anteroventral, AM: anteromedial, AD: anterodorsal; CA: Cornu Ammonis.
Oscillatory activity emerges with the maturation of synapses early after birth. One contribution to oscillatory development includes spontaneous network driven events, such as giant depolarizing potentials (GDPs) (Ben-Ari et al., 1989; Griguoli and Cherubini, 2017). GDPs have been shown to originate in the hilus and successively propagate to areas CA3 and CA1 (Strata et al., 1997). These events are mediated by the maturation of GABAergic and Glutamatergic synapses between interneurons and pyramidal neurons, respectively (Ben-Ari, 2001). Rodent hippocampal local field potential (LFP) gamma band (30–100Hz) oscillations, which are thought to transiently coordinate interactions of excitation and inhibition (Buzsáki and Wang, 2012), begin to emerge as early as P2 and have a marked increase in power as hippocampal theta band (7–12Hz) oscillations emerge around P8 (Mohns and Blumberg, 2008). Both theta and gamma LFP oscillations continue to increase in power until they become adult-like around P21 (Wills et al., 2010), which coincides with the ability to complete spatial memory tasks such as the hidden platform MWT (Brown and Whishaw, 2000; Carman and Mactutus, 2001; Rudy et al., 1987; Schenk, 1985), radial arm maze (Rauch and Raskin, 1984), and T-maze (Green and Stanton, 1989). Hippocampal theta oscillations are strongly correlated with spatial processing (Buzsáki, 2005; Hasselmo, 2005; Kahana et al., 1999), are largely entrained by entorhinal and medial septal inputs, and are internally generated by CA3 collaterals as well as voltage-dependent Ca2+ currents in pyramidal cell dendrites (Buzsáki, 2002; Buzsáki and Moser, 2013). After gamma oscillations emerge at P2, immature sharp wave ripples (140–200Hz) have been recorded as early as P4, and mature ripples emerge around P10 (Leinekugel, 2002). These ripples arise from the excitatory recurrent CA3 system are thought to assist in the transferring of compressed neuronal sequences to distributed circuits in order to support memory consolidation (Buzsáki, 2015).
Immature place cells with poor spatial stability have been observed in rat pups as early as P14 (Muessig et al., 2015) (Fig. 3E). Adult-like place cells that form stable place fields are present at P16. However, further development occurs following P16 as place fields are able to represent locations away from boundaries only after grid cells (discussed below) in the medial entorhinal cortex emerge at P21 (Muessig et al., 2015). Coinciding with the maturation of network activity discussed above, once place cells fully mature, animals are then able to complete tasks that require spatial memory (Brown and Whishaw, 2000; Carman and Mactutus, 2001; Green and Stanton, 1989; Rauch and Raskin, 1984; Rudy et al., 1987; Schenk, 1985).
During sleep, the hippocampus is thought to engage in a process of memory consolidation which involves reactivation or replay. During slow wave sleep, hippocampal place cell firing sequences observed during recent locomotion will “replay” in a temporally ordered and faster timescale in series of sharp wave bursts (Lee and Wilson, 2002; Nádasdy et al., 1999; Skaggs and McNaughton, 1996; Wilson and McNaughton, 1994). These compressed sequences of place cell firing are hypothesized to underlie the network mechanism of memory consolidation (Dupret et al., 2010; Ego-Stengel and Wilson, 2009; Girardeau et al., 2009; McNamara et al., 2014; van de Ven et al., 2016). Recent evidence suggests that hippocampal replay gradually emerges beginning on P17 and becomes mature or adult-like on P32 (Muessig et al., 2019). Before replay maturity, hippocampal replay is only able to represent single stationary locations, and young rats gradually acquire the ability to combine the spiking of multiple place cells into a coherent compressed “replay” sequence as they age.
Together, the cellular prenatal and functional postnatal development of the hippocampus occurs gradually in a sequence-like manner. Alcohol exposure at any point of development might disrupt this sequence which in turn may disrupt hippocampal spatial processing.
4.2. Hippocampal cellular changes after developmental alcohol exposure
Following prenatal alcohol exposure, there is evidence of cell loss throughout the hippocampus. One of the first studies examining hippocampal cell counts following alcohol exposure focused attention on dorsal hippocampal pyramidal (CA1-CA3) and DG granule cells in rats following 15.5 g/kg of alcohol per day (unknown blood alcohol content (BAC)) from G10-G21 (Barnes and Walker, 1981). While they found 20% fewer CA1-CA3 cells at P60 (early adulthood), DG cells were unaffected. This finding is consistent when taken into context with the development of the hippocampus. CA1-CA3 cells emerge on days G15-G20, and DG cells start to emerge on G20. Thus, given that alcohol was present during CA1-CA3 development to a much greater extent than during DG’s development, DG neurons were spared in adulthood. A subsequent study by Miller, (1995) using a slightly different exposure period (G6-G21) and amount of alcohol (BAC 144mg/dl) also found fewer CA1-CA3 cells and unaffected DG cells at ~P30 (adolescence) in rats. Recent studies suggest however that prenatal alcohol exposure may have a greater impact on cell density later in life. For instance, studies using an expanded exposure period (G1-G20) found no differences in CA1-CA3 and DG cell counts in rats at P10 (Livy et al., 2003; Maier and West, 2001). In addition, a decline in DG neurogenesis has been reported in adult rats after prenatal alcohol exposure (Gil-Mohapel et al., 2014). However, other evidence suggests that only CA1 is affected in adulthood following a P1-P22 exposure (BAC~300mg/dl) while CA3 and DG neurons in rats are unaffected (Tran and Kelly, 2003), and another study using guinea pigs has found decreased CA1 cell counts in early life (P10) (Gibson et al., 2000), which contrasts with the work above. Taken together, these results suggest that specific hippocampal cell populations might be susceptible during different prenatal periods, but the outcomes largely depend on many different factors such as alcohol dose, timing of administration, type of administration, the age of testing, and cell counting methodology.
There is also evidence of hippocampal cell loss following postnatal alcohol exposure. For instance, West et al., (1986) exposed rat pups from P4-P10 and found that a BAC of 380 mg/dl did not affect CA1 or CA3, but it did cause a curious 10% increase in the cell density of DG neurons. In a following study, Bonthius and West, (1990) similarly exposed rat pups from P4-P10 and found that while BACs of 39.2 & 190.7mg/dl produced no effect on hippocampal density, a BAC of 361.1mg/dl reduced CA1 cell density and left CA3 and DG unaffected. This finding, observed at P10, was also observed in rats at P90 indicating that CA1 cell loss was permanent and unable to be rescued by maturity (Bonthius and West, 1991). With similar findings, Tran and Kelly, (2003) exposed rat pups from P2-P10 and found that a BAC of 398.2mg/dl reduced CA1 cell counts while sparing CA3 and DG in adulthood (P112-P174) which further provides evidence of life long changes following postnatal alcohol exposure. Inconsistent with the ongoing trend of impaired CA1 neurons and unimpaired CA3 and DG cells, Livy et al., (2003) exposed rat pups from P4-P9 and found that a BAC of 359mg/dl did result in reduced CA1 cell numbers at P10, however CA3 and DG were also affected with reduced cell counts.
4.3. Hippocampal interneuron density and function after developmental alcohol exposure
A recent series of studies have focused attention on the activation of apoptotic pathways in GABAergic hippocampal interneurons in rodents exposed to alcohol in the third trimester (Bird et al., 2018; Ogievetsky et al., 2017). Ogievetsky et al., (2017) found that apoptotic interneurons were most densely found in area CA1 and the hilus after an acute high dose of intraperitoneally injected alcohol. In addition, using mice Bird et al., (2018) found significant increases in apoptotic interneurons in area CA3, with non-significant but increased apoptotic interneurons in the hilus and CA1. Furthermore, interneuron counts in area CA3 were reduced by 11.9% at P90 compared to control counts. Though the results of these studies are slightly different, both indicate that alcohol affects the distribution of interneurons within the hippocampus. Additionally, cortical regions, of which provide input into upstream regions of the hippocampus, such as the entorhinal cortex, nucleus reuniens and parahippocampal cortex, also exhibit significant alterations in GABAergic interneurons (Moore et al., 1997; Skorput et al., 2015; Smiley et al., 2015). For instances, alcohol exposure in utero increases the rate of migration of GABAergic interneurons in the medial prefrontal cortex (Skorput et al., 2015), while parvalbumin positive interneurons are decreased in the anterior cingulate cortices (Moore et al., 1998).
GABAergic interneuron dysfunction could be contributing to hippocampal hyper-excitability observed after postnatal alcohol exposure. For instance, GABAergic interneurons in the hippocampus are thought to provide inhibitory control of principal pyramidal cells and loss of this inhibition could lead to a state of hyper-excitability. Zakharov et al. (2016) found that postnatal alcohol exposure led to hyper-excitability of CA3 hippocampal cells with increased frequency of giant depolarizing potentials (GDPs) and reduced firing synchronization of CA3 neurons in rats. Although GDPs were not specifically measured, Krawczyk et al. (2016) found that first trimester exposure to alcohol also results in hyper-excitable CA3 neuron responses whereby sharp wave ripple amplitude and duration are increased in mouse P15–21 hippocampal slice preparations. Importantly, these changes may result in long-term changes to hippocampal functional connectivity. Wilson et al. (2011) found that postnatal alcohol exposed adult mice exhibited enhanced coherence between the piriform cortex and dorsal hippocampus, enhanced dorsal hippocampus spontaneous activity, and increased theta and delta band power in the hippocampus during odor-evoked stimulation under anesthesia. Furthermore, although both control and alcohol exposed mice had no trouble habituating to- or identifying odors, alcohol exposed mice were impaired on an object-place memory task, indicating that this circuit level dysfunction may be particularly harmful for spatial memory.
4.4. Hippocampal plasticity after developmental alcohol exposure
Emerging evidence suggests that prenatal or postnatal exposure in mice and rats prevents typical development of plasticity within the hippocampus. One function of the hippocampus is to act as a putative cellular information storage mechanism through the process of synaptic plasticity (Bliss and Collingridge, 1993). Mice that lack NMDA receptor-mediated synaptic currents and long-term potentiation in CA1 synapses show deficits in performing a Morris water maze hidden platform task, indicating that hippocampal synaptic plasticity is essential for effective spatial memory (Tsien et al., 1996). Activity-dependent synaptic plasticity can occur through several different mechanisms, but here we will describe the developmental impact of alcohol on short and long-term synaptic plasticity.
Generally, short-term synaptic plasticity involves short-lasting changes, on the order of milliseconds to minutes, in synaptic strength or transmitter release resulting from several processes which occur in the presynaptic neuron (Dolphin et al., 1982; Zucker and Regehr, 2002). One experimental method to study short-term plasticity is a paired-pulse stimulation paradigm which involves the administration of two stimuli (voltage change) around 10–100ms apart while simultaneously listening for responses. The response in the post-synaptic neuron to the second stimulus can be increased or decreased (paired-pulse facilitation, paired-pulse depression) relative to the first stimulus. One of the first studies to examine alterations in short-term synaptic plasticity following prenatal alcohol exposure found that paired-pulse facilitation in CA1 was enhanced relative to controls over a range of interstimulus-intervals from 5–100ms in rats (Hablitz, 1986). This finding suggests that, after alcohol exposure, CA1 synapses have a lower release probability, which may inhibit glutamate release onto CA1 neurons. A following study found a dose effect where following prenatal alcohol exposure, paired-pulse facilitation in CA1 was enhanced only after a high dose (35% ethanol-derived calories (ECD)) of alcohol, whereas there was no effect following a low dose (17% ECD) in rats (Tan et al., 1990). While prenatal alcohol exposure produces negative effects on short-term CA1 plasticity, there is evidence that postnatal alcohol exposure spares CA1 function which suggests that CA1 may be particularly vulnerable to alcohol exposure during the first two trimesters (Bellinger et al., 1999).
Long-term synaptic plasticity involves long-lasting changes, on the order of hours to days, in synaptic strength or neurotransmitter release resulting from several processes which occur in the pre- and postsynaptic neuron. While previous work has demonstrated that developmental ethanol exposure can produce reductions in long-term experience-dependent structural changes associated with plasticity (e.g., changes in dendritic complexity and spine density; see Berman and Hannigan, 2000), an extensive body of work has focused attention on the impact of developmental alcohol exposure on long-term potentiation (LTP) and long-term depression (LTD) (Bliss and Gardner-Medwin, 1973; Bliss and Lømo, 1973). LTP is a persistent strengthening of synapses based on recent patterns of activity, while LTD is the long-lasting decrease in synaptic strength. LTP and LTD are believed to be necessary for the encoding and consolidation of new information as well as recall of previously gained information (discussed in Redondo and Morris, 2011).
Hippocampal CA1 LTD has been proposed to be necessary for effective spatial memory. Rats with blocked CA1 LTD show impaired spatial memory on a Morris water maze task, suggesting that LTD is necessary for the consolidation of long-term spatial memory (Ge et al., 2010). There have been only a hand full of studies which examined prenatal alcohol’s effect on hippocampal LTD. Izumi et al., (2005), using a single exposure on P0 or P7, found that CA1 LTD was reduced, while more recent evidence suggests that exposure through all three trimesters enhanced CA1 LTD suggesting a delay in synapse maturation (Kervern et al., 2015). Further, there is evidence that aberrant CA1 LTD following developmental alcohol exposure is dose dependent with no effects after a moderate dose (87mg/dl) (Titterness and Christie, 2012) and impaired LTD after a high dose (20% v/v ethanol solution) throughout the first two trimesters (An and Zhang, 2013; An et al., 2013).
Hippocampal CA1 LTP has also been proposed to be important for effective spatial memory as Sakimura et al., (1995) found that mice with impaired CA1 LTP display deficient spatial learning on the MWT. One of the first investigations of LTP following prenatal alcohol exposure (3% v/v ethanol solution, G1-G22) found decreased CA1 LTP (Swartzwelder et al., 1988). Since this initial study, several other groups have investigated CA1 LTP, but have come to inconsistent results. Several studies have supported the original finding of decreased CA1 LTP (An et al., 2013; Izumi et al., 2005; Kervern et al., 2015; Richardson et al., 2002), while several others have found no effect (Bellinger et al., 1999; Byrnes et al., 2004; Krahl et al., 1999; Tan et al., 1990). Other evidence has suggested a sex difference in hippocampal plasticity where following prenatal exposure, males have decreased CA1 LTP and females have increased CA1 LTP (An and Zhang, 2013).
Few studies have investigated developmental alcohol’s effects on CA3 LTP. Hippocampal CA3 has been long known to be crucial for accurate spatial navigation and memory (Handelmann and Olton, 1981). While many studies have focused on the effects of alcohol exposure on NMDA-dependent and AMPA-mediated field excitatory postsynaptic potential LTP in CA1, Zucca and Valenzuela, (2010) examined the effects of postnatal alcohol exposure on the hippocampal CA3 GABAergic system. They found that third-trimester equivalent moderate alcohol exposure (BAC: 23mg/dl) abolished brain-derived neurotrophic factor dependent LTP-GABAA in CA3 pyramidal neurons (Zucca and Valenzuela, 2010). This finding indicates that CA3 is functionally affected by postnatal alcohol exposure which may lead to a hindrance of spatial navigation and memory. Further, because hippocampal theta oscillations are generated internally by CA3 (Buzsáki, 2002; Buzsáki and Moser, 2013), one might also find altered hippocampal theta rhythms in animals with developmental alcohol exposure.
Studies that have examined LTP in the DG following prenatal alcohol have provided more consistent observations. Sutherland et al., (1997) exposed rats prenatally to a moderate amount of alcohol (BAC: 83mg/dl) and waited until offspring were 120 to 150 days old in order to assess synaptic differences in adulthood. They then measured rat’s field excitatory postsynaptic potentials and population spikes from the DG in response to perforant path stimulation and found significantly reduced LTP. This evidence suggests that even under moderate doses, DG LTP is affected. Subsequent studies using an assortment of moderate to high doses of prenatal alcohol have identified similar results that LTP in DG cells from adult male rodents is reduced after prenatal alcohol exposure (Brady et al., 2013; Christie et al., 2005; Helfer et al., 2012; Patten et al., 2013a, 2013b; Sickmann et al., 2014; Sutherland et al., 1997; Titterness and Christie, 2012; Varaschin et al., 2010, 2014). In addition, work by Brady et al., (2013) has indicated that reductions in DG LTP may reflect alterations in the subunit composition of NMDA receptors after moderate prenatal alcohol exposure.
Lastly, some studies have reported sex differences in the effects of developmental alcohol exposure on DG LTP. Specifically, increased DG LTP has been reported in adolescent (P30-P35) female rats (Titterness and Christie, 2012) and control-like DG LTP in early adulthood (P55-P70) (Patten et al., 2013a, 2013b; Sickmann et al., 2014). This evidence suggests that female rodents might have some mechanism that causes a protective effect, but this mechanism is currently unknown.
4.5. Neuroimaging and hippocampal function after developmental alcohol exposure
Less invasive procedures such as magnetic resonance imaging (fMRI) and functional MRI have been utilized to examine hippocampal morphological features and activity after developmental alcohol exposure. For instance, Joseph et al., (2014) reported that children (mean age 11.6 years old) with FAS and partial FAS differed from control subjects with respect to the physical shape of their hippocampi. While some studies suggest that hippocampal volume in children is relatively intact following prenatal alcohol exposure (Archibald et al., 2001; Treit et al., 2013), others have reported smaller left hemisphere hippocampi in children with FAS (Riikonen et al., 2007; Willoughby et al., 2008).
Using fMRI, Rodriguez et al., (2016) scanned rats that were exposed to a moderate amount of prenatal alcohol (5% v/v ethanol solution BAC: 60.8mg/dl) and found reduced functional connectivity between the hippocampus and other brain areas, suggesting network abnormalities in individuals exposed to alcohol during development. There is also evidence of diminished activation in the temporal lobe from children (8–13 years old) who were exposed to high levels of prenatal alcohol which further indicates network abnormalities following prenatal alcohol exposure (Sowell et al., 2007). Additionally, Woods et al., (2018) scanned children with FASD in an fMRI while they passively watch a recording of a first person’s view of a virtual MWT. They found that, among other affected regions, the parahippocampal gyrus and temporal lobes had reduced activation compared with the control group. Together, there is a consistent finding that the functional hippocampal network and hippocampal activation is diminished following prenatal alcohol exposure which may account for observed behavioral differences.
4.6. Hippocampal place cells and hippocampal-dependent spatial behavior after developmental alcohol exposure
The sections above summarize considerable evidence linking changes in morphology, neural activity, and synaptic plasticity in the hippocampus after prenatal alcohol exposure. However, we are unaware of published findings on the impact of developmental alcohol exposure on hippocampal place cell firing. Despite the absence of systematic investigation on developmental alcohol and hippocampal place cell function, we can speculate on several possible outcomes. First, future work investigating the impact of developmental alcohol on population firing characteristics of hippocampal place cells will be critical. For instance, changes in the balance between excitatory and inhibitory signaling can result in the desynchronization of firing by hippocampal neural populations (Marissal et al., 2018). Secondly, as discussed above, pre-and postnatal alcohol exposure produce large reductions in hippocampal synaptic plasticity, especially between entorhinal-to-DG synapses. Importantly, there is evidence that reductions in hippocampal LTP are associated with a decrease in the stability of place fields, i.e., hippocampal place fields tend to change their firing locations, or “remap” between recording sessions (Agnihotri et al., 2004; Barnes, 1979; Barnes and McNaughton, 1980; Barnes et al., 2000; Dieguez and Barea‐Rodriguez, 2004; Rotenberg et al., 1996, 2000). The absence of consistent, or stable, hippocampal place cell firing when encountering familiar environments could be related to the consistent spatial memory deficits reported after developmental alcohol exposure (see Fig. 1). Third, impairments in hippocampal LTP, NMDA receptor function, and hyperactivity in hippocampal circuitry have been linked to an animal’s difficulty in discriminating between overlapping spatial stimuli or retrieving a spatial memory from partial sensory information; behaviors often linked to the computational processes of pattern separation and pattern completion, respectively (Wilson, 2004; Wilson et al., 2003; reviewed in Yassa and Stark, 2011). Pattern separation is hypothesized to be critical for an animal’s ability to distinguish events that may include overlapping sensory features and spatial locations. There has been much evidence suggesting a dependence of pattern separation on the integrity of DG (for review, see Kassab and Alexandre, (2018) & Yassa and Stark, (2011). Briefly, the DG, which is composed of a dense population of granule cells, is thought to generate a sparse code from entorhinal cortex inputs (Knierim and Neunuebel, 2016; Leutgeb et al., 2007). Pattern completion is thought to involve CA3 neurons which act as an autoassociative network due to its dense recurrent collaterals.
It might be expected that hippocampal-dependent pattern separation or pattern completion operations are disrupted after developmental alcohol exposure. Support for this possibility come from the reported discrimination impairments observed in the radial arm maze (Brady et al., 2012), and acquisition deficits reported in the object-place task after moderate alcohol exposure (Sanchez et al., 2019). Both tasks are known to require intact DG and CA3 (Gilbert and Kesner, 2003; Lee and Solivan, 2010; also see Patten et al., 2016). It is important to point out that these studies were performed without parametrically manipulating the similarity of spatial stimuli, making it difficult to draw firm conclusions regarding pattern separation/completion (for discussion see Yassa and Stark, 2011). Future studies at the behavioral and neural population level are needed to address these issues.
5. Anterior Thalamic Nuclei Development and Developmental Alcohol
A large body of behavioral and electrophysiological studies have linked the anterior thalamus (ATN) to spatial processing (Clark and Harvey, 2016). Central to this conclusion was the discovery of head direction (HD) cells, which fire as a function as an animals HD and are hypothesized to provide an animal with their sense of direction (Cullen and Taube, 2017). HD cells (Fig. 3B) have been identified in the ATN but have also been described in a broad network of cortical and thalamic limbic system including the postsubiculum, entorhinal cortex, retrosplenial cortex, posterior parietal cortex, and lateral mammillary nucleus (Fig. 2) (Chen et al., 1994; Giocomo et al., 2014; Lozano et al., 2017; Stackman and Taube, 1998; Taube and Muller, 1998; Taube et al., 1990a, 1990b; Wilber et al., 2014). HD-like signals have also been identified in human retrosplenial cortex, thalamus, and precuneus using fMRI and virtual navigation (Shine et al., 2016). Research on the role of head direction cell activity in spatial navigation is still developing, however, several studies have shown that damage to brain regions containing HD cell populations can produce impairments in navigation (Aggleton et al., 1996; Beracochea et al., 1989; Clark & Harvey, 2016; Harvey et al., 2017; Mair et al., 2003; Mitchell and Dalrymple-Alford, 2006; for review see Weiss and Derdikman, 2018). Though many areas distributed throughout the brain contain head direction cells, this review will focus on the anterior thalamic nuclei due to the strength of HD signaling in this region and due to the fact that intact anterior thalamic nuclei are necessary for HD cell firing in downstream cortical and parahippocampal regions (Clark and Taube, 2012; Winter et al., 2015).
5.1. ATN is composed of several subnuclei
The ATN is comprised of anterodorsal (AD), anteroventral (AV), anteromedial (AM), and lateral dorsal (LD) nuclei, all of which contain a high percentage of HD cells compared with other limbic areas (Fig. 2A,C). It is estimated that the AD, in particular, is comprised of approximately 60% HD cells (Taube, 1995). AV also contains HD cells which are strongly theta modulated (Tsanov et al., 2011); a possible consequence of receiving the majority of its inputs from the medial mammillary nuclei whose neurons are also theta modulated (Sharp and Turner-Williams, 2005). Similarly, the AM has been found to contain HD cells (Jankowski et al., 2015) and receives input from the medial mammillary nuclei (Sharp and Turner-Williams, 2005). LD, which has also been found to contain HD cells (Mizumori and Williams, 1993), is hypothesized to guide spatial orientation and establish trajectories based on visual-somatosensory stimuli and proximal landmarks (Clark and Harvey, 2016). When the ATN is lesioned or inactivated, direction-specific firing properties of HD cells in downstream areas such as the postsubiculum, entorhinal cortex and parasubiculum are severely disrupted, which indicates that the ATN is involved in the generation or maintenance of the HD signal throughout the forebrain (Fig. 2B) (Goodridge and Taube, 1997; Winter et al., 2015). Thus, it is important to identify when the ATN is formed in utero in order to make inferences about how the timing of exposure might affect the function of this component of the brain’s spatial processing system.
5.2. General overview of ATN development
Coinciding with the rat’s equivalent second-trimester (G11-G20), the LD is the first anterior thalamic nuclei to go through neurogenesis starting at G14, peaking on G15, and finishing at G16, followed by AD, AM, AV starting at ~G15 and finishing at ~G17 (Bayer et al., 1993) (Fig. 5). In humans, it is estimated that ATN neurogenesis extends from the 6th through the 8th week of development.
Following birth, the HD signal is expressed prior to other spatial representations. Though directionally unstable, HD cells in rats have been recorded in the postsubiculum as early as P11 (Bjerknes et al., 2015) and in AD as early as P12 (Tan et al., 2015) (Fig. 3E). Note that the HD signal comes online ~3–4 days before rat pups first open their eyes, and is thus supported only by self-motion and textural or tactile sensory cues such as the geometric contours of the environment (Taube, 2007). It is not until pups open their eyes and start receiving visual landmark cue control that HD cells become directionally stable at around P16 (Bjerknes et al., 2015; Tan et al., 2015). There is also recent evidence that HD cells can become directionally stable in rat pups around P13-P14 in smaller environments rich with geometric and textural cues, which suggests that HD cells are internally consistent or organized before visual cues are present (Bassett et al., 2018).
5.3. ATN cellular changes after developmental alcohol exposure
While much is known about hippocampal cellular changes following developmental alcohol exposure, fewer studies have examined the ATN. Granato et al., (1995) investigated the thalamo-cortical-loop following prenatal alcohol exposure in rats and found that cell counts from thalamic nuclei were equivalent between groups. A following study by Ikonomidou, (2000) investigated postnatal alcohol exposure’s effect on cell death in rats and found that, among several other affected regions, the laterodorsal and mediodorsal thalamus showed increased cell death. Farber et al., (2010) exposed macaques to third-trimester alcohol exposure and also found a large increase in cell death in the ATN. Lastly, Wozniak et al., (2004) exposed rats to a single day of third-trimester alcohol exposure and found severe damage in the ATN with the greatest damage in the AD which showed a 60% reduction of volume at P14, P30, and P90. Together, it can be concluded that developmental alcohol exposure negatively affects the ATN on a cellular level, but these deficits have not been associated to behavioral performance in spatial tasks (Hall et al., 1994; Reyes et al., 1989).
5.4. Neuroimaging and thalamic function after developmental alcohol exposure
Structural differences in the form of volumetric changes have also been found in humans following prenatal alcohol exposure. Using volumetric MRI on adolescents (5–15 years old) with FASD, Treit et al., (2013) found that, among other affected regions, thalamic volume was decreased by 14% compared with controls. Other similar studies have found a similar reduction in thalamic volume in children diagnosed with FASD (Nardelli et al., 2011; Roussotte et al., 2012). It is currently unknown if structural/volumetric difference in the ATN exists in adult humans with FASD. Thus, future studies are needed in order to better track structural/volumetric differences over a lifetime.
The thalamus has also been of interest in studies examining functional connectivity and functional activation using fMRI imaging of subjects exposed to alcohol prenatally. Male rats prenatally exposed to a moderate dose of alcohol (~60–80 mg/dl) displayed reduced functional connectivity in the thalamus, whereas females displayed increased functional connectivity in the thalamus (Rodriguez et al., 2016b). Similarly, Woods et al., (2018) found that FASD in human males was associated with increased path length and latency to the goal in a virtual water maze task and also had reduced activation in the thalamus, while females with FASD were unaffected. Together, there is a consistent finding that thalamic functional connectivity and thalamic activation is diminished following prenatal alcohol exposure, with males affected to a greater extent.
5.5. The anterior thalamic head direction cell signal and anterior thalamic-dependent spatial behavior after developmental alcohol exposure
Whether neural activity in the ATN, or more specifically HD cell activity, is altered following developmental alcohol exposure is currently unknown. However, given that developmental alcohol exposure has been associated with increases in ATN cell death, reductions in thalamic activation and functional connectivity as measured by fMRI, it seems likely that ATN HD cell signaling might also be affected. A large body of previous work has determined that the preferred direction of ATN HD cells (the direction of maximal firing) can be controlled by environmental landmarks, but also by cues associated with an animal’s self-motion such as vestibular cues, optic flow, and proprioception (Clark and Taube, 2012; Taube, 2007). Deficits in the landmark control of HD cells, and modulation of HD cell firing by self-motion cues, has been associated with impairments in accurate navigation (e.g., Butler et al., 2017; Clark and Taube, 2009; Clark et al., 2013; Yoder and Taube, 2011). Thus, future research should be directed at determining the impact of developmental alcohol on the sensory control of ATN HD cells.
A number of behavioral tasks that are dependent on intact ATN have been used to assess the impact of prenatal alcohol exposure (Fig. 1). For instance, radial arm maze performance has been hypothesized to be guided by the HD signal, which may be utilized in discriminating between the different directional orientations of the maze arms (Aggleton et al., 1996; Alexinsky, 2001; Dudchenko and Taube, 1997; Harvey et al., 2017; Mair et al., 2003; Mitchell and Dalrymple-Alford, 2005, 2006). For example, Dudchenko and Taube, (1997) recorded HD cells from the ATN while rats completed a radial arm maze and found that rats were impaired at completing the task when HD cell populations became un-anchored with the room cues. This finding indicates that the HD signal is necessary for accurate radial maze navigation. Similarly, inactivation of the ATN in a radial arm maze impairs performance despite extensive training with the task and the environment which further implicates the involvement of HD cells in spatial behavior (Harvey et al., 2017). Further, other work has similarly demonstrated the ATN and HD signal’s involvement with radial maze performance (Aggleton et al., 1996; Alexinsky, 2001; Mair et al., 2003; Mitchell and Dalrymple-Alford, 2005, 2006). Provided that developmental alcohol produces impairments radial arm maze performance, deficits in HD cell signaling might also be detected following alcohol exposure.
6. Entorhinal Cortex Development and Developmental Alcohol
The entorhinal cortex is a multi-layered structure within the greater hippocampal formation which projects to, and receives major connections from, the hippocampus proper (CA1-CA3, dentate gyrus) (Fig. 2A,E). The entorhinal cortex can be subdivided into medial and lateral zones on the basis of cytoarchitectural differences and connectivity differences (Witter et al., 2017). While the lateral entorhinal cortex (LEC) has been associated with the processing of local environmental and object-related features (Connor and Knierim, 2017), the medial entorhinal cortex (MEC) is thought to have a central role in spatial processing (Hardcastle et al., 2017). Notably, the MEC has been found to contain grid cells which fire in a grid formation covering the environment (Hafting et al., 2005) (Fig. 3C), border cells which fire in proximity to environmental boundaries (Solstad et al., 2008) (Fig. 3D), head direction cells (Giocomo et al., 2014) (Fig. 3B), as well as neurons that have firing correlates with a conjunctive of position, direction, and velocity (Sargolini et al., 2006). Other than rodents, evidence of MEC grid-like cells (Jacobs et al., 2013) and other grid-like signals (Doeller et al., 2010; Kim and Maguire, 2018) have been discovered in humans.
One function of the MEC and grid cells might be to perform the essential underlying computations to support path integration (Fiete et al., 2008; Gil et al., 2018; Hafting et al., 2005; McNaughton et al., 2006), which involves the use of idiothetic cues (internal linear and angular cues generated by the animal’s movements encoded by vestibular cues, somatosensory cues, sensory flow, or efference copy of movement commands) to calculate the current position of the animal in relation to its start location. In practice, path integration allows animals to plot their trajectory back to where it initiated, make direct trajectories back to the start of the trajectory (dead reckoning), and plan direct novel trajectories through unexplored regions of the environment in order to effectively reach goal locations (vector-based navigation) (Bush et al., 2015; Erdem and Hasselmo, 2012; Fiete et al., 2008). Interestingly, researchers have recently trained artificial recurrent neural networks to perform path integration (Banino et al., 2018). Over the course of training, the network independently developed representations that strikingly resembled MEC grid cells which then allowed the artificial agent to effectively navigate and create shortcuts to goal locations in its virtual environment.
Border cells, which fire when an animal is close to one or multiple environmental boundaries such as walls of a recording box (Fig. 3D), are found within all layers of the MEC as well as within the adjacent parasubiculum (Solstad et al., 2008). In a naturalistic sense, environments are not continuous open spaces but are usually divided into smaller areas with boundaries such as walls, rivers, mountain ranges, and other natural or unnatural boundaries. Border cells are thought to aid in the coding of these boundaries and might also function as a reference frame to support other spatially selective cells such as place and grid cells.
As noted above, the LEC is frequently linked to the processing of features within the environment such as object-related information (Connor and Knierim, 2017). However, recent work suggests that both the LEC and MEC contain neurons that fire in relation to the animal’s body orientation to environmental objects (Høydal et al., 2019; Wang et al., 2018). Høydal et al., (2019) reported that MEC neurons establish spiking patterns that resemble vectors to objects. In other words, these neurons are tuned to the animal’s direction and distance relative to objects. Lastly, recent research by Tsao et al., (2018) has discovered LEC cells that demonstrate a unique temporal signal that encodes time across multiple scales from seconds to hours and across different environmental contexts. These temporal signals have been hypothesized to feed into the hippocampus as part of a greater what-when-where representation of experience (Tsao et al., 2018).
6.1. General overview of entorhinal development
The multi-layered entorhinal cortex develops with a deep-to-superficial and a lateral-to-medial gradient with neurons in layer V-VI generated mostly on G15, layers II and IV forming mostly on G15–16, and layer III mostly forming on G17 (Bayer, 1980a; Bayer et al., 1993) (Fig. 5). Neurogenesis in the human entorhinal cortex is estimated to occur between the 5th through 9th week after fertilization.
MEC border cells appear adult-like on P16 (Bjerknes et al., 2014). However, more research is needed to determine the development of MEC border cells as the youngest rat used in the only developmental MEC border cell study was 16 days old. Border cells may very well emerge before P16 as younger rat pup have already developed HD and place cells as discussed above.
One of the last spatial representations to emerge is the grid cell (Fig. 3E). Adult-like grid cells appear around P20 and obtain near adult levels of spatial stability by P22 (Wills et al., 2010). It is hypothesized that the emergence of the grid signal is dependent on stable velocity inputs derived from a combination of vestibular, optic flow, motor efference, and proprioceptive cues (Widloski and Fiete, 2014). Thus, once these self-motion signals mature, their coordination can help produce a stable grid signal.
6.2. Entorhinal cellular changes after developmental alcohol exposure
Like the ATN, there are few studies that examine the entorhinal cortex following developmental alcohol exposure. Angelucci et al., (1998) exposed mice to prenatal alcohol (3.95 g/kg) from G8-G14 and found altered entorhinal cortex neuronal growth and differentiation. Museridze and Gegenava, (2010) similarly found a decrease in entorhinal cortex total neuron count at P3, P7, P15, P21, and P30 following alcohol exposure (15% v/v alcohol solution) throughout pregnancy and lactation. Together, it can be concluded that developmental alcohol exposure does indeed negatively affect neuron development in the entorhinal cortex.
6.3. Entorhinal plasticity after developmental alcohol exposure
The entorhinal cortex is the major source for the cortical information necessary for the hippocampus to carry out its functions. Considerable evidence indicates that moderate prenatal alcohol exposure impairs LTP between the entorhinal perforant pathway and DG (Galindo et al., 2004; Savage et al., 2010; Sutherland et al., 1997; Varaschin et al., 2010, 2014). However, the impact of developmental alcohol and propensity for experience-dependent synaptic plasticity within the entorhinal cortex has not been examined.
6.4. Neuroimaging and entorhinal function after developmental alcohol exposure
In human adults, Coles et al., (2011) found that while hippocampal volumes were reduced, entorhinal cortex volumes were largely unaffected by prenatal alcohol exposure. Golub et al., 2015) examined children (~10 years old) with heavy prenatal alcohol and found a decreased fMRI measured activation in the left medial temporal lobe which contains the entorhinal cortex. Collectively, these findings indicate volumetric and functional impairment following developmental alcohol exposure in humans, however, more research is needed to confirm and expand on these findings.
6.5. Entorhinal spatial representations and entorhinal-dependent spatial behavior after developmental alcohol exposure
There are currently no studies that have examined entorhinal spatial representations such as grid, border, head direction, and other conjunctive spatial cell types following developmental alcohol exposure. Given that developmental alcohol exposure has been associated with altered entorhinal neuronal growth, differentiation, decreased cell counts, decreased functional activation, and impaired entorhinal-to-DG plasticity, it is possible that disruptions to entorhinal neural activity may also be observed after developmental alcohol exposure. The entorhinal cortex serves as the largest input/output relationship with the hippocampus (Fig. 2B) (Witter et al., 2017). Thus, morphological changes in hippocampal circuitry after developmental alcohol exposure are likely to have an impact on entorhinal function. Indeed, previous work has shown that reductions in hippocampal neural activity can alter entorhinal grid cell firing (Bonnevie et al., 2013). In addition, the ATN provides an important input into the parahippocampal region (Fig. 2B) (Nassar et al., 2018; Winter et al., 2015), and alcohol induced alterations to the ATN region may also influence entorhinal function. Supporting this possibility are the findings that reductions in ATN neural activity produce considerable disruptions to entorhinal grid cell and HD signaling (Winter et al., 2015). Postnatal binge-like alcohol exposure can also dysregulate GABA receptors (Hsiao et al., 1998) and prenatal exposure can reduce neuron counts in the medial septum (Moore et al., 1997), which serves an important input for normal grid cell functioning (Brandon et al., 2011; Koenig et al., 2011). Reductions in septal interneurons, which form large projections to the entorhinal cortex (Fuchs et al., 2016), have been reported after prenatal alcohol exposure (Moore et al., 1997). Because septal inputs are thought to influence the entrainment of entorhinal neurons to theta rhythm (Brandon et al., 2011), investigation into whether oscillatory activity in the entorhinal cortex is disrupted after prenatal alcohol exposure is particularly warranted. Additionally, the finding that sharp wave ripple duration is increased after ethanol exposure points to possible oscillatory dysfunction in the entorhinal cortex or dentate gyrus as these regions have been shown to influences the timing of sharp wave ripples in the hippocampus (Sullivan et al., 2011). Cross-frequency coupling measures, such as theta-gamma phase amplitude coupling measured in hippocampal region CA1 may also be helpful in elucidating the consequences of alcohol exposure on entorhinal cortex function (Colgin et al., 2009; Fernández-Ruiz et al., 2017; Oliva et al., 2018).
Our summary above indicates that developmental alcohol exposure can impair performance on spatial tasks often associated with entorhinal function (see Fig. 1). For instance, previous work has reported that damage to the entorhinal cortex can produce spatial memory deficits in the MWT and deficits in tasks involving processing object-spatial relationships (Burwell, 2004; Steffenach et al., 2005; Van Cauter et al., 2013). Moderate prenatal alcohol exposure can produce acquisition impairments in an object-place paired associate task (Sanchez et al., 2019), pointing to the possibility that conjunctive coding of object and spatial information might be disrupted in the entorhinal cortex. Patten et al., (2016) reported impairments after moderate prenatal alcohol exposure on a discrimination task that requires retaining the temporal relationship between sequential exposures to objects. Although this task has been linked to CA3 processing, the discrimination between objects and their temporal relationship may also require entorhinal processing. Lastly, it is generally theorized that the entorhinal cortex, particularly grid cells in the MEC, play a critical role in accurate path integration, and damage to the MEC produces impairments in tasks in which animals are required to navigate to spatial locations on the basis of idiothetic cues (Gil et al., 2018; Van Cauter et al., 2013). To date, no study has examined whether path integration is impaired following developmental alcohol and should be considered in future studies.
7. Attenuating the effects of developmental alcohol exposure on spatial processing
The development of treatments targeting spatial memory impairments after developmental alcohol exposure has not been investigated. However, a number of interventions have been explored with respect to their impact on hippocampal processing which may ultimately have a significant impact on spatial behavior. Non-pharmacological interventions such as behavioral training and environmental enrichment can influence hippocampal synaptic plasticity and spatial learning and memory (Christie et al., 2005; Hall et al., 1994; Hannigan et al., 2007; Kleiber et al., 2011; Lee and Rabe, 1999; Wozniak et al., 2004) and exercise (Christie et al., 2005). For example, Christie et al., (2005) found that LTP and Morris water maze deficits were rescued in rats with prenatal alcohol exposure following access to a running wheel for only 12 days. Consistent with this observation, many of the behavioral studies reviewed above show that rodents with developmental alcohol exposure achieve control-like spatial performance with only a small amount of extra training (a few more trials or days of training).
Dietary supplementation may also provide benefits to the neurobiological basis of spatial processing. For instance, supplementation of N-acetylcysteine from P23 to P60 was able to completely restore DG LTP in rats with prenatal alcohol exposure (Patten et al., 2013b). Several studies report that choline supplementation also attenuates spatial learning deficits observed following developmental alcohol exposure (Idrus and Thomas, 2011; Monk et al., 2012; Otero et al., 2012; Ryan et al., 2008; Schneider and Thomas, 2016; Thomas, 2004; Thomas et al., 2000, 2007, 2010), possibly through alterations in hippocampal miRNA and gene expression (Balaraman et al., 2017). Lastly, administration of a histamine receptor antagonist (ABT-239), which is thought to improve glutamate and acetylcholine signaling, has been shown to improve spatial memory as well as DG LTP (Savage et al., 2010; Varaschin et al., 2010). Thus, pharmacological manipulation of histamine neurotransmission may serve as a potential therapeutic target for hippocampal-dependent spatial deficits (Davies et al., 2019).
Together, be it through behavioral training, environmental enrichment, exercise, or pharmacological therapies, there is some evidence suggesting that spatial deficits and hippocampal-dependent processing after prenatal alcohol exposure can be ameliorated. However, whether the interventions described above have a similar influence on other neural systems such as the anterior thalamus and entorhinal cortex, is currently unknown and should be a focus of future investigation. Finally, it is important to point out that while our review has centered on behavioral and neural systems-level changes accompanying developmental alcohol exposure, it will be important for future studies to develop a better understanding of changes at molecular and receptor level within this circuitry. A better understanding of how interventions might influence a broad network of neural systems involved in spatial behavior, and at multiple levels of analysis, will be critical toward the development of circuit-specific and targeted interventions.
8. Conclusion
A consistent behavioral consequence of developmental alcohol exposure has been the reported impairments in spatial behavior (Fig. 1). A central aim of the present review was to explore the hypothesis that damage to a combination of hippocampal and limbic-thalamic regions is likely responsible for these deficits in spatial processing. In summary, developmental alcohol exposure causes detrimental effects, be it cell loss, structural changes, changes in neural activity, a loss in synaptic plastic, or a combination of changes to the hippocampus, thalamic, and entorhinal cortex, but in a clear dose-dependent manner. Although the various alcohol administration paradigms and diverse dosing periods have made the synthesis of these studies challenging, the findings are striking in that developmental alcohol exposure has a clear regional, dose, and timing effect on the neurobiology of spatial processing.
A major aim of the present review was to identify gaps in the literature and highlight avenues of new research. Thus, elucidating whether spatial signals represented within hippocampal, thalamic and entorhinal circuitry are altered after developmental alcohol exposure will be of critical importance in future studies. In addition, it will be critical that future work be committed to investigating the impact of developmental alcohol exposure on a broad range of hippocampal-thalamic-entorhinal dependent behavioral tasks. The range of behavioral assessments known to be dependent on the spatial processing circuitry described in this review should be systematically evaluated in future work. Because spatial processing depends on the coordinated activity between many different areas, it is critical to examine other structures in order to create a complete picture of the effects of developmental alcohol exposure.
Highlights.
The consumption of alcohol during gestation is detrimental to spatial processing
Alcohol negatively affects the hippocampus, thalamic, & entorhinal cortex
Future work should explore alcohol’s effect on the thalamus & entorhinal cortex
Representations within the hippocampal formation & thalamus should be investigated
Acknowledgments
This work was supported by grants from NIAAA (R21 AA024983, P50 AA022534 and T32 AA014127-15).
References
- Adams-Chapman I (2009). Insults to the developing brain and impact on neurodevelopmental outcome. J. Commun. Disord 42, 256–262. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, and Neave N (1996). The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav. Brain Res 81, 189–198. [DOI] [PubMed] [Google Scholar]
- Agnihotri NT, Hawkins RD, Kandel ER, and Kentros C (2004). The long-term stability of new hippocampal place fields requires new protein synthesis. Proc. Natl. Acad. Sci 101, 3656–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen K, Andersen A-MN, Olsen J, and Grønbæk M (2004). Alcohol Consumption during Pregnancy and the Risk of Preterm Delivery. Am. J. Epidemiol 159, 155–161. [DOI] [PubMed] [Google Scholar]
- Alexinsky T (2001). Differential effect of thalamic and cortical lesions on memory systems in the rat. Behav. Brain Res 122, 175–191. [DOI] [PubMed] [Google Scholar]
- Amaral DG, and Witter MP (1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 31, 571–591. [DOI] [PubMed] [Google Scholar]
- An L, and Zhang T (2013). Spatial cognition and sexually dimorphic synaptic plasticity balance impairment in rats with chronic prenatal ethanol exposure. Behav. Brain Res 256, 564–574. [DOI] [PubMed] [Google Scholar]
- An L, Yang Z, and Zhang T (2013). Imbalanced Synaptic Plasticity Induced Spatial Cognition Impairment in Male Offspring Rats Treated with Chronic Prenatal Ethanol Exposure. Alcohol. Clin. Exp. Res 37, 763–770. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Fiore M, Cozzari C, and Aloe L (1998). Prenatal Ethanol Effects on NGF Level, NPY and ChAT Immunoreactivity in Mouse Entorhinal Cortex: A Preliminary Study. 11. [DOI] [PubMed]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, and Jernigan TL (2001). Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol 43, 148–154. [PubMed] [Google Scholar]
- Aronson M, Kyllerman M, Sabel K-G, Sandin B, and Olegård R (1985). Children of Alcoholic Mothers: Developmental, Perceptual and Behavioural Characteristics as Compared to Matched Controls. Acta Paediatr. 74, 27–35. [DOI] [PubMed] [Google Scholar]
- Balaraman S, Idrus NM, Miranda RC, and Thomas JD (2017). Postnatal choline supplementation selectively attenuates hippocampal microRNA alterations associated with developmental alcohol exposure. Alcohol 60, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banino A, Barry C, Uria B, Blundell C, Lillicrap T, Mirowski P, Pritzel A, Chadwick MJ, Degris T, Modayil J, et al. (2018). Vector-based navigation using grid-like representations in artificial agents. Nature. [DOI] [PubMed] [Google Scholar]
- Barnes CA (1979). Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol 93, 74–104. [DOI] [PubMed] [Google Scholar]
- Barnes CA, and McNaughton BL (1980). Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J. Physiol 309, 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, and Walker DW (1981). Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Dev. Brain Res 1, 333–340. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, and Houston FP (2000). LTP induction threshold change in old rats at the perforant path–granule cell synapse. Neurobiol. Aging 21, 613–620. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Wills TJ, and Cacucci F (2018). Self-Organized Attractor Dynamics in the Developing Head Direction Circuit. Curr. Biol 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA (1980a). Development of the hippocampal region in the rat I. Neurogenesis examined with3H-thymidine autoradiography. J. Comp. Neurol 190, 87–114. [DOI] [PubMed] [Google Scholar]
- Bayer SA (1980b). Development of the hippocampal region in the rat II. Morphogenesis during embryonic and early postnatal life. J. Comp. Neurol 190, 115–134. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, and Zhang X (1993). Timetables of Neurogenesis in the Human Brain Based on Experimentally Determined Patterns in the Rat. Neurotoxicology 14, 62. [PubMed] [Google Scholar]
- Bellinger FP, Bedi KS, Wilson P, and Wilce PA (1999). Ethanol exposure during the third trimester equivalent results in long-lasting decreased synaptic efficacy but not plasticity in the CA1 region of the rat hippocampus. Synapse 31, 51–58. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y (2001). Developing networks play a similar melody. Trends Neurosci. 24, 353–360. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, and Gaiarsa JL (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol 416, 303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beracochea DJ, Jaffard R, and Jarrard LE (1989). Effects of anterior or dorsomedial thalamic ibotenic lesions on learning and memory in rats. Behav. Neural Biol 51, 364–376. [DOI] [PubMed] [Google Scholar]
- Berkowitz LE, Harvey RE, Drake E, Thompson SM, and Clark BJ (2018). Progressive impairment of directional and spatially precise trajectories by TgF344-Alzheimer’s disease rats in the Morris Water Task. Sci. Rep 8, 16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, and Hannigan JH (2000). Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 10, 94–110. [DOI] [PubMed] [Google Scholar]
- Bird CW, Taylor DH, Pinkowski NJ, Chavez GJ, and Valenzuela CF (2018). Long-term Reductions in the Population of GABAergic Interneurons in the Mouse Hippocampus following Developmental Ethanol Exposure. Neuroscience 383, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes TL, Moser EI, and Moser M-B (2014). Representation of Geometric Borders in the Developing Rat. Neuron 82, 71–78. [DOI] [PubMed] [Google Scholar]
- Bjerknes TL, Langston RF, Kruge IU, Moser EI, and Moser M-B (2015). Coherence among Head Direction Cells before Eye Opening in Rat Pups. Curr. Biol 25, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Riley EP, and Hannigan JH (1987). Deficits on a Spatial Navigation Task Following Prenatal Exposure to Ethanol. Neurotoxicol. Teratol. 9, 253–258. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, and Collingridge GL (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, and Gardner-Medwin AR (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. J. Physiol 232, 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, and Lømo T (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, et al. (2018). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, and Moser M-B (2013). Grid cells require excitatory drive from the hippocampus. Nat. Neurosci 16, 309–317. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, and West JR (1990). Alcohol-Induced Neuronal Loss in Developing Rats: Increased Brain Damage with Binge Exposure. Alcohol. Clin. Exp. Res 14, 107–118. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, and West JR (1991). Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology 44, 147–163. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, and Caldwell KK (2012). A Limited Access Mouse Model of Prenatal Alcohol Exposure that Produces Long-Lasting Deficits in Hippocampal-Dependent Learning and Memory: A LIMITED ACCESS MOUSE MODEL OF PAE. Alcohol. Clin. Exp. Res 36, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, and Caldwell KK (2013). Moderate Prenatal Alcohol Exposure Reduces Plasticity and Alters NMDA Receptor Subunit Composition in the Dentate Gyrus. J. Neurosci 33, 1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, and Hasselmo ME (2011). Reduction of Theta Rhythm Dissociates Grid Cell Spatial Periodicity from Directional Tuning. Science 332, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, and Clark RE (2004). Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci 101, 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, and Whishaw IQ (2000). Similarities in the development of place and cue navigation by rats in a swimming pool. Dev. Psychobiol 37, 238–245. [DOI] [PubMed] [Google Scholar]
- Burwell RD (2004). Corticohippocampal Contributions to Spatial and Contextual Learning. J. Neurosci 24, 3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, Barry C, Manson D, and Burgess N (2015). Using Grid Cells for Navigation. Neuron 87, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WN, Smith KS, van der Meer MAA, and Taube JS (2017). The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation. Curr. Biol 27, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2002). Theta Oscillations in the Hippocampus. Neuron 33, 325–340. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2005). Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827–840. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2015). Hippocampal sharp wave‐ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, and Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci 16, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, and Wang X-J (2012). Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci 35, 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes ML, Richardson DP, Brien JF, Reynolds JN, and Dringenberg HC (2004). Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt–prenatal ethanol exposure. Neurotoxicol. Teratol 26, 543–551. [DOI] [PubMed] [Google Scholar]
- Carman HM, and Mactutus CF (2001). Ontogeny of spatial navigation in rats: A role for response requirements? Behav. Neurosci 115, 870–879. [PubMed] [Google Scholar]
- Ceranik K, Deng J, Heimrich B, Lübke J, Zhao S, Förster E, and Frotscher M (1999). Hippocampal Cajal-Retzius cells project to the entorhinal cortex: retrograde tracing and intracellular labelling studies: Projection of hippocampal Cajal-Retzius cells. Eur. J. Neurosci 11, 4278–4290. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin L-H, and Green EJ (1994). Head-direction cells in the rat posterior cortex. Exp. Brain Res 16. [DOI] [PubMed] [Google Scholar]
- Christie BR, and Cameron HA (2006). Neurogenesis in the adult hippocampus. Hippocampus 16, 199–207. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, and Webber A (2005). Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur. J. Neurosci 21, 1719–1726. [DOI] [PubMed] [Google Scholar]
- Chudley AE (2005). Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Can. Med. Assoc. J 172, S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, and Harvey RE (2016). Do the anterior and lateral thalamic nuclei make distinct contributions to spatial representation and memory? Neurobiol. Learn. Mem 133, 69–78. [DOI] [PubMed] [Google Scholar]
- Clark BJ, and Taube JS (2009). Deficits in landmark navigation and path integration after lesions of the interpeduncular nucleus. Behav. Neurosci 123, 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, and Taube JS (2012). Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Front. Neural Circuits 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Rice JP, Akers KG, Candelaria-Cook FT, Taube JS, and Hamilton DA (2013). Lesions of the dorsal tegmental nuclei disrupt control of navigation by distal landmarks in cued, directional, and place variants of the Morris water task. Behav. Neurosci 127, 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Hong NS, Bettenson DJ, Woolford J, Horwood L, and McDonald RJ (2015). Maintained directional navigation across environments in the Morris water task is dependent on vestibular cues. J. Exp. Psychol. Anim. Learn. Cogn 41, 301–308. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Simmons CM, Berkowitz LE, and Wilber AA (2018). The Retrosplenial-Parietal Network and Reference Frame Coordination for Spatial Navigation. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausing P, Ferguson SA, Holson RR, Allen RR, and Paule MG (1995). Prenatal ethanol exposure in rats: Long-lasting effects on learning. Neurotoxicol. Teratol 17, 545–552. [DOI] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, and Hu X (2011). Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 75, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser M-B, and Moser EI (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357. [DOI] [PubMed] [Google Scholar]
- Connor CE, and Knierim JJ (2017). Integration of objects and space in perception and memory. Nat. Neurosci 20, 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, LeBlanc N, Loock CA, Lutke J, et al. (2016). Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ 188, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronise K, Marino MD, Tran TD, and Kelly SJ (2001). Critical periods for the effects of alcohol exposure on learning in rats. Behav. Neurosci 115, 138–145. [DOI] [PubMed] [Google Scholar]
- Cullen KE, and Taube JS (2017). Our sense of direction: progress, controversies and challenges. Nat. Neurosci 20, 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen CL, Burne THJ, Lavidis NA, and Moritz KM (2014). Low Dose Prenatal Alcohol Exposure Does Not Impair Spatial Learning and Memory in Two Tests in Adult and Aged Rats. PLOS ONE 9, e101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Ballesteros-Merino C, Allen NA, Porch MW, Pruitt ME, Christensen KH, Rosenberg MJ, and Savage DD (2019). Impact of moderate prenatal alcohol exposure on histaminergic neurons, histidine decarboxylase levels and histamine H2 receptors in adult rat offspring. Alcohol Fayettev. N 76, 47–57. [DOI] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, and Larkby C (2002). Prenatal Alcohol Exposure Predicts Continued Deficits in Offspring Size at 14 Years of Age. Alcohol. Clin. Exp. Res 26, 1584–1591. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, and Rawlins JNP (2006). T-maze alternation in the rodent. Nat. Protoc 1, 7–12. [DOI] [PubMed] [Google Scholar]
- Dieguez D, and Barea‐Rodriguez EJ (2004). Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse 52, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, and Sands J (1978). Comparative aspects of the brain growth spurt. Early Hum. Dev 79–83. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Barry C, and Burgess N (2010). Evidence for grid cells in a human memory network. Nature 463, 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC, Errington ML, and Bliss TVP (1982). Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature 297, 496–497. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, and Taube JS (1997). Correlation between head direction cell activity and spatial behavior on a radial arm maze. Behav. Neurosci 111, 3. [DOI] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, and Csicsvari J (2010). The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci 13, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun I, Jakubowskadogru E, and Uzbay T (2006). Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol. Biochem. Behav 85, 345–355. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, and Wilson MA (2009). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus NA-NA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, and Ranganath C (2007). The Medial Temporal Lobe and Recognition Memory. Annu. Rev. Neurosci 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, and Fried I (2003). Cellular networks underlying human spatial navigation. Nature 425, 184–188. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Patai EZ, Julian JB, and Spiers HJ (2017). The cognitive map in humans: spatial navigation and beyond. Nat. Neurosci 20, 1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem UM, and Hasselmo M (2012). A goal-directed spatial navigation model using forward trajectory planning based on grid cells: Forward linear look-ahead trajectory model. Eur. J. Neurosci 35, 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA, and National Birth Defects Prevention Study (2009). Alcohol Consumption by Women Before and During Pregnancy. Matern. Child Health J 13, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraji J, Karimi M, Lawrence C, Mohajerani MH, and Metz GAS (2018). Non-diagnostic symptoms in a mouse model of autism in relation to neuroanatomy: the BTBR strain reinvestigated. Transl. Psychiatry 8, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, Creeley CE, and Olney JW (2010). Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol. Dis 40, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, and Buzsáki G (2017). Entorhinal-CA3 Dual-Input Control of Spike Timing in the Hippocampus by Theta-Gamma Coupling. Neuron 93, 1213–1226.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR, Burak Y, and Brookings T (2008). What Grid Cells Convey about Rat Location. J. Neurosci 28, 6858–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Neitz A, Pinna R, Melzer S, Caputi A, and Monyer H (2016). Local and Distant Input Controlling Excitation in Layer II of the Medial Entorhinal Cortex. Neuron 89, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo R, Frausto S, Wolff C, Caldwell KK, Perrone‐Bizzozero NI, and Savage DD (2004). Prenatal Ethanol Exposure Reduces mGluR5 Receptor Number and Function in the Dentate Gyrus of Adult Offspring. Alcohol. Clin. Exp. Res 28, 1587–1597. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, and Kempermann G (2009). Adult-Generated Hippocampal Neurons Allow the Flexible Use of Spatially Precise Learning Strategies. PLOS ONE 4, e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Dong Z, Bagot RC, Howland JG, Phillips AG, Wong TP, and Wang YT (2010). Hippocampal long-term depression is required for the consolidation of spatial memory. Proc. Natl. Acad. Sci 107, 16697–16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring TV, Luksys G, Sandi C, and Vasilaki E (2015). Detailed classification of swimming paths in the Morris Water Maze: multiple strategies within one trial. Sci. Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C (1990). Rats exposed prenatally to alcohol exhibit impairment in spatial navigation test. Behav. Brain Res 36, 217–228. [DOI] [PubMed] [Google Scholar]
- Gibson MAS, Butters NS, Reynolds JN, and Brien JF (2000). Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal guinea pig. Neurotoxicol. Teratol 22, 183–192. [DOI] [PubMed] [Google Scholar]
- Gil M, Ancau M, Schlesiger MI, Neitz A, Allen K, Marco RJD, and Monyer H (2018). Impaired path integration in mice with disrupted grid cell firing. Nat. Neurosci 21, 81. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, and Kesner RP (2003). Localization of Function Within the Dorsal Hippocampus: The Role of the CA3 Subregion in Paired-Associate Learning. Behav. Neurosci 117, 1385–1394. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Titterness AK, Patten AR, Taylor S, Ratzlaff A, Ratzlaff T, Helfer J, and Christie BR (2014). Prenatal ethanol exposure differentially affects hippocampal neurogenesis in the adolescent and aged brain. Neuroscience 273, 174–188. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Stensola T, Bonnevie T, Van Cauter T, Moser M-B, and Moser EI (2014). Topography of head direction cells in medial entorhinal cortex. Curr. Biol. CB 24, 252–262. [DOI] [PubMed] [Google Scholar]
- Girard TA, and Wainwright PE (2002). Testing the spatial-versus object-learning distinction: water-maze performance of male rats exposed to ethanol during the brain growth spurt. Behav. Brain Res 134, 493–503. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, and Zugaro MB (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci 12, 1222–1223. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, and Johnson TB (1997). Neonatal Binge Ethanol Exposure Using Intubation: Timing and Dose Effects on Place Learning. Neurotoxicol. Teratol 19, 435–446. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Kelly SJ, and West JR (1987). Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiology 15, 64–74. [Google Scholar]
- Goodridge JP, and Taube JS (1997). Interaction between the Postsubiculum and Anterior Thalamus in the Generation of Head Direction Cell Activity. J. Neurosci 17, 9315–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato A, Santarelli M, Sbriccoli A, and Minciacchi D (1995). Multifaceted alterations of the thalamo-cortico-thalamic loop in adult rats prenatally exposed to ethanol. Anat. Embryol. (Berl.) 191. [DOI] [PubMed] [Google Scholar]
- Graziano A, Petrosini L, and Bartoletti A (2003). Automatic recognition of explorative strategies in the Morris water maze. J. Neurosci. Methods 130, 33–44. [DOI] [PubMed] [Google Scholar]
- Green RJ, and Stanton ME (1989). Differential ontogeny of working memory and reference memory in the rat. Behav. Neurosci 103, 98–105. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, and Reynolds JN (2009). Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J. Child Psychol. Psychiatry 50, 688–697. [DOI] [PubMed] [Google Scholar]
- Gressens P, Lammens M, Picard J, and Evard P (1992). Ethanol-induced disturbances of gliogenesis and neuronogenesis in the developing murine brain: an in vitro and in vivo immunohistochemical and ultrastructural study. Alcohol Alcohol. [PubMed] [Google Scholar]
- Griguoli M, and Cherubini E (2017). Early Correlated Network Activity in the Hippocampus: Its Putative Role in Shaping Neuronal Circuits. Front. Cell. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, and Sanchis R (1985). Acetaldehyde and alcohol levels in pregnant rats and their fetuses. Alcohol 2, 267–270. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, and Riley EP (2009). Foetal Alcohol Spectrum Disorders and Alterations in Brain and Behaviour. Alcohol Alcohol 44, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz JJ (1986). Prenatal exposure to alcohol alters short-term plasticity in hippocampus. Exp. Neurol 93, 423–427. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, and Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. [DOI] [PubMed] [Google Scholar]
- Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, and Clark RE (2014). Medial Entorhinal Cortex Lesions Only Partially Disrupt Hippocampal Place Cells and Hippocampus-Dependent Place Memory. Cell Rep. 9, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL, Church MW, and Berman RF (1994). Radial arm maze deficits in rats exposed to alcohol during midgestation. Psychobiology 22, 181–185. [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, and Savage DD (2003). Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav. Brain Res 143, 85–94. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, and Sutherland RJ (2007). How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J. Exp. Psychol. Anim. Behav. Process 33, 100–114. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Sutherland RJ, Weisend MP, and Redhead ES (2008). The relative influence of place and direction in the Morris water task. J. Exp. Psychol. Anim. Behav. Process 34, 31–53. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, and Savage DD (2014). Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav. Brain Res 269, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelmann GE, and Olton DS (1981). Spatial memory following damage to hippocampal CA3 pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res. 217, 41–58. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, O’Leary-Moore SK, and Berman RF (2007). Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neurosci. Biobehav. Rev 31, 202–211. [DOI] [PubMed] [Google Scholar]
- Hardcastle K, Ganguli S, and Giocomo LM (2017). Cell types for our sense of location: where we are and where we are going. Nat. Neurosci 20, 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RE, Thompson SM, Sanchez LM, Yoder RM, and Clark BJ (2017). Post-training Inactivation of the Anterior Thalamic Nuclei Impairs Spatial Performance on the Radial Arm Maze. Front. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME (2005). What is the function of hippocampal theta rhythm?—Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15, 936–949. [DOI] [PubMed] [Google Scholar]
- Helfer JL, White ER, and Christie BR (2012). Enhanced Deficits in Long-Term Potentiation in the Adult Dentate Gyrus with 2nd Trimester Ethanol Consumption. PLOS ONE 7, e51344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen TB, Hjollund NH, Jensen TK, Bonde JP, Andersson A-M, Kolstad H, Ernst E, Giwercman A, Skakkebæk NE, and Olsen J (2004). Alcohol Consumption at the Time of Conception and Spontaneous Abortion. Am. J. Epidemiol 160, 661–667. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Dannenberg H, Alexander AS, and Hasselmo ME (2018). Neural mechanisms of navigation involving interactions of cortical and subcortical structures. J. Neurophysiol 119, 2007–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori E, Nishio Y, Kazui K, Umeno K, Tabuchi E, Sasaki K, Endo S, Ono T, and Nishijo H (2005). Place-related neural responses in the monkey hippocampal formation in a virtual space. Hippocampus 15, 991–996. [DOI] [PubMed] [Google Scholar]
- Høydal ØA, Skytøen ER, Andersson SO, Moser M-B, and Moser EI (2019). Object-vector coding in the medial entorhinal cortex. Nature. [DOI] [PubMed] [Google Scholar]
- Hsiao S-H, Mahoney JC, West JR, and Frye GD (1998). Development of GABAA receptors on medial septumrdiagonal band ŽMSrDB. neurons after postnatal ethanol exposure. 14. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Chen V, Tran GT, and Kesner RP (2013). The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: A test of the binding ofitems and context model. Hippocampus 23, 380–391. [DOI] [PubMed] [Google Scholar]
- Idrus NM, and Thomas JD (2011). Fetal Alcohol Spectrum Disorders: Experimental Treatments and Strategies for Intervention. Alcohol Res. Health 34, 76–85. [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C (2000). Ethanol-Induced Apoptotic Neurodegeneration and Fetal Alcohol Syndrome. Science 287, 1056–1060. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Dringenberg HC, Brien JF, and Reynolds JN (2004). Chronic prenatal ethanol exposure alters hippocampal GABAA receptors and impairs spatial learning in the guinea pig. Behav. Brain Res 150, 117–125. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, and Zorumski CF (2005). Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136, 509–517. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei X-X, Suthana N, Sperling MR, Sharan AD, Fried I, et al. (2013). Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci 16, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Passecker J, Islam MN, Vann S, Erichsen JT, Aggleton JP, and O’Mara SM (2015). Evidence for spatially-responsive neurons in the rostral thalamus. Front. Behav. Neurosci. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Warton C, Jacobson SW, Jacobson JL, Molteno CD, Eicher A, Marais P, Phillips OR, Narr KL, and Meintjes EM (2014). Three-dimensional surface deformation-based shape analysis of hippocampus and caudate nucleus in children with fetal alcohol spectrum disorders: Analysis of Hippocampus and Caudate Nucleus in Children. Hum. Brain Mapp 35, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaemingk KL, and Halverson PT (2000). Spatial Memory Following Prenatal Alcohol Exposure: More than a Material Specific Memory Deficit. Child Neuropsychol. 6, 115–128. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, and Madsen JR (1999). Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784. [DOI] [PubMed] [Google Scholar]
- Kassab R, and Alexandre F (2018). Pattern separation in the hippocampus: distinct circuits under different conditions. Brain Struct. Funct 223, 2785–2808. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, and West JR (1988). Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav. Brain Res 27, 247–257. [DOI] [PubMed] [Google Scholar]
- Kervern M, Silvestre de Ferron B, Alaux-Cantin S, Fedorenko O, Antol J, Naassila M, and Pierrefiche O (2015). Aberrant NMDA-dependent LTD after perinatal ethanol exposure in young adult rat hippocampus: LTD After In Utero Ethanol. Hippocampus 25, 912–923. [DOI] [PubMed] [Google Scholar]
- Kesmodel U, Olsen SF, and Secher NJ (2000). Does Alcohol Increase the Risk of Preterm Delivery? Epidemiology 11, 512–518. [DOI] [PubMed] [Google Scholar]
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, and Secher NJ (2002). Moderate Alcohol Intake during Pregnancy and the Risk of Stillbirth and Death in the First Year of Life. Am. J. Epidemiol 155, 305–312. [DOI] [PubMed] [Google Scholar]
- Kim M, and Maguire EA (2018). Can we study 3D grid codes non-invasively in the human brain? Methodological considerations and fMRI findings. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JPJ, and Weinberg J (1997). Object-Recognition and Spatial Learning and Memory in Rats Prenatally Exposed to Ethanol. 11. [DOI] [PubMed] [Google Scholar]
- Kleiber ML, Wright E, and Singh SM (2011). Maternal voluntary drinking in C57BL/6J mice: Advancing a model for fetal alcohol spectrum disorders. Behav. Brain Res 223, 376–387. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, and Hamilton DA (2011). Framing Spatial Cognition: Neural Representations of Proximal and Distal Frames of Reference and Their Roles in Navigation. Physiol. Rev 91, 1245–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, and Neunuebel JP (2016). Tracking the Flow of Hippocampal Computation: Pattern Separation, Pattern Completion, and Attractor Dynamics. Neurobiol. Learn. Mem 129, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, and Leutgeb S (2011). The Spatial Periodicity of Grid Cells Is Not Sustained During Reduced Theta Oscillations. Science 332, 592–595. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, and Sutherland RJ (1994). Dissociation of the Medial Prefrontal, Posterior Parietal, and Posterior Temporal Cortex for Spatial Navigation and Recognition Memory in the Rat. Cereb. Cortex 4, 664–680. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Berman RF, and Hannigan JH (1999). Electrophysiology of Hippocampal CA1 Neurons After Prenatal Ethanol Exposure. Alcohol 17, 125–131. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Ramani M, Dian J, Florez CM, Mylvaganam S, Brien J, Reynolds J, Kapur B, Zoidl G, Poulter MO, et al. (2016). Hippocampal hyperexcitability in fetal alcohol spectrum disorder: Pathological sharp waves and excitatory/inhibitory synaptic imbalance. Exp. Neurol 280, 70–79. [DOI] [PubMed] [Google Scholar]
- Lalonde R (2002). The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev 26, 91–104. [DOI] [PubMed] [Google Scholar]
- Lantz CL, Pulimood NS, Rodrigues-Junior WS, Chen C-KJ, Kalatsky VA, Manhaes AC, and Medina AE (2014). Visual deficits in a mouse model of Fetal alcohol spectrum disorders. Front. Pediatr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, and Wilson MA (2002). Memory of Sequential Experience in the Hippocampus during Slow Wave Sleep. Neuron 36, 1183–1194. [DOI] [PubMed] [Google Scholar]
- Lee I, and Kim J (2010). The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus. Learn. Mem 17, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, and Solivan F (2010). Dentate gyrus is necessary for disambiguating similar object-place representations. Learn. Mem 17, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, and Rabe A (1999). Infantile Handling Eliminates Reversal Learning Deficit in Rats Prenatally Exposed to Alcohol. Alcohol 18, 49–53. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Clark BJ, and Whishaw IQ (2007). Similar development of cued and learned home bases in control and hippocampal-damaged rats in an open field exploratory task. Hippocampus 17, 370–380. [DOI] [PubMed] [Google Scholar]
- Leinekugel X (2002). Correlated Bursts of Activity in the Neonatal Hippocampus in Vivo. Science 296, 2049–2052. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M-B, and Moser EI (2007). Pattern Separation in the Dentate Gyrus and CA3 of the Hippocampus. Science 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Rangananth C, and Redish AD (2017). Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nature 20, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, and West JR (2003). Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol. Teratol 25, 447–458. [DOI] [PubMed] [Google Scholar]
- Lozano YR, Page H, Jacob P-Y, Lomi E, Street J, and Jeffery K (2017). Retrosplenial and postsubicular head direction cells compared during visual landmark discrimination. Brain Neurosci. Adv 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, and West JR (2001). Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol 23, 49–57. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, and Porter MC (2003). Impairment of radial maze delayed nonmatching after lesions of anterior thalamus and parahippocampal cortex. Behav. Neurosci 117, 596–605. [DOI] [PubMed] [Google Scholar]
- Marissal T, Salazar RF, Bertollini C, Mutel S, De Roo M, Rodriguez I, Müller D, and Carleton A (2018). Restoring wild-type-like CA1 network dynamics and behavior during adulthood in a mouse model of schizophrenia. Nat. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, and Brigman JL (2016). The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol 51, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PD, and O’Keefe J (1998). Place field dynamics and directionality in a spatial memory task. Brain Res. 783, 249–261. [DOI] [PubMed] [Google Scholar]
- Matthews DB, and Simson PE (1998). Prenatal Exposure to Ethanol Disrupts Spatial Memory: Effect of the Training–Testing Delay Period. Physiol. Behav 64, 63–67. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, and Riley EP (1999). Executive Functioning in Children With Heavy Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res 23, 1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund Å, Autti-Rämö I, Jones KL, May PA, Adnams CM, Konovalova V, and Riley EP (2010). Towards a Neurobehavioral Profile of Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res 34, 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, and Dupret D (2014). Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci 17, 1658–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, and Moser M-B (2006). Path integration and the neural basis of the “cognitive map.” Nat. Rev. Neurosci 7, 663–678. [DOI] [PubMed] [Google Scholar]
- Miller MW (1995). Generation of Neurons in the Rat Dentate Gyrus and Hippocampus: Effects of Prenatal and Postnatal Treatment with Ethanol. Alcohol. Clin. Exp. Res 19, 1500–1509. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, and Dalrymple-Alford JC (2005). Dissociable memory effects after medial thalamus lesions in the rat. Eur. J. Neurosci 22, 973–985. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, and Dalrymple-Alford JC (2006). Lateral and anterior thalamic lesions impair independent memory systems. Learn. Mem 13, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJY, and Williams JD (1993). Directionally Selective Mnemonic Properties of Neurons in the Lateral Dorsal Nucleus of the Thalamus of Rats. 13, 4015–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, and Blumberg MS (2008). Synchronous Bursts of Neuronal Activity in the Developing Hippocampus: Modulation by Active Sleep and Association with Emerging Gamma and Theta Rhythms. J. Neurosci 28, 10134–10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BR, Leslie FM, and Thomas JD (2012). The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus 22, 1750–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DB, Ruygrok AC, Walker DW, and Heaton MB (1997). Effects of Prenatal Ethanol Exposure on Parvalbumin-Expressing GABAergic Neurons in the Adult Rat Medial Septum. Alcohol. Clin. Exp. Res 21, 849–856. [PubMed] [Google Scholar]
- Moore DB, Quintero MA, Ruygrok AC, Walker DW, and Heaton MB (1998). Prenatal ethanol exposure reduces parvalbumin-immunoreactive GABAergic neuronal number in the adult rat cingulate cortex. Neurosci. Lett 249, 25–28. [DOI] [PubMed] [Google Scholar]
- Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, and O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser M-B, and McNaughton BL (2017). Spatial representation in the hippocampal formation: a history. Nat. Neurosci 20, 1448. [DOI] [PubMed] [Google Scholar]
- Muessig L, Hauser J, Wills TJ, and Cacucci F (2015). A Developmental Switch in Place Cell Accuracy Coincides with Grid Cell Maturation. Neuron 86, 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muessig L, Lasek M, Varsavsky I, Cacucci F, and Wills TJ (2019). Coordinated Emergence of Hippocampal Replay and Theta Sequences during Post-natal Development. Curr. Biol 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Museridze DP, and Gegenava LG (2010). Effects of Ethanol on Neuron Density in the Limbic Cortex of the Brain and Correction of Evoked Changes with the Antioxidant Dolivin. Neurosci. Behav. Physiol 40, 553–556. [DOI] [PubMed] [Google Scholar]
- Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, and Buzsáki G (1999). Replay and Time Compression of Recurring Spike Sequences in the Hippocampus. J. Neurosci 19, 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, and Beaulieu C (2011). Extensive Deep Gray Matter Volume Reductions in Children and Adolescents with Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res no–no. [DOI] [PubMed] [Google Scholar]
- Nassar XM, Simonnet XJ, Huang XL-W, Mathon XB, Cohen I, Bendels MHK, Beraneck XM, Miles R, and Fricker XD (2018). Anterior Thalamic Excitation and Feedforward Inhibition of Presubicular Neurons Projecting to Medial Entorhinal Cortex. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, and Bor W (2003). Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years of age: a longitudinal study. Early Hum. Dev 71, 137–148. [DOI] [PubMed] [Google Scholar]
- Ogievetsky E, Lotfullina N, Minlebaeva A, and Khazipov R (2017). Ethanol-Induced Apoptosis of Interneurons in the Neonatal GAD67-GFP Mouse Hippocampus. BioNanoScience 7, 151–154. [Google Scholar]
- O’Keefe J (1976). Place units in the hippocampus of the freely moving rat. Exp. Neurol 51, 78–109. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, and Nadel L (1978). The Hippocampus as a Cognitive Map (Oxford: Clarendon Press; ). [Google Scholar]
- O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, and Hannigan JH (2006). Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol 38, 99–110. [DOI] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Oliveira E.F. de, and Buzsáki G (2018). Origin of Gamma Frequency Power during Hippocampal Sharp-Wave Ripples. Cell Rep. 25, 1693–1700.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Becker JT, and Handelmann GE (1979). Hippocampus, space, and memory. Behav. Brain Sci 2, 313–322. [Google Scholar]
- Otero NKH, Thomas JD, Saski CA, Xia X, and Kelly SJ (2012). Choline Supplementation and DNA Methylation in the Hippocampus and Prefrontal Cortex of Rats Exposed to Alcohol During Development. Alcohol. Clin. Exp. Res 36, 1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J, Bakker R, Irving H, Jaddoe VWV, Malini S, and Rehm J (2011). Dose–response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)—a systematic review and meta-analyses. BJOG Int. J. Obstet. Gynaecol 118, 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Gil-Mohapel J, Wortman RC, Noonan A, Brocardo PS, and Christie BR (2013a). Effects of Ethanol Exposure during Distinct Periods of Brain Development on Hippocampal Synaptic Plasticity. Brain Sci. 3, 1076–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Brocardo PS, Sakiyama C, Wortman RC, Noonan A, Gil-Mohapel J, and Christie BR (2013b). Impairments in hippocampal synaptic plasticity following prenatal ethanol exposure are dependent on glutathione levels: Glutathione and Synaptic Plasticity in Fasd. Hippocampus 23, 1463–1475. [DOI] [PubMed] [Google Scholar]
- Patten AR, Sawchuk S, Wortman RC, Brocardo PS, Gil-Mohapel J, and Christie BR (2016). Prenatal ethanol exposure impairs temporal ordering behaviours in young adult rats. Behav. Brain Res 299, 81–89. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Probst C, Gmel G, and Rehm J (2017). Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob. Health 5, e290–e299. [DOI] [PubMed] [Google Scholar]
- Popović M, Caballero-Bleda M, and Guerri C (2006). Adult rat’s offspring of alcoholic mothers are impaired on spatial learning and object recognition in the Can test. Behav. Brain Res 174, 101–111. [DOI] [PubMed] [Google Scholar]
- Poulter S, Hartley T, and Lever C (2018). The Neurobiology of Mammalian Navigation. Curr. Biol 28, R1023–R1042. [DOI] [PubMed] [Google Scholar]
- Rauch SL, and Raskin LA (1984). Cholinergic mediation of spatial memory in the preweanling rat: Application of the radial arm maze paradigm. Behav. Neurosci 98, 35–43. [DOI] [PubMed] [Google Scholar]
- Redondo RL, and Morris RGM (2011). Making memories last: the synaptic tagging and capture hypothesis. Nat. Rev. Neurosci 12, 17–30. [DOI] [PubMed] [Google Scholar]
- Reyes E, Wolfe J, and Savage DD (1989). The effects of prenatal alcohol exposure on radial arm maze performance in adult rats. Physiol. Behav 46, 45–48. [DOI] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, and Dringenberg HC (2002). Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig: Chronic prenatal ethanol exposure: effects on learning. Eur. J. Neurosci 16, 1593–1598. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, and Verho S (2007). Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev. Med. Child Neurol 41, 652–659. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR, and Baldwin J (1979). Response perseveration in rats exposed to alcohol prenatally. Pharmacol. Biochem. Behav 10, 255–259. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, and Warren KR (2011). Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychol. Rev 21, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Magcalas CM, Barto D, Fink BC, Rice JP, Bird CW, Davies S, Pentkowski NS, Savage DD, and Hamilton DA (2016a). Effects of sex and housing on social, spatial, and motor behavior in adult rats exposed to moderate levels of alcohol during prenatal development. Behav. Brain Res 313, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Davies S, Calhoun V, Savage DD, and Hamilton DA (2016b). Moderate Prenatal Alcohol Exposure Alters Functional Connectivity in the Adult Rat Brain. Alcohol. Clin. Exp. Res 40, 2134–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Mayford M, Hawkins RD, Kandel ER, and Muller RU (1996). Mice Expressing Activated CaMKII Lack Low Frequency LTP and Do Not Form Stable Place Cells in the CA1 Region of the Hippocampus. Cell 87, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Abel T, Hawkins RD, Kandel ER, and Muller RU (2000). Parallel Instabilities of Long-Term Potentiation, Place Cells, and Learning Caused by Decreased Protein Kinase A Activity. J. Neurosci 20, 8096–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, and Sowell ER (2012). Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum. Brain Mapp 33, 920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubert G, Miñana R, Pascual M, and Guerri C (2006). Ethanol exposure during embryogenesis decreases the radial glial progenitorpool and affects the generation of neurons and astrocytes. J. Neurosci. Res 84, 483–496. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Stadler-Morris S, and Albert P (1987). Ontogeny of spatial navigation behaviors in the rat: Dissociation of ‘proximal’- and ‘distal’-cue-based behaviors. Behav. Neurosci 101, 62–73. [DOI] [PubMed] [Google Scholar]
- Ruediger S, Spirig D, Donato F, and Caroni P (2012). Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat. Neurosci 15, 1563–1571. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, and Thomas JD (2008). Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res. 1237, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. (1995). Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ε1 subunit. Nature 373, 151–155. [DOI] [PubMed] [Google Scholar]
- Sanchez L, Benthem S, Johnson S, Turner S, Savage D, Burke S, and Clark BJ (2018). The effect of moderate prenatal alcohol exposure on object discrimination by adult rats (San Diego, CA). [Google Scholar]
- Sanchez LM, Goss J, Wagner J, Davies S, Savage DD, Hamilton DA, and Clark BJ (2019). Moderate prenatal alcohol exposure impairs performance by adult male rats in an object-place paired-associate task. Behav. Brain Res 360, 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, and Moser EI (2006). Conjunctive Representation of Position, Direction, and Velocity in Entorhinal Cortex. Science 312, 758–762. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, Torre AJ, and Sutherland RJ (2002). Dose-Dependent Effects of Prenatal Ethanol Exposure on Synaptic Plasticity and Learning in Mature Offspring. Alcohol. Clin. Exp. Res 26, 1752–1758. [DOI] [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El‐Emawy A, Staples MC, Varaschin RK, Wright CA, Seidel JL, Caldwell KK, et al. (2010). Effects of a Novel Cognition-Enhancing Agent on Fetal Ethanol-Induced Learning Deficits. Alcohol. Clin. Exp. Res 34, 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F (1985). Development of place navigation in rats from weaning to puberty. Behav. Neural Biol 43, 69–85. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, and Nolan MF (2017). Synaptic integrative mechanisms for spatial cognition. Nat. Neurosci 20, 1483–1492. [DOI] [PubMed] [Google Scholar]
- Schneider RD, and Thomas JD (2016). Adolescent Choline Supplementation Attenuates Working Memory Deficits in Rats Exposed to Alcohol During the Third Trimester Equivalent. Alcohol. Clin. Exp. Res 40, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, and Milner B (1957). LOSS OF RECENT MEMORY AFTER BILATERAL HIPPOCAMPAL LESIONS. J. Neurol. Neurosurg. Psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE, and Turner-Williams S (2005). Movement-Related Correlates of Single-Cell Activity in the Medial Mammillary Nucleus of the Rat During a Pellet-Chasing Task. J. Neurophysiol 94, 1920–1927. [DOI] [PubMed] [Google Scholar]
- Shine JP, Valdés-Herrera JP, Hegarty M, and Wolbers T (2016). The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame. J. Neurosci 36, 6371–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann HM, Patten AR, Morch K, Sawchuk S, Zhang C, Parton R, Szlavik L, and Christie BR (2014). Prenatal ethanol exposure has sex-specific effects on hippocampal long-term potentiation: Sex Differences in Ltp in a Fasd Model. Hippocampus 24, 54–64. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, and McNaughton BL (1996). Replay of Neuronal Firing Sequences in Rat Hippocampus During Sleep Following Spatial Experience. Science 271, 1870–1873. [DOI] [PubMed] [Google Scholar]
- Skorput AGJ, Gupta VP, Yeh PWL, and Yeh HH (2015). Persistent Interneuronopathy in the Prefrontal Cortex of Young Adult Offspring Exposed to Ethanol In Utero. J. Neurosci 35, 10977–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Jamot L, Bertholet J-Y, and Crusio WE (2005). Prenatal exposure to alcohol does not affect radial maze learning and hippocampal mossy fiber sizes in three inbred strains of mouse. Behav. Brain Funct 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Saito M, Bleiwas C, Masiello K, Ardekani B, Guilfoyle DN, Gerum S, Wilson DA, and Vadasz C (2015). Selective reduction of cerebral cortex GABA neurons in a late gestation model of fetal alcohol spectrum disorder. Alcohol 49, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser M-B, and Moser EI (2008). Representation of Geometric Borders in the Entorhinal Cortex. Science 322, 1865–1868. [DOI] [PubMed] [Google Scholar]
- Soriano E, Cobas A, and Fairén A (1989a). Neurogenesis of glutamic acid decarboxylase immunoreactive cells in the hippocampus of the mouse. I: Regio superior and regio inferior: NEUROGENESIS OF GAD-POSITIVE CELLS IN MOUSE HIPPOCAMPUS. J. Comp. Neurol 281, 586–602. [DOI] [PubMed] [Google Scholar]
- Soriano E, Cobas A, and Fairén A (1989b). Neurogenesis of glutamic acid decarboxylase immunoreactive cells in the hippocampus of the mouse. II: Area dentata: NEUROGENESIS OF GAD-POSITIVE CELLS IN MOUSE AREA DENTATA. J. Comp. Neurol 281, 603–611. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O??Hare ED, McCourt ST, Mattson SN, O??Connor MJ, and Bookheimer SY (2007). Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure: NeuroReport 18, 635–639. [DOI] [PubMed] [Google Scholar]
- Squire LR (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev 99, 195–231. [DOI] [PubMed] [Google Scholar]
- Stackman RW, and Taube JS (1998). Firing Properties of Rat Lateral Mammillary Single Units: Head Direction, Head Pitch, and Angular Head Velocity. J. Neurosci. Off. J. Soc. Neurosci 18, 9020–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Lora JC, and Williams SB (2012). Directional Responding of C57BL/6J Mice in the Morris Water Maze Is Influenced by Visual and Vestibular Cues and Is Dependent on the Anterior Thalamic Nuclei. J. Neurosci 32, 10211–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenach H-A, Witter M, Moser M-B, and Moser EI (2005). Spatial Memory in the Rat Requires the Dorsolateral Band of the Entorhinal Cortex. Neuron 45, 301–313. [DOI] [PubMed] [Google Scholar]
- Strata F, Atzori M, Molnar M, Ugolini G, Tempia F, and Cherubini E (1997). A Pacemaker Current in Dye-Coupled Hilar Interneurons Contributes to the Generation of Giant GABAergic Potentials in Developing Hippocampus. J. Neurosci 17, 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, and Barr HM (1989). Neurobehavioral Effects of Prenatal Alcohol: Part III. PLS Analyses of Neuropsychologic Tests I. Neurotoxicol. Teratol 11, 493–507. [DOI] [PubMed] [Google Scholar]
- Streissguth P, Randels SP, LaDue RA, and Smith DF (1991). Fetal Alcohol Syndrome in Adolescents and Adults. 7. [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, and Buzsaki G (2011). Relationships between Hippocampal Sharp Waves, Ripples, and Fast Gamma Oscillation: Influence of Dentate and Entorhinal Cortical Activity. J. Neurosci 31, 8605–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, and Soriano E (1994). The organization of the embronic and early postnatal murine hippocampus. II. Development of entorhinal, commissural, and septal connections studied with the lipophilic tracer DiI: DEVELOPMENT OF HIPPOCAMPAL CONNECTIONS. J. Comp. Neurol 344, 101–120. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, and Savage DD (1997). Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus 7, 232–238. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, and Savage DD (2000). Prenatal exposure to moderate levels of ethanol can have long-lasting effects on learning and memory in adult offspring. Psychobiology 28, 532–539. [DOI] [PubMed] [Google Scholar]
- Swanson L (2004). Brain Maps: Structure of the Rat Brain (Gulf Professional Publishing; ). [Google Scholar]
- Swartzwelder HS, Farr KL, Wilson WA, and Savage DD (1988). Prenatal exposure to ethanol decreases physiological plasticity in the hippocampus of the adult rat. Alcohol 5, 121–124. [DOI] [PubMed] [Google Scholar]
- Tan HM, Bassett JP, O’Keefe J, Cacucci F, and Wills TJ (2015). The Development of the Head Direction System before Eye Opening in the Rat. Curr. Biol 25, 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SE, Berman RF, Abel EL, and Zajac CS (1990). Prenatal alcohol exposure alters hippocampal slice electrophysiology. Alcohol 7, 507–511. [DOI] [PubMed] [Google Scholar]
- Taube JS (1995). Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci 15, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS (2007). The Head Direction Signal: Origins and Sensory-Motor Integration. Annu. Rev. Neurosci 30, 181–207. [DOI] [PubMed] [Google Scholar]
- Taube JS, and Muller RU (1998). Comparisons of head direction cell activity in the postsubiculum and anterior thalamus of freely moving rats. Hippocampus 8, 87–108. [DOI] [PubMed] [Google Scholar]
- Taube J, Muller R, and Ranck J (1990a). Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J, Muller R, and Ranck J (1990b). Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci 10, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J (2004). Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol 26, 35–45. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, and Goodlett CR (1996). Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: Importance of developmental timing and number of episodes. Dev. Psychobiol 29, 433–452. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Weinert SP, Sharif S, and Riley EP (1997). MK-801 Administration During Ethanol Withdrawal in Neonatal Rat Pups Attenuates Ethanol-Induced Behavioral Deficits. Alcohol. Clin. Exp. Res 21, 8. [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VRE, and Riley EP (2000). Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol. Teratol 22, 703–711. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, and Dominguez HD (2007). Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav. Neurosci 121, 120–130. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, and Dominguez HD (2010). Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birt. Defects Res. A. Clin. Mol. Teratol 88, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, and Riley EP (1998). Comparison of Social Abilities of Children with Fetal Alcohol Syndrome to Those of Children with Similar IQ Scores and Normal Controls. Alcohol. Clin. Exp. Res 22, 528–533. [PubMed] [Google Scholar]
- Thompson SM, Berkowitz LE, and Clark BJ (2018). Behavioral and Neural Subsystems of Rodent Exploration. Learn. Motiv 61, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titterness AK, and Christie BR (2012). Prenatal ethanol exposure enhances NMDAR-dependent long-term potentiation in the adolescent female dentate gyrus. Hippocampus 22, 69–81. [DOI] [PubMed] [Google Scholar]
- Tran T (2000). Critical periods for the effects of alcohol exposure on brain weight, body weight, activity and investigation. Behav. Brain Res 116, 99–110. [DOI] [PubMed] [Google Scholar]
- Tran TD, and Kelly SJ (2003). Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol. Teratol 25, 519–528. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, and Beaulieu C (2013). Longitudinal MRI Reveals Altered Trajectory of Brain Development during Childhood and Adolescence in Fetal Alcohol Spectrum Disorders. J. Neurosci 33, 10098–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Vann SD, Reilly R, Erichsen JT, Aggleton JP, and O’Mara SM (2011). Theta-modulated head direction cells in the rat anterior thalamus. J. Neurosci. Off. J. Soc. Neurosci 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser M-B, and Moser EI (2018). Integrating time from experience in the lateral entorhinal cortex. Nature. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, and Tonegawa S (1996). The Essential Role of Hippocampal CA1 NMDA Receptor–Dependent Synaptic Plasticity in Spatial Memory. Cell 87, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Uecker A, and Nadel L (1996). Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia 34, 209–223. [DOI] [PubMed] [Google Scholar]
- Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, and Save E (2013). Distinct Roles of Medial and Lateral Entorhinal Cortex in Spatial Cognition. Cereb. Cortex 23, 451–459. [DOI] [PubMed] [Google Scholar]
- Varaschin RK, Akers KG, Rosenberg MJ, Hamilton DA, and Savage DD (2010). Effects of the Cognition-Enhancing Agent ABT-239 on Fetal Ethanol-Induced Deficits in Dentate Gyrus Synaptic Plasticity. J. Pharmacol. Exp. Ther 334, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaschin RK, Rosenberg MJ, Hamilton DA, and Savage DD (2014). Differential Effects of the Histamine H3 Receptor Agonist Methimepip on Dentate Granule Cell Excitability, Paired-Pulse Plasticity and Long-Term Potentiation in Prenatal Alcohol-Exposed Rats. Alcohol. Clin. Exp. Res 38, 1902–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven GM, Trouche S, McNamara CG, Allen K, and Dupret D (2016). Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples. Neuron 92, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernescu RM, Adams RJ, and Courage ML (2012). Children with fetal alcohol spectrum disorder show an amblyopia-like pattern of vision deficit. Dev. Med. Child Neurol 54, 557–562. [DOI] [PubMed] [Google Scholar]
- Wagner JL, Zhou FC, and Goodlett CR (2014). Effects of one- and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol 48, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE, Ward GR, Winfield D, Huang Y-S, Mills DE, Ward RP, and McCutcheon D (1990). Effects of Prenatal Ethanol and Long-Chain n-3 Fatty Acid Supplementation on Development in Mice. 1. Body and Brain Growth, Sensorimotor Development, and Water T-Maze Reversal Learning. Alcohol. Clin. Exp. Res 14, 405–412. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen X, Lee H, Deshmukh SS, Yoganarasimha D, Savelli F, and Knierim JJ (2018). Egocentric coding of external items in the lateral entorhinal cortex. 5. [DOI] [PMC free article] [PubMed]
- Weinberg J, Sliwowska JH, Lan N, and Hellemans KGC (2008). Prenatal Alcohol Exposure: Foetal Programming, the Hypothalamic-Pituitary-Adrenal Axis and Sex Differences in Outcome. J. Neuroendocrinol 20, 470–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, and Derdikman D (2018). Role of the head-direction signal in spatial tasks: when and how does it guide behavior? J. Neurophysiol 120, 78–87. [DOI] [PubMed] [Google Scholar]
- West JR, Hamre KM, and Cassell MD (1986). Effects of Ethanol Exposure during the Third Trimester Equivalent on Neuron Number in Rat Hippocampus and Dentate Gyrus. Alcohol. Clin. Exp. Res 10, 190–197. [DOI] [PubMed] [Google Scholar]
- Widloski J, and Fiete IR (2014). A Model of Grid Cell Development through Spatial Exploration and Spike Time-Dependent Plasticity. Neuron 83, 481–495. [DOI] [PubMed] [Google Scholar]
- Wigal T, and Amsel A (1990). Behavioral and Neuroanatomical Effects of Prenatal, Postnatal, or Combined Exposure to Ethanol in Weanling Rats. 104, 116–126. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Forster TC, Tatsuno M, and McNaughton BL (2014). Interaction of Egocentric and World-Centered Reference Frames in the Rat Posterior Parietal Cortex. J. Neurosci 34, 5431–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, and Rovet J (2008). Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J. Int. Neuropsychol. Soc 14, 1022. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, and O’Keefe J (2010). Development of the Hippocampal Cognitive Map in Preweanling Rats. Science 328, 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA (2004). Cognitive Aging and the Hippocampus: How Old Rats Represent New Environments. J. Neurosci 24, 3870–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, and McNaughton B (1994). Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Peterson J, Basavaraj BS, and Saito M (2011). Local and Regional Network Function in Behaviorally Relevant Cortical Circuits of Adult Mice Following Postnatal Alcohol Exposure. Alcohol. Clin. Exp. Res 35, 1974–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, and Tanila H (2003). Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol. Aging 24, 297–305. [DOI] [PubMed] [Google Scholar]
- Winter SS, Clark BJ, and Taube JS (2015). Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science 347, 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, and Bussey TJ (2004). Double Dissociation between the Effects of Peri-Postrhinal Cortex and Hippocampal Lesions on Tests of Object Recognition and Spatial Memory: Heterogeneity of Function within the Temporal Lobe. J. Neurosci 24, 5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Doan TP, Jacobsen B, Nilssen ES, and Ohara S (2017). Architecture of the Entorhinal Cortex A Review of Entorhinal Anatomy in Rodents with Some Comparative Notes. Front. Syst. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer DP, and Lipp H-P (2000). Dissecting the Behaviour of Transgenic Mice: Is it the Mutation, the Genetic Background, or the Environment? Exp. Physiol 85, 627–634. [PubMed] [Google Scholar]
- Woods KJ, Thomas KGF, Molteno CD, Jacobson JL, Jacobson SW, and Meintjes EM (2018). Prenatal alcohol exposure affects brain function during place learning in a virtual environment differently in boys and girls. Brain Behav. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak D, Hartman R, Boyle M, Vogt S, Brooks A, Tenkova T, Young C, Olney J, and Muglia L (2004). Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol. Dis 17, 403–414. [DOI] [PubMed] [Google Scholar]
- Yang Q, Witkiewicz BB, Olney RS, Liu Y, Davis M, Khoury MJ, Correa A, and Erickson JD (2001). A Case-Control Study of Maternal Alcohol Consumption and Intrauterine Growth Retardation. Ann. Epidemiol 11, 497–503. [DOI] [PubMed] [Google Scholar]
- Yartsev MM, and Ulanovsky N (2013). Representation of Three-Dimensional Space in the Hippocampus of Flying Bats. Science 340, 367–372. [DOI] [PubMed] [Google Scholar]
- Yassa MA, and Stark CEL (2011). Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, and Taube JS (2011). Projections to the anterodorsal thalamus and lateral mammillary nuclei arise from different cell populations within the postsubiculum: Implications for the control of head direction cells. Hippocampus 21, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov A, Lotfullina N, and Khazipov R (2016). Impairments to the Giant Depolarizing Potentials After the Third Trimester Equivalent Ethanol Exposure in the Neonatal Rat. BioNanoScience 6, 523–527. [Google Scholar]
- Zimmerberg B, Mattson S, and Riley EP (1989). Impaired alternation test performance in adult rats following prenatal alcohol exposure. Pharmacol. Biochem. Behav 32, 293–299. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Sukel HL, and Stekler JD (1991). Spatial learning of adult rats with fetal alcohol exposure: deficits are sex-dependent. Behav. Brain Res 42, 49–56. [DOI] [PubMed] [Google Scholar]
- Zink M, Ferbert T, Frank ST, Seufert P, Gebicke‐Haerter PJ, and Spanagel R (2011). Perinatal exposure to alcohol disturbs spatial learning and glutamate transmission-related gene expression in the adult hippocampus. Eur. J. Neurosci 34, 457–468. [DOI] [PubMed] [Google Scholar]
- Zucca S, and Valenzuela CF (2010). Low Concentrations of Alcohol Inhibit BDNF-Dependent GABAergic Plasticity via L-type Ca2+ Channel Inhibition in Developing CA3 Hippocampal Pyramidal Neurons. J. Neurosci 30, 6776–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, and Regehr WG (2002). Short-Term Synaptic Plasticity. Annu. Rev. Physiol 64, 355–405. [DOI] [PubMed] [Google Scholar]