Abstract
Subtyping depression is important in order to further delineate biological causes of depressive syndromes. The aim of this study was to evaluate clinical and outcome characteristics of distinct subtypes of depression and to assess proportion and features of patients fulfilling criteria for more than one subtype. Melancholic, atypical and anxious subtypes of depression were assessed in a naturalistic sample of 833 inpatients using DSM‐IV specifiers based on operationalized criteria. Baseline characteristics and outcome criteria at discharge were compared between distinct subtypes and their overlap. A substantial proportion of patients (16%) were classified with more than one subtype of depression, 28% were of the distinct anxious, 7% of the distinct atypical and 5% of the distinct melancholic subtype. Distinct melancholic patients had shortest duration of episode, highest baseline depression severity, but were more often early improvers; distinct anxious patients had higher NEO‐Five Factor Inventory (NEO‐FFI) neuroticism scores compared with patients with unspecific subtype. Melancholic patients with overlap of anxious features had worse treatment outcome compared to distinct melancholic and distinct anxious subtype. Distinct subtypes differed in only few variables and patients with overlap of depression subtypes may have independent clinical and outcome characteristics. Studies investigating biological causes of subtypes of depression should take influence of features of other subtypes into account.
Keywords: anxiety, DSM, inpatients, major depressive disorder, mood disorders
1. INTRODUCTION
The classification of major depressive disorder (MDD) into depressive subtypes is important to further identify the biological basis of depressive syndromes and to predict outcome given the heterogeneity of depressive disorders (Prins, Olivier, & Korte, 2011). Several subtypes and models to classify depressive syndromes have been suggested including DSM‐IV and DSM‐5 specifiers of melancholic and atypical subtype and the recently more often studied subtype of anxious depression (Fava et al., 2004; Fava et al., 2008). The specifier “with anxious distress” has now been included as a new specifier in DSM‐5 [American Psychiatric Association (APA), 2013b].
Studies focussing on clinical or biological characteristics of subtypes of depression most often compared one subtype of depression with patients not exhibiting this subtype (Bandelow et al., 2014; Baune et al., 2008; Harkness & Monroe, 2006; Kaestner et al., 2005; Liu et al., 2016; Monzon et al., 2010; Papakostas, Fan, & Tedeschini, 2012; Paslakis et al., 2011; Pizzagalli et al., 2004; Quinn, Rennie, Harris, & Kemp, 2014; Seppala et al., 2012; Zaninotto et al., 2016). However, such approaches do not account for the underlying heterogeneity, since other subtypes were disregarded in dichotomous approaches. This might be one of the reasons why results on specific biological alterations in subtypes of MDD, mainly melancholic and atypical depression, including e.g. activation state of hypothalamus‐pituitary–adrenal (HPA) axis, rapid eye movement (REM) sleep latency, alterations in immune functions (for review see Antonijevic, 2006; Gold & Chrousos, 1999, 2002; Gold, Licinio, Wong, & Chrousos, 1995; Leventhal & Rehm, 2005; Stetler & Miller, 2011; Stewart, McGrath, Quitkin, & Klein, 2009) or differential involvement of prefrontal cortex (PFC) (Gold & Chrousos, 2002) are still contradictory to date. Furthermore, some studies found only marginal clinical differences in e.g. illness course of subtypes and low subtype stability across episodes (Melartin et al., 2004) or in poor outcome prediction in regard to treatment strategies (Uher et al., 2011). Due to these inconsistent results there is still a lot of controversy about the construct validity of subtypes of depression (Thase, 2009). Therefore, DSM‐5 criteria subtype classification of MDD still relies on mere clinical characteristics not taking biological aspects into account, and ICD‐11 criteria will most likely do so accordingly [APA, 2013b; World Health Organization (WHO), 2011]. In the beginning of the development process for DSM‐5, results of neuroscientific and genetic studies should have been taken into account, yet no genetic or other biological finding was established as a diagnostic criterion in MDD (APA, 2013a). Still, new specifiers (“with mixed features” and “with anxious distress”) were included (APA, 2013b). The beta draft of the ICD‐11 reveals MDDs with several symptom specifiers (“with psychotic symptoms”, “with prominent anxiety symptoms”, “with melancholia”), yet with no biological components (WHO, 2016).
Reasons for divergent results are numerous and apart from applied definitions for subtypes (categorical versus dimensional approach) further include the populations under study (e.g. inpatients versus outpatients, study populations versus naturalistic samples) or used comparison groups (Parker, 2000; Rasmussen, 2007). Bearing in mind that operationalized criteria sets allow combinations of symptom patterns that fulfil criteria for more than one subtype, a closer look at overlapping groups might teach us more on the nature of the subtypes. So before trying to further delineate biological causes of subtypes of depression it is of importance to examine most common subtypes at the same time in naturalistic patients in order to determine the potential overlap of melancholic, atypical and anxious subtypes of depression, because this overlap hampers conclusive research in respect to determination of biological causes (Parker et al., 1995; Rasmussen, 2007). In a recent analysis of the same patient sample, latent class analysis (LCA) based on the Hamilton Depression Rating Scale (HAMD) was used to identify categories of symptom profiles, and their outcome was further elucidated using linear mixed effects (LME) model (Buhler, Seemuller, & Lage, 2014). A solution with five classes named “suicide”, “melancholic”, “psychovegetative”, “dismayed” and “anxious” was obtained and these classes separated in time to remission in LME (Buhler et al., 2014). In the analysis at hand we used operationalized criteria using HAMD and AMDP (Association for Methodology and Documentation in Psychiatry) items to be able to adhere to DSM‐IV criteria of depression subtypes as closely as possible. We investigated clinical and diverse outcome parameters of patients fulfilling criteria for one or more than one subtype of depression in a large naturalistic sample of inpatients with MDD.
2. AIMS OF THE STUDY
Characterizing distinct subtypes and their overlap using operationalized criteria.
Reflecting a potential assignment of patients fulfilling criteria for more than one subtype (overlap groups) to one of the distinct subtypes.
Deducing implications for the recent developments of DSM‐5 and ICD‐11.
3. MATERIALS AND METHODS
3.1. Study overview
Data of this study were prospectively collected within the framework of the German Competence Network on Depression conducted in several university and district hospitals across Germany (university hospitals: Berlin: Campus Charité Mitte and Campus Benjamin‐Franklin, Düsseldorf, Halle, Heidelberg, Munich: Max‐Planck‐Institute (MPI) and LMU; district hospitals: kbo‐Inn‐Salzach‐Clinics Gabersee/Bavaria, kbo‐Isar‐Amper‐Clinics Haar/Bavaria, Berlin: Auguste‐Viktoria‐Hospital, St Joseph Hospital and St Hedwig Hospital).
3.2. Sample description and rating scales
Details and results of the acute inpatient phase for the whole patient sample were presented at length elsewhere (Seemuller et al., 2010).
Briefly, inclusion criteria comprised age between 18 and 65, signed written informed consent and ICD‐10 diagnostic criteria for any major depressive episode (ICD‐10: F31.3–5, F32, F33, F34, F38) or for a depressive disorder not otherwise specified (ICD‐10: F39). Exclusion criteria were organic causes of depression, insufficient knowledge of German language and long distance from home to study centre. Diagnosis of a depressive spectrum disorder and any relevant axis I or axis II comorbidities according to DSM‐IV were confirmed using the Structured Clinical Interview for DSM‐IV (SCID‐I and SCID‐II) (Wittchen, Wunderlich, Gruschwitz, & Zaudig, 1997) at baseline. The scale of clinical and socio‐demographic variables in psychiatry (BADO) (Cording, Gaebel, & Spengler, 1995) was used to record socio‐demographic and clinical variables. Psychopathological symptoms were assessed using the HAMD‐21 scale (Hamilton, 1967), and the 140‐item AMDP scale (Pietzcker & Gebhardt, 1983). To assess outcome the Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery & Asberg, 1979), the Global Assessment of Functioning (GAF) scale (APA, 2000b), the Social and Occupational Functioning Assessment Scale (SOFAS) (APA, 2000b) and the NEO‐Five Factor Inventory (NEO‐FFI) (Costa & McCrae, 1988) were administered. Ratings were assessed at baseline and every other week until discharge. All raters were experienced psychiatrists and received a standardized training for all scales prior to the study.
The protocol was approved by the respective local Ethics Committee of each participating site and followed the Declaration of Helsinki and subsequent revisions. All patients gave their written informed consent prior to inclusion into the study.
3.3. Treatment
Patients of this naturalistic study were treated at the discretion of the psychiatrist in charge taking into consideration the international clinical guidelines for the treatment of depression [APA, World Federation of Societies of Biological Psychiatry (WFSBP)] (APA, 2000a; Bauer, Whybrow, Angst, Versiani, & Moller, 2002; Bauer et al., 2007).
3.4. Definition of subtypes
Melancholic subtype of depression was defined according to DSM‐IV (APA, 2000b) specifier criteria using HAMD items. Used items to operationalize DSM‐IV criterion A (loss of pleasure in all, or almost all, activities and lack of reactivity to pleasurable stimuli – HAMD items 1 or 7 had to be scored greater than 2) and criterion B (three or more of the following: distinct quality of depressed mood, depression regularly worse in the morning, early morning awakening, marked psychomotor retardation or agitation, significant anorexia or weight loss, or excessive or inappropriate guilt – at least three of the remaining HAMD criteria had to be fulfilled) are depicted later.
Atypical depression, defined in DSM‐IV by (A) mood reactivity and (B) two or more of the following: significant weight gain or increase in appetite, hypersomnia, leaden paralyses and interpersonal rejection sensitivity (APA, 2000b) was operationalized using the AMDP scale as in a previous publication (Seemuller et al., 2008). Anxious subtype was defined as HAMD‐17 anxiety/somatization subscale of ≥7 (Fava et al., 2000; Fava et al., 2004; Fava et al., 2008).
Patients with distinct subtypes were defined as patients conforming to only one subtype of depression; patients meeting more than one subtype definition were subsumed in the corresponding overlap group. Patients not fulfilling any of the three subtype definitions were termed “unspecific”.
3.5. Definition of outcome criteria
As some items of the HAMD scale were used to classify subtypes of depression, all depression‐specific symptomatic outcomes relied on the MADRS: remission was defined as MADRS total score ≤ 10 and response as 50% baseline reduction of the MADRS total score at discharge. In addition to these binary criteria, the relative change of the MADRS total score from admission to discharge was calculated. Values ≤ ˗50% correspond to the response criterion. A reduction of ≥20% of the MADRS score two weeks after admission was defined as early improvement (Henkel et al., 2009). Further outcome criteria were the GAF and SOFAS at discharge and a treatment resistance index according to the “Maudsley Staging” method (Fekadu, Wooderson, Markopoulou, & Cleare, 2009). We also analysed NEO‐FFI subscores at discharge.
3.6. Statistical analyses
Patients were eligible for this post hoc analysis if at least complete ratings of HAMD, MADRS, BADO scores and AMDP items at baseline to define subtypes of depression and one post‐baseline MADRS score were available.
Descriptive statistics with absolute numbers and percentages or mean (median) values ± standard deviation (SD) or ± inter‐quartile range (IQR) as appropriate are displayed. All analyses were based on available cases. Missing data for individual comparisons are indicated in Tables 1, 2, 3.
Table 1.
Baseline demographic characteristics of the whole sample and distinct depressive subtypes, results of overlap groups are not presented
| Overall (n = 833) | Anxious (1) (n = 233) | Atypical (2) (n = 61) | Melancholic (3) (n = 43) | Unspecific (4) (n = 365) | p‐Valuea | Post‐testb | ||
|---|---|---|---|---|---|---|---|---|
| Gender | Female | 522 (63%) | 150 (64%) | 44 (72%) | 24 (56%) | 217 (59%) | 0.17 | |
| Age | Mean (median) ± SD | 45.4 (46.3) ± 11.93 | 46.3 (47.9) ± 12.78 | 44.7 (46.0) ± 11.48 | 46.6 (47.6) ± 11.04 | 44.0 (44.8) ± 11.78 | 0.12 | |
| Age of onset | Mean (median) ± SD [Missings] | 38.0 (38.0) ± 12.56 [29] | 38.8 (38.0) ± 13.01 [8] | 37.9 (37.5) ± 12.90 [1] | 39.5 (41.0) ± 11.46 [6] | 37.3 (37.0) ± 12.58 [12] | 0.29 | |
| Reason for admission | Psychological change | 438 (54%) | 142 (63%) | 30 (50%) | 22 (51%) | 177 (49%) | 0.29 | |
| Suicidality | 230 (28%) | 46 (20%) | 19 (32%) | 16 (37%) | 112 (31%) | |||
| Aggressiveness | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Psychosocial crisis | 41 (5%) | 10 (4%) | 2 (3%) | 1 (2%) | 24 (7%) | |||
| Treatment resistance | 84 (10%) | 22 (10%) | 9 (15%) | 2 (5%) | 37 (10%) | |||
| Adverse events | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Other | 20 (2%) | 5 (2%) | 0 (0%) | 2 (5%) | 8 (2%) | |||
| [missing] | [19] | [7] | [1] | [0] | [7] | |||
| Diagnostic type | Bipolar | 50 (6%) | 8 (3%) | 4 (7%) | 3 (7%) | 29 (8%) | 0.0078 | 1–4 (0.025) |
| Single episode | 342 (41%) | 113 (48%) | 17 (28%) | 20 (47%) | 150 (41%) | |||
| Recurrent depression | 441 (53%) | 112 (48%) | 40 (66%) | 20 (47%) | 186 (51%) | |||
| Psychotic depressionc | Yes | 61 (7%) | 17 (7%) | 4 (7%) | 3 (7%) | 17 (5%) | 0.56 | |
| Suicidalityd | Yes | 382 (46%) | 103 (44%) | 26 (43%) | 22 (51%) | 161 (44%) | 0.63 | |
| Any personality disorder | Yes | 235 (28%) | 62 (27%) | 21 (34%) | 13 (30%) | 104 (28%) | 0.48 | |
| Comorbid F1e | Yes | 84 (10%) | 22 (9%) | 9 (15%) | 4 (9%) | 39 (11%) | 0.91 | |
| Comorbid F4e | Yes | 94 (11%) | 31 (13%) | 10 (16%) | 2 (5%) | 29 (8%) | 0.018 | |
| Number of comorbidities | Mean (median) ± IQR | 0.4 (0) ± 1.0 | 0.4 (0) ± 1.0 | 0.7 (0) ± 1.0 | 0.4 (0) ± 1.0 | 0.4 (0) ± 1.0 | 0.44 | |
| Duration of episode | < 1 month | 117 (14%) | 28 (12%) | 13 (22%) | 11 (27%) | 54 (15%) | 0.00035 |
1–3 (0.005) 2–3 (0.022) 2–4 (0.017) 3–4 (0.022) |
| < 6 months | 440 (54%) | 121 (53%) | 20 (34%) | 27 (66%) | 201 (56%) | |||
| < 2 years | 210 (25%) | 64 (28%) | 18 (31%) | 3 (7%) | 84 (24%) | |||
| > 2 years | 54 (7%) | 15 (7%) | 8 (14%) | 0 (0%) | 17 (5%) | |||
| [missing] | [21] | [5] | [2] | [2] | [9] | |||
| Any previous treatment | Yes [missing] | 602 (73%) [9] | 169 (73%) [2] | 48 (80%) [1] | 27 (66%) [2] | 247 (68%) [4] | 0.44 | |
| Number of previous hospitalizations | Mean (median) ± IQR | 1.5 (1) ± 2.0 | 1.2 (0) ± 2.0 | 1.3 (1) ± 2.0 | 1.2 (1) ± 2.0 | 1.5 (0) ± 2.0 | 0.98 | |
| Partner | Yes [missing] | 422 (51%) [4] | 120 (52%) [1] | 28 (46%) [0] | 22 (51%) [0] | 189 (52%) [0] | 0.39 | |
| School qualification | No | 143 (18%) | 36 (16%) | 15 (25%) | 10 (24%) | 61 (17%) | 0.99 | |
| Secondary modern school qualification | 162 (20%) | 41 (18%) | 9 (15%) | 9 (21%) | 73 (20%) | |||
| O‐level | 239 (30%) | 68 (31%) | 16 (27%) | 12 (29%) | 105 (29%) | |||
| University‐entrance diploma | 264 (33%) | 77 (35%) | 20 (33%) | 11 (26%) | 119 (33%) | |||
| [missing] | [25] | [11] | [1] | [1] | [7] | |||
| Vocational qualification | No | 129 (16%) | 39 (17%) | 13 (22%) | 4 (9%) | 55 (16%) | 0.40 | |
| Apprenticeship | 359 (44%) | 85 (38%) | 24 (40%) | 23 (53%) | 170 (48%) | |||
| University of applied sciences degree | 159 (20%) | 45 (20%) | 15 (25%) | 9 (21%) | 64 (18%) | |||
| University degree | 161 (20%) | 56 (25%) | 8 (13%) | 7 (16%) | 65 (18%) | |||
| [missing] | [25] | [8] | [1] | [0] | [11] | |||
| Job situation | Employed (broadly defined) | 561 (70%) | 148 (66%) | 44 (77%) | 31 (76%) | 258 (73%) | 0.74 | |
| Unemployed | 107 (13%) | 33 (15%) | 4 (7%) | 3 (7%) | 50 (14%) | |||
| Retired | 129 (16%) | 44 (20%) | 9 (16%) | 7 (17%) | 44 (12%) | |||
| [missing] | [36] | [8] | [4] | [2] | [13] | |||
| Family history of psychiatric diseases | Yes [missing] | 423 (51%) [9] | 115 (50%) [4] | 29 (48%) [1] | 17 (40%) [0] | 194 (54%) [3] | 0.11 | |
| Early life stress before sixth year | Yes [missing] | 142 (17%) [9] | 47 (21%) [4] | 7 (12%) [1] | 9 (21%) [0] | 59 (16%) [3] | 0.39 | |
| Early life stress between sixth and 15th year | Yes [missing] | 165 (20%) [9] | 51 (22%) [4] | 11 (18%) [1] | 14 (33%) [0] | 68 (19%) [3] | 0.16 | |
| MADRS at baseline | Mean (median) ± SD [missing] | 29.8 (30.0) ± 7.50 | 31.3 (31.0) ± 7.15 | 25.8 (26.0) ± 6.18 | 33.3 (33.0) ± 5.61 | 27.3 (28.0) ± 7.03 | < 0.0001 |
1–2 (0.002) 1–3 (0.028) 1–4 (< 0.0001) 2–3 (< 0.0001) 3–4 (< 0.0001) |
| GAF at baseline | Mean (median) ± SD [missing] | 48.3 (50.0) ± 11.37 [7] | 47.5 (45.0) ± 11.59 [2] | 48.7 (50.0) ± 8.59 | 46.1 (48.0) ± 9.16 | 49.6 (50.0) ± 11.84 [4] | 0.42 | |
| SOFAS at baseline | Mean (median) ± SD [missing] | 50.5 (50.0) ± 12.97 [10] | 49.5 (50.0) ± 12.66 [4] | 51.4 (50.0) ± 9.70 | 49.0 (50.0) ± 12.54 [1] | 52.0 (50.0) ± 13.53 [4] | 0.72 | |
| NEO‐FFI at baseline | Mean (median) ± SD [missing] | |||||||
| Neuroticism | 2.6 (2.6) ± 0.62 [128] | 2.6 (2.6) ± 0.63 [45] | 2.5 (2.7) ± 0.65 [5] | 2.5 (2.6) ± 0.70 [9] | 2.5 (2.5) ± 0.60 [43] | 0.060 | 1–4 (0.046) | |
| Extraversion | 1.7 (1.8) ± 0.54 [128] | 1.7 (1.6) ± 0.54 [45] | 1.7 (1.5) ± 0.62 [4] | 1.8 (1.8) ± 0.65 [9] | 1.7 (1.8) ± 0.53 [43] | 0.34 | ||
| Openness | 2.2 (2.2) ± 0.51 [128] | 2.2 (2.2) ± 0.48 [45] | 2.3 (2.2) ± 0.53 [4] | 2.2 (2.2) ± 0.49 [9] | 2.2 (2.2) ± 0.54 [43] | 0.63 | ||
| Tolerance | 2.5 (2.5) ± 0.45 [128] | 2.5 (2.5) ± 0.44 [45] | 2.5 (2.5) ± 0.54 [4] | 2.6 (2.5) ± 0.44 [9] | 2.5 (2.4) ± 0.45 [43] | 0.37 | ||
| Conscientious | 2.4 (2.4) ± 0.60 [128] | 2.3 (2.3) ± 0.61 [45] | 2.5 (2.5) ± 0.54 [4] | 2.3 (2.2) ± 0.66 [9] | 2.4 (2.5) ± 0.60 [43] | 0.29 |
For categorical variables, the Cochran–Mantel–Haenszel Test was used, for all other variables the Wald‐test for the subtype in a mixed regression model using the centre as a random effect (assuming Poisson and negative binomial distribution for the numbers of comorbidities and previous hospitalizations, respectively, and normal distribution otherwise).
Pairwise comparisons were adjusted for multiple testing according to Holm's method.
Psychotic depression was defined according to ICD‐10 in the case of fulfilling criteria for bipolar affective disorder, current episode severe depression with psychotic symptoms (F31.5), severe depressive episode with psychotic symptoms (F32.3) or recurrent depressive disorder, current episode severe with psychotic symptoms (F33.3).
Suicidality was defined according to BADO items in the case of suicidality as reason for admission or suicidality or suicide attempt prior to admission.
Comorbid F1 covers all mental and behavioural disorders due to psychoactive substance use (ICD‐10: F10.x–F19.x) and comorbid F4 refers to the ICD‐10 chapter of neurotic, stress‐related and somatoform disorders.
Table 2.
Outcome at discharge of whole sample and distinct depressive subgroups; results of overlap groups are not presented
| Overall (n = 833) | Anxious (1) (n = 233) | Atypic (2) (n = 61) | Melancholic (3) (n = 43) | Unspecific (4) (n = 365) | p‐Valuea | Post‐testc | ||
|---|---|---|---|---|---|---|---|---|
| Early improvementc | Yes (%) | 546 (66%) | 139 (60%) | 37 (61%) | 39 (91%) | 246 (67%) | 0.0149 | 1–3 (0.022) |
| Responsec | Yes (%) | 595 (71%) | 166 (71%) | 42 (69%) | 37 (86%) | 259 (71%) | 0.39 | |
| Remissionc | Yes (%) | 465 (56%) | 125 (54%) | 35 (57%) | 25 (58%) | 223 (61%) | 0.93 | |
| Duration of hospital stayd (days) | Mean (median) ± IQR (range) | 63.3 (55) ± 47.0 (1–363) | 66.0 (56) ± 46.0 | 70.4 (56) ± 38.0 | 54.9 (44) ± 48.0 | 61.5 (50) ± 49.0 | 0.149$ | |
| MADRSb at discharge | Mean (median) ± SD | 11.2 (9.0) ± 8.83 | 12.1 (10.0) ± 9.20 | 11.1 (9.0) ± 8.86 | 9.4 (8.0) ± 8.69 | 10.1 (8.0) ± 8.34 | 0.267 | |
| Relative change of MADRSb | Mean (median) ± SD | –61.1 (−68.8) ± 31.8 | −60.1 (−69.7) ± 34.0 | −56.3 (−63.2) ± 35.0 | −71.2 (−76.9) ± 26.2 | −61.8 (−69.2) ± 31.8 | 0.369 | |
| Treatment resistance indexd (range 3–15) | Mean (median) ± IQR (range) [missing] | 7.0 (7) ± 2.0 (4–13) [117] | 7.0 (7) ± 2.0 [35] | 7.0 (7) ± 2.0 [6] | 7.0 (7) ± 2.0 [7] | 6.9 (7) ± 2.0 [40] | 0.743$ | |
| GAFb at discharge | Mean (median) ± SD [missing] | 69.9 (70.0) ± 11.4 [83] | 69.3 (70.0) ± 11.9 [23] | 67.4 (70.0) ± 9.3 [2] | 72.2 (70.0) ± 12.8 [3] | 71.4 (70.0) ± 10.8 [45] | 0.266 | |
| SOFASb at discharge | Mean (median) ± SD [missing] | 68.4 (70.0) ± 11.5 [89] | 68.3 (70.0) ± 11.2 [26] | 65.6 (65.0) ± 10.9 [4] | 70.8 (70.0) ± 12.0 [3] | 69.5 (70.0) ± 11.6 [47] | 0.368 | |
| NEO‐FFI neuroticismb at discharge | Mean (median) ± SD [missing] | 2.2 (2.2) ± 0.67 [297] | 2.3 (2.2) ± 0.67 [92] | 2.3 (2.2) ± 0.62 [21] | 2.0 (2.2) ± 0.63 [16] | 2.1 (2.1) ± 0.67 [127] | 0.258 | |
| NEO‐FFI extraversionb at discharge | Mean (median) ± SD [missing] | 1.9 (1.9) ± 0.50 [297] | 1.9 (1.9) ± 0.49 [92] | 1.8 (1.9) ± 0.54 [21] | 1.9 (1.9) ± 0.57 [16] | 2.0 (2.0) ± 0.52 [127] | 0.383 | |
| NEO‐FFI opennessb at discharge | Mean (median) ± SD [missing] | 2.3 (2.2) ± 0.48 [297] | 2.3 (2.2) ± 0.48 [92] | 2.3 (2.4) ± 0.51 [21] | 2.3 (2.2) ± 0.45 [16] | 2.3 (2.2) ± 0.49 [127] | 0.792 | |
| NEO‐FFI toleranceb at discharge | Mean (median) ± SD [missing] | 2.6 (2.5) ± 0.44 [297] | 2.6 (2.6) ± 0.45 [92] | 2.5 (2.5) ± 0.40 [21] | 2.6 (2.6) ± 0.46 [16] | 2.6 (2.5) ± 0.43 [127] | 0.838 | |
| NEO‐FFI conscientiousb at discharge | Mean (median) ± SD [missing] | 2.6 (2.6) ± 0.53 [297] | 2.5 (2.4) ± 0.56 [92] | 2.6 (2.7) ± 0.50 [21] | 2.7 (2.8) ± 0.56 [16] | 2.6 (2.6) ± 0.53 [127] | 0.0093 | 1–3 (0.01) |
Wald test for the subtype in a mixed regression model with centre as a random effect (logistic model for the three outcome criteria, Gaussian otherwise).
For these variables, statistical group comparisons are adjusted for baseline MADRS‐scores, other variables at discharge are adjusted for their respective baseline values.
With square root transformation of the duration to support a Gaussian model.
Pairwise comparisons were adjusted for multiple testing using Holm's method.
The treatment resistance index was calculated based on the “Maudsley Staging” method with some modifications due to missing data as described in the main text.
Table 3.
Comparisons of patients with distinct depressive subtypes and patients with an overlap summerized for all groups. Only significant differences of baseline and outcome variables of distinct subgroups were compared to corresponding overlap groups
| Anx.b (1) (n = 233) | Anx.‐Melan. (2) (n = 60) | Melan. (3) (n = 43) | Anx.‐Atyp. (4) (n = 60) | Atyp. (5) (n = 61) | Melan.‐Atyp. (6) (n = 21) | p‐Valuec | ||
|---|---|---|---|---|---|---|---|---|
| Duration of episode | < 1 month | 28 (12%) | 7 (12%) | 11 (27%) | 4 (8%) | 13 (22%) | 0 (0%) |
1–2 (0.33) 2–3 (0.038) 5–6 (0.018) 3–6 (0.015) |
| < 6 months | 121 (53%) | 35 (60%) | 27 (66%) | 22 (45%) | 20 (34%) | 14 (67%) | ||
| < 2 years | 64 (28%) | 10 (17%) | 3 (7%) | 15 (31%) | 18 (31%) | 7 (33%) | ||
| > 2 years | 15 (7%) | 6 (10%) | 0 (0%) | 8 (16%) | 8 (14%) | 0 (0%) | ||
| [missing] | [5] | [2] | [2] | [1] | [2] | [0] | ||
| Diagnostic type | Bipolar | 8 (3%) | 2 (3%) | 3 (7%) | 0 (0%) | 4 (7%) | 4 (19%) | 1–4 (0.022) |
| Single episode | 113 (48%) | 21 (35%) | 20 (47%) | 16 (32%) | 17 (28%) | 5 (24%) | ||
| Recurrent depression | 112 (48%) | 37 (62%) | 20 (47%) | 34 (68%) | 40 (66%) | 12 (57%) | ||
| MADRS at baseline | Mean (median) ± SD | 31.3 (31.0) ± 7.2 | 36.8 (37.0) ± 6.65 | 33.3 (33.0) ± 5.6 | 31.6 (31.0) ± 5.8 | 25.8 (26.0) ± 6.2 | 37.2 (37.0) ± 5.7 |
1–2 (< 0.0001) 2–3 (0.021) 1–4 (0.45) 4–5 (0.0002) 5–6 (< 0.0001) 3–6 (0.026) |
| Early improvementa | Yes | 139 (60%) | 40 (67%) | 39 (91%) | 29 (58%) | 37 (61%) | 16 (76%) |
1–2 (0.73) 2–3 (0.012) 5–6 (0.567) 3–6 (0.147) |
| NEO‐FFI conscientiousa at discharge | Mean (median) ± SD [Missings] | 2.5 (2.4) ± 0.56 [92] | 2.6 (2.7) ± 0.50 [19] | 2.7 (2.8) ± 0.56 [16] | 2.5 (2.7) ± 0.50 [21] | 2.6 (2.7) ± 0.50 [21] | 2.6 (2.8) ± 0.57 [5] |
1–2 (0.15) 2–3 (0.12) 5–6 (0.13) 3–6 (0.50) |
Statistical group comparisons are adjusted on respective baseline values.
Anx., distinct anxious; Anx.‐Melan., anxious‐melancholic overlap; Melan., distinct melancholic; Anx.‐Atyp., anxious‐atypical overlap; Atyp., distinct atypical; Melan.‐Atyp., melancholic‐atypical overlap regardless of concurrent anxious features.
Wald tests for the subtype in mixed regression models with centre as a random effect as in Table 2, here p‐values are not adjusted for multiple testing.
Proportional subtype allocation is illustrated using a Venn diagram (Wilkinson, 2012).
Baseline characteristics and outcome criteria were compared between distinct subtypes of depression using omnibus tests and pairwise post‐tests. Associations between subtypes and categorical variables were assessed using Cochran–Mantel–Haenszel tests with stratification by centre. For all other variables, mixed regression models were estimated using centre as a random effect, where the fixed effect of the subtype was assessed with a Wald test. We used Poisson or negative binomial models for count variables, logistic models for binary outcome criteria, and Gaussian models for other variables (with square root transformation, if appropriate). Models of outcome criteria were additionally adjusted for baseline values. The p‐values of post‐tests were adjusted for multiple comparisons using Holm's (1979) method.
Patients with an overlap of two subtypes were compared with those patients showing only one of the distinct subtypes assessing all variables with significant differences between the distinct subtypes. Concerning patients fulfilling criteria for both melancholic and atypical subtype, we compared the overlap group regardless of presence or absence of criteria for anxious subtype. We further compared means of the anxiety/somatization score in all patients fulfilling criteria for anxious depression with respect to the other subtypes using mixed regression models as mentioned earlier.
The treatment resistance index was calculated on the basis of the “Maudsley Staging” method (Fekadu et al., 2009). Due to some missing data the definition of treatment failures of the method of Fekadu et al. (2009) had to be slightly adapted. In detail the electroconvulsive therapy (ECT) was considered as fulfilled in all cases where ECT was applied, and not only in those patients with at least an eight session course. Secondly, antidepressive treatment trials and use of augmentation strategies were reckoned in all cases with at least one week of continuous antidepressive or augmentation treatment with the same agent. No minimum dosage was required. Given these changes the scoring was applied according to Fekadu et al. (2009) yielding a score ranging from 3 to 15.
All statistical analyses were performed using the statistical software environment R 3.3.2 (R Core Team, 2016) with the packages venneuler (for Figure 1), VIM (for Figure 2), lme4 (for mixed models), and multcomp (for adjusted post‐tests).
Figure 1.
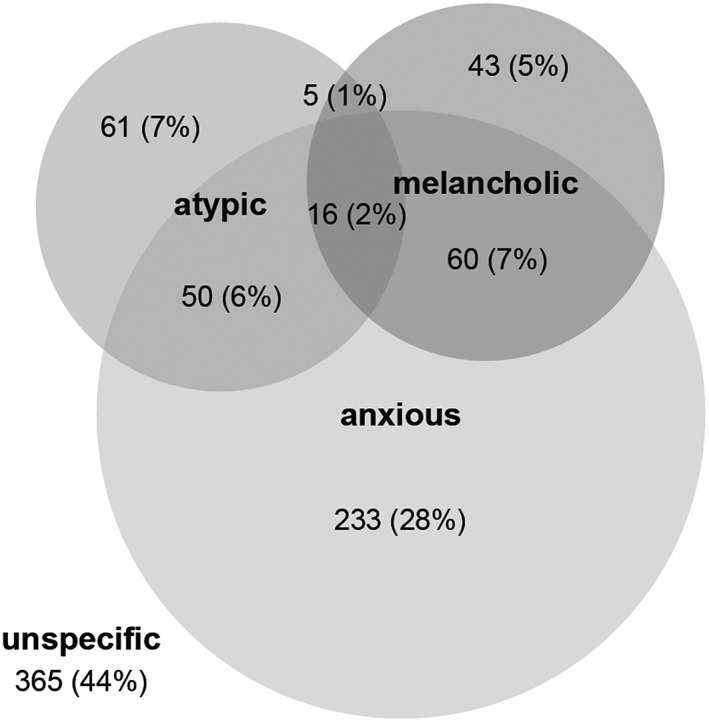
Venn diagram (90) showing proportional allocation of depression subtypes
Figure 2.
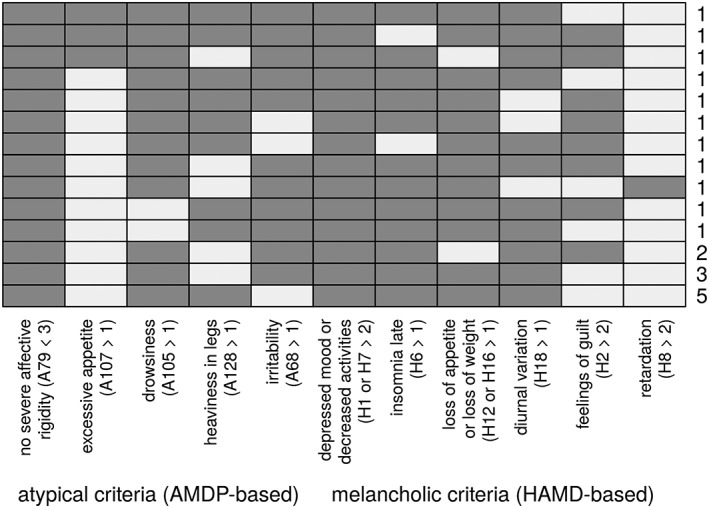
Frequency diagram of fulfilled criteria in the subgroup of melancholic‐atypical overlap group (n = 21). The frequency diagram shows all naturally occurring psychopathological patterns of patients assigned to the melancholic‐atypical overlap group. The exact number of patients fulfilling the respective pattern is displayed on the right. A, AMDP items; H, HAMD items; dark grey colour indicates criterion fulfilled, horizontal lines stand for combination of fulfilled items (e.g. all patients in this overlap group fulfilled criterion A79 < 3 [no severe affective rigidity] [column on the left]; criterion H8 > 2 [retardation] was least common [column on the right]. The most common pattern/combination of criteria is displayed in the bottom row, being fulfilled by five patients)
4. RESULTS
4.1. Sample characteristics and distribution of subtypes of depression
The German Research Network on Depression encompassed a total of 1073 patients. For this post hoc analysis 833 patients were available to define and compare subtypes of depression (59 patients were excluded due to missing baseline data; 78 patients refused inpatient treatment after start of the antidepressant treatment (mean treatment time 20.9 days (±21.97 days) and were classified as dropouts; 982 patients had minimum baseline data (BADO‐A & HAMD‐21 & ICD‐10), of which 881 were documented on those five AMDP items for definition of atypical subtype, of which 833 patients were available with baseline MADRS and at least one follow‐up MADRS score).
Absolute numbers and percentages of depression subtypes are highlighted in Figure 1. The majority of patients were assigned to at least one of the subtypes (56%); 44% had no assignment (unspecific), 43% were of the anxious, 16% of the atypical and 15% of the melancholic subtype; 16% were classified with more than one subtype of depression. Anxious depression was also the most frequent distinct subtype.
Demographic and clinical characteristics for the whole sample, the distinct subtypes and unspecific subgroup are shown in Table 1.
Overall, the majority of patients (80%) were treated in university hospitals. These patients were significantly more often diagnosed with a personality disorder (p = 0.0009) and had significantly longer index episodes (p = 0.048) compared to patients treated in district hospitals. Subtypes were unevenly distributed across the centres. To account for these centre‐effects we included centre as random‐effect in all subsequent analyses.
Patients with distinct melancholic subtype had the shortest durations of present episode compared to all other subtypes (versus anxious p = 0.005; versus atypical p = 0.022; versus unspecific p = 0.022), patients with distinct atypical subtype had greatest proportion of longer episodes (versus melancholic p = 0.022; versus unspecific p = 0.017), patients with distinct melancholic subtype had significantly higher MADRS scores at admission compared to all other subtypes (versus anxious p = 0.028; versus atypical p < 0.0001; versus unspecific p < 0.0001), patients with distinct anxious subtype had significantly higher MADRS scores at admission compared to atypical (p = 0.002) and unspecific subtype (p < 0.0001) and patients with distinct anxious subtype had higher NEO‐FFI neuroticism scores compared with patients with unspecific subtype (p = 0.046). For all other baseline characteristics, there was no evidence for differences among distinct subtypes of depression.
4.2. Treatment
After adjusting for any centre effect there were no differences in psychopharmacological interventions between the subtypes, including use of antipsychotics (stratified for typical, atypical), antidepressants [stratified for tricyclic antidepressants (TZAs), selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), other], tranquilizers, mood‐stabilizers and lithium, dopamine agonists, beta‐blockers and other psychopharmacological substances.
4.3. Outcome at discharge
Patients with distinct melancholic depression were significantly more often early improvers compared to the distinct anxious subtype (p = 0.022). NEO‐FFI conscientious subscore was significantly higher in patients with distinct melancholic subtype compared with the distinct anxious subtype (p = 0.01). All other outcome criteria did not differ between the groups (see Table 2).
4.4. Patients with subtype overlap
As was already shown in Figure 1, a substantial number of patients were assigned to more than one subtype of depression. Comparisons of patients with an overlap of subtypes with those patients exhibiting only one of these subtypes are shown in Table 3.
The anxious‐melancholic overlap group had significantly longer duration of current episode (p = 0.038) and lower proportion of patients with early improvement (p = 0.012) compared to patients with distinct melancholic subtype, but not compared to the distinct anxious subtype. The mean MADRS score was significantly higher in the overlap group compared to both distinct groups (versus distinct anxious p < 0.0001; versus distinct melancholic p = 0.021). The NEO‐FFI conscientious subscore at discharge of the overlap group was between the two distinct subgroups and did not differ significantly.
The anxious‐atypical overlap group resembled the distinct atypical subtype in terms of distribution of ICD‐10 diagnostic type of depression (but differed from the distinct anxious group, p = 0.022), but mean MADRS scores at baseline were similar to the distinct anxious subtype (and different from the atypical group, p = 0.0002) (see Table 3).
Patients with an overlap of melancholic and atypical criteria had significantly higher MADRS scores at admission compared with both distinct subgroups (versus melancholic p = 0.026; versus atypical p < 0.0001) (see Table 3). The overlap group was between the two distinct subgroups in terms of duration of current episode (distinct melancholic versus melancholic‐atypical p = 0.015; distinct atypical versus melancholic‐atypical p = 0.018).
The frequency of covered items to fulfill criteria for either subtype is shown in Figure 2.
Means of the anxiety/somatization scores increased from patients with distinct anxious subtype to the overlap groups resulting in overall significant differences (Wald‐test for group comparisons in a mixed regression model using centre as random effect: p = 0.031). Yet post hoc t‐tests were not significant with adjustment for multiple testing (anxiety/somatization subscore (mean ± SD): all patients with anxious depression: 8.8 ± 1.67; distinct anxious: 8.6 ± 1.51; anxious‐atypical: 8.6 ± 1.65; anxious‐melancholic 9.2 ± 2.05; anxious‐melancholic‐atypical: 9.6 ± 2.03).
5. DISCUSSION
The major finding of our post hoc analysis is on the one hand the fact that the investigated subtypes of depression differed only in four out of 25 studied baseline characteristics and in two out of 10 studied outcome criteria, and on the other hand that a considerable number of patients fulfil criteria for more than one subtype of MDD. The proportions of overlap were in similar magnitude to the results of the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) and iSPOT‐D (International Study to Predict Optimized Treatment – Depression) samples using a similar approach (Arnow et al., 2015). In both trials, fewer patients were assigned to no specific subtype (iSPOT 25% and STAR*D 33%); accordingly, more patients were classified to a distinct subtype or one of the overlap groups. The STAR*D sample subtype distribution clearly had the same emphasis on the anxious subtype and smaller proportions of overlap groups, just as our sample (distinct anxious 28%, distinct melancholic 6%, distinct atypical 7%, melancholic‐anxious 14%, melancholic‐atypical 1%, anxious‐atypical 8% and all three subtypes 3%) – whereas the iSPOT sample differed substantially with greater overlap groups (distinct anxious 13%, distinct melancholic 11%, distinct atypical 15%, melancholic‐anxious 5%, melancholic‐atypical 7%, anxious‐atypical 13% and all three subtypes 11%) (Arnow et al., 2015).
The prevalence of depression subtypes varies depending on applied definition, patient care setting and population under study. Our findings are in a comparable magnitude with previous results investigating all three subtypes (Arnow et al., 2015; Uher et al., 2011). Prevalence rates of anxious depression are in very good accordance with the results of others (Fava et al., 2004; Fava et al., 2006; Wiethoff et al., 2010).
Before discussing the clinical characteristics of patients with subtype overlap we want to put the findings of the distinct subtypes into perspective with results of the literature as our approach of studying distinct subtypes differs from previous studies.
5.1. Clinical characteristics of distinct depression subtypes
5.1.1. Distinct melancholic subtype
In agreement with our results most reports of the literature found significantly higher baseline severity in melancholic subtype compared to non‐melancholic patients (Kaestner et al., 2005; Khan et al., 2006; McGrath et al., 2008; Uher et al., 2011; Whiffen, Parker, Wilhelm, Mitchell, & Malhi, 2003), but not all (Fink et al., 2007; Rothermundt et al., 2001). Indeed, some authors suggested severity of depression as the only prerequisite of melancholic depression hypothesizing a threshold‐model (Kendler, 1997; Schotte, Maes, Cluydts, & Cosyns, 1997), while others highlighted psychomotor disturbances as the most important and distinct core feature (Parker et al., 1995; Parker et al., 2000). Using an LCA approach in the same patient sample Buhler et al. (2014) found higher HAMD‐17 baseline scores in patients who were characterized by anxious features compared to patients who displayed melancholic features.
Duration of current episode was further significantly shorter in patients with distinct melancholic depression compared to all other subtypes, which is in line with the findings of the large sample of the STAR*D trial (Khan et al., 2006).
With regard to outcome parameters, the more favourable aspects of patients with melancholic subtype being significantly more often early improvers (91%) and having highest GAF and SOFAS scores at discharge, the latter two though not being significantly different, could be due to the naturalistic treatment setting being poorly comparable with study populations showing e.g. worse treatment outcome in melancholic patients compared to all non‐melancholic patients in the case of treatment with SSRIs (McGrath et al., 2008; Uher et al., 2011).
Interestingly, NEO‐FFI conscientious scores were highest in patients with distinct melancholic subtype at discharge, and differences were significant compared to the distinct anxious subtype. This might be considered in accordance with results of other studies finding association of Tellenbach's typus melancholicus featuring perfectionism in patients with melancholic depression at the nadir of their episode (Furukawa et al., 1998; Rubino, Zanasi, Robone, & Siracusano, 2009).
Within the melancholic subtype male gender was more often represented compared to the other subtypes, which would be in accordance with previous results, yet our findings were not significant (Hildebrandt, Stage, & Kragh‐Soerensen, 2003).
Other interesting non‐significant results are the low rates of positive family history in patients with distinct melancholic subtype, challenging the hypothesized more biological background of this subtype. However, this is in accordance with previous findings not reporting any differences in family history of depressive disorders (Andreasen et al., 1986; Khan et al., 2006; McGuffin, Katz, & Bebbington, 1987; Parker et al., 2000).
Ourselves and others found no association of depression with psychotic features and melancholic subtype in comparison to non‐melancholic subtypes (Melartin et al., 2004) or other subtypes of depression (Duggan, Lee, & Murray, 1991). By contrast Parker et al. (2000) hypothesized psychotic depression to be a subtype of melancholic depression, showing most of endogeneity items and more severe psychomotor disturbances.
In summary, our results of distinct subtypes are in accordance with previous findings and overall patients with distinct melancholic subtype seem to have shorter, but more severe episodes of major depression, yet respond quite well to antidepressive treatment not restricting options to any specific agent.
5.1.2. Distinct atypical subtype
Most characteristics of distinct atypical subtype in our study are in agreement with previous findings such as longest durations of episode, most often treatment resistance as reason for admission, highest rates of any pretreatment and highest rates of recurrent depression. Though results were not significant in post hoc pairwise group comparisons, these findings might reflect the more chronic nature of illness course of patients with atypical depression (Stewart, McGrath, Rabkin, & Quitkin, 1993) showing also lower proportion of patients with full remission (APA, 2000b). Fittingly, patients with distinct atypical subtype had low rates of early improvement, longest duration of hospitalization, lowest relative change in MADRS scores and lowest GAF and SOFAS scores at discharge. Though not all results were significantly different it seems in summary that patients with distinct atypical depression have longer periods of major depression and do not respond very well to pharmacological treatment. Furthermore, we found a higher number of comorbidities using SCID‐I and SCID‐II interviews, though not being significantly different. In qualification, it should be stated that not all potentially co‐existing comorbidities in DSM‐IV are captured with these structured interviews.
More contradictory are the low MADRS baseline scores of patients with atypical depression. Though being in line with previous reports of our group investigating atypical features in primary care patients (Henkel et al., 2004), they seem to stand in contrast to results of the same sample exploring features of patients with atypical subtype and non‐atypical subtype (Seemuller et al., 2008) and also to the results of others (Novick et al., 2005; Parker et al., 2002; Posternak & Zimmerman, 2002). The reason for this discrepancy might lie in the fact that we compared distinct subtypes in the analysis at hand.
The same accounts for gender distribution, age and age at onset. While some found significant differences (Akiskal & Benazzi, 2005; Angst, Gamma, Sellaro, Zhang, & Merikangas, 2002; Novick et al., 2005; Parker et al., 2002; Seemuller et al., 2008; Uher et al., 2011), others did not (Henkel et al., 2004; Parker et al., 2002; Posternak & Zimmerman, 2002; Robertson et al., 1996). Thus our negative findings at least do not stand in contrast with previous results.
5.1.3. Distinct anxious subtype
Differences in baseline variables of patients with distinct anxious depression were somewhat between results of distinct melancholic and distinct atypical subtypes with respect to MADRS scores and duration of current episode. The only specific finding might be higher NEO‐FFI neuroticism scores. Yet results were only significant in comparison with the unspecific subgroup.
Thus the distinct anxious subtype of our study behaves different with regard to approaches comparing all patients with anxious depression versus non‐anxious‐depression, as some of these previous studies found anxious depressive patients to be associated with older age (Fava et al., 2004; Fava et al., 2006; Uher et al., 2011; Wiethoff et al., 2010), later age at onset (Uher et al., 2011), more severe depression (Fava et al., 2004; Fava et al., 2006; Uher et al., 2011; Wiethoff et al., 2010), greater functional impairment (Fava et al., 2004; Fava et al., 2006; Joffe, Bagby, & Levitt, 1993), chronicity (VanValkenburg, Akiskal, Puzantian, & Rosenthal, 1984), delayed response to treatment (Clayton et al., 1991; Fava et al., 2008), female gender (Fava et al., 2004; Fava et al., 2006), non‐single marital status (Fava et al., 2004; Fava et al., 2006), being unemployed (Fava et al., 2004; Fava et al., 2006), being less educated (Fava et al., 2004; Fava et al., 2006; Wiethoff et al., 2010), being of Hispanic origin (Fava et al., 2004; Fava et al., 2006) and having more often suicidal ideation (Fava et al., 2004; Fava et al., 2006; Tollefson, Rampey, Beasley Jr, Enas, & Potvin, 1994b).
We only found non‐significant tendencies which might be considered supporting previous findings as in our sample patients of the distinct anxious subtype had highest rates of unemployment, second highest MADRS scores at baseline, highest rates of female patients compared with distinct melancholic subtype and the unspecific group and were among the oldest.
In some aspects our results are in good accordance with previous findings also investigating an inpatient sample reporting no differences in age at onset, presence of comorbid personality disorder or suicidality (Wiethoff et al., 2010).
Regarding outcome parameters we found no differences in response and remission comparing distinct subtypes. Reports on overall responsiveness to antidepressant treatment are contradictory, as some found patients with anxious depression less likely to respond (Davidson, Meoni, Haudiquet, Cantillon, & Hackett, 2002; Wiethoff et al., 2010) while others did not (Nelson, 2010; Tollefson, Holman, Sayler, & Potvin, 1994a; Uher et al., 2011). In our sample patients with distinct anxious subtype of depression showed lowest proportion of patients with early improvement, which fits well with previous findings of delayed response to treatment (Clayton et al., 1991; Fava et al., 2008).
5.2. Characteristics of subtype overlap
There are only a few reports in the literature comparing distribution of depression subtypes and their overlap. We found equal extension of overlap between anxious subtype and atypical or melancholic subtype. This is in contrast to the results of Fava et al. (2004, 2006) finding patients with anxious depression to exhibit melancholic features more often, which seems surprising in light of the fact that patients with distinct atypical subtype in our study had highest comorbidity rates of any somatoform and stress‐related disorders, including panic disorder, generalized anxiety disorder or agoraphobia (see also Seemuller et al., 2008). Our results underline the close relationship between anxiety symptoms and atypical depression, as was also proposed by Roth, Gurney, Garside, and Kerr (1972). Differences in respect to the results of Fava et al. (2004, 2006) may be due to the fact that we compared primarily distinct subgroups and the sample consisted of inpatients.
5.2.1. Overlap with anxious features: Worsening of treatment outcome?
Our results evoke the impression that the addition of anxious features worsen treatment outcome in patients otherwise classified as either atypical or melancholic. Proportion of early improvement significantly dropped, duration of index episode was longer, MADRS scores at baseline were higher and diagnostic subtype more often recurrent compared to the distinct subtypes. As MADRS scores at baseline were highest in patients of distinct melancholic subtype and all results were adjusted for severity of depression, this may not seem to be solely related to a higher score in several HAMD‐items used to classify anxious subtype. These findings might be considered in line with previous results showing significantly lower response rates in patients with anxious and melancholic features compared to patients of mere melancholic subtype (Domschke, Deckert, Arolt, & Baune, 2010).
With respect to overlap of anxious features with melancholic or atypical subtypes, for clinical routine we therefore suggest screening patients thoroughly for symptoms of anxiety irrespective of other subtype classification as depression severity worsens, while for research purposes patients with anxious features should rather be excluded when an attempt is being made to delineate biological causes or differences of melancholic and atypical depression using a dichotomous approach.
5.2.2. The atypical‐melancholic overlap: More melancholic or more atypical depression?
The last overlap group of our study consisted of patients with melancholic and atypical features irrespective of presence of anxious features due to the small sample size. This group is somewhat artificial, since according to criterion C of DSM‐IV, and also of DSM‐5, patients must not be diagnosed with atypical specifier in the case of diagnosis of the melancholic subtype. Still, these 21 patients (3%) fulfilled criteria of both subtype definitions when operationalized criteria sets were used, underlining the difficulties in subtype classification based on an operational approach rather than on clinical judgement. These patients exhibited especially unfavourable clinical characteristics, having significantly longer duration of current episode and highest MADRS baseline scores compared to patients with distinct melancholic subtype. Also proportion of patients with early improvement was lower compared to the distinct melancholic subgroup, yet differences did not reach statistical significance. Based on a large (n = 1624) community sample the atypical‐melancholic overlap subgroup was as high as 12.2% of all depressed patients using DSM‐IV criteria with a non‐hierarchal approach (Rodgers et al., 2016).
As some clinical features of DSM‐IV (as well as of DSM‐5) melancholic and atypical specifier seem to contrast each other we were interested in details of combination of items leading to subtype definition (see Figure 2). Interestingly, some patients with melancholic‐atypical overlap were rated both high on HAMD item 12 or 16 (decrease in appetite or weight loss) and AMDP item 107 (increased appetite). This seems to be contradictory; however, a patient could have weight loss and increased appetite over the same time period. Still we cannot completely rule out rating errors. Furthermore, almost none of the patients in our sample with atypical‐melancholic overlap scored high on HAMD‐item 8 (retardation) and this item was never combined with high scores on HAMD item 2 (feelings of guilt). According to the concepts of Parker et al. (1995) psychomotor disturbances stand at the core of melancholic depression. Thus with respect to these entire differences one could argue that patients with melancholic‐atypical overlap were more likely patients with very severe atypical depression, challenging the hierarchal approach of DSM‐IV and DSM‐5 criteria giving melancholia priority and implying the development of better exclusion criteria for both subtypes in further revisions.
For the purpose of clarity we propose to either exclude patients fulfilling operationalized criteria for both atypical and melancholic features in studies trying to investigate biological causes of depression subtype or to closely monitor such contradictory symptom descriptions, respectively.
5.2.3. How to proceed with the largest subgroup?
Lastly, it would be worthwhile discussing characteristics of the remaining patients called “unspecific” depressive in the study at hand comprising 44% of patients and constituting the largest subgroup. Clinically these patients on average are shown as having neither best nor worst outcome. Having said this it seems necessary to try and further subdivide this large group in order to individualize treatment options and optimize outcome. Parker and colleagues extensively studied the large non‐melancholic subgroup and proposed characteristics and subtypes of this most encountered comparison group (Parker, Roy, Hadzi‐Pavlovic, Mitchell, & Wilhelm, 2003; Parker et al., 1998a; Parker et al., 1998b; Parker et al., 1999). However, as Parker and colleagues based their classification on a hierarchal model, which does not include anxious or atypical subtype, this approach and all the remaining patients are barely comparable with our approach. At this stage, using operationalized criteria sets based on common concepts we cannot meaningfully subdivide this large patient group. Statistical approaches using e.g. cluster analyses might be helpful in future studies.
5.3. Limitations
In interpreting the results of our study several limitations have to be borne in mind. The article reports post hoc‐analyses of prospective collected data. The study was not designed to investigate clinical features of depression subtypes or to adjust treatment modalities for the different subtypes. Furthermore, we followed a heuristic approach and the results have to be interpreted according to the exploratory nature of the multiple comparisons. Though raters were extensively trained in all applied scales in order to reduce overall interrater variability we found some differences across sites and accordingly adjusted all tests using centre as random effect. We did not adjust for any variables, as clear confounders for any of the distinctive subtypes or their overlap are yet to be established. We still hope to contribute to the identification of such confounders in future studies.
Although overall inclusion criteria were broad we had to exclude a substantial number of patients due to missing data of one of the scales to apply subtypes of depression criteria or at least one post‐baseline MADRS score reducing the total number of 1073 enrolled patients to the 833 available cases for our analyses. In addition, some individual comparisons were hampered by missing data exceeding a total of 50 data (NEO‐FFI at baseline and discharge, GAF and SOFAS at discharge and treatment resistance index).
Discrepancies of baseline and outcome parameters with previous results have to be reflected in the light of different comparison groups as most studies focussed on one subtype comparing this with all remaining patients. We used operationalized criteria for subtype definition, which may lead to more subtype overlap than using clinical judgement (Rasmussen, 2007). Numerous other criteria sets to define subtypes of depression would have been possible (Rush & Weissenburger, 1994), including the hierarchal approach of Parker (2000) and Parker et al. (2009). We assumed a categorical approach for atypical, melancholic and anxious depression and chose to investigate psychotic symptoms as independent dimension in all subtypes. The uniform prevalence of 7% of patients with psychotic depression in all three distinct subtypes apart from the unspecific group supports this notion. Yet this could be criticized in light of the model proposed by Parker (2000) and Parker et al. (2009).
All patients were hospitalized and may thus differ from outpatient populations, however, this fact facilitated the analyses of features like suicidality and psychotic symptoms. Nearly all patients were of Caucasian origin so we cannot generalize results to other ethnicities.
However, the strength of our study is clearly the broad applied inclusion criteria not excluding patients with bipolar, psychotic or catatonic depressive characteristics. Therefore, the picture drawn might be representative for inpatient samples of Caucasian origin.
Our attempt to further delineate the concepts of depression subtypes supports a more dimensional approach of subtypes. We found substantial symptom overlap of different depression subtypes using operationalized criteria. Studies trying to further ascertain biological causes of depression subtypes should therefore attempt to compare several groups, distinct and overlap groups. Simply comparing patients with melancholic versus non‐melancholic or atypical versus non‐atypical features will most likely not result in a clear picture due to heterogeneous comparison groups and might thus contribute to conflicting results (Rasmussen, 2007).
Larger studies involving different symptom‐based approaches in combination with biological markers seem to be necessary to further delineate any subtype patterns of depression.
DECLARATION OF INTEREST
The authors declare that over the past three years author Dr R. Musil has received research support from Janssen‐Cilag, Speaker Honoraria from Otsuka and has been on the advisory board of Roche Pharmaceuticals. Author PD Dr F. Seemüller has received research support from Lundbeck, Speaker Honoraria from Lundbeck, Servier and Ferrer. Author PD Dr Adli has received Grant/Research Support from the German Federal Ministry of Education and Research, German Federal Ministry of Health, the Volkswagen‐Foundation, Lundbeck, Bristol‐Myers Squibb, and esparma. He has received Speaker Honoraria from AstraZeneca, Eli Lilly & Company, Lundbeck, Bristol‐Myers Squibb, GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Sanofi, esparma, Wyeth Pharmaceuticals, and Deutsche Bank. He has been a consultant to Bristol‐Myers Squibb, esparma, and Lundbeck. Prof. Dr M. Bauer has received Grant/Research Support from the Deutsche Forschungsgemeinschaft (DFG), Bundesministeriums für Bildung und Forschung (BMBF), American Foundation of Suicide Prevention. He is a consultant for Allergan, Ferrer Internacional, Janssen, Lilly, neuraxpharm, Lundbeck, Otsuka, Servier, Takeda, and Novartis and has further received Speaker Honoraria from AstraZeneca, Pfizer, Lilly, Lundbeck, Otsuka, and Servier. Author Prof. Dr I. Heuser acted as consultant to GE Healthcare, AstraZeneca, Novartis and Bayer Healthcare. She received grants from the BMBF (German Federal Ministry for Education and Research) and the DFG (Deutsche Forschungsgemeinschaft). Author Prof. Dr W. Gaebel has received symposia support from Aristo Pharma, Janssen‐Cilag, Lilly and Servier. He is a member of the Faculty of Lundbeck International Neuroscience Foundation (LINF), Denmark. Author Prof. Dr Moeller has received honoraria for lectures or for advisory activities by the following pharmaceutical companies: Astra‐Zeneca, Eli Lilly, Janssen, Lundbeck, Pfizer, Schwabe, Servier, Otsuka and Takeda. He was president or on the Executive Board of the following organisations: CINP ECNP, WFSBP, EPA and chairman of the WPA‐section on Pharmacopsychiatry. Author Prof. Dr. M. Riedel has received grants/research support form Otsuka and Speaker Honoraria from Servier and Otsuka. Author Prof. Dr G. Laux has received grants, and acted as consultant, advisor, or speaker for the following companies: Astra‐Zeneca, Bayer, Boehringer Ingelheim, Janssen‐Cilag, Lilly, Lundbeck, Merz, Novartis, Organon, Pfizer, Servier, Steigerwald, Teva, Wyeth.
These affiliations have no relevance to the work covered in the manuscript. All other authors state that they have no conflicts of interest to declare.
ROLE OF FUNDING SOURCE
The authors declare that this work was performed within the framework of the German Research Network on Depression, which was funded by the German Federal Ministry for Education and Research BMBF (01GI0219). The BMBF had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
ACKNOWLEDGEMENTS
The study was part of the German research network, funded by the German Federal Ministry of Education and Research (BMBF) and was conducted in 12 psychiatric hospitals: Berlin Charité Campus Mitte (Andreas Heinz, Mazda Adli, Katja Wiethoff), Berlin Charité Campus Benjamin Franklin (Isabella Heuser, Gerd Bischof), Berlin Auguste Viktoria Klinik (Joachim Zeiler, Robert Fisher, Cornelia Fähser), Berlin St Hedwig (Florian Standfest), Berlin St Joseph (Dorothea Schloth), Düsseldorf (Wolfgang Gaebel, Joachim Cordes, Arian Mobascher), Wasserburg‐Gabersee (Gerd Laux, Sissi Artmann), Haar (Wolfram Bender, Nicole Theyson), Halle (Andreas Marneros, Dörthe Strube, Yvonne Reinelt), Heidelberg (Christoph Mundt, Klaus Kronmüller, Daniela Victor), München LMU (Hans‐Jürgen Möller, Ulrich Hegerl, Roland Mergel, Michael Riedel, Florian Seemüller, Florian Wickelmaier, Markus Jäger, Thomas Baghai, Ingrid Borski, Constanze Schorr, Roland Bottlender), München MPI (Florian Holsboer, Matthias Majer, Marcus Ising). The authors thank Thelma Coutts for assistance with language.
Musil R, Seemüller F, Meyer S, et al. Subtypes of depression and their overlap in a naturalistic inpatient sample of major depressive disorder. Int J Methods Psychiatr Res. 2018;27:e1569 10.1002/mpr.1569
REFERENCES
- Akiskal, H. S. , & Benazzi, F. (2005). Atypical depression: A variant of bipolar II or a bridge between unipolar and bipolar II? Journal of Affective Disorders, 84(2–3), 209–217. 10.1016/j.jad.2004.05.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2000a). Practice guideline for the treatment of patients with major depressive disorder (revision). American Journal of Psychiatry, 157(4 Suppl), 1–45. [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2000b). Diagnostic and Statistical Manual of Mental Disorders – DSM‐IV‐TR (fourth ed.). Washington, DC: APA. [Google Scholar]
- American Psychiatric Association (APA) . (2013a). From planning to publication: Developing DSM‐5. https://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=11&cad=rja&uact=8&ved=0ahUKEwjou6HuzM7PAhUDPxQKHRxABkUQFghXMAo&url=https%3A%2F%2Fwww.psychiatry.org%2FFile%2520Library%2FPsychiatrists%2FPractice%2FDSM%2FAPA_DSM-Development-of-DSM-5.pdf&usg=AFQjCNGeE1oiXbW8fwBLYRxM_N_JaZFH3A
- American Psychiatric Association (APA) (2013b). Diagnostic and Statistical Manual of Mental Disorders – DSM‐5 (fifth ed.). Arlington, VA: APA. [Google Scholar]
- Andreasen, N. C. , Scheftner, W. , Reich, T. , Hirschfeld, R. M. , Endicott, J. , & Keller, M. B. (1986). The validation of the concept of endogenous depression. A family study approach. Archives of General Psychiatry, 43(3), 246–251. [DOI] [PubMed] [Google Scholar]
- Angst, J. , Gamma, A. , Sellaro, R. , Zhang, H. , & Merikangas, K. (2002). Toward validation of atypical depression in the community: Results of the Zurich cohort study. Journal of Affective Disorders, 72(2), 125–138. [DOI] [PubMed] [Google Scholar]
- Antonijevic, I. A. (2006). Depressive disorders – Is it time to endorse different pathophysiologies? Psychoneuroendocrinology, 31(1), 1–15. 10.1016/j.psyneuen.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Arnow, B. A. , Blasey, C. , Williams, L. M. , Palmer, D. M. , Rekshan, W. , Schatzberg, A. F. , … Rush, A. J. (2015). Depression subtypes in predicting antidepressant response: A report from the iSPOT‐D trial. American Journal of Psychiatry, 172(8), 743–750. 10.1176/appi.ajp.2015.14020181 [DOI] [PubMed] [Google Scholar]
- Bandelow, B. , Bauer, M. , Vieta, E. , El‐Khalili, N. , Gustafsson, U. , Earley, W. R. , & Eriksson, H. (2014). Extended release quetiapine fumarate as adjunct to antidepressant therapy in patients with major depressive disorder: Pooled analyses of data in patients with anxious depression versus low levels of anxiety at baseline. World Journal of Biological Psychiatry, 15(2), 155–166. 10.3109/15622975.2013.842654 [DOI] [PubMed] [Google Scholar]
- Bauer, M. , Whybrow, P. C. , Angst, J. , Versiani, M. , & Moller, H. J. (2002). World Federation of Societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Acute and continuation treatment of major depressive disorder. World Journal of Biological Psychiatry, 3(1), 5–43. [DOI] [PubMed] [Google Scholar]
- Bauer, M. , Bschor, T. , Pfennig, A. , Whybrow, P. C. , Angst, J. , Versiani, M. , & Moller, H. J. (2007). World Federation of Societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders in primary care. World Journal of Biological Psychiatry, 8(2), 67–104. 10.1080/15622970701227829 [DOI] [PubMed] [Google Scholar]
- Baune, B. T. , Hohoff, C. , Roehrs, T. , Deckert, J. , Arolt, V. , & Domschke, K. (2008). Serotonin receptor 1A‐1019C/G variant: Impact on antidepressant pharmacoresponse in melancholic depression? Neuroscience Letters, 436(2), 111–115. 10.1016/j.neulet.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Buhler, J. , Seemuller, F. , & Lage, D. (2014). The predictive power of subgroups: An empirical approach to identify depressive symptom patterns that predict response to treatment. Journal of Affective Disorders, 163, 81–87. 10.1016/j.jad.2014.03.053 [DOI] [PubMed] [Google Scholar]
- Clayton, P. J. , Grove, W. M. , Coryell, W. , Keller, M. , Hirschfeld, R. , & Fawcett, J. (1991). Follow‐up and family study of anxious depression. American Journal of Psychiatry, 148(11), 1512–1517. 10.1176/ajp.148.11.1512 [DOI] [PubMed] [Google Scholar]
- Cording, C. , Gaebel, W. , & Spengler, A. (1995). Die neue psychiatrische Basisdokumentation. Eine Empfehlung der DGPPN zur Qualitätssicherung im (teil‐) stationären Bereich. [the new psychiatric basic documentation. A recommendation by the DGPPN for quality assurance in inpatient treatment]. Spektrum Psychiatrie Nervenheilkunde, 24, 3–41. [Google Scholar]
- Costa, P. T. Jr. , & McCrae, R. R. (1988). Personality in adulthood: A six‐year longitudinal study of self‐reports and spouse ratings on the NEO personality Inventory. Journal of Personality and Social Psychology, 54(5), 853–863. [DOI] [PubMed] [Google Scholar]
- Davidson, J. R. , Meoni, P. , Haudiquet, V. , Cantillon, M. , & Hackett, D. (2002). Achieving remission with venlafaxine and fluoxetine in major depression: Its relationship to anxiety symptoms. Depression and Anxiety, 16(1), 4–13. 10.1002/da.10045 [DOI] [PubMed] [Google Scholar]
- Domschke, K. , Deckert, J. , Arolt, V. , & Baune, B. T. (2010). Anxious versus non‐anxious depression: Difference in treatment outcome. Journal of Psychopharmacology, 24(4), 621–622. 10.1177/0269881108097723 [DOI] [PubMed] [Google Scholar]
- Duggan, C. F. , Lee, A. S. , & Murray, R. M. (1991). Do different subtypes of hospitalized depressives have different long‐term outcomes? Archives of General Psychiatry, 48(4), 308–312. [DOI] [PubMed] [Google Scholar]
- Fava, M. , Rosenbaum, J. F. , Hoog, S. L. , Tepner, R. G. , Kopp, J. B. , & Nilsson, M. E. (2000). Fluoxetine versus sertraline and paroxetine in major depression: Tolerability and efficacy in anxious depression. Journal of Affective Disorders, 59(2), 119–126. [DOI] [PubMed] [Google Scholar]
- Fava, M. , Alpert, J. E. , Carmin, C. N. , Wisniewski, S. R. , Trivedi, M. H. , Biggs, M. M. , … Rush, A. J. (2004). Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychological Medicine, 34(7), 1299–1308. [DOI] [PubMed] [Google Scholar]
- Fava, M. , Rush, A. J. , Alpert, J. E. , Carmin, C. N. , Balasubramani, G. K. , Wisniewski, S. R. , … Shores‐Wilson, K. (2006). What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: A replication and extension. Canadian Journal of Psychiatry, 51(13), 823–835. [DOI] [PubMed] [Google Scholar]
- Fava, M. , Rush, A. J. , Alpert, J. E. , Balasubramani, G. K. , Wisniewski, S. R. , Carmin, C. N. , … Trivedi, M. H. (2008). Difference in treatment outcome in outpatients with anxious versus nonanxious depression: A STAR*D report. American Journal of Psychiatry, 165(3), 342–351. 10.1176/appi.ajp.2007.06111868 [DOI] [PubMed] [Google Scholar]
- Fekadu, A. , Wooderson, S. C. , Markopoulou, K. , & Cleare, A. J. (2009). The Maudsley Staging method for treatment‐resistant depression: Prediction of longer‐term outcome and persistence of symptoms. Journal of Clinical Psychiatry, 70(7), 952–957. 10.4088/JCP.08m04728 [DOI] [PubMed] [Google Scholar]
- Fink, M. , Rush, A. J. , Knapp, R. , Rasmussen, K. , Mueller, M. , Rummans, T. A. , … Kellner, C. H. (2007). DSM melancholic features are unreliable predictors of ECT response: A CORE publication. Journal of ECT, 23(3), 139–146. 10.1097/yct.0b013e3180337344 [DOI] [PubMed] [Google Scholar]
- Furukawa, T. , Yamada, A. , Tabuse, H. , Kawai, K. , Takahashi, K. , Nakanishi, M. , & Hamanaka, T. (1998). Typus melancholicus in light of the five‐factor model of personality. European Archives of Psychiatry and Clinical Neuroscience, 248(2), 64–69. [DOI] [PubMed] [Google Scholar]
- Gold, P. W. , & Chrousos, G. P. (1999). The endocrinology of melancholic and atypical depression: Relation to neurocircuitry and somatic consequences. Proceedings of the Association of American Physicians, 111(1), 22–34. [DOI] [PubMed] [Google Scholar]
- Gold, P. W. , & Chrousos, G. P. (2002). Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Molecular Psychiatry, 7(3), 254–275. 10.1038/sj.mp.4001032 [DOI] [PubMed] [Google Scholar]
- Gold, P. W. , Licinio, J. , Wong, M. L. , & Chrousos, G. P. (1995). Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Annals of the New York Academy of Sciences, 771, 716–729. [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1967). Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology, 6(4), 278–296. [DOI] [PubMed] [Google Scholar]
- Harkness, K. L. , & Monroe, S. M. (2006). Severe melancholic depression is more vulnerable than non‐melancholic depression to minor precipitating life events. Journal of Affective Disorders, 91(2–3), 257–263. 10.1016/j.jad.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Henkel, V. , Mergl, R. , Coyne, J. C. , Kohnen, R. , Allgaier, A. K. , Ruhl, E. , … Hegerl, U. (2004). Depression with atypical features in a sample of primary care outpatients: Prevalence, specific characteristics and consequences. Journal of Affective Disorders, 83(2–3), 237–242. 10.1016/j.jad.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Henkel, V. , Seemuller, F. , Obermeier, M. , Adli, M. , Bauer, M. , Mundt, C. , … Riedel, M. (2009). Does early improvement triggered by antidepressants predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. Journal of Affective Disorders, 115(3), 439–449. 10.1016/j.jad.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Hildebrandt, M. G. , Stage, K. B. , & Kragh‐Soerensen, P. (2003). Gender differences in severity, symptomatology and distribution of melancholia in major depression. Psychopathology, 36(4), 204–212. https://doi.org/72791 [DOI] [PubMed] [Google Scholar]
- Holm, S. (1979). Simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Joffe, R. T. , Bagby, R. M. , & Levitt, A. (1993). Anxious and nonanxious depression. American Journal of Psychiatry, 150(8), 1257–1258. 10.1176/ajp.150.8.1257 [DOI] [PubMed] [Google Scholar]
- Kaestner, F. , Hettich, M. , Peters, M. , Sibrowski, W. , Hetzel, G. , Ponath, G. , … Rothermundt, M. (2005). Different activation patterns of proinflammatory cytokines in melancholic and non‐melancholic major depression are associated with HPA axis activity. Journal of Affective Disorders, 87(2–3), 305–311. 10.1016/j.jad.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Kendler, K. S. (1997). The diagnostic validity of melancholic major depression in a population‐based sample of female twins. Archives of General Psychiatry, 54(4), 299–304. [DOI] [PubMed] [Google Scholar]
- Khan, A. Y. , Carrithers, J. , Preskorn, S. H. , Lear, R. , Wisniewski, S. R. , John Rush, A. , … Fava, M. (2006). Clinical and demographic factors associated with DSM‐IV melancholic depression. Annals of Clinical Psychiatry, 18(2), 91–98. 10.1080/10401230600614496 [DOI] [PubMed] [Google Scholar]
- Leventhal, A. M. , & Rehm, L. P. (2005). The empirical status of melancholia: Implications for psychology. Clinical Psychology Review, 25(1), 25–44. 10.1016/j.cpr.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Yieh, L. , Yang, T. , Drinkenburg, W. , Peeters, P. , Steckler, T. , … Ye, J. (2016). Metabolomic biosignature differentiates melancholic depressive patients from healthy controls. BMC Genomics, 17, 669 10.1186/s12864-016-2953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, P. J. , Khan, A. Y. , Trivedi, M. H. , Stewart, J. W. , Morris, D. W. , Wisniewski, S. R. , … Rush, A. J. (2008). Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: A STAR*D report. Journal of Clinical Psychiatry, 69(12), 1847–1855. [DOI] [PubMed] [Google Scholar]
- McGuffin, P. , Katz, R. , & Bebbington, P. (1987). Hazard, heredity and depression. A family study. Journal of Psychiatric Research, 21(4), 365–375. [DOI] [PubMed] [Google Scholar]
- Melartin, T. , Leskela, U. , Rytsala, H. , Sokero, P. , Lestela‐Mielonen, P. , & Isometsa, E. (2004). Co‐morbidity and stability of melancholic features in DSM‐IV major depressive disorder. Psychological Medicine, 34(8), 1443–1452. [DOI] [PubMed] [Google Scholar]
- Montgomery, S. A. , & Asberg, M. (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Monzon, S. , Gili, M. , Vives, M. , Serrano, M. J. , Bauza, N. , Molina, R. , … Roca, M. (2010). Melancholic versus non‐melancholic depression: Differences on cognitive function. A longitudinal study protocol. BMC Psychiatry, 10, 48 10.1186/1471-244X-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, J. C. (2010). Anxiety does not predict response to duloxetine in major depression: Results of a pooled analysis of individual patient data from 11 placebo‐controlled trials. Depression and Anxiety, 27(1), 12–18. 10.1002/da.20632 [DOI] [PubMed] [Google Scholar]
- Novick, J. S. , Stewart, J. W. , Wisniewski, S. R. , Cook, I. A. , Manev, R. , Nierenberg, A. A. , … Rush, A. J. (2005). Clinical and demographic features of atypical depression in outpatients with major depressive disorder: Preliminary findings from STAR*D. Journal of Clinical Psychiatry, 66(8), 1002–1011. [DOI] [PubMed] [Google Scholar]
- Papakostas, G. I. , Fan, H. , & Tedeschini, E. (2012). Severe and anxious depression: Combining definitions of clinical sub‐types to identify patients differentially responsive to selective serotonin reuptake inhibitors. European Neuropsychopharmacology, 22(5), 347–355. 10.1016/j.euroneuro.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Parker, G. (2000). Classifying depression: Should paradigms lost be regained? American Journal of Psychiatry, 157(8), 1195–1203. 10.1176/appi.ajp.157.8.1195 [DOI] [PubMed] [Google Scholar]
- Parker, G. , Hadzi‐Pavlovic, D. , Austin, M. P. , Mitchell, P. , Wilhelm, K. , Hickie, I. , … Eyers, K. (1995). Sub‐typing depression, I. Is psychomotor disturbance necessary and sufficient to the definition of melancholia? Psychological Medicine, 25(4), 815–823. [DOI] [PubMed] [Google Scholar]
- Parker, G. , Gladstone, G. , Wilhelm, K. , Hickie, I. , Mitchell, P. , Hadzi‐Pavlovic, D. , … Eyers, K. (1998a). An aetiological model of non‐melancholic depression: Study design and validity of the measures. Australian & New Zealand Journal of Psychiatry, 32(1), 104–111. [DOI] [PubMed] [Google Scholar]
- Parker, G. , Hadzi‐Pavlovic, D. , Roussos, J. , Wilhelm, K. , Mitchell, P. , Austin, M. P. , … Eyers, K. (1998b). Non‐melancholic depression: The contribution of personality, anxiety and life events to subclassification. Psychological Medicine, 28(5), 1209–1219. [DOI] [PubMed] [Google Scholar]
- Parker, G. , Roy, K. , Wilhelm, K. , Mitchell, P. , Austin, M. P. , Hadzi‐Pavlovic, D. , & Little, C. (1999). Sub‐grouping non‐melancholic depression from manifest clinical features. Journal of Affective Disorders, 53(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Parker, G. , Roy, K. , Hadzi‐Pavlovic, D. , Mitchell, P. , Wilhelm, K. , Menkes, D. B. , … Schweitzer, I. (2000). Subtyping depression by clinical features: The Australasian database. Acta Psychiatrica Scandinavica, 101(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Parker, G. , Roy, K. , Mitchell, P. , Wilhelm, K. , Malhi, G. , & Hadzi‐Pavlovic, D. (2002). Atypical depression: A reappraisal. American Journal of Psychiatry, 159(9), 1470–1479. 10.1176/appi.ajp.159.9.1470 [DOI] [PubMed] [Google Scholar]
- Parker, G. , Roy, K. , Hadzi‐Pavlovic, D. , Mitchell, P. , & Wilhelm, K. (2003). Distinguishing early and late onset non‐melancholic unipolar depression. Journal of Affective Disorders, 74(2), 131–138. [DOI] [PubMed] [Google Scholar]
- Parker, G. , Fletcher, K. , Hyett, M. , Hadzi‐Pavlovic, D. , Barrett, M. , & Synnott, H. (2009). Measuring melancholia: The utility of a prototypic symptom approach. Psychological Medicine, 39(6), 989–998. 10.1017/s0033291708004339 [DOI] [PubMed] [Google Scholar]
- Paslakis, G. , Krumm, B. , Gilles, M. , Schweiger, U. , Heuser, I. , Richter, I. , & Deuschle, M. (2011). Discrimination between patients with melancholic depression and healthy controls: Comparison between 24‐h cortisol profiles, the DST and the Dex/CRH test. Psychoneuroendocrinology, 36(5), 691–698. 10.1016/j.psyneuen.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Pietzcker, A. , & Gebhardt, R. (1983). Depressive syndromes and scales in the AMDP‐system. Acta Psychiatrica Scandinavica, Supplement, 310, 65–84. [PubMed] [Google Scholar]
- Pizzagalli, D. A. , Oakes, T. R. , Fox, A. S. , Chung, M. K. , Larson, C. L. , Abercrombie, H. C. , … Davidson, R. J. (2004). Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Molecular Psychiatry, 9(4), 325), 393–405. 10.1038/sj.mp.4001469 [DOI] [PubMed] [Google Scholar]
- Posternak, M. A. , & Zimmerman, M. (2002). The prevalence of atypical features across mood, anxiety, and personality disorders. Comprehensive Psychiatry, 43(4), 253–262. [DOI] [PubMed] [Google Scholar]
- Prins, J. , Olivier, B. , & Korte, S. M. (2011). Triple reuptake inhibitors for treating subtypes of major depressive disorder: The monoamine hypothesis revisited. Expert Opinion on Investigational Drugs, 20(8), 1107–1130. 10.1517/13543784.2011.594039 [DOI] [PubMed] [Google Scholar]
- Quinn, C. R. , Rennie, C. J. , Harris, A. W. , & Kemp, A. H. (2014). The impact of melancholia versus non‐melancholia on resting‐state, EEG alpha asymmetry: Electrophysiological evidence for depression heterogeneity. Psychiatry Research, 215(3), 614–617. 10.1016/j.psychres.2013.12.049 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Rasmussen, K. G. (2007). Attempts to validate melancholic depression: Some observations on modern research methodology. Bulletin of the Menninger Clinic, 71(2), 150–163. 10.1521/bumc.2007.71.2.150 [DOI] [PubMed] [Google Scholar]
- Robertson, H. A. , Lam, R. W. , Stewart, J. N. , Yatham, L. N. , Tam, E. M. , & Zis, A. P. (1996). Atypical depressive symptoms and clusters in unipolar and bipolar depression. Acta Psychiatrica Scandinavica, 94(6), 421–427. [DOI] [PubMed] [Google Scholar]
- Rodgers, S. , Vandeleur, C. L. , Ajdacic‐Gross, V. , Aleksandrowicz, A. A. , Strippoli, M. P. , Castelao, E. , … Preisig, M. (2016). Tracing the associations between sex, the atypical and the combined atypical‐melancholic depression subtypes: A path analysis. Journal of Affective Disorders, 190, 807–818. 10.1016/j.jad.2015.10.067 [DOI] [PubMed] [Google Scholar]
- † Roth, M. , Gurney, C. , Garside, R. F. , & Kerr, T. A. (1972). Studies in the classification of affective disorders. The relationship between anxiety states and depressive illnesses, I. British Journal of Psychiatry, 121(561), 147–161. [DOI] [PubMed] [Google Scholar]
- Rothermundt, M. , Arolt, V. , Fenker, J. , Gutbrodt, H. , Peters, M. , & Kirchner, H. (2001). Different immune patterns in melancholic and non‐melancholic major depression. European Archives of Psychiatry and Clinical Neuroscience, 251(2), 90–97. [DOI] [PubMed] [Google Scholar]
- Rubino, I. A. , Zanasi, M. , Robone, C. , & Siracusano, A. (2009). Personality differences between depressed melancholic and non‐melancholic inpatients. Australian & New Zealand Journal of Psychiatry, 43(2), 145–148. 10.1080/00048670802607204 [DOI] [PubMed] [Google Scholar]
- Rush, A. J. , & Weissenburger, J. E. (1994). Melancholic symptom features and DSM‐IV. American Journal of Psychiatry, 151, 489–498. [DOI] [PubMed] [Google Scholar]
- Schotte, C. K. , Maes, M. , Cluydts, R. , & Cosyns, P. (1997). Cluster analytic validation of the DSM melancholic depression. The threshold model: Integration of quantitative and qualitative distinctions between unipolar depressive subtypes. Psychiatry Research, 71(3), 181–195. [DOI] [PubMed] [Google Scholar]
- Seemuller, F. , Riedel, M. , Wickelmaier, F. , Adli, M. , Mundt, C. , Marneros, A. , … Henkel, V. (2008). Atypical symptoms in hospitalised patients with major depressive episode: Frequency, clinical characteristics, and internal validity. Journal of Affective Disorders, 108(3), 271–278. 10.1016/j.jad.2007.10.025 [DOI] [PubMed] [Google Scholar]
- Seemuller, F. , Riedel, M. , Obermeier, M. , Bauer, M. , Adli, M. , Kronmuller, K. , … Moller, H. J. (2010). Outcomes of 1014 naturalistically treated inpatients with major depressive episode. European Neuropsychopharmacology, 20(5), 346–355. 10.1016/j.euroneuro.2009.11.011 [DOI] [PubMed] [Google Scholar]
- Seppala, J. , Vanhala, M. , Kautiainen, H. , Eriksson, J. , Kampman, O. , Mantyselka, P. , … Koponen, H. (2012). Prevalence of metabolic syndrome in subjects with melancholic and non‐melancholic depressive symptoms. a Finnish population‐based study. Journal of Affective Disorders, 136(3), 543–549. 10.1016/j.jad.2011.10.032 [DOI] [PubMed] [Google Scholar]
- Stetler, C. , & Miller, G. E. (2011). Depression and hypothalamic‐pituitary‐adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine, 73(2), 114–126. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- Stewart, J. W. , McGrath, P. J. , Rabkin, J. G. , & Quitkin, F. M. (1993). Atypical depression. A valid clinical entity? Psychiatric Clinics of North America, 16(3), 479–495. [PubMed] [Google Scholar]
- Stewart, J. W. , McGrath, P. J. , Quitkin, F. M. , & Klein, D. F. (2009). DSM‐IV depression with atypical features: Is it valid? Neuropsychopharmacology, 34(13), 2625–2632. 10.1038/npp.2009.99 [DOI] [PubMed] [Google Scholar]
- Thase, M. E. (2009). Atypical depression: Useful concept, but it's time to revise the DSM‐IV criteria. Neuropsychopharmacology, 34(13), 2633–2641. 10.1038/npp.2009.100 [DOI] [PubMed] [Google Scholar]
- Tollefson, G. D. , Holman, S. L. , Sayler, M. E. , & Potvin, J. H. (1994a). Fluoxetine, placebo, and tricyclic antidepressants in major depression with and without anxious features. Journal of Clinical Psychiatry, 55(2), 50–59. [PubMed] [Google Scholar]
- Tollefson, G. D. , Rampey, A. H. Jr. , Beasley, C. M. Jr. , Enas, G. G. , & Potvin, J. H. (1994b). Absence of a relationship between adverse events and suicidality during pharmacotherapy for depression. Journal of Clinical Psychopharmacology, 14(3), 163–169. [PubMed] [Google Scholar]
- Uher, R. , Dernovsek, M. Z. , Mors, O. , Hauser, J. , Souery, D. , Zobel, A. , … Farmer, A. (2011). Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. Journal of Affective Disorders, 132(1–2), 112–120. 10.1016/j.jad.2011.02.014 [DOI] [PubMed] [Google Scholar]
- VanValkenburg, C. , Akiskal, H. S. , Puzantian, V. , & Rosenthal, T. (1984). Anxious depressions. Clinical, family history, and naturalistic outcome – Comparisons with panic and major depressive disorders. Journal of Affective Disorders, 6(1), 67–82. [DOI] [PubMed] [Google Scholar]
- Whiffen, V. E. , Parker, G. B. , Wilhelm, K. , Mitchell, P. B. , & Malhi, G. (2003). Parental care and personality in melancholic and nonmelancholic depression. Journal of Nervous and Mental Disease, 191(6), 358–364. 10.1097/01.nmd.0000071583.32879.cc [DOI] [PubMed] [Google Scholar]
- Wiethoff, K. , Bauer, M. , Baghai, T. C. , Moller, H. J. , Fisher, R. , Hollinde, D. , … Adli, M. (2010). Prevalence and treatment outcome in anxious versus nonanxious depression: Results from the German algorithm project. Journal of Clinical Psychiatry, 71(8), 1047–1054. 10.4088/JCP.09m05650blu [DOI] [PubMed] [Google Scholar]
- Wilkinson, L. (2012). Exact and approximate area‐proportional circular Venn and Euler diagrams. IEEE Transactions on Visualization and Computer Graphics, 18(2), 321–331. 10.1109/tvcg.2011.56 [DOI] [PubMed] [Google Scholar]
- Wittchen, H.‐U. , Wunderlich, U. , Gruschwitz, S. , & Zaudig, M. (1997). Strukturiertes Klinisches Interview für DSM‐IV. Göttingen: Hogrefe. [Google Scholar]
- World Health Organization (WHO) . (2011). The international classification of diseases 11th revision. http://www.who.int/classifications/icd/revision/en/index.html
- World Health Organization (WHO) . (2016, 08.10.2016). ICD‐11 Beta Draft. http://apps.who.int/classifications/icd11/browse/f/en%20-%20/http%3a//id.who.int/icd/entity/1563440232%20%5b8%20October%202016%5d
- Zaninotto, L. , Solmi, M. , Veronese, N. , Guglielmo, R. , Ioime, L. , Camardese, G. , & Serretti, A. (2016). A meta‐analysis of cognitive performance in melancholic versus non‐melancholic unipolar depression. Journal of Affective Disorders, 201, 15–24. 10.1016/j.jad.2016.04.039 [DOI] [PubMed] [Google Scholar]


