Abstract
Body awareness and reactivity dysfunction are characteristic of a range of psychiatric disorders. Although the neural pathways communicating between the body and brain that contribute to these experiences involve the autonomic nervous system, few research tools for studying subjective bodily experiences have been informed by these neural circuits. This paper describes the factor structure, reliability, and convergent validity of the Body Awareness and Autonomic Reactivity subscales of the Body Perception Questionnaire‐Short Form (BPQ‐SF). Exploratory and confirmatory factor analyses were applied to data from three samples collected via the internet in Spain and the US and a college population in the US (combined n = 1320). Body awareness was described by a single factor. Autonomic reactivity reflected unique factors for organs above and below the diaphragm. Subscales showed strong reliability; converged with validation measures; and differed by age, sex, medication use, and self‐reported psychiatric disorder. Post hoc analyses were used to create the 12‐item Body Awareness Very Short Form. Results are discussed in relation to the distinct functions of supra‐ and sub‐diaphragmatic autonomic pathways as proposed by the Polyvagal Theory and their potential dysfunction in psychiatric disorders.
Keywords: autonomic nervous system, autonomic reactivity, body awareness, interoception, polyvagal theory
1. INTRODUCTION
Increasingly, the role of disordered body awareness and reactivity is described across a range of clinical problems including anxiety (Domschke, Stevens, Pfleiderer, & Gerlach, 2010; Mallorquí‐Bagué et al., 2013), depression (Harshaw, 2015;), post‐traumatic stress (van der Kolk, 2015), autism (DuBois, Ameis, Lai, Casanova, & Desarkar, 2016), schizophrenia (Wylie & Tregellas, 2010), and eating disorders (Kaye, Fudge, & Paulus, 2009). With rising interest and methodological availability, knowledge about how physiological and neural processes are related to subjective body experiences and psychiatric dysfunction has grown considerably (e.g., Craig, 2009; Critchley & Harrison, 2013; Damasio, 1999; Porges, 2009a). With these developments, there is a need for self‐report methods that can conceptually bridge subjective body experiences with neuroscience, physiology, and medicine. Self‐reports provide indispensable information about internal experiences in naturalistic settings and provide an important complement to laboratory‐based measures, physiological monitoring, and quantification of neural processes. However, few psychometrically‐tested self‐report measures informed by the neural links between body and brain are available to study the embodied experiences involved in typical individual differences and clinical populations.
1.1. Physiological circuits underlying body awareness and reactivity
Body awareness, sometimes called the “sixth sense”, is the subjective experience of information arising from within the body. It emerges from a complex network of afferent and efferent pathways and their feedback loops between body structures and the central nervous system (Cameron, 2001; Craig, 2002; Gellhorn, 1964; Mandler, Mandler, & Uviller, 1958; Mehling et al., 2009; Porges, 1993b; Sherrington, 1906). Signals about internal body functions originate in sensors that carry information from target organs and tissues, are integrated and propagated through afferent pathways that include the spinal and cranial nerves, are routed to brain structures that monitor incoming information, and are regulated by descending efferent signals. These efferent signals also regulate the function of individual tissues and organs in response to internal and external conditions. Many of these signals travel along autonomic pathways, a system that has traditionally been divided into two antagonistic components—the sympathetic nervous system and the parasympathetic nervous system (e.g., Langley, 1921; Wenger, 1966). However, contemporary views point toward autonomic function as reflecting multiple coordinated control systems, which are activated or dampened in response to internal and external situational demands. Though each innervated organ and tissue may have individual neural feedback loops that regulate its specific function, the physiological needs of an organism and functional integrative circuits can produce widespread changes across the body (e.g., Jänig, 2006).
The Polyvagal Theory (Porges, 1995, 2009b, 2011) provides a neurophysiological framework for the study of the organization of autonomic systems. Using evidence from comparative anatomy, neurophysiology and behavioral observations, this theory describes two distinct vagal circuits within the parasympathetic nervous system that form a ventral vagal complex (VVC) and a dorsal vagal complex (DVC). The organization of these individual circuits, along with the sympathetic nervous system, can affect subjective experiences of body awareness by modulation of signals that arise from the body by top‐down post‐processing, including cortical areas informed by the information traveling through the body‐integrative circuits of the brain (e.g., Craig, 2002).
First, the VVC regulates the striated muscles of the face and head (e.g., pharynx, larynx, mastication muscles, and middle ear muscles) and visceral organs above the diaphragm (e.g., heart and bronchi; see Porges 2001, Porges, 2009b, 2011) through efferent pathways that originate in the nucleus ambiguus in the brainstem. In addition to the efferent action of this system, these bodily structures also contain afferent fibers that route information about supradiaphragmatic organs and the striated muscles of face and head to the brain, with many of these sensory pathways terminating in the brainstem in the nucleus of the solitary tract (NTS).
Second, the sympathetic nervous system innervates many of the same organs throughout the body as the VVC (see above) and the DVC (see below) as well as additional efferent connections to skin, skeletal muscle, the trunk, and extremities. Some afferent signals involved in these functions are routed to uniquely pre‐sympathetic pathways such as A1 catecholaminergic cell group and the rostral ventrolateral medulla and others terminate in integrative brainstem sites that are shared with vagal afferents, including the NTS, parabrachial nucleus, and insula (Craig, 2002), and thus may share some functional integration with VVC and DVC activity.
Third, the DVC carries signals that regulate the organs below the diaphragm (e.g., stomach, liver, gallbladder, pancreas, and intestines), with efferent pathways originating in the dorsal nucleus of the vagus. Animal models show that induced activity in dorsal motor nucleus of the vagus (DVC) produces changes in digestive function via descending vagal pathways (Zhang, Ai, & Cui, 2006; Zhu, Chang, Xie, & Ai, 2016). Unlike the diverging efferent projection sites in the VVC and DVC, afferent vagal fibers from both the subdiaphgragmatic organs and supradiaphragmatic organs terminate largely in the same location, the NTS.
1.2. The Body Perception Questionnaire
Although many instruments have been introduced to measure subjective experiences of body awareness and the body's activation responses, Mehling et al. (2009) observed that few had been developed with attention to rigorous psychometric testing. Psychometric testing assesses the measurement properties of research instruments, without which reliability and validity is unknown. In the years since, several new psychometrically‐rigorous scales have been introduced (e.g., Mehling et al., 2012; Tove, Målfrid, & Liv Inger, 2012). However, those that have been shown to have strong psychometric properties have not been developed with a foundation in organization of peripheral neural pathways.
The Body Perception Questionnaire (BPQ; Porges, 1993a) was developed to assess the subjective experiences of the function and reactivity of target organs and structures that are innervated by the autonomic nervous system. The original BPQ, totaling 122 items, assessed body awareness, autonomic nervous system reactivity, cognitive‐emotional‐somatic stress response, body and cognitive stress response styles, and health history. Since its introduction, the BPQ has been used in more than 25 peer‐reviewed publications (see Supplemental Material Table S6) and has been translated into several languages. However, broader application of this instrument has been limited by a lack of psychometric testing and its extensive length. The BPQ aspects that have been of highest research interest are the body awareness and autonomic reactivity subscales, with studies often using only these subscales (e.g., Bernátová & Svetlak, 2017; Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004). In addition, many stress coping questionnaires and health history inventories are already widely available. Thus, with the aim of developing a shorter questionnaire, we focus here on the two subscales that may prove most useful for research purposes.
1.3. The present study
This paper examines the psychometric properties of the body awareness and autonomic reactivity BPQ subscales to create a shorter, psychometrically supported measurement instrument (BPQ‐Short Form) and examine the relations of the subscales to demographics and psychiatric illness diagnosis.
2. METHOD
2.1. Participants
Data were collected from three sources: a Spanish online sample, an undergraduate American college sample, and an American online sample. All procedures were approved by the IRB of the institution overseeing data collection.
First, a sample of Spanish‐speaking adults (n = 500) completed an online survey distributed through the University of Barcelona. Recruitment was conducted using Spanish‐language websites and online newspapers. Participants were excluded if they were younger than 18 years of age, reported taking psychotropic drugs and/or beta‐blockers, or did not complete the questionnaires. No incentive was provided for survey completion. The final sample consisted of 465 participants (Mean age = 33.91, SD = 12.26; 62% female). These participants completed the BPQ, criterion validity measures, and demographic questions. Fifty‐three randomly selected participants completed the BPQ a week after their initial responses for test–retest reliability.
A second dataset was collected as part of a larger study distributed through an online portal at Indiana University. English‐speaking American residents were recruited using Amazon's Mechanical Turk. Respondents received $.30 for survey completion. Responders were excluded if they did not complete the survey (n = 64), incorrectly answered attention‐testing questions (e.g., “Please select ‘Very much’ for this response”, n = 52), or submitted from duplicate IP addresses (n = 5). The final sample consisted of 540 participants (Mean age = 35.13, SD = 10.97; 63% female; 84% White, 7% African‐American/Black, 6% Asian, and 5% Hispanic or Latina/o).
A third dataset was collected at the University of Maryland from an English‐speaking undergraduate student population enrolled in an introductory psychology course (n = 315). Participants completed a paper version of the BPQ. All students present on the day of data collection participated and no incentive was provided for survey completion. Specific demographic information was not collected at the time of questionnaire administration. The freshman cohort at the time of collection was 53% male and 60% Caucasian (University of Maryland, 2017). Most participants were under the age of 20 but the exact age distribution is unavailable.
2.2. Measures
2.2.1. The Body Perception Questionnaire (BPQ)
Twenty‐eight items from the original 45‐item body awareness subscale were selected by the original questionnaire's author. Items were selected on the basis of the insight that has been gained about the relation of these experiences with autonomic circuits since the original BPQ was developed over 20 years ago. Items were retained on the basis of their precision in capturing aspects of direct functional control via autonomic pathways. For instance, the item “an urge to swallow” was retained due to its focus on the swallowing muscles and their feedback, which are innervated by vagal and glossopharyngeal pathways that have a well‐documented peripheral neural circuit (see Kolacz, Lewis, & Porges, in press). Items which bear relation to autonomic circuits but were “noisy” in their likely relation to complex extra‐autonomic underpinnings (e.g., “clumsiness or bumping into people”) were dropped. All items from the autonomic reactivity subscale were retained.
Item responses for both subscales are on a 5‐point ordinal scale spanning never (1) to always (5). Participants in the American samples completed the original English‐language version of the measure. Participants in the Spanish sample completed a version that was translated and back‐translated by native Spanish speakers fluent in English. Item wording in the back translation converged well with the original English‐language version, supporting translation fidelity.
2.2.2. Validation measures
In the Spanish sample, the Stress Reactivity Index (SRI; de Rivera, De las Cuevas, Monterrey, Rodriguez‐Pulido, & Gracia, 1993) and the Spanish‐language version of the SomatoSensory Amplification Scale (SSAS; Barsky, Wyshak, & Klerman, 1990; Nakao & Barsky, 2007) were used for testing convergent validity. The SRI consists of 32 Likert‐type questions regarding habitual reactions under stress or tension (e.g., digestive discomfort), which are summed to generate a global stress reactivity score. The SRI was developed in Spanish (Gonzalez de Rivera, Rodriguez‐Abunim, & Hernandez, 1996; Monterray, 1996) and captures both intra‐individual stability and stress reactivity (Monterrey, Gonzalez de Rivera, De las Cuevas, & Rodríguez, 1991). The SSAS consists of 10 Likert‐type items that assess the extent to which participants are bothered by uncomfortable visceral and somatic sensations that are not typical of serious disease. Its single‐factor dimensionality, reliability, and validity are documented in multiple studies (Barsky et al., 1990; Speckens, Spinhoven, Sloekers, Bolk, & van Hemert, 1996).
2.2.3. Demographic information
In the Spanish sample, participants self‐reported their sex, age, current medication use, and psychiatric disorder diagnosis.
2.3. Procedures
Data analysis was conducted using SPSS, R version 3.3.3 (R Core Team, 2017), RStudio version 1.0.136 (RStudio, Inc., 2009–2016), and Mplus 7.31 (Muthen & Muthen, 1998–2015).
2.3.1. Data preparation
Categorical exploratory factor analysis (EFA) using full item distributions resulted in solutions requiring untenably high numbers of factors and loadings with complex structure before adequate fit could be achieved. Thus, items were dichotomized (0 = never, 1 = occasionally or more often) to examine whether threshold‐based categorical responses could be better described by a simple factor structure. This threshold was to selected and to preserve acceptable response distribution cell sizes for factor analysis. Data on all items in the Spanish sample were complete. In the American online sample, missing values comprised less than 3% of any one item and .9% of all data. In the American undergraduate sample, missing values comprised less than 1% of any one item and less than 0.2% of all data. Inspection of these missing values did not reveal any systematic patterns of missingness.
2.3.2. Factor analysis
Subscale dimensionality was assessed by a combination of exploratory and confirmatory factor analysis. We applied a robust weighted least squares estimator (WLSMV; see Muthén & Muthén, 1998–2013), as recommended for models with discrete responses by Barendse, Oort, and Timmerman (2015). Exploratory factor retention was guided by model fit, factor loading simple structure, theoretical predictions, and scree plots (Cattell, 1966). Goodness of fit to the data was evaluated using the root mean squared error of approximation (RMSEA; Steiger & Lind, 1980; Steiger, 1990), the Tucker‐Lewis index (TLI; Tucker & Lewis, 1973); and the Comparative Fit Index (CFI; Bentler, 1990). As suggested by Hu and Bentler (1999), we considered good fit to be evidenced by an RMSEA value near .06 or lower as well as CFI and TLI values near .95 or greater. Scree plots were examined for the last substantial drop in eigenvalue magnitude (Fabrigar, Wegener, MacCallum, & Strahan, 1999). EFA results were subject to oblique rotation according to the geomin criterion (Yates, 1987), which produces solutions with simple interpretations when factor structure is not highly complex (Sass & Schmitt, 2010) and can reproduce correlated or uncorrelated factor structures (Fabrigar et al., 1999). EFA results from the Spanish dataset were then applied to the American datasets as confirmatory factor analysis (CFA) models and assessed for goodness of fit using the cut off values described above.
2.3.3. Reliability, validity, and relation to demographic variables
Subscale scores based on the observed factor structure were computed and used to assess reliability, validity, and relation to demographic variables in the Spanish sample.
3. RESULTS
3.1. Exploratory factor analysis
3.1.1. Body awareness
The EFA was conducted on the body awareness and autonomic reactivity items separately. Two items from the body awareness subscale were removed (“An urge to urinate” and “Fullness of my bladder”) due to their bivariate cross tables with other items resulting in unpopulated cells, which can produce unreliable results in factor analysis. The body awareness subscale results supported a one‐factor structure, as evidenced by the scree plot (Figure 1) and RMSEA (Table 1). CFI and TLI values approached good fit in the one‐factor solution but did not fully reach our criteria for good fit until a second factor was included in the model (Table 1). When examined, the two‐factor solution lacked a simple structure, with many items loading substantially on both factors. Thus, given the support for the one factor solution by the RMSEA, scree plot, and simple structure, the one‐factor solution was retained. Geomin rotated standardized loadings using this solution ranged from .57 to .76 (see Table 2).
Figure 1.
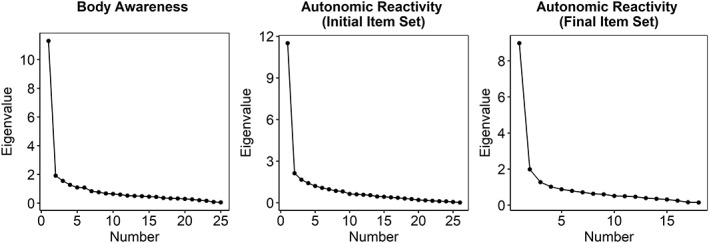
Exploratory factor analysis scree plots for body awareness and autonomic reactivity items in the Spanish internet sample
Table 1.
Exploratory factor analysis model fit statistics for the Spanish internet sample
| Factors | χ2 | df | RMSEA | RMSEA 90% confidence intervals | CFI | TLI | |
|---|---|---|---|---|---|---|---|
| Body awareness subscale | |||||||
| 1 | 647.20 | 299 | .050 | .045 | .055 | .94 | .93 |
| 2 | 433.87 | 274 | .035 | .029 | .042 | .97 | .97 |
| 3 | 330.28 | 250 | .026 | .018 | .034 | .99 | .98 |
| 4 | 253.56 | 222 | .016 | .000 | .026 | 1.00 | .99 |
| 5 | 194.19 | 205 | .000 | .000 | .016 | 1.00 | 1.00 |
| Autonomic reactivity subscale initial item set | |||||||
| 1 | 1330.49 | 324 | .082 | .077 | .086 | .85 | .84 |
| 2 | 909.58 | 298 | .066 | .062 | .071 | .91 | .89 |
| 3 | 703.02 | 273 | .058 | .053 | .064 | .94 | .92 |
| 4 | 490.55 | 249 | .046 | .040 | .052 | .97 | .95 |
| 5 | 362.89 | 226 | .036 | .029 | .043 | .98 | .97 |
| Autonomic reactivity subscale final item set | |||||||
| 1 | 728.75 | 170 | .084 | .078 | .090 | .89 | .87 |
| 2 | 327.96 | 151 | .050 | .043 | .058 | .96 | .96 |
| 3 | 210.52 | 133 | .035 | .026 | .044 | .98 | .98 |
| 4 | 148.29 | 116 | .024 | .010 | .035 | .99 | .99 |
| 5 | 107.00 | 100 | .012 | .000 | .028 | 1.00 | 1.00 |
RMSEA = root mean square error of approximation; CFI = Comparative Fix Index; TLI = Tucker‐Lewis Index.
Table 2.
Body awareness subscale exploratory (EFA) and confirmatory (CFA) factor analysis standardized factor loadings
| Item | Loading | ||
|---|---|---|---|
| EFA (Spanish, internet) | CFA (US, internet) | CFA (US, college) | |
| Swallowing frequently | .62 | .73 | .72 |
| An urge to cough to clear my throat | .57 | .74 | .64 |
| My mouth being dry* | .66 | .80 | .69 |
| How fast I am breathing* | .67 | .83 | .66 |
| Watering or tearing of my eyes | .70 | .82 | .60 |
| Noises associated with my digestion | .59 | .80 | .51 |
| A swelling of my body or parts of my body* | .71 | .83 | .67 |
| An urge to defecate | .70 | .76 | .56 |
| Muscle tension in my arms and legs* | .67 | .87 | .65 |
| A bloated feeling because of water retention* | .63 | .84 | .73 |
| Muscle tension in my face | .60 | .81 | .63 |
| Goose bumps* | .65 | .85 | .71 |
| Stomach and gut pains* | .68 | .90 | .82 |
| Stomach distension or bloatedness* | .66 | .86 | .80 |
| Palms sweating | .64 | .78 | .58 |
| Sweat on my forehead | .69 | .79 | .61 |
| Tremor in my lips* | .76 | .85 | .76 |
| Sweat in my armpits | .61 | .80 | .58 |
| The temperature of my face (especially my ears) | .58 | .81 | .65 |
| Grinding my teeth | .58 | .76 | .63 |
| General jitteriness | .59 | .85 | .66 |
| The hair on the back of my neck “standing up”* | .75 | .82 | .67 |
| Difficulty in focusing | .58 | .86 | .63 |
| An urge to swallow* | .75 | .87 | .74 |
| How hard my heart is beating* | .70 | .87 | .66 |
| Feeling constipated | .58 | .84 | .61 |
Items composing the Body Awareness Very Short Form
3.1.2. Autonomic reactivity
The autonomic reactivity EFA scree plot and fit indices did not clearly converge on one solution in the first iteration. The scree plot indicated one large eigenvalue followed by relatively low values that did not have a clear second substantial drop, pointing to a one‐factor solution (Figure 1); the RMSEA approached good fit in the three‐factor solution (Table 1); and the CFI and TLI suggested a 4‐factor solution (Table 1). Thus, the 1–3‐ and 4‐factor solutions were examined. One item was dropped due to its singular driving of a fourth factor (“I have difficulty adjusting my eyes to changes in illumination”), two items were dropped due to their substantial loadings on multiple factors (“I drool, especially when I am excited”, “I produce a lot of saliva even when I am not eating”), and two items were dropped due to their lack of substantial loadings on any factor (“My nose is runny, even when I am not sick”; “I have trouble focusing when I go into dimly or brightly illuminated places”). The resulting item pool was reanalyzed. The scree plot showed two deviating eigenvalues, indicating that one or two factors could be used to explain the data (Figure 1). Fit indices supported a two‐factor solution (RMSEA = .050 [90% CI: .041, .056], CFI = .96, TLI = .95). Thus, we accepted the two‐factor solution. All items but one (“I feel like vomiting”) demonstrated simple structure by loading substantially on only one factor.
The resulting factors corresponded with reactivity of organs above the diaphragm (supradiaphragmatic) and below the diaphragm (subdiaphragmatic). These factors were moderately correlated (r = .50). One item (81: “I get dizzy when urinating or having a bowel movement”) loaded onto the supradiaphragmatic factor but did not conceptually coalesce with the other items. Its removal did not substantially affect the factor loadings, factor correlations (r = .49), fit indices (Table 1), or eigenvalues (bottom right panel of Figure 1). Resulting factor loadings are presented in Table 3.
Table 3.
Autonomic reactivity exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) standardized loadings
| Item | Loadings | |||||
|---|---|---|---|---|---|---|
| EFA (Spain, internet) | CFA (US, internet) | CFA (US, college) | ||||
| Supradiaphragmatic reactivity | Subdiaphragmatic reactivity | Supradiaphragmatic reactivity | Subdiaphragmatic reactivity | Supradiaphragmatic reactivity | Subdiaphragmatic reactivity | |
| I have difficulty coordinating breathing and eating. | 0. 94 | −0.22 | 0.88 | 0.74 | ||
| When I am eating, I have difficulty talking. | 0.72 | −0.10 | 0.76 | 0.58 | ||
| My heart often beats irregularly. | 0.60 | 0.01 | 0.76 | 0.59 | ||
| When I eat, food feels dry and sticks to my mouth and throat. | 0.68 | 0.02 | 0.87 | 0.70 | ||
| I feel shortness of breath. | 0.79 | 0.04 | 0.84 | 0.79 | ||
| I have difficulty coordinating breathing with talking. | 0.89 | −0.21 | 0.87 | 0.67 | ||
| When I eat, I have difficulty coordinating swallowing, chewing, and/or sucking with breathing. | 1.02 | −0.24 | 0.95 | 0.75 | ||
| I have a persistent cough that interferes with my talking and eating. | 0.52 | 0.19 | 0.83 | 0.67 | ||
| I gag from the saliva in my mouth. | 0.49 | 0.13 | 0.85 | 0.75 | ||
| I have chest pains. | 0.58 | 0.12 | 0.78 | 0.57 | ||
| I gag when I eat. | 0.60 | 0.13 | 0.81 | 0.80 | ||
| When I talk, I often feel I should cough or swallow the saliva in my mouth. | 0.64 | 0.03 | 0.81 | 0.68 | ||
| When I breathe, I feel like I cannot get enough oxygen. | 0.73 | 0.10 | 0.84 | 0.70 | ||
| I have difficulty controlling my eyes. | 0.61 | 0.02 | 0.85 | 0.70 | ||
| I feel like vomiting. | 0.45 | 0.32 | 0.29 | 0.61 | 0.26 | 0.50 |
| I have “sour” stomach. | 0.21 | 0.53 | 0.88 | 0.65 | ||
| I am constipated. | 0.07 | 0.50 | 0.87 | 0.75 | ||
| I have indigestion. | −0.01 | 0.97 | 0.88 | 0.85 | ||
| After eating I have digestive problems. | 0.14 | 0.81 | 0.88 | 0.88 | ||
| I have diarrhea. | 0.29 | 0.43 | 0.81 | 0.82 | ||
3.2. Confirmatory factor analysis
The EFA results were tested using a CFA on the American datasets. This structure fit the data well in both the internet sample (RMSEA = .035 [90% CI: .032, .038], CFI = .98, TLI = .98) and the college sample (RMSEA = .029 [90% CI: .023, .034], CFI = .94, TLI = .94). CFA loadings were similar to EFA results (Table 2 and 3). The supradiaphragmatic reactivity factor was correlated with subdiaphragmatic reactivity in both confirmatory samples (US internet r = .78; US undergraduate r = .65). The body awareness factors were correlated with supradiaphragmatic reactivity (US online r = .72; US undergraduate r = .57) and with subdiaphragmatic reactivity (US online r = .70, US undergraduate r = .49).
3.3. Reliability and validity
3.3.1. Descriptive statistics
The BPQ‐SF was scored using the sum of dichotomized responses (0 = never, 1 = occasionally or more often) according to the factor structure described above, with “I feel like vomiting” included in both reactivity scales. Descriptive statistics for BPQ‐SF measures are presented in Table 4. Subscales deviated from normality, as assessed by skewness, kurtosis, visual examination of qq plots, and Shapiro–Wilk tests (all subscale scores p < .05). Thus, reliability and validity tests were conducted using measures that do not rely on normality assumptions.
Table 4.
Descriptive statistics for the Body Perception Questionnaire‐Short Form (BPQ‐SF) subscales and the Body Awareness Very Short Form
| Measure | Mean | Median | SD | Skew | Kurtosis | Min | Max |
|---|---|---|---|---|---|---|---|
| Spanish internet sample | |||||||
| BPQ‐SF Body Awareness | 16.83 | 17.00 | 6.17 | −.37 | −.64 | 0.00 | 26.00 |
| BPQ‐SF Supradiaphragmatic Reactivity | 5.79 | 5.00 | 4.13 | .49 | −.65 | 0.00 | 15.00 |
| BPQ‐SF Subdiaphragmatic Reactivity | 3.30 | 4.00 | 1.96 | −.28 | −1.18 | 0.00 | 6.00 |
| Body Awareness Very Short Form | 7.53 | 8.00 | 3.15 | −.36 | −.71 | 0.00 | 12.00 |
| American internet sample | |||||||
| BPQ‐SF Body Awareness | 16.95 | 19.00 | 8.24 | −.66 | −.83 | 0.00 | 26.00 |
| BPQ‐SF Supradiaphragmatic Reactivity | 5.00 | 4.00 | 4.75 | .78 | −.62 | 0.00 | 15.00 |
| BPQ‐SF Subdiaphragmatic Reactivity | 3.05 | 3.00 | 2.27 | −.08 | −1.49 | 0.00 | 6.00 |
| Body Awareness Very Short Form | 7.62 | 9.00 | 4.02 | −.57 | −1.02 | 0.00 | 12.00 |
| American college sample | |||||||
| BPQ‐SF Body Awareness | 21.97 | 23.00 | 4.35 | −1.31 | 1.48 | 3.00 | 26.00 |
| BPQ‐SF Supradiaphragmatic Reactivity | 6.49 | 6.00 | 4.04 | .34 | −.82 | 0.00 | 15.00 |
| BPQ‐SF Subdiaphragmatic Reactivity | 4.01 | 4.00 | 1.87 | −.73 | −.56 | 0.00 | 6.00 |
| Body Awareness Very Short Form | 10.10 | 11.00 | 2.27 | −1.48 | 1.95 | 0.00 | 12.00 |
3.3.2. Internal consistency
Internal consistency was assessed using the categorical omega coefficient (Green & Yang, 2009; Kelley & Pornprasertmanit, 2016) implemented in the MBESS R package (Kelley, 2017). This method provides internal consistency assessments superior to Cronbach's alpha when items are categorical and factor loadings are variable, as was the case with BPQ‐SF items. Like Cronbach's alpha, categorical omega ranges from 0 to 1 and its assessments of internal consistency for published psychometrically‐examined scales includes ranges from .68 to .97 (Roberson & Renshaw, 2017; Voskuil, Pierce, & Robbins, 2017; Zhu & Lowe, 2017). We computed 95% confidence intervals using bias‐corrected and accelerated bootstrapping with 1000 draws. Results are presented in Table 5. All internal consistency estimates were within a typical range compared to the psychometric studies cited above.
Table 5.
Internal consistency (categorical ω) for Body Perception Questionnaire‐Short Form subscales and Body Awareness Very Short Form; values in brackets are 95% confidence intervals
| Body Perception Questionnaire‐Short Form | Body Awareness Very Short Form | |||
|---|---|---|---|---|
| Body awareness | Supradiaphragmatic reactivity | Subdiaphragmatic reactivity | ||
| Spanish internet sample | .92 [.91–.93] | .89 [.86–.90] | .77 [.72–.80] | .86 [.82–.87] |
| American internet sample | .96 [.94–.97] | .94 [.92–.95] | .87 [.84–.89] | .91 [.88–.92] |
| American college sample | .92 [.88–.91] | .88 [.85–.89] | .78 [.71–.82] | .83[.68–.87] |
3.3.3. Test–retest reliability
Test–retest reliability was assessed using the intra‐class correlation coefficient (ICC) in the Spanish sample. Each subscale demonstrated high test–retest reliability (body awareness = .99; supradiaphragmatic reactivity = .97, subdiaphragmatic reactivity = .96).
3.3.4. Convergent validity
Spearman correlations were used to examine convergent validity in the Spanish internet sample (Table 6). SRI scores (M = 22.38, SD = 5.82) and SSAS scores (M = 7.40, SD = 1.98) were moderately correlated with all BPQ‐SF subscales.
Table 6.
Spearman correlation (Rho) table for Body Perception Questionnaire‐Short Form (BPQ‐SF) subscales, Body Awareness Very Short Form, Stress Reactivity Index (SRI), SomatoSensory Amplification Scale (SSAS), and age in the Spanish internet sample
| 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|
| 1. BPQ‐SF Body awareness | 0.67* | 0.57* | 0.94* | 0.57* | 0.51* | −0.14* |
| 2. BPQ‐SF Supradiaphragmatic reactivity | 0.57* | 0.66* | 0.65* | 0.46* | −0.05 | |
| 3. BPQ‐SF Subdiaphragmatic reactivity | 0.56* | 0.58* | 0.42* | 0.00 | ||
| 4. Body Awareness Very Short Form | 0.55* | 0.48* | −0.11* | |||
| 5. SRI | 0.51* | −0.03 | ||||
| 6. SSAS | −0.11* | |||||
| 7. Age |
p < .05.
3.4. BPQ‐SF relation to sample, demographics, and clinical variables
Wilcoxon‐Mann–Whitney U tests were used to test BPQ‐SF subscale differences among samples and between categorical demographic variables. Effect sizes were calculated using Cliff's d (Cliff, 1993), implemented in the orddom R package (Rogmann, 2013), and are included for relative comparison of effect strength and as a reference for planning future studies. Nearly all between‐sample contrasts were statistically significant (Figure 2). The American college sample deviated most substantially from the others, showing the highest scores on all subscales and deviating most strongly in body awareness.
Figure 2.
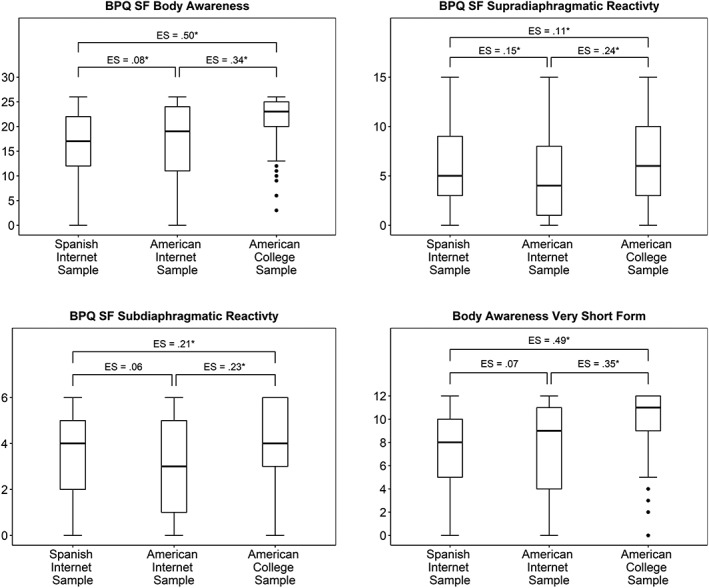
Body Perception Questionnaire‐Short Form (BPQ SF) and Body Awareness Very Short Form subscale comparisons between samples. Statistical significance [*p < .05] was computed using Wilcoxon‐Mann–Whitney U tests (see Supplementary Material Table S6 for test statistics). Effect sizes (ES) were computed using Cliff's d
Data from the Spanish sample was used to assess relations of BPQ‐SF subscales with demographics and self‐reported clinical variables. Body awareness was negatively associated with age (Table 6). Females scored higher on all three BPQ‐SF subscales and medication use predicted higher subdiaphragmatic reactivity scores (Table 7). Seventy participants self‐reported having a psychiatric disorder (15.15%). Of these, the most commonly reported were anxiety, depression, dysthymia, or their combination (n = 41); eating disorders (n = 5); and obsessive–compulsive disorder (n = 5). Participants who reported a psychiatric disorder had elevated scores on all BPQ‐SF subscales (Table 7). Too few participants reported disorders to permit sufficient power for assessing differences among specific diagnoses.
Table 7.
Differences in Body Perception Questionnaire‐Short Form (BPQ‐SF) subscales and Body Awareness Very Short Form by sex, medication use, and self‐reported psychiatric disorder in the Spanish internet sample. U = Wilcoxon‐Mann–Whitney U test statistic; p = statistical significance at alpha = .05; ES = effect size as measured by Cliff's d; Mdn = Median
| BPQ‐SF | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable / percent of sample | Body awareness | Supradiaphragmatic reactivity | Subdiaphragmatic reactivity | Body Awareness Very Short Form | ||||||||||||
| U | p | ES | Mdn | U | p | ES | Mdn | U | p | ES | Mdn | U | p | ES | Mdn | |
| Sex | 29162 | .008 | .15 | 28747 | .018 | .13 | 30572 | <.001 | .20 | 30901 | <.001 | .22 | ||||
| Female 62.15% | 18.00 | 6.00 | 4.00 | 8.00 | ||||||||||||
| Male 37.85% | 16.00 | 5.00 | 3.00 | 7.00 | ||||||||||||
| Medication use | 18799 | .851 | .01 | 17282 | .150 | .09 | 15861 | .008 | .17 | 18213 | .501 | .04 | ||||
| No 77.20% | 17.00 | 5.00 | 3.00 | 8.00 | ||||||||||||
| Yes 22.80% | 17.00 | 5.50 | 4.00 | 8.00 | ||||||||||||
| Self‐reported psychiatric disorder | 16279 | .013 | .19 | 17071 | .001 | .24 | 16792 | .003 | .22 | 16274 | .013 | .19 | ||||
| No 84.85% | 17.00 | 5.00 | 4.00 | 8.00 | ||||||||||||
| Yes 15.15% | 19.00 | 7.00 | 4.50 | 9.00 | ||||||||||||
3.5. Post hoc analysis: Body Awareness Very Short Form
Given the number of items included in the body awareness subscale for the measurement of a single factor, we examined whether the item count could be reduced for research applications in which scale brevity is of utmost concern. We assessed whether 10 to 15 items with the highest factor loadings across all datasets could be used to generate scores with high fidelity to the 26‐item score (criterion Rho = .90). The lowest item count that met our criteria was a 12‐item subscale (Spanish sample Rho = .94, US online Rho = .97, US undergraduate Rho = .91). The items that compose the resulting Body Awareness Very Short Form are marked with an asterisk in Table 2. Descriptive statistics are displayed in Table 4. Internal consistency was acceptable but lower than the full BPQ‐SF body awareness subscale (Table 5). Test–retest reliability was excellent (ICC = .97). Differences between samples, sex, medication use, and self‐reported psychiatric disorders, as measured by effect size, were very similar to the full BPQ‐SF body awareness subscale (Figure 2; Table 7).
4. DISCUSSION
The goal of this study was to assess the factor structure, reliability, convergent validity, and demographic variability of the BPQ‐SF. Results suggest that body awareness can be described using a single factor while items measuring autonomic reactivity cluster into subdiaphragmatic and supradiaphragmatic responses. This structure is consistent across a Spanish online sample as well as online and undergraduate college samples recruited in the United States, providing evidence that the structure of these BPQ‐SF subscales may be robust to cultural differences across these populations. Post hoc analyses showed that the 12‐item Body Awareness Very Short Form provides an alternative to the BPQ‐SF body awareness subscale for studies in which questionnaire length is particularly constrained.
The single body awareness factor may reflect the shared afferent targets of cranial and spinal pathways in the brainstem. Although some afferent pathways among these systems are unique, much of afferent cranial and spinal traffic is routed through shared integrative brainstem regions while traveling to higher brain structures (Craig, 2002). Conversely, the autonomic reactivity subscale structure was described by two factors, reflecting supra‐ and sub‐diaphragmatic responses. This clustering suggests distinct efferent control systems that give rise to individual differences in physiological responses in separate parts of the body. Supradiaphragmatic responses are likely driven by outflow from the VVC, which contains efferent source nuclei in the nucleus ambiguus, while responses below the diaphragm are likely coordinated with the enteric nervous system through efferent pathways via the unmyelinated vagal fibers that originate in the dorsal motor nucleus. Sympathetic efferent pathways innervate organs both above and below the diaphragm and thus the role of this system is difficult to interpret in light of the observed factor structure. It is likely that sympathetic reactivity contributes to both supra‐ and sub‐diaphragmatic reactivity and may contribute the strength of the association between the two autonomic reactivity factors observed in both samples. Notably, all items had substantial unique effects not described by common factors, likely reflecting unique feedback loops that regulate individual functions, in addition to capturing measurement error.
The results of the autonomic reactivity factor analysis showed that items have strong simple structure, with the exception of the supra‐ and sub‐diaphragmatic reactivity loadings of the item “I feel like vomiting” in the Spanish sample EFA. However, in the CFA factor loadings in the American samples, the item was associated more strongly with subdiaphragmatic reactivity than supradiaphragmatic reactivity. Although this could reflect cross‐cultural differences in the subscale structure, self‐reports of bodily reactivity have been found to show remarkable stability across cross‐cultural samples (e.g., Nummenmaa, Glerean, Hari, & Hietanen, 2014). Additional data are needed to examine whether this discrepancy can be replicated or may be attributable to random sampling variability.
The BPQ‐SF body awareness scores were positively related with the SomatoSensory Amplification Scale, supporting convergent validity that this subscale provides an assessment of the strength of perceived visceral and somatic sensations. Furthermore, both supra‐ and sub‐diaphragmatic scores converged with Stress Reactivity Index scores, indicating that both subscales provide information on bodily stress reactivity.
There was a small but substantial negative association between body awareness and age, which replicates previous work showing decreased interoception associated with age (e.g., Khalsa, Rudrauf, & Tranel, 2009; Murphy, Geary, Millgate, Catmur, & Bird, 2017) and is consistent with the American undergraduates, our youngest sample, showing highest levels of body awareness. Age‐related declines are also observed in cardiac autonomic regulation by the VVC, as measured by respiratory sinus arrythmia (Antelmi et al., 2004; Byrne, Fleg, Vaitkevicius, Wright, & Porges, 1996; Zhang, 2007), suggesting that the co‐occurring changes in body sensation may reflect dampened signal transmission between body and brain over time.
Women scored higher than men on all BPQ‐SF subscales. It is possible that these scores reflect physiological sex differences, though other studies have been inconclusive in this area. Respiratory sinus arrhythmia has been found to be both higher (Zhang, 2007) and lower (Ramaekers, Ector, Aubert, Rubens, & Van de Werf, 1998) in women compared to men (note these differences may be age‐dependent; see Byrne et al., 1996). It is probable that the higher rates of body awareness and autonomic reactivity in women in our study are the result of complex physiological and cultural interactions. However, women's elevated physiological reactivity in our study is in line with women's elevated clinical prevalence of anxiety (McLean, Asnaani, Litz, & Hofmann, 2011) and functional gastro‐intestinal disorders (Chang, 2004). Medication use was also related to elevated sub‐diaphragmatic reactivity, which may be caused by medication side effects or use specifically to reduce problems with subdiaphragmatic organ regulation.
All BPQ‐SF subscale scores were elevated in participants with a self‐reported psychiatric diagnosis. These results are consistent with previous clinical observations showing altered interoceptive functions across a range of psychiatric diagnoses (e.g., Harshaw, 2015; van der Kolk, 2015). While our small sample of self‐reported psychiatric diagnoses does not permit the assessment of altered function in specific disorders, there is abundant converging physiological and medical evidence from other studies to support altered efferent and afferent autonomic functions in specific diagnoses. Examples include Autism Spectrum Disorders (ASD), wherein reduced VVC control of the heart is inversely related to the severity of social impairment (Patriquin, Scarpa, Friedman, & Porges, 2013; Porges et al., 2013, 2014) and heightened risk of gastrointestinal disorders (Horvath & Perman, 2002) may be underpinned by DVC function. PTSD is related to elevated rates of cardio‐respiratory issues, which involve regulation via multiple autonomic circuits, and gastrointestinal issues, which are regulated in part by the DVC (Pacella, Hruska, & Delahanty, 2013). Patients with gastrointestinal problems have also been found to have elevated rates of anxiety and depression and the number of gastrointestinal symptoms highly increases the probability of an anxiety disorder (Mussell et al., 2008). More than half of the psychiatric self‐report diagnoses in our sample included anxiety and/or depression, and these disorders likely have an outsize role in the observed effects. Additional work with specific samples is needed to better elucidate the effects of individual disorders on BPQ‐SF subscales.
4.1. Limitations
The results of this study are based on self‐reported subjective experiences only. Though the clustering of autonomic reactivity items is consistent with predictions derived from neurophysiology, further research is needed to test whether subjective experiences are indicative of differences in autonomic control systems. Future studies will need to investigate how experiences of supradiaphragmatic and subdiaphragmatic reactivity relate to objective physiological measurements.
The internet and college recruitment methods also provide limitations for this study. Internet‐based recruitment and data collection introduces bias by being limited to respondents with internet access. Undergraduate sampling introduces bias due to a restricted age range and other population characteristics associated with advanced education, such as socioeconomic status. These features limit generalizability to broader populations. The lack of specific demographic information in the undergraduate dataset limits assessment of how these characteristics may have impacted results. However, the convergence of the factor structure across three samples provides support for the generalizability of BPQ‐SF dimensionality across populations and the large multi‐national, age‐diverse group of samples used in this study (combined n = 1320) provides a strong foundation for future work using more population‐representative samples.
Our assessment of convergent validity with the SRI was limited by the incomplete published psychometric information about that measure. Thus, its inclusion provides a weak test of convergent validity. However, its development as a Spanish‐language questionnaire offers an important benefit and complements the use of the SSAS, which has extensive psychometric information but is used in translated form.
Finally, our measurement of psychiatric problems in the Spanish internet sample was based on a simple self‐report, rather than diagnostic criteria, which may produce reporting bias or incomplete information. Participants may be unaware that they meet diagnostic criteria if they lack access to psychiatric services or have beliefs that prevent them from seeking services. Though our results of altered body awareness and autonomic reactivity in those with psychiatric diagnoses are consistent with previous research that utilized more stringent criteria, future studies should use more precise psychiatric disorder measurement.
5. CONCLUSION
Our results support the BPQ‐SF and Body Awareness Very Short Form as tools for the measurement of subjective experiences of autonomic state and reactivity. We found that body awareness of autonomically innervated organs is best described by a single factor, supporting neuroanatomical evidence that information from multiple afferent streams is integrated in the brain. We also found support for the individual perceptions of bodily reactivity to stress as organized according sub‐diaphragmatic and supra‐diaphragmatic regions, which may reflect functional organization via distinct autonomic circuits. Although the activation and function of autonomic circuits is not directly available to subjective awareness, the monitoring of the function of target organs can be individually observed and reported. Applying such self‐report methods with neurophysiologically informed organizing principles of bodily experiences may help identify the status of individual circuits that contribute to dysfunction and the development of novel interventions that can target specific system dysfunction.
DECLARATION OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Table S1. Comparison of original BPQ, BPQ‐Short Form, and Body Awareness Very Short Form (VAVSF) item numbers. BPQ items 1–45 compose the body awareness subscale, 56–82 compose the Autonomic Reactivity subscale(s).
Table S2. Supradiaphragmatic reactivity scoring for the BPQ and BPQ Short Form
Table S3. Subdiaphragmatic Reactivity scoring for the BPQ and BPQ Short Form
Table S4. Comparison of imputed and not imputed summary scores for samples with missing data
Table S5. Wilcoxon‐Mann–Whitney U tests for subscale differences among samples
Table S6. Studies that have used the Body Perception Questionnaire to date
ACKNOWLEDGEMENTS
The authors wish to thank Eric Lara, for his advice and computer support; A. Rafaela Castro, Narciso Cabrera, and Carlos Cabrera for their support and confidence in the project; Justin Garcia and Amanda Gesselman for their help in planning and gathering data for the US online sample; Katie Gates for her input on an early version of this manuscript; Danny Rahal for compiling a list of studies that have used the BPQ; and all our participants. This research complies with the APA ethical standards for research with human populations. The authors do not have any conflicts of interests to report.
Cabrera A, Kolacz J, Pailhez G, Bulbena‐Cabre A, Bulbena A, Porges SW. Assessing body awareness and autonomic reactivity: Factor structure and psychometric properties of the Body Perception Questionnaire‐Short Form (BPQ‐SF). Int J Methods Psychiatr Res. 2018;27:e1596 10.1002/mpr.1596
REFERENCES
- Antelmi, I. , De Paula, R. S. , Shinzato, A. R. , Peres, C. A. , Mansur, A. J. , & Grupi, C. J. (2004). Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. The American Journal of Cardiology, 93(3), 381–385. [DOI] [PubMed] [Google Scholar]
- Barendse, M. T. , Oort, F. J. , & Timmerman, M. E. (2015). Using exploratory factor analysis to determine the dimensionality of discrete responses. Structural Equation Modeling, 22(1), 87–101. [Google Scholar]
- Barsky, A. J. , Wyshak, G. , & Klerman, G. L. (1990). The somatosensory amplification scale and its relationship to hypochondriasis. Journal of Psychiatric Research, 24(4), 323–334. [DOI] [PubMed] [Google Scholar]
- Bentler, P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238. [DOI] [PubMed] [Google Scholar]
- Bernátová, T. , & Svetlak, M. (2017). Emotional and interoceptive awareness and its relationship to restriction in young women with eating disorders and healthy controls: A cascade from emotional to behavioral dysregulation. Activitas Nervosa Superior, 59(2), 78–86. 10.1007/s41470-017-0006-z [DOI] [Google Scholar]
- Byrne, E. A. , Fleg, J. L. , Vaitkevicius, P. V. , Wright, J. , & Porges, S. W. (1996). Role of aerobic capacity and body mass index in the age‐associated decline in heart rate variability. Journal of Applied Physiology, 81(2), 743–750. [DOI] [PubMed] [Google Scholar]
- Cameron, O. G. (2001). Interoception: The inside story—A model for psychosomatic processes. Psychosomatic Medicine, 63(5), 697–710. [DOI] [PubMed] [Google Scholar]
- Cattell, R. B. (1966). The scree test for the number of factors. Multivariate Behavioral Research, 1(2), 245–276. [DOI] [PubMed] [Google Scholar]
- Chang, L. (2004). Review article: Epidemiology and quality of life in functional gastrointestinal disorders. Alimentary Pharmacology & Therapeutics, 20(s7), 31–39. [DOI] [PubMed] [Google Scholar]
- Cliff, N. (1993). Dominance statistics: Ordinal analyses to answer ordinal questions. Psychological Bulletin, 114(3), 494. [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2009). How do you feel ‐ now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Öhman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. [DOI] [PubMed] [Google Scholar]
- Damasio, A. (1999). The feeling of what happens: Body and emotion in the making of consciousness. New York, NY: Harcourt Press. [Google Scholar]
- Domschke, K. , Stevens, S. , Pfleiderer, B. , & Gerlach, A. L. (2010). Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clinical Psychology Review, 30(1), 1–11. [DOI] [PubMed] [Google Scholar]
- DuBois, D. , Ameis, S. H. , Lai, M. C. , Casanova, M. F. , & Desarkar, P. (2016). Interoception in autism spectrum disorder: A review. International Journal of Developmental Neuroscience, 52, 104–111. [DOI] [PubMed] [Google Scholar]
- Fabrigar, L. R. , Wegener, D. T. , MacCallum, R. C. , & Strahan, E. J. (1999). Evaluating the use of exploratory factor analysis in psychological research. Psychological Methods, 4, 272–299. [Google Scholar]
- Gellhorn, E. (1964). Motion and emotion: The role of proprioception in the physiology and pathologyof the emotions. Psychological Review, 71(6), 457. [DOI] [PubMed] [Google Scholar]
- Gonzalez de Rivera, J. L. , Rodriguez‐Abunim, M. J. , & Hernandez, L. (1996): Inner consistency and testretest reliability of the stress reactivity index. Presented at the X World Congress of Psychiatry; Madrid, España. [Google Scholar]
- Green, S. B. , & Yang, Y. (2009). Reliability of summed item scores using structural equation modeling: An alternative to coefficient alpha. Psychometrika, 74, 155–167. 10.1007/s11336-008-9099-3 [DOI] [Google Scholar]
- Harshaw, C. (2015). Interoceptive dysfunction: Toward an integrated framework for understanding somatic and affective disturbance in depression. Psychological Bulletin, 141(2), 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, K. , & Perman, J. A. (2002). Autism and gastrointestinal symptoms. Current Gastroenterology Reports, 4(3), 251–258. [DOI] [PubMed] [Google Scholar]
- Hu, L. T. , & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. [Google Scholar]
- Jänig, W. (2006). The integrative action of the autonomic nervous system: Neurobiology of homeostasis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Kaye, W. H. , Fudge, J. L. , & Paulus, M. (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience, 10(8), 573. [DOI] [PubMed] [Google Scholar]
- Kelley, K . (2017). The MBESS R package (Version 4.3.0). https://CRAN.R-project.org/package=MBESS
- Kelley, K. , & Pornprasertmanit, S. (2016). Confidence intervals for population reliability coefficients: Evaluation of methods, recommendations, and software for composite measures. Psychological Methods, 21, 69–92. 10.1037/a0040086 [DOI] [PubMed] [Google Scholar]
- Khalsa, S. S. , Rudrauf, D. , & Tranel, D. (2009). Interoceptive awareness declines with age. Psychophysiology, 46(6), 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolacz, J. , Lewis, G. F. , & Porges, S. W. (in press). The integration of vocal communication and biobehavioral state regulation in mammals: A polyvagal hypothesis In Brudzynski S. M. (Ed.), Handbook of ultrasonic vocalization. New York: Elsevier Press. [Google Scholar]
- Langley, J. N. (1921). The autonomic nervous system. Cambridge, England: Heffer & Sons. [Google Scholar]
- Mallorquí‐Bagué, N. , Garfinkel, S. N. , Engels, M. , Eccles, J. A. , Pailhez, G. , Bulbena, A. , & Critchley, H. D. (2013). Neuroimaging and psychophysiological investigation of the link between anxiety, enhanced affective reactivity and interoception in people with joint hypermobility. Frontiers in Psychology, 5, 1162–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler, G. , Mandler, J. M. , & Uviller, E. T. (1958). Autonomic feedback: The perception of autonomic activity. The Journal of Abnormal and Social Psychology, 56(3), 367. [DOI] [PubMed] [Google Scholar]
- McLean, C. P. , Asnaani, A. , Litz, B. T. , & Hofmann, S. G. (2011). Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45(8), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling, W. E. , Gopisetty, V. , Daubenmier, J. , Price, C. J. , Hecht, F. M. , & Stewart, A. (2009). Body awareness: Construct and self‐report measures. PLoS One, 4(5), e5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling, W. E. , Price, C. , Daubenmier, J. J. , Acree, M. , Bartmess, E. , & Stewart, A. (2012). The multidimensional assessment of interoceptive awareness (MAIA). PLoS One, 7(11). e48230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterray, A. L. (1996). Psychophysiological validation of the stress reactivity index. Presented at the X World Congress of Psychiatry; Madrid, España. [Google Scholar]
- Monterrey, A. L. , Gonzalez de Rivera, J. L. , De las Cuevas, C. , & Rodríguez, P. F. (1991). El indice de reactividad al estrés (IRE):¿ Rasgo o estado? Rev. Psiquiatria Fac. Med. Berna, 18(1), 23–27. [Google Scholar]
- Murphy, J. , Geary, H. , Millgate, E. , Catmur, C. , & Bird, G. (2017). Direct and indirect effects of age on interoceptive accuracy and awareness across the adult lifespan. Psychonomic Bulletin & Review, 10.3758/s13423-017-1339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussell, M. , Kroenke, K. , Spitzer, R. L. , Williams, J. B. , Herzog, W. , & Löwe, B. (2008). Gastrointestinal symptoms in primary care: Prevalence and association with depression and anxiety. Journal of Psychosomatic Research, 64(6), 605–612. [DOI] [PubMed] [Google Scholar]
- Muthén, L. K. , & Muthén, B. O. (1998. ‐2013). Mplus User's Guide (Seventh ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nakao, M. , & Barsky, A. J. (2007). Clinical application of somatosensory amplification in psychosomatic medicine. BioPsychoSocial Medicine, 1(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa, L. , Glerean, E. , Hari, R. , & Hietanen, J. K. (2014). Bodily maps of emotions. PNAS, 111(2), 646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella, M. L. , Hruska, B. , & Delahanty, D. L. (2013). The physical health consequences of PTSD and PTSD symptoms: A meta‐analytic review. Journal of Anxiety Disorders, 27(1), 33–46. [DOI] [PubMed] [Google Scholar]
- Patriquin, M. A. , Scarpa, A. , Friedman, B. H. , & Porges, S. W. (2013). Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology, 55(2), 101–112. [DOI] [PubMed] [Google Scholar]
- Porges, S. W. (1993a). Body perception questionnaire. Laboratory of Developmental Assessment: University of Maryland. [Google Scholar]
- Porges, S. W. (1993b). The infant's sixth sense: Awareness and regulation of bodily processes. Zero to Three: Bulletin of the National Center for Clinical Infant Programs, 14, 12–16. [Google Scholar]
- Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32, 301–318. [DOI] [PubMed] [Google Scholar]
- Porges, S. W. (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42, 123–146. [DOI] [PubMed] [Google Scholar]
- Porges, S. W. (2009a). Reciprocal influences between body and brain in the perception and expression of affect: A polyvagal perspective In Fosha D., Siegel D. J., & Solomon M. F. (Eds.), The healing power of emotion: Affective neuroscience, development, clinical practice. New York: Norton. [Google Scholar]
- Porges, S. W. (2009b). The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine, 76(S2), S86–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges, S. W. (2011). The Polyvagal Theory: Neurophysiological foundations of emotions, attachment, communication, and self‐regulation. New York: Norton. [Google Scholar]
- Porges, S. W. , Bazhenova, O. V. , Bal, E. , Carlson, N. , Sorokin, Y. , Heilman, K. J. , … Lewis, G. F. (2014). Reducing auditory hypersensitivities in autistic Spectrum disorder: Preliminary findings evaluating the listening project protocol. Frontiers in Pediatrics, 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges, S. W. , Macellaio, M. , Stanfill, S. D. , McCue, K. , Lewis, G. F. , Harden, E. R. , … Heilman, K. J. (2013). Respiratory sinus arrhythmia and auditory processing in autism: Modifiable deficits of an integrated social engagement system? International Journal of Psychophysiology, 88(3), 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2017). R: A Language and Environment for Statistical Computing version 3.3.3. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org
- Ramaekers, D. , Ector, H. , Aubert, A. E. , Rubens, A. , & Van de Werf, F. (1998). Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? European Heart Journal, 19(9), 1334–1341. [DOI] [PubMed] [Google Scholar]
- de Rivera, J. L. G. , De las Cuevas, C. , Monterrey, A. L. , Rodriguez‐Pulido, F. , & Gracia, R. (1993). Stress reactivity in the general population. European Journal of Psychiatry, 7(1), 5–11. [Google Scholar]
- Roberson, A. J. , & Renshaw, T. L. (2017). Structural validity of the HBSC bullying measure: Self‐report rating scales of youth victimization and perpetration behavior. Journal of Psychoeducational Assessment. 0734282917696932 [Google Scholar]
- Rogmann, J. J. (2013). Ordinal dominance statistics (orddom): An R project for statistical computing package to compute ordinal, nonparametric alternatives to mean comparison (version 3.1). Available online from the CRAN website http://cran.r-project.org/.
- Sass, D. A. , & Schmitt, T. A. (2010). A comparative investigation of rotation criteria within exploratory factor analysis. Multivariate Behavioral Research, 45(1), 73–103. [DOI] [PubMed] [Google Scholar]
- Sherrington, C. S. (1906). The Integrative Action of the Nervous System. New Haven: Yale University Press. [Google Scholar]
- Speckens, A. E. , Spinhoven, P. , Sloekers, P. P. , Bolk, J. H. , & van Hemert, A. M. (1996). A validation study of the Whitely Index, the Illness Attitude Scales, and the Somatosensory Amplification Scale in general medical and general practice patients. Journal of Psychosomatic Research, 40(1), 95–104. [DOI] [PubMed] [Google Scholar]
- Steiger, J. H. (1990). Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research, 25(2), 173–180. [DOI] [PubMed] [Google Scholar]
- Steiger, J. H. & Lind, J. C. (1980). Statistically‐based tests for the number of common factors. Paper presented at the annual spring meeting of the Psychometric Society, Iowa City, IA. [Google Scholar]
- Tove, D. , Målfrid, R. , & Liv Inger, S. (2012). Body awareness rating questionnaire: Measurement properties. Physiotherapy Theory and Practice, 28(7), 515–528. [DOI] [PubMed] [Google Scholar]
- Tucker, L. R. , & Lewis, C. (1973). A reliability coefficient for maximum likelihood factor analysis. Psychometrika, 38, 1–10. [Google Scholar]
- University of Maryland (2017) Graph: Students by race and gender. Retrieved from: https://reports.umd.edu/reportHolder.html#StudentsbyRaceandGender/Graph
- Van der Kolk, B. A. (2015). The body keeps the score: Brain, mind, and body in the healing of trauma Penguin Books. [Google Scholar]
- Voskuil, V. R. , Pierce, S. J. , & Robbins, L. B. (2017). Comparing the psychometric properties of two physical activity self‐efficacy instruments in urban, adolescent girls: Validity, measurement invariance, and reliability. Frontiers in Psychology, 8, 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger, M. A. (1966). Studies of autonomic balance: A summary. Psychophysiology, 2(3), 173–186. [DOI] [PubMed] [Google Scholar]
- Wylie, K. P. , & Tregellas, J. R. (2010). The role of the insula in schizophrenia. Schizophrenia Research, 123(2), 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates, A. (1987). Multivariate exploratory data analysis: A perspective on exploratory factor analysis. Albany: State University of New York Press. [Google Scholar]
- Zhang, J. (2007). Effect of age and sex on heart rate variability in healthy subjects. Journal of Manipulative and Physiological Therapeutics, 30(5), 374–379. [DOI] [PubMed] [Google Scholar]
- Zhang, X. Y. , Ai, H. B. , & Cui, X. Y. (2006). Effects of nuclei ambiguus and dorsal motor nuclei of vagus on gastric H+ and HCO3‐secretion in rats. World Journal of Gastroenterology, 12(20), 3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Chang, L. , Xie, J. , & Ai, H. (2016). Arginine vasopressin injected into the dorsal motor nucleus of the vagus inhibits gastric motility in rats. Gastroenterology Research and Practice, 2016, 4618672. 10.1155/2016/4618672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q. , & Lowe, P. A. (2017). Examination of the psychometric properties of the revised children's manifest anxiety scale–second edition scores among Chinese secondary school students. Journal of Psychoeducational Assessment, 1–11. 10.1177/0734282917698302 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of original BPQ, BPQ‐Short Form, and Body Awareness Very Short Form (VAVSF) item numbers. BPQ items 1–45 compose the body awareness subscale, 56–82 compose the Autonomic Reactivity subscale(s).
Table S2. Supradiaphragmatic reactivity scoring for the BPQ and BPQ Short Form
Table S3. Subdiaphragmatic Reactivity scoring for the BPQ and BPQ Short Form
Table S4. Comparison of imputed and not imputed summary scores for samples with missing data
Table S5. Wilcoxon‐Mann–Whitney U tests for subscale differences among samples
Table S6. Studies that have used the Body Perception Questionnaire to date


