Abstract
Objectives
Substance use may influence study results in human subjects research. This study aims to report the concordance between self‐report and biochemical assessments of substance use and test the effect of methods to reduce false reports of abstinence in trauma‐exposed women participating in a research study.
Methods
In this pilot study, substance use was assessed during telephone prescreening and via self‐report and biochemical verification (i.e., urine toxicology and alcohol breathalyzer tests) at an in‐person evaluation. Due to the high number of participants who tested positive for substances despite self‐reporting abstinence during prescreening, study procedures were modified to disincentivize false self‐reports of substance use two thirds of the way through recruitment. New potential participants were explicitly informed during prescreening and informed consent that a positive drug or alcohol test during screening would result in exclusion from the study and withholding of payment.
Results
Prior to modifying study methods, 20% of participants who had reported abstinence during the telephone prescreen had a positive substance use test at the in‐person visit. Modifying study procedures resulted in an 81% decrease in positive substance use assessments.
Conclusions
Adoption of this methodology may decrease inadvertent confounding of clinical research outcomes by undetected and/or misreported substance use.
Keywords: biochemical verification, drug testing, post‐traumatic stress disorder, substance abuse, trauma
1. INTRODUCTION
Substance use is important to assess in psychological studies where use could affect outcomes. Past and current use, as well as the timing of recent use or the presence of withdrawal, can substantially impact a range of neurobiological, physiological, cognitive, emotional, and behavioral measures (American Psychiatric Association, 2013; Bonnet & Preuss, 2017; Evans & Cahill, 2016; Fernández‐Serrano, Pérez‐García, & Verdejo‐García, 2011). Substance use is common in the United States, with 10% of the U.S. adult population reporting past month illicit drug use and 56% reporting past month alcohol use (Substance Abuse and Mental Health Services Administration, 2016). For this reason, any research study including outcomes potentially impacted by substance use or withdrawal may benefit from rigorous substance use assessment.
Use of psychoactive substances can affect performance on a range of cognitive measures, including measures of episodic memory (cannabis, methamphetamine, 3,4‐methylenedioxy‐methamphetamine [MDMA], opioids, and alcohol), impulsivity (methamphetamine, MDMA, and alcohol), reasoning (heroin and alcohol), processing speed (cannabis and alcohol), cognitive flexibility (alcohol), and verbal fluency (MDMA; Fernández‐Serrano et al., 2011). Assessment of substance use is also essential to the integrity of neurobiology research (Rasmusson et al., 2017; Rasmusson, Vythilingam, & Morgan 3rd., 2003). Acute substance use may also affect clinical trial outcomes including treatment response and dropout (Baker et al., 2007; Mazza et al., 2009).
Treatment settings for individuals with substance use disorder (SUD) routinely assess for recent substance use and confirm self‐reported use with biochemical verification by urine toxicology tests and breathalyzer tests (Clancy, O'Connell, & Couto, 2013). Multimodal assessment (self‐report plus biochemical verification) is necessary as self‐reported substance use and biochemical verification can be discrepant and may be differentially related to treatment outcome (Decker et al., 2014; Haller et al., 2010; Hilario et al., 2015; Wish, Hoffman, & Nemes, 1997; Zatzick et al., 2012).
The validity of self‐reported versus biochemical assessment of substance use may vary based on a variety of contextual factors. When assessing patterns of use over the past month, self‐report may outperform biological assessment (Zatzick et al., 2012), which typically only captures substance use in the past 5 days. Accurate reporting may also vary depending on substance type. For example, when substance use was assessed at an intake interview, Wish et al. (1997) found that participants were more likely to report use of heroin than use of cocaine. Participants may also feel increased pressure to underreport substance use in situations where substance use may have direct aversive consequences such as when awaiting organ transplant (Haller et al., 2010) or when they perceived that acknowledgment of use would be negatively evaluated by others such as at the conclusion of SUD treatment (Wish et al., 1997). Finally, previous research has found differences in rates of underreporting as a function of participant characteristics, such as racial/ethnic background (Fendrich, Johnson, Wislar, Hubbell, & Spiehler, 2004). Because there are limitations to both self‐report and biochemical assessment of substance use, study procedures that fail to include both forms of assessment will likely inadvertently include individuals who recently used psychoactive substances and/or individuals with current SUDs and result in erratic, uncontrolled, and unquantified confounding influences of substance use on study outcomes.
Assessment of substance use is particularly important in research studies including participants with increased likelihood of recent substance use, such as those exposed to trauma and diagnosed with post‐traumatic stress disorder (PTSD; Fetzner, McMillan, Sareen, & Asmundson, 2011; Roberts, Roberts, Jones, & Bisson, 2016). However, research studies of individuals with trauma exposure or PTSD often fail to assess participants for recent substance use and those that do typically rely on participant self‐report. A review of all studies of adults that included face‐to‐face visits published in the 2016 volume of a trauma‐focused journal (N = 19) revealed that only nine (47%) assessed substance use by self‐report and two (11%) included biochemical verification of use.
In summary, chronic or episodic substance use during participation in clinical research may affect neurobiological and mental processes that can influence research findings (Fernández‐Serrano et al., 2011). Thus, methods to accurately measure and disincentivize substance use misreporting in clinical research studies are needed. In the current study, we report rates of discordance between self‐report and biochemical assessments of substance use in a study of trauma‐exposed women. The study also examined the effectiveness of methods developed to disincentivize misreporting of abstinence. Specifically, we modified the telephone prescreening script to inform participants that they would not qualify for the study or be compensated at the first in‐person screening evaluation if biochemical tests for substance use were positive. We hypothesized that this telephone prescreening modification would reduce the number of participants testing positive for substances at the in‐person screening evaluation. We also hypothesized that this modification would increase the rate of “no shows” and cancellations for the in‐person screening evaluation by participants who misreported substance abstinence during the telephone prescreening.
2. METHOD
2.1. Design
The current study used a pre‐post design (i.e., Phases 1 and 2) to examine the effectiveness of methods to reduce false reports of substance abstinence during eligibility assessment for a larger study (Nillni et al., 2015; Pineles, Blumenthal, et al., 2016; Pineles, Nillni, et al., 2016).
2.2. Sample
One hundred sixty women (aged 18–55 years) who had experienced a Diagnostic and Statistical Manual of Mental Disorders‐IV PTSD Criterion A traumatic event were included in this study (n = 114 in Phase 1 and n = 46 in Phase 2; mean age 34.63 [standard deviation = 9.91]; 38% Caucasian, 38% African American, and 24.4% of other races). The most frequently reported “worst” traumatic events experienced included sexual trauma (24%), unexpected and sudden death of a loved one (13%), and serious motor vehicle accident (8%). Exclusion criteria included substances and medications that would disrupt the menstrual cycle or affect measures of stress hormones, as these were main aims of the larger study. This included past 30‐day drug use or heavy alcohol use (defined as drinking four or more drinks on a single occasion at least twice a week), current infectious illnesses, history of organic brain disorder, schizophrenia, use of medications in the past 4 weeks, with very few exceptions (e.g., steroid inhaler), irregular menstrual cycle, oral contraceptive use, and perimenopausal or postmenopausal status.
2.3. Procedure
Study procedures were approved by the VA Boston Healthcare System Institutional Review Board (Protocol # 2107). Women who had experienced a traumatic event were recruited via flyers and online advertisements for a study of changes in physiology across the menstrual cycle. Flow charts of participants for Phases 1 and 2 of the study and reasons for study exclusion are presented in Figure 1.
Figure 1.
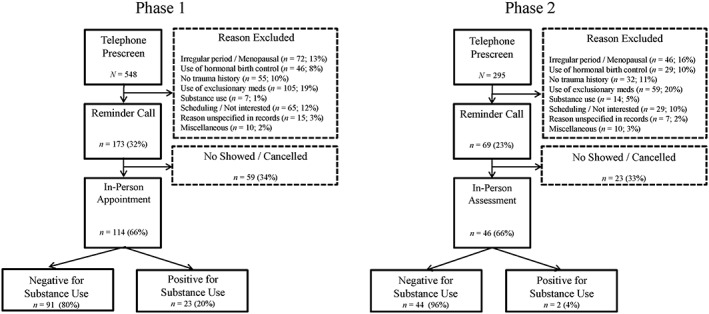
Participant flow for Phases 1 and 2 of the study
2.3.1. Phase 1
Interested women contacted the laboratory to indicate interest in the study. During a subsequent telephone prescreening session, women were asked about tobacco use (“Do you smoke? How much?”), alcohol use (“How often do you drink alcohol? And how much do you drink, on average, on the days that you drink?”), and substance use (“What drugs do you take? How often?”). Callers were informed that a urine drug test and an alcohol breathalyzer test would be performed at the in‐person screening evaluation. Although they were not explicitly informed that drug and alcohol use was exclusionary, potentially eligible callers were asked to agree to abstain from alcohol and drug use during the study. Those who passed the telephone prescreen were reminded of this request 24 hr before the in‐person screening evaluation.
At the in‐person screening evaluation, after completion of written informed consent, urine toxicology tests, alcohol breathalyzer tests, and self‐report assessments of substance use, depression, anxiety, and PTSD were performed. Regardless of eligibility for the remainder of the study, Phase 1 participants were compensated $50 for the in‐person screening evaluation.
2.3.2. Phase 2
Telephone prescreening methods were altered approximately 2/3 of the way through recruitment due to the high number of Phase 1 participants who tested positive for substances on urine testing despite self‐reporting abstinence on the telephone prescreen (see Section 3). As in Phase 1, interested women completed a telephone prescreening session. In contrast to Phase 1 procedures, Phase 2 callers were explicitly informed that substance use would result in exclusion from the study. To disincentivize false reports of abstinence, callers were informed that abstinence would be biochemically verified and that those testing positive for substance use at the in‐person evaluation would not be paid for the visit. Specifically, the following statements were added to the beginning and end of the phone prescreen, respectively:
If you test positive for any legal or illegal drugs (for example, cocaine, heroin, opiates, marijuana, benzodiazepines, antipsychotics, antidepressants, sedatives, stimulants, etc.), or any adulteration materials (for example, bleach, peroxide, water, etc.), you will be deemed ineligible for study participation and you will not be able to continue in the study; nor will you be compensated for the visit.
What drugs do you take? Again, it should be noted that if you test positive for drugs at any point you will be ineligible for the study and you will not be compensated.”
Those who passed the telephone prescreen were invited for an in‐person evaluation (n = 46 attended). As in Phase 1, participants provided written informed consent and completed urine toxicology and alcohol breathalyzer tests and self‐report assessments. Participants testing positive for drugs or alcohol on urine toxicology or breathalyzer tests were excluded from the study prior to completing the self‐report assessments. We did not request that participants who tested positive for drugs or alcohol complete self‐report assessments because they were not paid for the study visit and thus would not be compensated for the time required to complete the assessments. Those who tested negative for substance use on self‐report and biochemical measures were paid $50 for the screening visit.
2.4. Assessment of substance use
Participants completed a self‐report assessment of substance use (“Please list all medications, drugs, and alcohol you used within the past week and the past 24 hours.”; “How many cigarettes did you smoke in the last 24 hours? How many cigarettes do you smoke each day?”). Drug use was also measured with a urine toxicology test (RediTest Panel‐Dip 6 Panel Urine Test, Redwood Toxicology Laboratory, Inc.). The urine toxicology test detects tetrahydrocannabinol, cocaine, opiates, methamphetamine, oxycodone, and benzodiazepines. The length of time for which drug use is detectable by this test varies by substance. The shortest length of time was 1–3 days (analgesics and opiates); the longest was 30 days (long‐term marijuana use and phencyclidine). Alcohol use was verified using an alcohol breathalyzer test (Alco‐Sensor IV, Intoximeters, Inc.). Nicotine use was verified by a urine cotinine test (current use >200 ng/ml; Reditest; Redwood Toxicology, Santa Rosa, CA, USA; cotinine is detectable in urine for approximately 3 days after smoking).
2.5. Additional study measures
The Beck depression inventory‐II (Beck, Steer, & Brown, 1996) is a 21‐item scale assessing depressive symptoms on a four‐point severity scale (range: 0–63). Depression severity is calculated by summing all item scores.
The Trait subscale of the State–Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) is a 20‐item measure of trait anxiety (range: 0–80). A total score for anxiety is calculated by summing all item scores.
The PTSD Checklist (Weathers, Litz, Herman, Huska, & Keane, 1993) is a 17‐item self‐report measure of PTSD symptom severity (range: 17–85) as defined by Diagnostic and Statistical Manual of Mental Disorders‐IV (American PsychiatricAssociation, 2000). PTSD severity is calculated by summing all item scores.
2.6. Data analysis
All analyses were conducted using SPSS version 24. Descriptive statistics for demographic, psychosocial, and substance use variables were computed. Next, we used chi‐squared tests or one‐way analyses of variance to compare demographic measures, reported tobacco or alcohol use, and psychological symptoms between (a) individuals who reported no substance use and tested positive for substances on urine toxicology testing and (b) individuals who reported no substance use and tested negative on urine toxicology testing. If cells had counts under 5, we used Fisher's Exact test. Due to the small sample size and the fact that this is a pilot study, we did not adjust for multiple comparisons to minimize Type II error. To compare the rates of positive drug tests and no shows/cancellations between participants in Phase 1 (not explicitly disincentivized for substance use) and Phase 2 (explicitly disincentivized for substance use), Pearson's chi‐squared tests were used.
3. RESULTS
Of the 114 participants in Phase 1, 112 were asked about self‐reported drug and alcohol use on self‐report assessments at the in‐person visit. Two (2%) participants tested positive for drug use and were excluded before the questionnaires were completed. Of the 112, 8 (7%) endorsed past week drug use, and 5 of these 8 (63%) tested positive on the drug test. An additional 13 of the 112 (12%) denied past week drug and alcohol use but tested positive. One participant (1%) with a positive drug test also had a positive breathalyzer test. In total, 23 participants (21%) in Phase 1 tested positive for substance use (Figure 2). Of the 21 participants who had both self‐report and biochemical substance use assessments, 5 (24%) tested positive on both assessments, 3 (14%) self‐reported substance use but their biochemical assessment was negative, and 13 (62%) denied substance use on the self‐report assessment but had positive biochemical assessments of drug or alcohol use.
Figure 2.
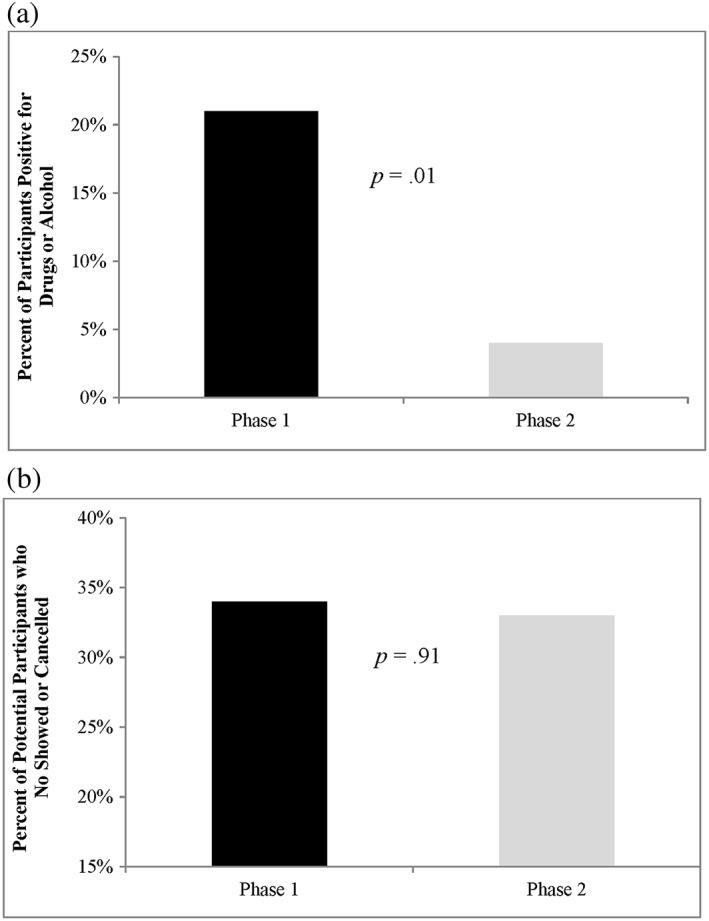
Percentage of Phases 1 and 2 participants (a) with positive drug tests (self‐reported and/or biochemical) and (b) who no showed or cancelled their in‐person appointment
In Phase 2 of the study, after the change to our telephone prescreening procedures, no participants self‐reported drug use, no participants had a positive breathalyzer test, and only two (4%) had a positive drug test (χ2 = 6.23, degrees of freedom [df] = 1, p = .01). Thus, the changes to our telephone prescreening methods resulted in an 81% decrease in the number of positive substance use assessments (Figure 2a).
We next assessed differences in the rate of missed in‐person evaluations (in possible anticipation of a positive drug test result) between participants who had or had not been disincentivized for substance use (Figure 2b). Missed appointments could be due to cancellations or no shows (if an appointment was scheduled but the participant did not cancel or show up). The percentage of participants who failed to attend the in‐person evaluation did not change following the changes to our screening procedures (Phase 1: 34%, Phase 2: 33%; χ2 = .01, df = 1, p = .91).
With regard to nicotine use, which was not an exclusion criterion for the study, 30 (26%) endorsed smoking in the past 24 hr in Phase 1. Of those, 25 (22% of the total sample) tested positive for cotinine and 5 (4%) tested negative for cotinine. In addition, 11 (10%) denied using cigarettes but tested positive for cotinine. With regard to concordance between self‐report and biochemical assessment, of the 41 people who tested positive for nicotine, 25 (61%) self‐reported cigarette smoking and tested positive for cotinine; 5 (12%) self‐reported smoking, but their cotinine assessment was negative; and 11 (27%) denied smoking but tested positive for cotinine. In Phase 2, 4 (9%) endorsed smoking and tested positive for cotinine, 0 endorsed smoking and tested negative for cotinine, and 3 (7%) denied smoking but tested positive for cotinine.
As an exploratory aim, we examined whether the demographic and psychosocial characteristics of Phase 1 participants who reported abstinence from drugs (n = 104) differed as a function of their urine drug test (i.e., positive or negative; see Table 1). Within this subsample, a positive urinalysis test was associated with self‐identification as a cigarette user, χ2 = 3.88, df = 1, p = .049, as well as with using more cigarettes per day, F(1, 100) = 7.22, p = .008. Among the 13 Phase 1 participants who reported substance abstinence but had a positive urine toxicology tests, 5 (45%) also reported abstinence from cigarettes but had a positive urine cotinine test. Urine drug test results were not associated with age, race, alcohol use, veteran status, severity of depression, PTSD, or trait anxiety.
Table 1.
Phase 1 participants who denied substance use (N = 104) as a function of biochemical verification results (i.e., negative or positive urinalysis)
| Negative urinalysis (n = 91) | Positive urinalysis (n = 13) | χ2/F | p | ||
|---|---|---|---|---|---|
| Age | M (SD) | 35.53 (10.61) | 36.54 (8.47) | 0.11 | .74 |
| Race | 2.34 | .80 | |||
| Caucasian | % | 41.8 | 38.5 | ||
| African American | % | 37.4 | 38.5 | ||
| Other | % | 20.9 | 23.1 | ||
| Veteran status | % | 5.5 | 7.7 | 0.10 | .75 |
| Daily tobacco use (yes/no) | % | 21.1 | 46.2 | 3.88 | .049 |
| Cigarettes per day | M (SD) | 1.70 (4.44) | 5.31 (5.07) | 7.22 | .008 |
| Alcohol use (days/month) | M (SD) | 2.33 (3.91) | 0.54 (1.05) | 2.68 | .10 |
| Alcohol use (drinks/month) | M (SD) | 6.00 (11.48) | 0.92 (1.93) | 2.53 | .12 |
| Depression | M (SD) | 16.60 (11.46) | 22.49 (11.40) | 2.80 | .10 |
| Anxiety | M (SD) | 86.89 (23.93) | 92.51 (26.10) | 0.61 | .44 |
| PTSD | M (SD) | 45.42 (15.35) | 51.38 (13.43) | 1.77 | .19 |
Note. Questions regarding age, race, veteran status, tobacco use, and alcohol use were asked during a telephone prescreen; psychological symptom scores were obtained during the in‐person appointment using the Beck depression inventory (depression symptoms), the State Trait Anxiety Inventory (anxiety symptoms), and the post‐traumatic stress disorder (PTSD) Checklist (PTSD symptoms). Drinks per month was calculated based on participants' answers to two demographic questions: “How many days did you drink this past month? How many drinks did you have on average on days that you drank alcohol?” SD = standard deviation; bold font indicates statistical significance and the α = .05 level
4. DISCUSSION
The primary aim of the current study was to examine the frequency of false abstinence reporting and inconsistency across self‐report and biochemical assessments of drug and alcohol use in a research study of trauma‐exposed women. The study also tested the effect of methodological procedures implemented to decrease substance use at screening evaluations (an exclusion criterion) and false reports of abstinence. In Phase 1 of the study, 21% of participants had a positive substance use assessment (either self‐reported or via urine toxicology test) during the initial intake appointment. All participants self‐reported drug and alcohol abstinence during the telephone prescreen and had agreed to remain abstinent during the study. Thus, a significant minority of participants either misrepresented their drug use at the prescreen and/or did not comply with study requirements to maintain abstinence between the prescreen and the in‐person evaluation (see Section 3).
Results also revealed that use of self‐report and biochemical assessments captured substance use among different participants. Among Phase 1 participants who tested positive for substance use and completed self‐report measures, only 24% were positive on both the self‐report and biochemical assessments. More than half (62%) tested positive on the biochemical assessment but denied substance use in the past week. An additional 14% reported substance use in the past week but did not test positive on the urinalysis or breathalyzer tests. With regard to nicotine use, of those in the Phase 1 sample who tested positive for smoking on self‐report or urine cotinine tests, 61% tested positive on both self‐report and biochemical assessment; 12% self‐reported smoking, but it was not detectible on the urine cotinine test; and 27% self‐reported not smoking but had a positive urine cotinine test. It is important to note that some substances are more readily detectable on urine drug and alcohol breathalyzer testing due to longer half‐lives (e.g., marijuana). In addition, use of some other substances was not assessed (e.g., K2 and spice). People who denied cigarette use on self‐reports could have been using other forms of nicotine or tobacco. Estimates of drug and alcohol use by biochemical measures are, therefore, a conservative estimate of drug use in this sample. Given the discrepant findings between methods of substance use testing, we recommend a multimethod approach to obtain as accurate an assessment of substance use as possible.
It is not surprising that many participants in this study had recently used substances given previous research demonstrating the high prevalence of substance abuse in trauma‐exposed populations (Roberts et al., 2016) and the high rates of substance use in the general population (Substance Abuse and Mental Health Services Administration, 2016). Findings are also consistent with research showing significant discordance between self‐report and biochemical assessments of substance use in other populations (Decker et al., 2014; Haller et al., 2010; Hilario et al., 2015; Wish et al., 1997; Zatzick et al., 2012).
Although this study demonstrated that the majority of participants accurately reported abstinence during the telephone prescreen, careful in‐person assessment and biochemical verification of substance use is needed to reveal the substantial minority of participants who misreport abstinence. As previously discussed, it is important to the integrity of many research studies to know the substance use status of participants, given that chronic or episodic drug or alcohol use may affect many types of study outcomes (Fernández‐Serrano et al., 2011). Although the current study focused on trauma‐exposed populations, the method for discouraging false reports of abstinence is nevertheless likely to be broadly applicable to all research studies that could be inadvertently confounded by undetected substance use. In studies wherein substance use is exclusionary, use of such methods will likely increase the appropriate exclusion of participants using substances; when substance use is not exclusionary, results of the multimodal assessment of substance use employed (self‐report plus biological verification) can be taken into account when interpreting the study data.
In the current study, informing participants about our intention to biochemically verify abstinence and disqualify participants with positive tests or signs of sample adulteration (Phase 2) significantly decreased false reports of substance abstinence. Contrary to our hypothesis, this methodological approach had no effect on scheduled in‐person evaluation attendance, indicating that it led either to greater honesty during the telephone prescreen or to opting out before the in‐person evaluation was scheduled. This explanation is supported by a slight increase in rates of substance use reporting on the telephone prescreen in Phase 2 (from 1% to 5% of prescreen callers reporting substance use; Figure 1). It is also possible that these methods increased the motivation of potential participants to avoid using substances in order to qualify.
Participants whose substance abstinence was not confirmed by biochemical tests did not differ from those whose abstinence was confirmed on self‐reported age, race, alcohol use, veteran status, depression, PTSD, or anxiety. Individuals who denied substance use, but tested positive on urine toxicology tests, were more likely to report smoking cigarettes and reported using more cigarettes per day. Tobacco use is highly comorbid with other SUDs (Donald, Chartrand, & Bolton, 2013). In addition, we found that several participants who misreported substance abstinence also had cotinine tests positive for tobacco use despite denying cigarette use. It is possible that these participants were using other noncigarette forms of tobacco, but it is also possible that they believed that all substance use was exclusionary and misreported all substance use in attempt to qualify for the study. Other traits (such as including infrequent use patterns, concerns regarding social desirability, or financial motives) not assessed in the current research may have also affected substance use reporting.
Study results should be interpreted with several limitations in mind. As discussed above, the urine drug tests included assessments of many, but not all, substances, and the substances assessed had different half‐lives. Moreover, because the sample was small and only a subset of participants tested positive for drug or alcohol use, some of our analyses were likely underpowered; replication of findings is encouraged. Finally, this was not an epidemiological sample: The study only examined substance use reporting among trauma‐exposed women, with regular menstrual cycles, not taking medications or hormonal contraceptives, who responded to advertisements about a clinical research study. Study findings need to be replicated in other clinical research participant samples. Because men have a greater prevalence of SUDs (American Psychiatric Association, 2013), careful assessment of substance use may be even more important for male participants.
In conclusion, many clinical research studies would benefit from collecting data on substance use to increase our knowledge of disease process, comorbidities, and treatment success. The current study demonstrated that a substantial minority of trauma‐exposed participants misrepresented their drug or alcohol use when screened for participation in a research study. Careful assessment of substance use including self‐report and biochemical verification is therefore essential when the research being conducted could be compromised by current drug or alcohol use. In addition, the methods investigated in this study appeared to substantially disincentivize false reporting of substance use and may have encouraged compliance with the study proscription against substance use.
ACKNOWLEDGEMENTS
Support for this work was provided by a VA Career Development Award (PI: Pineles) from the Clinical Sciences Research and Development Service, Department of Veterans Affairs. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The funding agency was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. A portion of this work was presented at the International Society for Traumatic Stress Studies annual meeting in November 2013.
Japuntich SJ, Arditte Hall KA, Joos CM, Rasmusson AM, Pineles SL. Methods to reduce false reporting of substance abstinence in clinical research. Int J Methods Psychiatr Res. 2018;27:e1603 10.1002/mpr.1603
REFERENCES
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders: DSM IV‐TR. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Baker, K. D. , Lubman, D. I. , Cosgrave, E. M. , Killackey, E. J. , Yuen, H. P. , Hides, L. , … Yung, A. R. (2007). Impact of co‐occurring substance use on 6 month outcomes for young people seeking mental health treatment. Austrialian and New Zealand Journal of Psychiatry, 41, 896–902. 10.1080/00048670701634986 [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Manual for Beck depression inventory‐II. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bonnet, U. , & Preuss, U. W. (2017). The cannabis withdrawal syndrome: Current insights. Subtance Abuse and Rehabilitation, 8, 9–37. 10.2147/SAR.S109576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy, Z. , O'Connell, K. , & Couto, J. (2013). The use of urine drug monitoring in chronic opiod therapy: An analysis of current clinician behavior. Journal of Opiod Management, 9, 121–127. 10.5055/jom.2013.0153 [DOI] [PubMed] [Google Scholar]
- Decker, S. E. , Frankforter, T. , Babuscio, T. , Nich, C. , Ball, S. A. , & Carroll, K. M. (2014). Assessment concordance and predictive validity of self‐report and biological assay of cocaine use in treatment trials. The American Journal on Addictions, 23, 466–474. 10.1111/j.1521-0391.2014.12132.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald, S. , Chartrand, H. , & Bolton, J. M. (2013). The relationship between nicotine cessation and mental disorders in a nationally representative sample. Journal of Psychiatric Research, 47, 1673–1679. 10.1016/j.jpsychires.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Evans, C. J. , & Cahill, C. M. (2016). Neurobiology of opioid dependence in creating addiction vulnerability. F1000 Research, 5 10.12688/f1000research.8369.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich, M. , Johnson, T. P. , Wislar, J. S. , Hubbell, A. , & Spiehler, V. (2004). The utility of drug testing in epidemiological research: Results from a general population survey. Addiction, 99, 197–208. 10.1111/j.1360-0443.2003.00632.x [DOI] [PubMed] [Google Scholar]
- Fernández‐Serrano, M. J. , Pérez‐García, M. , & Verdejo‐García, A. (2011). What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance. Neuroscience and Biobehavioral Reviews, 35, 377–406. 10.1016/j.neubiorev.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Fetzner, M. G. , McMillan, K. A. , Sareen, J. , & Asmundson, G. J. (2011). What is the association between traumatic life events and alcohol abuse/dependence in people with and without PTSD? Findings from a nationally representative sample. Depression and Anxiety, 28, 632–638. 10.1002/da.20852 [DOI] [PubMed] [Google Scholar]
- Haller, D. L. , Acosta, M. C. , Lewis, D. , Miles, D. R. , Schlano, T. , Shapiro, P. A. , … Newville, H. (2010). Hair analysis versus conventional methods of drug testing in substance abusers seeking organ transplantation. American Journal of Transplantation, 10, 1306–1311. 10.1111/j.1600-6143.2010.03090.x [DOI] [PubMed] [Google Scholar]
- Hilario, E. Y. , Griffin, M. L. , McHugh, R. K. , McDermott, K. A. , Connery, H. S. , Fitzmaurice, G. M. , & Weiss, R. D. (2015). Denial of urinalysis‐confirmed opioid use in prescription opioid dependence. Journal of Substance Abuse Treatment, 48, 85–90. 10.1016/j.jsat.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza, M. , Mandelli, L. , Di Nicola, M. , Harnic, D. , Catalano, V. , Tedeshi, D. , … Janiri, L. (2009). Clinical features, response to treatment and functional outcome of bipolar disorder patients with and without co‐occurring substance use disorder: 1‐year follow‐up. Journal of Affective Disorders, 115, 27–35. 10.1016/j.jad.2008.08.019 [DOI] [PubMed] [Google Scholar]
- Nillni, Y. I. , Pineles, S. L. , Patton, S. C. , Rouse, M. H. , Sawyer, A. T. , & Rasmusson, A. M. (2015). Menstrual cycle effects on psychological symptoms in women with PTSD. Journal of Traumatic Stress, 28, 1–7. 10.1002/jts.21984 [DOI] [PubMed] [Google Scholar]
- Pineles, S. L. , Blumenthal, T. D. , Curreri, A. J. , Nillni, Y. I. , Putnam, K. M. , Resick, P. A. , … Orr, S. P. (2016). Prepulse inhibition deficits in women with PTSD. Psychophysiology, 53, 1377–1385. 10.1111/psyp.12679 [DOI] [PubMed] [Google Scholar]
- Pineles, S. L. , Nillni, Y. I. , King, M. W. , Patton, S. C. , Bauer, M. R. , Mostoufi, S. M. , … Rasmusson, A. M. (2016). Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. Journal of Abnormal Psychology, 125, 349–355. 10.1037/abn0000138 [DOI] [PubMed] [Google Scholar]
- Rasmusson, A. M. , Marx, C. E. , Pineles, S. L. , Locci, A. , Scioli‐Salter, E. R. , Nillni, Y. I. , … Pinna, G. (2017). Neuroactive steroids and PTSD treatment. Neuroscience Letters, 649, 156–163. 10.1016/j.neulet.2017.01.054 [DOI] [PubMed] [Google Scholar]
- Rasmusson, A. M. , Vythilingam, M. , & Morgan, C. A. 3rd. (2003). The neuroendocrinology of posttraumatic stress disorder: New directions. CNS Spectrums, 8(651–656), 665–657. 10.1017/S1092852900008841 [DOI] [PubMed] [Google Scholar]
- Roberts, N. P. , Roberts, P. A. , Jones, N. , & Bisson, J. I. (2016). Psychological therapies for post‐traumatic stress disorder and comorbid substance use disorder. The Cochrane Database of Systematic Reviews. 10.1002/14651858.CD010204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. , Gorsuch, R. , Lushene, R. , Vagg, P. , & Jacobs, G. (1983). Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . (2016). Results from the 2015 National Survey on Drug Use and Health: Detailed tables. Downloaded from https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf [PubMed]
- Weathers, F. W. , Litz, B. T. , Herman, D. S. , Huska, J. A. , & Keane, T. M. (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the Meeting of the International Society for Traumatic Stress Studies, San Antonio, TX.
- Wish, E. D. , Hoffman, J. A. , & Nemes, S. (1997). The validity of self‐reports of drug use at treatment admission and at follow‐up: Comparisons with urinalysis and hair assays. NIDA Research Monograph, 167, 200–226. [PubMed] [Google Scholar]
- Zatzick, D. , Donovan, D. , Dunn, C. , Russo, J. , Wang, J. , Jurkovich, G. , … Gentilello, L. (2012). Substance use and posttraumatic stress disorder symptoms in trauma center patients receiving mandatory alcohol SBI. Journal of Substance Abuse Treatment, 43, 410–417. 10.1016/j.jsat.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


