Abstract
This study aims to quantify placebo response (PR) in children with attention deficit hyperactivity disorder (ADHD) as assessed by parents and teachers and to explore some of its determinants.
Five hundred and forty children with ADHD (ages 6–12) were recruited to a randomized, double‐blind, placebo‐controlled crossover trial with methylphenidate. The main outcome variable was Conners' Global Index (CGI), based on assessment of behaviour by parents (CGI‐P) and teacher (CGI‐T). PR was calculated as the difference between CGI‐P/T scores at baseline and placebo week.
There was a highly significant PR as assessed by the parents' and teachers' (p < 0.001). The magnitude of PR as assessed by parents was greater (10.57 points) compared to that assessed by teachers (3.93 points). The determinants of PR were different between parents and teachers. For parents, income, marital status, education, maternal smoking during pregnancy, and prior psychostimulant exposure (PPE) showed a significant effect on PR. For teachers, only ethnicity and PPE had an effect. The pattern of PR revealed two distinct profiles that may shed some light on the mechanisms involved in PR.
PR in children with ADHD varies depending on the setting of the observations and the evaluator. Several psychosocial factors have been identified as modulators of PR. This is relevant for the design and interpretation of clinical trials and for clinical practice.
Keywords: ADHD, parents, placebo response, predictors, teachers
1. INTRODUCTION
Modern interest in placebos stems from the discovery that one major source of treatment response is the propensity of subjects to feel better after receiving treatment, even when this treatment is devoid of active ingredients (Beecher, 1955). In addition to its relevance for clinical practice, this phenomenon is gaining increased attention in research given its importance in determining the outcome of clinical trials. Indeed, an increasing magnitude of placebo response (PR) over the years has been reported (Tuttle et al., 2015) and may explain, at least in part, the failure of several clinical trails (Kirsch et al., 2008). It is therefore important to study the determinants of PR in order to better understand the various factors underlying its variability, which in turn, can help with the design of clinical trials and reduce their rate of failure. This might be particularly important with psychiatric disorders given that the magnitude of PR, although variable from one condition to the other, is relatively high (Khan et al., 2005).
It is important to distinguish between PR and placebo effect (PE), given their interchangeable use in current scientific literature. PR represents the total improvement in a measured outcome after administration of placebo. It encompasses group measurement factors, such as regression to the mean, individual‐level factors such as the natural course of the illness and the PE proper. The PE represents the improvement in a measured outcome attributed specifically to the inactive substance or treatment, due to neurobiological and psychosocial factors, including expectations, classical conditioning, and non‐specific treatment effects such as contact with study personnel (Kirsch, 2013).
The mesostriatal dopamine system and prefrontal cortex play an important role in the PE (Murray & Stoessl, 2013). These same neural systems are also central to the pathophysiology of attention deficit hyperactivity disorder (ADHD) (Arnsten, 2006), thus making ADHD a fertile ground for exploring the placebo phenomenon. In ADHD, the PR rate has been estimated at 20–30% using various outcome measures, although there is heterogeneity between studies both at the level of methodology and results (Waschbusch, Pelham, Waxmonsky, & Johnston, 2009).
The moderators, mediators, and other predictors of PR were studied in a wide variety of conditions/disorders. Baseline demographic factors (such as sex, age, and household income), previous medication history, comorbidity, family factors (such as history of psychiatric illnesses, parent education), and severity of illness have been found to be potential predictors of PR (Arnold et al., 2010; Aslaksen, Bystad, Vambheim, & Flaten, 2011; Brown, Johnson, & Chen, 1992; Hunter, Cook, & Leuchter, 2010; Katja Weimer, Colloca, & Enck, 2015; King et al., 2013; Potkin et al., 2011). Some of these factors have been shown to account, at least in part, for PR in ADHD, including age, ethnicity, time since diagnosis, ADHD subtype, and comorbidity; however, inconsistant results have been obtained (Buitelaar et al., 2012; Newcorn et al., 2009; Waxmonsky, Waschbusch, Glatt, & Faraone, 2011). This lack of consistency may be due to various factors including different definitions of response (often categorical), reliance on different observers, assessment of the behaviours in different settings (home, school, clinic), different time frames to assess response and relatively small sample sizes.
The present study aims to expand the existing literature by examining PR and its determinants in a large (n = 540) randomized, placebo‐controlled one week trial of methylphenidate in children diagnosed with ADHD and assessed by parents at home and teachers at school.
2. METHODS
2.1. Ethics statement
The research protocol was approved by the Research Ethics Board of the Douglas Mental Health University Institute in Montreal, Canada and registered at http://clinlicaltrial.gov (NCT00483106). Informed written consent was obtained from parents, and verbal assent from children. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki.
2.2. Study design and participants
The data were collected from November 1999 to October 2012. Although designed as a randomized clinical trial (RCT), the trial does not aim to demonstrate the superiority of methylphenidate over placebo, given the well‐established benefits of methylphenidate (Schachter, Pham, King, Langford, & Moher, 2001). Rather, it is geared to investigate genetic predictors of response to methylphenidate and placebo. The present manuscript does not report on genetic findings, but focuses exclusively on the characteristics of PR and its determinants. The study protocol was described in detail previously (Sengupta et al., 2008). The flow of participants through the study is summarized in Figure 1.
Figure 1.
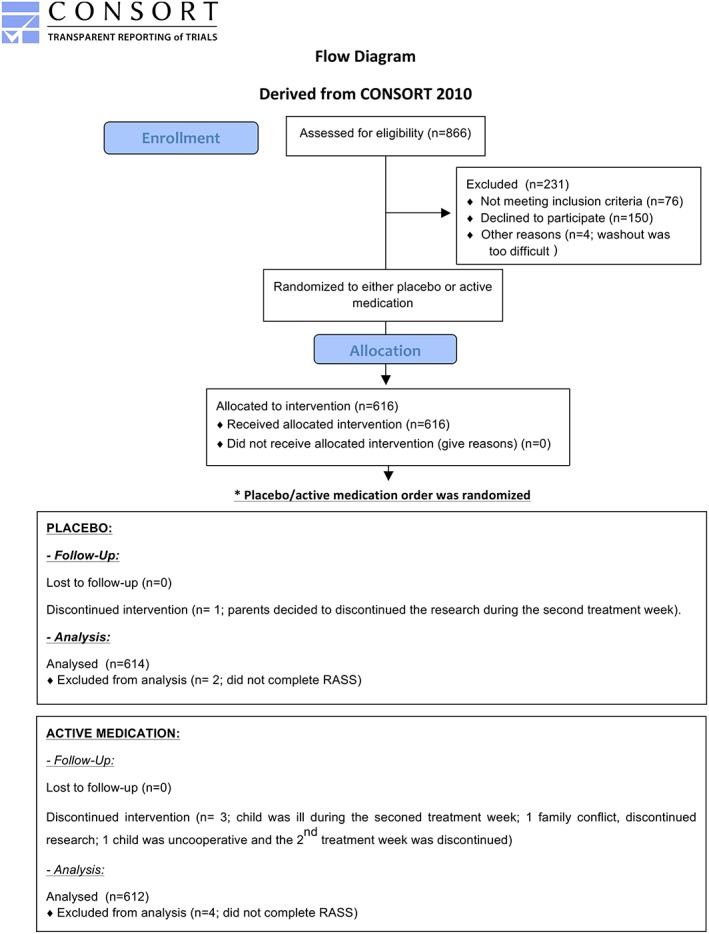
Study participants CONSORT flowchart
Five hundred and forty children aged 6 to 12 years, referred from the community, were included in the study if they meet DSM‐IV criteria for ADHD [based on a clinical interview with the child and parents by a psychiatrist, complemented by the structured Diagnostic Interview Schedule for Children (Shaffer, Fisher, Lucas, Dulcan, & Schwab‐Stone, 2000)]. Children with low IQ (< 70), a history of Tourette's syndrome or psychosis were excluded from the trial. Parents were told that their child will go through a study with active medication and placebo to measure their response to the treatment and its side effects and to give them “objective” information to help their decision on whether to use medication or not. The study design was also explained to the teachers. Both parents and teachers were aware that after one week of baseline observation (which served also as a washout period for children who were previously on medication), participants (in a blind order) received either one week of active medication (methylphenidate 0.25 mg/kg bid) followed by one week of placebo or the reverse order to assess their response to medication in an unbiased fashion.
To improve tolerability and minimize drop‐outs, there was no wash‐out between the two treatment periods. Methylphenidate and placebo were encapsulated into opaque gelatin capsules in weekly blister packs by a pharmacist not otherwise affiliated with the study. Their order of administration was determined by counterbalanced random assignment, using a computer‐generated randomization list prepared by a statistician not otherwise affiliated with the study.
2.3. Procedures and study outcomes
Parents and teachers completed the respective version of the Conners' Global Index (CGI) scale (Conners, Erhardt, & Sparrow, 1999) (CGI‐P and CGI‐T, respectively) at baseline (that was also the wash‐out week for the children who were previously on medication), at the end of the placebo and methylphenidate weeks. CGI‐P and CGI‐T are each comprised of two 10 items, each describing a specific behaviour that is rated on a 4‐point Likert scale from 0 (not at all true) to 3 (very much true). Factor analysis has shown that the CGI is composed of two factors, emotional lability and restless impulsive behaviours.
2.4. Factors associated with the PR
Based on previous literature investigating factors associated with PR in ADHD and other diorders, we elected to explore the the following factors with regard to their possible effect on PR: socio‐demographic variables [gender of the child, ethnicity, and parental socio‐economic status (SES)]; ADHD subtype and comorbidities [conduct disorder (CD), oppositional defiant disorder (ODD), mood disorders, and anxiety disorders (ADs)]; other factors [previous stimulant treatment, maternal smoking during pregnancy (MSDP), and order of treatment, i.e. placebo before or after methylphenidate].
2.5. Statistical analysis
Statistical analyses were conducted using SPSS version 20. A series of univariate repeated measure analysis of variance (ANOVA) were performed, with the measures of psychopathology (CGI‐P, CGI‐T) at baseline and during placebo treatment as the within‐subject factors, while various socio‐demographic and clinical factors were dichotomized and used as between‐subject factors. Interactions between socio‐demographic and clinical factors however, and changes in psychopathology ratings between the baseline and placebo weeks were also investigated.
The focus of the current paper is the PR and its determinants. However, to provide perspective for our results, we present data on the response to methylphenidate where appropriate. Given the exploratory nature of the study, no adjustment for multiple comparisons was performed (level of statistical significance was set at 5%). To deal with missing values, complete case analysis was used for each study outcome.
3. RESULTS
The demographic and clinical characteristics of the participants are summarized in (Table 1).
Table 1.
Demographic and clinical characteristics of children with ADHD
| Demographic characteristics | |
|---|---|
| Gender: Percentage of males | 78.3% |
| Age: Mean (standard deviation) | 9.1 years (1.8) |
| Ethnicity: Percentage Caucasian | 86.8% |
| Family characteristics | |
| Parental income: Percentage low income a | 40.1% |
| Marital status: Percentage single mothers | 43.2% |
| Maternal education: Percentage lower education b | 36.7% |
| Maternal smoking during pregnancy: | 38.3% |
| ADHD presentation | |
| Percentage predominantly inattentive | 37.0% |
| Percentage predominantly hyperactive/impulsive | 9.6% |
| Percentage combined presentation | 53.3% |
| Percentage previously treated | 37.1% |
| Comorbidity | |
| Percentage conduct disorder | 18.5% |
| Percentage oppositional defiant disorder | 41.4% |
| Percentage mood disorders | 7.5% |
| Percentage anxiety disorders | 42.6% |
Income was grouped into two categories: (a) low ≤ $30,000 and (b) high > $30,000 (Canadian dollars).
Mother's education level was divided in: (a) lower education ≤11 years (i.e. high school education or less in Quebec) and (b) high education >11 years.
A total of 540 participants completed the CGI‐P and 528 completed the CGI‐T. Participants received significantly improved ratings from parents and teachers (p < 0.001) during the placebo week, compared to baseline (Figure 2). The magnitude of PR in parents [10.57 points; standard deviation (SD) = 12.6] was two and a half times the magnitude of PR in teachers' (3.9 points; SD = 10.37) (p < 0.001).
Figure 2.
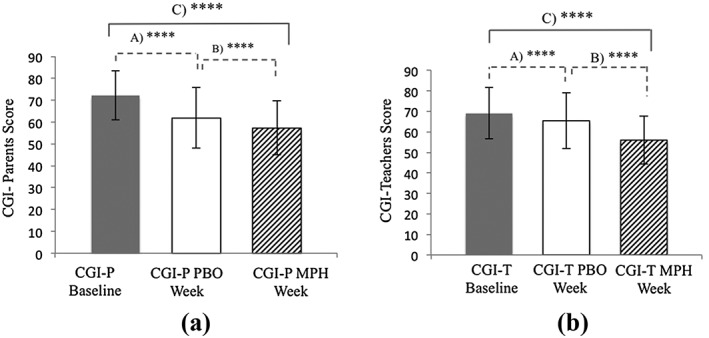
Changes in attention deficit hyperactivity disorder (ADHD) symptoms between baseline, placebo, and active‐medication week according to (a) the parents and (b) the teachers. A – Shows a significant difference between baseline week and placebo week scores (i.E. placebo response) as assessed by the Conners' Global Index for parents and teachers (CGI‐P and CGI‐T, respectively) (p < 0.001). B – Shows a significant difference between active‐medication week and placebo week scores (p < 0.001). C – Shows a significant difference between baseline week and acive‐medication week scores (i.E. treatment response) (p < 0.001). Error bars represent the standard deviation
As indicated in Table 2, for parents, low SES, single parental status, lower education level of mothers, and MSDP were associated with significantly higher PR (p = 0.001, p = 0.039, p = 0.008, and p = 0.029, respectively), while prior psychostimulant exposure (PPE) was associated with lower PR (p = 0.001). There was no significant interaction between PR as assessed by parents and participants' gender, order of treatment, ADHD subtype, or any comorbidity.
Table 2.
Factors associated with placebo response (PR) in the parents' Conners' Global Index (CGI‐P) ratings
| CGI‐P during baseline week, mean (SD) | CGI‐P during placebo week, mean (SD) | Interaction | |
|---|---|---|---|
| Statistic and p‐value, partial eta squared | |||
| Ethnicity | F 1,537 = 0.677, p = 0.411; 0.001 | ||
| Caucasian (N = 474) | 72.46 (10.98) | 61.72 (13.91) | |
| Others (N = 65) | 70.22 (11.77) | 60.85 (14.13) | |
| Gender | F 1,538 = 1.26, p = 0.261; 0.002 | ||
| Boys (N = 423) | 70.86 (10.54) | 60.61 (13.55) | |
| Girls (N = 117) | 77.01 (11.72) | 65.27 (14.62) | |
| Marital status a | F 1,485 = 4.28, p = 0.039; 0.009 | ||
| Single (N = 200) | 74.34 (10.39) | 62.12 (14.13) | |
| Couples (N = 287) | 70.01 (11.04) | 61.17 (13.73) | |
| Income b | F 1,511 = 10.28, p = 0.001; 0.020 | ||
| Low (N = 195) | 74.93 (9.97) | 61.91 (14.18) | |
| High (N = 318) | 70.68 (11.19) | 61.34 (13.65) | |
| Oppositional defiant disorder (ODD) | F 1,533 = 0.64, p = 0.43; 0.001 | ||
| No (N = 311) | 69.77 (11.48) | 59.58 (13.13) | |
| Yes (N = 224) | 75.67 (9.50) | 64.60 (14.3) | |
| Conduct disorder (CD) | F 1,532 = 0.88, p = 0.348; 0.002 | ||
| No (N = 443) | 71.07 (10.98) | 60.26 (13.55) | |
| Yes (N = 91) | 77.73 (9.77) | 68.27 (13.43) | |
| Stress disorders | F 1,504 = 1.75, p = 0.187; 0.003 | ||
| No (N = 470) | 71.89 (11.10) | 61.46 (13.66) | |
| Yes (N = 36) | 75.25 (10.33) | 61.92 (15.47) | |
| Anxiety disorders | F 1,504 = 2.09, p = 0.149; 0.004 | ||
| No (N = 293) | 70.48 (11.26) | 60.56 (13.71) | |
| Yes (N = 213) | 74.32 (10.39) | 62.76 (13.76) | |
| Time of giving placebo | F 1,537 = 0.77, p = 0.380; 0.001 | ||
| First week (N = 291) | 72.33 (11.19) | 61.32 (13.40) | |
| Second week (N = 248) | 72.04 (11.02) | 61.99 (14.54) | |
| ADHD subtype | F 2,536 = 1.70, p = 0.184; 0.006 | ||
| Inattentive (N = 209) | 67.85 (11.04) | 56.72 (12.19) | |
| Hyperactive (N = 51) | 72.37 (10.31) | 59.49 (13.28) | |
| Combined (N = 279) | 75.44 (10.15) | 65.72 (14.01) | |
| Smoking during pregnancy | F 1,494 = 4.82, p = 0.029; 0.010 | ||
| No (N = 317) | 71.06 (10.79) | 61.35 (13.67) | |
| Yes (N = 179) | 74.27 (11.07) | 62.00 (14.15) | |
| Mothers' level of education c | F 1,481 = 7.15, p = 0.008; 0.015 | ||
| Low (N = 166) | 74.36 (10.65) | 61.31 (14.77) | |
| High (N = 317) | 71.03 (10.71) | 61.20 (13.29) | |
| Previous medications | F 1,517 = 10.80, p = 0.001; 0.020 | ||
| No (N = 357) | 71.75 (10.92) | 59.9 (13.57) | |
| Yes (N = 162) | 73.66 (11.14) | 65.71 (13.90) | |
Marital status was grouped into two categories: (a) single, includes: separated/divorced, single or widow/windower and (b) couples, includes: married or living together.
Income was grouped into two categories: (a) low ≤ $30,000 and (b) high > $30,000 (Canadian dollars).
Mother's education level was divided in: (a) lower education ≤11 years (i.e. high school education or less in Quebec) and (b) high education >11 years.
In terms of PR assessed by teachers, Caucasian ethnicity (of the subject) was associated with a higher PR (p = 0.021), while PPE was associated with lower PR (p = 0.001). There was no significant interaction between PR as assessed by teachers and the other socio‐demographic and clinical variables (Table 3).
Table 3.
Factors associated with placebo response (PR) in the teachers' Conners' Global Index (CGI‐T) ratings
| CGI‐T during baseline week, mean (SD) | CGI‐T during placebo week, mean (SD) | Interaction statistic and p‐value, partial eta squared | |
|---|---|---|---|
| Income a | F 1,494 = 0.059, p = 0.808; 0.00 | ||
| Low (N = 197) | 71.91 (11.90) | 67.80 (13.26) | |
| High (N = 299) | 67.57 (12.17) | 63.68 (13.44) | |
| Marital status b | F 1,471 = 0.36, p = 0.55; 0.001 | ||
| Single (N = 208) | 71.39 (12.01) | 66.85 (13.93) | |
| Couples (N = 265) | 68.02 (12.30) | 64.06 (13.25) | |
| Gender | F 1,526 = 1.52, p = 0.217; 0.003 | ||
| Boys (N = 420) | 68.72 (11.40) | 65.08 (12.88) | |
| Girls (N = 108) | 71.56 (15.52) | 66.53 (15.85) | |
| Ethnicity | F 1,525 = 5.397, p = 0.021; 0.010 | ||
| Caucasian (N = 459) | 69.48 (12.27) | 65.14 (13.40) | |
| Others (N = 68) | 68.41 (13.07) | 67.19 (14.33) | |
| Stress disorders | F 1,491 = 0.010, p = 0.920; 0.000 | ||
| No (N = 456) | 69.11 (12.31) | 65.07 (13.43) | |
| Yes (N = 37) | 70.05 (13.19) | 65.84 (14.36) | |
| Conduct disorder (CD) | F 1,519 = 0.41, p = 0.523; 0.001 | ||
| No (N = 425) | 69.34 (12.56) | 65.18 (13.53) | |
| Yes (N = 96) | 69.20 (11.43) | 65.78 (13.38) | |
| Oppositional defiant disorder (ODD) | F 1,520 = 0.298, p = 0.586; 0.001 | ||
| No (N = 318) | 68.08 (12.11) | 63.86 (12.93) | |
| Yes (N = 204) | 71.31 (12.49) | 67.60 (14.07) | |
| Anxiety disorders | F 1,492 = 0.306, p = 0.580; 0.001 | ||
| No (N = 283) | 69.37 (12.24) | 65.6 (13.43) | |
| Yes (N = 211) | 68.91 (12.48) | 64.62 (13.62) | |
| Time of giving placebo | F 1,524 = 0.170, p = 0.680; 0.000 | ||
| First week (N = 279) | 68.94 (12.27) | 64.86 (13.43) | |
| Second week (N = 247) | 69.64 (12.57) | 65.93 (13.72) | |
| Mothers' level of education c | F 1,470 = 2.04, p = 0.154; 0.004 | ||
| Low (N = 170) | 72.06 (11.64) | 68.59 (13.15) | |
| High (N = 302) | 68.03 (12.24) | 63.14 (13.23) | |
| ADHD subtype | F 2,525 = 1.95, p = 0.143; 0.007 | ||
| Inattentive (N = 196) | 65.68 (13.11) | 61.55 (12.87) | |
| Hyperactive (N = 48) | 73.13 (10.69) | 66.63 (13.77) | |
| Combined (N = 284) | 71.15 (11. 56) | 67.80 (12.69) | |
| Previous medications | F 1,506 = 15.50, p = 0.000; 0.030 | ||
| No (N = 324) | 69.05 (12.23) | 63.78 (13.69) | |
| Yes (N = 184) | 70.05 (12.42) | 68.49 (12.81) | |
Income was grouped into two categories: (a) low ≤ $30,000 and (b) high > $30,000 (Canadian dollars).
Marital status was grouped into two categories: (a) single, includes: separated/divorced, single or widow/windower and (b) couples, includes: married or living together
Mother's education level was divided in: (a) lower education ≤11 years (i.e. high school education or less in Quebec) and (b) high education >11 years
Similar analyses were carried out for the two factors of CGI‐P and CGI‐T: restless‐impulsive behaviour and emotional lability, and the results were similar (data not shown).
The patterns of PR were examined more closely by stratifying participants in two groups according to the factors that were significantly associated with PR as outlined earlier (e.g. prior treatment). This revealed two different patterns of PR. When stratifying participants by the presence or not of prior treatment, a divergent pattern emerged (Figure 3B and C), in which groups with and without prior treatment had similar CGI‐P and CGI‐T scores at baseline (p = 0.067; p = 0.378, respectively), but diverged after placebo administration (p = 0.000; p = 0.000, respectively). Patients without prior treatment improved more according to both parents and teachers (average improvement mean = 11.8, SD = 12.77 versus mean = 5.26, SD = 9.95). A similar divergent pattern emerged in teachers' ratings when stratifying the children by ethnicity (Figure 3A), with Caucasian children showing greater improvement on placebo (average improvement mean = 4.33, SD = 0.482 versus mean = 1.22, SD = 1.25).
Figure 3.
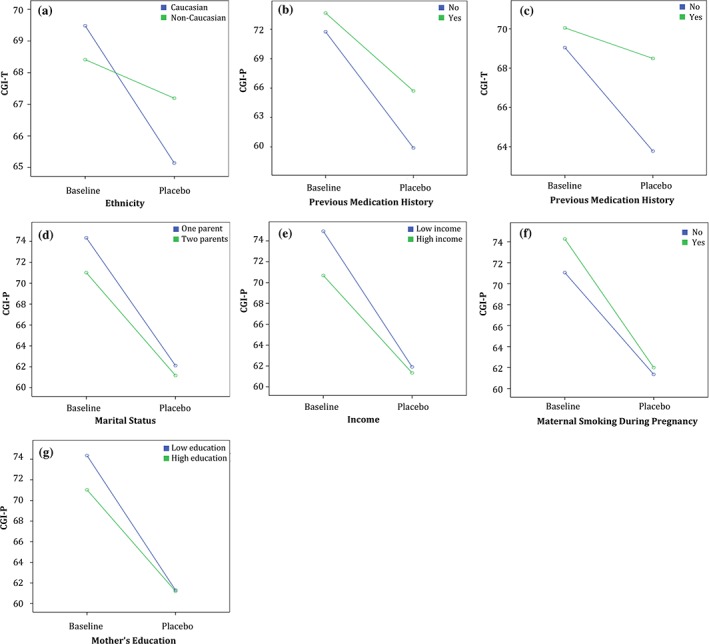
Differences in CGI‐P and CGI‐T trajectories in groups stratified by factors associated with the placebo response. (A) to (C) show significant interactions between ethnicity and previous treatment with medication, respectively. (D) to (G) show significant interactions between parental marital status, parental income, maternal smoking during pregnancy, and maternal education, respectively, and the change in parents' CGI‐P scores between the baseline and placebo weeks
The second pattern, which may be termed convergent, emerged when stratifying participants by parental income, mothers' level of education, marital status and MSDP (Figure 3D–G). Here, at baseline, participants with lower SES, lower maternal education, single parents, or a history of MSDP started with higher CGI‐P scores at baseline, but converged to similar CGI‐P scores during the placebo week compared to the groups with the alternate characteristics (e.g. with higher parental income).
There was no significant effect of order of treatment on the parents' or teachers' ratings (p > 0.05). To further safeguard against possible carry‐over effects, we repeated our analysis on the subgroup of children who received placebo in the first week (n = 323), obtaining very similar results.
4. DISCUSSION
We report here a highly statistically significant PR as assessed by parents and teachers, confirming previous studies showing a consistent PR in children with ADHD (Buitelaar et al., 2012; Newcorn et al., 2009; Waxmonsky et al., 2011). As noted in the introduction and Figure 4, we emphasize that our findings relate to a PR, rather than a PE. This is particularly relevant in this context given that the response to treatment is not assessed by the subject taking the treatment, but by observers who can be sensitive to the pacebo effects induced in the child, but also to their own expectations, biases and observers' skills. In fact, measuring PE requires specific neurobiological hypothesis that need to be probed and correlated with PR (in order to isolate a more specific PE) and our study design only allows us to study PR.
Figure 4.
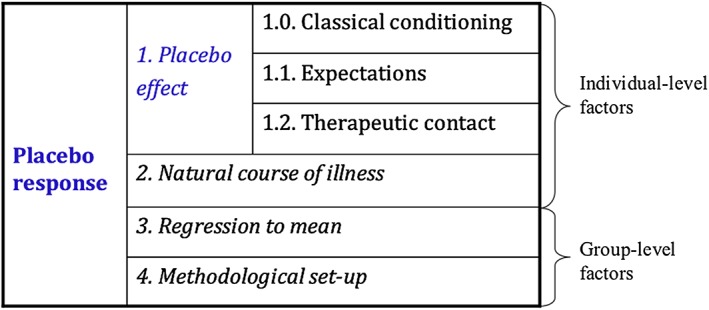
Components of placebo response
The first main finding in this study is the higher and highly significant PR with a larger effect size as assessed by parents compared to teachers. Several factors may account for this observation. First, the desire to improve is a known modulator of the PR (Price, Finniss, & Benedetti, 2008). The parents' desire for their children's behaviour to improve may exceed the desire of the teachers. Second, the capacity to generate expectations of improvement, an integral part of the PR (Price et al., 2008), may be more limited in teachers compared to parents. Alternatively, but not exclusively, it is possible that parents and teachers are attentive to different dimensions of the child behaviours that respond differentially to placebo in different environments (i.e. school and home). These observations imply that general statements regarding ‘the PR in ADHD’ might lack meaning without specifying who observed the response and in what context. Therefore, our results caution against the common practice in ADHD research of combining ratings from several sources into a single outcome variable (Newcorn et al., 2009). Rather than achieving greater precision, this practice may instead obscure opposing trends seen by different observers. A similar separation between PRs from different observers was reported in major depression (Mora, Nestoriuc, & Rief, 2011).
The second main finding from this study is the association between PR and several clinical and demographic characteristics of the child. Interestingly these associations were also specific to the environmental context of the assessment and who completed these assessments. Two factors were associated with smaller PR as assessed by teachers: previous exposure to medication and ethnicity. Previous exposure to medication was also associated with smaller PR as assessed by parents. This observation could be explained by the fact that both parents and teachers have a prior knowledge of how the child responded to active medication, which in case it was poor, will lower their expectations of PR. In contrast, in children without prior exposure to active treatment, the expectations of observers with regard to response might be anchored in more general expectations of treatment response without adjustment to the child prior history of treatment response. This observation replicates previous work showing lower PR after prior treatment (Newcorn et al., 2009; Sandler & Bodfish, 2008) and might have important implications for the design and interpretation of clinical trials in ADHD. The observation that Caucasians were assessed as more responsive to placebo by teachers is intriguing. Whether it is related to a true ethnic difference in PE or it represents a racial bias in evaluating PR is difficult to disentangle in this study and warrants further investigation.
In addition to its association with prior exposure to treatment, PR as assessed by parents was associated with many socio‐demographic characteristics of the child and his environment. In order to gain better understanding of the relation between these factors and PR, we stratified children according to these factors, and observed two patterns of PR – which we called convergent and divergent patterns. In the former, participants with lower parental income, lower maternal education, single parent status, and MSDP had more severe ratings at baseline, but experienced a higher PR, thus “catching up” (i.e. converging) with the rest of the sample during the placebo phase. All four of these factors, including MSDP (Lu, Tong, & Oldenburg, 2001), are associated with lower SES. The PR observed in the lower SES group appears to be the result of higher symptom scores at baseline according to the parent's assessment, rather than lower scores on placebo. These higher behavioural severity scores at baseline could be due to an intrinsically more severe psychopathology in the group of children with these psychosocial characteristics. This is in line with studies from our group (Thakur et al., 2013) and others (Langley, Holmans, van den Bree, & Thapar, 2007; Russell, Ford, & Russell, 2015; Sagiv, Epstein, Bellinger, & Korrick, 2013; Sciberras, Ukoumunne, & Efron, 2011; Thapar et al., 2003). Speculatively, there may also be a tendency of some parents to overestimate their child's psychopathology at entry in order to secure treatment, especially in an environment where access to care is scarce (Benedetti, 2009). Upon repeated measurement, the initially inflated ratings would tend to yield values closer to average, mimicking an improvement or reflecting the well known regression to the mean phenomenon. Previous exposure to treatment was associated with a divergent pattern of PR in both parents and teachers. Here previously‐treated participants started with similar ratings of psychopathology at baseline to those not previously‐treated, but showed a lower PR, thus ending up with significantly poorer ratings during the placebo phase compared with their counterparts. As mentioned earlier, this observation might have important implication for the design and interpretation of clinical trials. These observations were valid for both the “restless‐impulsive” and “emotional lability” factors of CGI‐P and CGI‐T. It is also important to note that the effect sizes associated with these factors are of relatively small and variable magnitude. However, these magnitudes, when compared to the magnitude of the total PR can reach substantial proportions (up to 23% for PPE in teachers). Also, it is important to note that the cumulative effect of all the statistically significant correlates of PR amount to 17.8% of PR in parents and 17.8% of PR in teachers. Given that these factors can be balanced in RCT, considering all of them when designing RCTs may be important to reduce the failure of clinincal trials.
The precise neurobiological mechanisms of the placebo effects in ADHD still await elucidation. Placebo may increase meso‐limbic dopamine release in Parkinson's disease (de la Fuente‐Fernandez, 2009), and perhaps in other conditions (Murray & Stoessl, 2013) including ADHD, so their effect may be neurobiologically similar to that of psychostimulants used to treat ADHD, which also increase dopamine levels in various brain regions (Arnsten, 2006). However, the possible deficits in mesolimbic dopamine in patients with ADHD (Volkow, Wang, Newcorn, et al., 2007) may result in lower positive effects of placebos in this condition. Similarly, the possible deficits in the reward system in ADHD, perhaps linked to dopaminergic dysfunction (Volkow et al., 2010), predict smaller benefit from placebo in ADHD patients, given the importance of reward mechanisms for the PE (de la Fuente‐Fernandez, 2009). Brain dopamine regulation is a complex phenomenon that is dependent on genetic and environmental factors. It is therefore possible that placebo effects will be modulated by the environmental conditions that affect the child in various ways and affect his dopamine, and other neurobiological pathways implicated in PE. Thus, it is not surprising that the factors associated with the PR differ across observers. Notwithstanding these biological mechanisms underlying PE, PR is a very complex trait that involves several other determinants. Studying the determinants, biological and otherwise, of PR could help understand how complex mental processes such as beliefs and expectation (that play a vital role in therapeutic outcome) affect brain functions and human behaviours (Benedetti, Mayberg, Wager, Stohler, & Zubieta, 2005; Finniss & Benedetti, 2005). On a more practical front, these studies are critical in optimizing the design of clinical trials and interpretation of the results. Indeed, the quality and validity of clinical trials can be improved significantly by understanding PR. One of the primary purposes of clinical trials is to isolate the real effects of medications from the background effect of placebo. This goal can be achieved by minimizing the placebo effects as much as possible, which, in turn, can increase the the statistical power of clinical trial (Enck, Bingel, Schedlowski, & Rief, 2013). This could be achieved either by selecting patients who are expected, according to their baseline characteristics, to be least responsive to placebo or by statistically controlling for these characteristics (Benedetti & Amanzio, 2011). Other innovative clinical trial designs exploiting a better knowledge of PR have been proposed (Benedetti, 2012).
4.1. Study's strengths and weaknesses
The present study was the first to compare parents' assessment of PR in children with ADHD with ratings by their teachers, treating both measures as separate outcomes in a large sample. The within‐subject cross‐over design increased precision in determining the PR in comparison to the parallel‐group design used in previous trials. Another advantage is the measurement of an acute PR at one week in comparison with the duration of previous studies, lasting typically one month or more. The PR has been shown to peak in short‐duration studies (Hrobjartsson & Gotzsche, 2001, 2004) thus a shorter study‐design, especially with a medication like methylphenidate, whose benefits are nearly immediate, may succeed in capturing the PR at its time of maximal expression. To avoid cutoffs used in previous studies to determine categorical measures of response, which may bias results and make it difficult to compare findings, we relied instead on quantitative measures.
The cross‐over design carries a risk of bias due to carry‐over effects of the interventions across treatment periods. We acknowledge that the absence of a wash‐out period between the two treatment weeks magnifies this risk. We note, however, that the pharmacokinetic properties of methylphenidate limit its duration of action to approximately 1–4 hours. The elimination half‐life is approximately 2–3 hours, so the drug is completely cleared within 24 hours, and a steady‐state plasma concentration is never reached (Kimko, Cross, & Abernethy, 1999). This concurs with clinical experience that a methylphenidate tablet taken one day no longer has any discernible effects by the next day. Additionally, clinical trials of stimulants have shown that symptoms return after cessation of the drug (Biederman et al., 2010). All these observations suggest that the risk of carry‐over effects is limited. Indeed, reassuringly, our analyses revealed no significant order‐of‐treatment effect, and restricting the calculations to children who received placebo first yielded very similar results.
As our trial lasted for two weeks, the result of response to placebo might not sustain. Although trials of depression showed that response to placebo was maintained for more than three months in 79% of the patients; however, it is unknown if this can be the case with ADHD (Khan, Redding, & Brown, 2008). Lastly, our sample included a larger number of boys than girls, which may limit the generalizability of our findings.
5. CONCLUSION
PR in children with ADHD is a complex phenomenon that varies across different observers and settings. In clinical trials, it is important to collect and analyse data separately from different observers while controlling (either statistically or by design) for factors that modulate the magnitude and shape (convergent or divergent) of the PR. These findings could contribute to a better understanding of PR; hence, clinical trials design and interpretation, and treatment approaches in clinical practice.
DECLARATION OF INTEREST STATEMENT
W. Fageera, S. M. Sengupta, A. Traicu, M. Fortier, Z. Choudhry, A. Labbe, and N. Grizenko declared no conflict of interest. R. Joober sits on the advisory boards and speakers' bureaus of Pfizer, Janssen Ortho, BMS, Sunovion, Otsuka and Lundbeck; he has received grant funding from them and from AstraZeneca. He has received honoraria from Janssen Canada, Shire, Lundbeck, Otsuka and from Pfizer Canada for CME presentations and royalties for Henry Stewart talks.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Serge Gallant for assistance with the statistical analysis and reviewing the manuscript, as well as the ADHD programme staff for their help with participant recruitment and evaluation. This work was supported in part by a grant from the Fond de Recherche du Québec and the Canadian Institutes of Health Research. Weam Fageera is a recipient of a PhD scholarship from the Ministry of Education of Saudi Arabia.
Fageera W, Traicu A, Sengupta SM, et al. Placebo response and its determinants in children with ADHD across multiple observers and settings: A randomized clinical trial. Int J Methods Psychiatr Res. 2018;27:e1572 10.1002/mpr.1572
This work was supported in part by a grant from the Fond de Recherche du Québec and the Canadian Institutes of Health Research.
Trial registration: ClinicalTrials.gov NCT00483106.
REFERENCES
- Arnold, L. E. , Farmer, C. , Kraemer, H. C. , Davies, M. , Witwer, A. , Chuang, S. , … Swiezy, N. B. (2010). Moderators, mediators, and other predictors of risperidone response in children with autistic disorder and irritability. Journal of Child and Adolescent Psychopharmacology, 20(2), 83–93. 10.1089/cap.2009.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten, A. F. (2006). Fundamentals of attention‐deficit/hyperactivity disorder: Circuits and pathways. Journal of Clinical Psychiatry, 67(Suppl. 8), 7–12. [PubMed] [Google Scholar]
- Aslaksen, P. M. , Bystad, M. , Vambheim, S. M. , & Flaten, M. A. (2011). Gender differences in placebo analgesia: Event‐related potentials and emotional modulation. Psychosomatic Medicine, 73(2), 193–199. 10.1097/PSY.0b013e3182080d73 [DOI] [PubMed] [Google Scholar]
- Beecher, H. K. (1955). The powerful placebo. Journal of the American Medical Association, 159(17), 1602–1606. [DOI] [PubMed] [Google Scholar]
- Benedetti, F. (2009). Placebo Effects: Understanding the Mechanisms in Health and Disease. Oxford: Oxford University Press. [Google Scholar]
- Benedetti, F. (2012). Placebo‐induced improvements: How therapeutic rituals affect the patient's brain. J Acupuncture and Meridian Studies, 5(3), 97–103. 10.1016/j.jams.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Benedetti, F. , & Amanzio, M. (2011). The placebo response: How words and rituals change the patient's brain. Patient Education and Counseling, 84(3), 413–419. 10.1016/j.pec.2011.04.034 [DOI] [PubMed] [Google Scholar]
- Benedetti, F. , Mayberg, H. S. , Wager, T. D. , Stohler, C. S. , & Zubieta, J. K. (2005). Neurobiological mechanisms of the placebo effect. Journal of Neuroscience, 25(45), 10390–10402. 10.1523/JNEUROSCI.3458-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman, J. , Mick, E. , Surman, C. , Doyle, R. , Hammerness, P. , Kotarski, M. , & Spencer, T. (2010). A randomized, 3‐phase, 34‐week, double‐blind, long‐term efficacy study of osmotic‐release oral system‐methylphenidate in adults with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology, 30(5), 549–553. 10.1097/JCP.0b013e3181ee84a7 [DOI] [PubMed] [Google Scholar]
- Brown, W. A. , Johnson, M. F. , & Chen, M. G. (1992). Clinical features of depressed patients who do and do not improve with placebo. Psychiatry Research, 41(3), 203–214. [DOI] [PubMed] [Google Scholar]
- Buitelaar, J. K. , Sobanski, E. , Stieglitz, R.‐D. , Dejonckheere, J. , Waechter, S. , & Schäuble, B. (2012). Predictors of placebo response in adults with attention‐deficit/hyperactivity disorder: Data from 2 randomized trials of osmotic‐release oral system methylphenidate. Journal of Clinical Psychiatry, 73(8), 1097–1102. [DOI] [PubMed] [Google Scholar]
- Conners, C. K. , Erhardt, D. , & Sparrow, E. (1999). Conners' Adult ADHD Rating Scales: Technical Manual. North Tonawanda, NY: Multi‐Health Systems (MHS) Incorporated. [Google Scholar]
- de la Fuente‐Fernandez, R. (2009). The placebo‐reward hypothesis: Dopamine and the placebo effect. Parkinsonism & Related Disorders, 15(Suppl. 3), S72–S74. 10.1016/s1353-8020(09)70785-0 [DOI] [PubMed] [Google Scholar]
- Enck, P. , Bingel, U. , Schedlowski, M. , & Rief, W. (2013). The placebo response in medicine: Minimize, maximize or personalize? Nature Reviews. Drug Discovery, 12(3), 191–204. 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- Finniss, D. G. , & Benedetti, F. (2005). Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain, 114(1–2), 3–6. 10.1016/j.pain.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Hrobjartsson, A. , & Gotzsche, P. C. (2001). Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. New England Journal of Medicine, 344(21), 1594–1602. 10.1056/NEJM200105243442106 [DOI] [PubMed] [Google Scholar]
- Hrobjartsson, A. , & Gotzsche, P. C. (2004). Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. Journal of Internal Medicine, 256(2), 91–100. 10.1111/j.1365-2796.2004.01355.x [DOI] [PubMed] [Google Scholar]
- Hunter, A. M. , Cook, I. A. , & Leuchter, A. F. (2010). Impact of antidepressant treatment history on clinical outcomes in placebo and medication treatment of major depression. Journal of Clinical Psychopharmacology, 30(6), 748–751. 10.1097/JCP.0b013e3181faa474. [DOI] [PubMed] [Google Scholar]
- Katja Weimer, P. , Colloca, L. , & Enck, P. (2015). Placebo effects in psychiatry: Mediators and moderators. Lancet Psychiatry, 2(3), 246–257. 10.1016/S2215-0366(14)00092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. , Kolts, R. L. , Rapaport, M. H. , Krishnan, K. R. , Brodhead, A. E. , & Browns, W. A. (2005). Magnitude of placebo response and drug‐placebo differences across psychiatric disorders. Psychological Medicine, 35(5), 743–749. [DOI] [PubMed] [Google Scholar]
- Khan, A. , Redding, N. , & Brown, W. A. (2008). The persistence of the placebo response in antidepressant clinical trials. Journal of Psychiatric Research, 42(10), 791–796. 10.1016/j.jpsychires.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Kimko, H. C. , Cross, J. T. , & Abernethy, D. R. (1999). Pharmacokinetics and clinical effectiveness of methylphenidate. Clinical Pharmacokinetics, 37(6), 457–470. 10.2165/00003088-199937060-00002 [DOI] [PubMed] [Google Scholar]
- King, B. H. , Dukes, K. , Donnelly, C. L. , Sikich, L. , McCracken, J. T. , Scahill, L. , … Hirtz, D. (2013). Baseline factors predicting placebo response to treatment in children and adolescents with autism spectrum disorders: A multisite randomized clinical trial. JAMA Pediatrics, 167(11), 1045–1052. 10.1001/jamapediatrics.2013.2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, I. (2013). The placebo effect revisited: Lessons learned to date. Complementary Therapies in Medicine, 21(2), 102–104. [DOI] [PubMed] [Google Scholar]
- Kirsch, I. , Deacon, B. J. , Huedo‐Medina, T. B. , Scoboria, A. , Moore, T. J. , & Johnson, B. T. (2008). Initial severity and antidepressant benefits: A meta‐analysis of data submitted to the Food and Drug Administration. PLoS Medicine, 5(2), e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley, K. , Holmans, P. A. , van den Bree, M. B. , & Thapar, A. (2007). Effects of low birth weight, maternal smoking in pregnancy and social class on the phenotypic manifestation of attention deficit hyperactivity disorder and associated antisocial behaviour: Investigation in a clinical sample. BMC Psychiatry, 7, 26 10.1186/1471-244X-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Tong, S. , & Oldenburg, B. (2001). Determinants of smoking and cessation during and after pregnancy. Health Promotion International, 16(4), 355–365. [DOI] [PubMed] [Google Scholar]
- Mora, M. S. , Nestoriuc, Y. , & Rief, W. (2011). Lessons learned from placebo groups in antidepressant trials. Philosophical Transactions of the Royal Society of London, Series B: Biological Scieance, 366(1572), 1879–1888. 10.1098/rstb.2010.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, D. , & Stoessl, A. J. (2013). Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions. Pharmacolpgy & Therapeutics, 140(3), 306–318. [DOI] [PubMed] [Google Scholar]
- Newcorn, J. H. , Sutton, V. K. , Zhang, S. , Wilens, T. , Kratochvil, C. , Emslie, G. J. , … Allen, A. J. (2009). Characteristics of placebo responders in pediatric clinical trials of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 48(12), 1165–1172. 10.1097/CHI.0b013e3181bc730d [DOI] [PubMed] [Google Scholar]
- Potkin, S. , Agid, O. , Siu, C. , Watsky, E. , Vanderburg, D. , & Remington, G. (2011). Placebo response trajectories in short‐term and long‐term antipsychotic trials in schizophrenia. Schizophrenia Research, 132(2–3), 108–113. 10.1016/j.schres.2011.07.028 [DOI] [PubMed] [Google Scholar]
- Price, D. D. , Finniss, D. G. , & Benedetti, F. (2008). A comprehensive review of the placebo effect: Recent advances and current thought. Annual Review of Psychology, 59, 565–590. [DOI] [PubMed] [Google Scholar]
- Russell, A. E. , Ford, T. , & Russell, G. (2015). Socioeconomic associations with ADHD: Findings from a mediation analysis. PloS One, 10(6), e0128248. DOI: 10.1371/journal.pone.0128248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv, S. K. , Epstein, J. N. , Bellinger, D. C. , & Korrick, S. A. (2013). Pre‐ and postnatal risk factors for ADHD in a nonclinical pediatric population. Journal of Attention Disorders, 17(1), 47–57. 10.1177/1087054711427563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler, A. D. , & Bodfish, J. W. (2008). Open‐label use of placebos in the treatment of ADHD: A pilot study. Child Care Health and Development, 34(1), 104–110. 10.1111/J.1365-2214.2007.00797.X [DOI] [PubMed] [Google Scholar]
- Schachter, H. M. , Pham, B. , King, J. , Langford, S. , & Moher, D. (2001). How efficacious and safe is short‐acting methylphenidate for the treatment of attention‐deficit disorder in children and adolescents? A meta‐analysis. CMAJ, 165(11), 1475–1488. [PMC free article] [PubMed] [Google Scholar]
- Sciberras, E. , Ukoumunne, O. C. , & Efron, D. (2011). Predictors of parent‐reported attention‐deficit/hyperactivity disorder in children aged 6–7 years: A national longitudinal study. Journal of Abnormal Child Psychology, 39(7), 1025–1034. 10.1007/s10802-011-9504-8 [DOI] [PubMed] [Google Scholar]
- Sengupta, S. , Grizenko, N. , Schmitz, N. , Schwartz, G. , Bellingham, J. , Polotskaia, A. , … Joober, R. (2008). COMT Val108/158Met polymorphism and the modulation of task‐oriented behavior in children with ADHD. Neuropsychopharmacology, 33(13), 3069–3077. 10.1038/npp.2008.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, D. , Fisher, P. , Lucas, C. P. , Dulcan, M. K. , & Schwab‐Stone, M. E. (2000). NIMH Diagnostic interview Schedule for children version IV (NIMH DISC‐IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Thakur, G. A. , Sengupta, S. M. , Grizenko, N. , Schmitz, N. , Page, V. , & Joober, R. (2013). Maternal smoking during pregnancy and ADHD: A comprehensive clinical and neurocognitive characterization. Nicotine & Tobacco Research, 15(1), 149–157. 10.1093/ntr/nts102 [DOI] [PubMed] [Google Scholar]
- Thapar, A. , Fowler, T. , Rice, F. , Scourfield, J. , van den Bree, M. , Thomas, H. , … Hay, D. (2003). Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. American Journal of Psychiatry, 160(11), 1985–1989. 10.1176/appi.ajp.160.11.1985 [DOI] [PubMed] [Google Scholar]
- Tuttle, A. H. , Tohyama, S. , Ramsay, T. , Kimmelman, J. , Schweinhardt, P. , Bennett, G. J. , & Mogil, J. S. (2015). Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain, 156(12), 2616–2626. 10.1097/j.pain.0000000000000333 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Wang, G. J. , Newcorn, J. , Telang, F. , Solanto, M. V. , Fowler, J. S. , … Swanson, J. M. (2007). Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry, 64(8), 932–940. 10.1001/archpsyc.64.8.932 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Wang, G.‐J. , Newcorn, J. H. , Kollins, S. H. , Wigal, T. L. , Telang, F. , … Logan, J. (2010). Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry, 16(11), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschbusch, D. A. , Pelham, W. E. Jr. , Waxmonsky, J. , & Johnston, C. (2009). Are there placebo effects in the medication treatment of children with attention‐deficit hyperactivity disorder? Journal of Developmental & Behavioral Pediatrics, 30(2), 158–168. [DOI] [PubMed] [Google Scholar]
- Waxmonsky, J. G. , Waschbusch, D. A. , Glatt, S. J. , & Faraone, S. V. (2011). Prediction of placebo response in 2 clinical trials of lisdexamfetamine dimesylate for the treatment of ADHD. Journal of Clinical Psychiatry, 72(10), 1366. [DOI] [PubMed] [Google Scholar]


