Abstract
Objectives
Real world implementation of proactive screening and brief intervention in health care is threatened by high cost. Using e‐health interventions and screening for multiple health risk factors may provide more efficiency. We describe methodological details of a proactive multipurpose health risk screening in health care settings and report on participation rates, participants' characteristics, and participation factors.
Methods
Patients between 18 and 64 years from ambulatory practices and hospitals were proactively approached by study assistants at three sites for a computerized screening on harmful alcohol and tobacco consumption, depressive symptoms, insufficient fruit/vegetable consumption, physical inactivity and overweight. On the basis of their health risk pattern, a computerized algorithm allocated patients to one of five studies each of them addressing a psychiatric research question.
Results
Among all eligible patients, 13,763 (86.5%) were screened. Younger age and being female predicted screening participation. Of those with complete data (n = 12,828), 82.9% reported at least two health risks and 34.0% were eligible for a study. Study participation ranged between 35.2% and 50.8%, and was associated with socio‐demographics and problem severity.
Conclusions
This study supports the use of systematic proactive screening for multiple health risks in health care settings as it is more resource‐saving than single focused screening.
Keywords: health care, health risk factor, proactive, recruitment strategy, screening and brief intervention
1. INTRODUCTION
There is increasing evidence that brief intervention in health care settings can be effective in modifying health risk factors (e.g., tobacco use and risky drinking; Kaner et al., 2018; Rosembaun, Rojas, Rodriguez, Barticevic, & Rivera Mercado, 2018). In that context, proactive recruitment is seen as the most suitable contacting to yield sufficient reach and produce significant effects on a population level (Velicer et al., 2000). In contrast with reactive approaches, every individual of the target population is approached. This results in higher participation rates and more representative samples. Another important advantage of proactive approaches is that higher proportions of individuals with no or low motivation to change behaviors can be reached (e.g., Hoving, Mudde, & de Vries, 2007). This is of high public health relevance as the majority of the general population engaging in health risk behaviors does not intend to change it in the future (John, Meyer, Rumpf, & Hapke, 2003; Velicer et al., 2000). Reactive approaches reach only those who are interested (e.g., via media invocations) and may miss those most in need (Ludden, van Rompay, Kelders, & van Gemert‐Pijnen, 2015). Despite the advantages of proactive contacting, it requires high personnel resources and thus has not yet been practiced sufficiently.
One way to improve cost–benefit ratio is the use of a systematic proactive screening approach aiming at multiple health risk factors. Especially when combined with computer‐based counseling systems, which provide an effective and timesaving alternative to face‐to‐face counseling by physicians (Krebs, Prochaska, & Rossi, 2010; Meyer et al., 2012), it may ease implementation into routine care. Screening and intervention on multiple health risk factors is highly adequate because the majority of the adult population reports multiple health risk factors (Fine, Philogene, Gramling, Coups, & Sinha, 2004; John, Hanke, & Freyer‐Adam, 2018). Clustering of health risk factors places individuals at increased risk due to synergistic negative influences on health and multiplies health care burden as, for example, in case of combined tobacco and alcohol use (Xu et al., 2007). Disparities exist by socio‐economic status: Individuals with low socioeconomic status are more likely to cluster health risks compared with those with higher socioeconomic status (John et al., 2018; Schuit, van Loon, Tijhuis, & Ocke, 2002). This is especially concerning as socioeconomic health inequalities have increased over the last years (Ding, Do, Schmidt, & Bauman, 2015).
Consequently, screening for and targeting change in multiple risks in health care settings offer the potential of increased health benefits and higher impact on public health than single risk screening and intervention approaches and may support reduction of health disparities (J. J. Prochaska & Prochaska, 2011; J. O. Prochaska, 2008).
The aims of the current paper were (a) to describe methodological details of a proactive multipurpose health risk screening in health care settings, and (b) to analyze which part of the population is reached through this screening approach, and for subsequent offered studies.
2. METHOD
2.1. Participants and screening procedure
The proactive screening procedure used in this study was tested within a pilot study between December 2015 and May 2016. Within that timeframe, 1,523 ambulatory care patients and 1,250 hospital care patients aged 18 and 64 years were screened for health risk factors (for more details see Krause et al., in preparation).
Data collection for the present study was conducted between January 2017 and March 2018 in ambulatory practices and hospitals across three German cities (Greifswald, Site 1; Tübingen, Site 2; and Lübeck, Site 3). Approvals by the ethical review boards of the universities were received (Site 1: BB 170/16, BB 161/16; Site 2: 598/2016BO2; and Site 3: 15–256). In both settings, patients aged 18 to 64 were proactively approached and asked to fill out an anonymous computerized questionnaire (via tablet computer) on health risk factors. As effective recruitment in health care requires reduction of clinician workload (Aspy et al., 2008; Ngune, Jiwa, Dadich, Lotriet, & Sriram, 2012) study assistants to conduct recruitment were present at each site.
2.1.1. Ambulatory care patients
In total, 144 ambulatory practices were systematically and proactively contacted by mail, fax, telephone, and/or personal visits to explain research rationale and to obtain recruitment permission. Selection of practices was based on their localization (inner city area; Sites 2 and 3), number of physicians per practice (at least two; site 3), and treatment focus (substance substitution; site 2). In Site 1, all existing general practices were contacted. Recruitment took place in 39 (27.1%) practices (16 from Site 1, 7 from Site 2 and 16 from Site 3) including 35 general practices, two practices for gynecology and two outpatient clinics (dermatology, oral, and maxillofacial surgery). During opening hours, consecutive patients were approached by a study assistant and screened for age. Patients consulting for prescription or referrals without direct physician contact were excluded from screening. Recruitment period per practice varied between three and 103 days; mean recruitment time per practice was 16.5 days. Although we aimed to cover the complete office hours of the practices, this could only be realized in Site 1. In Site 2, https://www.dict.cc/englisch-deutsch/the.html https://www.dict.cc/englisch-deutsch/level.html https://www.dict.cc/englisch-deutsch/of.html office hours https://www.dict.cc/englisch-deutsch/covered.html on recruitment days varied between 47.1% and 82.2% per practice (mean coverage: 65.5%) due to limited resources. Site 3 realized a complete coverage of office hours in all practices except two (coverage of 67.5% and 66.5%) because of extended office hours (up to 10 or 15 hr a day, respectively).
2.1.2. Hospital care patients
Selection of hospital departments was based on the consultability of patients, that is, we excluded intensive and critical care units as well as wards dealing with terminal illnesses (e.g., oncology).
The following types of departments were included: internal medicine, surgery, otorhinolaryngology, gynecology, dermatology, ophthalmology, orthopedics, urology, odontology, and neurology. Prior to recruitment, directors of departments were contacted and ask for cooperation. All departments consented to participate in the recruitment except for one because of conflicting project timeframes. A total of 26 departments covering 56 wards participated in the recruitment (Site 1: 4 departments covering 7 wards; Site 2: 11 departments covering 24 wards plus one waiting/registration area; Site 3: 11 departments covering 20 wards and further 5 interdisciplinary wards).
Only patients within the age range were visited at their hospital bed and asked to participate in the screening. In Sites 1 and 3, information from the central patient admission system was available. On all working days, study assistants got a list of newly admitted patients aged 18 to 64 from the preceding day.
In Site 2, recruitment was on the basis of room plans of the wards from the recruitment day. Because of time restrictions, every ward was visited once a week at this site.
2.2. Study target populations
On the basis of their health risk pattern, screening participants were allocated to one of five studies using a computerized scoring algorithm. Studies included four proof of concept trials testing the potential efficacy of computer‐based interventions aiming at depressive symptoms (Study 1), tobacco use (Study 2) and harmful alcohol consumption in co‐occurrence with either tobacco use (Study 3) or depressive symptoms (Study 4). Further, individuals with moderate to severe alcohol dependence were invited to an interview study about consumer's perspectives on alcohol related treatments (Study 5). Due to the defined inclusion and exclusion criteria, there was no multi‐eligibility. Detailed information on inclusion and exclusion criteria, design, planned incentives, and assessments as well as interventions delivered can be found in Table 1.
Table 1.
Overview of studies
| Study characteristic | Study 1 | Study 2 | Study 3 | Study 4 | Study 5 |
|---|---|---|---|---|---|
| Coordinating site | Greifswald (Site 1) | Tübingen (Site 2) | Tübingen (Site 2) | Greifswald (Site 1) | Lübeck (Site 3) |
| Study name | ActiLife (Activating primary medical care patients for a depression‐preventive lifestyle with individualized e‐health interventions) | CSI‐ITo (Reduction of harmful tobacco use in the general population by individualized e‐coach assisted computer or smartphone interventions) | CS‐I (Reduction of harmful alcohol and tobacco use in the general population by individualized e‐coach assisted computer or smartphone interventions) | ITE (Individually tailored E‐health Interventions for primary care patients with problematic alcohol use and co‐occurring depressive symptoms) | ART‐COPE (Alcohol related treatment: A consumer' s perspective) |
| Study aim | Testing the efficacy of an individualized e‐health intervention to promote a depression‐preventive lifestyle | Testing the efficacy of individualized e‐coach assisted computer or smartphone interventions to reduce harmful tobacco use | Testing the efficacy of individualized e‐coach assisted computer or smartphone interventions to reduce harmful alcohol and tobacco use | Testing the efficacy of an individualized e‐health intervention for patients with problematic alcohol use and co‐occurring depressive symptoms | Collecting qualitative data on consumer' perspectives on alcohol related treatment to identify barriers and attractors |
| Study design | Two‐armed RCT | Three‐armed RCT | Three‐armed RCT | Two‐armed RCT | Interview study |
| Planned n | 600 | 180 | 180 | 120 | 60 |
| Inclusion criteria | Subsyndromal depressive symptoms, major depression, or dysthymia a | Daily smoking of at least one cigarette per day |
Harmful alcohol consumption b
Daily smoking of at least one cigarette per day |
Harmful alcohol consumption b
Subsyndromal depressive symptoms, major depression, or dysthymia a |
Moderate to severe alcohol dependence d |
| Exclusion criteria |
Harmful alcohol consumption b
Moderate to severe alcohol dependence d
Severe episode of major depression within last 12 months c
No reachability via e‐mail and SMS |
Harmful alcohol consumption b
Moderate to severe alcohol dependence d
Subsyndromal depressive symptoms, major depression, or dysthymia a
No reachability via e‐mail |
Moderate to severe alcohol dependence d
Subsyndromal depressive symptoms, major depression, or dysthymia a
No reachability via e‐mail |
Moderate to severe alcohol dependence d
Severe episode of major depression within last 12 months c
No reachability via e‐mail and SMS |
None |
|
Intervention content |
Intervention group: Participants receive computer‐based individually tailored motivational feedback via weekly SMS or e‐mails, and six mailed letters over a period of 6 months.
Control group: Assessments only |
Intervention groups: Six weeks interaction with an online program containing psychoeducational and goalsetting features along with motivational feedback.
Participants receive either weekly automated e‐mails about their program progress (intervention arm 1) or have weekly e‐mail‐contact to an e‐coach (intervention arm 2).
Control group: Brief advice to quit smoking (once via e‐mail) |
Intervention groups: Six weeks interaction with an online program containing psychoeducational and goalsetting features along with motivational feedback.
Participants receive either weekly automated e‐mails about their program progress (intervention arm 1) or have weekly e‐mail‐contact to an E‐coach (intervention arm 2).
Control group: Brief advice to quit smoking and/or reduce alcohol consumption (once via e‐mail) |
Intervention group: Participants receive computer‐based individually tailored motivational feedback via weekly SMS or e‐mails, and six mailed letters over a period of 6 months.
Control group: Assessment only |
Not applicable |
| Assessments |
Telephone assessments at baseline, after 2, 4, 6, and 12 months
Control group scheduled only at baseline, after 6 and 12 months |
Online assessments at baseline, 6 weeks after baseline; 1, 3, 6, 9, and 12 months after the intervention was finished
Weekly online assessments during the intervention period for intervention group only |
Online assessments at baseline, 6 weeks after baseline; 1, 3, 6, 9, and 12 months after the intervention was finished
Weekly online assessments during the intervention period for intervention group only |
Telephone assessments at baseline, after 2, 4, 6, and 12 months
Control group scheduled only at baseline, after 6 and 12 months |
One 45–60 min interview |
| Incentive (maximum) | 10€ | 60€ | 100€ | 100€ | 50€ |
Note. SMS: short messenger service; RCT: randomized control trial.
Experiencing at least one cardinal symptom (depressed mood or anhedonia) as well as one additional depressive symptom on more than half of the days in the most severe episode in the past 12 months.
Average daily alcohol use exceeding 12 or 24 grams for women or men, respectively and/or binge drinking behavior (drinking five or more alcoholic drinks for men and four or more alcoholic drinks for women per occasion) at least once a month.
Individuals were excluded if the most severe episode in the last 12 months reached a Patient Health Questionnaire sum score ≥ 20 and if depressive symptoms of any severity were still present within the last 2 weeks.
Sum score of Alcohol Use Disorder Identification Test ≥ 20.
2.3. Informed consent procedure
Verbal informed consent was obtained for participation in the screening. Prior to data collection, participants were informed by a note on the tablet that by answering the screening questions, they give their permission for their answers to be analyzed for research purposes and for determining their eligibility for further studies. After completion of the screening questionnaire patients handed the tablet back to the study assistant. If a patient was eligible for any of the five studies, the study assistant explained the nature of the respective study and its procedures to the patient. Eligible patients were asked to give written informed consent for study participation.
2.4. Patients' safety
If the screening data suggested the presence of a moderate to severe alcohol dependence and/or a current severe depressive episode, patients received a message on the tablet. It said that their answers indicate they may have risky alcohol consumption and/or a depressive disorder, and if they wish professional advice, they may approach their treating physician. Study assistants further provided a list with contact addresses for counselling and treatment in a closed letter.
2.5. Quality assurance
All proof of concept trials have been registered within the German Clinical Trial Register (DRKS). An audit was conducted by the Institute for Community Medicine at the university medicine Greifswald, which was not involved in the conduction of the trials described. In that audit, completeness and consistence of the study documents, as well as the electronic data collection, data storage system, and data privacy were proven. All study assistants were trained prior to the start of data collection (e.g., collecting informed consent, documentation and data saving). On all recruitment sites, consistent study documents and standard operating procedures were used, for example, to collect informed consent or for documentation. During data collection, joint team supervisions were conducted via telephone conferences.
2.6. Screening measures
Data from the screening procedure (including number of people approached, excluded, and refused screenings) were collected using paper‐based forms and then transferred to computer‐based storage.
2.6.1. Socio‐demographics
Data on age, gender, relationship status, school education, and occupational status were assessed. Based on the German school system, years of schooling were categorized as less than 10, 10, and more than 10 years. Occupational status was categorized as full‐time (at least 35 hr per week) or part‐time employed (15–34 hr per week), unemployed, and not‐working (homemaker, retiree, student, or similar).
2.6.2. Alcohol consumption
The Alcohol Use Disorder Identification Test (AUDIT; Saunders, Aasland, Babor, de la Fuente, & Grant, 1993) was used to assess alcohol consumption patterns (Items 1 to 3) and negative consequences of alcohol consumption (Items 4 to 10). We modified the original AUDIT version as follows: The first two items (assessing the drinking occasions and number of drinks consumed per occasion in categories) were each followed by an additional item to specify the exact number of drinking occasions and drinks, respectively. Further, the third item (assessing binge drinking frequency) was assessed for men and woman separately: “How often do you drink five or more alcoholic drinks on one occasion” and “How often do you drink four or more alcoholic drinks on one occasion.” An alcoholic drink was defined as 0.25–0.3 L beer, 0.1–1.15 L (sparkling) wine, or 0.04 L spirits. Alcohol consumption in grams per day was calculated using a quantity‐frequency index on the basis of the exact number of drinking occasions and drinks. Harmful alcohol consumption was defined as an average daily alcohol use exceeding 24 g of alcohol for men and 12 g of alcohol for woman, and/or binge drinking defined as drinking five or more alcoholic drinks for men and four or more alcoholic drinks for women per occasion at least once a month (National Institute of Alcohol Abuse and Alcoholism, 2012). A moderate to severe alcohol dependence was assumed at an AUDIT score ≥ 20 (Donovan, Kivlahan, Doyle, Longabaugh, & Greenfield, 2006).
2.6.3. Tobacco consumption
Smoking status was assessed with the question “Do you currently smoke?” with four response options “Yes, I smoke everyday”; “Yes, I smoke occasionally”; “No, I quit smoking”; and “No, I never smoked”. The number of cigarettes usually smoked per day and the number of years individuals have been smoking regularly were assessed. Further, the first item of the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991; German version from Schumann, Rumpf, Meyer, Hapke, & John, 2010) assessing the time to the first cigarette after awaking (0 “After one hour”, 1 “Within one hour”, 2 “Within half an hour”, and 3 “Within five minutes”) was used as a measure of nicotine dependence (Baker et al., 2007).
2.6.4. Depressive symptoms
Three questions derived from the adapted German version of the DSM‐IV Composite International Diagnostic Interview (Wittchen et al., 1995) were used to screen for depressive symptoms: “In the past 12 months, have you had a period of two weeks or longer where you … “ (a) “felt sad, despondent, or depressed almost daily for most of the time?”, (b) “felt constantly tired, drawn, or exhausted, even when you had not been doing hard work or have been physically ill?”, and (c) “lost interest in most things? This means hobbies, leisure, and being together with friends for example, that is things you usually enjoy?“
Participants endorsing any of the three questions were presented the Patient Health Questionnaire (PHQ‐8) to assess symptoms of depression during patients' most severe episode in the past 12 months (Spitzer, Kroenke, & Williams, 1999). The PHQ‐8 assesses eight of the nine diagnostic criteria for major depression according to DSM‐IV: depressed mood, anhedonia, significant change in weight or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or guilt, and diminished ability to think or concentrate. The criterion regarding thoughts of death and suicidality is not assessed. The German translation of the items was retrieved from Löwe, Spitzer, Zipfel, and Herzog (2002). Items were assessed on a 4‐point Likert scale (0 “Not at all”, 1 “On several days”, 2 “More than half of the days”, 3 “Nearly every day”).
Subsyndromal depression was defined as experiencing at least two of the eight depressive symptoms including at least one cardinal symptom (depressed mood or anhedonia) on more than half of the days. Major depression was defined as experiencing at least five of the eight depressive symptoms including at least one cardinal symptom on more than half of the days (Kroenke et al., 2009). Further, a PHQ‐8 sum score was calculated.
2.6.5. Fruit and vegetable intake
Fruit and vegetable consumption was assessed by four questions of the World Health Organizations' STEPS instrument (WHO, n.d.): Patients were asked (a) “In a typical week, on how many days do you eat fruits/vegetables?” and (b) “How many servings of fruit/vegetables do you eat on one of those days?”. Questions were supplemented by an explanation of a serving (“A serving is, for example, an apple, one small bowl of salad, or a hand of vegetables (except potatoes). More than 0.2 L of fruit or vegetable juice counts as one serving”. The intake of less than two servings of fruit or less than three servings of vegetables are assumed to be insufficient (Wang et al., 2014).
2.6.6. Physical activity
Physical activity was assessed using the Godin and Shephard leisure‐time physical activity questionnaire (Godin, 2011). The English items were translated into German by translation and back‐translation. Prior to the presentation of the questionnaire items, the intensity levels assessed (light, moderate, and vigorous) were introduced based on relative and absolute intensity (heart rate, breathing, and example activities). Activity examples from the original questionnaire were adapted (e.g., alpine skiing and snow‐mobiling were dropped as these are uncommon in most German regions). Further, questionnaire items were supplemented by additional items assessing the usual amount of time per exercise in categories (less than 20 min, 21–30 min, 31–40 min, and so on, up to 171–180 min).
The number of exercises performed was multiplied by the usual amount of time per exercise (mean of the categories were used here,e.g., 35.5 for category 31–40) to calculate physical activity in minutes per week for each intensity level. Individuals were categorized as physically inactive if they performed less than 150 min of moderate‐to‐vigorous physical activity and less than 75 min of vigorous physical activity, or an equivalent combination, for example, 100 min of moderate and 25 min of vigorous activity (WHO, 2010).
2.6.7. Body mass index (BMI)
BMI was calculated by dividing self‐reported body weight in kilograms by self‐reported height in meters squared. Overweight and obesity were defined in accordance with the WHO (2018) as a BMI ≥ 25 and BMI ≥ 30, respectively.
2.6.8. Total number of health risks
An index ranging from zero to six was created for the number of health risks participants reported: harmful alcohol consumption, smoking (at least occasionally), at least subsyndromal depressive symptoms, insufficient fruit and vegetable consumption, physical inactivity, and overweight.
2.6.9. Self‐rated general health
Self‐rated general health was assessed with one item “In general, would you say your health is …” (McHorney, Ware, & Raczek, 1993) answered on a Likert scale ranging from 1 to 5 (excellent, very good, good, fair, and poor). Item‐scale values were recalibrated according to Ware, Snow, Kosinski, and Gandek (1993) resulting in scale values of 5.0 (excellent), 4.4 (very good), 3.4 (good), 2.0 (fair), and 1.0 (poor).
2.6.10. E‐mail and mobile phone use
To assess reachability via e‐mail and mobile phone two questions with a yes or no answer format were used: “Are you reachable via an e‐mail address at least once a week?” and “Do you use a mobile phone?”
3. ANALYSIS
First, the flow of approached patients was described: Numbers and percentages of individuals approached, fulfilling inclusion, and exclusion criteria, as well as participation rates were reported on screening and study level. Participation rate was defined as the proportion of eligibles that agreed to participate in the screening or one of the studies, respectively.
Second, descriptive statistics (means, standard deviations, and percentages) were used to describe screening participants' characteristics, that is, socio‐demographics and health risk factors. T‐tests and Chi‐squared tests (for continuous and categorical variables, respectively) were then used to explore differences by setting. Because of its highly skewed distribution, alcohol intake (grams per day) was described based on median and interquartile range, and the Wilcoxon rank‐sum test was used for comparison across settings.
Third, to identify participation factors within eligible patients, we compared participants to nonparticipants using logistic regression analyses. Screening participation factors were examined based on age and gender. Study participation factors were examined on the basis of all participants' characteristics, that is, socio‐demographics and health risk factors described above. On the study level, some characteristics assessed did not vary between participants and nonparticipants because of defined inclusion and exclusion criteria (e.g., all eligible patients for Study 3 report harmful alcohol consumption). Therefore, regression analysis for those variables was omitted. The statistical software Stata 14.2 (StataCorp 2015, College Station, Texas, USA) was used for analyses.
4. RESULTS
4.1. Screening participation rates
The flow of approached ambulatory care and hospital care patients is presented in Tables 2 and 3, respectively. A total of 56.3% of the 15,486 approached ambulatory care patients and 58.2% of the 12,366 hospital care patients were eligible for the computerized screening (see Tables 2 and 3 for inclusion and exclusion criteria). Screening participation varied across settings with lower participation rates in ambulatory care patients (83.1%; site variation 77.2–88.2%) compared with hospital care patients (90.6%; site variation 83.7–95.6%).
Table 2.
Inclusion criteria and participation rate for the screening of ambulatory care patients
| Ambulatory care patients | N (%) |
|---|---|
| Registered consultations a , b | 17,111 (100) |
| Thereof: | |
| Already approached | 1,625 (9.5) |
| Ambulatory care patients | 15,486 (90.5) |
| Thereof: | |
| Not eligible for screening | 6,763 (43.7) |
| Thereof: | |
| No contact to physician | 1,159 (17.1) |
| Not approached | 147 (2.2) |
| Not willing to reveal age | 394 (5.8) |
| Age < 18 or > 64 yearsc | 3,882 (57.4) |
| Too ill | 350 (5.2) |
| Insufficient language skills | 595 (8.8) |
| Sensory deficits | 89 (1.3) |
| Cognitive impairment | 108 (1.6) |
| Already recruited in hospital | 38 (0.6) |
| Project staff | 1 (0.01) |
| Eligible for screening | 8,723 (56.3) |
| Thereof: | |
| Refused screening participation | 1,478 (16.9) |
| Screening conducted | 7,245 (83.1) |
| Thereof: | |
| Data loss due to technical problem | 93 (1.3) |
| Screening incomplete | 491 (6.8) |
| Screening complete | 6,661 (91.9) |
Including 6,624 patients from site 1, 4,729 patients from Site 2 and 5,758 patients from Site 3.
Including 13 consultations that were not documented on the screening list but for whom.
One practitioner in Site 1 did not allow to ask the patients for their specific age, therefore patients in this practice were only asked if they were in the age range of 18 to 64; however, “age < 18 or > 64 years” was coded if that was denied.
Table 3.
Inclusion criteria and participation rate for the screening of hospital care patients
| Hospital care patients | N (%) |
|---|---|
| Registered hospital care patients between 18 and 64 years a , b | 15,432 (100) |
| Thereof: | |
| Already approached c | 3,066 (19.9) |
| Hospital care patients | 12,366 (80.1) |
| Thereof: | |
| Not eligible for screening | 5,170 (41.8) |
| Thereof: | |
| Never admitted | 262 (5.1) |
| Discharged or relocated to other ward | 1,890 (36.6) |
| Isolated (e.g., due to infection) | 284 (5.5) |
| Cognitive impairment | 121 (2.3) |
| Not reached | 1,039 (20.1) |
| Deceased | 1 (0.02) |
| Too ill | 955 (18.5) |
| Insufficient language skills | 530 (10.3) |
| Sensory deficits | 75 (1.5) |
| Already recruited in general practices | 12 (0.2) |
| Project staff | 1 (0.02) |
| Eligible for screening | 7,196 (58.2) |
| Thereof: | |
| Refused screening participation | 678 (9.4) |
| Screening conducted | 6,518 (90.6) |
| Thereof: | |
| Data loss due to technical problem | 47 (0.7) |
| Screening incomplete | 304 (4.7) |
| Screening complete | 6,167 (94.6) |
Including 5,538 patients from Site 1, 5,104 patients from Site 2 and 4,790 patients from Site 3.
In Sites 1 and 3 a list of new admissions between 18 and 64 years was generated automatically based on the hospital admission system; in Site 2 room plans were used.
Including n = 2,599 readmitted patients from Site 1 that were identified based on their patient id and excluded from recruitment.
4.2. Study participation rates
Of all individuals with full screening data (n = 12,828), 34.0% were eligible for one of the five studies (Figure 1). A total of 2,919 patients were approached for study participation, with study participation rates ranging from 35.2% to 50.8%.
Figure 1.
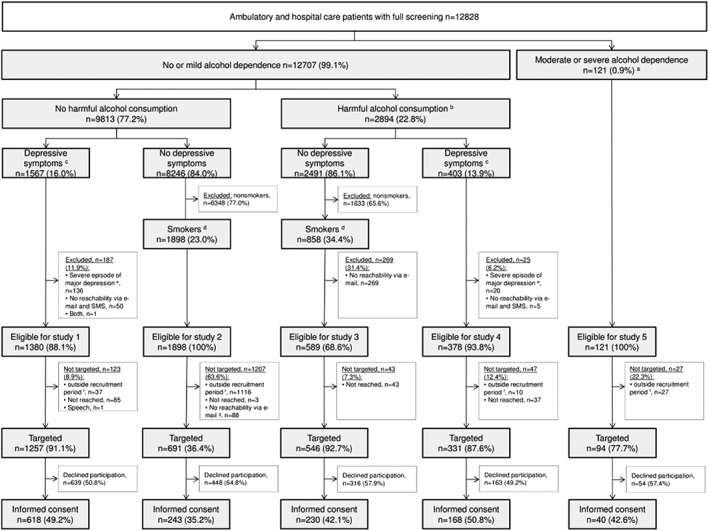
Participation flow. SMS: Short messenger service; reported percentages are fractions of the number in the next higher box.
a Sum score of Alcohol Use Disorder Identification Test ≥ 20.
b Average daily alcohol use exceeding 12 or 24 g for women or men respectively and/or binge drinking behavior (drinking five or more alcoholic drinks for men and four or more alcoholic drinks for women per occasion) at least once a month.
c Experiencing at least one cardinal symptom (depressed mood or anhedonia) as well as one additional depressive symptom on more than half of the days in the most severe episode in the past 12 months.
d Daily smoking of at least one cigarette per day.
e Individuals were excluded if the most severe episode in the last 12 months reached a Patient Health Questionnaire sum score ≥ 20 and if depressive symptoms of any severity were still present within the last two weeks.
f Whereas recruitment started in January 2017 for Studies 1, 3, 4, and 5, recruitment for Study 2 did not start until October 2017; further, due to awaiting of ethical approval, start of recruitment in Site 2 was delayed for Studies 1 and 4 (4 weeks delay), as well as for Study 5 (10 months delay).
g Due to a technical problem, for Study 2 the exclusion criterion reachability via e‐mail was not included in the computerized scoring algorithm used on the tablet. Instead, study assistants asked those within recruitment period and being eligible based on the scoring algorithm for reachability via e‐mail after screening completion.
4.3. Characteristics of screening participants
Characteristics of screening participants significantly differed across settings (Table 4). Ambulatory care patients were younger, fewer were married or in partnership, more were female, higher educated, and more were employed compared with hospital care patients.
Table 4.
Characteristics of screening participants
| Screening participant characteristic | Total (n = 12,828) | Ambulatory care patients (n = 6,661) | Hospital care patients (n = 6,167) | p |
|---|---|---|---|---|
| Socio‐demographics and general health status | ||||
| Age in years, M (SD) | 41.7 (14.1) | 37.8 (13.5) | 45.9 (13.5) | <0.001 |
| Gender: female, n (%) | 6,766 (52.7) | 3,930 (59.0) | 2,836 (46.0) | <0.001 |
| Married or in partnership, n (%) | 9,198 (71.7) | 4,683 (70.3) | 4,515 (73.2) | <0.001 |
| Educational level, n (%) | <0.001 | |||
| <10 years of schooling | 2,451 (19.1) | 975 (14.6) | 1,476 (23.9) | |
| 10 years of schooling | 4,675 (36.4) | 2,139 (32.1) | 2,536 (41.1) | |
| >10 years of schooling | 5,448 (42.5) | 3,416 (51.3) | 2,032 (32.9) | |
| Not classifiable | 254 (2.0) | 131 (2.0) | 123 (2.0) | |
| Occupational status a, n (%) | <0.001 | |||
| Fulltime employed | 6,785 (52.9) | 3,529 (53.0) | 3,256 (52.8) | |
| Part‐time employed | 2,272 (17.7) | 1,299 (19.5) | 973 (15.8) | |
| Unemployed | 887 (6.9) | 408 (6.1) | 479 (7.8) | |
| Not working b | 2,883 (22.5) | 1,424 (21.4) | 1,459 (23.7) | |
| General health status, M (SD) a , c | 3.2 (1.0) | 3.3 (0.9) | 3.1 (1.0) | <0.001 |
| Health risk factors | ||||
| Alcohol consumption | ||||
| Grams per day, Mdn (IQR) | 1.1 (0.4–3.4) | 1.1 (0.4–3.4) | 0.8 (0.4–3.4) | <0.001d |
| AUDIT sum score, M (SD) | 3.3 (3.9) | 3.4 (3.9) | 3.2 (4.0) | <0.001 |
| Harmful consumption e, n (%) | 3,011 (23.5) | 1,580 (23.7) | 1,431 (23.2) | 0.491 |
| Tobacco consumption | ||||
| Any smoking f, n (%) | 4,565 (35.6) | 2,352 (35.3) | 2,213 (35.9) | 0.497 |
| Never smoker, n (%) | 4810 (37.5) | 2,709 (40.7) | 2,101 (34.1) | <0.001 |
| Former smoker, n (%) | 3,453 (26.9) | 1,600 (24.0) | 1,853 (30.0) | |
| Occasionally, n (%) | 1,088 (8.5) | 626 (9.4) | 462 (7.5) | |
| Daily, n (%) | 3,477 (27.1) | 1,726 (25.9) | 1,751 (28.4) | |
| Cigarettes per day g, M (SD) | 15.8 (11.7) | 15.4 (12.0) | 16.1 (11.3) | 0.075 |
| Years of smoking g, M (SD) | 20.9 (12.5) | 17.8 (11.5) | 24.0 (12.7) | <0.001 |
| Nicotine dependence g , h, M (SD) | 1.4 (1.0) | 1.5 (1.1) | 1.3 (1.0) | < 0.001 |
| Depressive symptoms | ||||
| Subsyndromal depression, n (%) | 2,020 (15.7) | 1,258 (18.9) | 762 (12.4) | <0.001 |
| Major depression, n (%) | 1,098 (8.6) | 694 (10.4) | 404 (6.6) | <0.001 |
| PHQ i, M (SD) | 8.2 (5.1) | 8.5 (5.2) | 7.7 (4.8) | <0.001 |
| Insufficient fruit and vegetable consumption, n (%) | 12,060 (94.0) | 6,221 (93.4) | 5,839 (94.7) | <0.005 |
| Physical inactivity, n (%) | 4,639 (36.2) | 2,201 (33.0) | 2,438 (39.5) | <0.001 |
| BMI | <0.001 | |||
| <25, n (%) | 6,151 (47.9) | 3,559 (53.4) | 2,592 (42.0) | |
| ≥ 25 and < 30, n (%) | 3,937 (30.7) | 1,931 (29.0) | 2,006 (32.5) | |
| ≥ 30, n (%) | 2,740 (21.4) | 1,171 (17.6) | 1,569 (25.4) | |
| Total number of health risks, M (SD) | 2.6 (1.1) | 2.5 (1.1) | 2.6 (1.1) | <0.001 |
Note. M: Mean; SD: standard deviation; Mdn: Median; IQR: interquartile range; AUDIT: sum score of Alcohol Use Disorder Identification Test; PHQ‐8: sum score of patient health questionnaire; BMI: body mass index.
Based on n = 12,827 observations due to data loss during saving process.
Homemaker, retiree, student, or similar.
Coded from five (excellent) to one (poor).
Wilcoxon rank‐sum test.
Average daily alcohol use exceeding 12 or 24 g for women or men respectively and/or binge drinking behavior (drinking five or more alcoholic drinks for men and four or more alcoholic drinks for women per occasion) at least once a month.
Occasionally or daily smoking.
Of those who reported smoking at least one cigarette daily: n = 3,446; years of smoking contains two missings due to inconsistent answers: n = 3,444.
Coded from zero (low dependence) to three (high dependence).
Of those who had a period of at least 2 weeks in the last 12 months with sadness, tiredness and/or loss of interest: n = 7,375.
Ambulatory care patients also reported a significantly better general health and fewer health risk factors compared with hospital care patients including lower proportions of insufficient fruit and vegetable consumption, physical inactivity, overweight, and obesity. However, proportions of individuals reporting subsyndromal or major depression, as well as PHQ‐8 sum scores were higher in ambulatory care patients.
Over both settings, proportions of individuals with 0, 1, 2, 3, 4, 5, or 6 health risk factors were 1.7%, 15.4%, 31.8%, 31.1%, 15.7%, 4.0%, and 0.4%, respectively. The most prevalent health risk factors were insufficient fruit and vegetable consumption (94.0%) and being overweight or obese (52.1%). These two were also the most prevalent single risk combination with 13.5% in the total sample, followed by insufficient fruit and vegetable consumption combined with overweight and physical inactivity (8.5%).
4.4. Screening participation factors
Associations of age and gender with willingness to participate were analyzed based on ambulatory care patients (n = 8,723) and hospital care patients (n = 7,196) eligible for screening. However, in some cases, data were missing because of incomplete documentation and lack of permission to ask for exact age in one ambulatory practice. Younger age predicted screening participation in ambulatory care patients (Odds Ratio, OR = 0.97, 95% CI [0.97–0.98], n = 8,615) and hospital care patients (OR = 0.96, 95% CI [0.96–0.97], n = 7,186). Being female predicted screening participation only within ambulatory care patients (OR = 1.47, 95% CI [1.32–1.65], n = 8,709); within hospital care patients screening participation did not differ with respect to gender (OR = 1.02, 95% CI [0.87–1.20], n = 7,193).
4.5. Study participation factors
Recruitment site and setting, as well as socio‐demographics had significant effects on study participation. However, study participation factors largely differed between the studies (Table 5). The total number of health risk factors did not predict willingness to participate in any study. However, the presence of single health risk factors was associated with lower participation including physical inactivity in Study 5 and overweight in Study 3. Further, a higher severity level of depressive symptoms measured by the PHQ‐8 predicted higher participation in Studies 1 and 3. For Study 4 there were no differences between study participants and nonparticipants with respect to any of the assessed variables.
Table 5.
Study participation factors by study
| Participation factor | Odds ratio, 95% confidence interval | ||||
|---|---|---|---|---|---|
| Study 1 n = 1,257 | Study 2 n = 691 | Study 3 n = 546 | Study 4 n = 331 | Study 5 n = 94 | |
| Recruitment site and setting | |||||
| Recruitment site | |||||
| Site 1 | Reference | Reference | Reference | Reference | Reference |
| Site 2 | 0.68 (0.52–0.89)** | 1.11 (0.71–1.74) | 1.33 (0.86–2.04) | 0.79 (0.46–1.38) | 1.01 (0.32–3.20) |
| Site 3 | 0.93 (0.71–1.22) | 1.18 (0.82–1.69) | 0.64 (0.42–0.95)* | 1.14 (0.65–1.98) | 1.16 (0.47–2.87) |
| Setting: Ambulatory care patients | 1.07 (0.86–1.34) | 1.53 (1.08–2.17)* | 0.82 (0.58–1.15) | 1.08 (0.68–1.70) | 0.29 (0.12–0.70)** |
| Socio‐demographics and general health status | |||||
| Age in years | 0.99 (0.99–1.00) | 1.00 (0.98–1.01) | 0.99 (0.98–1.00) | 0.99 (0.97–1.00) | 1.00 (0.97–1.03) |
| Gender: female | 1.07 (0.85–1.34) | 0.88 (0.64–1.20) | 1.23 (0.86–1.77) | 1.22 (0.80–1.89) | 2.77 (1.02–7.53)* |
| Married or in partnership | 1.04 (0.82–1.31) | 0.89 (0.63–1.26) | 0.95 (0.66–1.36) | 0.82 (0.53–1.27) | 0.72 (0.31–1.64) |
| Educational level | |||||
| <10 years of schooling | Reference | Reference | Reference | Reference | Reference |
| 10 years of schooling | 1.01 (0.74–1.38) | 1.07 (0.73–1.56) | 1.11 (0.71–1.72) | 0.99 (0.50–1.94) | 1.46 (0.55–3.89) |
| >10 years of schooling | 1.09 (0.81–1.47) | 1.12 (0.74–1.71) | 1.71 (1.08–2.71)* | 1.11 (0.58–2.09) | 1.77 (0.63–4.94) |
| Not classifiable | 1.57 (0.65–3.81) | 0.71(0.22–2.30) | 0.98 (0.27–3.51) | ‐ | ‐ |
| Occupational status | |||||
| Fulltime employed | Reference | Reference | Reference | Reference | Reference |
| Part‐time employed | 0.59 (0.43–0.81)*** | 0.94 (0.61–1.43) | 0.98 (0.58–1.63) | 1.16 (0.63–2.12) | 0.48 (0.05–4.81) |
| Unemployed | 0.76 (0.52–1.11) | 1.46 (0.83–2.57) | 1.52 (0.79–2.95) | 2.19 (0.96–4.99) | 0.83 (0.29–2.35) |
| Not working a | 0.84 (0.65–1.10) | 1.28 (0.80–2.06) | 1.19 (0.72–1.98) | 0.93 (0.55–1.55) | 5.73 (1.76–18.59)** |
| General health status b | 0.93 (0.84–1.03) | 1.06 (0.88–1.27) | 1.06 (0.88–1.28) | 1.23 (0.99–1.52) | 0.95 (0.64–1.39) |
| Health risks factors | |||||
| Alcohol consumption | |||||
| Grams per day | 1.00 (0.95–1.04) | 0.99 (0.94–1.04) | 1.00 (0.99–1.01) | 0.98 (0.96–1.00) | 1.00 (0.99–1.01) |
| AUDIT sum score | 1.02 (0.97–1.07) | 1.05 (0.98–1.12) | 1.00 (0.95–1.04) | 1.02 (0.97–1.07) | 0.97 (0.89–1.06) |
| Harmful consumption c | omitted | omitted | omitted | omitted | omitted |
| Tobacco consumption | |||||
| Any smoking d | 0.98 (0.78–1.24) | omitted | omitted | 0.81 (0.52–1.25) | 0.92 (0.37–2.32) |
| Cigarettes per day e | 0.99 (0.97–1.01) | 1.02 (1.00–1.03) | 0.98 (0.96–1.00) | 1.00 (0.99–1.02) | 0.98 (0.44–1.01) |
| Years of smoking e | 0.99 (0.97–1.01) | 1.00 (0.99–1.02) | 1.00 (0.99–1.01) | 0.99 (0.97–1.02) | 0.99 (0.94–1.04) |
| Nicotine dependence e , f | 0.99 (0.81–1.21) | 1.10 (0.95–1.29) | 0.87 (0.74–1.02) | 1.22 (0.86–1.73) | 0.87 (0.51–1.48) |
| Depressive symptoms | |||||
| Subsyndromal depression | omitted | omitted | omitted | omitted | 0.97 (0.42–2.23) |
| Major depression | 1.36 (1.09–1.69)** | omitted | omitted | 1.27 (0.82–1.96) | 0.99 (0.40–2.46) |
| PHQ‐8 g | 1.05 (1.02–1.08)*** | 1.0 (0.93–1.07) | 1.10 (1.01–1.20)* | 1.04 (0.98–1.10) | 1.05 (0.97–1.13) |
| Insufficient fruit and vegetable consumption | 0.75 (0.47–1.20) | 0.69 (0.25–1.88) | 0.85 (0.28–2.55) | 1.12 (0.51–2.46) | 3.12 (0.34–29.04) |
| Physical inactivity | 0.87 (0.70–1.09) | 0.81 (0.59–1.11) | 0.83 (0.57–1.19) | 0.98 (0.63–1.53) | 0.27 (0.11–0.69)** |
| BMI | |||||
| <25 | Reference | Reference | Reference | Reference | Reference |
| ≥25 and < 30 | 1.01(0.77–1.31) | 0.73 (0.51–1.04) | 0.86 (0.59–1.27) | 0.87 (0.51–1.47) | 0.42 (0.15–1.14) |
| ≥30 | 1.08 (0.82–1.43) | 1.13 (0.75–1.70) | 0.57 (0.35–0.92)* | 1.00 (0.56–1.79) | 0.38 (0.13–1.15) |
| Total number of health risks | 0.95 (0.85–1.07) | 0.83 (0.67–1.03) | 0.80 (0.63–1.01) | 0.93 (0.75–1.16) | 0.69 (0.46–1.03) |
Note. AUDIT: sum score of Alcohol Use Disorder Identification Test; PHQ‐8: sum score of patient health questionnaire; BMI: body mass index.
p < 0.001.
p < 0.01.
p < 0.05.
Homemaker, retiree, student, or similar.
Coded from five (excellent) to one (poor).
Average daily alcohol use exceeding 12 or 24 g for women or men respectively and/or binge drinking behavior (drinking five or more alcoholic drinks for men and four or more alcoholic drinks for women per occasion) at least once a month.
Occasionally or daily smoking.
Of those who reported smoking at least one cigarette daily: n = 341, 691, 546, 129, and 57 for the Studies 1 to 5 respectively.
Coded from zero (low dependence) to three (high dependence).
Of those who had a period of at least 2 weeks in the last 12 months with sadness, tiredness, and/or lost interest: n = 1,257, 402, 278, 331, and 77 for the Studies 1 to 5 respectively.
5. DISCUSSION
This paper aimed at describing methodological details of a multipurpose health risk screening in different health care settings. Strengths of the present study include the systematic and proactive screening approach in a multicenter sample. The screening approach was found feasible and efficient with high screening participation rates and over 30% of screened patients being eligible for one of the subsequent studies.
The second aim of the present study was to analyze reach of participants. Analyses of reach are essential to evaluate the potential public health impact (Glasgow, Vogt, & Boles, 1999) as sample composition and intervention effectiveness may vary by recruitment strategy (Boshuizen, Viet, Picavet, Botterweck, & van Loon, 2006; Winhusen, Winstanley, Somoza, & Brigham, 2012). So far, there have been only few studies examining reach of proactive strategies in health care settings (e.g., Freyer‐Adam et al., 2016; Guertler et al., 2017; Krist et al., 2014 ; Meyer et al., 2008). The present study allows comparison of reach across different recruitment settings and target populations.
5.1. Screening level
It is necessary to reach patients (individual level) and clinicians (organizational level) for participation in prevention efforts (Glasgow et al., 1999). In the present study, the proportion of hospital departments that were willing to conduct screening (adoption) was high. However, adoption within ambulatory practices was lower than in previous research reporting adoption rates of 60–87% (Guertler et al., 2017; Krist et al., 2014; Meyer et al., 2008). Beyond potential barriers identified by previous research (Bower et al., 2009; Hummers‐Pradier et al., 2008), one main reason for lower adoption was the low level of proactivity when contacting most ambulatory practices in Site 2 (i.e., sending a letter and waiting for answer). This stresses the need for highly proactive approaches when recruiting clinicians (e.g., telephone or in person contact). Further, some practices in Site 3 were not selected for recruitment (even though they may have agreed to) because of high proportions of patients >64 years or low patient flow in general. However, due to lack of documentation, these practices were handled as if they had declined participation.
On the patient level, more than 83% of eligible ambulatory care patients and 90% of hospital care patients could be reached through the proactive screening. These participation rates are comparable with the previous studies in health care settings in Germany using proactive screening approaches (Freyer‐Adam et al., 2016; Meyer et al., 2008). However, length of screening may also have affected participation rates as the screening used in Meyer et al. (2008) consisted only of one question to identify smoking individuals in ambulatory practices, which resulted in participation of nearly all patients (99.6%).
Regarding the characteristics of the patients reached, hospital care patients reported lower socioeconomic status and general health as well as more health risk factors compared with ambulatory care patients. However, prevalence of depressive symptoms was highest in ambulatory practices, underlining the potential of this setting to improve patients' care for mental health. In total, 98% of all participants reported at least one health risk factor; thus, nearly all patients could have been approached for an intervention aiming at one or more health risk factors. Further, 83% of patients reported at least two and 51% at least three health risk factors, confirming that the majority of adult population has multiple health risk factors (John et al., 2018). In line with previous research (John et al., 2018), nutrition and energy balance associated factors (fruit and vegetable consumption, overweight, and physical inactivity) were the most prevalent health risk cluster. Although selection bias may be reduced within proactive screening procedures (Velicer et al., 2000), we were not able to reach males at the same rate as females in ambulatory practices. This result is in line with previous research showing that females are more likely to be interested in health, health information seeking, and participation in scientific studies than men (Ek, 2015; Galea & Tracy, 2007). Younger age predicted participation in both settings. However, literature about the effect of age on participation is less consistent (Galea & Tracy, 2007). Further, it cannot be precluded that other factors like socioeconomic status (Bender, Jorgensen, Helbech, Linneberg, & Pisinger, 2014) and health behaviors (Schneider, Schulz, Pouwels, de Vries, & van Osch, 2013) have influenced screening participation.
5.2. Study level
So far, only few trials in health care settings addressed multiple health risk factors in a single intervention (e.g., Goldstein, Whitlock, & DePue, 2004) and it is unclear whether multifocus interventions may differ in reach compared with single focused interventions. This is of particular interest as the number of health risks is linearly associated with mortality risk (Ford, Bergmann, Boeing, Li, & Capewell, 2012). We found no evidence that targeting two health risks lowers intervention participation compared with targeting only one risk. Instead, targeting smoking in conjunction with harmful alcohol consumption resulted in higher participation rates compared with targeting smoking only. As different health risk combinations may pose different recruitment challenges, analyzes of reach regarding other risk combinations would be of interest, for example, depressive symptoms in combination with smoking.
In general, our proactive approach led to relatively high proportions of patients willing to participate in the studies in comparison with reactive approaches (Velicer et al., 2000). However, intervention participation rates were lower compared with other studies using proactive recruitment in health care settings that resulted in participation rates over 80% (Freyer‐Adam et al., 2016; Meyer et al., 2008). Differences in participation rates may be a result of progressing proliferation of research studies during the last years (Galea & Tracy, 2007). Another likely explanation relates to the complexity of the recruitment procedure for multiple studies that may lead to an overload for the study assistants when offering study participation. A detailed examination of reasons why individuals choose not to participate could further inform efforts to increase reach.
Results on participation factors on study level reflect known biases including that males and those with low education are harder to reach for prevention efforts (Galea & Tracy, 2007; Stanczyk et al., 2014). Recruitment site and setting did also affect participation. Participation rates were most favorable when recruitment took place in the coordinating study site. This might be the result of a higher identification of potential study participants with one's home region and a higher motivation of the study team for engaging patients in their “own” study. Whereas smokers were reached better in ambulatory practices, individuals with at least moderate alcohol dependence were reached better in general hospitals. Overall, participation factors clearly differed between the studies. A large part of these differences are probably because of the different characteristics of patients eligible for each study. Further, the type of study (intervention and interview) may also have affected willingness to participate.
As little is known about how to address multiple health risks in an efficient manner (Meader et al., 2017; J. J. Prochaska & Prochaska, 2011) more research is needed on intervention strategies for patients presenting multiple health risks. Further, it is known that tailoring intervention content to the needs of participants can enhance intervention impact (Kreuter, Strecher, & Glassman, 1999). It has to be examined whether offering of study participation may also need to be framed differently, for example, depending on risk factor combination, socioeconomic status, and motivation to change in order to increase participation rates and to reduce selection bias. Lastly, multifocus interventions may have the potential to be framed in a less‐stigmatizing way compared with single‐focus interventions, for example, aiming at a healthy lifestyle in general instead at a single undesired and stigmatized behavior, for example, alcohol use (Barney, Griffiths, Jorm, & Christensen, 2006).
6. LIMITATIONS
First, assessment of participants' characteristics within the screening was solely based on self‐report, therefore social desirability bias cannot be ruled out. However, objective measurements were not feasible, for example, no extra room for height and weight measurements.
Second, the results on study participation only refer to the initial dropout of patients. It would be important to analyze factors related to retention and differential dropout in intervention arms (Glasgow et al., 2007).
Lastly, being a multicenter study, implementation of screening had to be adjusted to local conditions, which did not allow implementing the exact same screening procedure at all sites. Thus, samples acquired may be biased based on procedures used and may not be fully comparable with each other, for example, those with longer hospital stays and with more ambulatory practice visits may be overrepresented in the sample acquired in Site 2 because of nondaily recruitment. Although single‐center studies have the advantage of more uniform procedures, multicenter studies yield a higher external validity because of the heterogonous study population and implementation (Meinert & Tonascia, 1986).
7. CONCLUSION
This study supports the use of a systematic proactive screening for multiple health risks in different health care settings as it is more resource‐saving than a single focused screening and allows combining different research questions in one screening. However, resources to address critical findings have to be available. Implementing systematic screening into routine care may be fostered by training of practice or hospital staff or by providing additional staff to organize screening and adequate interventions.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests.
ACKNOWLEDGEMENTS
This study is embedded in the research consortium AERIAL (Addiction: Early Recognition and Intervention Across the Lifespan) of the Bundesministerium für Bildung und Forschung (German Federal Ministry of Education and Research, BMBF) within the Research Network on Mental Disorders (Forschungsnetz zu psychischen Erkrankungen), Grant No. 01EE1406F; 01EE1406E; 01EE1406H.
Guertler D, Moehring A, Krause K, et al. Proactive multipurpose health risk screening in health care settings: Methods, design, and reach. Int J Methods Psychiatr Res. 2019;28:e1760 10.1002/mpr.1760
German Clinical Trial Register (DRKS): DRKS0001163; DRKS00011637; DRKS00013057
REFERENCES
- Aspy, C. B. , Mold, J. W. , Thompson, D. M. , Blondell, R. D. , Landers, P. S. , Reilly, K. E. , & Wright‐Eakers, L. (2008). Integrating screening and interventions for unhealthy behaviors into primary care practices. American Journal of Preventive Medicine, 35, S373–S380. 10.1016/j.amepre.2008.08.015 [DOI] [PubMed] [Google Scholar]
- Baker, T. B. , Piper, M. E. , McCarthy, D. E. , Bolt, D. M. , Smith, S. S. , Kim, S. Y. , … Transdisciplinary tobacco use research center (TTURC) tobacco dependence phenotype workgroup (2007). Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research, 9(Suppl 4), S555–S570. 10.1080/14622200701673480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barney, L. J. , Griffiths, K. M. , Jorm, A. F. , & Christensen, H. (2006). Stigma about depression and its impact on help‐seeking intentions. Australian and New Zealand Journal of Psychiatry, 40, 51–54. 10.1111/j.1440-1614.2006.01741.x [DOI] [PubMed] [Google Scholar]
- Bender, A. M. , Jorgensen, T. , Helbech, B. , Linneberg, A. , & Pisinger, C. (2014). Socioeconomic position and participation in baseline and follow‐up visits: The Inter99 study. European Journal of Preventive Cardiology, 21, 899–905. 10.1177/2047487312472076 [DOI] [PubMed] [Google Scholar]
- Boshuizen, H. C. , Viet, A. L. , Picavet, H. S. , Botterweck, A. , & van Loon, A. J. (2006). Non‐response in a survey of cardiovascular risk factors in the Dutch population: Determinants and resulting biases. Public Health, 120, 297–308. 10.1016/j.puhe.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Bower, P. , Wallace, P. , Ward, E. , Graffy, J. , Miller, J. , Delaney, B. , & Kinmonth, A. L. (2009). Improving recruitment to health research in primary care. Family Practice, 26, 391–397. 10.1093/fampra/cmp037 [DOI] [PubMed] [Google Scholar]
- Ding, D. , Do, A. , Schmidt, H. M. , & Bauman, A. E. (2015). A widening gap? Changes in multiple lifestyle risk behaviours by socioeconomic status in New South Wales, Australia, 2002‐2012. PLoS One, 10 10.1371/journal.pone.0135338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, D. M. , Kivlahan, D. R. , Doyle, S. R. , Longabaugh, R. , & Greenfield, S. F. (2006). Concurrent validity of the alcohol use disorders identification test (AUDIT) and AUDIT zones in defining levels of severity among out‐patients with alcohol dependence in the COMBINE study. Addiction, 101, 1696–1704. 10.1111/j.1360-0443.2006.01606.x [DOI] [PubMed] [Google Scholar]
- Ek, S. (2015). Gender differences in health information behaviour: A Finnish population‐based survey. Health Promotion International, 30, 736–745. 10.1093/heapro/dat063 [DOI] [PubMed] [Google Scholar]
- Fine, L. J. , Philogene, G. S. , Gramling, R. , Coups, E. J. , & Sinha, S. (2004). Prevalence of multiple chronic disease risk factors. 2001 National Health Interview Survey. American Journal of Preventive Medicine, 27, 18–24. 10.1016/j.amepre.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Ford, E. S. , Bergmann, M. M. , Boeing, H. , Li, C. Y. , & Capewell, S. (2012). Healthy lifestyle behaviors and all‐cause mortality among adults in the United States. Preventive Medicine, 55, 23–27. 10.1016/j.ypmed.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer‐Adam, J. , Baumann, S. , Haberecht, K. , Tobschall, S. , Schnuerer, I. , Bruss, K. , … Gaertner, B. (2016). In‐person and computer‐based alcohol interventions at general hospitals: Reach and retention. European Journal of Public Health, 26, 844–849. 10.1093/eurpub/ckv238 [DOI] [PubMed] [Google Scholar]
- Galea, S. , & Tracy, M. (2007). Participation rates in epidemiologic studies. Annals of Epidemiology, 17, 643–653. 10.1016/j.annepidem.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Glasgow, R. E. , Nelson, C. C. , Kearney, K. A. , Reid, R. , Ritzwoller, D. P. , Strecher, V. J. , … Wildenhaus, K. (2007). Reach, engagement, and retention in an internet‐based weight loss program in a multi‐site randomized controlled trial. Journal of Medical Internet Research, 9 10.2196/jmir.9.2.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow, R. E. , Vogt, T. M. , & Boles, S. M. (1999). Evaluating the public health impact of health promotion interventions: The RE‐AIM framework. American Journal of Public Health, 89, 1322–1327. 10.2105/Ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin, G. (2011). Commentary—The Godin‐Shepard leisure‐time physical activity questionnaire. Health & Fitness Journal of Canada, 4, 18–22. Retrived from http://new-hfjc.library.ubc.ca/index.php/html/article/viewFile/82/49 [Google Scholar]
- Goldstein, M. G. , Whitlock, E. P. , & DePue, J. (2004). Multiple behavioral risk factor interventions in primary care. Summary of research evidence. American Journal of Preventive Medicine, 27, 61–79. 10.1016/j.amepre.2004.04.023 [DOI] [PubMed] [Google Scholar]
- Guertler, D. , Meyer, C. , Dorr, M. , Braatz, J. , Weymar, F. , John, U. , … Ulbricht, S. (2017). Reach of individuals at risk for cardiovascular disease by proactive recruitment strategies in general practices, job centers, and health insurance. International Journal of Behavioral Medicine, 24, 153–160. 10.1007/s12529-016-9584-5 [DOI] [PubMed] [Google Scholar]
- Heatherton, T. F. , Kozlowski, L. T. , Frecker, R. C. , & Fagerstrom, K. O. (1991). The fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction, 86, 1119–1127. Retrieved from https://pdfs.semanticscholar.org/1174c1118/dd1144c488807e488054a488805ed488711f488806bc488807b488802fbeaea.pdf [DOI] [PubMed] [Google Scholar]
- Hoving, C. , Mudde, A. N. , & de Vries, H. (2007). Effect of recruitment method and setting on the composition of samples consisting of adult smokers. Patient Education and Counseling, 65, 79–86. 10.1016/j.pec.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Hummers‐Pradier, E. , Scheidt‐Nave, C. , Martin, H. , Heinemann, S. , Kochen, M. M. , & Himmel, W. (2008). Simply no time? Barriers to GPs' participation in primary health care research. Family Practice, 25, 105–112. 10.1093/fampra/cmn015 [DOI] [PubMed] [Google Scholar]
- John, U. , Hanke, M. , & Freyer‐Adam, J. (2018). Health risk behavior patterns in a national adult population survey. International Journal of Environmental Research and Public Health, 15 10.3390/ijerph15050873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, U. , Meyer, C. , Rumpf, H. J. , & Hapke, U. (2003). Relation among stage of change, demographic characteristics, smoking history, and nicotine dependence in an adult German population. Preventive Medicine, 37, 368–374. 10.1016/S0091-7435(03)00149-X [DOI] [PubMed] [Google Scholar]
- Kaner, E. F. S. , Beyer, F. R. , Muirhead, C. , Campbell, F. , Pienaar, E. D. , Bertholet, N. , … Burnand, B. (2018). Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database of Systematic Reviews.. 10.1002/14651858.CD004148.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, K. , Guertler, D. , Moehring, A. , Batra, A. , Rumpf, H. J. , Bischof, G. , … Meyer, C. (in preparation). A computer based expert system targeting harzrdous alcohol consumption and depressive symptoms—Results of a pilot study.
- Krebs, P. , Prochaska, J. O. , & Rossi, J. S. (2010). A meta‐analysis of computer‐tailored interventions for health behavior change. Preventive Medicine, 51, 214–221. 10.1016/j.ypmed.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter, M. W. , Strecher, V. J. , & Glassman, B. (1999). One size does not fit all: The case for tailoring print materials. Annals of Behavioral Medicine, 21, 276–283. 10.1007/Bf02895958 [DOI] [PubMed] [Google Scholar]
- Krist, A. H. , Phillips, S. M. , Sabo, R. T. , Balasubramanian, B. A. , Heurtin‐Roberts, S. , Ory, M. G. , … Glasgow, R. E. (2014). Adoption, reach, implementation, and maintenance of a behavioral and mental health assessment in primary care. Annals of Family Medicine, 12, 525–533. 10.1370/afm.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. , Strine, T. W. , Spitzer, R. L. , Williams, J. B. W. , Berry, J. T. , & Mokdad, A. H. (2009). The PHQ‐8 as a measure of current depression in the general population. Journal of Affective Disorders, 114, 163–173. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Löwe, B. , Spitzer, R. L. , Zipfel, S. , & Herzog, W. (2002). PHQ‐D manual Pfizer . Retrieved from https://www.klinikum.uni‐heidelberg.de/fileadmin/Psychosomatische_Klinik/download/PHQ_Manual1.pdf.
- Ludden, G. D. S. , van Rompay, T. J. L. , Kelders, S. M. , & van Gemert‐Pijnen, J. E. W. C. (2015). How to increase reach and adherence of web‐based interventions: A design research viewpoint. Journal of Medical Internet Research, 17 10.2196/jmir.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney, C. A. , Ware, J. E. Jr. , & Raczek, A. E. (1993). The MOS 36‐item short‐form health survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31, 247–263. Retrieved from https://www.jstor.org/stable/3765819?seq=3765811#page_scan_tab_contents [DOI] [PubMed] [Google Scholar]
- Meader, N. , King, K. , Wright, K. , Graham, H. M. , Petticrew, M. , Power, C. , … Sowden, A. J. (2017). Multiple risk behavior interventions: Meta‐analyses of RCTs. American Journal of Preventive Medicine, 53, E19–E30. 10.1016/j.amepre.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Meinert, C. L. , & Tonascia, S. (1986). Single‐center versus multicenter trials In Meinert C. L. (Ed.), Clinical Trials: Design, Conduct and Analysis. Oxford: Oxford University Press; 10.1093/acprof:oso/9780195035681.003.0004 [DOI] [Google Scholar]
- Meyer, C. , Ulbricht, S. , Gross, B. , Kastel, L. , Wittrien, S. , Klein, G. , … John, U. (2012). Adoption, reach and effectiveness of computer‐based, practitioner delivered and combined smoking interventions in general medical practices: A three‐arm cluster randomized trial. Drug and Alcohol Dependence, 121, 124–132. 10.1016/j.drugalcdep.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Meyer, C. , Ulbricht, S. , Schumann, A. , Rüge, J. , Rumpf, H.‐J. , & John, U. (2008). Proactive smoking interventions to foster smoking cessation in the general medical practice. Prävention und Gesundheitsförderung, 3, 25–30. 10.1007/s11553-007-0092-y [DOI] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism . (2012). Drinking levels defined. Retrieved from https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- Ngune, I. , Jiwa, M. , Dadich, A. , Lotriet, J. , & Sriram, D. (2012). Effective recruitment strategies in primary care research: A systematic review. Quality in Primary Care, 20, 115–123. Retrieved from https://pdfs.semanticscholar.org/dc134/107ac135c176a153e587c893a116dafe166ec116d118aa.pdf [PubMed] [Google Scholar]
- Prochaska, J. J. , & Prochaska, J. O. (2011). A review of multiple health behavior change interventions for primary prevention. American Journal of Lifestyle Medicine, 5 10.1177/1559827610391883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska, J. O. (2008). Multiple health behavior research represents the future of preventive medicine. Preventive Medicine, 46(3), 281–285. 10.1016/j.ypmed.2008.01.015 [DOI] [PubMed] [Google Scholar]
- Rosembaun, A. , Rojas, P. , Rodriguez, M. V. , Barticevic, N. , & Rivera Mercado, S. (2018). Brief interventions to promote behavioral change in primary care settings, a review of their effectiveness for smoking, alcohol and physical inactivity. Medwave, 18, e7148 10.5867/medwave.2018.01.7148 [DOI] [PubMed] [Google Scholar]
- Saunders, J. B. , Aasland, O. G. , Babor, T. F. , de la Fuente, J. R. , & Grant, M. (1993). Development of the alcohol use disorder identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction, 88, 791–804. http://www.automesure.com/library/pdf/8329970.pdf [DOI] [PubMed] [Google Scholar]
- Schneider, F. , Schulz, D. N. , Pouwels, L. H. L. , de Vries, H. , & van Osch, L. A. D. M. (2013). The use of a proactive dissemination strategy to optimize reach of an internet‐delivered computer tailored lifestyle intervention. BMC Public Health, 13 10.1186/1471-2458-13-721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit, A. J. , van Loon, A. J. M. , Tijhuis, M. , & Ocke, M. C. (2002). Clustering of lifestyle risk factors in a general adult population. Preventive Medicine, 35, 219–224. 10.1006/pmed.2002.1064 [DOI] [PubMed] [Google Scholar]
- Schumann, A. , Rumpf, H. J. , Meyer, C. , Hapke, U. , & John, U. (2010). German version of the Fagerström‐test for nicotine dependence (FTND‐G) and the heaviness of smoking index (HSI‐G) In Glöckner‐Rist A., & Rist F. (Eds.), Electronic handbook of assessment instruments in substance use (EHES). (Version 4.00). Bonn: GESIS. [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , & Williams, J. B. W. (1999). Validation and utility of a self‐report version of PRIME‐MD: The phq primary care study. Journal of the American Medical Association, 282, 1737–1744. Retrieved from https://www.klinikum.uni-heidelberg.de/fileadmin/medizinische_klinik/Abteilung_1732/pdf/JAMAphq1999.pdf [DOI] [PubMed] [Google Scholar]
- Stanczyk, N. E. , Bolman, C. , Smit, E. S. , Candel, M. J. J. M. , Muris, J. W. M. , & de Vries, H. (2014). How to encourage smokers to participate in web‐based computer‐tailored smoking cessation programs:A comparison of different recruitment strategies. Health Education Research, 29, 23–40. 10.1093/her/cyt104 [DOI] [PubMed] [Google Scholar]
- Velicer, W. F. , Prochaska, J. O. , Fava, J. L. , Rossi, J. S. , Redding, C. A. , Laforge, R. G. , & Robbins, M. L. (2000). Using the transtheoretical model for population‐based approaches to health promotion and disease prevention. Homeostasis in Health and Disease, 40, 174–195. 10.1207/S15324796ABM2502_08 [DOI] [Google Scholar]
- Wang, X. , Ouyang, Y. , Liu, J. , Zhu, M. , Zhao, G. , Bao, W. , & Hu, F. B. (2014). Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose‐response meta‐analysis of prospective cohort studies. BMJ, 349, g4490 10.1136/bmj.g4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. , Snow, K. , Kosinski, M. , & Gandek, B. (1993). SF‐36 health survey manual and interpretation guide. Boston: MA; Retrieved from http://czresearch.com/info/SF36_healthsurvey_ch6.pdf [Google Scholar]
- WHO . (2010). Global recommendations on physical activity for health. Retrieved from http://apps.who.int/iris/bitstream/handle/10665/44399/9789241599979_eng.pdf;jsessionid=DE68057A309C8B10C0F4259D8BEF1DC5?sequence=1 [PubMed]
- WHO . (2018). Fact sheet Obesity and overweight. Retrieved from http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- WHO . (n.d.). The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS) Intrument. Retrieved from http://www.who.int/chp/steps/instrument/STEPS_Instrument_V3.1.pdf?ua=1
- Winhusen, T. , Winstanley, E. L. , Somoza, E. , & Brigham, G. (2012). The potential impact of recruitment method on sample characteristics and treatment outcomes in a psychosocial trial for women with co‐occurring substance use disorder and PTSD. Drug and Alcohol Dependence, 120, 225–228. 10.1016/j.drugalcdep.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen, H. , Beloch, E. , Garczynski, E. , Holly, A. , Lachner, G. , Perkonigg, A. , … Zieglgänsberger, S. (1995). Munich composite international diagnostic interview (M‐CIDI). München: Max‐Planck‐Institut für Psychiatrie. [Google Scholar]
- Xu, W. H. , Zhang, X. L. , Gao, Y. T. , Xiang, Y. B. , Gao, L. F. , Zheng, W. , & Shu, X. O. (2007). Joint effect of cigarette smoking and alcohol consumption on mortality. Preventive Medicine, 45, 313–319. 10.1016/j.ypmed.2007.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]


