Abstract
Objective:
The goal of this study was to optimize robust PID control for propofol anesthesia in children aged 5–10 years to improve performance, particularly to decrease the time of induction of anesthesia while maintaining robustness.
Methods:
We analyzed results of a previous study conducted by our group to identify opportunities for system improvement. Allometric scaling was introduced to reduce the interpatient variability and a new robust PID controller was designed using an optimization based method. We evaluated this optimized design in a clinical study involving 16 new cases.
Results:
The optimized controller design achieved the performance predicted in simulation studies in the design stage. Time of induction of anesthesia was median [Q1, Q3] 3.7 [2.3, 4.1] minutes and the achieved global score was 13.4 [9.9, 16.8].
Conclusion:
Allometric scaling reduces the interpatient variability in this age group, and allows for improved closed-loop performance. The uncertainty described by the model set, the predicted closed-loop responses and the predicted robustness margins are realistic. The system meets the design objectives of improved speed of induction of anesthesia while maintaining robustness, improving clinically relevant system behavior.
Significance:
Control system optimization and ongoing system improvement are essential to the development of a clinically relevant commercial device. This paper demonstrates the validity of our approach, including system modeling, controller optimization and pre-clinical testing in simulation.
Keywords: Anesthesia, Clinical trials, Medical control systems, PID control, Robust control
I. Introduction
Propofol is an intravenous hypnotic agent used for general anesthesia in the operating room. It is commonly co-administered with an analgesic, for example remifentanil, and for certain procedures a muscle relaxant. The effect of propofol on depth of hypnosis (DOH) is characterized by large inter-patient variability, and drug requirements can further change in certain situations, for example as a result of surgical blood loss. While the uncertainty introduced by this inter-patient variability is large, performance requirements are limited; in current clinical practice, drug dosing is controlled manually by the anesthesiologist. Initial dosing is estimated primarily based on patient age and weight, and adjusted based on feedback from clinical signs of DOH (for example changes in heart rate or blood pressure). Closed-loop control can reduce variability, optimize the anesthesiologists workload, improve control of the clinical state and ultimately improve safety and quality of clinical care [1].
Closed-loop anesthesia has been shown to outperform manually controlled drug infusion in terms of time in range of adequate anesthesia [2], [3], and reduce recovery time [3]. Closed-loop intravenous anesthesia has successfully been evaluated in hundreds of cases in clinical trials, e.g. [4], [5], [6], [7], [8]. To obtain regulatory approval for a commercial closed-loop medical device, demonstration of safety of ad-hoc systems will be challenging. Designs that support robustness and safety analysis have been proposed, using various methods including fuzzy PID [9], adaptive MPC [10], robust predictive control [11], adaptive predictive control [12] and internal model control (for isoflurane anesthesia) [13].
In this problem setting, with large variability, relatively simple drug dynamics and low performance criteria, robust PID control can be expected to perform adequately. We have shown feasibility of robust PID control of propofol in both adults and children [14], [15], [16]. We have demonstrated that uncertainty is indeed an important factor limiting the performance, and that limited performance improvement can be expected from controllers with more complexity, unless strategies that reduce the uncertainty are implemented [17]. This paper illustrates how previous clinical results can be exploited for system improvement, in this case for control of propofol infusion in young children (aged 5–10 years).
We analyzed the results of a previous study, conducted by our group, for the target population [16]: The median time to complete induction of anesthesia was 4.38 minutes for patients aged 6–10 years, compared to 3.18 minutes for patients aged 11–17 years. Induction of anesthesia took longer than five minutes for over 25% of the younger patients. This prolonged induction of anesthesia is undesirable for these young patients, where fast induction is required to limit discomfort and stress. Propofol induction and maintenance doses were higher in the younger group. This result reflects higher drug requirements in younger children.
We present an optimized robust PID controller for children aged 5–10 years, with the goal of reducing the induction time in this target population. We reduced variabiliy in the drug response by introducing allometric scaling, and redesigned a robust PID controller using optimization-based tuning [18]. The new control system was evaluated in a clinical study, and propofol infusion was closed-loop controlled in 16 cases.
Additional details of the available data from the previous clinical study [16], and available models identified from this data, are summarized in Section II. Section III describes the allometric scaling, the controller design for children aged 5–10 years, and presents simulations used in the design process prior to clinical evaluation. Section IV summarizes the results of the new clinical study. The paper is concluded in Section V.
II. Analysis of available clinical data
A. System performance for target population with previous design
In a previous study conducted by our group, closed-loop control of propofol infusion was evaluated in 71 patients using the final controller design as described in [16] and [19], including 25 patients aged 6–10 years (2 patients were 6 years old, 7 patients were 7, 7 were 8, 3 were 9 and 6 patients were 10 years old) and 46 patients aged 11–17 years. A summary of the performance is given in Table I.
TABLE I.
Summary of system performance for target population. Results are presented as median [Q1,Q3] (min, max)
| Induction time [min] | 4.38 [3.56, 5.42] (1.28, 6.12) | 3.18 [2.65, 4.15] (1.80, 5.42) |
| Propofol induction dose [mg/kg] | 2.93 [2.42, 3.87] (1.29, 5.33) | 1.99 [1.69, 2.40] (0.97, 3.82) |
| Propofol maintenance dose [mcg/kg/min] | 329 [252, 377] (194, 579) | 180 [145, 218] (90, 447) |
Time to induction of anesthesia, defined as the start of propofol infusion until the time when the WAVCNS first achieves < 60 and remains < 60 for 30 seconds, was longer in the younger group. Propofol induction and maintenance doses reflect known drug requirements in these age groups; pharmacokinetic clearances and volumes of distribution are known to be larger in children than in adults, including the central volume [20]. Children therefore require larger per kilogram induction doses and maintenance infusion rates.
B. Modeling the effect of propofol
From the data described in [16], 47 models were identified [21]. The effect of propofol on the DOH is commonly described by a three-compartment pharmacokinetic (PK) model, followed by a pharmacodynamic (PD) model described by a first-order system and the nonlinear Hill function [22]. Clinical responses to propofol infusion can show a time delay, which has been included in these PD models used for the purpose of controller design [23].
This model set was shown to describe the interpatient variability adequately, and was validated for robust controller design. Twenty of these models describe the dynamics of children aged 6–10 years, 27 describe the dynamics of 11–16 year olds (no models were identified for 17 year old patients).
Figure 1 shows the Bode diagrams of the models identified by [21], and compares the response from children aged 6–10 to that of the older patients aged 11–16 years. The gain of the models for the older group is higher than the gain of the models for the target population, reflecting the higher drug requirements of the younger children.
Fig. 1.
Bode diagram of pediatric model set linearized for induction of anesthesia. Patients aged 6–10 years are shown in red, patients aged 11–16 years in blue. Input and output units are ml/kg/h and 100 − WAVCNS respectively.
III. Optimized robust PID design
Based on the clinical evidence described above, the controller was retuned, with the objective to improve the speed of induction of anesthesia for the target population (aged 5–10 years) and particularly the younger children in this age group.
A. Reducing variability
We introduced allometric scaling to reduce the variability in this population, and to improve the response of the younger children specifically:
| (1) |
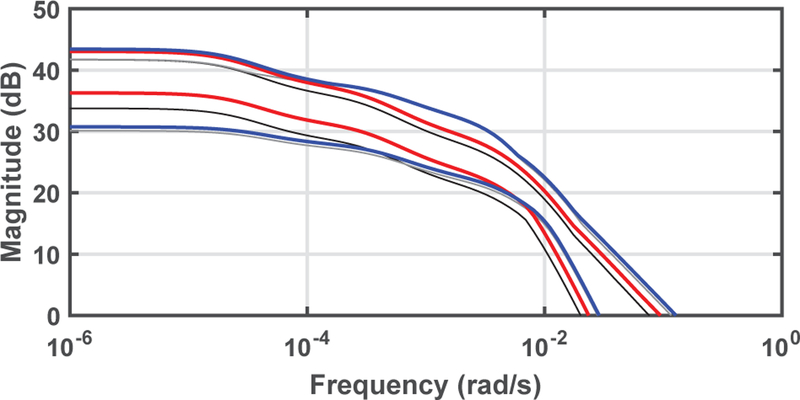
where walo is the allometrically scaled weight, bwt is the body weight in kg and the scaling of the normalized body weight of 70 kg is used to facilitate comparisons with previous designs based on body weight. Figure 2 compares the Bode magnitude diagrams of the models scaled using allometric scaling to the Bode magnitude when scaled per body weight. The gain of the younger group increased, while the gain of the older group remained almost identical. The system gain of the younger target population now shows increased overlap with the older group.
Fig. 2.
Bode diagram of allometrically scaled pediatric model set, linearized for induction of anesthesia, compared to the set scaled by body weight. The minimal and maximal Bode magnitude of the set scaled with walo is shown for patients aged 6–10 in red, and patients aged 11–16 in blue. The minimal and maximal magnitudes for the model set scaled with body weight are shown in black (aged 6–10) and grey (aged 11–16).
B. Controller design
A robust PID controller was designed for the target population using the optimization-based tuning method described in [18]. Of the twenty models for children aged 6–10 years, available for controller design, one describes outlier behavior with a time delay of 120 seconds. This model was identified from closed-loop data where occlusion of the propofol infusion line occured during the start of induction of anesthesia. Consequently, the time delay was likely overestimated and this model was discarded for controller design. The controller design was based on a multi-model uncertainty description containing 19 individual patient models.
The controller tuning followed the method for multi-model uncertainty described in [18]. Parameters [kp, ki, kd] of a continuous time PID controller, C(s) = kp + ki/s + kds, were optimized to minimize the integral of the error following a load step disturbance. This is equivalent to maximizing the integral gain ki. Robustness is enforced by constraining Ms, the maximum sensitivity magnitude: Ms ≤ 1.8. This is a convex-concave optimization problem, that can be solved efficiently using convex relaxation. The convex programs were solved using CVX in MATLAB1.
The optimal controller (with previously mentioned input and output units) is given by:
For children with allometrically scaled weight walo similar to their bwt, the gain of this controller is similar to the gain of the manually tuned controller for children aged 6–17 years, with [kp, ki, kd] = [1.1, 0.0061, 66]. For children with higher walo than bwt, the gain is higher.
Note that this optimization based controller tuning approach ensures robustness, because the maximum amplitude of the sensitivity function (Ms) is constrained. Post-hoc robustness analysis is therefore not required.
A two-degree of freedom implementation using set-point weighting in the derivative term was used, where the reference contribution to the derivative action was weighted by c. This set-point weight c affects the initial dose administered upon the step change in the reference signal at the start of induction of anesthesia. It was tuned to achieve clinically relevant doses for induction of anesthesia, as evaluated by an expert anesthesiologist, and was set to c = 1. Note that with this choice of c, the two-degree of freedom implementation corresponds to a one-degree of freedom controller. The controller was discretized with sampling time Ts = 5 s. A second order noise filter with a 15 second time constant was used to filter both the feedback and reference signal.
C. Simulation results
Performance of the controller was evaluated in simulation for the complete model set of 47 (nonlinear) patient models described in Section II-B. Figure 3 shows induction of anesthesia of the target population, and compares the result with the induction profile using the controller tuned for children aged 6–17 years (see Section II). The optimized design improves the speed of induction of anesthesia as required. Tuning of the set-point weight c = 1 assures adequate propofol infusion at the start of the case to mitigate pain due to slow propofol infusion. This is achieved with a minimal increase in the overshoot.
Fig. 3.
Simulation results for 20 models describing the response to propofol infusion in the target population. Simulated induction of anesthesia using the controller optimized for children aged 5–10 (red) and the available controller tuned for children aged 6–17 (black).
Figure 4 shows the simulated response to induction of anesthesia for children aged 11–16 using the controller optimized for younger children. Since the design uses allometric scaling, the gain of the controller is similar to the original design for this group, the controller achieves similar performance and sufficient robustness margins are maintained.
Fig. 4.
Simulation results for 27 models describing the response to propofol infusion for children aged 11–16. Simulated induction of anesthesia using the controller optimized for children aged 5–10 (blue) and the available controller tuned for children aged 6–17 (black).
Note that due to the significant uncertainty, sufficiently fast induction of anesthesia cannot be achieved for all patients without some overshoot. In the urological surgeries considered in this study, patients were not required to maintain spontaneous breathing, and the predicted overshoot is clinically acceptable. A consequent undershoot is predicted for some patient models, due to the time delay. This is expected to represent a worst case scenario as the time delay of these models reflects the delay and some nonlinear behavior observed during induction of anesthesia. The delay during maintenance of anesthesia is expected to be smaller. The predicted undershoot is clinically insignificant.
IV. Clinical evaluation
This clinical study was the closed-loop total intravenous anesthesia (TIVA) arm of a two-arm study, where the other arm used inhaled anesthesia. This paper focuses on the evaluation of performance of the control system design and reports the control-related results of the closed-loop arm.
Following approval from the local research ethics board (H15–01532) and Health Canada testing authorization (243057), the study took place at BC Children’s Hospital in Vancouver, BC, Canada. Healthy children or children with mild systemic disease (ASA I-II) , ⩾ 5 and < 11 years of age undergoing elective urological surgeries, lasting at least 45 minutes, were included in the study. Patients with developmental delay, neurological injury or psychiatric diagnosis, anxiety in the pre-operative period requiring sedative medication, contra-indication to total intravenous anesthesia, < 3rd or > 97th percentile weight for age, or previous anesthetic complications were excluded.
Propofol was controlled by the closed-loop system with the controller described above. The WAVCNS measure of DOH (NeuroSENSE monitor) was used for feedback, the sampling time of the system was 5 seconds. Remifentanil was administered manually; an initial bolus of 1 mcg/kg followed by a fixed rate infusion of 0.1 mcg/kg/min. For additional analgesic relief during the procedure, the attending anesthesiologist could administer fentanyl in boluses of 1 mcg/kg (rounded to the nearest 25 mcg). Data were collected every second from the control system, the pumps controlling the propofol and remifentanil infusions, the NeuroSENSE monitor and the patient monitor. Significant events were recorded by a research assistant.
Study data:
After informed written consent/assent, 20 patients were enrolled in the study. In one case, closed-loop control of propofol infusion was not possible due to a device malfunction. In three cases, intravenous access could not be obtained in a timely fashion and sevoflurane was required for induction of anesthesia. These four cases were excluded. In one case, the control mode was switched to manual and target controlled infusion (TCI) for part of the case due to sustained EEG artifacts and invalid feedback. In another case, the control mode was briefly switched to TCI due to EEG artifacts and invalid feedback.
Demographics:
Sixteen cases were included in this study (14 males, 2 females). Median [Q1, Q3] age was 8 [6, 9] (in years), weight (in kg) 28.3 [21.9, 35.4] and height (in cm) 130.0 [120.4, 138.5].
Results:
Results of the clinical study are summarized in Table II. The WAVCNS-index calculated in real-time was valid for median [Q1,Q3] 98 [89 ,99] % of the time. In one patient, a valid WAVCNS-index was obtained for only 39 % of the time. Large slow wave EEG amplitude in children under propofol anesthesia can cause the monitor, in the configuration used for this study (NS-701, v.2.0.0.3), to flag valid EEG segments as artifacts and invalidate them, predominantly at relatively deep levels of anesthesia (WAVCNS-index≈40). In two cases, the control mode was temporarily switched to TCI or manual as a result of this invalid feedback signal.
TABLE II.
Summary of results. Results are presented as median [Q1,Q3], unless stated differently.
| Closed-loop TIVA [n = 16] |
|
|---|---|
| Valid WAV measure [% time] | 98 [89, 99] |
| Length of anesthesia [min] | 44.2 [34.0, 67.3] |
| Minimum WAV during case | 33.8 [31.5, 37.1] |
| Occurrence of burst suppression [yes/no] | 0/16 |
| Induction of anesthesia | |
| Time to induction [min] | 3.7 [2.3, 4.1] |
| Propofol induction dose [mg/kg] | 2.8 [1.9, 3.1] |
| Remifentanil Induction dose [ng/kg] | 0.4 [0.2, 0.4] |
| Propofol Ce at Induction [mcg/ml] | 3.2 [2.2, 3.6] |
| Remifentanil Ce at Induction [ng/ml] | 2.8 [2.6, 3.6] |
| Maintenance of anesthesia | |
| Length of maintenance of anesthesia [min] | 41.4 [30.9, 64.1] |
| Mean propofol dose [mcg/kg/min] | 177.2 [150.1, 227.8] |
| Mean remifentanil dose [mcg/kg/min] | 0.1 [0.1, 0.1] |
| Mean propofol Ce [mcg/ml] | 3.2 [2.7, 3.9] |
| Mean remifentanil Ce [ng/ml] | 2.0 [1.9, 2.1] |
| WAV > 60 [% of WAV valid time] | 0 [0, 2] |
| WAV < 40 [% of WAV valid time] | 6 [3, 20] |
| Time in range [% of WAV valid time] | 93 [85, 96] |
| MDPE | −5.9 [−9.1, −2.6] |
| MDAPE | 7.1 [4.5, 9.7] |
| Wobble | 4.9 [4.2, 6.3] |
| Global Score | 13.4 [9.9, 16.8] |
The maintenance phase was defined as the time from induction complete to the end of the propofol infusion. Population average predicted plasma concentrations Ce were calculated for both propofol and remifentanil using the Paedfusor [24] and the Rigby-Jones [25] models respectively. The occurrence of burst suppression was defined as any recorded suppression ratio > 1 % during propofol infusion.
Time in range was calculated as the percentage of time within 10 units of the setpoint for the valid WAVCNS measurements during maintenance of anesthesia. Periods of light (WAVCNS > 60) and deep (WAVCNS < 40) anesthesia are quantified as % of time. Performance measures commonly used for closed-loop anesthesia are included to facilitate comparison to other studies: the median prediction error (MDPE), median absolute prediction error (MDAPE), Wobble and Global Score are calculated according to [4]:
Induction time was reduced compared to the previous clinical study [16], see Figure 5 (Median difference, −1.22 minutes [95% confidence interval: −1.83 to −0.33, p = 0.006], Wilcoxon rank sum test). Note that this study was not designed to test this hypothesis, and the sample size is small. More importantly, induction time reduced to below five minutes for all patients. This is clinically important for this patient group, to decrease patient discomfort and stress for both the patient and parents. Furthermore, this result illustrates that the new system achieved the design objective.
Fig. 5.
Induction time achieved with the new design, compared to the induction time for children aged 6–10 years in the previous clinical study [16].
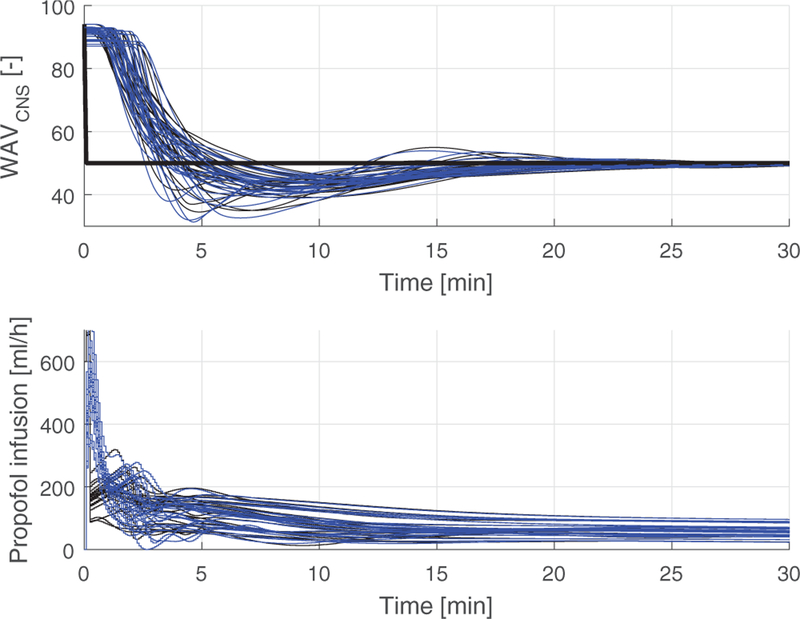
Figure 6 shows the predicted closed-loop response from the simulation study performed prior to clinical evaluation, as well as the closed-loop response of the 16 clinical closed-loop cases. The closed-loop system performed as designed. In some cases the WAVCNS remained below the setpoint following a period of fallback operation where no valid WAVCNS was available, as discussed above. The additional bolus infusions as indicated in the bottom graph were administered in two cases, for movement, as reported by the surgeon, and high blood pressure.
Fig. 6.
Simulation results for 20 models compared to results from clinical evaluation in 16 cases. Top: Simulated (predicted) closed-loop response (red) and measured WAVCNS (black) and setpoint (thick black line). Bottom: Simulated propofol infusion (red) and propofol infusion in clinical evaluation (black).
V. Conclusion
This paper describes the design and clinical evaluation of a closed-loop propofol system for children aged 5–10 years. We describe the analysis of previous clinical evidence, reduction of variability through scaling, robust PID design, pre-clinical testing in simulation and results of clinical evaluation. The results indicate that the expected uncertainty described by the model set is realistic and the predicted closed-loop responses and robustness margins are realistic. The control system performed as designed, reducing induction time while providing adequate robustness for the target population to ensure safe closed-loop anesthesia.
This paper illustrates our methodology for ongoing development and optimization of system performance, ensuring that patient safety can be demonstrated: Initial control system design relied on limited clinical data, and robust PID designs focused on ensuring sufficient margins. This initial robust design was evaluated in a clinical trial. Data from this initial clinical trial were used to analyze system performance and identify opportunities for system improvement. The data were also used to identify and validate additional patient models to support continuing development. This allows for the redesign and optimization of the control system. The results of clinical evaluation of the new redesigned controller indicate that the optimized controller design achieved the performance improvement predicted in simulation studies in the design stage.
Acknowledgment
The authors would like to thank the anaesthetists, surgeons, operating room staff, PACU nurses and surgical day care nurses at BC Children’s hospital for their interest and willingness to support study procedures, James Gaynor for his support in the clinical evaluation, and the patients for their participation in the study.
The research reported herein was supported in part by the National Institute of General Medical of the National Institutes of Health under award number R44GM112358. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Support from NeuroWave Systems Inc. (Cleveland Heights, OH, USA) is also gratefully acknowledged.
Control technology developed by the authors is part of a licencing agreement between UBC and NeuroWave Systems Inc. G. Dumont is a co-inventor of the NeuroSENSE monitor).
Footnotes
CVX: a Matlab-based convex modeling framework, www.cvxr.com.
References
- [1].Dumont GA and Ansermino JM, “Closed-loop control of anesthesia: a primer for anesthesiologists,” Anesthesia & Analgesia, vol. 117, no. 5, pp. 1130–1138, 2013. [DOI] [PubMed] [Google Scholar]
- [2].Brogi E, Cyr S, Kazan R, Giunta F, and Hemmerling TM, “Clinical performance and safety of closed-loop systems: A systematic review and meta-analysis of randomized controlled trials.” Anesthesia & Analgesia, 2016. [DOI] [PubMed] [Google Scholar]
- [3].Pasin L, Nardelli P, Pintaudi M, Greco M, Zambon M, Cabrini L, and Zangrillo A, “Closed-loop delivery systems versus manually controlled administration of total IV anesthesia: A meta-analysis of randomized clinical trials.” Anesthesia & Analgesia, 2016. [DOI] [PubMed] [Google Scholar]
- [4].Liu N, Chazot T, Genty A, Landais A, Restoux A, McGee K, Laloë P-A, Trillat B, Barvais L, and Fischler M, “Titration of propofol for anesthetic induction and maintenance guided by the bispectral index: Closed-loop versus manual control: A prospective, randomized, multicenter study,” Anesthesiology, vol. 104, no. 4, pp. 686–695, 2006. [DOI] [PubMed] [Google Scholar]
- [5].Liu N, Chazot T, Hamada S, Landais A, Boichut N, Dussaussoy C, Trillat B, Beydon L, Samain E, Sessler DI, and Fischler M, “Closed-loop coadministration of propofol and remifentanil guided by bispectral index: A randomized multicenter study,” Anesthesia & Analgesia, vol. 112, no. 3, pp. 546–557, 2011. [DOI] [PubMed] [Google Scholar]
- [6].Puri GD, Mathew PJ, Biswas I, Dutta A, Sood J, Gombar S, Palta S, Tsering M, Gautam P, Jayant A et al. , “A multicenter evaluation of a closed-loop anesthesia delivery system: A randomized controlled trial,” Anesthesia & Analgesia, vol. 122, pp. 106–114, 2016. [DOI] [PubMed] [Google Scholar]
- [7].Struys MMRF, De Smet T, Versichelen LFM, Van de Velde S, Van den Broecke R, and Mortier EP, “Comparison of closed-loop controlled administration of propofol using bispectral index as the controlled variable versus “standard practice” controlled administration,” Anesthesiology, vol. 95, no. 1, pp. 6–17, 2001. [DOI] [PubMed] [Google Scholar]
- [8].Zaouter C, Hemmerling TM, Lanchon R, Valoti E, Remy A, Leuillet S, and Ouattara A, “The feasibility of a completely automated total IV anesthesia drug delivery system for cardiac surgery.” Anesthesia & Analgesia, vol. 123, no. 4, 2016. [DOI] [PubMed] [Google Scholar]
- [9].Janda M, Simanski O, Bajorat J, Pohl B, Noeldge-Schomburg GFE, and Hofmockel R, “Clinical evaluation of a simultaneous closed-loop anaesthesia control system for depth of anaesthesia and neuromuscular blockade,” Anaesthesia, vol. 66, no. 12, pp. 1112–1120, 2011. [DOI] [PubMed] [Google Scholar]
- [10].Sawaguchi Y, Furutani E, Shirakami G, Araki M, and Fukuda K, “A model-predictive hypnosis control system under total intravenous anesthesia,” IEEE Trans. Biomed. Eng, vol. 55, no. 3, pp. 874–887, 2008. [DOI] [PubMed] [Google Scholar]
- [11].Ionescu CM, De Keyser R, Torrico BC, De Smet T, Struys MM, and Normey-Rico JE, “Robust predictive control strategy applied for propofol dosing using BIS as a controlled variable during anesthesia,” IEEE Trans. Biomed. Eng, vol. 55, no. 9, pp. 2161–2170, Sept. 2008. [DOI] [PubMed] [Google Scholar]
- [12].Niño J, De Keyser R, Syafiie S, Ionescu C, and Struys M, “EPSAC-controlled anesthesia with online gain adaptation,” International Journal of Adaptive Control and Signal Processing, vol. 23, no. 5, pp. 455–471, 2009. [Google Scholar]
- [13].Gentilini A, Rossoni-Gerosa M, Frei CW, Wymann R, Morari M, Zbinden AM, and Schnider TW, “Modeling and closed-loop control of hypnosis by means of bispectral index (BIS) with isoflurane,” IEEE Trans. Biomed. Eng, vol. 48, no. 8, pp. 874–889, 2001. [DOI] [PubMed] [Google Scholar]
- [14].Dumont GA, Liu N, Petersen C, Chazot T, Fischler M, and Ansermino JM, “Closed-loop administration of propofol guided by the neurosense: Clinical evaluation using robust proportional-integral-derivative design,” in American Society of Anesthesiologists Annual Meeting, Chicago, IL, 2011. [Google Scholar]
- [15].West N, van Heusden K, Görges M, Brodie S, Rollinson A, Petersen CL, Dumont GA, Ansermino JM, and Merchant RN, “Design and evaluation of a closed-loop anesthesia system with robust control and safety system.” Anesthesia & Analgesia, 2017. [DOI] [PubMed] [Google Scholar]
- [16].West N, Dumont GA, van Heusden K, Petersen CL, Khosravi S, Soltesz K, Umedaly A, Reimer E, and Ansermino JM, “Robust closed-loop control of induction and maintenance of propofol anesthesia in children,” Pediatric Anesthesia, vol. 23, pp. 712–719, 2013. [DOI] [PubMed] [Google Scholar]
- [17].van Heusden K, Ansermino JM, and Dumont GA, “Performance of robust PID and Q-design controllers for propofol anesthesia,” in IFAC Conference on Advances in PID Control, Ghent, Belgium, 2018. [Google Scholar]
- [18].Soltesz K, van Heusden K, Hast M, Ansermino JM, and Dumont GA, “A synthesis method for automatic handling of inter-patient variability in closed-loop anesthesia,” in American Control Conference, 2016. [Google Scholar]
- [19].van Heusden K, Dumont GA, Soltesz K, Petersen CL, Umedaly A, West N, and Ansermino JM, “Design and clinical evaluation of robust PID control of propofol anesthesia in children,” IEEE Trans. Contr. Syst. Technol, vol. 22, no. 2, pp. 491–501, 2014. [Google Scholar]
- [20].Rigby-Jones AE and Sneyd JR, “Propofol and children–what we know and what we do not know,” Pediatric Anesthesia, vol. 21, no. 3, pp. 247–254, 2011. [DOI] [PubMed] [Google Scholar]
- [21].van Heusden K, Ansermino JM, Soltesz K, Khosravi S, West N, and Dumont GA, “Quantification of the variability in response to propofol administration in children,” IEEE Trans. Biomed. Eng, vol. 60, no. 9, pp. 2521–2529, 2013. [DOI] [PubMed] [Google Scholar]
- [22].Bibian S, Ries C, Huzmezan M, and Dumont G, “Introduction to automated drug delivery in clinical anesthesia,” European Journal of Control, vol. 11, pp. 535–557, 2005. [Google Scholar]
- [23].Bibian S, Dumont GA, Huzmezan M, and Ries CR, “Patient variability and uncertainty quantification in clinical anesthesia: Part I – PKPD modeling and identification,” in IFAC Symp. Modelling and Control in Biomedical Systems, Reims, France, 2006. [Google Scholar]
- [24].Absalom A and Kenny G, “‘Paedfusor’ pharmacokinetic data set,” British Journal of Anaesthesia, vol. 95, no. 1, p. 110, 2005. [DOI] [PubMed] [Google Scholar]
- [25].Rigby-Jones A, Priston M, Sneyd J, McCabe A, Davis G, Tooley M, Thorne G, and Wolf A, “Remifentanil–midazolam sedation for paediatric patients receiving mechanical ventilation after cardiac surgery,” British Journal of Anaesthesia, vol. 99, no. 2, pp. 252–261, 2007. [DOI] [PubMed] [Google Scholar]