Introduction
Prior to the introduction of targeted therapy, cytoreductive nephrectomy (CN) was considered the standard of care for metastatic renal cell carcinoma (mRCC) after randomized, controlled trials demonstrated a survival benefit of upfront CN followed by cytokine therapy.1,2 With the advent of tyrosine kinase inhibitors (TKIs), however, the role and timing of CN has been questioned. Although large, retrospective series have demonstrated a continued role for CN followed by TKIs, this practice has not been supported by the recent CARMENA trial. This randomized, controlled trial enrolled 450 patients with intermediate- and poor-risk Memorial Sloan-Kettering Cancer Center (MSKCC) criteria over 10 years across multiple centers in Europe and randomly assigned patients to receive either sunitinib alone (n=224) or to undergo CN followed by sunitinib (n=226). Sunitinib alone (without CN) was shown to be non-inferior to upfront CN followed by sunitinib. In fact, median overall survival (OS) was longer among patients who were randomized to receive sunitinib alone (18.4 months) compared to CN then sunitinib (13.9 months).3 This randomized, controlled trial suggests that patients who present with intermediate- or poor-risk criteria should not have CN upfront and should have TKI therapy first. The role for delayed CN was not an endpoint in the trial.
The SURTIME trial is another pivotal trial that was recently published providing evidence for delayed CN over immediate CN.4 Although initially designed to recruit 458 patients, due to poor accrual this trial only enrolled 99 patients (22%) over a period of 5.7 years across the Netherlands, Canada, U.K., and Belgium, and randomized these patients to have sunitinib before and after CN (n=49) or sunitinib after CN (n=50).4 Median OS was greater in patients who received sunitinib before and after CN compared to sunitinib after upfront CN (32.4 vs. 15.0 months, respectively; p=0.03). The results from both trials were landmark, with some suggesting a paradigm shift in the management of mRCC, stating that upfront CN should no longer be considered the standard. Critics of CN highlight that surgery is associated with unnecessary morbidity and may delay time to medical therapy, allowing metastatic sites to progress.5 In light of these recent trials and associated controversies, we evaluated our experience with the management of mRCC with CN in the era of TKIs, with specific emphasis on the timing of CN.
Methods
We performed a retrospective analysis at our institution of consecutive patients with mRCC who underwent CN and received TKIs between 2009 and 2016. We included any patients who underwent CN, received ≥1 month of TKI and had ≥1 year followup or until death, whichever preceded. We had two distinct groups: patients who underwent upfront CN followed by TKI and patients who received upfront TKI followed by CN. Our primary outcome was OS, defined as the time elapsed from mRCC diagnosis to death, and was modelled using the Kaplan-Meier method. Secondary outcomes included the analysis of prognostic factors for OS using univariable and multivariable analyses with Cox proportional hazards regression. Data was analyzed using IBM® SPSS Statistics, version 18.0.
Results
We included 54 consecutive, non-randomized patients in this study; 32 in the upfront CN group and 22 in the upfront TKI group. Table 1 demonstrates patient characteristics, operative information, and pathologial features. Patient characteristics were similar between the two groups. According to International Metastatic RCC Database Consortium (IMDC) risk-stratification, 45 patients (83%) had intermediate-risk disease and nine (17%) had poor-risk disease. Median OS was similar between the two groups but there was a non-significant trend favoring upfront TKI compared to upfront CN (36.9 vs. 30.7 months; p=0.09).
Table 1.
Patient demographic, operative, and pathology factors
| Characteristic | Overall (n=54) | Upfront TKI (n=22) | Upfront CN (n=32) | p |
|---|---|---|---|---|
| Median age at mRCC diagnosis, years (IQR) | 59.3 (52.6–69.2) | 63.3 (56.8–68.7) | 56.6 (51.6–69.3) | 0.880 |
| Male gender, n (%) | 44 (81.5) | 16 (72.7) | 28 (87.5) | 0.203 |
| History of smoking, n (%) | 41 (75.9) | 19 (86.4) | 22 (68.8) | 0.344 |
| History of cardiovascular disease, n (%) | 8 (14.8) | 5 (22.7) | 3 (9.4) | 0.068 |
| Clinical T stage, n (%) | 0.969 | |||
| T1 | 11 (20.4) | 5 (22.7) | 6 (18.8) | |
| T2 | 20 (37.0) | 9 (40.9) | 11 (34.4) | |
| T3 | 19 (35.2) | 7 (31.8) | 12 (37.5) | |
| T4 | 4 (7.4) | 1 (4.5) | 3 (9.4) | |
| Karnofsky performance status <80%, n (%) | 13 (24.1) | 4 (18.1) | 9 (28.1) | 0.340 |
| Time from mRCC diagnosis to treatment <1 year, n (%) | 47 (87.0) | 21 (95.5) | 26 (81.3) | 0.127 |
| IMDC risk, n (%) | 0.747 | |||
| Intermediate risk | 45 (83.3) | 17 (77.3) | 28 (87.5) | |
| Poor risk | 9 (16.7) | 5 (22.7) | 4 (12.5) | |
| Surgical approach, n (%) | 0.292 | |||
| Open | 14 (26.0) | 8 (36.4) | 6 (18.7) | |
| Laparoscopic | 40 (74.1) | 14 (63.6) | 26 (81.3) | |
| Lymph node dissection, n (%) | 36 (66.7) | 14 (63.6) | 22 (68.8) | 0.657 |
| Lymph node involvement, n (%) | 9 (16.7) | 2 (9.1) | 7 (21.9) | 0.003 |
| Thrombectomy, n (%) | 9 (16.7) | 5 (22.7) | 4 (12.5) | 0.076 |
| Margin status, n (%) | 0.622 | |||
| Negative | 45 (83.3) | 20 (90.9) | 25 (78.1) | |
| Positive | 9 (16.7) | 2 (9.1) | 7 (21.9) | |
| Median estimated blood loss, mL (IQR) | 200 (100–475) | 200 (100–550) | 175 (100–425) | 0.684 |
| Median length of hospital stay, days (IQR) | 4.0 (3.0–5.0) | 4.0 (3.0–5.5) | 3.5 (3.0–5.0) | 0.197 |
| Histology, n (%) | ||||
| Clear-cell | 52 (96.3) | 22 (100) | 30 (93.8) | 0.113 |
| Non-clear-cell | 2 (3.7) | 0 (0) | 2 (6.2) | 0.423 |
| Grade, n (%) | 0.057 | |||
| 1 or 2 | 22 (40.7) | 8 (36.4) | 14 (43.8) | |
| 3 or 4 | 30 (55.6) | 14 (63.6) | 16 (50.0) | |
| Unknown | 2 (3.7) | 0 (0) | 2 (9.1) | |
| Median tumour size, cm (IQR) | 8.5 (6.4–11.0) | 8.5 (5.5–11.3) | 8.5 (7.0–10.9) | 0.934 |
| Stage, n (%) | 0.436 | |||
| pT1 | 6 (11.1) | 3 (13.6) | 3 (9.4) | |
| pT2 | 7 (13.0) | 3 (13.6) | 4 (12.5) | |
| pT3 | 34 (63.0) | 15 (68.2) | 19 (59.4) | |
| pT4 | 7 (13.0) | 1 (4.5) | 6 (18.8) |
CN: cytoreductive nephrectomy; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; IQR: interquartile range; mRCC: metastatic renal cell carcinoma; TKI: tyrosine kinase inhibitor.
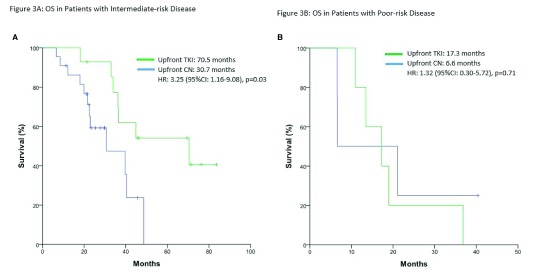
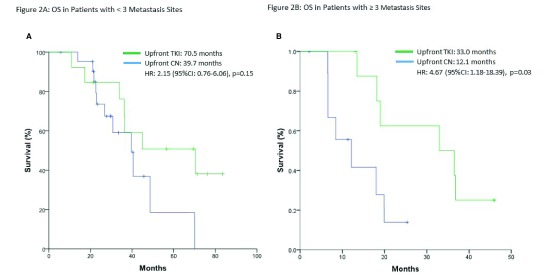
When patients were stratified by number of metastatic sites (<3 vs. ≥3 sites), OS was significantly longer in the upfront TKI group with ≥3 metastasis sites (33.0 vs. 12.1 months; p=0.03) (Fig. 1). Table 2 outlines prognosticators of survival using univariate and multivariate analyses. On multivariate analysis, only ≥3 sites of metastasis (p=0.014) and poor-risk category (p=0.001) were found to be independent predictors of worse OS. In the intermediate-risk group only, the upfront TKI group experienced a significantly longer median OS (70.5 vs. 39.7 months; p=0.03) (Fig. 2A). This difference was not evident in patients with poor-risk disease (p=0.71) (Fig. 2B).
Fig. 1.
Overall survival (OS) in (A) patients with <3 metastasis; and (B) patients with ≥3 metastasis. CI: confidence interval; CN: cytoreductive nephrectomy; HR: hazard ratio; TKI: tyrosine kinase inhibitor.
Table 2.
Univariable and multivariable analysis of possible prognostic factors
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | HR | 95% CI | p | HR | 95% CI | p |
| Age (<65/>65 years) | 1.03 | 0.50–2.14 | 0.927 | |||
| Hemoglobin (<LLN/>LLN) | 3.36 | 1.54–7.31 | 0.322 | |||
| Neutrophils (<ULN/>ULN) | 1.11 | 0.38–3.24 | 0.318 | |||
| Platelets (<ULN/>ULN) | 1.72 | 0.65–4.57 | 0.868 | |||
| Time from mRCC diagnosis to treatment (<1/>1 year) | 1.42 | 0.49–4.09 | 0.127 | 9.89 | 1.43–68.66 | 0.056 |
| Karnofsky performance status (<80%/>80%) | 2.1 | 0.91–4.89 | 0.340 | |||
| Risk category (Intermediate/poor) | 3.76 | 1.58–8.96 | 0.045 | 12.9 | 2.82–59.14 | 0.001 |
| Number of metastasis (<3/>3) | 2.68 | 1.17–8.42 | 0.001 | 4.44 | 1.36–14.54 | 0.014 |
| Grade (1–2/3–4) | 2.07 | 0.24–17.93 | 0.057 | 1.22 | 1.05–14.67 | 0.346 |
| Surgical approach (open/laparoscopic) | 1.35 | 1.05–2.73 | 0.292 | |||
| LN involvement (yes/no) | 2.15 | 0.81–5.71 | 0.090 | 3.40 | 0.77–15.01 | 0.106 |
CI: confidence interval; HR: hazard ratio; LLN: lower limits of normal; LN: lymph node; mRCC: metastatic renal cell carcinoma; ULN: upper limits of normal.
Fig. 2.
Overall survival (OS) in (A) patients with intermediate-risk disease; and (B) patients with poor-risk disease. CI: confidence interval; CN: cytoreductive nephrectomy; HR: hazard ratio; TKI: tyrosine kinase inhibitor.
Discussion
The CARMENA trial has stimulated much debate over the current role of upfront CN. However, there are certain criticisms and inherent biases of the trial when the methodology and patient demographics are examined more closely. First, the study was designed to detect non-inferiority only and thus, it cannot be concluded that TKI without CN is superior. Next, although this was a randomized, controlled trial, the groups were not balanced. Compared with the sunitinib-only group, more patients in the nephrectomy-sunitinib group had locally advanced disease of stage T3 or T4 (70% vs. 51%) and N1 disease (30% vs. 19%), potentially affecting surgical outcomes. There were also evident deviations from the intention-to-treat compared to the treatment patients actually received; 18% of patients randomized to receive CN followed by sunitinib did not actually receive sunitinib compared to 5% in the sunitinib-only group, and 17% of patients in the “sunitinib-only group” ended up undergoing CN. Furthermore, there is concern over patients included in this study, as nearly half the cohort (44%) in the trial were MSKCC poor-risk.3 There was poor accrual in this trial, as it took over seven years to recruit. Patients were recruited at the discretion of the surgeon, which introduced bias in the study population. Patients who were good surgical candidates were likely not offered to participate in this randomized study, as they would undergo CN as general standard of care. Previous evidence has shown CN benefits mainly favorable-risk patients with good performance status and fails to provide any survival benefit in poor-risk patients (e.g., ≥4 IMDC prognosticators).6 As these patients are already destined to have a poor prognosis, CN was already not recommended in this group.6–8
Given that the CARMENA trial was weighted toward poor-risk patients, it is unsurprising that the non-inferiority endpoint was reached. Our retrospective series demonstrated an OS trend in favor of upfront TKI followed by CN as opposed to upfront CN in patients with predominately intermediate-risk disease. This benefit was statistically significant in patients with intermediate-risk disease only and in patients with ≥3 sites of metastasis. Rather than performing immediate CN, it may be beneficial in these selected patients to undergo a trial of TKI. If they have a favorable response, they may subsequently benefit from undergoing consolidative surgery with deferred CN. This strategy would spare some patients the morbidity of CN and does not delay time to systemic therapy. Although there is significant selection bias, changing the timing of CN after a trial of TKI may be a decent litmus test for patients.
The particular timing of TKI therapy in the context of CN has been investigated by the recently published SURTIME trial. Most patients had MSKCC intermediate-risk disease (88%) and baseline characteristics were balanced between groups. Using an intention-to-treat analysis, median OS was significantly greater in patients who received sunitinib before and after CN compared to sunitinib after CN (32.4 vs. 15.0 months, respectively; p=0.03).4 However, this finding was no longer significant with a per-protocol analysis. It is also important to note 18% of patients were ineligible for the trial and 13% of patients in the immediate CN arm did not subsequently receive sunitinib, while all patients in the delayed CN arm received sunitinib. This may be due to reasons related to immediate CN that prevented the start of TKI therapy, which suggests immediate CN may be a risk in select patients. Upon further analysis, it was found that patients had poor prognosis if disease progression occurred before surgery in the deferred CN arm or within 16 weeks after surgery in the immediate CN arm. Delayed CN provides clinicians the opportunity to identify patients with TKI resistance, who are unlikely to benefit from surgery. These findings also support conclusions from the CARMENA trial, suggesting immediate CN is not ideal. Overall, the SURTIME trial provides evidence to support delayed CN in intermediate-risk patients without disease progression.
Patient selection for CN based on established risk models is of paramount importance and multimodal treatment remains critical for the management of mRCC.9 Rather than completely abandoning CN, the decision should be made on an case-by-case basis, taking into account baseline patient factors, risk features, tumor characteristics, and surgical operability.9 Select patients may benefit from initial systemic therapy as a litmus test, followed by delayed nephrectomy in the TKI era. Recent randomized trials for mRCC in the first-line setting have also demonstrated impressive responses, including OS, with newer targeted therapies such as cabozantinib (CABOSUN trial),10 as well as immunotherapies with checkpoint blockade, including ipilimumab with nivolumab (Checkmate 214 trial).11 The current standard treatment for mRCC patients with intermediate- and poor-risk criteria has recently been established as nivolumab in combination with ipilimumab based upon Checkmate 214. With the re-introduction of immunotherapy in this patient population, the role of CN in the management of these patients with mRCC will continue to evolve.
Footnotes
Competing interests: Dr. Kapoor has been an advisory board member for and received honoraria from BMS, Eisai, Ipsen, Merck, Novartis, Pfizer, and Roche; and has been a speakers’ bureau member for Eisai, Ipsen, Novartis, and Roche. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 2.Mickisch GHJ, Garin A, Van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomized trial. Lancet. 2001;358:966–70. doi: 10.1016/S0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 3.Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med. 2018;379:417–27. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 4.Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs. deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib. JAMA Oncol. 2018;5:164–70. doi: 10.1001/jamaoncol.2018.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silberstein JL, Adamy A, Maschino AC, et al. Systematic classification and prediction of complications after nephrectomy in patients with metastatic renal cell carcinoma (RCC) BJU Int. 2012;110:1276–82. doi: 10.1111/j.1464-410X.2012.11103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heng DYC, Rini BI, Beuselinck B, et al. Cytoreductive nephrectomy (CN) in patients with synchronous metastases from renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) J Clin Oncol. 2014;32:396. doi: 10.1200/jco.2014.32.4_suppl.396. [DOI] [PubMed] [Google Scholar]
- 7.Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;34:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and followup. Ann Oncol. 2014;25:iii49–56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Russo P. Cytoreductive nephrectomy — patient selection is key. N Engl J Med. 2018;379:481–2. doi: 10.1056/NEJMe1806331. [DOI] [PubMed] [Google Scholar]
- 10.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib vs. sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35:591–7. doi: 10.1200/JCO.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab vs. sunitinib in advanced renal cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]