Abstract
Introduction
We aimed to evaluate the impact of thrombo-embolic-deterrent + intermittent pneumatic compression (TED + IPC) vs. muscle pump activator (MPA) on incisional wound healing in kidney and simultaneous pancreas-kidney (SPK) transplant recipients.
Methods
We conducted a single-center, randomized, controlled trial in which 104 patients (kidney n=94; SPK n=10) were randomly assigned to wear TED + IPC (n=52) or MPA (n=52) for the first six days following surgery. Patient demographics, postoperative outcomes, and incisional wound images were taken using a Health Insurance Portability and Accountability Act (HIPAA)-compliant application on postoperative days (POD) 3, 5, and 30, and assessed using the validated Southampton Wound Care Score.
Results
There were no demographic differences between the groups. The MPA group had a significant improvement in wound healing on POD 3 (p=0.04) that persisted until POD 5 (p=0.0003). At POD 30, both groups were similar in wound healing outcomes (p=0.51). Bayesian inferential analysis revealed that the use of TED + IPC following transplantation had inferior outcomes compared to the use of MPA with sequential moderate evidence. The rate of complex wound infections was significantly greater in the TED + IPC group compared to the MPA group (29% vs. 12%, respectively; p=0.03). Patients were more satisfied with the use of a MPA device than TED + IPC. No major complications were encountered in either group.
Conclusions
The use of a MPA device in the immediate postoperative period leads to a significant improvement in immediate and early wound healing, and decreased number of complex wound infections following kidney and SPK transplantation compared to standard TED + IPC therapy. Patients were more satisfied with the use of a MPA device than TED + IPC.
Introduction
Transplantation is the optimal mode of renal replacement therapy for patients suffering from end-stage renal disease. Wound infections contribute to postoperative morbidity after transplantation1 and are attributable to both the mandatory immunosuppression, as well as to patient comorbidities at the time of surgery. The incidence of overall infectious complications during the first year after renal transplantation has been reported to be as high as 49%,2 with wound infection rate as high as 27%.3–5 In the case of simultaneous kidney and pancreas (SPK) transplantation, surgical site infections are higher (>75%).6–8 Various risk factors contribute to impaired wound healing following kidney or SPK transplantation, including recipient age greater than 60 years, patient dialysis pre-transplantation, a body mass index >30 kg/m2, the need for postoperative plasmapheresis, use of thymoglobulin as induction therapy or its use in acute rejection, the use of mycophenolate mofetil or sirolimus for maintenance immunosuppression, and delayed graft function.9,10
Enhancing blood flow to a surgical site is known to improve wound healing. Postoperative fluid overload is common in patients following renal or SPK transplantation, as ensuring adequate vascular pre-load to the graft is paramount. Unfortunately, this often leads to significant edema, which could compromise micro-capillary blood flow to the wound. Common practices to decrease edema include limb elevation;11 activating the calf muscle pump, which requires reciprocating ankle plantar flexion and/or dorsiflexion;12 and mechanical devices such as compression stockings13 or intermittent pneumatic compression (IPC) devices.14,15 Unfortunately, these strategies may not be effective in some patients with comorbid conditions that limit limb movement or in patients with contraindications to adequate compression, such as skin infections and vascular insufficiency. In addition, current mechanical compression devices can be difficult to apply and uncomfortable, which decreases compliance. A method to increase blood flow involves transcutaneous direct muscular stimulation, which uses electrical stimulation via electrodes applied to the skin to stimulate the calf muscle pump and promote lower limb venous blood flow.16–18 However, because of the high voltage intensity required to stimulate the muscle and subsequent discomfort, these devices have not been widely adopted in routine clinical use. Geko™ device is an alternative to direct electrical muscle stimulation for activating the calf muscle pump.19 It is a self-contained neuromuscular stimulation device that adheres to the skin over the common peroneal nerve and delivers a low-voltage stimulus at a frequency of 1 Hz, thereby activating the calf and foot musculature of the lower limb without the voltage-related discomfort. The muscle pump activator (MPA) device has previously been shown to increases superficial femoral vein blood flow and velocity,19 reduce leg edema,20 and improve chronic wound healing by enhancing transcutaneous oxygen tension (a predictor of tissue viability and ischemic wound healing).19,21
Promoting wound healing in the transplant population is challenging, given the chronic immunosuppression and patient factors that contribute heavily to postoperative wound complications. The use of a MPA in enhancing wound healing in this population has never been evaluated. Herein, we report the outcomes of a randomized, controlled trial testing whether the use of the MPA device compared to traditional stockings thrombo-embolic-deterrent + intermittent pneumatic compression (TED +IPC) would enhance wound healing and surgical site infection rates following kidney and SPK transplantation.
Methods
Study design
This study was single-center, open-label, randomized, clinical trial. All patients undergoing renal and SPK transplant were recruited in the study. The trial followed the CONSORT 2010 guidelines (Fig. 1). The protocol was approved by the University of Western Ontario Research Ethics Board (protocol number 103618).
Fig. 1.
CONSORT diagram for the trial (protocol #103618).
Participants
Patients aged >18 years undergoing kidney or SPK transplantation between January 1, 2016 and December 31, 2017 were included in the study. The exclusion criteria were age younger than 18 years, history of deep vein thrombosis, history of leg amputation, and patients with intra-cardiac defibrillators. At the time of surgery, both groups of patients were placed on TED + IPC (Covidien and Flowtron Excel). On postoperative day (POD) 1, patients were randomly assigned to their assigned groups. The MPA device (Geko Plus, Perfuse Med, U.K.) was replaced daily, as per the manufacturer’s instructions to maintain battery performance. Both groups of patients were maintained on either TED + IPC or MPA for six days following surgery, at which time the devices were removed. Incisional wound healing images were taken using a Health Insurance Portability and Accountability Act (HIPAA)-compliant mobile app (MODICA, Clearwater Clinical Limited, Canada) on POD 3, 5, and 30. We collected demographic and medical information on all patients and evaluated their wounds using the validated Southampton Wound Scoring System.22 The questionnaire was used to assess patient satisfaction with the use of the devices on the sixth day post-transplant. There are six questions that relate to wound swelling, device comfort during the period, the intent to use the device in case of future surgeries, and devices’ influence on patients’ mobility and sleep (Table 1).
Table 1.
Questionnaire to assess patient satisfaction with the use of the various devices (participants asked to check one reply for each question)
| To which random group were you placed? | IPC + TED stockings MPA device |
| How comfortable are the device? | Extremely comfortable Moderately comfortable Average Moderately uncomfortable Extremely uncomfortable |
| What is the extent of the wound swelling? | Extremely increased Slightly increased No change Slightly reduced Extremely decreased |
| What was the device’s influence on sleep patterns? | Extremely positive Moderately positive No effect Moderately negative Extremely positive |
| What is the device’s mobility after surgery? | Extremely difficult Moderately difficult No change Moderately easy Extremely easy |
| Would you want to use the same device if you had another surgery? | Yes No |
MPA: muscle pump activator; TED + IPC: thrombo-embolic-deterrent + intermittent pneumatic compression.
Randomization
Before randomization, surgeons determined each patient’s preoperative frailty and functional status, as well as proposed postoperative care, including the need to go to the intensive care unit. The patients were randomized into the intervention arm (MPA) or control arm (TED + IPC) in a ratio of 1:1 using sealed, sequentially labelled randomization envelopes opened by the recipient coordinator.
Sample size estimation
Based on data from a previous pilot study at our institution, which examined the impact of a MPA on incisional wound healing compared to standard stockings and compression devices in kidney and kidney-pancreas transplant recipients, the sample size calculation was a priori performed with G*Power V.3.1.2. To detect a moderate effect size of 0.6 (mean difference of 3 units, standard deviation [SD] 5) with a power of 80% and α set at 0.05, a minimum of 42 participants in each group were required. To account for attrition and the likelihood of increasing pediatric transplant practice at our institution, our accrual target was 50 participants in each of the two study arms (MPA vs. TED + IPC).
Statistical analysis
The data was analyzed using the Statistical Package for the Social Sciences (SPSS, Version 23.0, IBM, U.S.). A Pearson’s Chi-square correlation was run to assess the relationship of wound score between TED + IPC and MPA cohorts. The normalcy of distribution of demographics and patient characteristics with outlier detection were assessed by Shapiro-Wilk’s test. P value of <0.05 was considered to be significant. On Bayesian inferential analysis, there was evidence that MPA has superior outcomes compared to TED + IPC. The null hypothesis, “MPA leads to better wound scores compared to TED + IPC” was accepted with sequential strong/moderate evidence in favor of MPA, H0>H1 at POD 3 and 5.
Results
The demographic and outcomes characteristics in both cohorts are listed in Table 2. A total of 104 kidney and kidney-pancreatic transplant recipients were randomly assigned to wear TED + IPC (n=52) and MPA device (n=52). Ninety-four patients underwent kidney transplantation and 10 underwent SPK transplantation. The median age of the groups were 49 and 51 years, with a similar distribution of body mass index (27.5 vs. 25.8) in the TEC + IPC and MPA cohorts, respectively. On average, kidney transplant patients were hospitalized for 6.5 days and SPK patients for 10 days post-transplant. There were no significant differences between the two groups with respect to age, gender, body mass index, or length of hospital stay. In addition, all patients received maintenance immunosuppressive regimen consisting of prednisone, tacrolimus, and mycophenolic acid. Levels were monitored closely in both groups and adjusted according to accepted practices (trough levels, lymphocyte counts under thymoglobulin therapy). Induction treatment was used and consisted of antithymocyte globulin (ATG 5–8 mg/kg total) for the majority of the patients and basiliximab (20 mg IV day 0 and 4) was given to low-immunological-risk kidney transplant recipients. Preoperative antimicrobial prophylaxis was standardized and consisted of cefazolin in kidney recipients, with added metronidazole in SPK recipients, all of which was administered up to two days following transplantation. Our standard protocol following kidney/pancreas transplantation is to place two drains (one in the retroperitoneal space near the kidney [left] and another in the abdomen near the pancreas [right]). In kidney transplant patients, we placed a single drain in the retroperitoneal space and the majority of these drains was taken out on POD 2 or 3 if output was less than 50 cc in 24 hours; otherwise, they would be removed in clinic during followup. Both cohorts of patients were treated equally throughout the process and had similar rates of early and late drain removal. Our fluid protocol included 0.45% NS 30 ml/hour + previous hour urine output and 0.9% NS according to central venous pressure (CVP) protocol, if CVP <5 rate is 250 ml/30 minutes and recheck CVP, 5–9 CVP rate is 100 ml/hour, >9 CVP 30 ml/hour. Routinely, we do not use postoperative diuretic. Trimethoprim/sulfamethoxazole was given as prophylaxis for both urinary tract infection and pneumocystis pneumonia three times a week indefinitely. Antifungal prophylaxis was routinely used in kidney-pancreas transplantation for a period of three months.
Table 2.
Demographics and patient characteristics
| Type of recipients | |||
|---|---|---|---|
|
|
|||
| IPC + TED | MPA | p | |
| Number of patients | 52 | 52 | – |
| Age, years (range) | 49 (27–65) | 51 (40–69) | 0.98 |
| Male: Female | 34:18 | 30:22 | 0.89 |
| BMI (kg/m2) | 27.5±4.2 | 25.8±4.6 | 0.26 |
| Weight (kg) | 89.2±6 | 86.6±5 | 0.69 |
| Height (cm) | 160.0±5.02 | 158.9±2.92 | 0.43 |
| Superficial wound infection (%) | 15 (29) | 6 (12) | 0.03 |
| Kidney transplant | 46 | 48 | – |
| Length of stay (days) | 6.9±1.5 | 6.6±1.4 | 0.85 |
| DM | 20 | 22 | 0.91 |
| HTN | 10 | 12 | 0.75 |
| Smoking | 8 | 10 | 0.78 |
| Peripheral vascular disease | 8 | 6 | 0.18 |
| Induction immunosuppression | |||
| Thymoglobulin | 30 | 35 | 0.65 |
| Basiliximab | 16 | 13 | 0.71 |
| Maintenance immunosuppression | |||
| Tacrolimus/mycophenolic acid/prednisone | 52 | 52 | 0.84 |
| Superficial wound infection (%) | 11 (24) | 4 (8) | 0.04 |
| Kidney + pancreas transplant | 6 | 4 | – |
| Length of stay (days) | 10.2±1.5 | 9.6±1.7 | 0.94 |
| DM | 5 | 4 | 0.99 |
| HTN | 3 | 2 | 0.45 |
| Smoking | 3 | 2 | 0.75 |
| Peripheral vascular disease | 2 | 1 | 0.24 |
| Induction immunosuppression | |||
| Thymoglobulin | 6 | 4 | 0.99 |
| Maintenance immunosuppression | |||
| Tacrolimus/mycophenolic acid/prednisone | 6 | 4 | 0.64 |
| Superficial wound infection (%) | 4 (67) | 2 (50) | 0.59 |
Data are presented as median ± standard deviation. BMI: body mass index; DM: diabetes mellitus; HTN: hypertension; MPA: muscle pump activator; TED + IPC: thrombo-embolic-deterrent + intermittent pneumatic compression.
Several recipient risk factors known for the development of wound infections, including hypertension, diabetes mellitus, smoking, and peripheral vascular disease, were evaluated in both groups and found to be non-significant between cohorts. Overall, 15 (29%) patients in the TED + IPC cohort and seven (13%) in the MPA cohort developed superficial wound infections (p=0.03). There were 15 (22%) patients who developed superficial wound infections following kidney transplantation (11 patients in TED + IPC and four in MPA; p=0.04). In the SPK transplant patient, six (60%) developed superficial infections, of which four were in the TED + IPC and two in the MPA cohorts (p=0.59). All superficial wound infections were managed conservatively with penicillin-based antibiotics for 10 days; a few cases were managed by wound packing and drain insertion.
Incisional wound healing images were taken using the MODICA app and scored using the Southampton Wound Score System (Figs. 2–5, Table 3). On POD day 3, TED + IPC cohort had significantly higher wound score in comparison to the MPA cohort (Pearson Chi-square 4.1; p= 0.04; SD ±2.48 for TED + IPC group compared to MPA) (Fig. 6). By POD 5, TED + IPC cohort still had significantly higher wound score (≥2) in comparison to MPA cohort (Pearson Chi-square 6.88; p =0.0003; SD ±1.81 for TED + IPC group compared to MPA) (Fig. 7). At 30 days following transplant, MPA and TED + IPC cohorts had similar wound scores (Pearson Chi-square 6.20; p=0.51; SD ±1.84 for MPA compared to TED + IPC cohort) (Fig. 8). The likelihood of significant wound scores (>2 on Southampton Wound Score) were <1 for MPA group compared to TED + IPC group at day 3 and day 5, further proving the efficacy of MPA over TED + IPC (Table 4). We did evaluate mobilization in both groups of patients and found that patients wearing the MPA device were more apt to mobilize early compared to the TED + IPC group, but there were no statistical differences with the small numbers evaluated here. Regardless of mobilization, both groups of patients were maintained on their respective treatments until POD 6.
Fig. 2.
Muscle pump activator (MPA) group patient post-kidney transplantation. Wound images taken at (a) 3; (b) 5; and (c) 30 postoperative days (POD) show serial improvement in wound scores.
Fig. 3.
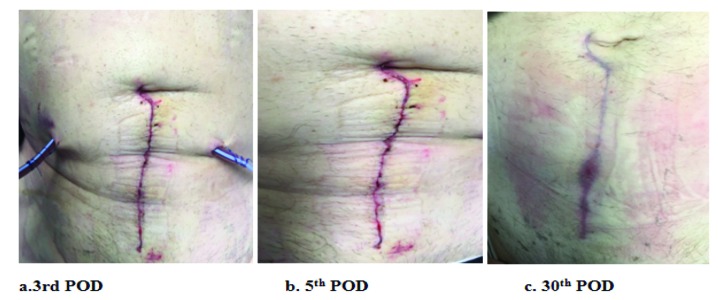
Thrombo-embolic-deterrent + intermittent pneumatic compression (TED + IPC) group patient post-kidney transplantation. Wound images taken at (a) 3; (b) 5; and (c) 30 postoperative days (POD) show interval improvement in wound scores.
Fig. 4.
Muscle pump activator (MPA) group patient post-kidney-pancreas transplantation. Wound images taken at (a) 3; (b) 5; and (c) 30 postoperative days (POD) show serial improvement in wound scores.
Fig. 5.
Thrombo-embolic-deterrent + intermittent pneumatic compression (TED + IPC) group patient post-kidney-pancreas transplantation. Wound images taken at (a) 3; (b) 5; and (c) 30 postoperative days (POD) show interval improvement in wound scores.
Table 3.
Southampton Wound Scoring System24
| Southampton scoring system | ||
|---|---|---|
|
| ||
| Grade | Appearance | Assigned numerical score |
| 0 | Normal healing | 0 |
| I - Normal healing with mild bruising or erythema | 1 | |
| A | Some bruising | 2 |
| B | Considerable bruising | 3 |
| C | Mild erythema | 4 |
| II - Erythema plus other signs of inflammation | 5 | |
| A | At one point | 6 |
| B | Around sutures | 7 |
| C | Along wound | 8 |
| D | Around wound | 9 |
| III - Clear or hemoserous discharge | 10 | |
| A | At one point only (<2 cm) | 11 |
| B | Along wound (>2 cm) | 12 |
| C | Large volume | 13 |
| D | Prolonged (>3 days) | 14 |
| IV - Pus | 15 | |
| A | At one point only (<2 cm) | 16 |
| B | Along wound (>2 cm) | 17 |
| V - Deep or severe wound infection with or without tissue breakdown; hematoma requiring aspiration | 18 | |
Fig. 6.
At postoperative day (POD) 3, the thrombo-embolic-deterrent + intermittent pneumatic compression (TED + IPC) cohort had significantly higher wound score difference in comparison to the muscle pump activator (MPA) cohort (Pearson Chi-square 4.1; p=0.04; standard deviation [SD] ± 2.48 for TED + IPC group compared to MPA). Day 3 H0>H1 null hypothesis MPA leads to better wound scores compared to TED + IPC. Bayesian inferential analysis: strong evidence in favor of MPA.
Fig. 7.
At postoperative day (POD) 5, the thrombo-embolic-deterrent + intermittent pneumatic compression (TED + IPC) cohort had significantly higher wound score (≥2) in comparison to the muscle pump activator (MPA) cohort (Pearson Chi-square 6.88; p=0.0003; standard deviation [SD] ± 1.81 for TED + IPC group compared to MPA). Day 5 H0>H1 null hypothesis MPA leads to better wound scores compared to TED + IPC. Bayesian inferential analysis: moderate evidence in favor of MPA.
Fig. 8.
At postoperative day (POD) 30, the muscle pump activator (MPA) cohort had equivalent wound score in comparison to thrombo-embolic-deterrent + intermittent pneumatic compression (TED + IPC) cohort (Pearson Chi-square 6.20; p=0.51; standard deviation [SD] ± 1.84 for MPA compared to TED + IPC). Day 30 H0>H1 null hypothesis MPA leads to better wound scores compared to TED + IPC. Bayesian inferential analysis: equivalent evidence for either group.
Table 4.
Likelihood of significant wound score >2 comparing MPA vs. TED + IPC cohort
| Likelihood of significant wound score >2 | 3 days | 5 days | 30 days |
| MPA vs. TED + IPC | 0.18 (95% CI 0.06–0.27) | 0.01 (95% CI 0.002–0.13) | NS |
CI : confidence interval; MPA: muscle pump activator; NS: non-significant; TED + IPC: thrombo-embolic-deterrent + intermittent pneumatic compression.
Patient satisfaction
The answers from patients in both arms were recorded and presented as follows:
The first question asked of the patient was, “How comfortable are the devices?” Of the 104 participants, 52 took part in the TED + IPC study while 52 were fitted with MPA device. When level of discomfort was evaluated in TED+IPC patients, 57% reported some level of discomfort, 29% reported no effect on comfort, and 14% reported comfort. In contrast, the reports were skewed towards being more comfortable in the MPA arm, with 13%, 23%, and 64%, being responses for discomfort, no effect on comfort, and comfortable, respectively. The Pearson Chi-square showed that there is a significant difference in comfort between the two groups (p<0.003). In response to second question, “What is the extent of the wound swelling?” 52% of TED + IPC patients had an increase in wound swelling, 17% had no change, and 31% recorded a decrease in swelling, while MPA device participants recorded 22%, 30%, and 48%, respectively for the same questions. This suggests that patients who wore the MPA device had the perception of improved wound edema compared to those patients who were on standard therapy. The Pearson Chi-square showed that there is a significant difference in the wound swelling between the two groups (p<0.001).
In response to third question, “What was the device’s influence on sleep patterns?” 49% of TED + IPC participants indicated no change in sleep patterns compared to 50% in the MPA arm. However, 31% reported a negative change in the TED + IPC compared to only 16% in the MPA group. Interestingly, 20% of patients reported that they had an easier time going to sleep in the TED + IPC group, whereas this number was 34% in the MPA arm. The Pearson Chi-square showed that there is a significant difference in influence on sleep patterns between the two groups (p<0.02).
In response to the fourth question, “What is the device’s mobility after surgery?” after undergoing surgery, 29% of patients fitted with TED + IPC reported no effect on mobility, while 28% and 43% reported difficulty and improvement in mobility, respectively. On the other hand, the MPA device created a 10% mobility difficulty, with 17% reporting no change effect and 73% reporting a free and improved mobility. This becomes increasingly important in patient mobility after major surgery and could have a significant impact on patient convalescence and length of stay in hospital. The Pearson Chi-square showed that there is a significant difference in mobility after surgery between the two groups (p<0.001).
The final question was, “Would you want to use the same device if you had another surgery?” Fifty-seven percent of TED + IPC participants acknowledged that they would use it in comparison to MPA device, whose participants gave it 83%.
Discussion
This is the first report of a randomized, controlled trial evaluating the effect of a MPA on wound healing in transplant patients. We found that there was a significant improvement in wound healing and infection rates compared to standard TED + IPC, suggesting that this novel therapy may be an alternative strategy at improving patient outcomes in the peri-operative period in this high wound complication risk group.
The use of the MPA device in the immediate postoperative period lead to a significant improvement in wound healing at POD 3 (p=0.04) and 5 (p<0.0003) in both kidney and SPK transplant recipients compared to standard TED + IPC (Fig. 9). Previous studies have reported the incidence of infectious complications after renal transplantation to be 49%,2 with wound infections comprising up to 27% of these cases.3,4 As expected, infection rates are higher in SPK transplants (75%).6,7 Linhares et al reported a higher prevalence of bacterial infections (71%) was observed after transplantation, of which 44% were by Gram-negative rods and 27% by Gram-positive cocci.8 In the current study, 15 (29%) patients in the TED + IPC cohort and seven (13%) in the MPA cohort developed superficial wound infections (p=0.03). There were 15 (16%) patients who developed superficial wound infections following kidney transplantation, whereas in SPK transplant recipients, six patients (60%) developed superficial infections. While these rates are slightly lower than expected from the literature, they are quite significant in these populations. Although there was a reduction in wound infections observed in the SPK group, this was not significant, likely due to the low number of patients in this cohort. However, despite this, rates of superficial wound infections were found to be reduced considerably in the MPA group compared to the expected rate in the literature, suggesting that a larger trial would likely find significance. Given that recipient risk factors, including hypertension, diabetes mellitus, smoking, and peripheral vascular disease were evaluated in both groups and found not to be significant between the treatment cohorts, these findings are likely attributable to the use of the MPA device in the early perioperative period following transplantation. The shortcoming of this study is the relatively short followup of 30 days. It could help explain why so many of the known risk factors for wound complications, including drain placement and duration, fell out as statistically insignificant in our study. A larger, multicenter trial would be needed to further strengthen our findings Physiologically, electrical stimulation is believed to accelerate wound healing by imitating the natural electrical current that occurs in injured skin. Electrical field, along with chemotaxis and injury stimulation aids epithelial cell migration during wound healing.23 Zhao et al24 demonstrated that epithelial cells cultured in the presence of an electrical field demonstrate an increase in the distance of cell movement, thus exhibiting rapid response towards wound healing. In a study by Xu et al,25 electrical stimulation was also shown to be an effective adjunctive therapy in reducing bacterial loads and clinical infections in diabetic ulcer. Increased perfusion associated with electrical stimulation may also be associated with increased secretion of vascular endothelial growth factor (VEGF).26 In addition to increased skin perfusion, electrical stimulation therapy has been shown to improve venous flow, which can also positively contribute to wound healing through increasing capillary emptying.27 Whether the mechanism of positive action of the MPA device is via these pathways is unknown and requires further evaluation.
Fig. 9.
At postoperative day (POD) 3, the muscle pump activator (MPA) cohort had significantly lower wound score in comparison to thromboembolic-deterrent + intermittent pneumatic compression (TED + IPC) cohort (p=0.04). At POD 5, the MPA cohort had significantly lower wound score in comparison to TED + IPC cohort (p=0.0003). At POD 30, there was no significant difference between both groups (p=0.051). Null hypothesis MPA leads to better wound scores compared to TED + IPC. Bayesian inferential analysis: overall moderate in favor of MPA.
MPA is a self-powered neuromuscular stimulation device that stimulates the common peroneal nerve, which in turn, stimulates lower limb musculature to gently contract at a set frequency; this mechanism of action has been shown to increase femoral vein velocity and lower limb blood flow.20 Increasing blood circulation has been shown to enhance transcutaneous oxygen tension (TCpO2), which is a predictor of tissue viability and ischemic wound healing.28 There have been various studies demonstrating that the MPA device increases venous, arterial and microcirculatory blood flow in the lower limbs, reduces edema and increases TCpO2, thus creating favorable conditions for wound healing. In this regard, Clarke Moloney et aldemonstrated an increase in venous velocity using electrical stimulation as a treatment adjunct for venous ulceration.29 A meta-analysis by Gardner et al reported a 13% net healing rate per week with electrical stimulation, equating to a 144% increase over the control population.30 The MPA device has been shown to augment arterial flow, 19 as well as microcirculation, whereas compression therapy generally has a beneficial effect on venous flow only, and may reduce arterial and microcirculatory flows. Where an ulcer has an arterial component to its etiology, MPA device would be expected to be more efficacious than compression, by virtue of the augmentation of arterial inflow. Additionally, since healing any ulcer requires perfusion at the wound bed, the augmentation of microcirculatory flow brought about by the MPA device is expected to be beneficial for patients with chronic leg ulcers.
Lower limb edema is commonly experienced by post-kidney transplant surgery patients. Numerous studies have investigated how motor electrical stimulation, achieved by stimulating lower limb muscle activity, affects blood and lymphatic flow, both of which can reduce lower limb edema. Various studies have shown positive effects of motor electrical stimulation on increasing blood flow in human31 and animal32 models. In the surgical literature, Faghri et al reported that electrically stimulated contractions activate the skeletal muscle pump, thereby promoting limb blood flow, and may be effective in reducing venous pooling and edema in arthroplasty patients.33 In addition, lymphatic flow has been shown to increase during muscular exercise, highlighting the notion that lymphatic flow can be externally influenced by muscle contraction.34 In light of this, MPA demonstrated that the increase blood flow and velocity in the superficial femoral vein provides a tolerable and safe method for lower limb and wound tissue edema treatment. In addition, we demonstrated that patients had much higher satisfaction scores wearing the MPA device compared to the TED + IPC, especially in terms of improved comfort, perception of decreased edema, improved postoperative sleep, and enhanced early mobility.
To validate the MPA device’s appropriateness and effectiveness, it is necessary that the device pass the comfort test and preferably outperform the control set and other available devices. In our study, the MPA device had a cumulative positive comfort feedback of 64% vs. TED + IPC’s 14%. Additionally, the discomfort percentage for the MPA device was 13% vs. 57% for TED + IPC. In this regard, the MPA device is designed to offer very high levels of comfort to the patients.
The MPA device also recorded higher mobility rates of 73% (when adding the “somewhat easy” and “very easy” responses) in relation to TED + IPC’s 43%. This indicates that most patients could move easily without any negative effect from the device. The “very difficult” group accounted for 3%.
The positive feedback in relation to effect on sleep pattern and future use in case of requirement (83% for MPA vs. 17% for TED + IPC) indicate a high acceptability of MPA from the patients. Various studies support this outcome by showing both improvement in blood flow and a high patient satisfaction rate.19,20
Conclusions
The use of a MPA device in the immediate postoperative period leads to an improvement in wound healing vs. standard use TED + IPC as early as three days following kidney or SPK transplantation. In addition, we report for the first time that the use of a MPA leads to a significant reduction in wound infection rates in transplant patients who are at high risk for wound complications. Patients were more satisfied with the use of the MPA device than with TED + IPC. These findings should be evaluated in larger, multicenter studies.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Lynch RJ, Ranney DN, Shijie C, et al. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg. 2009;250:1014–20. doi: 10.1097/SLA.0b013e3181b4ee9a. [DOI] [PubMed] [Google Scholar]
- 2.Sousa SR, Galante NZ, Barbosa DA, et al. [Incidence of infectious complications and their risk factors in the first year after renal transplantation]. J Bras Nefrol. 2010;32:75–82. doi: 10.1590/S0101-28002010000100013. [DOI] [PubMed] [Google Scholar]
- 3.Roine E, Bjork IT, Oyen O. Targeting risk factors for impaired wound healing and wound complications after kidney transplantation. Transplant Proc. 2010;42:2542–6. doi: 10.1016/j.transproceed.2010.05.162. [DOI] [PubMed] [Google Scholar]
- 4.Khoury JA, Brennan DC. Infectious complications in kidney transplant recipients: Review of the literature. Saudi J Kidney Dis Transpl. 2005;16:453–97. [PubMed] [Google Scholar]
- 5.Fortun J, Martin-Davila P, Pascual J, et al. Immunosuppressive therapy and infection after kidney transplantation. Transpl Infect Dis. 2010;12:397–405. doi: 10.1111/j.1399-3062.2010.00526.x. [DOI] [PubMed] [Google Scholar]
- 6.Baktavatsalam R, Little DM, Connolly EM, et al. Complications relating to the urinary tract associated with bladder-drained pancreatic transplantation. Br J Urol. 1998;81:219e223. doi: 10.1046/j.1464-410X.1998.00517.x. [DOI] [PubMed] [Google Scholar]
- 7.Gettman MT, Levy JB, Engen DE, et al. Urological complications after kidney pancreas transplantation. J Urol. 1998;159:38e42. doi: 10.1016/S0022-5347(01)64005-9. discussion 42e43. [DOI] [PubMed] [Google Scholar]
- 8.Linhares MM, Gonzalez AM, Triviño T, et al. Simultaneous pancreas kidney transplantation: Infectious complications and microbiological aspects. Transplant Proc. 2004;36:980–1. doi: 10.1016/j.transproceed.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 9.Santangelo M, Clemente M, Spiezia S, et al. Wound complications after kidney transplantation in non-diabetic patients. Transplant Proc. 2009;41:1221–3. doi: 10.1016/j.transproceed.2009.03.098. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Walker M. The effects of intermittent pneumatic compression on the arterial and venous system of the lower limb: A review. J Tissue Viability. 2002;12:58–65. doi: 10.1016/S0965-206X(02)80015-8. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Own A, Scurr JH, Coleridge Smith PD. Effect of leg elevation on the skin microcirculation in chronic venous insufficiency. J Vasc Surg. 1994;20:705–10. doi: 10.1016/S0741-5214(94)70157-1. [DOI] [PubMed] [Google Scholar]
- 12.Padberg FT, Jr, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: A randomized trial. J Vasc Surg. 2004;39:79–87. doi: 10.1016/j.jvs.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 13.O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(21):CD000265. doi: 10.1002/14651858.CD000265.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Partsch H, Flour M, Smith PC International Compression Club. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence: Under the auspices of the IUP. Int Angiol. 2008;27:193–219. [PubMed] [Google Scholar]
- 15.Comerota AJ. Intermittent pneumatic compression: Physiologic and clinical basis to improve management of venous leg ulcers. J Vasc Surg. 2011;53:1121–9. doi: 10.1016/j.jvs.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 16.Browse NL, Negus D. Prevention of postoperative leg vein thrombosis by electrical muscle stimulation: An evaluation with 125I-labelled fibrinogen. Br Med J. 1970;3:615–8. doi: 10.1136/bmj.3.5723.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan RE, Czyrny JJ, Fung TS, et al. Electrical foot stimulation and implications for the prevention of venous thromboembolic disease. Thromb Haemost. 2002;88:200–4. doi: 10.1055/s-0037-1613187. [DOI] [PubMed] [Google Scholar]
- 18.Clarke Moloney M, Lyons GM, Breen P, et al. Hemodynamic study examining the response of venous blood flow to electrical stimulation of the gastrocnemius muscle in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 2006;31:300–5. doi: 10.1016/j.ejvs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Tucker A, Maass A, Bain D, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19:e31–7. doi: 10.1055/s-0031-1278361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alharbi B, Ali O, Saha M, et al. Neuromuscular stimulation leads to improved lower limb edema and blood flow compared to standard compression devices following kidney and pancreatic transplantation. [abstract] Am J Transplant. 2016;16(suppl 3) [Google Scholar]
- 21.Quigley FG, Faris IB. Transcutaneous oxygen tension measurements in the assessment of limb ischaemia. Clin Physiol. 1991;11:315–20. doi: 10.1111/j.1475-097X.1991.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 22.Bailey IS, Karran SE, Toyn K, et al. Community surveillance of complications after hernia surgery. BMJ. 1992;304:469–71. doi: 10.1136/bmj.304.6825.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakral G, LaFontaine J, Najafi B, et al. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4:22081. doi: 10.3402/dfa.v4i0.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–60. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, McLennan SV, Lo L, et al. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care. 2007;30:37880. doi: 10.2337/dc06-1383. [DOI] [PubMed] [Google Scholar]
- 26.Kanno S, Oda N, Abe M, et al. Establishment of a simple and practical procedure applicable to therapeutic angiogenesis. Circulation. 1999;99:26827. doi: 10.1161/01.CIR.99.20.2682. [DOI] [PubMed] [Google Scholar]
- 27.Doran FS, White HM. A demonstration that the risk of postoperative deep venous thrombosis is reduced by stimulating the calf muscles electrically during the operation. Br J Surg. 1967;54:686–9. doi: 10.1002/bjs.1800540805. [DOI] [PubMed] [Google Scholar]
- 28.Williams KJ, et al. Poster. Vascular Society Annual Scientific Meeting; Glasgow. November 2014. [Google Scholar]
- 29.Clarke-Moloney M, Lyons GM, Burke PE, et al. A review of technological approaches to venous ulceration. Crit Rev Biomed Eng. 2005;3:511–56. doi: 10.1615/CritRevBiomedEng.v33.i6.10. [DOI] [PubMed] [Google Scholar]
- 30.Gardner SE, Frantz RA, Schmidt FL. Effect of electric al stimulation on chronic wound healing: A meta-analysis. Wound Repair Regen. 1999;7:495–503. doi: 10.1046/j.1524-475X.1999.00495.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller BF, Gruben KG, Morgan BJ. Circulatory responses to voluntary and electrically induced muscle contractions in humans. Phys Ther. 2000;80:53–60. [PubMed] [Google Scholar]
- 32.Clemente FR, Matulionis DH, Barron KW, et al. Effect of motor neuromuscular electrical stimulation on microvascular perfusion of stimulated rat skeletal muscle. Phys Ther. 1991;71:397–404. doi: 10.1093/ptj/71.5.397. [DOI] [PubMed] [Google Scholar]
- 33.Faghri PD, Van Meerdervort HF, Glaser RM, et al. Electrical stimulation induced contraction to reduce blood stasis during arthroplasty. IEEE Trans Rehabil Eng. 1997;5:62–9. doi: 10.1109/86.559350. [DOI] [PubMed] [Google Scholar]
- 34.Olszewski W, Engeset A, Jaeger PM, et al. Flow and composition of leg lymph in normal men during venous stasis, muscular activity, and local hyperthermia. Acta Physiol Scand. 1977;99:149–55. doi: 10.1111/j.1748-1716.1977.tb10365.x. [DOI] [PubMed] [Google Scholar]