Abstract
Background
Instrumented lumbar surgeries, such as lumbar fusion and lumbar disc replacement, are increasingly being used in the United States for low back pain, with utilization rates approaching those of total joint arthroplasty. It is unknown whether there is a similar pattern in Canada. We sought to determine utilization rates and total medical costs of instrumented lumbar surgeries in a single-payer system and to compare these with the rates and costs of total hip and knee replacements.
Methods
We included Ontarians aged 20 years and older who underwent instrumented lumbar surgery or total knee or total hip replacement between April 1993 and March 2012. Utilization and medical cost of the procedures were evaluated and compared using linear regression in a time-series analysis. Instrumented lumbar surgical procedures were stratified by age and main indication for surgery.
Results
Utilization of instrumented lumbar surgeries rose from 6.2 to 14.2 procedures per 100 000 population between 1993 and 2012 (p < 0.001), well below the utilization of knee and hip arthroplasties. Patients were younger than 50 years for 29.2% of all instrumented lumbar surgery cases; annual procedure rates among those older than 80 years rose 7.6-fold. Direct medical costs of instrumented lumbar surgeries from 2002 to 2012 totaled $176 million. Spinal stenosis and spondylolisthesis were the most common indications for instrumented lumbar surgeries.
Conclusion
Use of instrumented lumbar surgeries in Ontario’s single-payer system has increased rapidly, especially among patients older than 80 years. In contrast to the situation in the United States, these rates were well below those of total joint arthroplasties. These data provide useful insights about resource allocation for surgical treatment of lumbar degenerative disorders.
Abstract
Contexte
Les chirurgies lombaires instrumentées, telles que l’arthrodèse ou la prothèse discale lombaires, sont de plus en plus utilisées aux États-Unis pour le traitement de la lombalgie, leurs taux d’utilisation s’approchant de ceux de l’arthroplastie totale. On ignore si la tendance est la même au Canada. Nous avons voulu mesurer les taux d’utilisation et les coûts médicaux totaux des chirurgies lombaires instrumentées et les comparer aux taux et aux coûts de l’arthroplastie totale de la hanche et du genou.
Méthodes
Nous avons inclus les Ontariens de 20 ans et plus ayant subi une chirurgie lombaire instrumentée ou une arthroplastie totale du genou ou de la hanche entre avril 1993 et mars 2012. L’utilisation et les coûts médicaux des interventions ont été évalués et comparés par analyse de régression linéaire des séries chronologiques. Les chirurgies lombaires ont été stratifiées selon l’âge et la principale indication.
Résultats
Le recours aux chirurgies lombaires instrumentées a augmenté de 6,2 à 14,2 interventions par 100 000 de population entre 1993 et 2012 (p < 0,001), ce qui reste bien inférieur au recours à l’arthroplastie du genou et de la hanche. Les patients avaient moins de 50 ans pour 29,2 % de tous les cas de chirurgies lombaires instrumentées; le taux annuel d’interventions chez les patients de plus de 80 ans a augmenté selon un facteur de 7,6. Les coûts médicaux directs des chirurgies lombaires instrumentées ont totalisé 176 millions de dollars entre 2002 et 2012. La sténose rachidienne et le spondylolisthésis étaient les plus fréquentes indications des chirurgies lombaires instrumentées.
Conclusion
L’utilisation de la chirurgie lombaire instrumentée pour le régime d’assurance santé à payeur unique ontarien a augmenté rapidement, particulièrement chez les patients de plus de 80 ans. Comparativement à la situation qui prévaut aux États-Unis, ces taux sont bien inférieurs aux taux d’arthroplasties totales. Ces données sont intéressantes du point de vue de l’allocation des ressources pour le traitement chirurgical de la dégénérescence discale lombaire.
Chronic back pain is a disabling condition that affects a significant proportion of the general population: epidemiologic surveys suggest two-thirds of Americans have back pain at some point in their lives, while 15% have frequent or prolonged backache.1 The prevalence of back pain is also higher with lower levels of education and income,1 which, combined with the high economic costs of this condition to patients,2 represents a significant burden of illness. Indeed, the Global Burden of Disease study suggests low back pain has risen to become the sixth leading cause of morbidity worldwide, accounting for more than 83 million disability-adjusted life-years lost.3
One of the procedures increasingly employed in chronic back pain management is fusion of the lumbar vertebrae.4–7 Originally devised as a treatment for symptoms of spinal tuberculosis,8 spinal fusions have become widely used for the treatment of fractures, deformity and degenerative conditions of the spine. More recently, artificial lumbar disc replacement was approved by the Food and Drug Administration in the United States for treatment of degenerative disease, expanding the armamentarium of surgical treatment for back pain. In the US, a 6-fold increase in the use of lumbar fusion for the treatment of degenerative lumbar conditions was observed from 1993 to 2011, with an increasing trend of instrumentation that includes insertion of screw-rod constructs and/or interbody cages.5,6 Meanwhile, use of artificial lumbar disc replacement has lagged, accounting for only 2.7% of lumbar degenerative disc disease cases from 2005 to 2009.9 Concomitant with increased utilization, the cost of fusions has risen drastically, reaching a median total hospital charge of over US$30 000 in 2001.5 This trend has translated into a dramatic burden in total spending on fusion surgeries; in the US, $40 billion is spent annually on spinal fusions, more than on any other hospital-based surgery.10
Despite this drastic increase in both rates and cost, evidence is limited for the efficacy and effectiveness of surgery as a treatment for low back pain associated with degenerative disc disease,11–15 the most frequently cited diagnoses for the use of the procedure.7 A 2009 review of evidence for an American Pain Society clinical practice guideline concluded that spinal fusion was no more effective than intensive rehabilitation with cognitive behavioural therapy in the treatment of nonradicular back pain.16 Curiously, rates of spinal fusion in the US now approach those of hip and knee replacements,7,17 2 procedures with a more established evaluation of efficacy.18,19 Currently, there is little information on the trend and cost of instrumented lumbar surgeries (ILS) such as lumbar fusion and lumbar disc replacement in Canada, which has a single-payer health system. Furthermore, it is unclear whether the indications for the procedures in Canada are similar to those observed in the US. In this study, we sought to determine the longitudinal utilization rates associated with ILS in Ontario, Canada’s largest province, with a population of 13.6 million, and to compare these with the rates of total hip and knee replacements to evaluate whether they mirror trends observed in the US. Further, we aimed to evaluate the direct medical costs of, and the main surgical indications for, ILS in Ontario.
Methods
Identification of patients and procedures
Using administrative databases housed at ICES, we identified all patients aged 20 years and older who underwent ILS, total knee arthroplasty or total hip replacement between Apr. 1, 1993, and Mar. 31, 2012. A case of ILS was defined using a modified algorithm of Canadian Classification of Health Interventions (CCI) procedural codes, International Classification of Diseases (ICD) diagnostic codes (see below for details) and Ontario Health Insurance Plan (OHIP) procedural codes (Appendix 1, available at canjsurg.ca/017016-a1).20 Claim codes pertaining to spinal diagnoses have been validated against the gold standard of physician review; interrater κ coefficients for disc herniation, spinal stenosis and acquired spondylolisthesis were 0.88, 0.74 and 0.73, respectively.21 Canadian case definitions of total knee arthroplasty and total hip replacement for this study were established previously (Appendix 1).22 People with fracture, tumour, infection, inflammation or a primary diagnosis of nonlumbar spinal pathology were excluded. In 2002, the diagnostic coding system was changed from the International Classification of Diseases, 9th revision (ICD-9) to the enhanced Canadian version of the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10-CA) system; the Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures (CCP) was changed to the CCI during the same time. Data through Mar. 31, 2002, were coded using the ICD-9/CCP system, and ICD-10CA/CCI codes were used from Apr. 1, 2002, onward.
Data sources
Four databases housed at ICES were linked for this study: the Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD), CIHI’s Same-Day Surgery Database (SDS), the OHIP database and the Registered Persons Database (RPDB). The CIHI-DAD and SDS databases provide data on all visits to acute, rehabilitation, chronic and day surgery institutions in Ontario. The quality of the data in CIHI-DAD was validated through a reabstraction and interrater reliability study in 2002–2004.23 A high level of agreement was found for patient demographic data. For Main Responsible Diagnosis coding, overall agreement was 85% in the reabstraction study and there was a κ score of 0.81 in inter-rater reliability. 23 Of note, orthopedic diagnoses such as hip arthrosis, knee arthrosis and femur fractures have greater than 90% agreement and are among the diagnostic codes with the highest rates of agreement in the study.24 The CIHI-DAD database was also used to calculate resource intensity weight-adjusted inpatient costs associated with the procedure. The OHIP database was used in an algorithm with DAD/SDS to identify eligible cases, as well as physician reimbursement for these procedures. The RPDB contains basic demographic information for everyone to whom an Ontario health card has been issued.
Baseline data
Baseline demographic information (age, sex) was collected. Postal code information was used to assign each patient to their local health integration network. Charlson comorbidity index scores were compiled on the basis of ICD diagnoses in CIHI-DAD. The RPDB was used to identify additional demographic information for the patients, such as their postal code, which served as a proxy for the location of residence. Other demographic information, such as urban/rural status, was obtained from the Canadian census on the basis of postal codes.
Statistical analysis
The annual rates of ILS, total knee arthroplasty and total hip replacement were determined relative to the population. Linear regression was used to determine trends in surgical rates in a time-series analysis. In addition, age-standardized analysis was carried out to account for demographic changes over the span of cohort accrual during the time period of the study. ILS rates were further subdivided into their components and stratified by the indications for which they were performed. Annual procedural costs, presented in 2011 Canadian dollars, were adjusted for inflation using the Bank of Canada’s inflation calculator (http://www.bankofcanada.ca/rates/related/inflation-calculator/). Procedural costs of the 3 procedures were derived using the resource intensity weight method via the %getcost macro developed by ICES to provide person-level costing in Ontario.24 CIHI-DAD classifies all acute discharges by case mix groups; this classification system was developed to categorize groups of patients with similar clinical and resource utilization patterns. Standardized inpatient costs within a case mix group are then adjusted by the resource intensity weight, an indicator representing the level of resources used relative to a reference case (cost per standardized case × resource intensity weight), to estimate the specific case cost of a given hospital admission.24 The %getcost macro draws upon databases pertaining to inpatient hospital admissions, emergency and ambulatory visits, same-day surgeries, inpatient rehabilitation, home care and physician services to generate case costs associated with each health care encounter.24 In the inpatient setting, these costs primarily involve acute inpatient utilization and physician services. Socioeconomic status was calculated through income quintiles using postal codes and income information available from dissemination areas of the Canadian census data available from Statistics Canada. Geographic location and rurality were determined via Statistical Area Classification (SAC) codes available from Statistics Canada. The Charlson Comorbidity Index was used to account for comorbidity. A p value of 0.05 was used to define a statistically significant difference. All analyses were done using SAS statistical software.
Ethics
The study was approved by the Health Sciences and Affiliated Teaching Hospitals Research Ethics Board at Queen’s University.
Results
Over the study period, 16 363 ILS procedures were performed in Ontario; by comparison, 233 281 knee arthroplasties and 154 381 total hip replacements were completed over the same period. Patients undergoing lumbar procedures were younger, had lower comorbidity and were more likely to have undergone the procedure at a large teaching hospital than patients undergoing the other 2 types of procedures (Table 1). The majority of the procedures were completed by orthopedic surgeons.
Table 1.
Baseline clinical and utilization characteristics
| Characteristic | Group; no. (%)* | ||
|---|---|---|---|
|
| |||
| Knee arthroplasty n = 233 281 |
Total hip replacement n = 154 381 |
Lumbar fusion n = 16 363 |
|
| Age at index date; mean ± SD and median [IQR] | 68.66 ± 9.44 | 67.09 ± 11.94 | 58.14 ± 14.68 |
| 69 [62–76] | 69 [60–76] | 60 [47–70] | |
|
| |||
| Female sex | 145 538 (62.4) | 86 754 (56.2) | 9258 (56.6) |
|
| |||
| Charlson Comorbidity Index score = 0 | 151 720 (65.0) | 107 434 (69.6) | 11 871 (72.5) |
|
| |||
| Neighbourhood income quintile | |||
|
| |||
| 1 | 42 891 (18.4) | 25 899 (16.8) | 2918 (17.8) |
|
| |||
| 2 | 48 177 (20.7) | 29 845 (19.3) | 3237 (19.8) |
|
| |||
| 3 | 47 205 (20.2) | 30 112 (19.5) | 3207 (19.6) |
|
| |||
| 4 | 46 624 (20.0) | 31 569 (20.4) | 3384 (20.7) |
|
| |||
| 5 | 47 431 (20.3) | 36 272 (23.5) | 3546 (21.7) |
|
| |||
| Missing | 953 (0.4) | 684 (0.4) | 71 (0.4%) |
|
| |||
| Rural | |||
|
| |||
| No | 191 610 (82.1) | 127 249 (82.4) | 13 656 (83.5) |
|
| |||
| Yes | 41 462 (17.8) | 27 001 (17.5) | 2678 (16.4) |
|
| |||
| Missing | 209 (0.1) | 131 (0.1) | 29 (0.2) |
|
| |||
| 2008 Rurality Index for Ontario; mean ± SD and median [IQR] | 13.73 ± 19.08 | 13.35 ± 18.71 | 13.17 ± 18.71 |
| 4 [0–24] | 4 [0–24] | 4 [0–22] | |
|
| |||
| OHIP provider (specialty) | |||
|
| |||
| General | ≤ 5 (0.0)† | 0 (0.0) | 0 (0.0) |
|
| |||
| Neuro | 0 (0.0) | 0 (0.0) | 3004 (18.4) |
|
| |||
| Ortho | 233 235 (100.0) | 154 334 (100.0) | 13 352 (81.6) |
|
| |||
| Other | 41–45 (0.0)† | 47 (0.0) | 7 (0.0) |
|
| |||
| Hospital type | |||
|
| |||
| Teaching | 178 603 (76.6) | 108 259 (70.1) | 9547 (58.3) |
|
| |||
| Nonteaching | 54 678 (23.4) | 46 121 (29.9) | 6816 (41.7) |
|
| |||
| Missing | 0 (0.0) | ≤ 5 (0.0)‡ | 0 (0.0) |
|
| |||
| Hospital size | |||
|
| |||
| Missing | 554 (0.2) | 156 (0.1) | 6 (0.0) |
|
| |||
| Large | 118 233 (50.7) | 90 025 (58.3) | 12 792 (78.2) |
|
| |||
| Small | 114 494 (49.1) | 64 200 (41.6) | 3565 (21.8) |
|
| |||
| Hospital rurality | |||
|
| |||
| Yes | 41 496 (17.8) | 27 051 (17.5) | 2682 (16.4) |
|
| |||
| No | 191 578 (82.1) | 127 199 (82.4) | 13 657 (83.5) |
|
| |||
| Missing | 207 (0.1) | 131 (0.1) | 24 (0.1) |
|
| |||
| No. of OHIP claim days 1 yr earlier; mean ± SD and median [IQR] | 37.78 ± 23.78 | 34.98 ± 25.06 | 40.97 ± 26.26 |
| 33 [22–47] | 29 [19–44] | 35 [24–51] | |
|
| |||
| Yes† | 41 496 (17.8) | 27 051 (17.5) | 2,682 (16.4) |
|
| |||
| No. of CIHI claim days 1 yr earlier; mean ± SD and median [IQR] 1.50 ± 0.92 | 1.56 ± 1.06 | 1.53 ± 0.98 | |
| 1 [1–2] | 1 [1–2] | 1 [1–2] | |
CIHI = Canadian Institute for Health Information; IQR = interquartile range; OHIP = Ontario Health Insurance Plan; SD = standard deviation.
Unless indicated otherwise.
No. (%) of patients with any OHIP claim within 1 yr of index date.
Cell sizes with < 5 cases were suppressed in concordance with ICES reidentification risk policy.
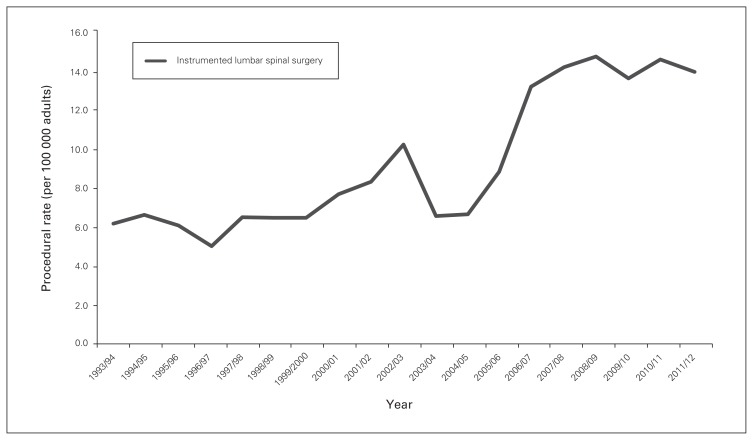
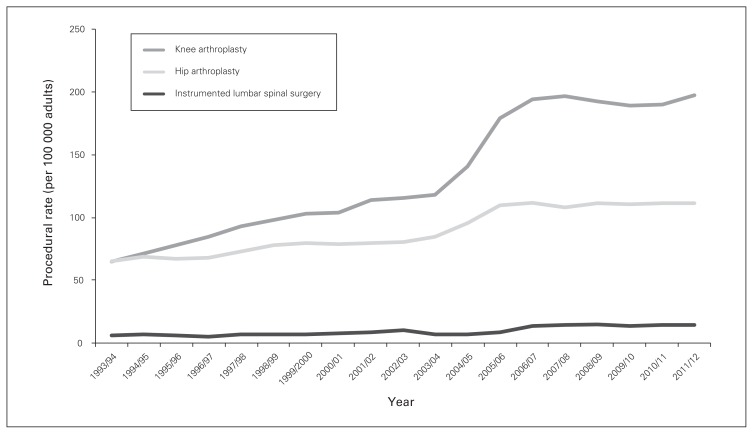
Utilization of ILS rose from 6.2 to 14.2 procedures per 100 000 population between 1993 and 2012 (p < 0.001) (Fig. 1); this was maintained with age standardization to account for demographic changes over time. These rates were well below the rates of knee and total hip arthroplasty procedures (knee: 64.9–197.4 cases per 100 000; hip: 65.2–111.6 cases per 100 000, p < 0.001 for comparison) (Fig. 2). Patients aged 60–69 years were the highest users of ILS (24.3% of all cases) among age deciles, followed by those aged 70–79 years (21.6%). Meanwhile, 29.2% of all cases involved patients younger than 50 years. The number of procedures completed on patients 80 years of age and older rose from 13 in 1993 to 99 in 2012.
Fig. 1.
Rates of instrumented lumbar spinal surgery in Ontario per 100 000 adults.
Fig. 2.
Rates of total knee replacement, total hip replacement and instrumented lumbar spinal surgery in Ontario per 100 000 adults.
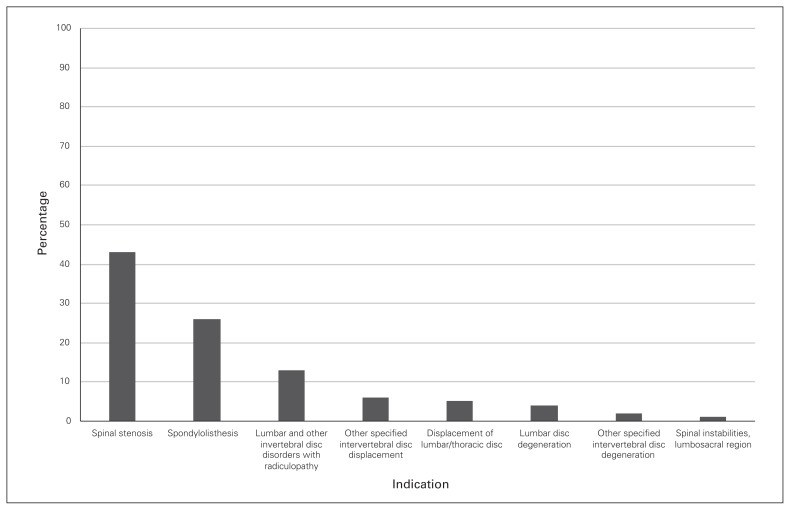
Lumbar stenosis and spondylolisthesis were the most common main diagnoses among the lumbar surgical interventions performed, accounting for 69% of the procedures. Lumbar disc degeneration was the diagnosis for only 4% of procedures (Fig. 3).
Fig. 3.
Distribution of indications involving instrumental lumbar surgical procedures in Ontario.
Length of hospital admission for ILS steadily declined from 8 to 4 days and from 8 to 5 days for men and women, respectively, over the study period. There was little difference across provider type, hospital type (teaching v. nonteaching) or geographic location. Stratification by age revealed the largest reduction in length of stay among patients aged 80–89 years, from 17 days in 1993 to 5 days in 2012, followed by patients aged 70–79 years, for whom length of stay fell from 11 days to 5 days (Table 2).
Table 2.
Median length of stay (in days) for lumbar surgery stratified by age group
| Year | Age group; median (IQR)* | |||||||
|---|---|---|---|---|---|---|---|---|
| 20–29 yr (n = 466) | 30–39 yr (n = 1663) | 40–49 yr (n = 2650) | 50–59 yr (n = 3300) | 60–69 yr (n = 3984) | 70–79 yr (n = 3530) | 80–89 yr (n = 763) | ≥90 yr (n = 7) | |
| 1993/94 | 7 (6–8) | 7 (5–9) | 7 (5–8) | 8 (6–10) | 9 (7–12) | 11 (8–15) | 17 (10–24) | |
| 1994/95 | 7 (6–7) | 7 (5–8) | 7 (6–9) | 8 (6–10) | 8 (7–11) | 10 (8–12) | 14 (10–20) | |
| 1995/96 | 7 (5–10) | 7 (5–9) | 7 (6–8) | 7 (6–9) | 8 (6–10) | 9 (7–13) | 8 (3–12) | |
| 1996/97 | 5 (5–8) | 6 (4–8) | 6 (5–7) | 7 (6–9) | 7 (6–10) | 8 (7–11) | 9 (9–10) | |
| 1997/98 | 5 (5–6) | 5 (4–6) | 6 (5–7) | 6 (5–8) | 6 (5–8) | 8 (6–10) | 11 (8–17) | |
| 1998/99 | 6 (4–7) | 5 (4–7) | 6 (4–7) | 6 (5–7) | 7 (5–8) | 8 (6–11) | 7 (6–12) | |
| 1999/2000 | 5 (4–6) | 5 (4–6) | 6 (4–7) | 6 (5–8) | 7 (5–8) | 7 (6–9) | 9 (7–13) | |
| 2000/01 | 5 (5–7) | 5 (4–6) | 5 (4–7) | 6 (4–7) | 7 (5–8) | 7 (5–10) | 9 (7–13) | |
| 2001/02 | 4 (3–6) | 5 (4–6) | 5 (4–6) | 6 (4–7) | 6 (5–8) | 7 (5–9) | 8 (6–13) | |
| 2002/03 | 6 (4–7) | 5 (4–6) | 5 (4–6) | 6 (4–7) | 6 (4–7) | 7 (5–8) | 7 (5–12) | |
| 2003/04 | 4 (3–6) | 5 (3–6) | 4 (3–6) | 4 (3–6) | 5 (4–7) | 6 (4–8) | 6 (5–9) | |
| 2004/05 | 5 (4–7) | 5 (3–6) | 5 (3–6) | 5 (4–7) | 6 (4–7) | 6 (4–8) | 7 (5–11) | |
| 2005/06 | 5 (3–7) | 4 (3–6) | 4 (3–5) | 5 (4–6) | 5 (4–7) | 5 (4–7) | 5 (4–7) | |
| 2006/07 | 4 (3–5) | 4 (3–5) | 4 (3–5) | 5 (4–6) | 5 (4–7) | 6 (4–8) | 7 (5–11) | |
| 2007/08 | 4 (3–5) | 4 (2–5) | 4 (3–5) | 4 (3–5) | 5 (3–6) | 5 (4–7) | 5 (4–8) | |
| 2008/09 | 4 (2–5) | 4 (2–5) | 4 (2–5) | 4 (3–5) | 5 (3–6) | 5 (3–7) | 6 (4–8) | |
| 2009/10 | 3 (2–4) | 3 (2–5) | 4 (2–5) | 4 (3–5) | 5 (3–6) | 5 (4–7) | 5 (4–7) | |
| 2010/11 | 3 (2–5) | 4 (2–5) | 3 (2–5) | 4 (3–5) | 5 (3–6) | 5 (3–7) | 6 (4–7) | |
| 2011/12 | 3 (2–5) | 4 (2–5) | 3 (2–5) | 4 (3–5) | 5 (3–6) | 5 (4–7) | 5 (3–7) | |
| Total | 5 (3–6) | 5 (3–6) | 5 (3–6) | 5 (4–7) | 5 (4–7) | 6 (4–8) | 6 (5–10) | 7 (6–11) |
IQR = interquartile range.
Cell sizes with < 6 cases were suppressed.
The direct medical cost of the 2 lumbar interventions rose from $13 million in 2002 to $24 million in 2012; overall, $176 million in medical costs accrued during this period (Table 3).
Table 3.
Direct medical cost of lumbar surgery between 2002 and 2011
| Year | Total cost, $ |
|---|---|
| 2002/03 | 13 398 511.89 |
| 2003/04 | 8 108 019.65 |
| 2004/05 | 9 146 695.93 |
| 2005/06 | 11 436 852.60 |
| 2006/07 | 16 971 239.82 |
| 2007/08 | 21 482 207.86 |
| 2008/09 | 23 049 605.23 |
| 2009/10 | 23 532 079.67 |
| 2010/11 | 24 372 346.98 |
| 2011/12 | 24 055 931.22 |
| Cumulative 2002–2011 | 175 553 490.90 |
Discussion
In this population-based study of surgical interventions for degenerative lumbar conditions, we found moderate increases in ILS rates, with the highest growth among patients aged 80 years and older. The overall utilization, of ILS, however, was well below the utilization of knee and total hip arthroplasty.
The rates of ILS utilization were markedly lower in this study than those reported in a similar population-based US study. Rajaee and colleagues reported that annual rates of lumbar fusion increased from 64.5 to 135.5 per 100 000 adults from 1998 to 2008, an increase approximately 10-fold higher in absolute terms than reported here.7 Our finding is consistent with work by Lavis and colleagues, who noted a 3.4-fold higher rate of spinal fusion for mechanical neck and back pain in the US compared with Ontario.25 Our lumbar surgery rate estimates are slightly lower than those reported in a 2009 study using similar databases, which demonstrated a 40% increase in the rate of procedures between 1995 and 2001 in Ontario.26 This was probably because Bederman and colleagues’ cohort was restricted to people under the age of 50 years, resulting in an enriched population with a higher prevalence of lumbar surgery.
Although rates of spinal fusion in the US now approach those of hip and knee replacement,7,17 similar trends were not seen in Ontario, where ratios of ILS to hip and knee replacements were approximately 1:8 and 1:14, respectively. Several health system and policy factors probably contributed to this divergence, especially resource allocation decisions in Canada’s single-payer system. In response to the 2003 First Ministers’ Accord on Health Care Renewal and the 10-Year Plan to Strengthen Health Care signed during the following year, $5.5 billion was allocated over 10 years to reduce wait times in Canada.27 Strategic priority was given to 5 clinical areas, 1 of which was hip and knee arthroplasties. In a setting where hospitals receive global operating room budgets, it is expected that targeted orthopedic procedures would receive higher utilization at the expense of alternative surgeries. It is important to note, however, that the level of evidence associated with the effectiveness of joint replacement is much stronger than that associated with spinal fusions: for example, 2 randomized controlled trials of unilateral total knee replacement among patients with moderate to severe osteoarthritis demonstrated markedly improved pain relief and functional improvement at 12 months.28,29 The role of spinal fusion for low back pain associated with spinal stenosis or spondylolisthesis remains uncertain, especially in the context of recent trials.30 Publication of the Swedish Spinal Stenosis Study demonstrated no clinical benefit associated with adjunctive fusion in addition to decompression for spinal stenosis with or without spondylolisthesis.31 Similarly, the Greenwich Lumbar Stenosis SLIP trial, performed among patients with stable spondylolisthesis, demonstrated potentially more favourable value with decompression alone given the costs of instrumented fusion, and the inability of moderate gains in general quality- of-life outcome to translate to improvement in disease-specific measures.32 The impact of these 2 recent trials on changes in fusion surgery trends would be crucial to reconcile with data from the earlier Spine Patient Outcomes Research Trial (SPORT),33 to ensure that the knowledge gained from these pragmatic comparative effectiveness trials is translated and disseminated in routine care. A recent Ontario comparative outcome and cost-utility study with a retrospective cohort design compared focal lumbar spinal stenosis surgery to total hip and knee arthroplaties and found that the 3 types of procedures offered durable and favourable cost-utility ratios. Similar evaluations of these health technologies in the real-world context, using methodologies to account for residual confounding, are needed to expand the evidence base for ILS in settings other than randomized controlled trials.37
Additionally, we found that close to 1 in 3 patients who received ILS in Ontario were younger than 50 years, and the annual rate of procedures for those aged 80 years and older grew close to 8-fold. The increasing utilization of spinal fusion in the very elderly mirrors trends observed in the US, where age-adjusted rates of fusion increased from 40 to 102 procedures per 100 000 persons.34 Although the rate of adverse events of lumbar fusion among the very elderly was noted to be low, in-hospital mortality was nonetheless higher in this age group than in patients aged 65–79 years.34 Furthermore, this group was poorly represented in the clinical trials in which the efficacy of lumbar fusion was evaluated.31–33
The major strength of our study is its extended time-frame spanning nearly 2 decades, which provides important longitudinal context and secular trends by which to evaluate more recent changes in the case mix of spinal surgical interventions. In addition, our use of administrative data in a jurisdiction with universal medical coverage captures comprehensive, population-based trends. Meanwhile, the high quality of these routinely audited databases, with codes previously validated in the spine population,26 provides confidence about the robustness of our findings.
Limitations
Several limitations need to be acknowledged. First, it is recognized that several methods of case-costing exist, including direct use of data from the Ontario Case Costing Initiative (OCCI), which are used to generate standardized per-case cost in the province. However, OCCI costs are not recorded for all hospitals, and the %getcost macro was used to encompass all hospitals performing ILS in the province. Second, outcomes of ILS were outside the scope of this study, including postsurgical opioid use. Although reductions in opioid analgesic use have been used in randomized controlled trials of spinal devices to assess clinical outcomes,35,36 there has been no efficacy evaluation of spinal fusion surgery at the population-level using administrative medication databases, an important area of exploration. Third, although surgical indications for ILS procedures, including spondiloliesthsis and lumbar stenosis, are generally associated with radicular pain, the nature of administrative databases precluded confirmation of symptoms in the preoperative setting. Similarly, the database we used is unable to definitively separate isthmic and degenerative spondylolisthesis, despite patients with fractures having been excluded from our study. Finally, administrative data are inherently susceptible to miscoding, and errors between interbody fusion and lumbar disc replacement remain possible given the novelty of the latter procedure. Therefore, rates of fusion and lumbar disc replacement were not separately analyzed; rather, these 2 types of procedures were unified as instrumental lumbar surgeries.
Conclusion
In a population-based cohort spanning 19 years, there was a modest increase in utilization of ILS among Ontario adults. Use of these lumbar procedures has grown rapidly among the very elderly, a group poorly represented in the randomized trials in which the efficacy of the procedures was rigorously evaluated. In contrast to US trends, however, utilization was well below that observed for joint arthroplasties. The observed decline in length of stay associated with ILS was offset by increased utilization that contributed to the rise in overall direct medical costs for these procedures. Given our finding of increasing utilization of and spending for ILS in Ontario, research aimed at determining both the clinical effectiveness and the cost-effectiveness of these procedures in the long-term, real-world setting35 using patient-oriented outcomes is urgently needed to form an evidence base to inform practice and policy.
Footnotes
Competing interests: None declared.
Contributors: Y. Xu, D. Yen and A. Johnson designed the study. Y. Xu, M. Whitehead, J. Xu and A. Johnson acquired the data, which Y. Xu, D. Yen, J. Xu and A. Johnson analyzed. Y. Xu wrote the article, which all authors reviewed and approved for publication. All authors agreed to be accountable for all aspects of the work.
References
- 1.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine. 2006;31:2724–7. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 2.Ekman M, Jonhagen S, Hunsche E, et al. Burden of illness of chronic low back pain in Sweden: a cross-sectional, retrospective study in primary care setting. Spine. 2005;30:1777–85. doi: 10.1097/01.brs.0000171911.99348.90. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JN, Lurie JD, Olson PR, et al. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31:2707–14. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441, 5. doi: 10.1097/01.brs.0000166503.37969.8a. discussion 1446–7. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res. 2006;443:139–46. doi: 10.1097/01.blo.0000198726.62514.75. [DOI] [PubMed] [Google Scholar]
- 7.Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27:E9. doi: 10.3171/2009.7.FOCUS09121. [DOI] [PubMed] [Google Scholar]
- 9.Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015;15:265–71. doi: 10.1016/j.spinee.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA. Fusion surgery for lumbar degenerative disc disease: still more questions than answers. Spine J. 2015;15:272–4. doi: 10.1016/j.spinee.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Brox JI, Reikerås O, Nygaard Ø, et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: a prospective randomized controlled study. Pain. 2006;122:145–55. doi: 10.1016/j.pain.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Brox JI, Nygaard OP, Holm I, et al. Four-year follow-up of surgical versus non-surgical therapy for chronic low back pain. Ann Rheum Dis. 2010;69:1643–8. doi: 10.1136/ard.2009.108902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brox JI, Sørensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine. 2003;28:1913–21. doi: 10.1097/01.BRS.0000083234.62751.7A. [DOI] [PubMed] [Google Scholar]
- 14.Fairbank J, Frost H, Wilson-MacDonald J, et al. Spine Stabilisation Trial Group. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ. 2005;330:1233. doi: 10.1136/bmj.38441.620417.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou R1, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidencebased clinical practice guideline from the American Pain Society. Spine. 2009;34:1066–77. doi: 10.1097/BRS.0b013e3181a1390d. [DOI] [PubMed] [Google Scholar]
- 16.Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine. 2009;34:1094–109. doi: 10.1097/BRS.0b013e3181a105fc. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery — the case for restraint. N Engl J Med. 2004;350:722–6. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi R, Perruccio AV, Mahomed NN. Surgical management of hip osteoarthritis. CMAJ. 2014;186:347–55. doi: 10.1503/cmaj.121584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Technologies for osteoarthritis of the knee: an evidence-based analysis. [Internet] Toronto, ON: Ontario Health Technology Advisory Committee; 2005. [accessed 2019 Aug 29]. Available from: http://www.hqontario.ca/english/providers/program/mas/tech/reviews/pdf/rev_ostknee_100105.pdf. [Google Scholar]
- 20.Bederman SS, Coyte PC, Kreder HJ, et al. Who’s in the driver’s seat? The influence of patient and physician enthusiasm on regional variation in degenerative lumbar spinal surgery: a population-based study. Spine. 2011;36:481–9. doi: 10.1097/brs.0b013e3181d25e6f. [DOI] [PubMed] [Google Scholar]
- 21.Faciszewski T, Broste SK, Fardon D. Quality of data regarding diagnoses of spinal disorders in administrative databases. A multicenter study. J Bone Joint Surg Am. 1997;79:1481–8. doi: 10.2106/00004623-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Paterson JM, Williams JI, Kreder HJ, et al. Provider volumes and early outcomes of primary total joint replacement in Ontario. Can J Surg. 2010;53:175–83. [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Clinical Evaluative Sciences. Canadian Institute for Health Information Discharge Abstract Database: a validation study. Toronto: ICES; 2006. [Google Scholar]
- 24.Wodchis WP, Bushmeneva K, Nikitovic M, et al. Guidelines on person-level costing using administrative databases in Ontario. Vol. 1. Toronto: Health System Performance Research Networ; 2013. (Working Paper Series). [Google Scholar]
- 25.Lavis JN, Malter A, Anderson GM, et al. Trends in hospital use for mechanical neck and back problems in Ontario and the United States: discretionary care in different health care systems. CMAJ. 1998;158:29–36. [PMC free article] [PubMed] [Google Scholar]
- 26.Bederman SS, Kreder HJ, Weller I, et al. The who, what and when of surgery for the degenerative lumbar spine: a population-based study of surgeon factors, surgical procedures, recent trends and reoperation rates. Can J Surg. 2009;52:283–90. [PMC free article] [PubMed] [Google Scholar]
- 27.Wading through wait times: What do meaningful reductions and guarantees mean? Toronto, ON: Health Council of Canada; 2007. [accessed 2019 Aug 29]. Available from: http://publications.gc.ca/collections/collection_2007/hcc-ccs/H174-7-2007E.pdf. [Google Scholar]
- 28.Johnston L, MacLennan G, McCormack K, et al. The Knee Arthroplasty Trial (KAT) design features, baseline characteristics, and two-year functional outcomes after alternative approaches to knee replacement. J Bone Joint Surg Am. 2009;91:134–41. doi: 10.2106/JBJS.G.01074. [DOI] [PubMed] [Google Scholar]
- 29.Skou ST, Roos EM, Laursen MB, et al. Randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373:1597–606. doi: 10.1056/NEJMoa1505467. [DOI] [PubMed] [Google Scholar]
- 30.Peul WC, Moojen WA. Fusion for lumbar spinal stenosis — Safeguard or superfluous surgical implant? N Engl J Med. 2016;374:1478–9. doi: 10.1056/NEJMe1600955. [DOI] [PubMed] [Google Scholar]
- 31.Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374:1413–23. doi: 10.1056/NEJMoa1513721. [DOI] [PubMed] [Google Scholar]
- 32.Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374:1424–34. doi: 10.1056/NEJMoa1508788. [DOI] [PubMed] [Google Scholar]
- 33.Tosteson AN, Lurie JD, Tosteson TD, et al. SPORT Investigators. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med. 2008;149:845–53. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96:1185–91. doi: 10.2106/JBJS.M.01126. [DOI] [PubMed] [Google Scholar]
- 35.Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;32:1155, 62. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 36.Blumenthal S1, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Part I: Evaluation of clinical outcomes. Spine (Phila Pa 1976) 2005;30:1565, 75. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 37.Rampersaud YR, Tso P, Walker KR, et al. Comparative outcomes and cost-utility following surgical treatment of focal lumbar spinal stenosis compared with osteoarthritis of the hip or knee: Part 2 —estimated life-time incremental cost-utility ratios. Spine J. 2014;14:244–54. doi: 10.1016/j.spinee.2013.11.011. [DOI] [PubMed] [Google Scholar]