Abstract
Histatin, a salivary protein, affects oral homeostasis through preservation of tooth integrity and protection against caries and fungal infections. However, the effects of histatin in the generation of oxidative stress induced by reactive oxygen species and in the oral cavity remain unclear. In this study, the effects of histatin on direct reactive oxygen species scavenging activity were examined using electron spin resonance. We demonstrated, for the first time, that histatin exhibits antioxidant activity against hydroxyl radicals generated by Fenton’s reaction by metal chelation or binding. The direct antioxidant effects of histatin, along with its antimicrobial activity, may be important in the oral protection of salivary proteins.
Keywords: saliva, histatin, oxidative stress, reactive oxygen species, antioxidant
Introduction
Salivary proteins play crucial roles in oral health as well as several lifestyle-related diseases through multiple host defense functions.(1) These include homeostatic processes, lubrication, antimicrobial activity, and tooth demineralization/remineralization. These salivary elements may function as active indicators of both local and systemic disorders.(2) Hyposalivation may also be a risk factor for acute respiratory infection.(3) Because a number of salivary proteins have been identified, and their primary structures determined, it has become possible to explore their structure/function relationships. Saliva (oral fluid) is a biofluid with a perceived role in the protection of oral cavity surfaces against chemical, mechanical, and microbial attacks.(4) Involved in this protective role is a complex mixture of proteins and peptides derived from salivary glands, gingival exudate, and cellular debris.(5–9) Of these components, approximately 30% are low molecular weight proteins, commonly referred to as salivary peptides,(10,11) which are assigned to four main classes: cystatins, histatins, statherin, and proline-rich proteins. These salivary peptides impact oral cavity homeostasis through preservation of tooth integrity,(12) protection from dental caries,(13) and avoidance of fungal infections.(14)
Reactive oxygen species (ROS) is a collective term for radical species of O2•−, HO•, nitric oxide, and non-radical oxygen derivatives. ROS production is a normal part of cellular metabolism. However, ROS overproduction disrupts tissue redox balance, inducing oxidative damage to DNA, lipids, and proteins.(15,16) Increased ROS level or reduced antioxidant function, including ROS overproduction or impaired ROS removal, is referred to as oxidative stress, and may lead to several conditions.(16–18) ROS is toxic to cells via enzyme inactivation, protein denaturation, DNA destruction, and lipid peroxidation.(16–18) These events increase reactive aldehyde levels and lead to cell membrane damage.(16–18)
Oxidative stress is implicated in various lifestyle-related diseases, including atherosclerosis, myocardial infarction, cerebrovascular disease, diabetes mellitus, cancer, and osteoporosis.(16–18) Furthermore, ROS causes loss of salivary antioxidant capacity, leading to the development of oral cancer in tobacco chewers and smokers.(19,20) Antioxidant systems including antioxidant enzymes and antioxidants play a protective role by scavenging ROS.(21–23)
Histatin belongs to a family of slightly basic 3–4 kDa peptides containing multiple histidine residues.(4,24) These peptides are secreted by parotid, submandibular, and sublingual glands, and were first characterized in the early 1970s as peptides that enhance the glycolytic activity of microorganisms.(25,26) It was later reported that they have bactericidal and fungicidal properties.(27,28) Structure-function studies on these proteins have identified distinct domains with specific functional properties. They display antifungal activity against a broad range of pathogens, including Candida albicans, Cryptococcus neoformans, and Aspergillus fumigates, and have antibacterial properties based on their killing and growth-inhibitory activity against various species of oral bacteria.(29) The main human histatins are histatin 1, 3, and 5.(30) Like other phosphorylated salivary proteins, histatin 1 is involved in the maintenance of tooth enamel mineral and pellicle formation.(31) Among the histatins, histatin 5 displays the highest antifungal activity,(30) and antifungal domains have been located in its N-terminal and middle regions. A segment spanning residues 4–15, designated P-113, has been evaluated for therapeutic efficacy in in vivo oral candidiasis.(32,33) Recently, it has been reported that histatins 1 and 3, but not histatin 5, exhibit wound closure activities in vitro.(34) The inactivity of histatin 5, comprising the 24 N-terminal residues of histatin 3, indicated that the C-terminal 8 residues in histatin 3 are essential for this activity. Because the last 7 of these 8 residues are homologous with the C terminus of histatin 1, this segment is possibly responsible for the wound-healing properties of histatins 1 and 3. Histatins also show affinity for mineral surfaces, reduce calcium phosphate precipitation, and maintain tooth integrity.(35)
The identification of functional regions within salivary proteins is critical to the development of artificial saliva. However, questions remain regarding the roles of salivary proteins, especially histatin, in ROS generation and oxidative stress in the oral cavity. Few studies have investigated the antioxidant effects of salivary proteins by measuring SOD level(36,37) or lipid peroxidation.(38) While the effect of copper-mediated oxidation of histatin 8 on the generation of HO•, as evaluated by electron spin resonance (ESR), has been reported,(39) the direct effects of histatin on ROS generation have not been investigated. In the current study, we used ESR to investigate the effects of histatin on ROS scavenging effects. Our results provide the first direct evidence of the antioxidant properties of histatin.
Materials and Methods
Reagents
Xanthine oxidase (XO) [grade III: from buttermilk, chromatographically purified suspension in 2.3 M (NH4)2SO4, 10 mM sodium phosphate buffer (pH 7.8), containing 1 mM EDTA and 1 mM sodium salicylate], xanthine, and superoxide dismutase were obtained from Sigma (St. Louis, MO). Hydrogen peroxide (H2O2) and FeSO4 were obtained from Wako Chemical (Osaka, Japan). 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) was purchased from Labotec (Tokyo, Japan).
Histatin
Synthetic histatin 1, 3, and 5 were obtained from the American Peptide Company (Sunnyvale, CA) and from Quality Controlled Biochemicals (Hopkinton, MA). Human parotid secretion (HPS) was collected from five healthy volunteers ranging in age from 25 to 38 years. Informed consent was obtained according to approved protocols of the Institutional Review Board at Boston University Medical Center. HPS was collected with a Curby cup device positioned over the orifice of the Stensen’s duct. HPS flow was stimulated with hard sour candies and the secretion was collected in graduated cylindrical tubes placed on ice.(40) A 25-µl aliquot of PS was plated on blood agar (Hardy Diagnostics) to verify the sterility of the collected PS secretion (absence of water-soluble contamination). Dialyzed and lyophilized PS proteins were dissolved in buffer A consisting of 50 mM Tris/HCl and 50 mM NaCl, (pH = 8), applied to a MonoQ HR16/10 column (Amersham Biosciences, Uppsala, Sweden), and eluted at a flow rate of 2 ml/min with buffer B containing 50 mM Tris/HCl and 1 M NaCl (pH = 8) using the following gradient steps: 0–38 min: 0–13% buffer B; 38–233 min: 13–22% buffer B; 233–250 min: 22–40% buffer B. The purity of the synthetic histatins was verified by cationic, anionic PAGE, and reversed-phase analysis.(41)
Determination of protein concentrations
Sample protein concentrations were measured using a micro-bicinchoninic acid (BCA) protein assay (Pierce Chemical, Co., Rockford, IL), with bovine serum albumin used as a protein standard.
In vitro ESR measurement
HO• was generated by the Fenton reaction (H2O2 plus FeSO4 or CuSO4) as described previously.(21,22) The reaction mixtures comprised H2O2 (20 µM) and FeSO4 (20 µM) or CuSO4 (20 µM) in 0.1 M phosphate-buffered saline (pH 7.2) containing 50 mM 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as spin trap, with or without salivary protein pretreatment, respectively. Mixtures were transferred to a cell and the DMPO-OH spin adduct was measured using ESR.
For generation of HO• by UV irradiation of H2O2, a reaction mixture containing 10 mM DMPO and H2O2 (20 mM) in 0.1 M phosphate-buffered saline (pH 7.2), with or without salivary protein pretreatment, was used. Mixtures were transferred to a cell and illuminated at 365 nm, 40 mW using a PAN UV lamp. After 20 s, the DMPO-OH spin adduct was measured with ESR.(21,22)
O2•− was generated using the xanthine-XO system, as described previously.(21,22) O2•− was generated from XO (0.1 U/ml) and xanthine (362 µM) in 0.1 µM phosphate-buffered saline (pH 7.2) containing 50 mM DMPO with or without salivary protein pretreatment, respectively. The mixtures were transferred to a cell and the DMPO-OOH spin adduct was measured with ESR.
ESR observations were performed with a JES-RE 3X, X-band spectrometer (JEOL, Tokyo, Japan) connected to a WIN-RAD ESR Data Analyzer (Radical Research, Tokyo, Japan) at the following instrument settings: microwave power, 8.00 mW; magnetic field, 334.8 ± 5 mT; field modulation width, 0.079 mT; receiver gain, 400; sweep time, 1 min; and time constant, 0.03 s. Hyperfine coupling constants were calculated based on resonance frequency, measured with a microwave frequency counter, and on resonance field, measured with a JEOL ES-FC5 field measurement unit. ESR spectra were used to quantify the detected spin adducts for manganese oxide standards. After recording ESR spectra, their signal intensities, expressed as relative height, were normalized against the signal intensity of the manganese oxide standard.(21,22) All experiments were repeated a minimum of four times.
Statistical analysis
Statistical analyses were performed using Dunnett (OMS, Saitama, Japan). Data were tested for normality. Results are presented as mean ± SD. Two-way analysis of variance was used to compare the averages of three or four concentration levels. P values <0.05 were considered statistically significant.
Results
Effects of histatin 1, 3, and 5 on HO• generation by the Fenton system
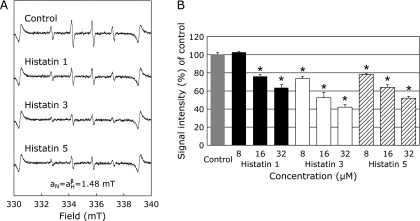
The effects of histatin 1, 3, and 5 on HO• generated from the Fenton reaction were investigated by ESR spin trapping with DMPO. As reported previously,(21,22) after adding H2O2 to FeSO4, a characteristic DMPO-OH spin adduct spectrum with hyperfine splitting giving rise to four resolved peaks was observed (Fig. 1A, control). Results indicate that the DMPO-OH signal was significantly reduced in a dose-dependent manner, except for the pretreatment of a final concentration of 8 µM of histatin 1 (Fig. 1A and B).
Fig. 1.
Effects of histatin 1, 3, and 5 (8, 16, and 32 µM) on HO• generation from the Fenton reaction using FeSO4. (A) ESR spin trapping measurement of HO• generated from H2O2 and FeSO4 in 0.1 M PBS, 50 mM DMPO as spin trap in the absence of histatin (control), and with histatin 1, 3, and 5 pretreatment at 32 µM, respectively. (B) Dose-response of histatins or control on HO• generation from the Fenton reaction using FeSO4. *Significantly different (p<0.05) from the corresponding control value.
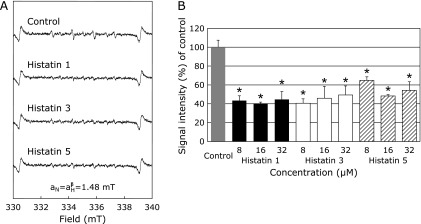
The effects of histatin 1, 3, and 5 on HO• generated using CuSO4, instead of FeSO4, were also investigated. Though the peaks were smaller than those generated using FeSO4, a characteristic DMPO-OH spin adduct spectrum with hyperfine splitting giving rise to four resolved peaks was observed following addition of H2O2 to CuSO4, (Fig. 2A, control). With histatin 1, 3, and 5 (8, 16, and 32 µM) pretreated with CuSO4 and subsequent addition of H2O2, the DMPO-OH signal was significantly reduced in comparison to the control (Fig. 2A and B).
Fig. 2.
Effects of histatin 1, 3, and 5 (8, 16, and 32 µM) or control on HO• generation from the Fenton reaction using CuSO4. (A) ESR spin trapping measurement of HO• generation from H2O2 and CuSO4 in 0.1 M PBS, 50 mM DMPO as spin trap in the absence of histatin (control), or with histatin 1, 3, and 5 pretreatment at 32 µM, respectively. (B) Dose-response of histatins 1 or control on HO• generation from the Fenton reaction using CuSO4. *Significantly different (p<0.05) from the corresponding control value.
Effects of histatin 1, 3, and 5 on HO• generation by ultraviolet irradiation of H2O2
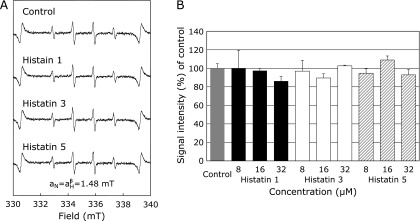
The effects of histatin 1, 3, and 5 on HO• generated from UV irradiation of H2O2 were investigated by ESR spin trapping with DMPO. As reported previously,(21,22) following UV irradiation of H2O2, a characteristic DMPO-OH spin adduct spectrum with hyperfine splitting giving rise to four resolved peaks (Fig. 3A, control) was observed. When H2O2 was pretreated with histatin 1, 3, and 5, (8, 16, and 32 µM) and followed by UV irradiation, the DMPO-OH signal was not significantly reduced (Fig. 3A and B).
Fig. 3.
Effects of histatins 1, 3, and 5 (8, 16, and 32 µM) or control on HO• generation from ultraviolet (UV) irradiation and H2O2. ESR spin trapping measurement of HO• generation from UV irradiation and H2O2 in 0.1 M PBS, 50 mM DMPO as spin trap in the absence of histatins (control), or with histatin 1, 3, and 5 pretreatment at 32 µM, respectively. (B) The dose-response of histatins and control on HO• generation from UV irradiation and H2O2 is represented.
Effects of histatin 1, 3, and 5 on O2•− generation
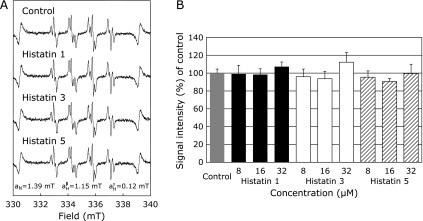
The effects of histatin 1, 3, and 5 on XO-mediated O2•− generation were determined using ESR spin trapping with DMPO. As reported previously,(21,22,42) after addition of xanthine to XO, a characteristic DMPO-OOH adduct spectrum with hyperfine splitting giving rise to 12 resolved peaks was observed (Fig. 4A). These signals were quenched by 150 U/ml superoxide dismutase, confirming that they were derived from O2•− (data not shown). With histatin 1, 3, and 5 pretreatment of XO and subsequent addition of xanthine, however, alteration of the DMPO-OOH signal was not observed at any histatin concentration (Fig. 4A and B).
Fig. 4.
Effects of histatin 1, 3, and 5 (8, 16, and 32 µM) on O2•− generation from xanthine oxidase (XO) and xanthine. (A) Electron spin resonance spin trapping measurement of O2•− generation from XO (0.1 U/ml) and xanthine (362 µM) in 0.1 M PBS, 440 mM DMPO as spin trap in the absence of histatins (control), or with histatin 1, 3, and 5 pretreatment at a final concentration of 32 µM, respectively. (B) Dose-response of histatin 1, 3, and 5 or control on O2•− generation from XO and xanthine.
Discussion
Histatin a human salivary protein, has antifungal activity and is susceptible to enzymatic degradation when released into the oral cavity(43) Histatin 5 exhibits an antifungal effect by decreasing cell metabolism in Candida albicans.(44,45) Histatin is unique to saliva and is rich in basic amino acid residues, which bind to the cell membranes of yeast and fungi, destroying their membrane structure. While mitochondrial-derived ROS is known to be involved in this antifungal action,(43,44) the direct effect of histatin on ROS production has not been reported. In this study, we used ESR to evaluate the effect of histatin on ROS generation, showing that it reduced HO• generation, but did not decrease O2•− generation, from xanthine-XO (Fig. 1–4).
First, we confirmed that histatin 1, 3, and 5 suppressed HO• generation by the Fenton reaction in a dose-dependent manner (Fig. 1A and C). Pathophysiological Fenton responses due to iron overload are reported by the ability of iron chelators, such as Desferal, to reduce high-intensity signals from DMPO-OH spin adducts, which are suggestive of HO• formation.(46,47) The production of HO• from Fenton’s reaction in the living body is important in various ROS-induced diseases, including oral diseases.(16,47,48)
All histatins are enriched in histidine, an amino acid capable of complexing with divalent metal ions. Histatin is also activated by complexation of its three N-terminal amino acids (NH2-Asp-Ser-His-) with Cu2+ ions through modification of the ATCUN motif as metal binding site.(49,50) Therefore, in order to investigate the effects on Cu2+ ion-mediated ROS production, we examined the effects of histatin on HO• produced from Cu2+ and H2O2. Interestingly, histatin significantly inhibited HO• generation (Fig. 2). Furthermore, in Fenton’s reaction with CuSO4, histatin 1, 3, and 5 suppressed the production of HO•, even at low concentrations (Fig. 2). These results suggest that histatin may have two physiological effects: antifungal action activated by Cu2+, and antioxidant effects by scavenging Cu2+ ion-related ROS.
In biological systems, homeostatic balance is maintained between ROS production and removal. This occurs even in the oral cavity, and disruption of this balance due to increased production of ROS increases the risk of oral disease.(51,52) Additionally, oxidative stress due to balance modulation of ROS and antioxidants increased production of ROS related to oral diseases such as periodontitis, and to systemic diseases such diabetes and cardiovascular disease(48,53) ROS is one of the most effective pathogenic mechanisms of chronic inflammation caused by bacteria, and undoubtedly leads to bone resorption.(54,55) Neutrophils obtained from the peripheral blood of acute apical periodontitis (AAP) patients show increased production of ROS, particularly in response to treatment of chronic periapical granuloma.(56) In addition, antioxidant salivary vitamins are known to be effective against oxidative stress caused by oral diseases such as oral lichen planus.(57) We confirmed that histatin suppressed the production of HO• from the Fenton reaction using FeSO4 or CuSO4 with H2O2 (Fig. 1 and 2). These results are the first direct evidence of the antioxidative effects by histatin. HO• is generated by the biological Fenton reaction, as shown below in equations 1–3, and is critical in oxidative stress-induced diseases, including oral diseases.(16,46,47) The finding that histatin scavenges HO• produced by this reaction is an interesting evidence of a new role for the salivary oral defense system.
Fe2+ + H2O2 → Fe3+ + OH− + HO• (1)
Cu2+ + H2O2 → Cu+ + HOO• + H+ (2)
Cu+ + H2O2 → Cu2+ + HO− + HO• (3)
We also investigated the effects of salivary proteins on other HO• generating systems using UV irradiation of H2O2, a well-known Fe2+-independent reaction.(21,22) Histatins did not affect HO• generation by this system (Fig. 3). In our previous reports, Desferal, an iron chelator, reduced the high intensity signal of the ESR DMPO-OH spin adduct, which indicates the production of HO•.(45,47) Therefore, combined with the results presented in Fig. 1–3, it appears that histatin’s HO• scavenging effect is due to chelation of Fe2+ or Cu2+ ions, and not to direct scavenging of HO• (Fig. 3), although the possibility of the oxidation of Fe2+ to Fe3+ by the reaction with histatin is not ruled out. In this study, it was confirmed that Fe2+ and Cu2+ inhibit HO• formation via their chelating action (Fig. 2 and 3) by directly interacting with the Zn/Cu binding motif and/or amino acids within histatin 5.(49,50) Further detailed studies on the influence of the formation of ROS by the reaction with histatin motif, amino acids, and metal ions are needed.
The amount of HO• formed by CuSO4 was much smaller than that by FeSO4 (Fig. 1 and 2) because the rate constant of HO• formation by Cu+ ions is larger than that by Cu2+ ions (equations 1–3). Although we should have used HO• formation by Cu+ to examine the effect of histatin on HO• formation in this study, we used the HO• generation system using Cu2+ since it was reported that histatin has a binding site for divalent metal ions (Cu2+ and Zn2+).(48,49) In the future, we would study the effects of histatin on HO• formation, including the biokinetics of Cu (I, II).
Histatins did not affect O2•− generation from XO and xanthine (Fig. 4). O2•− and nitric oxide, either alone or through interaction, are responsible for the physiological, pathophysiological, biological role in vivo. Histatin does not directly scavenge O2•−, but reduces HO• generation by Fenton’s reaction (Fig. 1, 2, and 4). While O2•−, which plays a critical physiological role and has weak oxidizing power, was not eliminated, histatin displayed excellent antioxidant effects against HO• (Fig. 1–4). This showed that histatin functions as an effective defense against oral oxidative stress. Saliva is known to possess a variety of physiologically active substances, some of which have bactericidal effects. The antimicrobial action of histatin could be linked to the antioxidant effect observed in this study (Fig. 1–4).
In conclusion, ESR was used to demonstrate, for the first time, that histatin 1, 3, and 5 display antioxidant activity against HO• by chelation or direct binding of Fe2+ or Cu2+. Direct evidence of histatin’s effects suggest that the antioxidant action of salivary protein may be important to antibacterial action in the oral cavity.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (grant number 23593049 to TK & ML; grant number 26463125 to TK & ML; grant number 16K11901 to ML & TK; grant number 19K10295 to ML & TK) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (JSPS KAKENHI).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nishioka H, Nishi K, Kyokane K. Human saliva inactivates mutagenicity of carcinogens. Mutat Res 1981; 85: 323–333. [DOI] [PubMed] [Google Scholar]
- 2.Burbelo PD, Bayat A, Lebovitz EE, Iadarola MJ. New technologies for studying the complexity of oral diseases. Oral Dis 2012; 18: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwabuchi H, Fujibayashi T, Yamane GY, Imai H, Nakao H. Relationship between hyposalivation and acute respiratory infection in dental outpatients. Gerontology 2012; 58: 205–211. [DOI] [PubMed] [Google Scholar]
- 4.Schenkels LC, Veerman EC, Nieuw Amerongen AV. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med 1995; 6: 161–175. [DOI] [PubMed] [Google Scholar]
- 5.Amado FM, Vitorino RM, Domingues PM, Lobo MJ, Duarte JA. Analysis of the human saliva proteome. Expert Rev Proteomics 2005; 2: 521–539. [DOI] [PubMed] [Google Scholar]
- 6.Cabras T, Pisano E, Boi R, et al. Age-dependent modifications of the human salivary secretory protein complex. J Proteome Res 2009; 8: 4126–4134. [DOI] [PubMed] [Google Scholar]
- 7.Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J Dent Res 2007; 86: 680–693. [DOI] [PubMed] [Google Scholar]
- 8.Lendenmann U, Grogan J, Oppenheim FG. Saliva and dental pellicle--a review. Adv Dent Res 2000; 14: 22–28. [DOI] [PubMed] [Google Scholar]
- 9.Schipper RG, Silletti E, Vingerhoeds MH. Saliva as research material: biochemical, physicochemical and practical aspects. Arch Oral Biol 2007; 52: 1114–1135. [DOI] [PubMed] [Google Scholar]
- 10.Beeley JA. Fascinating families of proteins: electrophoresis of human saliva. Biochem Soc Trans 1993; 21: 133–138. [DOI] [PubMed] [Google Scholar]
- 11.Vitorino R, Lobo MJ, Duarte JR, Ferrer-Correia AJ, Domingues PM, Amado FM. The role of salivary peptides in dental caries. Biomed Chromatogr 2005; 19: 214–222. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim FG, Salih E, Siqueira WL, Zhang W, Helmerhorst EJ. Salivary proteome and its genetic polymorphisms. Ann N Y Acad Sci 2007; 1098: 22–50. [DOI] [PubMed] [Google Scholar]
- 13.Rudney JD, Staikov RK, Johnson JD. Potential biomarkers of human salivary function: a modified proteomic approach. Arch Oral Biol 2009; 54: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Salih E, Oppenheim FG, Helmerhorst EJ. Kinetics of histatin proteolysis in whole saliva and the effect on bioactive domains with metal-binding, antifungal, and wound-healing properties. FASEB J 2009; 23: 2691–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 1994; 344: 721–724. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 2007; 35 (Pt 5): 1147–1150. [DOI] [PubMed] [Google Scholar]
- 17.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011; 283: 65–87. [DOI] [PubMed] [Google Scholar]
- 18.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 2015; 4: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beevi SS, Rasheed AM, Geetha A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jpn J Clin Oncol 2004; 34: 379–385. [DOI] [PubMed] [Google Scholar]
- 20.Nagler R, Dayan D. The dual role of saliva in oral carcinogenesis. Oncology 2006; 71: 10–17. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Yoshino F, Takahashi SS, et al. Direct assessments of the antioxidant effects of propofol medium chain triglyceride/long chain triglyceride on the brain of stroke-prone spontaneously hypertensive rats using electron spin resonance spectroscopy. Anesthesiology 2008; 109: 426–435. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Maehata Y, Kawamura Y, et al. Direct assessments of the antioxidant effects of the novel collagen peptide on reactive oxygen species using electron spin resonance spectroscopy. J Pharmacol Sci 2011; 116: 97–106. [DOI] [PubMed] [Google Scholar]
- 23.Patel BP, Rawal UM, Shah PM, et al. Study of tobacco habits and alterations in enzymatic antioxidant system in oral cancer. Oncology 2005; 68: 511–519. [DOI] [PubMed] [Google Scholar]
- 24.Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res 2000; 14: 16–21. [DOI] [PubMed] [Google Scholar]
- 25.De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 2005; 27: 1337–1347. [DOI] [PubMed] [Google Scholar]
- 26.Holbrook IB, Molan PC. A further study of the factors enhancing glycolysis in human saliva. Arch Oral Biol 1973; 18: 1275–1282. [DOI] [PubMed] [Google Scholar]
- 27.MacKay BJ, Denepitiya L, Iacono VJ, Krost SB, Pollock JJ. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun 1984; 44: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock JJ, Denepitiya L, MacKay BJ, Iacono VJ. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun 1984; 44: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gusman H, Travis J, Helmerhorst EJ, Potempa J, Troxler RF, Oppenheim FG. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect Immun 2001; 69: 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu T, Levitz SM, Diamond RD, Oppenheim FG. Anticandidal activity of major human salivary histatins. Infect Immun 1991; 59: 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppenheim FG, Yang YC, Diamond RD, Hyslop D, Offner GD, Troxler RF. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem 1986; 261: 1177–1182. [PubMed] [Google Scholar]
- 32.Rothstein DM, Helmerhorst EJ, Spacciapoli P, Oppenheim FG, Friden P. Histatin-derived peptides: potential agents to treat localised infections. Exp Opin Emerg Drugs 2002; 7: 47–59. [DOI] [PubMed] [Google Scholar]
- 33.Rothstein DM, Spacciapoli P, Tran LT, et al. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother 2001; 45: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oudhoff MJ, Bolscher JG, Nazmi K, et al. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J 2008; 22: 3805–3812. [DOI] [PubMed] [Google Scholar]
- 35.Lamkin MS, Oppenheim FG. Structural features of salivary function. Crit Rev Oral Biol Med 1993; 4: 251–259. [DOI] [PubMed] [Google Scholar]
- 36.Singh H, Shetty P, Sreelatha SV, Patidar M. Analysis of salivary antioxidant levels in different clinical staging and histological grading of oral squamous cell carcinoma: noninvasive technique in dentistry. J Clin Diagn Res 2014; 8: ZC08–ZC11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang PS, Huang WC, Chen SY, et al. Scaling-stimulated salivary antioxidant changes and oral-health behavior in an evaluation of periodontal treatment outcomes. ScientificWorldJournal 2014; 2014: 814671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metgud R, Bajaj S. Evaluation of salivary and serum lipid peroxidation, and glutathione in oral leukoplakia and oral squamous cell carcinoma. J Oral Sci 2014; 56: 135–142. [DOI] [PubMed] [Google Scholar]
- 39.Houghton EA, Nicholas KM. In vitro reactive oxygen species production by histatins and copper (I, II). J Biol Inorg Chem 2009; 14: 243–251. [DOI] [PubMed] [Google Scholar]
- 40.Jensen JL, Xu T, Lamkin MS, et al. Physiological regulation of the secretion of histatins and statherins in human parotid saliva. J Dent Res 1994; 73: 1811–1817. [DOI] [PubMed] [Google Scholar]
- 41.Helmerhorst EJ, Alagl AS, Siqueira WL, Oppenheim FG. Oral fluid proteolytic effects on histatin 5 structure and function. Arch Oral Biol 2006; 51: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 42.Lee CI, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem 2000; 275: 9369–9376. [DOI] [PubMed] [Google Scholar]
- 43.Helmerhorst EJ, Troxler RF, Oppenheim FG. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc Natl Acad Sci U S A 2001; 98: 14637–14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helmerhorst EJ, Breeuwer P, van't Hof W, et al. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem 1999; 274: 7286–7291. [DOI] [PubMed] [Google Scholar]
- 45.Komatsu T, Salih E, Helmerhorst EJ, Offner GD, Oppenheim FG. Influence of histatin 5 on Candida albicans mitochondrial protein expression assessed by quantitative mass spectrometry. J Proteome Res 2011; 10: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiyose M, Lee CI, Okabe E. Inhibition of skeletal sarcoplasmic reticulum Ca2+-ATPase activity by deferoxamine nitroxide free radical. Chem Res Toxicol 1999; 12: 137–143. [DOI] [PubMed] [Google Scholar]
- 47.Lee MC, Kawai Y, Shoji H, et al. Evidence of reactive oxygen species generation in synovial fluid from patients with temporomandibular disease by electron spin resonance spectroscopy. Redox Rep 2004; 9: 331–336. [DOI] [PubMed] [Google Scholar]
- 48.Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett 1992; 307: 108–112. [DOI] [PubMed] [Google Scholar]
- 49.Grogan J, McKnight CJ, Troxler RF, Oppenheim FG. Zinc and copper bind to unique sites of histatin 5. FEBS Lett 2001; 491: 76–80. [DOI] [PubMed] [Google Scholar]
- 50.Melino S, Gallo M, Trotta E, Mondello F, Paci M, Petruzzelli R. Metal-binding and nuclease activity of an antimicrobial peptide analogue of the salivary histatin 5. Biochemistry 2006; 45: 15373–15383. [DOI] [PubMed] [Google Scholar]
- 51.Kesarwala AH, Krishna MC, Mitchell JB. Oxidative stress in oral diseases. Oral Dis 2016; 22: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol 2017; 8: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamaki N, Yoshino F, Fukui M, et al. Relationship among salivary antioxidant activity, cytokines, and periodontitis: the Nagasaki Island study. J Clin Periodontol 2015; 42: 711–718. [DOI] [PubMed] [Google Scholar]
- 54.Sugiyama S, Takahashi SS, Tokutomi FA, et al. Gingival vascular functions are altered in type 2 diabetes mellitus model and/or periodontitis model. J Clin Biochem Nutr 2012; 51: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tokutomi F, Wada-Takahashi S, Sugiyama S, et al. Porphyromonas gingivalis-induced alveolar bone loss is accelerated in the stroke-prone spontaneously hypertensive rat. Arch Oral Biol 2015; 60: 911–918. [DOI] [PubMed] [Google Scholar]
- 56.Minczykowski A, Woszczyk M, Szczepanik A, Lewandowski L, Wysocki H. Hydrogen peroxide and superoxide anion production by polymorphonuclear neutrophils in patients with chronic periapical granuloma, before and after surgical treatment. Clin Oral Investig 2001; 5: 6–10. [DOI] [PubMed] [Google Scholar]
- 57.Abdolsamadi H, Rafieian N, Goodarzi MT, et al. Levels of salivary antioxidant vitamins and lipid peroxidation in patients with oral lichen planus and healthy individuals. Chonnam Med J 2014; 50: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]