Abstract
Carbapenem-resistant (CR) Gram-negative infections, including those caused by Enterobacteriaceae and the non-fermenters, represent the greatest unmet need for new effective treatments. The clinical development of new antibiotics for the treatment of CR infections is challenging and should focus on the individual pathogens irrespective of the infection site. However, the drug approval pathway is generally infection-site specific and rarely includes such drug-resistant pathogens. To overcome this limitation, a streamlined clinical development program may include a pathogen-focused clinical study, such as the CREDIBLE-CR study, to meet the expectations of some health authorities (ie, the European Medicines Agency [EMA]) and the medical community. Cefiderocol is a novel siderophore cephalosporin designed to target CR pathogens, including CR strains of Enterobacteriaceae (CRE), Pseudomonas aeruginosa, Acinetobacter baumannii, and also Stenotrophomonas maltophilia, which is intrinsically CR. The CREDIBLE-CR study was planned to evaluate cefiderocol in patients with CR Gram-negative infections regardless of species or infection-site source. Rapid diagnostic testing and/or selective media were provided to facilitate detection of CR pathogens to rapidly enroll patients with nosocomial pneumonia, bloodstream infection/sepsis, or complicated urinary tract infection. Patients were randomized 2:1 to receive cefiderocol or best available therapy. There were no pre-specified statistical hypotheses for this study, as the sample size was driven by enrollment feasibility and not based on statistical power calculations. The objective of the CREDIBLE-CR study was to provide descriptive evidence of the efficacy and safety of cefiderocol for the target population of patients with CR infections, including the non-fermenters. The CREDIBLE-CR study is currently the largest pathogen-focused, randomized, open-label, prospective, Phase 3 clinical study to investigate a new antibiotic in patients with CR Gram-negative infections. Here we describe the design of this pathogen-focused study and steps taken to aid patient enrollment into the study within an evolving regulatory environment.
ClinicalTrials.gov registration
EUDRA-CT registration
2015-004703-23.
Keywords: best available therapy, cefiderocol, carbapenem resistance, pathogen-focused study design, rapid diagnostics, streamlined/limited clinical development
Introduction
The increased burden of drug-resistant bacteria on healthcare systems1 and patients in terms of quality of life2 is well recognized globally, with carbapenem-resistant (CR) strains of Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae being listed by the World Health Organization as the highest priority for new antibiotics development.3 Furthermore, Stenotrophomonas maltophilia, which is intrinsically resistant to carbapenems, and CR strains of Burkholderia spp. are difficult-to-treat non-fermenter pathogens in certain patient populations.4,5 These CR pathogens can cause serious infections such as nosocomial pneumonia, bacteremia, sepsis, urinary tract infections, intra-abdominal infections, and even skin and soft tissue infections,6,7 and represent a high unmet medical need.2
Patients infected by CR pathogens often present with significant comorbidities and a history of prior hospitalization, require admission to the intensive care unit (ICU),7–9 and are at an increased risk of mortality merely due to the severity of their illness.7,10–12 One pooled analysis of several studies has shown an attributable mortality rate of approximately 26%–44% due to carbapenem-resistant Enterobacteriaceae (CRE) in patients diagnosed with sepsis, bacteremia, and/or nosocomial pneumonia,13 while the in-hospital all-cause mortality rate approached 48%–69%.
Cefiderocol is a novel siderophore cephalosporin developed by Shionogi with the purpose of broadly treating CR Gram-negative infections, including those caused by both the family Enterobacteriaceae and non-fermenters such as A. baumannii, P. aeruginosa, and S. maltophilia,14,15 regardless of the underlying carbapenem resistance mechanism. As a siderophore, cefiderocol sequesters iron from the host and efficiently crosses the outer membrane utilizing the bacteria’s active iron transport channels, and its specific side chains provide structural stability against all Ambler classes of β-lactamases, including metallo-β-lactamases (MBLs).14,16–18 The active transport via the outer membrane means that cefiderocol overcomes resistance related to loss of porin channel function or upregulation of efflux pumps.15 Cefiderocol has demonstrated potent in vitro activity against Gram-negative bacteria,15,19,20 which has been confirmed in the ongoing global SIDERO-WT surveillance studies (ie, 99% of the isolates with a minimum inhibitory concentration of ≤4 μg/mL), including carbapenem- or meropenem-non-susceptible strains.21–24 The broad spectrum of Gram-negative activity provides an opportunity to develop cefiderocol as a Gram-negative antibiotic, not restricted by species or mechanism of carbapenem resistance.
Cefiderocol has been investigated in extensive pre-clinical and Phase 1 development programs to describe its pharmacokinetic and pharmacodynamic (PK/PD) profile and to establish the dosing regimens for the treatment of patients with infections, including those with underlying renal disease.25–31 A first-in-human infection Phase 2 study in patients with complicated urinary tract infection (cUTI) in a patient population at risk for multidrug-resistant infections has been completed and demonstrated non-inferiority to imipenem-cilastatin in the primary endpoint and additionally superiority in the composite and microbiological responses in a post hoc analysis.32 To date, cefiderocol is the only siderophore cephalosporin that has reached late-stage clinical development, with two randomized Phase 3 studies, one of which is the pathogen-focused CREDIBLE-CR study described in this article.
Including target CR pathogens when developing new antibiotic agents may be challenging primarily due to the fact that they are still relatively uncommon pathogens in relation to the overall disease burden in all diagnoses.2,6,33,34 The recently approved β-lactam–β-lactamase inhibitor (BL-BLI) combination drugs (eg, ceftazidime-avibactam, meropenem-vaborbactam, or ceftolozane-tazobactam) demonstrate in vitro activity against many CREs or CR P. aeruginosa, respectively, but have limited potency against those strains expressing MBLs or the non-fermenters.12,35,36 These drugs have been investigated in traditional infection site-specific randomized, double-blind, non-inferiority, Phase 3 clinical studies, in which the proportions of CR strains were negligible.37–42 Previous open-label superiority studies addressing mainly CR A. baumannii infections investigated colistin ± rifampicin or ± meropenem but failed to show superiority of the combination regimens, and were associated with high treatment failure (73%–79%) and mortality (43%–45%) rates, demonstrating the lack of consistent benefit of these agents in monotherapy or in combination.10,43,44
The early detection of CR pathogens is of paramount importance because delaying appropriate antibiotic therapy against resistant pathogens (ie, >48 hrs) significantly increases the risk of treatment failure and mortality.45,46 This clinical fact results in immediate empiric use of broad-spectrum antibiotics, including antibiotics that might be active against CR pathogens (eg, colistin). This in turn makes it difficult to enroll patients into prospective randomized clinical trials of new antibiotics, where a limited time window of 24–36 hrs of prior active antibiotics excludes patients from the study as assessment of treatment effect may be confounded. Enrollment of patients with CR infections without delaying effective antibiotic therapy might be facilitated by the use of rapid diagnostic methods, which can detect CREs directly from different biospecimens.47–49 Indeed, recent evidence suggests that implementation of rapid diagnostics into clinical practice can reduce the time to appropriate antibiotic therapy with a favorable impact on patient outcomes.47,50–52 This suggests that rapid diagnostics could be a valuable tool in clinical investigations of new antibiotics.
The selection of the comparator antibiotic for a double-blind, randomized, prospective study is also problematic when the target pathogens include both CRE and CR non-fermenters. Standard-of-care treatment often involves a combination of agents even in the absence of in vitro activity and are also selected based on the specific pathogen and the site of infection.53 An antibiotic which might be considered an appropriate choice for KPC-producing Klebsiella pneumoniae might be inappropriate for A. baumannii. This is further complicated by the availability of different antibiotics in different countries (eg, intravenous fosfomycin), and by availability of new antibiotics during the enrollment period of the study (eg, ceftazidime-avibactam). The antibiotic options for the treatment of CR infections are limited to older antibiotics (eg, colistin, fosfomycin, aminoglycosides, tigecycline) and the recently approved BL-BLI combination drugs when the pathogens show susceptibility.54,55 Therefore, selection of a fixed single or combination antibiotic as the comparator for a double-blind, randomized, international, multicenter clinical investigation is not feasible when susceptibility rates to these agents of CR Enterobacteriaceae, P. aeruginosa, and A. baumannii vary greatly across countries and only best available (mono or combo) therapy would be prescribed for patients as standard of care.
This article describes the design of the CREDIBLE-CR study and the key elements that address the challenges to recruitment of patients into pathogen-focused clinical trials under an evolving regulatory environment. The objective of the randomized, international, open-label, parallel-group, Phase 3 CREDIBLE-CR study was to assess the efficacy and safety (and PK parameters) of cefiderocol or best available therapy (BAT) in hospitalized patients with evidence of CR infections, including patients with hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), healthcare-associated pneumonia (HCAP), or bloodstream infections (BSI) or sepsis, or cUTI caused by CR Gram-negative bacteria. The study has been conducted under a streamlined/limited clinical development program, which enabled the enrollment of patients with various diagnoses and a broad range of CR bacteria according to the guidance outlined by the European Medicines Agency (EMA).56–59
Methods And Patients
Overall Study Design
The CREDIBLE-CR study is a prospective, international, multicenter, open-label, parallel-group, randomized, Phase 3 clinical study (NCT02714595;60 2015-004703-2361), which enrolled patients with HAP/VAP/HCAP, BSI or sepsis (with a primary source that was not bacteremia), and cUTI caused by documented CR Gram-negative pathogens, including A. baumannii, P. aeruginosa, S. maltophilia, K. pneumoniae, E. coli, or other species in the family of Enterobacteriaceae (and in the updated order of Enterobacterales62,63). Patients meeting eligibility criteria were randomized 2:1 to receive either intravenous (IV) cefiderocol 2 g, q8h, infused over 3 hrs (with one adjunctive antibiotic with Gram-negative coverage permitted if considered necessary, except for cUTI), or IV BAT which included up to three antibiotics used in combination for 7–14 days, although treatment duration could be extended up to 21 days at the discretion of the investigator.
Ethics
The study was conducted according to the requirements outlined by institutional review boards or institutional ethics committees of the participating clinical sites (Supplemental Table 1), and according to the principles of the International Conference on Harmonization Good Clinical Practice and those outlined in the Declaration of Helsinki. Prior to participation in the study, patients or their legal representatives provided signed informed consent. Additional informed consent was required from patients or their legal representatives if rapid diagnostic testing was used to confirm the presence of CR Gram-negative bacteria. The study protocol, the general informed consent form, and the rapid diagnostics informed consent form were approved by the institutional review boards or local ethics committees (Supplemental Table 1).
Participants
Adult patients who had an infection caused by a documented CR Gram-negative pathogen and were expected to require hospitalization and IV antibiotic therapy for 7–14 days were eligible for enrollment. Patients had to be at least 18 years old at the time of providing informed consent.
Inclusion Criteria
Patients who were diagnosed with HAP/VAP/HCAP, BSI or sepsis, or cUTI were enrolled into the study. Each clinical diagnosis had specific inclusion criteria, as shown in Table 1. Additionally, all enrolled patients had an evidence of CR Gram-negative infection at the primary infection site that could be confirmed by at least one of the following five methods: (1) documented treatment failure while on empiric antibiotic therapy with a CR Gram-negative pathogen confirmed by the respective culture/susceptibility testing; (2) rapid diagnostics used on an appropriate clinical biospecimen to confirm a CR pathogen; (3) the local hospital antibiogram demonstrated >90% rate of carbapenem resistance among local pathogens and the sample obtained from the patient was confirmed to contain at least one of these resistant pathogens; (4) the pathogen was confirmed as S. maltophilia, which has intrinsic resistance to carbapenems; or (5) the patient was confirmed to be colonized at the site of the primary infection with CR Gram-negative bacteria within 72 hrs prior to enrollment and randomization.
Table 1.
CREDIBLE-CR Study Inclusion And Exclusion Criteria By Clinical Diagnosis
| HAP/VAP/HCAP | BSI/Sepsis | cUTI | |
|---|---|---|---|
| Definition | HAP is defined as an acute bacterial pneumonia in a patient hospitalized for ≥48 hrs or developing pneumonia within 7 days following discharge from a hospital. Patients may suffer from acute respiratory failure and require mechanical ventilation (ventilated-HAP). VAP is defined as acute bacterial pneumonia in a patient who is already receiving mechanical ventilation for ≥48 hrs. HCAP is defined as an acute bacterial pneumonia in a patient who required hospitalization in an acute care hospital for ≥48 hrs within 90 days prior to current infection; or resided in a nursing home or long-term care facility; or received IV antibiotic treatment, chemotherapy, or wound care; or attended hemodialysis clinic within 30 days prior to current infection. |
The BSI/sepsis category includes bacteremia or sepsis caused by infections other than HAP/VAP/HCAP or cUTI. Documented BSI caused by a CR Gram-negative pathogen. Systemic response to infection, meeting the clinical criteria of SIRS and an identified infection source (eg, severe skin infection, intra-abdominal infection) caused by a CR Gram-negative pathogen. |
cUTI is defined as a clinical syndrome characterized by pyuria and a documented microbial pathogen on urine culture, accompanied by local and systemic signs and symptoms including fever, chills, malaise, flank pain, back pain, and/or costovertebral angle pain or tenderness that occur in the presence of a functional or anatomical abnormality of the urinary tract or in the presence of catheterization and requiring hospitalization for the parenteral (IV) treatment of cUTI. |
| Inclusion criteria | All patients must have at least one of the following clinical features:
All patients must have at least one of the following signs:
All patients must have a chest radiograph or lung CT scan within 48 hrs prior to randomization showing the presence of new or progressive infiltrate(s) suggestive of bacterial pneumonia. |
BSI-specific inclusion criteria:
Sepsis-specific inclusion criteria Patients defined for SIRS, indicated by having two or more of the following responses:
|
All patients must have a cUTI with a history of at least one of the following:
All patients must have at least two of the following signs or symptoms:
All patients must have evidence of pyuria on urinalysis demonstrated by:
Patients who had a positive urine culture within 48 hrs prior to randomization containing ≥105 CFU/mL of a CR Gram-negative uropathogen are eligible for this study. (Note: patients may be randomized prior to the results of the urine culture if they have evidence of a CR pathogen.) Patients receiving antibiotic prophylaxis for cUTI who present with signs and symptoms consistent with an active new cUTI may be enrolled provided all other eligibility criteria are met including obtaining a pre-treatment qualifying urine culture. |
| Exclusion criteria | Patients who meet any of the following criteria at Screening will be excluded from the study:
|
BSI-specific exclusion criteria Patients who meet any of the following criteria at Screening will be excluded from the study:
Sepsis-specific exclusion criteria Patients who meet any of the following criteria at Screening will be excluded from the study:
|
Patients who meet any of the following criteria at Screening will be excluded from the study:
|
Abbreviations: HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; HCAP, healthcare-associated pneumonia; BSI, bloodstream infection; cUTI, complicated urinary tract infection; CR, carbapenem-resistant; SIRS, systemic inflammatory response syndrome; IV, intravenous; ABG, arterial blood gas; BUN, blood urea nitrogen; WBC, white blood cell; CT, computed tomography; CFU, colony-forming unit.
Exclusion Criteria
Patients were not eligible for enrollment if any of the following applied: they had received a potentially effective antibiotic regimen for the current CR infection within 72 hrs prior to randomization for a continuous duration of ≥24 hrs in cUTI, or ≥36 hrs in HAP/VAP/HCAP or BSI/sepsis; they had moderate or severe hypersensitivity or allergic reaction to any β-lactam antibiotic; they required >3 systemic antibiotics for the treatment of the current infection if randomized to the BAT arm; they had concurrent invasive aspergillosis, mucormycosis or other species of lethal mold infection; if the infection was expected to require therapy for >21 days (eg, bone and joint infection, endocarditis); they had cystic fibrosis or moderate or severe bronchiectasis; they had refractory septic shock not responding to fluid resuscitation and/or vasopressors; severe neutropenia (ie, <100 cells/μL blood); pregnancy; Acute Physiology and Chronic Health Evaluation II (APACHE II) score was >30; they had any condition that according to the investigator would have compromised the safety of the patient or the quality of the study data; they had previous exposure to cefiderocol in this trial or previous exposure to another investigational study drug within 30 days prior to enrollment and randomization; peritoneal dialysis; they met diagnosis-specific exclusion criteria. Each clinical diagnosis had specific exclusion criteria, as shown in Table 1.
Randomization To Treatment And Stratification
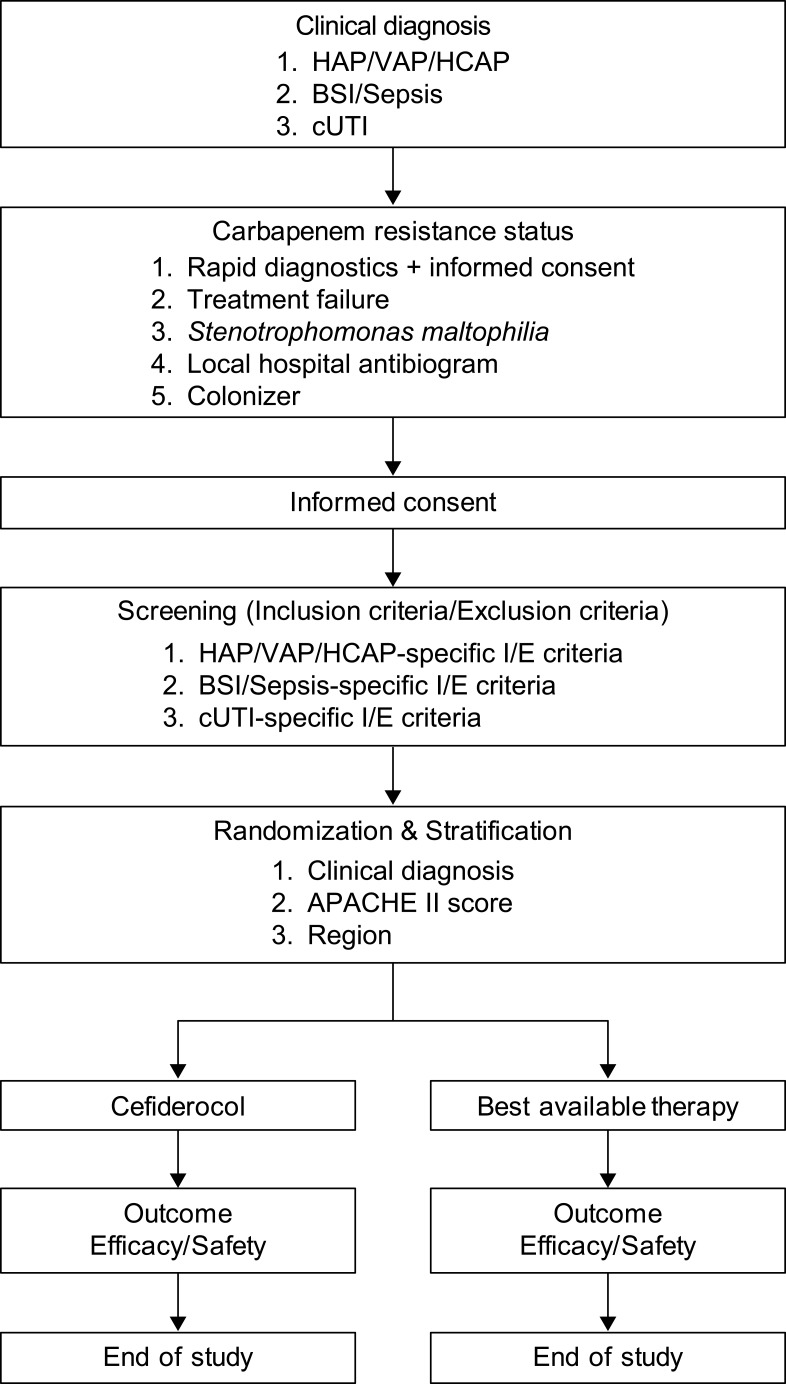
Patients were randomized 2:1 to receive IV therapy with either cefiderocol 2 g, q8h, or BAT, and were stratified by primary clinical diagnosis (HAP/VAP/HCAP, BSI/sepsis, cUTI), APACHE II score (≤15 or ≥16–≤30), and region (North America, South America region, Europe, Asia-Pacific) (Figure 1). An interactive web or voice response system was used to assign patients to identification numbers for which treatment had already been randomly assigned. The population eligible for enrollment was expected to result in approximately 50% of the patients with HAP/VAP/HCAP, 30% with cUTI, and 20% with BSI/sepsis.56 The APACHE II score was calculated based on the measurements of individual components to establish the severity of the disease, and patients were stratified as having APACHE II score ≤15 or ≥16. Patients were enrolled in approximately 100 study sites in the USA, Asia-Pacific, Europe, and North America and South America region.
Figure 1.
Patient flow in the CREDIBLE-CR study.
Treatment Regimens
The study drug, cefiderocol 2 g, q8h, was infused over 3 hrs in 100 mL solution. The dose was adjusted for patients with renal impairment or augmented creatinine clearance (Table 2). Patients with cUTI received cefiderocol as monotherapy, whereas patients with HAP/VAP/HCAP or BSI/sepsis were permitted to receive cefiderocol plus one additional antibiotic (adjunctive therapy) belonging to one of the following classes: aminoglycoside, macrolide, fluoroquinolone, penicillin (including piperacillin-tazobactam, piperacillin, or ampicillin-sulbactam), fosfomycin, tetracyclines (including tigecycline). Polymyxin (colistin or polymyxin B), cephalosporins, including new combinations with β-lactamase inhibitors or carbapenems were not permitted as adjunctive therapy with cefiderocol. The treatment duration was expected to be 7–14 days for HAP/VAP/HCAP or BSI/sepsis, and a minimum of 5 days for cUTI. The treatment duration could be extended up to 21 days if deemed necessary by the investigator.
Table 2.
Dose Adjustment Of Cefiderocol In Patients With Augmented Creatinine Clearance Or Renal Impairment.
| Augmented renal function (MDRD-eGFR ≥90 mL/min/1.73 m2 and CrCL ≥120 mL/min)a |
2 g, q6h, 3-hr infusion |
| Normal renal function (MDRD-eGFR ≥90 mL/min/1.73 m2 and CrCL <120 mL/min)a |
2 g, q8h, 3-hr infusion |
| Mild renal impairment (MDRD-eGFR 60 to <90 mL/min/1.73 m2) |
2 g, q8h, 3-hr infusion |
| Moderate renal impairment (MDRD-eGFR 30 to <60 mL/min/1.73 m2) |
1.5 g, q8h, 3-hr infusion |
| Severe renal impairment (MDRD-eGFR 15 to <30 mL/min/1.73 m2) |
1 g, q8h, 3-hr infusion |
| ESRD (MDRD-eGFR <15 mL/min/1.73 m2) | 0.75 g, q12h, 3-hr infusion |
| Patient with intermittent hemodialysis | 0.75 g, q12h, 3-hr infusionb |
| CVVH | 1 g, q12h, 3-hr infusionc |
| CVVHD or CVVHDF | 1.5 g, q12h, 3-hr infusionc |
Notes: aCrCL was calculated by Cockcroft–Gault equation at Screening. Urine measured CrCL was calculated by using timed urine collections of 2–8 hrs at early assessment (EA). bCefiderocol is hemodialysable, thus on dialysis days, a supplemental dose of 0.75 g was administered as a 3-hr infusion after the completion of intermittent hemodialysis. If the supplemental dose overlapped with the next regular dose, the investigator could consider skipping either the next regular q12h dose or the supplemental dose to avoid an excessive exposure and complexity of clinical operation. cThe dose was determined based on MDRD-eGFR on non-dialysis days. Copyright © American Society for Microbiology, [Antimicrobial Agents Chemotherapy, 61, 2017, e01381-16. doi: 10.1128/AAC.01381-16.29
Abbreviations: MDRD-eGFR, modification of diet in renal disease-estimated glomerular filtration rate calculated with the MDRD equation; CrCL, creatinine clearance; ESRD, end-stage renal disease; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; q6h, every 6 hrs; q8h, every 8 hrs; q12h, every 12 hrs.
BAT was the standard of care for CR infections at each enrolling study site and could include up to three antibiotics with Gram-negative coverage used in combination. BAT was determined by the site investigator based on the assessment of the patient’s clinical condition and had to be pre-specified prior to randomization. BAT included locally approved antibiotics (maximum three antibiotics in combination) for the treatment of the CR Gram-negative infection. The dosage of BAT was adjusted according to the local country-specific label. If the investigator used colistin or polymyxin B, the dosing recommendations outlined by the EMA had to be followed.64 De-escalation of BAT was allowed (eg, by reducing the number of antibiotics given) based on the assessment made at the early assessment (EA) time point.
Adjunctive aerosolized antibiotic treatment or step-down to oral treatment was not allowed in the study. Concomitant antibiotics were allowed if the patients had a confirmed/suspected Gram-positive or anaerobic co-infection (eg, vancomycin, daptomycin, linezolid, clindamycin, or metronidazole).
Clinical Assessments
Physical examination was performed, and vital signs, Sequential Organ Failure Assessment (SOFA) score, oxygenation status, and chest radiographs and Clinical Pulmonary Infection Score (CPIS) in HAP/VAP/HCAP patients were assessed at EA, end of therapy (EOT), test of cure (TOC), and follow-up (FUP) time points. Creatinine clearance was determined at screening and at the EA time point to confirm whether dose adjustment for cefiderocol was required.
Microbiological Assessments
Appropriate clinical specimens were collected from patients within 48 hrs prior to the start of infusion of the first dose of study treatment. For patients who were enrolled on the basis of treatment failure, clinical specimens collected within 72 hrs prior to randomization could be used as baseline screening culture. Clinical specimens were sent to the local microbiology laboratory for culture and susceptibility testing to document evidence of CR pathogens. Once randomized, additional clinical specimens were obtained at EA, EOT, TOC, and FUP time points. Additionally, two blood samples were collected from separate venipunctures regardless of the primary site of infection within 48 hrs prior to the first dose of study treatment. Subsequent blood cultures were required if the baseline blood culture was positive.
From HAP/VAP/HCAP patients, sputum, bronchoalveolar lavage, or protected brush specimen samples were appropriate.
For BSI patients, the blood sample collected at screening had to be positive; for sepsis patients (defined by the Systemic Inflammatory Response Syndrome), the source of infection had to be an anatomical location different from the source associated with HAP/VAP/HCAP or cUTIs.
For cUTI patients, the urine sample had to be positive for bacteria with clinical signs and symptoms of infection present.
In order to expedite the enrollment of patients with CR Gram-negative pathogens and initiate appropriate treatment before culture and susceptibility results were available, rapid diagnostic methods or selective chromogenic media could be used to identify patients with CR pathogens. Additionally, cultures of appropriate sites were performed for species identification and antibiotic susceptibility testing in the local microbiology laboratory.
All isolated pathogens were required to be frozen, stored, and later sent to the central laboratory (International Health Management Associates, Schaumburg, Illinois) for confirmation of species identification, antibiotic susceptibility, and molecular characterization of the mechanisms of carbapenem resistance.
Safety Assessments
Safety assessment in the safety population (defined below) included physical examination, adverse event reporting, laboratory investigations, vital signs assessment, and mortality up to the end of study visit (ie, EOT + 28 days [± 3 days]). Mortality included those events that occurred due to serious adverse events, which were ongoing at EOS visit. Adverse events were recorded by System Organ Class and Preferred Term according to MedDRA. The severity of adverse events was graded as mild, moderate, or severe, and its relationship to study treatment was determined by the investigator. Clinical laboratory investigations included hematology, blood chemistry, and urinalysis at subsequent time points during the study. Additional laboratory testing was performed to assess iron homeostasis parameters such as serum iron, hepcidin level, transferrin iron saturation, and total iron-binding capacity. Vital signs (daily) and 12-lead electrocardiogram (at screening) were also recorded.
Outcome Definitions
Clinical outcomes assessed by the investigator were defined for each diagnosis at EA (Day 3–4), EOT (last day of treatment), TOC (EOT + 7 days [± 2 days]), and FUP (EOT + 14 days [± 3 days]) time points. Clinical outcomes were categorized as clinical cure, failure, or indeterminate for each diagnosis at EA, EOT, and TOC time points. At FUP, patients could have sustained clinical cure, relapse, or indeterminate clinical outcome. The definitions of the clinical outcomes are included in Table 3.
Table 3.
Definitions Of Clinical Outcome (response) Parameters
| HAP/VAP/HCAP | BSI/Sepsis | cUTI | |
|---|---|---|---|
| EA, EOT, TOC | |||
| Clinical cure | Resolution or substantial improvement of baseline signs and symptoms of pneumonia including a reduction in SOFA and CPIS scores, and improvement or lack of progression of chest radiographic abnormalities such that no antibacterial therapy is required for the treatment of the current infection. | Resolution or substantial improvement of baseline signs and symptoms including a reduction in SOFA score, such that no antibacterial therapy is required for the treatment of BSI/sepsis. Patients with bacteremia must have eradication of bacteremia caused by the Gram-negative pathogen. | Resolution or substantial improvement of baseline signs and symptoms of cUTI, or return to pre-infection baseline if known, such that no antibacterial therapy is required for the treatment of the current infection. |
| Clinical failure | No apparent response to therapy; persistence or worsening of baseline signs and/or symptoms of pneumonia; reappearance of signs and/or symptoms of pneumonia; development of new signs and/or symptoms of pneumonia requiring antibiotic therapy other than, or in addition to, study treatment therapy; progression of chest radiographic abnormalities; or death due to pneumonia. | No apparent response to therapy; persistence or worsening of baseline signs and/or symptoms, reappearance of signs and/or symptoms; development of new signs and/or symptoms requiring antibiotic therapy other than, or in addition to, study treatment therapy; or death due to BSI/sepsis. | No apparent response to therapy; persistence or worsening of baseline signs and/or symptoms of cUTI; or reappearance of signs and/or symptoms of cUTI; development of new signs and/or symptoms of cUTI requiring antibiotic therapy other than, or in addition to, study treatment therapy; or death due to cUTI. |
| Indeterminate | Lost to follow-up such that a determination of clinical cure/failure cannot be made. | Lost to follow-up such that a determination of clinical cure/failure cannot be made. | Lost to follow-up such that a determination of clinical cure/failure cannot be made. |
| FUP | |||
| Sustained clinical cure | Continued resolution or substantial improvement of baseline signs and symptoms of pneumonia, such that no antibacterial therapy is required for the treatment of pneumonia in a patient assessed as cured at TOC. | Continued resolution or substantial improvement of baseline signs and symptoms associated with reduction in SOFA score, such that no antibacterial therapy is required for the treatment of the original BSI/sepsis in a patient assessed as cured at TOC. | Continued resolution or improvement of baseline signs and symptoms of cUTI, or return to pre-infection baseline if known, in a patient assessed as cured at TOC. |
| Relapse | Recurrence of signs and/or symptoms of pneumonia, appearance of new signs and/or symptoms of pneumonia, new chest radiographic evidence of pneumonia, or death due to pneumonia in a patient assessed as cured at TOC. | Recurrence of signs and/or symptoms of BSI/sepsis, appearance of new signs and/or symptoms of the original BSI/sepsis, or death due to BSI/sepsis in a patient assessed as cured at TOC. | Recurrence of signs and/or symptoms of cUTI, or appearance of new signs and/or symptoms of cUTI in a patient assessed as cured at TOC. |
| Indeterminate | Lost to follow-up such that a determination of clinical sustained cure/relapse cannot be made, or patient received additional antibacterial therapy for the treatment of the current infection. | Lost to follow-up such that a determination of clinical sustained cure/relapse cannot be made, or patient received additional antibacterial therapy for the treatment of the current infection. | Lost to follow-up such that a determination of clinical sustained cure/relapse cannot be made, or patient received additional antibacterial therapy for the treatment of the current infection. |
Abbreviations: HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; HCAP, healthcare-associated pneumonia; BSI, bloodstream infection; cUTI, complicated urinary tract infection; EA, early assessment; EOT, end of therapy; TOC, test of cure; SOFA, Sequential Organ Failure Assessment; CPIS, Clinical Pulmonary Infection Score.
An overall per-patient microbiological outcome was determined based on individual microbiological outcomes for each baseline pathogen. Microbiological outcomes were categorized as eradication, persistence, or indeterminate for each clinical diagnosis. At FUP, patients could have sustained eradication, recurrence, or indeterminate microbiological outcome. The definitions of the microbiological outcomes are included in Table 4. Additionally, any new pathogens isolated from appropriate biospecimens were categorized as superinfection (ie, isolation of a new pathogen from the primary infection site) or new infection (ie, isolation of a new pathogen from a different infection site).
Table 4.
Definitions Of Microbiological Outcome (Response) Parameters
| HAP/VAP/HCAP | BSI/Sepsis | cUTI | |
|---|---|---|---|
| EA, EOT, TOC | |||
| Eradication | Absence of the baseline Gram-negative pathogen from an appropriate clinical specimen. If it is not possible to obtain an appropriate clinical culture and the patient has a successful clinical outcome, the response will be presumed to be eradication. | Absence of the baseline Gram-negative pathogen from a blood culture and/or other primary source as applicable. In the case of sepsis, if the patient has a successful clinical outcome and it is not possible to obtain an appropriate clinical culture, the response will be presumed to be eradication. | A urine culture shows the baseline Gram-negative uropathogen found at entry at ≥105 CFU/mL are reduced to <103 CFU/mL. |
| Persistence | Continued presence of the baseline Gram-negative pathogen from an appropriate clinical specimen. | Continued presence of the baseline Gram-negative pathogen from a blood culture or other primary source. | A urine culture shows that the baseline Gram-negative uropathogen found at entry at ≥105 CFU/mL grows ≥103 CFU/mL. |
| Indeterminate | No culture obtained from an appropriate clinical specimen or additional antibacterial therapy for the treatment of the current infection including missed sampling. | No culture obtained or additional antibacterial therapy for the treatment of the current infection including missed sampling. | No urine culture obtained or additional antibacterial therapy for the treatment of the current infection including missed sampling. |
| FUP | |||
| Sustained eradication | Absence of the baseline Gram-negative pathogen from an appropriate clinical specimen after TOC. If it is not possible to obtain an appropriate clinical culture and the patient has a successful clinical response after TOC, the response will be presumed to be eradication. | Absence of the baseline Gram-negative pathogen from a blood culture or other primary source after TOC as applicable. In the case of sepsis, if the patient has a successful clinical outcome after TOC and it is not possible to obtain an appropriate clinical culture, the response will be presumed to be sustained eradication. | A culture taken any time after documented eradication at TOC, and a urine culture obtained at FUP shows that the baseline uropathogen found at entry at ≥105 CFU/mL remains <103 CFU/mL. |
| Recurrence | Recurrence of the baseline Gram-negative pathogen from an appropriate clinical specimen taken after TOC, and the TOC culture is negative. | Recurrence of the baseline Gram-negative pathogen from a blood culture or other primary source after TOC, and the TOC culture is negative. | A culture taken any time after documented eradication at TOC, up to and including FUP that grows the baseline uropathogen ≥103 CFU/mL. |
| Indeterminate | No culture obtained from an appropriate clinical specimen or patient received additional antibacterial therapy for the treatment of the current infection including missed sampling. | No culture or patient received additional antibacterial therapy for the treatment of the current infection including missed sampling. | No urine culture or patient received additional antibacterial therapy for the treatment of the current infection including missed sampling. |
Abbreviations: HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; HCAP, healthcare-associated pneumonia; BSI, bloodstream infection; cUTI, complicated urinary tract infection; EA, early assessment; EOT, end of therapy; TOC, test of cure; CFU, colony-forming unit.
Patient Populations
The intent-to-treat (ITT) population included all randomized patients who received at least one dose of study drug. The Microbiological ITT (Micro-ITT) population included all ITT patients with a confirmed Gram-negative pathogen at baseline from an appropriate clinical biospecimen. The CR-Micro-ITT (CR-MITT) included all Micro-ITT patients who had a confirmed CR Gram-negative pathogen at baseline. The CR-Microbiologically evaluable (CR-ME) population included all CR-Micro-ITT patients who had ≥5 days of IV treatment (unless the patient was classified as treatment failure), completed the study, and had valid assessment at TOC, and had no major protocol violation. The safety population included all randomized patients who received at least one dose of study drug, and safety of the actual treatment received was assessed. The PK population included all patients who received cefiderocol treatment and had a plasma sample collected for PK evaluation of cefiderocol.
Endpoints (Diagnosis Specific)
The primary endpoints of the study were: 1) clinical cure rates at TOC in adult patients with HAP/VAP/HCAP or BSI/sepsis caused by CR Gram-negative pathogens in the CR-Micro-ITT population; 2) microbiological outcomes at TOC in adult patients with cUTI caused by CR Gram-negative pathogens in the CR-Micro-ITT population. Secondary endpoints included: clinical outcome (EOT, FUP), microbiological outcome (EOT, TOC, FUP), and all-cause mortality at Day 14 and Day 28 for patients with HAP/VAP/HCAP or BSI/sepsis; and microbiological outcome (EOT, FUP), clinical outcome (EOT, TOC, FUP), and composite clinical and microbiological outcome (EOT, TOC, FUP) for patients with cUTI. The safety of cefiderocol or BAT was also investigated for all diagnoses. Other secondary endpoints included the composite endpoint of survival without the need to change antibiotic treatment due to lack of efficacy or presence of drug-related toxicity (TOC); SOFA score (EOT, TOC, FUP); and CPIS (EOT, TOC, FUP) in patients with HAP/VAP/HCAP.
Blood Sample Collection For PK Analysis Of Cefiderocol
All patients who received cefiderocol treatment provided blood samples for sparse PK analysis of cefiderocol. The purpose was to determine any relationship between actual drug exposure and clinical or microbiological outcomes (ie, PK/PD analyses). The blood samples were taken on Day 3 during therapy just prior to infusion, 1 hr after the start of infusion, at the end of infusion, and 1 hr after the end of infusion. Patients assessed with non-stable renal function at the EA time point who required dose adjustment for cefiderocol provided additional blood samples within 24–72 hrs after dose adjustment, with four blood samples being taken (ie, just prior to infusion, 1 hr after the start of infusion, at the end of infusion, and 1 hr after the end of infusion).
Statistical Analysis
As the CREDIBLE-CR study was not designed as a non-inferiority or superiority study, no formal inferential analysis was planned. During discussions with regulatory agencies (ie, EMA), enrollment of approximately 100 patients treated with cefiderocol was recommended. Due to feasibility considerations for total study enrollment, a 2:1 randomization ratio was adopted to provide only 50 patients in the control arm (BAT), providing a total sample size of approximately 150 enrolled patients.
The statistical analysis will be descriptive. The summary statistics for continuous variables will include the number of subjects, arithmetic mean, standard deviation, minimum, median, and maximum values. Categorical variables will be summarized by using frequency count and the percentage of patients in each category. No hypotheses have been pre-specified and no inferential testing has been planned for primary or secondary outcomes. Missing data for individual data points will remain as missing unless otherwise stated in the Statistical Analysis Plan. SAS version 9.2 or later will be used to analyze the data.
Discussion
Evolving Regulatory Environment
The global spread of carbapenem resistance in Gram-negative bacteria has remained a great concern for the past 15–20 years.65–67 The Infectious Diseases Society of America has published its “10 × ‘20” initiative for the urgent development of 10 new antibiotic agents,68,69 but this initiative could not be adopted specifically for CR infections under the regulatory framework in either the USA (Food and Drug Administration [FDA]) or Europe (EMA) at the time; thus, new guidance was needed.70,71 Both the FDA and EMA introduced a new guidance and/or changed their approach to enable pharmaceutical companies to develop new antibiotics targeting resistant pathogens, which are infrequently included in large, double-blind, randomized, non-inferiority, active-controlled studies designed for infection site-specific indications.56–58 Ongoing discussions led to the alternative streamlined (or limited) clinical development approach,56–59 which meant that the efficacy and safety of a new candidate drug could be investigated in different indications simultaneously, with appropriate endpoints being defined for each individual indication.56–58 One important requirement of the US FDA is that the safety of the drug must be supported by a “safety database” including information of at least 300 patients.56 This has been achieved in the randomized, double-blind, non-inferiority, Phase 2 APEKS-cUTI study.32 In the end, based on the discussions with the EMA Scientific Advisory Committee (ie, Committee for Medicinal Products for Human Use [CHMP]) in Europe,58 Shionogi gained acceptance of their strategy for the clinical investigation of cefiderocol to specifically address CR infections, making the CREDIBLE-CR study pivotal for regulatory approval in Europe.60
Rationale For Study Design And Patient Population
The CREDIBLE-CR study is an open-label, randomized, prospective, multinational, parallel-group, Phase 3 clinical study to assess the safety and efficacy of cefiderocol for the treatment of infections caused by CR bacteria where the patient population is primarily defined by the presence of these pathogens rather than the infection types, hence, the term “pathogen-focused study.” Because of the enrollment of a broad range of CR Gram-negative bacteria and infection types, the control arm (BAT) was not fixed and patients were allowed to receive up to three antibiotics to be used in combination, making the study particularly relevant to current real-world clinical practice.
While the cUTI study (APEKS-cUTI32) and the recently completed nosocomial pneumonia study (APEKS-NP; NCT03032380) are both randomized, double-blind, monotherapy clinical trials with inferential testing of a hypothesis, they both exclude patients known to have CR infections because the control drug is a carbapenem (imipenem-cilastatin or meropenem, respectively). The CREDIBLE-CR study has made an effort to include the CR infections that were excluded from the above-mentioned studies and to provide at least descriptive evidence of efficacy against the target pathogens.
The CREDIBLE-CR study accurately reflects the patient population with high unmet medical need that is most likely to benefit from treatment with cefiderocol. In conventional Phase 3 clinical trials, many comorbidities, immunosuppression, and short life expectancy are exclusion criteria, which was not the case in the CREDIBLE-CR study, and patients with such conditions could be enrolled. Thus, the design of the study met the investigators’ expectations in terms of the target population.
The CREDIBLE-CR study has enrolled approximately 150 patients who were severely ill with HAP, VAP, HCAP, BSI, sepsis, or cUTI, which were caused by CR pathogens. The enrollment of patients with various diagnoses made it possible to enrich the study population for a broad range of CR pathogens. Patients were stratified by their baseline diagnosis group (HAP/VAP/HCAP, BSI/sepsis, or cUTI), region, and APACHE II score (Figure 1). These three main stratification criteria will facilitate the comparison of outcomes by treatment group in patients with less or more severe conditions based on the APACHE II scores, and across geographic regions with different rates of carbapenem resistance in all indications.
These diagnoses are reported with different underlying all-cause mortality rates in real-world clinical practice (eg, pneumonia with ~40%–60% rate and UTI with 5%–8.5% rate),72–74 and all present a great challenge for physicians because of the lack of uniformly effective and relatively safe antibiotics for the treatment of CR pathogens. Due to varying rates of expected all-cause mortality and/or attributable mortality across the diagnoses, mortality was not selected as the primary efficacy endpoint because a single non-inferiority margin could not be selected for all sites of infection. A superiority study design to investigate all-cause mortality was an option to overcome the lack of a single non-inferiority margin, but this was viewed as non-realistic considering that these pathogens are rare and the enrollment of a very large number of patients into the study would have been necessary to demonstrate a treatment benefit of cefiderocol over the comparator arm for all-cause mortality. This feasibility issue was also observed during the TANGO II and CARE pathogen-focused Phase 3 clinical trials that addressed CRE infections. These studies enrolled a relatively low number of patients who were infected by confirmed CRE (ie, TANGO II: n=47; CARE: n=37) over a period of 2 to 3 years.75,76 Thus, setting a target of at least 150 patients was a priority in the CREDIBLE-CR study in order to ensure successful completion of enrollment and to collect adequate data on efficacy and safety as well as thorough PK data for patients treated with cefiderocol. Therefore, based on the discussions with the EMA, clinical cure rate was selected as the primary endpoint for patients with pneumonia or BSI/sepsis, and microbiological eradication rate was selected for patients with cUTI, with an overall lower patient number at a 2:1 randomization ratio providing 100 patients who were treated with cefiderocol.
Due to the lack of a pre-specified hypothesis, the study was not powered to detect statistically significant differences for the primary endpoints; the planned sample size was aligned with the guidance from the EMA and only descriptive statistics will be used. If such a pathogen-focused study is conducted as a registration trial without inferential statistical testing, the approved European label of the agent will be restricted to the treatment of patients infected by specific drug-resistant pathogens or pathogens that have limited treatment options.58 Despite the fact that the descriptive statistical analysis follows the guidance of the EMA CHMP,58 we have found that this approach within a study protocol and subsequent execution of a clinical study needed additional strong scientific support for ethics committee approval on a country-by-country basis. Thus, designing future multi-national pathogen-focused studies should be aligned with local institutional board and ethics committee’s strategies.
Evidence Of Carbapenem Resistance
Evidence for carbapenem resistance was the key criterion for enrollment into the CREDIBLE-CR study. Patients with only suspected CR infections could not be enrolled. Efforts to enrich patient populations in other pathogen-focused studies without objective evidence of the target pathogens have proven problematic.75 In the CREDIBLE-CR study, the evidence for carbapenem resistance could be provided by one of the five different pathways. If a patient’s infection was a treatment failure and the causative pathogen was confirmed as CR in routine culture and susceptibility testing, then the patient could be enrolled. To broaden the origin of CR infections, alternative pathways were also included: the use of rapid diagnostics that allowed identification of carbapenemase genes directly from patient biospecimens (eg, Gene Xpert CARBA-R), or selective media that identified carbapenem resistance, neither of which has been proactively utilized in previous randomized clinical trials. Rapid diagnostic methods can detect genes encoding carbapenemases within 1 hr prior to obtaining results from routine culture and susceptibility testing, with a positive impact on therapeutic decision.47,49,50,52 However, significant coordination between surgeons, infectious disease physicians, pulmonologists, nurses or hospital staff was required prior to requesting informed consent from patients or their family members for the use of rapid diagnostics, particularly when patients were very ill in the ICU. This could represent a challenge to rapid enrollment into this type of study. The use of selective chromogenic media for sputum and blood specimens, or other specimen types, was slightly more problematic because of the longer time required for confirmation (ie, overnight incubation of the specimens).77 The use of these methods expedited enrollment of patients within the allowed time window (ie, ≤24 hrs for cUTI patients and ≤36 hrs for other diagnoses) specified in the study protocol. In contrast, some previous studies allowed the enrollment of patients who could have received potentially effective empiric antibiotic therapy (ie, colistin-based regimens) for up to 96 hrs prior to randomization.10,43,44 Initiating appropriate antibiotic therapy early is crucial; thus, selecting the time window of prior empiric antibiotic treatment when designing a randomized clinical study to investigate the efficacy and safety of a potential new treatment option may have implications on outcomes. The additional pathways included surveillance cultures identifying carbapenem resistance from the same site of infection or the identification of A. baumannii (or another multidrug-resistant species) where the carbapenem resistance likelihood was >90% based on the hospital’s antibiogram, or S. maltophilia, which is intrinsically resistant to carbapenems.
Use Of Best Of Available Therapy
Unlike previous pathogen-focused studies (eg, RESTORE-IMI,78 CARE76), which selected colistin-based comparator regimens, the CREDIBLE-CR study used the “best available therapy” in each enrolling country as a “control” group, similarly to the TANGO II study.75 BAT was selected prior to randomization by the investigator at each study site from locally approved antibiotics and based on all available information of the patient’s infection site and pathogen, including susceptibility data. It was not feasible or appropriate to select one specific comparator agent for the entire study because of the multiple diagnoses and species involved, and up to three agents were allowed in the BAT treatment group. Because BAT could not be standardized across geographical regions and could have been different even among hospitals in the same country, blinding was not possible and the study was designed as an open-label study. In addition, the availability of new antibiotics (eg, the ceftazidime–avibactam combination approved by the US FDA, which has activity against certain CREs) meant that BAT could have changed during the study period in a participating hospital; thus, an open-label design seemed more reasonable. The use of BAT as a “control” instead of a fixed comparator agent was well received by the investigators and likely facilitated enrollment because they have extensive experience with the currently available agents for the treatment of such high-risk patients with CR infections. The selection of BAT, which was supported by scientific evidence, was also in accordance with the regulatory guidance by the EMA.58
Conclusion
This pioneering study will provide guidance for designing future pathogen-focused studies. It is likely that clinicians will come to expect similar pathogen-focused studies that inform their clinical decision-making in other clinical development programs for new antibiotics moving forward. Once a satisfactory pathway from regulatory agencies has been established and validated, patients and investigators may be more likely to participate in pathogen-focused studies rather than infection site-specific studies for the development of new antibiotics for rare pathogens. It is our hope that the experience gained through the CREDIBLE-CR study might serve as the basis for additional studies, and potentially will help to design future studies with the objectives of addressing more specific clinical questions, for example, the role of combination therapy, the use of empiric therapy, and for defining treatment and management algorithms in addition to registration studies.
Acknowledgments
The authors thank Ann Howell, Basil Houdali, and Marueen Silverman for their valuable review and critique of the manuscript. Editorial support was provided by Highfield, Oxford, United Kingdom, sponsored by Shionogi Inc., Florham Park, NJ, USA. The study was funded by Shionogi Inc., Florham Park, NJ, USA.
Data Sharing
Data sharing is not applicable to this manuscript because no participants’ data are included.
Disclosure
Outside the submitted work, MB has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, Bayer, Basilea, Biomerieux, Cidara, Gilead, Menarini, MSD, Nabriva, Paratek, Pfizer, Roche, Melinta, Shionogi, Tetraphase, VenatoRx and Vifor, and has received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead, Pfizer and MSD. YD has received grant support from The Medicines Company, Accelerate Diagnostics, Pfizer, MSD, Shionogi, Astellas, Kanto Chemical, the National Institutes of Health, the Japan Society for the Promotion of Science and AMED, has served on advisory boards for Allergan, The Medicines Company, Meiji, Roche, Pfizer, Tetraphase, Recida, Fedora, VenatoRx, and has received speaking honorarium from Pfizer, MSD, and Shionogi. BB, YM, KT are employees of Shionogi Inc., Florham Park, NJ, USA. TDN, MA are employees of Shionogi & Co., Ltd., Osaka, Japan. RME is a consultant for Shionogi Inc., Florham Park, NJ, USA. The authors report no other conflicts of interest in this work.
References
- 1.Bartsch SM, McKinnell JA, Mueller LE, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23:48.e9–48.e16. doi: 10.1016/j.cmi.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Global priority of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. Available from: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf Accessed February8, 2019.
- 4.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. Antimicrobial susceptibility of Acinetobacter calcoaceticus–acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis. 2019;6(Suppl 1):S34–S46. doi: 10.1093/ofid/ofy293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Chakhtoura NG, Saade E, Iovleva A, et al. Therapies for multidrug resistant and extensively drug-resistant non-fermenting gram-negative bacteria causing nosocomial infections: a perilous journey toward ‘molecularly targeted’ therapy. Expert Rev Anti Infect Ther. 2018;16:89–110. doi: 10.1080/14787210.2018.1425139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis. 2017;4:ofx176. doi: 10.1093/ofid/ofx176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible Gram-negative pathogens in ICU and non-ICU Settings in US Hospitals in 2017: a multicenter study. Open Forum Infect Dis. 2018;5:ofy241. doi: 10.1093/ofid/ofy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim T, Lee EJ, Park SY, et al. Natural prognosis of carbapenem-resistant Acinetobacter baumannii bacteremia in patients who did not receive appropriate antibiotic treatment: a retrospective multicenter study in Korea. Medicine (Baltimore). 2018;97:e12984. doi: 10.1097/MD.0000000000012984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, Kim SI, Hong KW, Kim YR, Park YJ, Kang MW. Risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia: impact of appropriate antimicrobial therapy. J Korean Med Sci. 2012;27:471–475. doi: 10.3346/jkms.2012.27.5.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349–358. doi: 10.1093/cid/cit253 [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Sun J, Wu X, Zhang L. Determinants of mortality in patients with nosocomial Acinetobacter baumannii bacteremia in Southwest China: a five-year case–control study. Can J Infect Dis Med Microbiol. 2018;2018:1–9. doi: 10.1155/2018/3150965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katchanov J, Asar L, Klupp EM, et al. Carbapenem-resistant Gram-negative pathogens in a German university medical center: prevalence, clinical implications and the role of novel β-lactam/β-lactamase inhibitor combinations. PLoS One. 2018;13(4):e0195757. doi: 10.1371/journal.pone.0195757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20:1170–1175. doi: 10.3201/eid2007.121004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki T, Yoshizawa H, Yamawaki K, et al. Cefiderocol (S-649266), a new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other Gram-negative pathogens including multi-drug resistant bacteria: structure activity relationship. Eur J Med Chem. 2018;155:847–868. doi: 10.1016/j.ejmech.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 15.Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother. 2017;62:e01454–17. doi: 10.1128/AAC.01454-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito-Horiyama T, Ishii Y, Ito A, et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother. 2016;60:4384–4386. doi: 10.1128/AAC.03098-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito A, Nishikawa T, Ota M, et al. Stability and low induction propensity of cefiderocol against chromosomal AmpC β-lactamases of Pseudomonas aeruginosa and Enterobacter cloacae. J Antimicrob Chemother. 2018;73:3049–3052. doi: 10.1093/jac/dky317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel L, Kieffer N, Nordmann P. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int J Antimicrob Agents. 2018;52:866–867. doi: 10.1016/j.ijantimicag.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Ito A, Nishikawa T, Matsumoto S, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60:7396–7401. doi: 10.1128/AAC.01405-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito A, Kohira N, Bouchillon SK, et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother. 2016;71:670–677. doi: 10.1093/jac/dkv402 [DOI] [PubMed] [Google Scholar]
- 21.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother. 2017;61:pii:e00093-17. doi: 10.1128/AAC.00093-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother. 2018;62:pii:e01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In vitro activity of cefiderocol, a siderophore cephalosporin, against Gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015–2016:SIDERO-WT-2015. Int J Antimicrob Agents. 2019;53:456–466. doi: 10.1016/j.ijantimicag.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents. 2019;53:177–184. doi: 10.1016/j.ijantimicag.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto S, Singley CM, Hoover J, et al. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother. 2017;61(9):pii:e00700-17. doi: 10.1128/AAC.00700-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura R, Ito-Horiyama T, Takemura M, et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother. 2019;63(9):pii: e02031-18. doi: 10.1128/AAC.02031-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a siderophore cephalosporin for Gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol. 2017;57:584–591. doi: 10.1002/jcph.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsube T, Miyazaki S, Narukawa Y, Hernandez-Illas M, Wajima T. Drug–drug interaction of cefiderocol, a siderophore cephalosporin, via human drug transporters. Eur J Clin Pharmacol. 2018;74:931–938. doi: 10.1007/s00228-018-2458-9 [DOI] [PubMed] [Google Scholar]
- 29.Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother. 2017;61:e01381–16. doi: 10.1128/AAC.01381-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for Gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother. 2018;62:e02163–17. doi: 10.1128/AAC.02163-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki S, Katsube T, Shen H, Tomek C, Narukawa Y. Metabolism, excretion, and pharmacokinetics of [14C]-Cefiderocol (S-649266), a siderophore cephalosporin, in healthy subjects following intravenous administration. J Clin Pharmacol. 2019;59:958–967. doi: 10.1002/jcph.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18:1319–1328. doi: 10.1016/S1473-3099(18)30554-1 [DOI] [PubMed] [Google Scholar]
- 33.Cai B, Echols R, Rudin D, Morgan G, Nagata T. Risk factors for carbapenem‐resistant Gram‐negative bloodstream infections (BSI) in US Hospitals (2010–2015). Open Forum Infect Dis. 2018;5(Suppl 1):S638. doi: 10.1093/ofid/ofy210.1819 [DOI] [Google Scholar]
- 34.Echols R, Cai B, Corvino F, Lodise T. Epidemiology and outcomes of patients with carbapenem‐resistant bloodstream infection in United States (US) hospitals, 2010–2015. Poster presented at: IDWeek 2018; October 2–6, 2018; San Francisco, CA; Poster 681. [Google Scholar]
- 35.van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis. 2016;63:234–241. doi: 10.1093/cid/ciw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tillotson GS. Trojan horse antibiotics – a novel way to circumvent Gram-negative bacterial resistance? Infect Dis (Auckl). 2016;9:45–52. doi: 10.4137/IDRT.S31567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319:788–799. doi: 10.1001/jama.2018.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kollef M, Novacek M, Kivistik U, et al. ASPECT-NP: a randomized, double-blind, Phase 3 trial comparing efficacy and safety of ceftolozane/tazobactam vs meropenem in patients with ventilated nosocomial pneumonia. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); April 13–16, 2019; Amsterdam, Netherlands; Poster 1917. [Google Scholar]
- 39.Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62:1380–1389. doi: 10.1093/cid/ciw133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis. 2015;60:1462–1471. doi: 10.1093/cid/civ097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet. 2015;385:1949–1956. doi: 10.1016/S0140-6736(14)62220-0 [DOI] [PubMed] [Google Scholar]
- 42.Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63:754–762. doi: 10.1093/cid/ciw378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickstein Y, Lellouche J, Dalak Amar MB, et al. Treatment outcomes of colistin and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis. 2019;69(5):769–776. doi: 10.1093/cid/ciy988 [DOI] [PubMed] [Google Scholar]
- 44.Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi: 10.1016/S1473-3099(18)30099-9 [DOI] [PubMed] [Google Scholar]
- 45.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Predictors of hospital mortality among septic ICU patients with Acinetobacter spp. bacteremia: a cohort study. BMC Infect Dis. 2014;14:572. doi: 10.1186/s12879-014-0572-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lodise TP, Zhao Q, Fahrbach K, Gillard PJ, Martin A. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: how long is too long? BMC Infect Dis. 2018;18:625. doi: 10.1186/s12879-018-3109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burillo A, Marín M, Cercenado E, et al. Evaluation of the Xpert Carba-R (Cepheid) assay using contrived bronchial specimens from patients with suspicion of ventilator-associated pneumonia for the detection of prevalent carbapenemases. PLoS One. 2016;11(12):e0168473. doi: 10.1371/journal.pone.0168473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tato M, Ruiz-Garbajosa P, Traczewski M, et al. Multisite evaluation of Cepheid Xpert Carba-R Assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol. 2016;54(7):1814–1819. doi: 10.1128/JCM.00341-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banerjee R, Humphries R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8:427–439. doi: 10.1080/21505594.2016.1185577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buss BA, Baures TJ, Yoo M, et al. Impact of a multiplex PCR assay for bloodstream infections with and without antimicrobial stewardship intervention at a cancer hospital. Open Forum Infect Dis. 2018;5(10):ofy258. doi: 10.1093/ofid/ofy258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulido MR, Moreno-Martínez P, González-Galán V, et al. Application of BioFire FilmArray blood culture identification panel for rapid identification of the causative agents of ventilator-associated pneumonia. Clin Microbiol Infect. 2018;24(11):1213.e1–1213.e4. doi: 10.1016/j.cmi.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 52.DiDiodato G, Bradbury N. Cerebrospinal fluid analysis with the BioFire FilmArray meningitis/encephalitis molecular panel reduces length of hospital stay in patients with suspected central nervous system infections. Open Forum Infect Dis. 2019;6(4):ofz119. doi: 10.1093/ofid/ofz125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Front Med (Lausanne). 2019;6:74. doi: 10.3389/fmed.2019.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents. 2016;47:269–285. doi: 10.1016/j.ijantimicag.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 56.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Draft guidance for industry on antibacterial therapies for patients with unmet medical need for the treatment of serious bacterial diseases. Draft July 2013. Available from: https://www.govinfo.gov/content/pkg/FR-2013-07-02/pdf/2013-15783.pdf Accessed March3, 2019.
- 57.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Limited population pathway for antibacterial and antifungal drugs. Guidance for industry. June 2018. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM610498.pdf Accessed April11, 2019.
- 58.European Medicines Agency Committee for Human Medicinal Products (CHMP)/Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. EMA/CHMP/351889/2013. October 24, 2013:16, paragraph ii.
- 59.Cox E, Nambiar S, Baden L. Needed: antimicrobial development. N Engl J Med. 2019;380:783–785. doi: 10.1056/NEJMe1901525 [DOI] [PubMed] [Google Scholar]
- 60.Shionogi Inc. Study of S-649266 or best available therapy for the treatment of severe infections caused by carbapenem-resistant Gram-negative pathogens (CREDIBLE – CR). Available from: https://clinicaltrials.gov/ct2/show/NCT02714595 Accessed October11, 2019.
- 61.EU Clinical Trials Register. A multicenter, randomized, open-label clinical study of S-649266 or best available therapy for the treatment of severe infections caused by carbapenem-resistant Gram-negative pathogens. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2015-004703-23 Accessed October11, 2019.
- 62.Adeolu M, Alnajar S, Naushad S, Gupta R. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol. 2016;66(12):5575–5599. doi: 10.1099/ijsem.0.001485 [DOI] [PubMed] [Google Scholar]
- 63.Munson E, Carroll KC. An update on the novel genera and species and revised taxonomic status of bacterial organisms described in 2016 and 2017. J Clin Microbiol. 2019;57(2):pii:e01181–18. doi: 10.1128/JCM.01181-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.European Medicines Agency. European Medicines Agency completes review of polymyxin-based medicines. EMA/643444/2014. 2014. Available from: https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-completes-review-polymyxin-based-medicines_en.pdf Accessed May8, 2019.
- 65.Rice LB, Bonomo RA. beta-Lactamases: which ones are clinically important? Drug Resist Updat. 2000;3(3):178–189. doi: 10.1054/drup.2000.0144 [DOI] [PubMed] [Google Scholar]
- 66.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thaden JT, Pogue JM, Kaye KS. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):403–416. doi: 10.1080/21505594.2016.1207834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Infectious Diseases Society of America. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55:1031–1046. doi: 10.1093/cid/cis688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 x ‘20 Progress – development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Echols RM. Understanding the regulatory hurdles for antibacterial drug development in the post-Ketek world. Ann N Y Acad Sci. 2011;1241:153–161. doi: 10.1111/j.1749-6632.2011.06300.x [DOI] [PubMed] [Google Scholar]
- 71.Echols RM. A long and winding road; evolution of antimicrobial drug development – crisis management. Expert Rev Anti Infect Ther. 2012;10:1311–1319. doi: 10.1586/eri.12.131 [DOI] [PubMed] [Google Scholar]
- 72.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S120–S125. doi: 10.1086/653060 [DOI] [PubMed] [Google Scholar]
- 73.Gharbi M, Drysdale JH, Lishman H, et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all cause mortality: population based cohort study. BMJ. 2019;364:l525. doi: 10.1136/bmj.l42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomila A, Carratalà J, Eliakim-Raz N, et al. Risk factors and prognosis of complicated urinary tract infections caused by Pseudomonas aeruginosa in hospitalized patients: a retrospective multicenter cohort study. Infect Drug Resist. 2018;11:2571–2581. doi: 10.2147/IDR.S185753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wunderink R, Giamarellos-Bourboulis E, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7:439–455. doi: 10.1007/s40121-018-0214-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med. 2019;380:791–793. doi: 10.1056/NEJMc1807634 [DOI] [PubMed] [Google Scholar]
- 77.Alizadeh N, Rezaee MA, Kafil HS, et al. Detection of carbapenem-resistant Enterobacteriaceae by chromogenic screening media. J Microbiol Methods. 2018;153:40–44. doi: 10.1016/j.mimet.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 78.Motsch J, de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind, comparator-controlled trial comparing the efficacy and safety of imipenem/relebactam versus colistin plus imipenem in patients with imipenem-non-susceptible bacterial infections, abstr O0427. Abstract presented at: 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); April 21–24, 2018; Madrid, Spain. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization (WHO). Global priority of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. Available from: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf Accessed February8, 2019.