Abstract
Purpose
The purpose of our study was to evaluate the influence of two PPIs (omeprazole (OME) and lansoprazole (LANSO)) on weakly basic anticancer drug doxorubicin (DOX) and pegylated liposomal doxorubicin (PLD) delivery to monolayer-cultured 4T1 murine breast cancer cells and tumor spheroids.
Methods
The effect of PPIs on cell viability was evaluated by MTT assay. 3D cell cultures (spheroids) were formed using 3D bioprinting method. DOX and PLD penetration into cancer cells and spheroids at pH 6.0 and 7.4 was assessed using fluorescence microscopy.
Results
Both OME and LANSO did not reduce the viability of 4T1 cells at 100 μM and lower concentrations, and therefore, in further experiments, 100 μM of PPIs was used. At pH 7.4, both tested PPIs did not enhance DOX (5 µM) and PLD (concentration corresponding to 5 µM DOX) delivery into 2D cell cultures. However, in acidic conditions, both PPIs increased the amount of drug in cancer cells and their nucleus. At physiological pH they were not effective at improving DOX delivery into spheroids, but after 2 hrs of incubation, OME slightly increased PLD delivery into edge and middle zones. At pH 6.0, both tested PPIs significantly enhanced DOX and PLD transport into spheroids, but the positive effect on delivery was observed only within the first 4 hrs of incubation.
Conclusion
Both OME and LANSO increased DOX and PLD penetration into monolayer-cultured cells at acidic conditions but did not show a positive effect on drug delivery at physiological pH. Also, pretreatment with tested PPIs slightly increased DOX and PLD delivery in the edge and middle zones of tumor spheroids. Thus, OME and LANSO are promising transport modulators of weakly basic drugs.
Keywords: vacuolar-H+-ATPase, drug delivery, spheroids, extracellular acidity, multidrug resistance
Introduction
Cancer is the second most common cause of death worldwide.1 The most important factors that lead to a high mortality rate are the delayed diagnosis and cancer resistance to chemotherapeutic drugs. One of the main mechanisms of this resistance is related to the tumor microenvironment pH. Extracellular fluid in a tumor is usually acidic and may vary from 6.2 to 7.2.1 This acidity is caused by increased glycolysis and lactic acid production in tumor tissues and proton exchangers that continuously carry protons outside the cell and into lysosomal vesicles.2
At the end of the last century, it was observed that the acidic environment limits weakly basic drug penetration into tumor tissue.3 Acidic microenvironment in tumor causes the ionisation of basic anticancer drugs, such as anthracyclines or alkaloids. Positive charge reduces drug ability to permeate the cellular membrane and reach the target site, thus limiting drug efficacy.4 It is called “ion trapping” phenomenon.
On the contrary, the decrease of extracellular acidity enhances weakly basic drug delivery into cells. Raghunand et al found out that raising pHe from 6.8 to 7.4 increases the amount of DOX into tumor cells by 2.25-fold.3 In this study tumor, alkalinisation was caused using sodium bicarbonate. This method is criticized because of its toxicity. Administration of sodium bicarbonate may induce various side effects such as metabolic alkalosis and electrolyte imbalance.5 Therefore, an increasing attention was given to the development of other approaches to prevent extracellular acidity.
One of the strategies to decrease pHe is an inhibition of vacuolar-H+-ATPase (V-ATPase). It is a complex of proteins that catalyses the active transport of protons from the cell cytoplasm to the extracellular fluid and acidifies the lumen of lysosomes and the endosomal vesicles.6 This results in extracellular acidification and increased pH of lysosomes. It was reported that V-ATPase genes are overexpressed in many types of cancer such as breast,7 brain,8 pancreatic,9 ovarian,10 lung cancer,11 melanoma12 and hepatocellular carcinoma.13
Proton pump inhibitors (PPIs) are a group of drugs that are used to reduce gastric acid production by inhibiting hydrogen potassium ATPase in gastric parietal cells.14 These drugs do not exert severe systemic toxicity even when are administered prolonged time and at high dosages, for example, in a case of Zollinger–Ellison syndrome.15 Recent studies show that they can reduce extracellular acidity and increase basic drug delivery to cancer cells, thus increasing the efficacy of chemotherapy.16
The aim of our study was to evaluate the influence of two PPIs OME and LANSO on weakly basic anticancer drug DOX and PLD delivery to monolayer-cultured triple-negative 4T1 murine breast cancer cells and tumor spheroids. It has been postulated that PPIs also exhibit a cytotoxic effect against tumor cells.17 Among all PPIs, LANSO exerts the greatest antitumor activity which is advantageous when combining this compound with anticancer drugs.17 In order to compare the results, we chose to test the first known PPI OME which is one of the most commonly prescribed members of PPIs group.18,19 DOX is one of the main drugs to treat triple-negative breast cancer,20 it is a weakly basic drug that tends to ionize in an acidic environment, and due to its fluorescence, it is easy to estimate its delivery using fluorescence microscopy. Compared to non-liposomal DOX, PLD has prolonged circulation time in the blood, passive targeting into a tumor, reduced exposure to normal tissues and relatively improved delivery to tumor tissues.21 The experiments were performed at two different pH values (6.0 that represents the acidic tumor environment, and 7.4). According to our knowledge, this paper is the first study to compare the effects of PPIs on drug transport at two different pH values and to test the efficacy of PPIs on drug delivery into tumor spheroids.
Materials And Methods
Materials
DOX hydrochloride was bought from Abcam (Cambridge, UK). PLD was purchased from FormuMax Scientific Inc. (Palo Alto, CA, USA). OME and LANSO were bought from Sigma-Aldrich Co. (St Louis, MO, USA).
Cell Cultures
A triple-negative murine breast cancer cell line 4T1 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in Roswell Park Memorial Institute 1640 GlutaMAX medium, supplemented with 10,000 U/mL penicillin, 10 mg/mL streptomycin, and 10% fetal bovine serum. Media and supplements were purchased from Gibco (Carlsbad, CA, USA). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Cell Viability
To determine the effect on cell viability, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Co.) assay was performed. 4T1 cells were seeded in 96-well plates in a volume of 100 μL (5,000 cells/well). After 24-hr preincubation, the cells were treated with 100 μL of different concentrations of statins. Only medium without cells was used as a positive control, and the medium with 0.5% DMSO (Sigma-Aldrich Co.) served as a negative control. After 4, 8, 12 and 24 hrs, the cells were incubated for 3 hrs with the MTT solution (Sigma-Aldrich Co.). The absorbance was measured at wavelengths of 570 and 630 nm.
Drug Delivery In Monolayer-Cultured Cells
4T1 cells were seeded in 24-well plates on collagen-coated coverslips in a volume of 500 μL (50,000 cells/well) and in a humidified atmosphere containing 5% CO2 at 37°C. After 24 hrs,the medium was replaced by a new medium of pH 6.0 or 7.4 and incubated for 1 hr. Then, cells were incubated with a new medium of the same pH containing 100 µM OME or LANSO or 0.2% DMSO. After 2 hrs of incubation, the medium was replaced with a new medium of the same pH containing 1 or 5 µM of DOX or PLD. After 0.5, 1, 2 and 4 hrs, the cells were washed with PBS and fixed with 4% paraformaldehyde (Thermo Scientific, Waltham, MA, USA) solution in PBS and stained with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Scientific). DOX and PLD penetration into whole cells and their nucleus was assessed using fluorescence microscopy and ImageJ software (National Institutes of Health).
Drug Delivery In Cell Spheroids
The spheroids were formed using 3D Bioprinting method.22 4T1 cells were incubated with nanoparticles NanoShuttle™ (Nano3D Biosciences Inc., Houston, TX, USA) for 8 hrs. After that, cells were resuspended and seeded into ultra-low attachment 96-well plates in a volume of 100 μL (800 cells per well). The plate was placed on a magnetic drive and incubated in a humidified atmosphere containing 5% CO2 at 37°C. After 2 days of incubation, the magnetic drive was removed, and the medium was replaced by a new medium of pH 6.0 or 7.4 and incubated for 1 hr. Then, cells were incubated with a new medium of the same pH containing 100 µM OME or LANSO or 0.2% DMSO. After 2 hrs of incubation, the medium was replaced with a new medium of the same pH containing 20 µM of DOX or PLD. After 1, 2, 4 and 8 hrs, spheroids were washed with PBS and fixed with 4% paraformaldehyde (Thermo Scientific, Waltham, MA, USA) solution in PBS. DOX and PLD penetration into spheroids was assessed using fluorescence microscopy and ImageJ software (National Institutes of Health) by evaluating fluorescence every one degree from the spheroid center to the edge around the whole spheroid.
Statistical Analysis
Statistical analysis was performed using Microsoft Office Excel 2016 software (Microsoft Corporation, Redmond, WA, USA). All the experiments were done in at least triplicate independent measurements and the obtained values were reported as mean ± standard deviation. Student’s t-test was used and p-values were calculated. A value of p < 0.05 was considered as the level of significance.
Results
Effect Of PPIs On 4T1 Cell Viability
Both tested PPIs did not reduce 4T1 cell viability at 100 μM and lower concentrations; therefore, in further experiments, 100 μM PPIs were used. DOX and PLD values after 12 hrs of incubation were >150 μM; hence, 1, 5 and 20 µM concentrations of DOX and corresponding concentrations of PLD that are below toxicity level were chosen to use in the research.
Effect Of PPIs On DOX And PLD Delivery In Cancer Cells
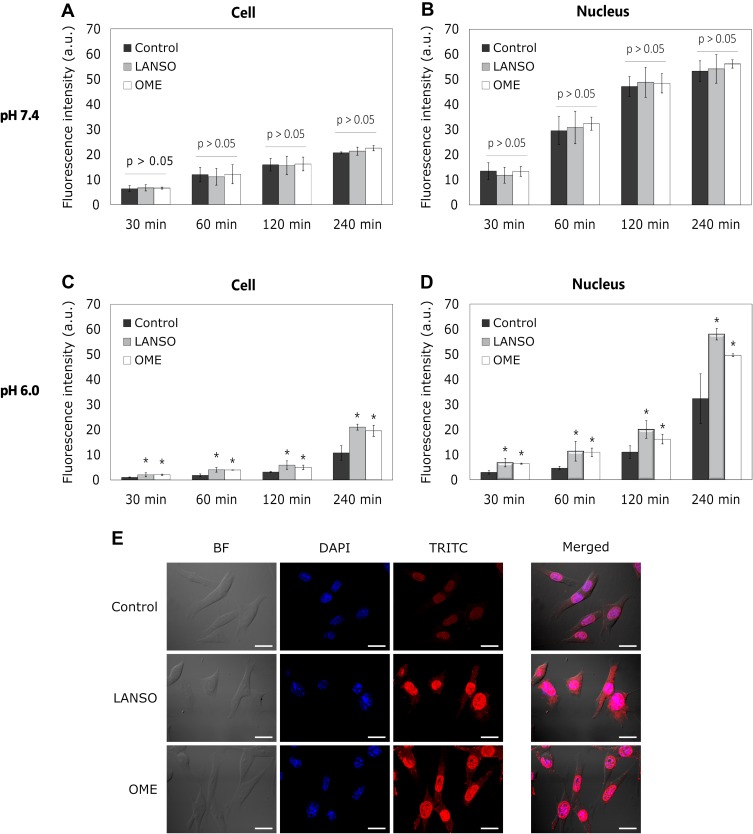
OME and LANSO did not enhance the delivery of 1 and 5 µM DOX at pH 7.4 (Figure 1A and B). Both PPIs did not exert a positive effect on 1 µM DOX penetration into cells at pH 6.0 as well. However, OME and LANSO increased 5 µM DOX delivery in cell and nucleus at the time period from 30 mins to 4 hrs in acidic conditions (Figure 1C–E).
Figure 1.
The effect of proton pump inhibitors on doxorubicin (DOX) delivery into monolayer-cultured cells at different pH values. (A) DOX fluorescence intensity in cells at different time periods at pH 7.4. (B) DOX fluorescence intensity in the cell nucleus at different time periods at pH 7.4. (C) DOX fluorescence intensity in cells at different time periods at pH 6.0. (D) DOX fluorescence intensity in the cell nucleus at different time periods at pH 6.0. (E) Representative images of cells after 4 hrs of incubation with DOX at pH 6.0. Magnification 600×. Scale bar = 50 µm. The asterisks (*) indicate p < 0.05.
Abbreviations: LANSO, lansoprazole; OME, omeprazole.
During the first 2 hrs, there was no difference in the amount of DOX in cells affected by OME and LANSO. After 4 hrs of incubation, the efficacy of LANSO on DOX delivery was slightly higher than the effect of OME (OME increased the amount of DOX in cell nucleus approximately 1.5-fold and LANSO 1.8-fold compared to control). As expected, the amount of DOX in cell and nucleus ant acidic conditions was lower than at physiological pH.
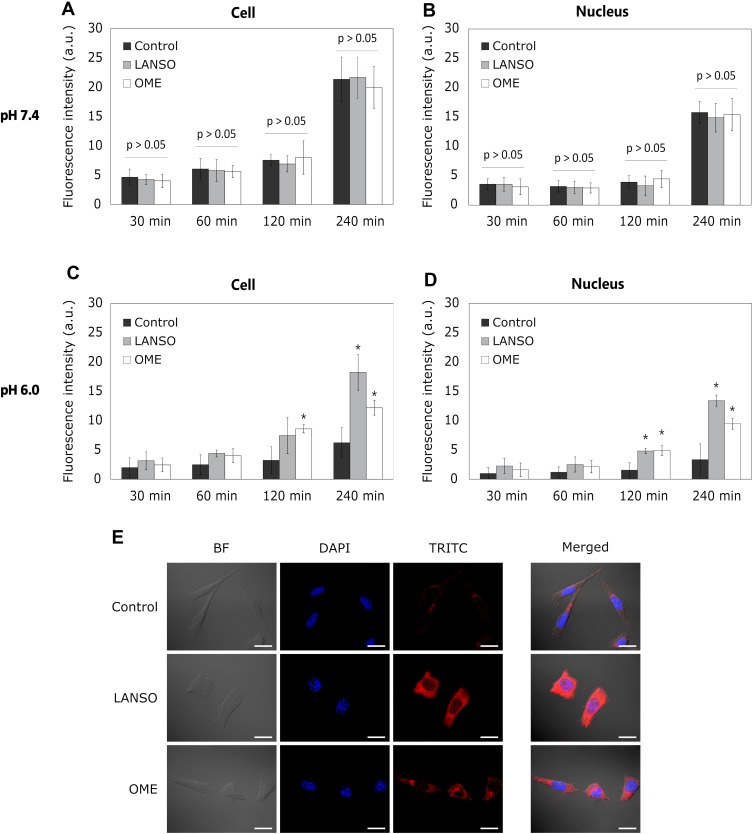
Both tested PPIs were less effective in improving PLD delivery in 2D cells compared to the results observed on DOX delivery. OME and LANSO did not increase the amount of PLD in cells at physiological pH (Figure 2A and B). Both PPIs showed no positive effect on PLD delivery in cells after 30 mins and 1 hr of incubation at pH 6.0 as well. However, OME and LANSO significantly enhanced PLD penetration into cancer cells and nucleus after prolonged incubation (Figure 2C and D). After 4 hrs of incubation, the efficacy of LANSO on PLD delivery was higher than the effect of OME (LANSO increased the amount of PLD in cell nucleus approximately 4-fold and OME 2.8-fold compared to control). As observed at the experiments with DOX, acidic pH decreased PLD penetration into 2D cancer cells and their nucleus.
Figure 2.
The effect of proton pump inhibitors on pegylated liposomal doxorubicin (PLD) delivery into monolayer-cultured cells at different pH values. (A) PLD fluorescence intensity in cells at different time periods at pH 7.4. (B) PLD fluorescence intensity in the cell nucleus at different time periods at pH 7.4. (C) PLD fluorescence intensity in cells at different time periods at pH 6.0. (D) DOX fluorescence intensity in the cell nucleus at different time periods at pH 6.0. (E) Representative images of cells after 4 hrs of incubation with DOX at pH 6.0. Magnification 600×. Scale bar = 50 µm. The asterisks (*) indicate p < 0.05.
Abbreviations: LANSO, lansoprazole; OME, omeprazole.
Effect Of PPIs On DOX And PLD Delivery In Spheroids
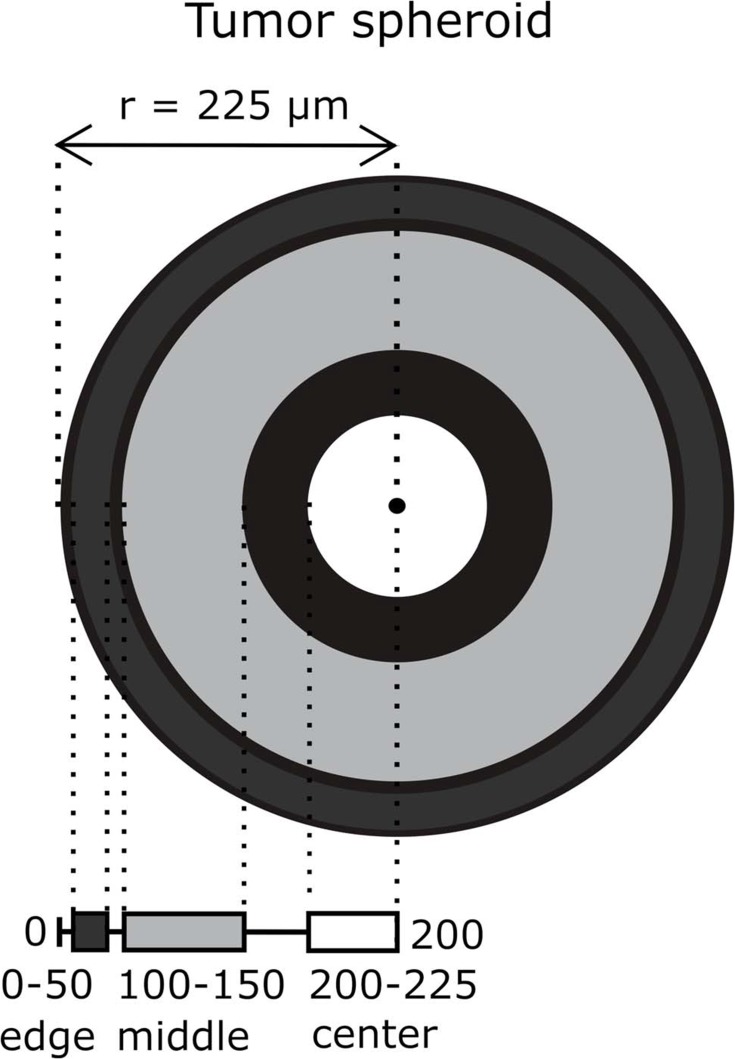
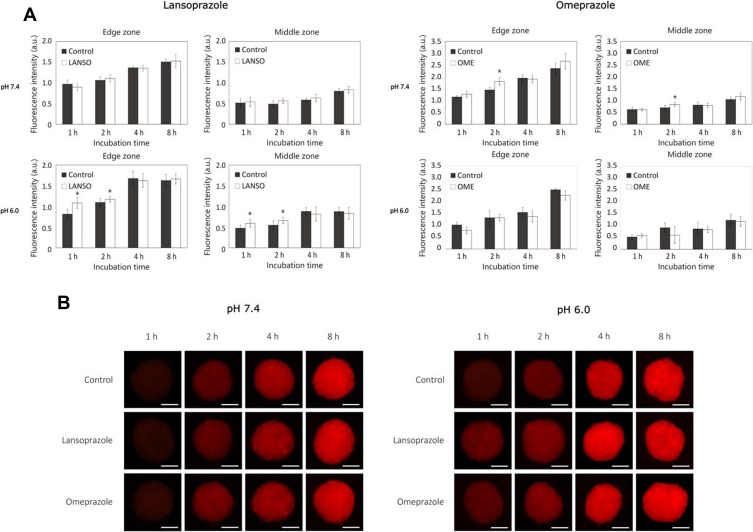
In order to evaluate the effect of PPIs on DOX and PLD delivery in 3D cancer cell cultures, tumor spheroids were distinguished into three zones – edge zone (0–50 µM), middle zone (100–150 µM) and center zone (200–225 µM) (Figure 3).
Figure 3.
A schematic representing spheroid division into edge (0–50 µM from the spheroid edge), middle (100–150 µM) and center (200–225 µM) zones. The mean spheroid radius was 225 µM.
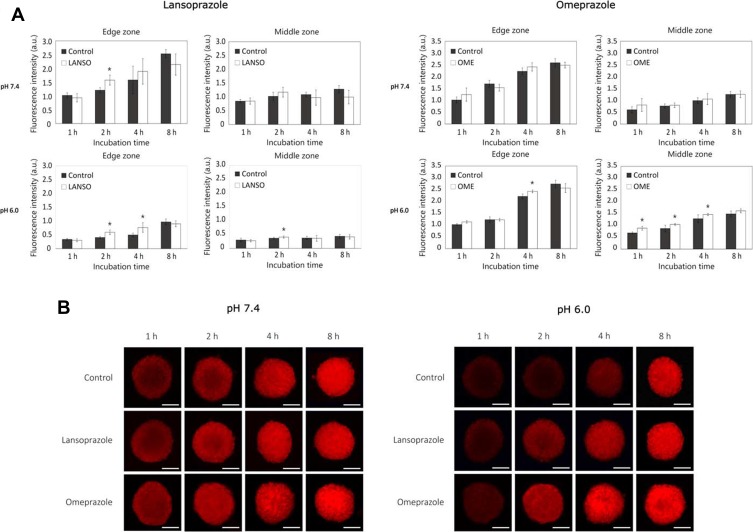
At pH 7.4, both tested PPIs did not increase DOX delivery both in middle and in central zones (Figure 4A and B). Nevertheless, after 2 hrs of incubation, LANSO increased DOX delivery into the edge zone by approximately 23.3 ± 11.4% compared to control. At pH 6.0, after 2 hrs of incubation, LANSO increased DOX delivery into the edge and middle zones by approximately 31.9 ± 13.6% and 10.4 ± 9.7%, respectively. After 4 hrs of incubation, LANSO increased DOX delivery only in the middle zone by 35.3 ± 18.3%. At acidic pH, after 1 hr of incubation, OME increased DOX penetration into middle and center zones by 22.7 ± 9.2% and 32.4 ± 11.5%, respectively; after 2 hrs of incubation – into middle zone by 16.6 ± 3.7%; and after 4 hrs of incubation – into edge and middle zones by 8.8 ± 2.4% and 12.2 ± 3.3%, respectively.
Figure 4.
The effect of proton pump inhibitors on doxorubicin (DOX) delivery into tumor spheroids at different pH values. (A) Fluorescence intensity of DOX in spheroids affected with lansoprazole and omeprazole at pH 7.4 and 6.0. (B) Representative images of spheroids after different times of incubation with DOX at pH 7.4 and 6.0. Magnification 100×. Scale bar = 200 µm. The asterisks (*) indicate p < 0.05.
Abbreviations: LANSO, lansoprazole; OME, omeprazole.
Similar results were obtained from the experiments with PLD (Figure 5A and B). At physiological pH, LANSO did not increase PLD delivery into any spheroid zone, whereas OME after 2 hrs of incubation slightly increased the amount of PLD into edge and middle zones by 19.8 ± 8.4% and 16.5 ± 11.3%, respectively. At acidic pH, after 1 and 2 hrs of incubation, LANSO increased PLD penetration into the edge and middle zones (after 1 hr the amount of PLD increased by 25.0 ± 12.1% and 20.0 ± 16.6%, respectively, and after 2 hrs – by 6.4 ± 6.0% and 17.2 ± 12.2%). At the same pH, the positive effect of OME on PLD delivery was observed only after 1 hr of incubation – the amount of PLD in center zone increased by 30.9 ± 20.8% compared to control. After a longer period (8 hrs), neither LANSO nor OME had no positive effect on DOX and PLD delivery in tumor spheroids.
Figure 5.
The effect of proton pump inhibitors on pegylated liposomal doxorubicin (PLD) delivery into tumor spheroids at different pH values. (A) Fluorescence intensity of PLD in spheroids affected by lansoprazole and omeprazole at pH 7.4 and 6.0. (B) Representative images of spheroids after different times of incubation with PLD at pH 7.4 and 6.0. Magnification 100×. Scale bar = 200 µm. The asterisks (*) indicate p < 0.05.
Abbreviations: LANSO, lansoprazole; OME, omeprazole.
Discussion
In this study, we showed that pretreatment with OME and LANSO enhances DOX and PLD delivery into 2D and 3D cell cultures at acidic conditions. Meanwhile, at physiological pH, both tested PPIs do not exert drug transport enhancing effect into 2D cell cultures, and this effect is weak in tumor spheroids. These results are in agreement with Yu et al's findings who showed that LANSO enhanced DOX delivery into multilayered cell cultures and increased DOX distribution in solid tumors.23 Luciani et al found that OME impaired the acidification of extracellular tissue. Furthermore, pretreatment with OME, esomeprazole and pantoprazole sensitized cancer cells to the cytotoxic effect of cisplatin, vinblastine, 5-fluorouracil.24 There is evidence that pantoprazole increases endosomal pH, thus reducing endosomal sequestration of DOX and increasing its nuclear uptake in multi-layered murine and human breast cancer cells. Pantoprazole also increases DOX efficacy in vivo in mice bearing MCF-7 xenografts.16 We did not observe a significant difference in LANSO and OME efficacy to improve DOX and PLD delivery into cancer cell cultures. The chemical activation of LANSO is slightly faster compared to the activation of OME.25
As we predicted, the amount of DOX and PLD in 2D and 3D cancer cells at acidic pH was lower than at physiological conditions. pKa value of DOX is 9.93 and at pH 7.4 around 80% of the molecules of this compound exist in cationic form.26 As claimed by our hypothesis, by increasing pHe, PPIs should prevent weakly basic drugs from protonation and enhance their transport to cells. However, according to our results, at physiological pH, tested PPIs did not increase DOX and PLD delivery into cancer cells. PPIs are weakly basic substituted 2-pyridyl-methylsulfinyl benzimidazole prodrugs. They are activated in the acidic environment after the protonation of the nitrogen atoms in the pyridine and benzimidazole rings.27 Based on these data, it can be hypothesised that at physiological pH, the majority of PPIs stay in unprotonated form and the number of ionized molecules is not sufficient to effectively inhibit V-ATPases in cancer cells. Wang et al estimated that pH 6.75 is suitable for the protonation of pantoprazole. Scientists showed that at this slightly acidic pH, pantoprazole efficiently chemosensitizes colorectal cancer cells to 5-fluorouracil. Pantoprazole also increases 5-fluorouracil's ability to inhibit tumor growth in mice.28
It is also important to mention that PLD resistance mechanism is different from free DOX. Besides pH, other factors, such as particle size and formulation, lipid composition, surface charge, also influence DOX release profile from PLD and its uptake.29 However, Shibata et al showed that pH affects DOX release from PLD as well, i.e., DOX release increases with an increase of solution pH.29 Our data correspond with the results of Shibata et al. In our study, we also showed that DOX uptake from PLD at pH 7.4 is higher than at pH 6.0. Since DOX release from PLD also depends on the characteristics of the liposomal formulation, the effect of PPIs on PLD transport was lower than of free DOX. This may explain why OME did not increase PLD delivery into spheroids at pH 6.0
There is still a lack of clinical studies proving the application of PPIs to modulator anticancer drug delivery. Ferrari et al conducted a Phase II study with a comparison with historical control among patients with osteosarcoma (n=98). Two days prior to the administration of methotrexate, cisplatin or doxorubicin, patients received 60 mg/day esomeprazole. For 61% of PPI-pretreated patients, percentage tumor necrosis was equal or higher than 90% while in the control group the rate of such response was 25%.30 A second Phase II trial was started in 2012 (clinicaltrials.gov, NCT02013453). The aim of this study was to evaluate the effect of pantoprazole on the activity and safety of docetaxel in men with castration-resistant prostate cancer. This trial is still ongoing, and the results are not published yet. In 2013, another Phase II study (clinicaltrials.gov, NCT02013453) evaluating the use of omeprazole in combination with 5-fluorouracil, paclitaxel or pemetrexed in patients with head and neck cancers was started. However, it was withdrawn due to the lack of funding.
Since V-ATPases are located in every eukaryotic cell,31 in order to increase the efficacy of inhibitors, it is important to target drugs directly to neoplastic cells. Recently, Bhattacharya designed a dual nanocarrier system loaded with paclitaxel and LANSO. These nanoparticles significantly increase the release kinetics and enhance in vitro cytotoxicity against human breast cancer cells.3,2 Such targeted delivery systems would let to diminish the overall drug exposure to normal tissues, reduce systemic toxicity and increase the efficacy of chemotherapy.
These findings and the results of our study support the application of PPIs as chemosensitizers of weakly basic anticancer drugs.
Conclusion
Both OME and LANSO increased DOX and PLD penetration into monolayer-cultured cells at acidic conditions but did not show a positive effect on drug delivery at physiological pH. Also, pretreatment with tested PPIs slightly increased DOX and PLD delivery in the edge and middle zones of tumor spheroids. Thus, OME and LANSO are promising transport modulators of weakly basic drugs.
Acknowledgment
The research was supported by Science Foundation of Lithuanian University of Health Sciences project “Application of transport to the cell modulators to improve doxorubicin and its pegylated formulation delivery in 2D and 3D cell cultures”, 2018.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2(4):343–366. doi: 10.1016/S0167-8140(84)80077-8 [DOI] [PubMed] [Google Scholar]
- 2.Alfarouk KO, Verduzco D, Rauch C, et al. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience. 2014;1(12):777–802. doi: 10.18632/oncoscience.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghunand N, He X, Van Sluis R, et al. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999;80(7):1005–1011. doi: 10.1038/sj.bjc.6690455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8(6):2032–2038. doi: 10.1021/mp200292c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faes S, Dormond O. Systemic buffers in cancer therapy: the example of sodium bicarbonate; stupid idea or wise remedy? Med Chem (Los Angeles). 2016;5(12):540–544. doi: 10.4172/2161-0444.1000314 [DOI] [Google Scholar]
- 6.Maxson ME, Grinstein S. The vacuolar-type H+-ATPase at a glance - more than a proton pump. J Cell Sci. 2014;127(23):4987–4993. doi: 10.1242/jcs.158550 [DOI] [PubMed] [Google Scholar]
- 7.Sennoune SR, Bakunts K, Martínez GM, et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 2004;286(6):C1443–C1452. doi: 10.1152/ajpcell.00407.2003 [DOI] [PubMed] [Google Scholar]
- 8.Gleize V, Boisselier B, Marie Y, Poëa-Guyon S, Sanson M, Morel N. The renal v-ATPase a4 subunit is expressed in specific subtypes of human gliomas. Glia. 2012;60(6):1004–1012. doi: 10.1002/glia.22332 [DOI] [PubMed] [Google Scholar]
- 9.Chung C, Mader CC, Schmitz JC, et al. The vacuolar-ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab Investig. 2011;91:732–743. doi: 10.1038/labinvest.2011.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulshrestha A, Ibrahim S, Pamarthy S, et al. Vacuolar ATPase isoform exhibits distinct cell surface accumulation and modulates matrix metalloproteinase activity in ovarian cancer. Oncotarget. 2015;6(6). doi: 10.18632/oncotarget.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Q, Lu S, Huang L, et al. The expression of V-ATPase is associated with drug resistance and pathology of non-small-cell lung cancer. Diagn Pathol. 2013;8:145. doi: 10.1186/1746-1596-8-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasui N, Sun-Wada G-H, Wada Y, et al. The a3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res. 2011;9(7):845–855. doi: 10.1158/1541-7786.mcr-10-0449 [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Xie R, Liu X, et al. Expression and functional role of vacuolar H+-ATPase in human hepatocellular carcinoma. Carcinogenesis. 2012;33(12):2432–2440. doi: 10.1093/carcin/bgs277 [DOI] [PubMed] [Google Scholar]
- 14.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528–534. doi: 10.1007/s11894-008-0098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005;11(35):5423–5432. doi: 10.3748/wjg.v11.i35.5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel KJ, Lee C, Tan Q, Tannock IF. Use of the proton pump inhibitor pantoprazole to modify the distribution and activity of doxorubicin: a potential strategy to improve the therapy of solid tumors. Clin Cancer Res. 2013;19(24):6766–6776. doi: 10.1158/1078-0432.CCR-13-0128 [DOI] [PubMed] [Google Scholar]
- 17.Lugini L, Federici C, Borghi M, et al. Proton pump inhibitors while belonging to the same family of generic drugs show different anti-tumor effect. J Enzyme Inhib Med Chem. 2016;31(4):538–545. doi: 10.3109/14756366.2015.1046062 [DOI] [PubMed] [Google Scholar]
- 18.Gawron AJ, Feinglass J, Pandolfino JE, Tan BK, Bove MJ, Shintani-Smith S. Brand name and generic proton pump inhibitor prescriptions in the United States: insights from the National Ambulatory Medical Care Survey (2006–2010). Gastroenterol Res Pract. 2015;2015:1–7. doi: 10.1155/2015/689531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hálfdánarson Ó, Pottegård A, Björnsson ES, et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol. 2018;11:175628481877794. doi: 10.1177/1756284818777943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeichner SB, Terawaki H, Gogineni KA. Review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl). 2016;10:25–36. doi: 10.4137/BCBCR.S32783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Lionberger R, Yu LX. In vitro and in vivo characterizations of PEGylated liposomal doxorubicin. Bioanalysis. 2011;3(3):333–344. doi: 10.4155/bio.10.204 [DOI] [PubMed] [Google Scholar]
- 22.Tseng H, Gage JA, Shen T, et al. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci Rep. 2015;5(1):13987. doi: 10.1038/srep13987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M, Lee C, Wang M, Tannock IF. Influence of the proton pump inhibitor lansoprazole on distribution and activity of doxorubicin in solid tumors. Cancer Sci. 2015;106(10):1438–1447. doi: 10.1111/cas.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96(22):1702–1713. doi: 10.1093/jnci/djh305 [DOI] [PubMed] [Google Scholar]
- 25.Kromer W, Krüger U, Huber R, Hartmann M, Steinijans VW. Differences in pH-dependent activation rates of substituted benzimidazoles and biological in vitro correlates. Pharmacology. 1998;56(2):57–70. doi: 10.1159/000028183 [DOI] [PubMed] [Google Scholar]
- 26.Faes S, Dormond O. Systemic buffers in cancer therapy: the example of sodium bicarbonate; stupid idea or wise remedy? Med Chem (Los Angeles). 2015;5(12):540–544. doi: 10.4172/2161-0444.1000314 [DOI] [Google Scholar]
- 27.Roche VF. The chemically elegant proton pump inhibitors. Am J Pharm Educ. 2006;70(5):101. doi: 10.5688/aj7005101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Liu C, Wang J, Fan Y, Wang Z. Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget. 2017;8(35):58801–58808. doi: 10.18632/oncotarget.18522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari S, Perut F, Fagioli F, et al. Proton pump inhibitor chemosensitization in human osteosarcoma: from the bench to the patients’ bed. J Transl Med. 2013;11(1):1. doi: 10.1186/1479-5876-11-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyenbach KW. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209(4):577–589. doi: 10.1242/jeb.02014 [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya S, Khanam J, Sarkar P, Pal TK. A chemotherapeutic approach targeting the acidic tumor microenvironment: combination of a proton pump inhibitor and paclitaxel for statistically optimized nanotherapeutics. RSC Adv. 2019;9(1):240–254. doi: 10.1039/C8RA08924H [DOI] [PMC free article] [PubMed] [Google Scholar]