Abstract
Background: Toxoplasma gondii is a protozoan parasite that chronically infects nearly one-third of the world's human population. In immunosuppressed individuals and fetus, infection with T. gondii contributes to a series of devastating conditions, including toxoplasmic encephalitis (TE), which is characterized by neuron damage in the central nervous system (CNS). Astrocyte polarization is currently found in some neurodegenerative diseases, and A1 subtype of astrocyte leads to neuron apoptosis. However, little information has been available on the role of astrocyte polarization in TE.
Methods: In the present study, we established a mouse model to study TE and detected A1 astrocyte in the brains of mice with TE. Expression level of A1 astrocyte-specific marker C3 was evaluated using indirect fluorescent assay (IFA) and Western blotting. Primary mouse astrocytes were incubated with different concentrations of T. gondii excreted-secreted antigens (TgESAs) in vitro. Expression level of C3 and A1 astrocyte-specific transcription levels were assessed using Western blotting and qRT-PCR, respectively. Bay11-7082 was used to study nuclear factor (NF) κB pathway in TgESA-induced astrocyte polarization.
Results: In mice with TE, the proportion of A1 astrocyte (GFAP+C3+) increased significantly. The results of in vitro study showed that TgESAs induced astrocyte polarization to A1 subtype. Blocking of NFκB pathway by Bay11-7082 inhibited TgESA-induced astrocyte polarization.
Conclusions: Our preliminary study showed the involvement of A1 astrocyte in the process of TE in mice, and TgESAs could trigger astrocyte to polarize to A1 subtype. These findings suggest a new mechanism underlying the neuropathogenesis induced by T. gondii infection.
Keywords: Toxoplasma gondii, encephalitis, astrocyte, NFκB pathway, neuron
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite that chronically infects the central nervous system (CNS) of up to one-third of the human population in the world (1). Humans get infected with such disease by ingesting water or food contaminated with oocysts shed by cats or consumption of raw or undercooked meat containing a tissue cyst or congenitally by transplacental transmission of tachyzoites (2). Upon infection with T. gondii, fast-replicating tachyzoites infect a wide range of host cells, including neurons. Tachyzoites convert into slow-replicating bradyzoites, which persist as cysts in neurons under the pressure of the immune response.
Although most infected persons show no clinical symptoms, chronic T. gondii infection could impair host neuron function and structure (3–5), which may alter the behavior of humans or even increase the risk for neurodegenerative and psychiatric disorders (6, 7). In the developing fetus and immunocompromised individuals, such as AIDS patients or organ transplant recipients, Toxoplasma infection can cause a devastating neurologic disease. Symptomatic brain infection with Toxoplasma is known as toxoplasmic encephalitis (TE) and can clinically present with dizziness, headaches, and seizures. Currently, TE occurs in untreated or undiagnosed AIDS patients and in patients on new immunomodulants (8). In TE, T. gondii bradyzoites within cysts switch to tachyzoites, which infect and destroy brain-resident cells. Previous in vitro and in vivo evidences suggest that neurons serve as primary target cells for T. gondii tachyzoites and bradyzoites (1, 9).
An in vitro culture of neurons with T. gondii tachyzoites at a low multiplicity of infection (MOI) as previously described (10) induced the formation of a large cyst rather than the lysis of neurons. However, the in vivo TE mouse model showed that the neuronal damage was increased in the brain, and T. gondii infection induced activated microglia, which contributed to neuronal apoptosis (11). In addition, T. gondii excreted-secreted antigens (ESAs) induce apoptosis of the neural stem cells (NSCs) through endoplasmic reticulum stress (ERS) signaling pathways and inhibit differentiation of C17.2 neural stem cells through Wnt/β-catenin signaling pathway (12, 13). Whether other CNS resident cells are involved in neuron loss in TE is still an enigma.
Astrocytes are the most common glial cells within the cerebral cortex, which provide trophic support for neurons, promote formation and function of synapses, and prune synapses by phagocytosis (14–16). These cells also perform a diversity of functions, including participation in the immune response of the brain and undergo a pronounced transformation called reactive astrocytosis after brain injuries and neurodegenerative diseases (17).
Recent studies have demonstrated that proinflammatory microglia induce the formation of a subtype of astrocytes (termed A1 astrocytes), which are characterized by highly upregulated classical complement cascade genes (i.e., C3) shown to be destructive to synapses and are strongly neurotoxic and rapidly kill neurons (18). A1 astrocytes are abundant in various human neurodegenerative diseases, including Alzheimer's disease, Huntington's disease, Parkinson's disease, amyotrophic lateral sclerosis, and multiple sclerosis (18). A1-like astrocyte reactivity is induced in normal aged brains that are vulnerable to injury and cognitive function declines (19). However, whether T. gondii infection induces astrocyte polarization to A1 and the role of A1 astrocytes in neuron death in TE are still not clear. In the present study, we aimed to investigate the effects of the ESAs of T. gondii (Tg-ESAs) on astrocyte polarization and assess the involvement of nuclear factor (NF) κB signaling pathway in Tg-ESAs-induced astrocyte polarization. This study provides insight into the underlying molecular mechanisms that regulate neuropathogenesis in TE.
Materials and Methods
Cell and Parasite
T. gondii Wh6 strain (avirulent strain) with genotype Chinese 1 (ToxoDB#9) was isolated as previously described (20). Cysts were maintained in the brain of chronically infected mice for in vivo infection. To collect cysts, brains from infected mice were mechanically homogenized in 1-ml sterile phosphate-buffered saline (PBS). Cyst numbers were counted in a 10-μl brain suspension using a light microscope (21). Tachyzoites of T. gondii were passaged in human foreskin fibroblast (HFF) monolayers for in vitro experiments. Mouse primary astrocytes were purchased from FenghuiShengwu (Changsha, China) and cultured in Dulbecco's modified Eagle medium (DMEM) medium supplemented with 10% fetal bovine serum.
Mice and Infection
Mice were divided into three groups (three mice/group): control group (non-infection group), chronic group (chronic infection without cyclophosphamide treatment), and TE group (chronic infection with cyclophosphamide treatment). The Wh6 strain cysts were prepared by homogenization of the brain tissues in phosphate-buffered saline (PBS). Seven-week-old female BALB/c mice were intragastrically administered with 30 cysts. After 6 weeks, mice with latent infection were intraperitoneally injected with cyclophosphamide (50 mg/kg; Baxter Oncology GmbH, Germany) to induce recurrence of toxoplasmosis as previously described (11). Seven days later, all mice of the three groups were euthanized for collection of the brain tissues for further experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Anhui Medical University.
Treatment of Astrocyte With TgESAs
ESAs from T. gondii were prepared as described previously (12). Tachyzoites of T. gondii were harvested as described above.
After resuspension with serum-free DMEM, 2 × 107 freshly collected tachyzoites were added into HFF monolayers. T. gondii-infected HFFs were further cultured in the serum-free DMEM medium at 37°C in 5% CO2 for another 48 h. The supernatants of the infected HFFs were collected by centrifugation at 12,000 g for 10 min at 4°C and then filtered through a 0.22-mm membrane filter. Protein concentration in the supernatants was determined by BCA kits according to the manufacturer's instructions (Thermo-Fisher, Boston, MA). Protein samples were stored at −80°C until use. Non-infected HFFs in serum-free DMEM were used as a negative control.
Mouse primary astrocytes were seeded in six-well cell culture plates. Cells were treated with different doses of TgESAs (0, 0.10, 0.15, and 0.30 mg/ml) for 24 h when the cell confluence reached approximately 70%. Then, C3 protein expression level and A1-specific gene transcription levels were detected by Western blotting and quantitative real-time PCR (qRT-PCR), respectively. In some experiments, astrocytes were pretreated with NFκB inhibitor BAY11-7082 (1 and 5 μM) (22). After 12 h, TgESAs (0.30 mg/ml) was added, and cells were co-cultured for further 24 h.
Immunofluorescence Assays
Mice were anesthetized with 1% pentobarbital and transcardially perfused with 20 ml ice-cold 4% paraformaldehyde after an initial flush with 20 ml ice-cold 0.01 M PBS. Brains were removed and post-fixed with 4% paraformaldehyde for 12 h. Brain tissues were subsequently dehydrated in 30% sucrose in 0.01 M PBS for 48 h. Tissues were embedded in optimal cutting temperature compound (OCT Compound, SAKURA, USA) and then sliced coronally (10–20 μm) on a cryostat microtome (CM3050S, Leica, Germany). For immunofluorescence staining, the samples were blocked with 5% bovine serum albumin (BSA) and 0.5% Triton X-100 (containing 0.02% normal goat serum) for 2 h at room temperature. The samples were incubated with primary antibodies overnight at 4°C and then with the appropriate fluorescent secondary antibodies for 2 h at room temperature. Primary antibodies included anti-GFAP (1:50, Abcam) and anti-C3 (1:400, Abcam). Fluorescent images (astrocytes in mouse cortex) were captured using an Olympus BX53 fluorescence microscope (Olympus, Tokyo, Japan) and processed using ImageJ software (ImageJ, National Institutes of Health, Bethesda, MD) for quantification of florescence intensity.
ELISA
Mouse brain tissues (mainly from cortex, 100 mg) were homogenized intensively and centrifuged at 12,000 g for 15 min at 4°C. Concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-1α in the mouse brain were evaluated using commercial kits according to the manufacturer's instructions (BioLegend, USA).
Western Blotting
Proteins extracted from mouse brains (mainly from cortex) or astrocytes were separated using SDS-PAGE electrophoresis and then transferred to a PVDF membrane (Millipore, USA). After blocking with 5% BSA for 1 h at room temperature, PVDF membrane was incubated with primary antibodies overnight at 4°C and then with the fluorescent secondary antibodies for 1 h at room temperature. Primary antibodies included anti-GFAP (1:1,000, Abcam), anti-C3 (1:2,000, Abcam), anti-β-actin (1:5,000, Abcam), and anti-Neu-N (1:1,000, Cell Signaling Technology). Fluorescent images were captured by the Tacon 5200 (Biotanon, China) and analyzed using ImageJ software.
Quantitative Real-Time PCR Assay
Total RNA was extracted from astrocytes using Trizol reagent (Tiangen Biotech, China) according to the manufacturer's protocols. The concentrations of the extracted RNA were measured using NanoDrop 2000c (ThermoFisher, USA) (23–25). Total RNA (1 μg) was reverse-transcribed to cDNA using a reverse transcription kit (TaKaRa, Japan). QRT-PCR was performed on the QuantStudio® 6 Flex real-time PCR instrument (Applied Biosystems, USA) using SYBR™ Green qPCR Master Mix (ThermoFisher, USA). Gene expression levels were normalized to β-tubulin levels using the 2−ΔΔCt method. All primers for A1 astrocyte-specific genes used in the present study are listed in Table 1 (18).
Table 1.
Primers used for quantitative real-time PCR in the present study.
| Gene | Forward | Reverse | Length (bp) |
|---|---|---|---|
| Amigo2 | GAGGCGACCATAATGTCGTT | GCATCCAACAGTCCGATTCT | 263 |
| Fbln5 | CTTCAGATGCAAGCAACAA | AGGCAGTGTCAGAGGCCTTA | 281 |
| Ggta1 | GTGAACAGCATGAGGGGTTT | GTTTTGTTGCCTCTGGGTGT | 115 |
| H2-D1 | TCCGAGATTGTAAAGCGTGAAGA | ACAGGGCAGTGCAGGGATAG | 204 |
| H2-T23 | GGACCGCGAATGACATAGC | GCACCTCAGGGTGACTTCAT | 212 |
| Iigp1 | GGGGCAATAGCTCATTGGTA | ACCTCGAAGACATCCCCTTT | 104 |
| Psmb8 | CAGTCCTGAAGAGGCCTACG | CACTTTCACCCAACCGTCTT | 121 |
| Serping1 | ACAGCCCCCTCTGAATTCTT | GGATGCTCTCCAAGTTGCTC | 299 |
Statistical Analysis
All statistical analyses were performed using SPSS (Version 24, IBM, USA). All data are expressed as mean ± SEM (standard error of the mean). Differences between groups were assessed by one-way ANOVA followed by Student-Newman-Keuls (SNK) multiple comparison posttest or Student's t test. Differences were considered statistically significant when P < 0.05.
Results
Establishment of a Murine Model of TE
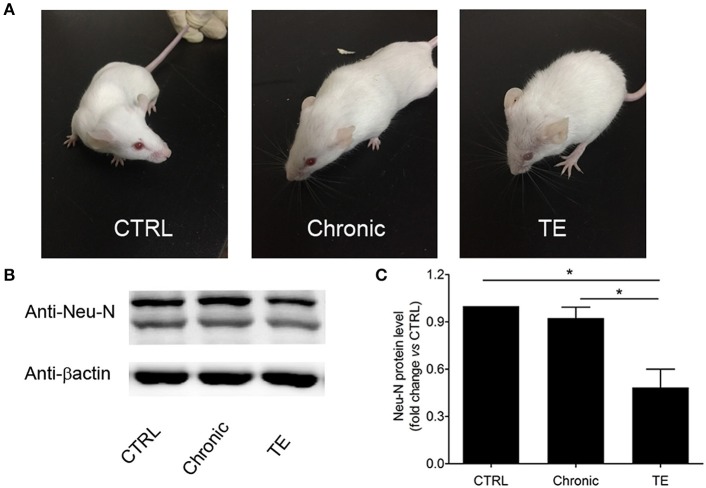
To establish a mouse model of TE, BALB/c mice were orally infected with Wh6 tissue cysts. Six weeks later, Toxoplasma-infected mice were immunosuppressed by intraperitoneal injection with cyclophosphamide to reactivate chronic T. gondii infection. As we can see in Figure 1A, mice with TE showed piloerection and hunching posture, which are typical physical characteristics of acute toxoplasmosis. Western blotting results showed that expression levels of neuron marker Neu-N in mice with TE decreased significantly compared to those in the control group (Figures 1B,C, Ctrl group vs. TE group, 1.00 ± 0.00 vs. 0.52 ± 0.09, P < 0.05). These results indicated that neurons in the central nervous system (CNS) were damaged when the mouse brains were infected with tachyzoites of T. gondii.
Figure 1.
Establishment of a murine model of toxoplasmic encephalitis (TE) (A) and identification of neuron damage in the brain of mice with TE (B,C). *P < 0.05.
Astrocyte Polarization to A1 in the Mouse Brain With TE
The proportion of A1 astrocyte (GFAP+C3+) was significantly higher in the TE group compared to the controls (Figure 2A) as detected by immunofluorescent assay (IFA).
Figure 2.
Identification of A1 astrocyte (GFAP+C3+) in the brain of mice with toxoplasmic encephalitis (TE) using indirect fluorescent assay (IFA) (A) and Western blotting analysis of C3 expression level in the mouse brain (B,C). Evaluation of interleukin (IL)-1α and tumor necrosis factor (TNF)-α in the mouse brain using commercial ELISA Kits (D,E). Bar, 50 μm, *P < 0.05 vs. control group.
The expression level of A1-specific protein C3 was evaluated using Western blotting. Consistent with IFA result, the expression level of C3 in brains of the TE group increased dramatically (Figures 2B,C, Ctrl group vs. TE group, 1.00 ± 0.00 vs. 6.98±1.06, P < 0.05), although the expression level of C3 in the chronic group was apparently higher than that of the control group. The concentrations of A1 cytokine inducers (TNF-α and IL-1α) were subsequently measured in the mouse brain using ELISA. The results indicated that TNF-α (Ctrl group vs. TE group, 56.20 ± 19.49 vs. 616.4 ± 104.8, P < 0.05) and IL-1α (Ctrl group vs. TE group, 198.2 ± 75.34 vs. 3,291 ± 260.0, P < 0.05) expression levels were remarkably enhanced in mice with TE (Figures 2D,E).
TgESAs Induced Astrocyte Polarization to A1 via the NFκb Pathway
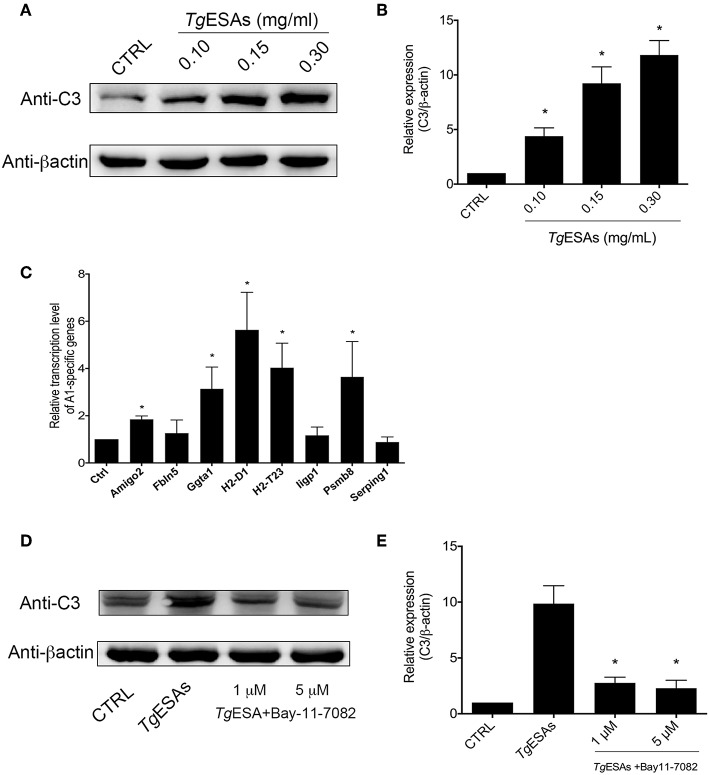
Since T. gondii tachyzoites can manipulate cells in the mouse brain that they do not productively invade (26), the non-colocalization of tachyzoites and A1 astrocytes prompted us to hypothesize that TgESAs induced astrocyte polarization. To test this hypothesis, the mouse primary astrocyte was incubated in vitro with TgESAs. Western blotting results showed that the expression level of C3 was robustly elevated in the TgESA group (Ctrl group vs. 0.30 mg/ml TgESA group, 1.00 ± 0.00 vs. 11.83 ± 1.32, P < 0.05) (Figures 3A,B). Then, the transcription levels of A1-specific genes were evaluated using qRT-PCR (18). As shown in Figure 3C, after incubation with TgESAs, the transcription levels of Amigo2, Ggta1, H2-D1, H2-T23, and Psmb8 genes were enhanced apparently, while there was no statistical difference in the transcription levels of Fbln5, Iigp1, and Serping1 genes between the control and TgESA groups. Interestingly, when primary astrocytes were pretreated with NFκB inhibitor BAY11-7082, the C3 expression level decreased significantly comparing to the non-inhibitor treatment group (TgESA group). Differences in the C3 expression level between the control and BAY11-7082 groups (TgESA group vs. TgESA + 5 μM BAY group, 9.87 ± 1.60 vs. 2.30 ± 0.70, P < 0.05) suggested that TgESAs polarized astrocytes to A1 subtype via NFκB signaling pathway (Figures 3D,E). These results indicated that TgESAs induced mouse primary astrocyte polarization to A1 subtype in vitro through activation of NFκB signaling pathway.
Figure 3.
T. gondii excreted-secreted antigen (TgESA) induction of astrocyte to A1 subtype via nuclear factor (NF)κB pathway. (A,B) TgESA treatment increased C3 expression level of astrocyte in a dose-dependent manner. (C) TgESA treatment changed A1-specific gene transcript levels. (D,E) Blockage of NFκB pathway inhibited TgESA-induced expression of C3 of astrocyte. *P < 0.05 vs. CTRL in (B,C); *P < 0.05 vs. TgESAs in (E).
Discussion
Reactivation of chronic T. gondii infection can cause life-threatening TE in immunocompromised individuals. Here, we show for the first time that T. gondii infection in CNS leads to astrocyte polarization to A1 subtype, which potentially harms neurons.
A1 astrocytes have been reported in the lipopolysaccharide (LPS)-induced CNS inflammation model (18), traumatic brain injury (TBI), and prion diseases (27, 28). In murine models of TE, astrocyte activation and proliferation are prominent, and these cells produce chemokines that can influence the recruitment of T cells and dendritic cells (DCs) as well as microglial cell activation (29). In this study, we found that the majority of activated astrocytes in mice with TE were A1 subtype (C3 positive), indicating that activated astrocytes may function as a double-edged sword in the development of TE. A1 subtype can act as chemokine producers for inflammatory cell recruitment. Additionally, A1 subtype may also release unidentified chemicals toxic for neurons, which needs to be addressed in further studies.
A previous study has demonstrated reported that activated microglia secrete IL-1α, TNF-α, and C1q in LPS-induced astrocyte polarization. These cytokines were essential for astrocyte polarization to A1 subtype (18). In the present study, increased expression levels of IL-1α and TNF-α were observed. Furthermore, IL-1α and TNF-α could be produced by mouse CNS-resident immune cells, such as CD11b+ microglia cells, since mice were immunosuppressed using cyclophosphamide. The cellular origin of these two cytokines and the cross talk between microglia and astrocytes should be determined in future studies.
A previous report demonstrated that the expression levels of cerebral cortical C1q were significantly elevated during T. gondii chronic infection (30). In this study, we found that the expression level of C3 was enhanced in acute TE. Based on our in vitro experiment results, the exposure of astrocyte to TgESAs may contribute to the enhancement of C3. C1q and C3 were mainly expressed by activated astrocytes. We can hypothesize that T. gondii chronic infection causes host behavioral changes partially through C1q activation and interaction with bradyzoite cysts. If bradyzoite cysts were disrupted, egressed parasites secrete effector proteins to trigger A1 astrocytes (C3+) resulting in TE. Thus, it is worthy to further study the detailed mechanism of how astrocyte shifts its expression from C1q to C3 at different stages of T. gondii infection.
In spinal cord injury (SCI), exosomes derived from mesenchymal stem cells (MSCs) reduced A1 astrocytes via downregulation of NFκB pathway (31). Similarly, in this study, we found that inhibition of NFκB pathway by BAY11-7082 significantly reduced the TgESA-induced C3 expression level in astrocytes. T. gondii-derived profilin recognized macrophage TLR-11 and induced the expressions of macrophage chemotactic protein 1 (MCP-1), IL-12, and interferon gamma (IFN-γ) through NFκB activation (32). The culture supernatant of T. gondii may inhibit THP-1 cell and arrest the cell cycle of THP-1 cells at G0/G1 phase mainly by regulating the expression of gene NFκB, cyclin D1 (33), while ROP16 of T. gondii could regulate NFκB pathway of A549 cells (34). The effector proteins in TgESAs linking NFκB activation and astrocyte plasticity need to be determined in further experiments.
In summary, we reported for the first time that neurotoxic A1 astrocytes were involved in TE, and TgESAs induced astrocyte polarization through NFκB activation. Our results provide new insights into the role of resident cells in the neuropathogenesis in brain toxoplasmosis.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Anhui Medical University.
Author Contributions
YJin, YY, and JT performed the experiments and analyzed the data. SE-A and YJi wrote the manuscript. YJi and JS designed the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was financially supported by the National Natural Science Foundation of China (YJi, Grant # 81802026) and Anhui Provincial Natural Science Foundation (YJi, Project # 1708085QH222).
References
- 1.Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. (2016) 12:e1005447. 10.1371/journal.ppat.1005447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Ashram S, Yin Q, Barta JR, Khan J, Liu X, Suo X. Immunoproteomic technology offers an extraordinary diagnostic approach for Toxoplasma gondii infection. J Microbiol Methods. (2015) 119:18–30. 10.1016/j.mimet.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 3.Parlog A, Harsan LA, Zagrebelsky M, Weller M, von Elverfeldt D, Mawrin C, et al. Chronic murine toxoplasmosis is defined by subtle changes in neuronal connectivity. Dis Model Mech. (2014) 7:459–69. 10.1242/dmm.014183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks JM, Carrillo GL, Su J, Lindsay DS, Fox MA, Blader IJ. Toxoplasma gondii infections alter GABAergic synapses and signaling in the central nervous system. MBio. (2015) 6:e01428–15. 10.1128/mBio.01428-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David CN, Frias ES, Szu JI, Vieira PA, Hubbard JA, Lovelace J, et al. GLT-1-dependent disruption of CNS glutamate homeostasis and neuronal function by the protozoan parasite Toxoplasma gondii. PLoS Pathog. (2016) 12:e1005643. 10.1371/journal.ppat.1005643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol. (2013) 29:156–63. 10.1016/j.pt.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. (2012) 38:642–7. 10.1093/schbul/sbs043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiderlen TR, Liesenfeld O, Schurmann D, Schneider T. Toxoplasmic encephalitis in AIDS-patients before and after the introduction of highly active antiretroviral therapy (HAART). Eur J Clin Microbiol Infect Dis. (2011) 30:1521–5. 10.1007/s10096-011-1254-6 [DOI] [PubMed] [Google Scholar]
- 9.Schluter D, Deckert M, Hof H, Frei K. Toxoplasma gondii infection of neurons induces neuronal cytokine and chemokine production, but gamma interferon- and tumor necrosis factor-stimulated neurons fail to inhibit the invasion and growth of T. gondii. Infect Immun. (2001) 69:7889–93. 10.1128/IAI.69.12.7889-7893.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka N, Ashour D, Dratz E, Halonen S. Use of human induced pluripotent stem cell-derived neurons as a model for Cerebral Toxoplasmosis. Microbes Infect. (2016) 18:496–504. 10.1016/j.micinf.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 11.Zhang YH, Chen H, Chen Y, Wang L, Cai YH, Li M, et al. Activated microglia contribute to neuronal apoptosis in Toxoplasmic encephalitis. Parasit Vectors. (2014) 7:372. 10.1186/1756-3305-7-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan X, Zhang X, Cheng Z, Chen L, Ding X, Du J, et al. Toxoplasma gondii inhibits differentiation of C17.2 neural stem cells through Wnt/beta-catenin signaling pathway. Biochem Biophys Res Commun. (2016) 473:187–93. 10.1016/j.bbrc.2016.03.076 [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Gan X, Wang Y, Zhang X, Ding X, Chen L, et al. Toxoplasma gondii prevalent in China induce weaker apoptosis of neural stem cells C17.2 via endoplasmic reticulum stress (ERS) signaling pathways. Parasit Vectors. (2015) 8:73. 10.1186/s13071-015-0670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. (2013) 14:311–21. 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. (2013) 504:394–400. 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddelow S, Barres B. SnapShot: astrocytes in health and disease. Cell. (2015) 162:1170–e1. 10.1016/j.cell.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 17.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. (2010) 119:7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. (2017) 541:481–7. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA. (2018) 115:E1896–905. 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZW, Gao JM, Huo XX, Wang L, Yu L, Halm-Lai F, et al. Genotyping of Toxoplasma gondii isolates from cats in different geographic regions of China. Vet Parasitol. (2011) 183:166–70. 10.1016/j.vetpar.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 21.Biswas A, French T, Dusedau HP, Mueller N, Riek-Burchardt M, Dudeck A, et al. Behavior of neutrophil granulocytes during Toxoplasma gondii infection in the central nervous system. Front Cell Infect Microbiol. (2017) 7:259. 10.3389/fcimb.2017.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. (2010) 285:9792–802. 10.1074/jbc.M109.082305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Ashram S, Al Nasr I, El-Kemary M, Mehmood R, Hu M, Suo X. Early and late gene expression profiles of the ovine mucosa in response to Haemonchus contortus infection employing Illumina RNA-seq technology. Parasitol Int. (2017) 66:681–92. 10.1016/j.parint.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 24.El-Ashram S, Li C, Abouhajer F, Mehmood R, Al Nasr I, Zhang Y, et al. An ex vivo abomasal ovine model to study the immediate immune response in the context of Haemonchus contortus larval-stage. Vet Parasitol. (2018) 254:105–13. 10.1016/j.vetpar.2018.02.042 [DOI] [PubMed] [Google Scholar]
- 25.Abouhajer F, El-Ashram S, Karama M, Huang S, Liu JF. An ex vivo ruminal ovine model to study the immediate immune response in the context of bacterial lipopolysaccharide. Funct Integr Genomics. (2018) 18:277–85. 10.1007/s10142-018-0589-9 [DOI] [PubMed] [Google Scholar]
- 26.Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA, et al. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. (2012) 8:e1002825 10.1371/journal.ppat.1002825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark DPQ, Perreau VM, Shultz SR, Brady RD, Lei E, Dixit S, et al. Inflammation in traumatic brain injury: roles for toxic A1 astrocytes and microglial-astrocytic crosstalk. Neurochem Res. (2019) 44:1410–24. 10.1007/s11064-019-02721-8 [DOI] [PubMed] [Google Scholar]
- 28.Hartmann K, Sepulveda-Falla D, Rose IVL, Madore C, Muth C, Matschke J, et al. Complement 3(+)-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. Acta Neuropathol Commun. (2019) 7:83. 10.1186/s40478-019-0735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. (2010) 120:1368–79. 10.1172/JCI41911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J, Li Y, Gressitt KL, He H, Kannan G, Schultz TL, et al. Cerebral complement C1q activation in chronic Toxoplasma infection. Brain Behav Immun. (2016) 58:52–6. 10.1016/j.bbi.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Pei S, Han L, Guo B, Li Y, Duan R, et al. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFkappaB P65 subunit in spinal cord injury. Cell Physiol Biochem. (2018) 50:1535–59. 10.1159/000494652 [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Zhu W, Liu Z, Yan K, Zhao S, Han D. Toll-like receptor 11-initiated innate immune response in male mouse germ cells. Biol Reprod. (2014) 90:38. 10.1095/biolreprod.113.114421 [DOI] [PubMed] [Google Scholar]
- 33.Ge P, Peng J, Li C, Liu Y, Ye B. Culture supernatant of Toxoplasma gondii inhibits the proliferation of human acute monocytic leukemia cell line THP-1. Basic Clin Med. (2011) 31:1129–33 (in Chinese). [Google Scholar]
- 34.Su Y, Dong H, Wang Y, Liu X, Zhao Z. Differential expression profiling of A549 cells induced by toxoplasma polymorphism effector ROP16. Chin J Zoonoses. (2018) 34:200–206 (in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.