Abstract
Background
Mounting evidence from both experimental and epidemiological studies suggest that exposure to the endocrine disruptor bisphenol A (BPA) has a role in metabolic disorders. The aim of the present study was to assess whether urinary BPA concentrations were associated with dyslipidaemia in children (≤17 years old) and adults (≥18 years old) by performing a meta-analysis of data from six cycles (2003–2014) in the National Health and Nutrition Examination Survey (NHANES).
Methods
We conducted a meta-analysis of data from 4604 children and 10 989 adult participants who were part of a substudy of urinary BPA measurements from six NHANES cycles from 2003 to 2014. Linear regression models conducted in each cycle were used to perform a meta-analysis to investigate associations between urinary BPA and serum levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides (TG) and apolipoprotein B (ApoB).
Results
The meta-analysis did not disclose any significant associations between urinary BPA concentrations and LDL-C, HDL-C, TC, TG and ApoB in children. In adults, the meta-analysis revealed negative regression coefficients for all five lipid variables. However, no associations were significant following Bonferroni correction for multiple tests.
Conclusions
In the present meta-analysis of cross-sectional data from NHANES, no associations were found between urinary BPA and the five different lipid variables when investigated in both children and adults. However, considering the cross-sectional nature of the present study, results should be clarified in carefully designed longitudinal cohort studies with repeated BPA measurements.
Keywords: epidemiology, bisphenol A (BPA, lipid levels, meta-analysis, NHANES
Introduction
The ‘obesogen hypothesis’ and ‘metabolism-disrupting hypothesis’ suggest that exposure to endocrine-disrupting chemicals (EDCs) found in the environment may predispose some individuals to the development of obesity and associated health conditions, such as type 2 diabetes and cardiovascular diseases. Bisphenol A (BPA) is one of the most commonly reported obesogens and metabolism-disrupting chemicals.1 2
BPA is a high-volume chemical extensively used in epoxy resins lining in food and beverage containers and as a monomer in polycarbonate plastics in many consumer products. Its use is still abundant and environmental contamination is apparent in waters, sediments, soil, air, wildlife and humans.3 Exposure to BPA, primarily via food, but also through dental sealants, thermal paper (receipts and tickets), dermal exposure and inhalation of household dust, is evident from the detectable levels of urinary BPA in humans.4–8 BPA has also been detected in adipose tissue,9 and urinary BPA concentrations do not decline rapidly with fasting time, implying that BPA partly accumulates in adipose tissue and other tissue compartments.10 11
BPA can interfere with multiple hormone actions,12 including the activity of membrane and nuclear receptors (eg, oestrogen receptors), thyroid hormone receptor, androgen receptor and orphan receptors (eg, aryl hydrocarbon receptor). The diversity of plausible mechanisms of BPA, in addition to the dose in relation to exposure during sensitive windows, may be an explanation for the effects of low-dose BPA exposure on endocrine systems.13
In humans, BPA exposure has been associated with the metabolic syndrome14 and its components hypertension,15 diabetes mellitus,16 17 insulin resistance18 and obesity.19 20 Especially troublesome is that associations between BPA exposure and metabolic diseases are observed also in children,20–23 indicating that metabolic disturbances due to BPA exposure start at a young age.
Because of the intense focus on BPA, several studies using National Health and Nutrition Examination Survey (NHANES) data to address the link between BPA and metabolic disorders such as obesity and diabetes already exist. However, to our knowledge, studies investigating the relation between BPA exposure and dyslipidaemia are limited.16 23 Thus, the aim of the present study was to investigate potential associations between urinary BPA concentrations and dyslipidaemia in US children and adults by performing a meta-analysis of data from six NHANES cycles from 2003 to 2014. We hypothesised that urinary BPA, as a surrogate biomarker for BPA exposure, is associated with a disturbed lipid metabolism.
Methods
Study population
NHANES is a continuous cross-sectional surveillance programme administered by the National Center for Health Statistics, which is part of Centers for Disease Control and Prevention (CDC). The aim of NHANES is to assess the health and nutritional status of the general US population for both children and adults. A detailed description of the NHANES study design and methods is available elsewhere.24 Results in the present study are based on data from the following six NHANES cycles containing BPA measurements: NHANES 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012 and 2013–2014. Data from the demographics, dietary, questionnaire, laboratory and physical examination components of each NHANES cycle were downloaded from the NHANES website https://www.cdc.gov/nchs/nhanes/index.htm. For the present study, 4604 children, aged 6–17 years, and 10 989 adults, aged 18 years or older, with BPA measurement data in urine, were eligible.
Urinary BPA
A spot urine sample was collected from a one-third random subset of participants of each NHANES cycle that were then analysed for BPA concentration. Total urinary BPA (free and conjugated; nanograms per millilitre) concentrations were measured using online solid-phase extraction coupled to high performance liquid chromatography-isotope dilution tandem mass spectrometry at the Division of Environmental Health Laboratory Sciences (National Center for Environmental Health, CDC). Comprehensive quality assurance and quality control was performed to ensure that samples were not contaminated during handling, storage and analysis.4 Urinary creatinine was included in all multivariate models to correct for urinary dilution of BPA, as done in previous publications.4 25
Lipid measures
Total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) were analysed enzymatically in serum using spectrophotometric measurement of the colour of a reaction byproduct. The colour intensity is directly proportional to the concentration of the respective lipid. Low-density lipoprotein cholesterol (LDL-C) levels were calculated from measured values of TC, TG and HDL-C based on the Friedwald equation ([LDL-C]=[TC]−[HDL-C]−[TG/5]), which is valid for individuals with TC levels <400 mg/dL.26 Apolipoprotein B (ApoB) was measured with an immunochemical light spectrometry method. Details of analyte extraction and measurement are available under each of the six different cycles under ‘Laboratory Methods’ at the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
Demographic and lifestyle factors
Information on demographic and lifestyle factors of study participants was collected using standardised questionnaires. Potential confounders were identified in the current literature20 22 23 27 28 and included after theoretical structured ordering of factors by use of directed acyclic graphs (online supplementary figure 1). Since obesity could have a big impact on lipid levels, we assumed the causal pathway to be: BPA exposure->obesity->dyslipidaemia. We therefore did not adjust for BMI or other markers of obesity, since we regard obesity to be on the casual pathway between BPA and lipid disturbances, that is, obesity is regarded as an intermediate in the present study. Sex was categorised as male or female. Race/ethnicity was categorised into five groups: Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black and other. Self-reported education/caregiver educational attainment was categorised into four levels: <9th grade, 9–11th grade, high school or general educational development and some college. Smoking was characterised using serum cotinine, a major metabolite of nicotine that is used as a marker for both active smoking and as an index of environmental tobacco smoke exposure or ‘passive smoking’. Income-to-poverty ratio is a continuous measure calculated using the Department of Health and Human Services poverty guidelines. For children, physical activity was characterised as the number of times per week the participant play or exercise hard. For adults, physical activity was categorised as having performed vigorous activity during the past 30 days or not. Statins was characterised as currently taking prescribed medicine (adults only). Alcohol intake was characterised as how often the participants (adults only) have drunk alcohol over past 12 months. Energy intake was obtained as mean total caloric intake (kcal) per day.
jech-2019-212555supp001.pdf (7.8MB, pdf)
Statistical analysis
Separate analyses were performed for adults (≥18 years old) and children (≤17 years old), which is the most commonly used approach in this type of study. The reason for this is that the children were likely more or less exposed even before birth, while the adults at different ages would be very heterogeneous in that respect.
Due to a right-skewed distribution, urinary BPA concentrations, TG and urinary creatinine were log-transformed. Linear regression analysis was performed with the lipid variables (LDL-C, HDL-C, TC, TG and ApoB) as dependent variables in separate models for each lipid variable and with BPA as the main independent variable.
In the first set of models, adjustment was performed for age, sex, urinary creatinine and race. In the second set of models, we also included other a priori chosen confounders because of their biological relevance to both the exposure and outcome: education/caregiver education, smoking (serum cotinine), income-to-poverty ratio, physical activity, alcohol intake (adults only), caloric intake, statins (adults only) and pregnancy (adults only). In a third set of models, an interaction term between sex and BPA was included to evaluate possible sex differences. In a fourth set of models, a squared term was included for BPA to evaluate possible non-linear relationships.
Missing data on education, alcohol intake, smoking (serum cotinine), statin use, physical activity and energy intake were imputed by using multiple imputation (Markov-Chain Monte Carlo) with 20 imputations. Each examination cycle was analysed separately and the results from all examination cycles were meta-analysed using the inverse-variance weighted fixed effect technique.
A sensitivity analysis was performed by excluding all individuals taking statins. A Bonferroni adjustment was used to account for multiple testing (critical level of significance (0.05/10)=0.005). STATA V.15 was used for calculations.
Results
During the study period (2003–2014), 25 172 children (<18 years of age) and 35 915 adults (>18 years of age) participated in NHANES. Our analytic sample comprised the 4604 children and 10 989 adult participants randomly selected for measurement of urinary BPA concentrations. Tables 1 and 2 present descriptive basic characteristics for children and adults of each of the six different NHANES cycles from 2003 to 2014. Over the whole study period, the mean levels of urinary BPA varied from 2.21 ng/mL to 6.10 ng/mL in children and from 2.97 ng/mL to 4.89 ng/mL in adults. For children, mean urinary BPA levels were highest in the 2003–2004 examination cycle and then decreased over time, with the lowest mean value for the 2013–2014 cycle. For adults, no such pattern was seen.
Table 1.
Study population (children) characteristics for the six different NHANES cycles from 2003 to 2014
| Cycle | NHANES 2003–2004 |
NHANES 2005–2006 |
NHANES 2007–2008 |
NHANES 2009–2010 |
NHANES 2011–2012 |
NHANES 2013–2014 |
||||||
| Characteristic | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Age | 852 | 12.33 (3.4) | 896 | 12.13 (3.41) | 690 | 11.25 (3.35) | 727 | 11.09 (3.40) | 689 | 11.02 (3.37) | 750 | 11.2 (3.5) |
| Sex | ||||||||||||
| Female | 424 | 49.8% | 429 | 47.9% | 336 | 48.7% | 349 | 48.0% | 348 | 50.5% | 375 | 50.0% |
| Male | 428 | 50.2% | 467 | 52.1% | 354 | 51.3% | 378 | 52.0% | 341 | 49.5% | 375 | 50.0% |
| Race/ethnicity | ||||||||||||
| Mexican American | 262 | 30.8% | 307 | 34.3% | 180 | 26.1% | 199 | 27.4% | 146 | 21.2% | 180 | 24.0% |
| Other Hispanic | 21 | 2.5% | 21 | 2.3% | 98 | 14.2% | 95 | 13.1% | 69 | 10.0% | 75 | 10.0% |
| Non-Hispanic white | 231 | 27.1% | 246 | 27.5% | 189 | 27.4% | 236 | 32.4% | 168 | 24.4% | 191 | 25.5% |
| Non-Hispanic black | 302 | 35.4% | 268 | 29.9% | 197 | 28.5% | 146 | 20.1% | 195 | 28.3% | 191 | 25.5% |
| Other | 36 | 4.2% | 54 | 6.0% | 26 | 3.8% | 51 | 7.0% | 111 | 16.1% | 113 | 15.0% |
| Caregiver education | ||||||||||||
| <9th grade | 102 | 12.3% | 112 | 13.0% | 83 | 12.5% | 78 | 11.0% | 72 | 10.8% | 75 | 10.3% |
| 9–11th grade | 177 | 21.4% | 158 | 18.4% | 138 | 20.9% | 123 | 17.4% | 118 | 17.7% | 112 | 15.4% |
| High school or GED | 197 | 23.9% | 195 | 22.7% | 157 | 23.7% | 170 | 24.0% | 150 | 22.6% | 166 | 22.8% |
| Some college | 227 | 27.5% | 246 | 28.7% | 176 | 26.6% | 198 | 28.0% | 177 | 26.6% | 229 | 31.4% |
| College graduate or above |
123 | 14.9% | 148 | 17.2% | 108 | 16.3% | 139 | 19.6% | 148 | 22.3% | 147 | 20.1% |
| Smoking (serum cotinine (ng/mL)) | 778 | 6.7 (36.9) | 780 | 9.0 (49.5) | 597 | 3.9 (23.5) | 628 | 3.6 (24.1) | 585 | 3.9 (28.4) | 643 | 1.95 (15.9) |
| No. of times/week you play or exercise hard | 314 | 6.5 (5.8) | 355 | 5.7 (2.9) | 340 | 5.5 (2.3) | 414 | 6.0 (1.9) | 395 | 5.9 (1.8) | 638 | 5.0 (2.3) |
| Caloric intake (kcal) | 813 | 2190.2 (928.1) | 843 | 2174.3 (1077.9) | 660 | 1933.4 (778.7) | 703 | 1965.9 (797.5) | 639 | 1936.4 (734.1) | 657 | 1944.7 (838.1) |
| Urinary BPA (ng/mL) | 852 | 6.10 (9.06) | 896 | 4.38 (7.59) | 690 | 4.18 (9.01) | 727 | 3.67 (10.36) | 689 | 3.38 (9.89) | 750 | 2.21 (2.85) |
| Urinary creatinine (µmol/L) | 850 | 12 293.8 (7841.2) | 896 | 12 543.3 (7236.3) | 690 | 10 845.1 (6562.0) | 727 | 10 564.4 (6521.3) | 688 | 10 068.7 (6718.6) | 750 | 10 780.4 (6822.6) |
| LDL-C (mmol/L) | 350 | 2.31 (0.65) | 239 | 2.20 (0.60) | 112 | 2.29 (0.63) | 128 | 2.36 (0.67) | 146 | 2.22 (0.65) | 149 | 2.17 (0.69) |
| HDL-C (mmol/L) | 778 | 1.41 (0.33) | 778 | 1.41 (0.33) | 602 | 1.35 (0.33) | 628 | 1.36 (0.34) | 582 | 1.38 (0.31) | 644 | 1.39 (0.34) |
| TC (mmol/L) | 501 | 4.17 (0.77) | 487 | 4.15 (0.71) | 270 | 4.11 (0.76) | 285 | 4.14 (0.76) | 268 | 3.95 (0.75) | 297 | 4.05 (0.77) |
| TG (mmol/L) | 350 | 0.93 (0.50) | 239 | 0.91 (0.44) | 112 | 0.94 (0.49) | 128 | 0.93 (0.53) | 146 | 0.93 (0.59) | 150 | 0.89 (0.64) |
| Apolipoprotein B (mg/dL) | NA | NA | 241 | 72.9 (17.4) | 112 | 69.6 (18.5) | 128 | 71.2 (19.4) | 147 | 65.7 (17.9) | 150 | 65.9 (18.2) |
BPA, bisphenol A; GED, general educational development; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey; TC, total cholesterol; TG, triglycerides.
Table 2.
Study population (adults) characteristics for the six different NHANES cycles from 2003 to 2014
| Cycle | NHANES 2003–2004 |
NHANES 2005–2006 |
NHANES 2007–2008 |
NHANES 2009–2010 |
NHANES 2011–2012 |
NHANES 2013–2014 |
||||||
| Characteristic | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Age | 1665 | 46.8 (20.7) | 1652 | 44.8 (19.8) | 1914 | 48.74 (18.6) | 2022 | 47.18 (18.7) | 1800 | 46.65 (18.5) | 1936 | 47.62 (18.6) |
| Sex | ||||||||||||
| Female | 864 | 51.9% | 849 | 51.4% | 974 | 50.9% | 1001 | 49.5% | 882 | 49.0% | 1026 | 53.0% |
| Male | 801 | 48.1% | 803 | 48.6% | 940 | 49.1% | 1021 | 50.5% | 918 | 51.0% | 910 | 47.0% |
| Race/ethnicity | ||||||||||||
| Mexican American | 351 | 21.1% | 330 | 20.0 | 351 | 18.3% | 367 | 18.1% | 170 | 9.5% | 258 | 13.3% |
| Other Hispanic | 61 | 3.7% | 51 | 3.1 | 204 | 10.7% | 198 | 9.8% | 186 | 10.3% | 177 | 9.1% |
| Non-Hispanic white | 844 | 50.7% | 792 | 47.9 | 888 | 46.4% | 970 | 48.0% | 645 | 35.8% | 797 | 41.2% |
| Non-Hispanic black | 333 | 20.0% | 410 | 24.8 | 400 | 20.9% | 370 | 18.3% | 470 | 26.1% | 418 | 21.6% |
| Other | 76 | 4.5% | 69 | 4.2 | 71 | 3.7% | 117 | 5.8% | 329 | 18.3% | 286 | 14.8% |
| Education | ||||||||||||
| <9th grade | 215 | 14.5% | 165 | 11.1% | 237 | 13.1% | 239 | 12.5% | 169 | 9.9% | 144 | 8.0% |
| 9-11th grade | 219 | 14.7% | 226 | 15.2% | 302 | 16.6% | 307 | 16.1% | 234 | 13.8% | 287 | 15.8% |
| High school or GED | 362 | 24.4% | 365 | 24.5% | 468 | 25.8% | 459 | 24.0% | 336 | 19.7% | 408 | 22.5% |
| Some college | 397 | 26.7% | 428 | 28.8% | 464 | 25.6% | 508 | 26.6% | 518 | 30.4% | 534 | 29.4% |
| College graduate or above |
292 | 19.7% | 304 | 20.4% | 343 | 18.9% | 397 | 20.8% | 446 | 26.2% | 440 | 24.3% |
| Smoking (serum cotinine (ng/mL)) | 1581 | 56.4 (133.6) | 1561 | 60.1 (129.4) | 1811 | 64.0 (137.8) | 1925 | 58.3 (123.6) | 1694 | 49.7 (122.3) | 1870 | 57.6 (127.0) |
| Vigorous activity over past 30 days | ||||||||||||
| Yes | 489 | 29.4% | 600 | 36.3% | 403 | 21.1% | 407 | 20.1% | 435 | 24.2% | 437 | 22.6% |
| No | 1176 | 70.6% | 1052 | 63.7% | 1511 | 78.9% | 1615 | 79.9% | 1365 | 75.8% | 1499 | 77.4% |
| Caloric intake (kcal) | 1584 | 2199.5 (1021.0) | 1591 | 2194.5 (1009.5) | 1824 | 2064.0 (1057.9) | 1934 | 2125.7 (994.3) | 1642 | 2169.0 (1026.8) | 1766 | 2121.5 (1068.2) |
| Now taking statins | ||||||||||||
| Yes | 212 | 12.7% | 212 | 12.8% | 342 | 17.9% | 350 | 17.3% | 330 | 18.3% | 372 | 19.2% |
| No | 1453 | 87.3% | 1440 | 87.2% | 1572 | 82.1% | 1672 | 82.7% | 1470 | 81.7% | 1564 | 80.8% |
| Urinary BPA (ng/mL) | 1665 | 4.69 (6.72) | 1652 | 4.03 (12.70) | 1914 | 3.67 (6.73) | 2022 | 4.89 (30.01) | 1800 | 3.10 (7.76) | 1936 | 2.97 (18.94) |
| Urinary creatinine (µmol/L) | 1664 | 11 953.7 (7527.5) | 1652 | 134.38 (81.11) | 1914 | 124.72 (78.07) | 2022 | 111 263.0 (7039.9) | 1799 | 10 694.6 (7209.9) | 1934 | 11 451.0 (7389.3) |
| LDL-C (mmol/L) | 733 | 2.98 (0.94) | 732 | 2.88 (0.96) | 862 | 2.89 (0.86) | 920 | 2.91 (0.87) | 808 | 2.95 (0.90) | 920 | 2.84 (0.93) |
| HDL-C (mmol/L) | 1583 | 1.41 (0.43) | 1561 | 1.43 (0.43) | 1811 | 1.34 (0.41) | 1922 | 1.35 (0.41) | 1687 | 1.36 (0.38) | 1868 | 1.36 (0.41) |
| TC (mmol/L) | 1572 | 5.19 (1.15) | 1554 | 5.09 (1.15) | 1803 | 5.06 (1.07) | 1919 | 4.95 (1.06) | 1681 | 4.89 (1.05) | 1863 | 4.89 (1.06) |
| TG (mmol/L) | 752 | 1.67 (1.38) | 749 | 1.59 (1.39) | 875 | 1.49 (1.19) | 937 | 1.47 (1.17) | 823 | 1.39 (1.08) | 930 | 1.32 (0.90) |
| Apolipoprotein B (mg/dL) | NA | NA | 752 | 99.5 (29.3) | 874 | 91.2 (24.0) | 937 | 90.1 (24.1) | 823 | 88.6 (24.6) | 930 | 89.3 (25.8) |
ApoB, apolipoprotein B; BPA, bisphenol A;GED, general educational development; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol;NHANES, National Health and Nutrition Examination Survey; TC, total cholesterol; TG, triglycerides.
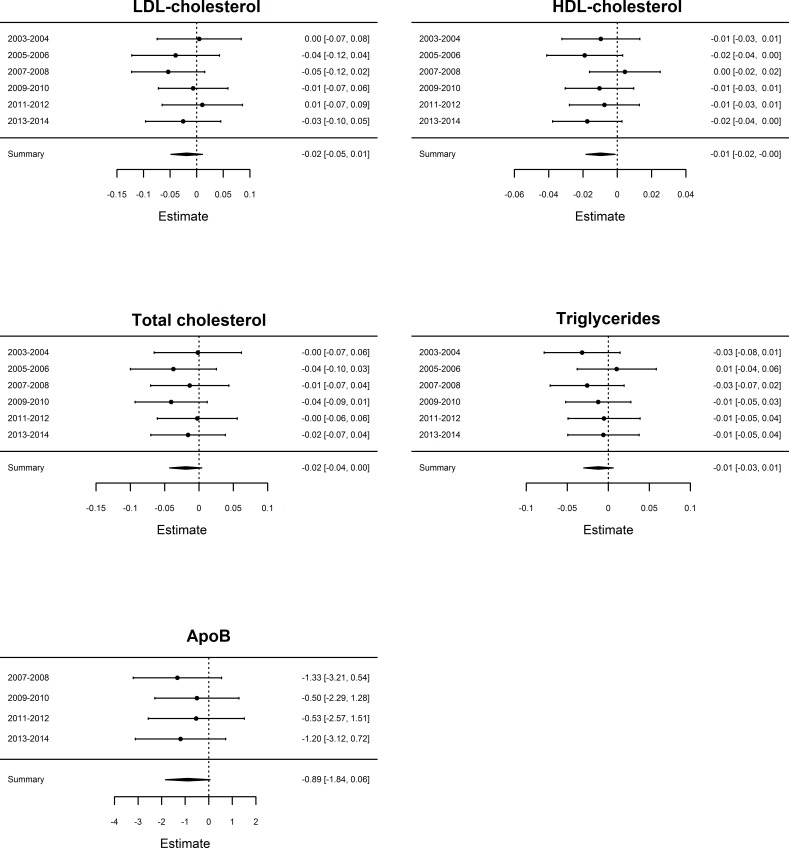
In children, the meta-analysis showed negative associations between urinary BPA concentrations and ApoB, LDL-C and HDL-C, and positive associations for TC and TG, in models adjusting for age, sex, race and urinary creatinine (table 3). In adults, the meta-analysis showed negative associations between urinary BPA concentrations and all five evaluated lipid variables (figure 1, table 3). However, none of the investigated associations were significant following Bonferroni adjustment (p>0.005), either in children or in adults. Following further adjustment for systolic blood pressure and fasting glucose to the standard adjusted model in adults the relationships between BPA and lipids were generally attenuated, as can be seen in online supplemental table 1.
Table 3.
Meta-analysis results of associations between urinary BPA and five different lipids in children and adults, when analysing women and men together using linear regression models
| Standard adjusted model* | Fully adjusted model† | |||||
| β | 95% CI | P value | β | 95% CI | P value | |
| Children | ||||||
| LDL-C | −0.005 | −0.05 to 0.05 | 0.98 | 0.003 | −0.05 to 0.05 | 0.91 |
| HDL-C | −0.01 | −0.02 to 0.002 | 0.09 | −0.01 | −0.02 to 0.002 | 0.10 |
| TC | 0.008 | −0.03 to 0.05 | 0.70 | 0.01 | −0.03 to 0.05 | 0.62 |
| TG | 0.01 | −0.02 to 0.05 | 0.51 | 0.01 | −0.02 to 0.05 | 0.48 |
| ApoB | −0.48 | −2.1 to 1.2 | 0.57 | −0.54 | −2.3 to 1.2 | 0.54 |
| Adults | ||||||
| LDL-C | −0.02 | −0.05 to 0.01 | 0.22 | −0.02 | −0.05 to 0.01 | 0.23 |
| HDL-C | −0.01 | −0.02 to 0.001 | 0.025 | −0.006 | −0.01 to 0.003 | 0.18 |
| TC | −0.02 | −0.04 to 0.004 | 0.11 | −0.02 | −0.01 to 0.003 | 0.09 |
| TG | −0.01 | −0.03 to 0.006 | 0.19 | −0.02 | −0.03 to 0.0002 | 0.053 |
| ApoB | −0.89 | −1.8 to 0.06 | 0.066 | −0.91 | −1.8-(−0.02) | 0.044 |
*Adjusted for urinary creatinine, age, sex and race.
†Adjusted for urinary creatinine, sex, age, race, education/caregiver education, smoking (serum cotinine), income to poverty ratio, physical activity, alcohol intake (adults only), caloric intake, statins (adults only) and pregnancy (adults only).
ApoB, apolipoprotein B; BPA, bisphenol A; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Figure 1.
Results of the meta-analysis of the relationship between urinary BPA and five different lipid variables in adult participants in models adjusting for age, sex, race and urinary creatinine. The estimates and 95% CI are given (right side of each figure) for each of the six NHANES examination cycles (specified in the left side of each figure) together with a summarised estimate. ApoB was not measured in the first two NHANES examination cycles. ApoB, apolipoprotein B; BPA, bisphenol A; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
Further adjustment for lifestyle factors changed the above-mentioned associations only marginally (table 3). However, again, none of the associations were significant following Bonferroni adjustment (p>0.005). Including a squared term for BPA in the models did not disclose any significant non-linear relationships, either in children or in adults. Neither did the sensitivity analysis, by excluding individuals taking statins, result in any significant relationships.
Including an interaction term between sex and BPA in the models, to evaluate possible sex differences, did not disclose any sex interactions with a statistical value of p<0.005 for any of the lipids in either children or adults (table 4).
Table 4.
Standard adjusted meta-analysis of associations between urinary BPA and different lipids in children and adults, when analysing women and men separately using linear regression models
| Female | Male | Interaction term | |||||
| β | 95% CI | P value | β | 95% CI | P value | P value | |
| Children | |||||||
| LDL-C | 0.04 | −0.04 to 0.11 | 0.32 | −0.03 | −0.09 to 0.04 | 0.40 | 0.36 |
| HDL-C | −0.01 | −0.03 to 0.007 | 0.22 | −0.01 | −0.03 to 0.003 | 0.10 | 0.57 |
| TC | −0.02 | −0.08 to 0.04 | 0.49 | 0.02 | −0.04 to 0.07 | 0.55 | 0.38 |
| TG | −0.02 | −0.07 to 0.03 | 0.44 | 0.04 | −0.003 to 0.09 | 0.07 | 0.13 |
| ApoB | −0.18 | −2.9 to 2.6 | 0.90 | −0.72 | −2.8 to 1.4 | 0.50 | 0.90 |
| Adults | |||||||
| LDL-C | −0.01 | −0.05 to 0.03 | 0.56 | −0.02 | −0.07 to 0.02 | 0.27 | 0.27 |
| HDL-C | −0.01 | −0.03 to 0.0002 | 0.053 | −0.008 | −0.02 to 0.004 | 0.18 | 0.061 |
| TC | −0.02 | −0.05 to 0.02 | 0.30 | −0.02 | −0.05 to 0.01 | 0.23 | 0.12 |
| TG | −0.01 | −0.04 to 0.01 | 0.37 | −0.01 | −0.04 to 0.02 | 0.46 | 0.37 |
| ApoB | −0.98 | −2.3 to 0.4 | 0.16 | −0.66 | −1.9 to 0.6 | 0.32 | 0.79 |
ApoB, apolipoprotein B; BPA, bisphenol A; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Discussion
In the present study, we aimed to assess whether urinary BPA concentrations were associated with dyslipidaemia by performing a meta-analysis of data on children and adults that participated in the 2003–2014 NHANES, as we have previously reported altered blood lipids after developmental exposure to BPA in rats.29 The present study did not reveal any major associations between urinary BPA levels and dyslipidaemia in children or adults despite the large sample sizes achieved by the meta-analysis performed using several examination cycles of NHANES data. In adults, we found a consistent pattern of an inverse relationship between BPA and all five investigated lipids, but none of the associations were significant following Bonferroni adjustment.
Two previous studies have used NHANES data to investigate the relationship between BPA exposure and lipid levels. In a study of adults, no significant relationships between urinary BPA and serum LDL-C or TG were found.16 However, that study used only the first (2003–2004) examination cycle and might therefore have limited power to detect any significant associations. In another study performed in children, no significant relationships between urinary BPA and serum TC, HDL-C, LDL-C or TG were observed.23 That study used pooled data from all NHANES examinations performed from 2003 to 2010. Our data are in agreement with those two studies, but in our case, we had better power to detect any potential relationships. Especially in the adults (n>10 000), we had the power to detect relationships for which the variation in BPA levels explained <0.05% of the variation in lipid levels. Such relationships would be statistically significant but would be of doubtful clinical significance.
Several previous studies that have used data from some NHANES cycles have found a positive association between BPA exposure and obesity in both children20 23 30 and adults,19 and diabetes in adults,17 31 whereas we did not find any associations between BPA and dyslipidaemia in the present meta-analysis. However, the earlier studies have only used some NHANES cycles compared with our meta-analysis where we have included six NHANES cycles, which of course result in a larger investigated population. It could be that only a few cycles stand out and drive the positive associations found in the earlier studies. BPA is a confirmed endocrine disrupting chemical, and it is quite evident from the literature that it can also disturb lipid metabolism, especially from experimental animal studies; therefore, we believe that there is a true association between BPA and obesity, diabetes and dyslipidaemia, but there is a need for better well-planned cohort studies in order to truly confirm these associations. In addition, when it comes to the relation between BPA exposure and obesity in particular, it is not possible to rule out the possibility that obese individuals ingest food with higher BPA content or have greater adipose stores of BPA.
A review evaluating epidemiological studies on BPA and cardiometabolic health outcomes showed that nearly all used a cross-sectional design and relied on a single measure of BPA exposure, which may result in serious exposure misclassification. In addition, the result of the review could conclude that for all outcomes, results across studies were inconsistent.32 Factors underlying the differing results observed among studies are multifactorial and likely the result of differences in study protocol, unaccounted for confounding factors or reverse causality (eg, individuals who are obese have a different dietary pattern that increases their exposure risk).
Previous studies conducted on data from NHANES often use pooled data from different survey cycles. It has been shown, in the context of correlation analysis, that pooling can generate false-positive findings and also that effects that do exist within the subgroups can be concealed.33 In the present study, we chose to handle each cycle as a different study population and thereafter performing a meta-analysis to avoid those pitfalls.
In our standard model, we adjusted for age, sex, race and urinary creatinine. Further adjustment for systolic blood pressure and fasting glucose generally attenuated the relationships between BPA and the lipids in the adults (online supplementary table 1). Both blood pressure and glucose are related to lipid levels, but it is not known if these two major risk factors could affect BPA levels by means of changing the metabolism or absorption of BPA. Therefore, it is not clear if blood pressure or glucose are confounders or mediators in the BPA versus lipid relationship, as BPA has been linked to diabetes and hypertension.15–17
The major strength of this study is the large meta-analysis of a nationally representative sample with high-quality measurements of BPA. Moreover, the use of rigorous study methods to collect the data and the availability of extensive data on confounders further strengthen the study. However, given the cross-sectional design, it is not possible to rule out reverse causality. Another major limitation is the reliance of a single spot urine sample to characterise BPA exposure and the unavailability of BPA measurements on children younger than 6 years of age. It is especially important to acquire repeated measures of EDCs with a short half-life, such as bisphenols. Thus, in future studies, BPA levels need to be measured at more than one time point to truly reflect BPA exposure. Also, in epidemiological studies, it is difficult to assess associations between low exposure levels and outcomes. Instead, it is increasing levels that are investigated with regard to different association outcomes. In addition, epidemiological studies fail to consider exposure relative to critical periods of differentiation (susceptibility windows), which has been identified as especially important for EDCs. Another limitation of the study is that Tanner stage was not assessed for children enrolled in NHANES. Therefore, we were unable to account for the potential impact of puberty on lipid concentrations in our analyses. However, all regression models were adjusted for children’s age, which is a significant predictor of onset of puberty.
Conclusions
Despite the large sample size of the present meta-analysis and the thorough statistical analysis performed, no associations were found between urinary BPA levels and the five different lipid variables investigated in children and adults. However, considering the cross-sectional nature of the present study and earlier studies showing associations between BPA and obesity and diabetes, these results should be clarified in carefully designed longitudinal cohort studies with repeated BPA measurements. It is especially important to acquire repeated measures of endocrine disrupting chemicals with a short half-life, such as bisphenols.
What is already known on this subject.
It has become evident from experimental animal studies that the endocrine disrupting chemical bisphenol A (BPA) also can act as a metabolism disrupting chemical, that is, that it can interfere with different metabolic processes that can increase the individual’s susceptibility to metabolic disease. This is, however, not as clear from reports from epidemiological studies, where both negative and positive associations have been reported between urinary BPA and metabolic diseases, mainly obesity.
What this study adds.
In the present study, we have performed a comprehensive meta-analysis using data from six National Health and Nutrition Examination (NHANES) cycles (children: n=4604 and adults: n=10 989) to investigate associations between urinary BPA exposure and serum lipids. Our hypothesis was that urinary BPA would be associated with dyslipidaemia since previous studies using NHANES data have reported associations between urinary BPA and obesity and diabetes. Despite these earlier reported associations, we could not find any associations between BPA and dyslipidaemia in the present meta-analysis of this particular cohort. However, the earlier studies have only used some NHANES cycles compared with our meta-analysis where we have included six NHANES cycles which, of course, result in a larger investigated population. It could be that only a few cycles stand out and drive the positive associations found in the earlier studies. BPA is a confirmed endocrine-disrupting chemical, and it is quite evident from the literature that it can also disturb lipid metabolism, especially from experimental animal studies; therefore, we believe that there is a true association between BPA and obesity, diabetes and dyslipidaemia, but there is a need for better well-planned cohort studies in order to truly confirm these associations. Our conclusion is therefore that despite the large sample size of the present meta-analysis and the thorough statistical analysis performed, there are no associations between BPA and dyslipidaemia in the particular cohort investigated (NHANES). It is important that the design of future studies are longitudinal and include several BPA measurements in order to make the exposure more representative of the real life scenario. It is especially important to acquire repeated measures of endocrine disrupting chemicals with a short half-life, such as bisphenols.
Footnotes
Contributors: LD and LL conceived the project, planned and performed the statistical analyses. LD wrote the initial draft of the manuscript and revised the manuscript. All authors discussed the results, read and contributed to the final manuscript.
Funding: This work was supported by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, FORMAS (grant number 216-2012-475) and by research funding from Uppsala University Hospital (ALF).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval and consent to participate: National Health and Nutrition Examination Survey (NHANES) is a publicly available data set and all participants in NHANES provide written informed consent, consistent with approval from the National Center for Health Statistics Research Ethics Review Board (NCHS ERB) (protocol #98-12 for NHANES cycle 2003-2004, protocol #2005-06 for NHANES cycles 2005-2006, 2007-2008 and 2009-2010, protocol #2011-17 for NHANES cycles 2011-2012 and 2013-2014).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The datasets used and analysed during the current study are available in the NHANES repository (https://www.cdc.gov/nchs/nhanes/index.htm).
References
- 1. Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol 2009;304:19–29. 10.1016/j.mce.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heindel JJ, Blumberg B, Cave M, et al. . Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 2017;68:3–33. 10.1016/j.reprotox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corrales J, Kristofco LA, Steele WB, et al. . Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response 2015;13 10.1177/1559325815598308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calafat AM, Kuklenyik Z, Reidy JA, et al. . Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005;113:391–5. 10.1289/ehp.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calafat AM, Ye X, Wong L-Y, et al. . Exposure of the U.S. Population to Bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 2008;116:39–44. 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vandenberg LN, Hauser R, Marcus M, et al. . Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–77. 10.1016/j.reprotox.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 7. Scinicariello F, Buser MC. Serum testosterone concentrations and urinary bisphenol A, benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011-2012. Environ Health Perspect 2016;124:1898–904. 10.1289/EHP150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Fang J, Ren L, et al. . Urinary bisphenol analogues and triclosan in children from South China and implications for human exposure. Environ Pollut 2018;238:299–305. 10.1016/j.envpol.2018.03.031 [DOI] [PubMed] [Google Scholar]
- 9. Fernandez MF, Arrebola JP, Taoufiki J, et al. . Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol 2007;24:259–64. 10.1016/j.reprotox.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 10. Stahlhut RW, Welshons WV, Swan SH. Bisphenol a data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect 2009;117:784–9. 10.1289/ehp.0800376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Csanády GA, Oberste-Frielinghaus HR, Semder B, et al. . Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein. Arch Toxicol 2002;76:299–305. 10.1007/s00204-002-0339-5 [DOI] [PubMed] [Google Scholar]
- 12. Reif DM, Martin MT, Tan SW, et al. . Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect 2010;118:1714–20. 10.1289/ehp.1002180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acconcia F, Pallottini V, Marino M. Molecular mechanisms of action of BPA. Dose Response 2015;13 10.1177/1559325815610582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: results from NHANES. Int J Endocrinol 2012;2012:598180 10.1155/2012/598180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bae S, Kim JH, Lim Y-H, et al. . Associations of bisphenol a exposure with heart rate variability and blood pressure. Hypertension 2012;60:786–93. 10.1161/HYPERTENSIONAHA.112.197715 [DOI] [PubMed] [Google Scholar]
- 16. Lang IA, Galloway TS, Scarlett A, et al. . Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008;300:1303–10. 10.1001/jama.300.11.1303 [DOI] [PubMed] [Google Scholar]
- 17. Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab 2011;96:3822–6. 10.1210/jc.2011-1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang T, Li M, Chen B, et al. . Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012;97:E223–E227. 10.1210/jc.2011-1989 [DOI] [PubMed] [Google Scholar]
- 19. Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res 2011;111:825–30. 10.1016/j.envres.2011.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol a concentration and obesity prevalence in children and adolescents. JAMA 2012;308:1113–21. 10.1001/2012.jama.11461 [DOI] [PubMed] [Google Scholar]
- 21. Khalil N, Ebert JR, Wang L, et al. . Bisphenol A and cardiometabolic risk factors in obese children. Sci Total Environ 2014;470-471:726–32. 10.1016/j.scitotenv.2013.09.088 [DOI] [PubMed] [Google Scholar]
- 22. Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol 2013;177:1263–70. 10.1093/aje/kws391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eng DS, Lee JM, Gebremariam A, et al. . Bisphenol A and chronic disease risk factors in US children. Pediatrics 2013;132:e637–45. 10.1542/peds.2013-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson CL P-RR, Ogden CL. The National health and nutrition examination survey: Analytic guidelines 1999-2010. Hyattsville, Maryland, 2013. [PubMed] [Google Scholar]
- 25. Barr DB, Wilder LC, Caudill SP, et al. . Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005;113:192–200. 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 27. Verstraete SG, Wojcicki JM, Perito ER, et al. . Bisphenol A increases risk for presumed non-alcoholic fatty liver disease in Hispanic adolescents in NHANES 2003-2010. Environ Health 2018;17 10.1186/s12940-018-0356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells EM, Jackson LW, Koontz MB. Association between bisphenol A and waist-to-height ratio among children: National health and nutrition examination survey, 2003-2010. Ann Epidemiol 2014;24:165–7. 10.1016/j.annepidem.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 29. Lejonklou MH, Dunder L, Bladin E, et al. . Effects of low-dose developmental bisphenol a exposure on metabolic parameters and gene expression in male and female Fischer 344 rat offspring. Environ Health Perspect 2017;125:067018 10.1289/EHP505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol 2013;50:625–31. 10.1007/s00592-013-0472-z [DOI] [PubMed] [Google Scholar]
- 31. Silver MK, O'Neill MS, Sowers MR, et al. . Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One 2011;6:e26868 10.1371/journal.pone.0026868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol 2014;44:121–50. 10.3109/10408444.2013.860075 [DOI] [PubMed] [Google Scholar]
- 33. Bravata DM, Olkin I. Simple pooling versus combining in meta-analysis. Eval Health Prof 2001;24:218–30. 10.1177/01632780122034885 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jech-2019-212555supp001.pdf (7.8MB, pdf)