Abstract
We aimed to evaluate efficiency and safety of transplantation of limbal stem cells (LSC) cultured on human amniotic membrane with no feeders and to compare cultured LSC with limbal tissue transplantation. Thirty eyes with stage III LSC deficiency were treated with autologous (autoLSC) or allogeneic (alloLSC) cultured LSC transplantation (prospective phase II clinical trial; average follow‐up time, 72 months) or autologous (autoLT) or allogeneic (alloLT) limbal tissue transplantation (retrospective control group; average follow‐up time, 132 months) between 1993 and 2014. The 5‐year graft survival defined by absence of recurrence of the clinical signs of limbal deficiency was 71% for autoLSC, 0% for alloLSC, 75% for autoLT, and 33% for alloLT. Visual acuity improved by 9.2 lines for autoLSC and 3.3 lines for autoLT. It decreased by 0.7 lines for alloLSC and 1.9 lines for alloLT. Adverse events were recorded in 1/7 autoLSC, 7/7 alloLSC, 6/8 autoLT, and 8/8 alloLT patients. Corneal epithelial defect was the only adverse event recorded after autoLSC, whereas severe sight‐threatening adverse events were recorded in the remaining three groups. Compared with failed grafts, successful grafts featured greater decrease in fluorescein staining, greater superficial vascularization‐free corneal area, lower variability of the corneal epithelial thickness, and higher corneal epithelial basal cell density. Autologous cultured LSC transplantation was associated with high long‐term survival and dramatic improvement in vision and was very safe. Autologous limbal tissue transplantation resulted in similar efficiency but was less safe. Cadaver allogeneic grafts resulted in low long‐term success rate and high prevalence of serious adverse events. stem cells translational medicine 2019;8:1230&1241
Keywords: Adverse events, Cornea, Limbal stem cell, Survival, Transplantation, Visual acuity
Autologous cultured limbal stem cell transplantation was associated with high long‐term survival, dramatic improvement in vision and was very safe. Autologous limbal tissue transplantation resulted in similar efficiency but was less safe. Cadaver allogeneic grafts resulted in low long‐term success rate and high prevalence of serious adverse events.
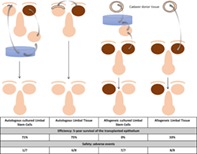
Lessons Learned.
Transplantation of autologous cultured limbal stem cells was associated with high long‐term survival, dramatic improvement in vision, and high safety.
Transplantation of autologous limbal tissue resulted in similar efficiency but lower safety.
Cadaver allogeneic grafts resulted in low long‐term success rate and high prevalence of serious adverse events.
Significance Statement.
Autologous cultured limbal stem cell transplantation was associated with high long‐term survival, vision improvement, and safety, whereas limbal grafts featured lower safety and allogeneic grafts featured low success rate and serious adverse events.
Introduction
Limbal stem cell (LSC) deficiency results from impaired function of the corneal epithelial stem cells that leads to impaired homeostasis of the corneal epithelium. LSC deficiency syndrome is characterized by invasion of the corneal surface by an epithelium with a conjunctival differentiation and, clinically, by opacification and vascularization of the corneal epithelium with impaired corneal epithelial healing and corneal ulcers that may lead to corneal perforation, in addition to ocular surface inflammation and scarring 1. When total, this syndrome results in severe disability. Vision decreases to well below the threshold of legal blindness. Overall, the most frequent etiology by far is the complete loss of LSCs induced by severe ocular burns. Until recently, no treatment for this pathology was available. Progress in understanding of the physiology of corneal epithelial renewal made it possible to introduce therapeutic approaches, that is, transplantation of limbal tissue (limbal tissue transplantation) or cultured LSCs (cultured LSC transplantation) retrieved from the healthy contralateral eye (autograft, unilateral diseases) or from a cadaveric donor eye/donor tissue from a live relative (allograft, bilateral diseases or unique eye) 2.
Cell therapy techniques were first described in Italy, Asia, and the U.S. with positive clinical results 3, 4, 5. Various processes exist for the preparation of the cell product to be transplanted, although none meet the safety criteria required by French legislation. Allogeneic limbal tissue transplantation carries a high risk of rejection and usually requires systemic immunosuppression 2. This particular immunological condition is associated with transplantation of tissue rich in donor dendritic cells, lymphocytes, blood cells, and vascular endothelial cells. We hypothesized that transplantation of cultured epithelial cells with no immune cells would be less likely to trigger a rejection reaction.
We developed a process for preparing a cell therapy product, which was accepted by the French regulation agency (AFSSaPS) for a clinical trial (TC181) that began in 2007 6, 7. The aims of this clinical trial were (a) evaluation of the clinical results of this technique in terms of improvement of visual function, reduction in handicap, improvement of the anatomical condition of the ocular surface, and restitution of the physiological function of the limbal epithelium and (b) evaluation of its possible side effects. We compared the results of cultured LSC transplantation with those of a retrospective group of patients who underwent autologous or allogeneic limbal tissue transplantation at our institution.
Materials and Methods
Prospective Phase II Interventional Clinical Trial (Transplantation of Allogeneic or Autologous Limbal Epithelial Stem Cells Cultured on Human Amniotic Membrane with no Feeders)
The study design was a biphasic, monocentric, noncomparative prospective clinical trial that included, according to the clinical responses observed during the first phase (plane of Gehan, β = 10%), between 14 and 50 patients with unilateral or bilateral total limbal deficiency treated with autologous or allogeneic cultured LSC transplantation. Patients were operated on and followed up at the French National Ophthalmology Hospital (Centre Hospitalier National d'Ophtalmologie des 15‐20, Paris, France). They were treated with autologous cultured LSC transplantation when a contralateral healthy eye was available (i.e., unilateral or very asymmetric diseases, autoLSC group) and allogeneic cultured LSC transplantation if no contralateral healthy eye was available (i.e., bilateral disease or unique eye, alloLSC group). The minimal patient follow‐up was 3 years. Patients were included between 2007 and 2014. The expected minimal success rate was 20% for allografts and 40% for the autografts. External monitoring was ensured by URC‐Est (Paris, France). The grafts were prepared by culture of autologous or allogeneic limbal epithelial cells from limbal explants on human amniotic membrane. The graft preparation process met medical safety requirements related to transplantation of tissues and cells, and the cell therapy products were secured at each stage of their preparation by conventional bacteriological and fungal tests and viral and bacterial polymerase chain reaction. The graft quality was controlled before transplantation. The main outcome measure was survival of the grafted epithelium (Kaplan–Meier method) defined by absence of recurrence of the clinical signs of limbal deficiency in the central cornea.
Inclusion criteria were the following: patient age between 18 and 70 years; informed consent; unilateral or bilateral total limbal deficiency (i.e., stage III LSC deficiency) 8 with diffuse opacification of the corneal epithelium, and superficial (or superficial and deep) corneal vascularization, and irregular corneal epithelial surface under slit‐lamp examination with fluorescein characterized by late fluorescein staining of the corneal epithelium, either associated with chronic epithelial defects; visual acuity of the eye to be treated ≤20/200; absence of keratinization of the ocular surface; Schirmer test >0 at 3 minutes; negative serology for HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV) for autografts. Stage III limbal deficiency (LSD) deficiency corresponds to limbal deficiency where the whole corneal surface is affected (i.e., superficial corneal vascularization and irregular corneal epithelial surface characterized by late fluorescein staining observed in 100% of the limbal circumference and in the central corneal zone) 8.
Exclusion criteria included partial limbal deficiency (i.e., healthy corneal epithelium in at least one corneal zone), previous treatment of limbal deficiency by limbal transplantation or amniotic membrane transplantation during the last 12 months, conjunctival stem cell deficiency (xerophthalmia, keratinization of the ocular surface), immune keratitis not controlled by medical treatment, ocular burn during the last 2 weeks, corneal anesthesia, pregnancy, breastfeeding, allergy to steroid eyedrops, active fungal keratitis, and, for autografts, risk factor for rabies or Creutzfeldt‐Jacob disease and positive serology for HIV, HCV, or HBV.
Retrospective Control Group (Allogeneic or Autologous Limbal Tissue Transplantation)
This group included all patients who underwent autologous or allogeneic limbal tissue transplantation for unilateral or bilateral limbal deficiency in the same institution between 1993 and 2006 with postoperative follow‐up longer than 12 months. They were treated with autologous limbal tissue transplantation when a contralateral healthy eye was available (i.e., unilateral diseases, autoLT group) and allogeneic limbal tissue transplantation if no contralateral healthy eye was available (i.e., bilateral disease or unique eye, alloLT group).
Inclusion criteria were the following: patient age between 18 and 70 years; informed consent; unilateral or bilateral total limbal deficiency (i.e., stage III LSD deficiency) with diffuse opacification of the corneal epithelium, and superficial (or superficial and deep) corneal vascularization, and irregular corneal epithelial surface at slit‐lamp examination with late fluorescein staining, either associated with chronic epithelial defects; visual acuity of the eye to be treated ≤20/200; absence of keratinization of the ocular surface; Schirmer test >0 at 3 minutes.
Ethics
Institutional review board approval was obtained from the CCPPRB Paris‐Saint Antoine, and authorization was obtained from the AFSSaPS. All patients gave informed consent before inclusion. The clinical trial is registered and publicly available at clinicalTrials.gov public site (clinicalTrials.gov identifier: NCT01619189). The described research adhered to the tenets of the Declaration of Helsinki.
Graft Preparation
For autologous transplantation, a superficial limbal biopsy was taken in the healthy contralateral eye of the patient under local or general anesthesia. The size of the limbal biopsy was one‐third of the limbal circumference for limbal tissue transplantation and 1 mm for cultured LSC transplantation. For limbal tissue transplantation, the biopsy was taken during the transplantation procedure, and it was immediately grafted onto the recipient eye 9, 10. For cultured LSC transplantation, the biopsy was taken 2–3 weeks before transplantation. It was secured on a human amniotic membrane fixed on a plastic lamella (320 mm2; Thermanox, Nunc, Illkirch, France) epithelial side up with 10/0 Vicryl suture, transferred to a 6‐well (907 mm2) plate (Becton Dickinson, Rungis, France) with 2 ml of medium and immediately sent, at room temperature, to the eye bank for culture.
For allogeneic transplantation, a cadaver donor corneal graft was used. For limbal tissue transplantation, a circular superficial limbal graft was prepared as follows. The graft was first trephined at 8 mm. A peripheral superficial dissection was performed with a crescent blade, and the scleral tissue was removed with scissors. The graft was taken during the transplantation procedure, and it was immediately grafted onto the recipient eye 11, 12. For cultured LSC transplantation, superficial limbal biopsies were taken on the donor graft under laminar flow. A stromal dissection between the anterior and the mid stroma was performed using a 15° blade, and the sclera was carefully removed with scissors, resulting in a superficial limbal rim. Six explants with homogeneous length (4 mm) were obtained using scissors. Five explants were secured on a human amniotic membrane fixed on a plastic lamella epithelial side up with 10/0 Vicryl suture and transferred to a 6‐well plate with 2 ml of medium and cultured at 31°C (5% CO2) in the eye bank. The remaining sixth explant was cultured at 31°C (5% CO2) with no amniotic membrane in a separate dish (control dish).
LSC Culture
LSCs were grown under feeder‐free conditions for 14–21 days. The only animal product used to grow cells was irradiated fetal calf serum originating from New Zealand and approved for corneal graft organ culture by the AFSSaPS. Culture assessment criteria were established by the AFSSaPS.
Limbal explants were cultured in cholera toxin‐free Green's medium. The medium was composed of a 3:1 mixture of calcium‐free Dulbecco's modified Eagle's medium (Dutscher, Brumath, France) and Ham F12 medium (Invitrogen, Cergy Pontoise, France) with 10% fetal bovine serum (Invitrogen), 1 mM/ml HEPES buffer (Invitrogen), 5 μg/ml human recombinant insulin (Actrapid; Novo Nordisk, Paris, France), 0.4 μg/ml hydrocortisone (Pharmacia, Pfizer, Paris, France), 4 μM/ml l‐glutamine (Invitrogen), 2 pM/ml tri‐iodo thyronine (Sigma, Saint Quentin en Yvelines, France), 200 nM/ml adenine (Sigma), 100 IU/ml penicillin (Invitrogen), 100 μg/ml streptomycin (Invitrogen), 0.25 μg/ml amphotericin B (Invitrogen), and 10 ng/ml human recombinant epithelial growth factor (Sigma). The medium was renewed three times a week, and cells were cultured for 2–3 weeks at 37°C with 5% CO2.
Morphological analysis (Supporting Information Fig. S1) of allogeneic and autologous LSC cultures was performed twice a week with a phase contrast light microscope. A sheet of cells covering the whole amniotic membrane at the end of culture with more than 50% of polygonal epithelial cells and less than 50% of fibroblasts was required to accept the cultured LSC grafts for transplantation.
Morphological analysis was performed on the control dish of allogeneic LSC cultures during the third week of culture. Image J software (National Institutes of Health, Bethesda, MD) was used to assess morphometry after individual contours of at least 100 cells were segmented manually. The mean values for cell area, Feret diameter, and percentage of small cells (smaller than 16 μm2) were assessed for each well. Criteria for accepting grafts for transplantation were the following: mean circularity >0.6, mean Feret diameter <40 μm, and >50% of cells featuring Feret diameter <50 μm and circularity >0.5.
Immunocytochemistry was performed on the control dish of allogeneic LSC cultures during the third week of culture. Immunochemical staining was performed to evaluate the expression of cytokeratin 3 (primary antibody, Clone AE‐5; Dako, Trappes, France). At least 200 cells from four different fields were assessed and classified as follows: (a) no staining; (b) moderate staining; and (c) strong staining. Grafts were accepted for transplantation if the percentage of cells in each of the three classes was higher than 10%.
Transplantation
Transplantation procedures were performed under general or local anesthesia. A circular limbal peritomy was made with scissors, and the epithelium and subepithelial fibrosis covering the corneal surface were removed. Limbal tissue grafts were secured in the limbal region with 10/0 Nylon interrupted sutures. Cultured LSC grafts were protected with sodium hyaluronate and sutured on the entire corneal surface with 10/0 Nylon interrupted sutures. The conjunctiva was then sutured in the limbal region with 10/0 Vicryl. A therapeutic contact lens was placed on the eye at the end of surgery and removed when the cornea was re‐epithelialized.
Postoperative treatment included topical dexamethasone and ofloxacin, four times daily, for all eyes, and 2% topical cyclosporine, four times daily, for eyes with allogeneic grafts. Systemic immunosuppression (oral cyclosporine, steroids, or chloraminophen) was used during the first postoperative year only in patients with limbal tissue allografts. The eye drop regimen was modified according to ocular surface inflammation and progressively diminished.
Patient Follow‐Up
Patients were hospitalized up to the time of graft re‐epithelialization. They were then examined prospectively at 1 and 2 weeks, 1, 3, 6, 9, 12, 18, 24, 30, and 36 months, and 3, 4, and 5 years after surgery. Manifest refraction (with spectacle correction), symptoms, fluorescein staining, and corneal vascularization were assessed at each examination. Slit‐lamp photographs of the cornea were taken at each examination. Cobalt blue light was used to visualize fluorescein staining. The criteria for graft failure were recurrence of the clinical signs of limbal deficiency (i.e., opacification of the corneal epithelium, irregularity of the corneal epithelium with late fluorescein staining, superficial corneal vascularization) in the central cornea. At various postoperative time points, the eyes with cultured LSC transplantation were evaluated using a Schirmer test, in vivo confocal microscopy (Heidelberg Retina Tomograph III; Heidelberg Engineering GmbH, Heidelberg, Germany), and spectral domain optical coherence tomography (RTVue‐100; Optovue Inc., Fremont, CA). Morphometric analysis of slit‐lamp images was performed with ImageJ software (NIH, Madison, WI; Fig. 1). The corneal area free of superficial vessels was measured with ImageJ software using the green channel of the native bright light slit‐lamp photograph. To assess fluorescein staining, we used the green channel of the native blue cobalt slit‐lamp photograph. Zones with bright reflex were discarded, and the region of interest corresponding to the visible corneal surface was defined. The fluorescein staining level was defined as the mean grayscale level of the region of interest. This figure quantifies all kinds of fluorescein staining including late fluorescein staining, fluorescein pooling, punctate keratitis, and corneal epithelial defects.
Figure 1.
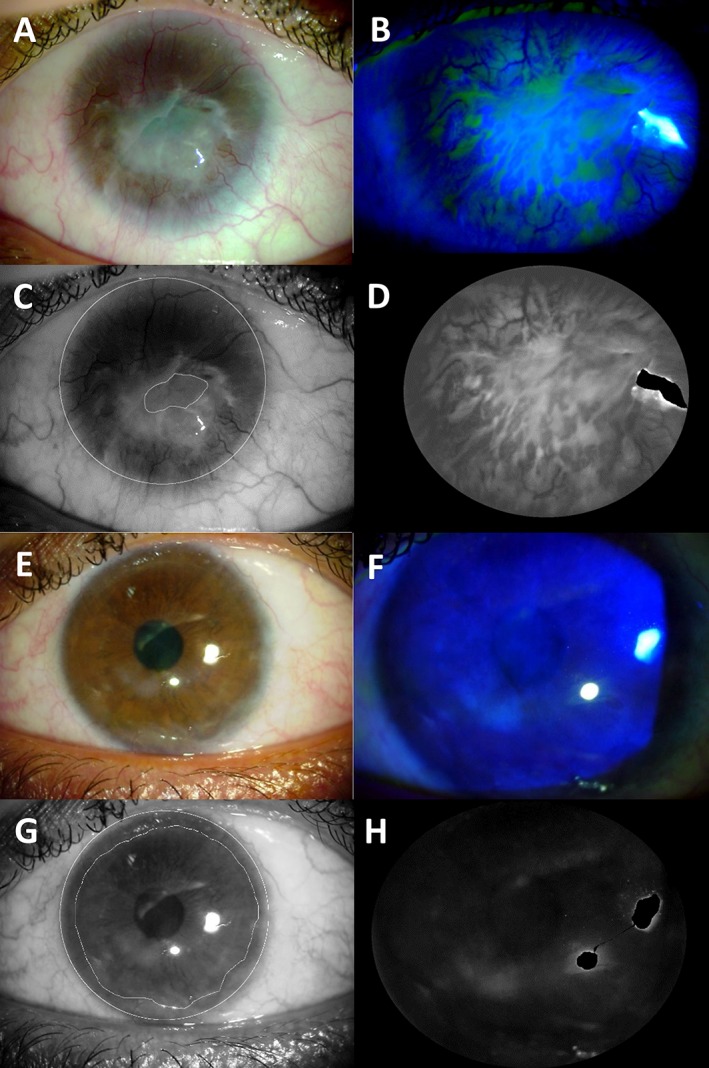
Morphometric analysis of slit‐lamp images of a patient with stage III limbal stem cell deficiency before (A–D) and 36 months after transplantation of autologous limbal stem cells cultured on human amniotic membrane (E–H). The corneal area free of superficial vessels (C, G) was measured with ImageJ software using the green channel of the native bright light slit‐lamp photograph (A, E). The green channel (D, H) of the native blue cobalt slit‐lamp photograph (B, F) was used to assess fluorescein staining. LSC transplantation was successful in this eye. Visual acuity improved from 20/3,991 preoperatively to 20/50 36 months after transplantation (+19 lines), fluorescein staining decreased from 97.7 to 22.3, and the corneal area free of superficial vessels increased from 21% to 74% of the total corneal area.
The primary outcome measure was survival of the transplanted epithelium defined by absence of recurrence of the clinical signs of limbal deficiency (opacification of the corneal epithelium, irregularity of the corneal epithelium with late fluorescein staining, superficial corneal vascularization) in the central cornea.
The secondary outcome measures included visual acuity, symptoms, and morphometric analysis of the ocular surface. A subjective score was calculated as the mean of quantitative evaluations of redness, pain, itching, foreign body sensation, and blurred vision.
Statistical Analysis
Nonparametric tests (chi‐square, Spearman rank correlation coefficient, Kruskal–Wallis analysis of variance [ANOVA], and Mann–Whitney U test) were used for statistical analysis with the Statistica 6.1 software (StatSoft Inc., Maisons‐Alfort, France). When Kruskal–Wallis ANOVA showed significant differences among groups, ANOVA with posthoc analysis was used to compare individual groups. p < .05 was considered statistically significant.
Results
Inclusions and Patient Follow‐Up
Prospective Phase II Interventional Clinical Trial
Fourteen patients were included and followed for at least 40 months (Table 1), of whom seven were in the alloLSC group and seven were in the autoLSC group. All study eyes presented a conjunctival differentiation of the epithelium covering cornea both in preoperative in vivo confocal microscopy and by histological examination of the corneal pannus retrieved during cultured LSC transplantation. Presence of goblet cells in the epithelium covering cornea was found in 13 out of 14 eyes. The average follow‐up time was 72 months (range 40–116 months). The inclusions were discontinued by the end of the first phase because the percentage of success was higher than expected in the autoLSC group and the frequency of serious adverse events was considered too high in the alloLSC group. All LSC cultures were successful in the autoLSC group (i.e., no limbal biopsies were lost for transplantation). In the alloLSC group, 20 cultures were initiated. Nine were discarded during culture because of positive infectious markers in the donors or donor tissues (corneas and amniotic membranes), four failed, and seven were successful and met all the criteria for transplantation.
Table 1.
Characteristics of 30 patients with stage III limbal deficiency treated with transplantation of limbal stem cells
| Case | Age (years) | Corneal disorder | Baseline LogMAR visual acuity | Group | Additional procedures | Result | Follow‐up (months) | Final LogMAR visual acuity |
|---|---|---|---|---|---|---|---|---|
| 1 | 69 | Stevens–Johnson syndromea | 2.30 | autoLSC | PK + AMT (M42) | S | 90 | 2.00 |
| 2 | 48 | Burn | 2.30 | autoLSC | PK + cataract + AMT (M41) | S | 85 | 0.70 |
| 3 | 34 | Burn | 2.30 | autoLSC | None | S | 40 | 0.40 |
| 4 | 25 | Burn | 2.00 | autoLSC | None | S | 43 | 0.85 |
| 5 | 38 | Burn | 2.00 | autoLSC | PK + AMT (M11) | F | 73 | 2.00 |
| 6 | 62 | Burn | 2.30 | autoLSC | None | F | 48 | 2.00 |
| 7 | 46 | Burn | 1.22 | autoLSC | None | S | 46 | 0.05 |
| 8 | 63 | Severe infectious keratitis | 2.00 | alloLSC | PK (M1, M2) PK + AMT (M3, M30) AMT (M46) |
F | 60 | 1.22 |
| 9 | 57 | Burn | 3.00 | alloLSC | PK during alloLSC cataract + AMT + tarsorraphy (M9) PK + AMT (M15) AMT + tarsorraphy (M23) | F | 78 | 3.00 |
| 10 | 42 | Burn | 1.70 | alloLSC | DALK during alloLSC PK + AMT (M6) AMT (M12, M17, M20) |
F | 116 | 2.30 |
| 11 | 47 | Multiple surgeries involving the limbus | 3.00 | alloLSC | PK + AMT (M11) | F | 96 | 2.30 |
| 12 | 57 | Aniridia | 2.30 | alloLSC | PK + AMT (M12, M28) | F | 91 | 3.00 |
| 13 | 60 | Burn | 2.30 | alloLSC | PK + AMT (M13) | F | 72 | 3.00 |
| 14 | 64 | Aniridia | 2.00 | alloLSC | None | F | 70 | 2.00 |
| 15 | 44 | Burn | 2.00 | autoLT | PK (M24) | F | 264 | 3.00 |
| 16 | 48 | Burn | 2.00 | autoLT | PK (M9) | F | 13 | 2.00 |
| 17 | 76 | Severe pterygium with multiple surgeries | 1.30 | autoLT | PK (M4) | S | 165 | 0.22 |
| 18 | 55 | Burn | 1.30 | autoLT | None | S | 25 | 0.30 |
| 19 | 24 | Burn | 2.00 | autoLT | PK (M7) | S | 134 | 0.49 |
| 20 | 82 | Severe infectious keratitis | 1.00 | autoLT | PK (M3) | S | 29 | 0.30 |
| 21 | 48 | Severe infectious keratitis | 2.00 | autoLT | PK (M13) | F | 221 | 3.00 |
| 23 | 62 | Multiple surgeries involving the limbus | 1.00 | autoLT | PK (M4) | S | 220 | 0.60 |
| 22 | 59 | Burn | 1.30 | alloLT | None | F | 223 | 3.00 |
| 24 | 54 | Burn | 2.30 | alloLT | PK (M8) | S | 31 | 2.30 |
| 25 | 74 | Mucus membrane pemphigoid | 2.30 | alloLT | None | F | 25 | 2.00 |
| 26 | 68 | Severe infectious keratitis | 2.30 | alloLT | PK (M3) | S | 79 | 1.70 |
| 27 | 32 | Stevens–Johnson syndrome | 2.30 | alloLT | PK + AMT (M9) | F | 184 | 2.30 |
| 28 | 24 | Burn | 2.30 | alloLT | PK + AMT (M10) | F | 206 | 2.30 |
| 29 | 38 | Burn | 2.30 | alloLT | AMT during alloLT PK + cataract+AMT (M5) | S | 206 | 2.30 |
| 30 | 14 | Burn | 2.30 | alloLT | AMT during alloLT PK (M1.5) | F | 92 | 3.00 |
Very asymmetric eye involvement.
Abbreviations: alloLSC, allogeneic cultured LSC transplantation; alloLT, allogeneic limbal tissue transplantation; AMT, amniotic membrane transplantation; autoLSC, autologous cultured LSC transplantation; autoLT, autologous limbal tissue transplantation; DALK, deep anterior lamellar keratoplasty; F, failure; LogMAR, logarithm of the minimum angle of resolution; LSC, limbal stem cell; PK, penetrating keratoplasty; S, success.
Retrospective Control Group
Sixteen patients were included in the control group, of whom eight were in the alloLT group and eight were in the autoLT group (Table 1). All study eyes presented a conjunctival differentiation of the epithelium covering cornea by histological examination of the corneal pannus retrieved during transplantation. The average follow‐up time was 132 months (range 13–264 months).
Survival of the Transplanted Epithelium
The 3‐year and 5‐year survival estimates were, respectively, 100% and 71% for the autoLSC group, 29% and 0% for the alloLSC group, 75% and 75% for the autoLT group, and 50% and 33% for the alloLT group (p = .02; Fig. 2). Survival was significantly better for autografts than allografts (p = .004), whereas no significant differences were found between limbal tissue grafts and cultured LSC grafts (p = .39).
Figure 2.
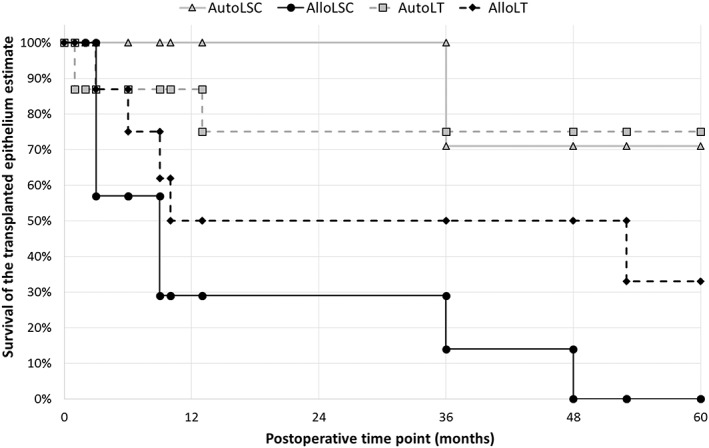
Survival of the transplanted epithelium after LSC transplantation. The primary outcome measure was defined as absence of recurrence of the clinical signs of LSC deficiency (opacification of the corneal epithelium, irregularity of the corneal epithelium with late fluorescein staining, superficial corneal vascularization) in the central cornea. Survival was significantly better for autografts than allografts, whereas no significant differences were found between limbal tissue grafts and cultured LSC grafts. Abbreviations: alloLSC, allogeneic cultured LSC transplantation; alloLT, allogeneic limbal tissue transplantation; autoLSC, autologous cultured LSC transplantation; autoLT, autologous limbal tissue transplantation; LSC, limbal stem cell.
Visual Recovery
Visual acuity data are shown in Tables 1 and 2 and Figure 3. Baseline visual acuity was significantly better in the autoLT group than in the alloLT group (p = .047) and in the alloLSC group (p = .01). Other posthoc tests were not significant.
Table 2.
Visual acuity before and after transplantation of limbal stem cells
| Variable | Autologous cultured LSC transplantation (autoLSC group) | Allogeneic cultured LSC transplantation (alloLSC group) | Autologous limbal tissue transplantation (autoLT group) | Allogeneic limbal tissue transplantation (alloLT group) | p value |
|---|---|---|---|---|---|
| n | 7 | 7 | 8 | 8 | |
| Follow‐up time (months) | 61, 48 [41–80] | 83, 78 [66–101] | 134, 149 [50–218] | 131, 138 [62–200] | .38 |
| LogMAR baseline visual acuity | 2.1 (20/2,301), 2.3 (20/4,000) [1.7–2.4] | 2.3 (20/4,263), 2.3 (20/4,000) [1.9–2.8] | 1.6 (20/752), 1.7 (20/894) [1.2–2.0] | 2.2 (20/3,000), 2.3 (20/4,000) [1.9–2.5] | .01 |
| LogMAR visual acuity at last exam | 1.1 (20/278), 0.9 (20/143) [0.4–1.9] | 2.4 (20/5,063), 2.3 (20/4,000) [1.8–3.0] | 1.2 (20/348), 0.6 (20/71) [0.2–1.2] | 2.4 (20/4,612), 2.3 (20/4,000) [2.0–2.7] | .02 |
| Change in visual acuity from baseline to last exam, lines | +9.2, +11.5 [+2.5 to +15.9] | −0.7, 0.0 [−6.6 to +5.1] | +3.3, +5.5 [−4.5 to +11.2] | −1.9, 0.0 [−7.8 to +4.1] | .08 |
Mean, median, and [95% confidence interval] are shown.
Abbreviations: LogMAR, logarithm of the minimum angle of resolution; LSC, limbal stem cell.
Figure 3.
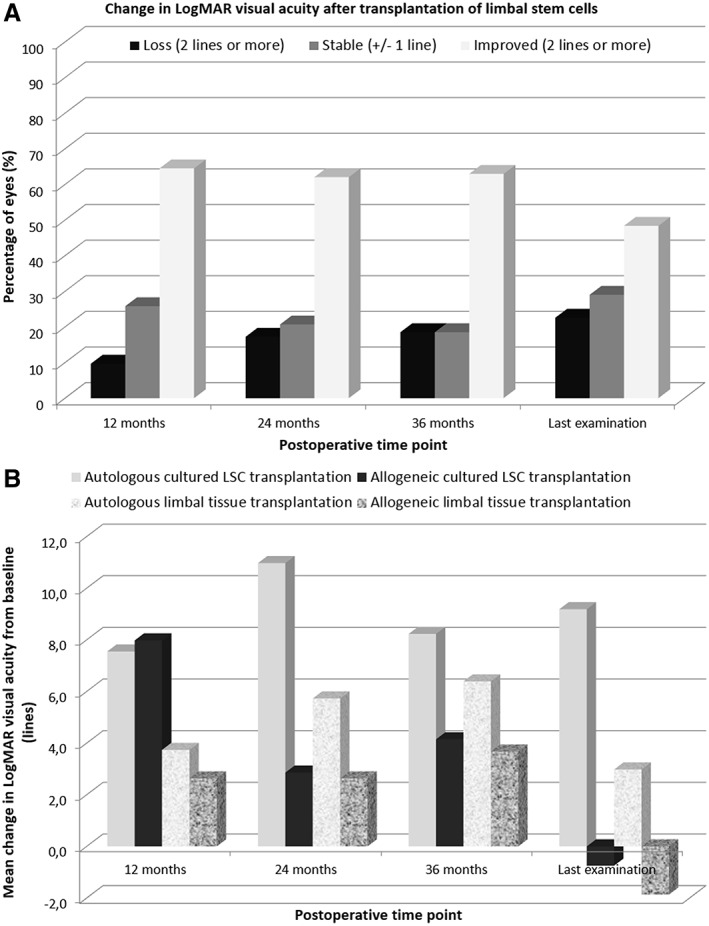
Change in visual acuity as a function of postoperative time point in 30 eyes with stage III limbal deficiency treated with transplantation of limbal stem cells. Overall vision improved after transplantation (A). From baseline to last visit, visual acuity improved by an average of 9.2 lines in the autoLSC group and 3.3 lines in the autoLT group, whereas it decreased by 0.7 lines in the alloLSC group and 1.9 lines in the alloLT group (B). Abbreviations: LogMAR, logarithm of the minimum angle of resolution; LSC, limbal stem cell.
Among 30 eyes, visual acuity significantly improved at all postoperative time points compared with preoperative values (p < .002). At last follow‐up visit, visual acuity was significantly better in the autoLSC group than in the alloLT and alloLSC groups (p = .01) and significantly better in the autoLT group than in the alloLT and alloLSC groups (p = .01). Other posthoc tests were not significant. From baseline to last visit, visual acuity improved by an average of 9.2 lines in the autoLSC group and 3.3 lines in the autoLT group, whereas it decreased by 0.7 lines in the alloLSC group and 1.9 lines in the alloLT group. At last visit, six of seven patients in the autoLSC group had improved their visual acuity by two lines or more. This figure was five of eight in the autoLT group, two of seven in the alloLSC group, and two of eight in the alloLT group. At last visit, none of the seven patients in the autoLSC group presented a loss of visual acuity of two lines or more. This figure was two of eight in the autoLT group, three of seven in the alloLT group, and two of eight in the alloLT group.
Among 13 eyes with successful grafts, 11 had improved vision by two lines or more at last examination and 2 had stable vision compared with preoperative visual acuity. Among 17 eyes with failed grafts, 7 had decreased vision at last follow‐up examination, 6 had stable vision, and 4 had better vision compared with preoperative value.
Adverse Events
Adverse events (Table 3) were recorded in one of seven patients in the autoLSC group, seven of seven patients in the alloLSC group, six of eight patients in the autoLT group, and eight of eight patients in the alloLT group. Corneal epithelial defect requiring topical treatment was the only adverse event recorded in the autoLSC group, whereas severe sight‐threatening adverse events were recorded in the remaining three groups.
Table 3.
Adverse events after transplantation of limbal stem cells
| Variable | Autologous cultured LSC transplantation (autoLSC group) | Allogeneic cultured LSC transplantation (alloLSC group) | Autologous limbal tissue transplantation (autoLT group) | Allogeneic limbal tissue transplantation (alloLT group) |
|---|---|---|---|---|
| Total no. of adverse events | 2 | 40 | 16 | 45 |
| No. of eyes with at least one adverse event | 1/7 | 7/7 | 6/8 | 8/8 |
| Phthisis bulbi | 0 | 1 | 1 | 1 |
| Corneal perforation requiring transplantation | 0 | 4 | 0 | 4 |
| Herpes keratitis requiring transplantation | 0 | 1 | 0 | 0 |
| Bacterial keratitis requiring transplantation | 0 | 3 | 0 | 0 |
| Bacterial keratitis treated medically | 0 | 3 | 0 | 3 |
| Fungal keratitis treated medically | 0 | 0 | 0 | 1 |
| Epithelial defect requiring AMT and/or tarsorrhaphy | 0 | 6 | 0 | 5 |
| Epithelial defect treated topically | 2 | 11 | 4 | 15 |
| Superficial punctate keratitis | 0 | 2 | 0 | 0 |
| Corneal transplantation rejection | 0 | 3 | 5 | 6 |
| Retinal detachment | 0 | 0 | 0 | 1 |
| Acute retinal necrosis | 0 | 0 | 0 | 1 |
| Vitreous hemorrhage | 0 | 0 | 0 | 1 |
| Epiretinal membrane | 0 | 0 | 0 | 1 |
| Cataract requiring surgery | 0 | 2 | 2 | 3 |
| Elevated IOP | 0 | 3 | 2 | 0 |
| Symblepharon | 0 | 0 | 0 | 2 |
| Trichiasis | 0 | 0 | 1 | 1 |
| Lagophthalmos | 0 | 0 | 1 | 0 |
| Quinolone‐related tendinopathy | 0 | 1 | 0 | 0 |
Shown are the number of adverse events that occurred (i.e., one eye may experience the same adverse event several times).
Abbreviations: AMT, amniotic membrane transplantation; IOP, intraocular pressure; LSC, limbal stem cell.
Subjective Symptoms and Schirmer Test after Cultured LSC Transplantation
Compared with the baseline value, subjective symptoms significantly improved after cultured LSC transplantation at all postoperative time points (p < .05; Fig. 4A). Conversely, no significant differences in subjective score were observed between the alloLSC and the autoLSC groups at all time points including baseline (p > .15). Compared with the baseline value, no significant changes in the Schirmer test value were observed after cultured LSC transplantation at all postoperative time points (p > .35; Fig. 4B). Similarly, no significant differences were observed between the alloLSC and the autoLSC groups at all time points including baseline (p > .10).
Figure 4.
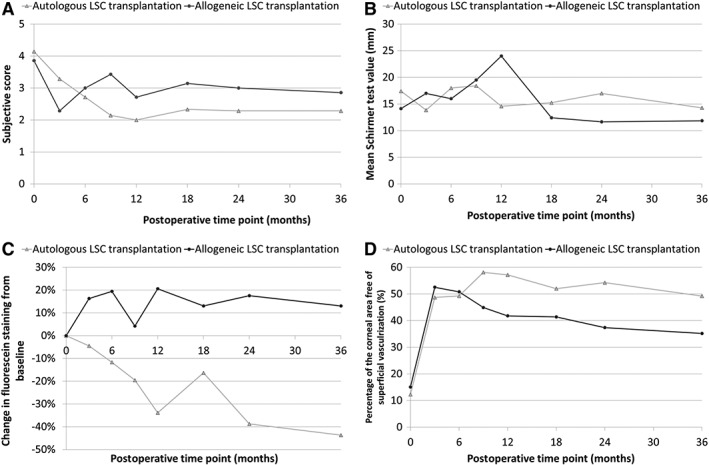
Subjective score (A), Schirmer test (B), fluorescein staining (C), and superficial vascularization‐free corneal area (D) as function of postoperative time point after cultured LSC transplantation. The subjective score was calculated as the mean of quantitative evaluations of redness, pain, itching, foreign body sensation, and blurred vision. Fluorescein staining was assessed with blue cobalt slit‐lamp images as the mean grayscale level of the green channel. We show the change in fluorescein staining from baseline. This decreased with postoperative time in the autoLSC group but not in the alloLSC group. Superficial vascularization‐free corneal area was assessed with the green channel of white slit‐lamp image. We show the percentage of the corneal area that was free of superficial vascularization. Abbreviation: LSC, limbal stem cell.
Morphometric Analysis of the Ocular Surface after Cultured LSC Transplantation
Fluorescein staining decreased with postoperative time point in the autoLSC group, whereas it increased from baseline value in the alloLSC group (Fig. 4C). All five successful grafts had decreased fluorescein staining 36 months after transplantation. Among nine failed grafts, five had increased fluorescein staining and four had decreased fluorescein staining 36 months after transplantation. Three years after transplantation, the average percentage of the corneal area free of superficial vascularization was 49.2% in the autoLSC group and 39.2% in the alloLSC group (Fig. 4D). The average increase in this figure from baseline to 36 months was 51.3% in successful grafts and 15.9% in failed grafts.
Compared with allogeneic grafts, autologous grafts featured significantly greater decrease in fluorescein staining from baseline, higher minimal corneal epithelial thickness, lower variability of the corneal epithelial thickness, and higher corneal epithelial basal cell density 3 years after transplantation (Table 4). Compared with failed grafts, successful grafts featured significantly greater decrease in fluorescein staining from baseline, greater superficial vascularization‐free corneal area, lower variability of the corneal epithelial thickness, and higher corneal epithelial basal cell density 3 years after transplantation (Table 4).
Table 4.
Morphometric analysis of the ocular surface 36 months after transplantation of cultured limbal stem cells
| Parameter | Autologous cultured LSC transplantation (autoLSC group) | Allogeneic cultured LSC transplantation (alloLSC group) | p value | Successful grafts | Failed grafts | p value |
|---|---|---|---|---|---|---|
| n | 7 | 7 | 5 | 9 | ||
| Change in fluorescein staining from baseline (%) | −44 ± 20 | +13 ± 44 | .006 | −47 ± 23 | +2 ± 44 | .03 |
| Corneal area free of superficial vascularization (mm2) | 49.2 ± 29.5 | 35.2 ± 19.9 | .41 | 62.4 ± 22.9 | 31.0 ± 19.3 | .04 |
| SD‐OCT central corneal thickness (μm) | 522 ± 80 | 612 ± 124 | .09 | 516 ± 93 | 593 ± 112 | .08 |
| SD‐OCT minimal corneal thickness (μm) | 431 ± 87 | 402 ± 98 | .47 | 410 ± 103 | 422 ± 87 | .85 |
| SD‐OCT central epithelial thickness (μm) | 54 ± 8 | 52 ± 11 | .63 | 54 ± 10 | 53 ± 10 | .87 |
| SD‐OCT minimal epithelial thickness (μm) | 38 ± 10 | 17 ± 14 | .04 | 35 ± 10 | 22 ± 15 | .14 |
| SD‐OCT central epithelial thickness SD (μm) | 10 ± 4 | 20 ± 4 | .008 | 9 ± 4 | 17 ± 5 | .03 |
| SD‐OCT maximum–minimum central epithelial thickness (μm) | 41 ± 14 | 79 ± 15 | .007 | 38 ± 16 | 70 ± 19 | .02 |
| IVCM basal epithelial cell density (cells per mm2) | ||||||
| Donor eye | 7,614 ± 1,846 | Not available | ||||
| Preoperative | 4,689 ± 1,405 | 2,809 ± 686 | .02 | 5,372 ± 955 | 2,852 ± 590 | .003 |
| Postoperative | 5,949 ± 2,278 | 2,784 ± 2,049 | .02 | 6,558 ± 1,155 | 3,254 ± 2,582 | .03 |
Mean ± SD is shown. Significant differences are shown by bolded values.
Abbreviations: IVCM, in vivo confocal microscopy; LSC, limbal stem cell; SD‐OCT, spectral domain optical coherence tomography.
Discussion
The primary endpoint of the present study was survival of the transplanted LSC. It was defined clinically as the absence of recurrence of the clinical signs of LSC deficiency (i.e., opacification of the corneal epithelium, irregularity of the corneal epithelium with late fluorescein staining, superficial corneal vascularization) in the central cornea. This endpoint has been widely used in clinical studies reporting the success rate of LSC transplantation 3, 4, 5, 13. The weakness of this endpoint is that it is subjective. We therefore used other outcome measurements including visual acuity and objective morphometric measurements for cultured LSC transplantation. We found a strong association between graft success and visual acuity improvement. In addition, graft success correlated with decrease in fluorescein staining in the prospective group. However, a few failed grafts had improved vision or decreased fluorescein staining, showing the primary endpoint could underestimate the success rate of LSC transplantation.
In the prospective study group, survival of the transplanted cultured autologous LSC was estimated to be 71% at 5 years. This figure was not significantly different from what we observed in our retrospective control group of autologous limbal tissue transplantation. It is similar to previously reported survival rates after autologous cultured LSC transplantation 5, 13, 14. In a series of 116 eyes, dissociated limbal epithelial cells cultured on fibrin with a murine feeder layer were reported to restore the ocular surface in 68.2% of cases after one graft and 76.6% after regraft in case of failure of the first graft 14. Transplantation of autologous LSC cultured on human amniotic membrane was reported to restore a stable corneal surface in 142 of 200 (71%) eyes at a mean follow‐up of 3 years with a two‐line improvement in visual acuity in 60.5% of eyes 13. In pediatric patients with LSC deficiency after ocular burn, autologous cultured LSC transplantation resulted in completely epithelialized, avascular, and stable ocular surfaces in 46.7% of 107 eyes after one or two grafts and a mean follow‐up of 3.4 years, with 58 eyes (54.2%) featuring improvement in visual acuity of two lines or more 15.
The success rate we observed in the autoLT group is similar to that reported in previous studies 9, 16. Interestingly the average follow‐up time of our patients in this group exceeded 10 years, which is a very long follow‐up for LSC transplantation studies. Regarding restoration of the corneal surface after autologous LSC transplantation, from current available studies, major differences between limbal tissue transplantation and cultured LSC transplantation are unlikely.
Despite dramatic differences in culture techniques, autologous cultured LSC transplantation appears to be associated with similar success rates in various published studies 13, 14, 15. Of note, with the dissociated LSC culture technique, the graft quality is assessed before transplantation and the success rate is associated with presence of holoclones expressing DeltaNp63alpha in culture 17, 18. This technology permits further graft to be performed from the original limbal biopsy as LSC are subcultured and stored as frozen dissociated cells. The drawbacks are high cost and a long procedure (i.e., several months are needed between biopsy of the healthy eye and transplantation of the diseased eye). With the limbal tissue culture technique, the graft corresponds to the primary culture of the limbal biopsy on human amniotic membrane. No assessment of graft quality other than morphology is possible, and only one graft can be performed. However, the procedure is quicker (2 or 3 weeks), and the cost is lower.
Interestingly, six of seven patients in the autoLSC group had improved their visual acuity at last visit and none had decreased vision. Among our four groups of patients, the autoLSC group featured the greatest change in visual acuity. However, the comparison of the autoLSC group with the autoLT group did not reach significance. In terms of safety, in the autoLSC group, no eyes had decreased vision at last follow up, adverse events were uncommon and only minor, and the size of the limbal biopsy taken in the healthy contralateral donor eye was very small (i.e., 1 mm). Conversely, in the autoLT group, two of nine eyes had decreased vision at last follow‐up, adverse events were common and potentially sight threatening, and the size of the limbal biopsy taken in the healthy contralateral donor eye was much larger. Whereas both groups were similar in terms of treatment efficiency, autologous cultured LSC transplantation appears to be safer than autologous limbal tissue transplantation.
We recorded all adverse events even if they were not directly related to LSC transplantation. In the autoLT group, most adverse events were related to subsequent keratoplasty rather than primary limbal transplantation (i.e., corneal transplantation rejection, cataract, and elevated intraocular pressure). However, seven of eight patients required keratoplasty to improve vision in this group. This figure was only three of seven in the autoLSC group (Table 1). We found a high incidence of infectious keratitis after transplantation of allogeneic LSC but not after transplantation of autologous LSC. Not only are eyes with stage III LSC deficiency at high risk of infectious keratitis, but failure of LSC transplantation to restore the corneal surface, extended contact lens wear, additional ocular surgical procedures, systemic immunosuppression, and use of corticosteroid or cyclosporine eye drops further increase the risk of infection 19, 20. Lastly, corneal perforation was common after allogeneic transplantation whereas it was not observed after autologous transplantation. This adverse event is a direct consequence of LSC graft failure.
Survival of the transplanted corneal epithelium appears to decrease rapidly with postoperative time in allogeneic cases despite good short‐term results 21, 22, 23. A previous study reported rapid deterioration of the therapeutic effect after the first postoperative year in eyes treated with allogeneic cultured LSC transplantation 24. The poor results obtained after transplantation of allogeneic cultured LSCs, which were similar to those obtained after allogeneic limbal tissue transplantation, do not support our primary hypothesis concerning the expected difference in the risk of rejection between tissue and cultured cells. We used cadaver donor tissue for transplantation in allogeneic cases with no human leukocyte antigen matching between donor and recipient. This may explain why our success rate was poor due to rejection of transplanted LSC. Another option is to use allogeneic donor tissue from a living relative. This option could provide better results 25. In fact, in patients with bilateral LSC deficiency after ocular burn, cultured LSC transplantation from a living relative donor was able to restore a stable corneal surface in 71.4% of cases after a mean follow‐up of 4.8 years 26. Final visual acuity improved to 20/60 or better in 67.8% of 20 transplanted eyes. Similarly, good results have been reported after allogeneic limbal tissue transplantation from a living relative donor 27. In fact, the success rate was reported to be 89% for living related donor allografts and 33% for cadaver donor allografts among 15 allolimbal transplants followed for an average of 2.5 years. The difference between living related and cadaver donor allografts could be explained by better tissue matching for the former as well as better quality of the graft (i.e., higher number of healthy LSC at the time of transplantation). Finally, prolonged systemic immunosuppression can also be used to increase the success rate of allogeneic LSC transplantation 28. The limitation is linked to the potential severe adverse events related to prolonged systemic immunosuppression.
The dramatic difference observed between allogeneic grafts and autologous grafts is likely to be the result of rejection of transplanted allogeneic LSC 29, 30. We still lack clear criteria for the diagnosis of LSC rejection. Inflammation and neovascularization might be either the features of rejection or the consequences of loss of LSC function. A difference in LSC impairment could also explain the difference between autologous and allogeneic cases. The former cases correspond to unilateral cases often secondary to ocular burns and the latter to bilateral cases with usually systemic immune or genetic disorders. Whereas all our patients had stage III LSC deficiency, poor vision, and conjunctivalization of the corneal surface evidenced by in vivo confocal microscopy and histology, the preoperative epithelial basal cell density was lower in the alloLSC group than in the autoLSC group despite similar preoperative visual acuity impairment. We could hypothesize that autologous cases could retain buried limbal crypts that are insufficient to maintain limbal function but that could help the transplanted cells to restore limbal function. Precise assessment of the limbal niche before LSC transplantation would be useful to further understand the therapeutic effect of LSC transplantation and to determine which patients should benefit from this treatment 31.
Study limitations include the size of the study population, use of a retrospective control group, and the absence of both a simple limbal epithelial transplantation (SLET) group and a cultivated autologous oral mucosal epithelial cell transplantation group 32, 33, 34, 35. The SLET technique is a challenging one for cell therapy technologies. It requires only one surgical procedure with no need for ex vivo cell expansion and low cost compared with autologous cultured LSC transplantation. It features a success rate similar to that of autologous cultured LSC transplantation, that is, a 76% success rate after a median postoperative follow‐up of 1.5 years 32. One limitation of this technique could be the risk of loss of some transplanted limbal explants due to weak adhesive properties of the fibrin glue used to maintain explants on the amniotic membrane. This adverse event is not expected with the cell therapy technology because the explant is secured by the surgeon on the amniotic membrane at the time of limbal biopsy and cells are grown for 2–3 weeks before transplantation. Further studies are needed to compare SLET with cell therapy techniques. Cultivated autologous oral mucosal epithelial cell transplantation could be an interesting alternative to allogeneic cultured LSC transplantation if long‐term follow‐up of patients confirms the good short‐term results.
Conclusion
Autologous cultured LSC transplantation using human amniotic membrane with no feeders was associated with high long‐term survival of the transplanted epithelium, dramatic improvement in visual acuity, and very low frequency of adverse events. Autologous limbal tissue transplantation resulted in similar efficiency but poorer safety. Allogeneic cultured LSC transplantation or limbal tissue transplantation from cadaver donor resulted in low long‐term success rates and high prevalence of serious adverse events.
Author Contributions
V.M.B.: conception and design, financial support, administrative support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.G.: conception and design, manuscript writing, final approval of manuscript; C.G., H.R.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.B.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; C.d.S.: collection and/or assembly of data, final approval of manuscript; A.L.: conception and design, administrative support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
V.M.B. declared consultant/advisory role with Chiesi (Parma, Italy; consulting), Dompe (Milan, Italy; consulting), and Optovue (Fremont, CA; consulting). The other authors indicated no potential conflicts of interest.
Supporting information
Supplemental Figure 1 Morphology of different grades of cultured limbal stem cells with various percentages of epithelial cells and fibroblasts. A sheet of cells covering the whole amniotic membrane at the end of culture with more than 50% polygonal epithelial cells and less than 50% fibroblasts was required to accept the cultured LSC grafts for transplantation. Stars show the limbal explants. The culture in A was discarded whereas the cultures in B‐D were accepted for transplantation.
Appendix S1: Supplementary File
Acknowledgments
External monitoring of the prospective trial: Dr. Tabassom Simon (URCEst, Paris, France). Preparation of the authorization process: Dr. Céline Fuchs. Manuscript preparation: Dr. Kate Grieve. This work was supported by the DGOS, Paris, France (PHRC 2001 AOM 01 086, PHRC 2009 AOM 09 005). The sponsor or funding organization had no role in the design or conduct of this research.
Data Availability Statement
Data available upon request because of privacy/ethical restrictions.
References
- 1. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf 2018;16:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dua HS, Azuara‐Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol 2000;44:415–425. [DOI] [PubMed] [Google Scholar]
- 3. Pellegrini G, Traverso CE, Franzi AT et al. Long‐term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990–993. [DOI] [PubMed] [Google Scholar]
- 4. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 2000;343:86–93. [DOI] [PubMed] [Google Scholar]
- 5. Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc 1999;97:891–986. [PMC free article] [PubMed] [Google Scholar]
- 6. Ghoubay‐Benallaoua D, Basli E, Goldschmidt P et al. Human epithelial cell cultures from superficial limbal explants. Mol Vis 2011;17:341–354. [PMC free article] [PubMed] [Google Scholar]
- 7. Ghoubay‐Benallaoua D, Sandali O, Goldschmidt P et al. Kinetics of expansion of human limbal epithelial progenitors in primary culture of explants with no feeders. PLoS One 2013;8:e81965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng SX, Borderie V, Chan CC et al. Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea 2019;38:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989;96:709–722. [DOI] [PubMed] [Google Scholar]
- 10. Moldovan SM, Borderie V, Baudrimont M et al. Treatment of unilateral limbal stem cell deficiency syndrome by limbal autograft. J Fr Ophtalmol 1999;22:302–309. [PubMed] [Google Scholar]
- 11. Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology 1996;103:29–36. [DOI] [PubMed] [Google Scholar]
- 12. Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc 1996;94:677–743. [PMC free article] [PubMed] [Google Scholar]
- 13. Sangwan VS, Basu S, Vemuganti GK et al. Clinical outcomes of xeno‐free autologous cultivated limbal epithelial transplantation: A 10‐year study. Br J Ophthalmol 2011;95:1525–1529. [DOI] [PubMed] [Google Scholar]
- 14. Rama P, Matuska S, Paganoni G et al. Limbal stem‐cell therapy and long‐term corneal regeneration. N Engl J Med 2010;363:147–155. [DOI] [PubMed] [Google Scholar]
- 15. Sejpal K, Ali MH, Maddileti S et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol 2013;131:731–736. [DOI] [PubMed] [Google Scholar]
- 16. Dua HS, Azuara‐Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol 2000;84:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellegrini G, Rama P, Matuska S et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen Med 2013;8:553–567. [DOI] [PubMed] [Google Scholar]
- 18. Corradini F, Venturi B, Pellegrini G et al. Methods for characterization/manipulation of human corneal stem cells and their applications in regenerative medicine. Methods Mol Biol 2012;916:357–372. [DOI] [PubMed] [Google Scholar]
- 19. Sandali O, Gaujoux T, Goldschmidt P et al. Infectious keratitis in severe limbal stem cell deficiency. Ocul Immunol Inflamm 2012;20:182–189. [DOI] [PubMed] [Google Scholar]
- 20. Cheung AY, Sarnicola E, Eslani M et al. Infectious keratitis after ocular surface stem cell transplantation. Cornea 2018;37:1395–1399. [DOI] [PubMed] [Google Scholar]
- 21. Koizumi N, Inatomi T, Suzuki T et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001;108:1569–1574. [DOI] [PubMed] [Google Scholar]
- 22. Shimazaki J, Aiba M, Goto E et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology 2002;109:1285–1290. [DOI] [PubMed] [Google Scholar]
- 23. Shortt AJ, Secker GA, Rajan MS et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology 2008;115:1989–1997. [DOI] [PubMed] [Google Scholar]
- 24. Shortt AJ, Bunce C, Levis HJ et al. Three‐year outcomes of cultured limbal epithelial allografts in aniridia and Stevens‐Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Translational Medicine 2014;3:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang T, Wang Y, Zhang H et al. Limbal allografting from living‐related donors to treat partial limbal deficiency secondary to ocular chemical burns. Arch Ophthalmol 2011;129:1267–1273. [DOI] [PubMed] [Google Scholar]
- 26. Basu S, Fernandez MM, Das S et al. Clinical outcomes of xeno‐free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol 2012;96:1504–1509. [DOI] [PubMed] [Google Scholar]
- 27. Miri A, Al‐Deiri B, Dua HS. Long‐term outcomes of autolimbal and allolimbal transplants. Ophthalmology 2010;117:1207–1213. [DOI] [PubMed] [Google Scholar]
- 28. Holland EJ, Mogilishetty G, Skeens HM et al. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10‐year experience. Cornea 2012;31:655–661. [DOI] [PubMed] [Google Scholar]
- 29. Qi X, Xie L, Cheng J et al. Characteristics of immune rejection after allogeneic cultivated limbal epithelial transplantation. Ophthalmology 2013;120:931–936. [DOI] [PubMed] [Google Scholar]
- 30. Ang AY, Chan CC, Biber JM et al. Ocular surface stem cell transplantation rejection: Incidence, characteristics, and outcomes. Cornea 2013;32:229–236. [DOI] [PubMed] [Google Scholar]
- 31. Banayan N, Georgeon C, Grieve K et al. Spectral domain optical coherence tomography in limbal stem cell deficiency. A case control study. Am J Ophthalmol 2018;190:179–190. [DOI] [PubMed] [Google Scholar]
- 32. Sangwan VS, Basu S, MacNeil S et al. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol 2012;96:931–934. [DOI] [PubMed] [Google Scholar]
- 33. Basu S, Sureka SP, Shanbhag SS et al. Simple limbal epithelial transplantation: Long‐term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology 2016;123:1000–1010. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura T, Inatomi T, Sotozono C et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol 2004;88:1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishida K, Yamato M, Hayashida Y et al. Corneal reconstruction with tissue‐engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 2004;351:1187–1196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Morphology of different grades of cultured limbal stem cells with various percentages of epithelial cells and fibroblasts. A sheet of cells covering the whole amniotic membrane at the end of culture with more than 50% polygonal epithelial cells and less than 50% fibroblasts was required to accept the cultured LSC grafts for transplantation. Stars show the limbal explants. The culture in A was discarded whereas the cultures in B‐D were accepted for transplantation.
Appendix S1: Supplementary File
Data Availability Statement
Data available upon request because of privacy/ethical restrictions.


