Abstract
Procaine directly triggers pH-dependent cytokinesis in equine oocytes and induces hypermotility in stallion spermatozoa, an important event during capacitation. However, procaine-induced hyperactivated motility is abolished when sperm is washed to remove the procaine prior to sperm-oocyte co-incubation. To understand how procaine exerts its effects, the external Ca2+ and Na+ and weak base activity dependency of procaine-induced hyperactivation in stallion spermatozoa was assessed using computer-assisted sperm analysis. Percoll-washed stallion spermatozoa exposed to Ca2+-depleted (+2 mM EGTA) procaine-supplemented capacitating medium (CM) still demonstrated hyperactivated motility, whereas CM without NaCl or Na+ did not. Both procaine and NH4Cl, another weak base, were shown to trigger a cytoplasmic pH increase (BCECF-acetoxymethyl (AM)), which is primarily induced by a pH rise in acidic cell organelles (Lysosensor green dnd-189), accompanied by hypermotility in stallion sperm. As for procaine, 25 mM NH4Cl also induced oocyte cytokinesis. Interestingly, hyperactivated motility was reliably induced by 2.5–10 mM procaine, whereas a significant cytoplasmic cAMP increase and tail-associated protein tyrosine phosphorylation were only observed at 10 mM. Moreover, 25 mM NH4Cl did not support the latter capacitation characteristics. Additionally, cAMP levels were more than 10× higher in boar than stallion sperm incubated under similar capacitating conditions. Finally, stallion sperm preincubated with 10 mM procaine did not fertilize equine oocytes. In conclusion, 10 mM procaine causes a cytoplasmic and acidic sperm cell organelle pH rise that simultaneously induces hyperactivated motility, increased levels of cAMP and tail-associated protein tyrosine phosphorylation in stallion spermatozoa. However, procaine-induced hypermotility is independent of the cAMP/protein tyrosine phosphorylation pathway.
Keywords: procaine, hyperactivated motility, cytokinesis, pH, cAMP, equids
Summary sentence: Procaine induces cAMP dependent tail-associated protein tyrosine phosphorylation in stallion spermatozoa at 10 mM as a result of its weak base activity, whereas hypermotility is induced over a much broader concentration range namely, 2.5–10 mM.
Introduction
Only two foals have ever been born as a result of conventional in vitro fertilization (IVF) with equine gametes [1, 2]. Subsequent attempts to establish a repeatable protocol for conventional equine IVF have not been successful [3–5], and it has been suggested that the primary deficit is the inability to adequately induce capacitation of stallion spermatozoa in vitro (reviewed by Leemans et al. [6, 7]). More specifically, the absence of hyperactivated sperm motility under standard in vitro capacitation conditions has been proposed to explain why IVF fails in the horse [8]. However, while adding either an alkaline follicular fluid fraction [9] or procaine to capacitation medium [8, 10, 11] has been shown to reliably induce hyperactivated motility in stallion spermatozoa, neither was able to support fertilization.
Initially, procaine appeared to have a promising effect on equine IVF, with two independent groups reporting high fertilization/cleavage results after co-incubating equine oocytes and spermatozoa in the presence of procaine. The sperm was first primed by incubating in capacitating medium (CM) exposed to air, to induce tail-associated protein tyrosine phosphorylation, followed by addition of procaine to induce hyperactivated motility [8, 12]. In 2015, we demonstrated that, while procaine does reliably induce hyperactivated motility of stallion spermatozoa, it also has direct detrimental effects on equine oocytes. In particular, we observed procaine-induced, sperm-independent oocyte cytokinesis, with the resulting cleaved oocytes failing to develop beyond the 8–16 cell stage; moreover, the “daughter cells” either lacked nuclei or contained fragmented and condensed chromatin bodies rather than normal nuclei [10].
Despite the adverse effects on equine oocytes, the ability of procaine to induce hyperactivated motility of equine sperm remains an interesting phenomenon, and the underlying mechanisms are the topic of the present study. In various mammalian species, hyperactivated motility (reviewed by Suarez [13]; and Lishko et al. [14]) and oocyte activation (reviewed by Ferrer-Buitrago et al. [15]) require a rise in intracellular Ca2+ to trigger downstream capacitation/fertilization events. In this respect, procaine has been reported to induce hyperactivated sperm motility in the horse independent of extracellular Ca2+ at medium pH 7.25 [11], but does not trigger a rise in cytoplasmic Ca2+ in equine oocytes [10]. On the contrary, in pig [16, 17] and cattle [18, 19] oocytes, procaine is able to inhibit the activation associated with cytoplasmic Ca2+ rise.
On the other hand, procaine is best known as a local anesthetic that induces a neuromuscular block, primarily via actions on the voltage-gated Na+ channels [20]. In this respect, Takei and Fujinoki [21] reported that extracellular Na+ suppresses hyperactivated motility in hamster spermatozoa by reducing intracellular Ca2+. Conversely, reducing extracellular Na+ in the incubation medium supported hyperactivated motility in a tyrosine phosphorylation-independent manner. Similarly in stallion sperm, 2.5–5 mM procaine induces hyperactivated motility in a protein tyrosine phosphorylation-independent way [10].
Procaine is also known to act as a weak base that, at 2.5–5 mM, stimulates a significant elevation in cytoplasmic pH in equine sperm [11] and oocytes [10]. Increased medium and cytoplasmic pH have also been reported to induce tail-associated protein tyrosine phosphorylation [9, 22–24], and an association between elevated cytoplasmic pH and hyperactivated motility [9–11] in stallion spermatozoa has been reported. In this respect, a pH-gated influx of Ca2+ through CatSper channels is an obligatory step in the induction of hyperactivated motility in several mammalian species [25]. Moreover, weak bases are able to partition into acidic cell organelles, alkalinizing them and releasing Ca2+ from them [26, 27]. Recently, Chávez et al. [28] reported a similar action of weak bases resulting in the acrosome reaction in mouse and human sperm. In contrast, Loux et al. [11] suggested that the relationship between hyperactivated motility and Ca2+ influx is likely to be weak in stallion sperm cells because analysis of the equine CatSper1 protein revealed species-specific differences in the structure of the pH sensor region.
Since the inclusion of procaine in equine IVF media directly stimulated sperm hyperactivated motility, but also caused abnormal cleavage of nonfertilized equine oocytes [10], the first aim of this study was to determine whether stallion sperm retained the procaine-induced hyperactivated motility after a washing step, to avoid subsequent direct exposure of equine oocytes to the toxic effects of procaine. Next, the effect of extracellular Ca2+ on procaine-induced hyperactivated motility at pH 7.4 and 7.9 was examined. Dependency of hyperactivated sperm motility and oocyte cytokinesis on extracellular Na+ and cytoplasmic pH elevation was then tested. Finally, procaine concentration-dependent effects on cytoplasmic pH, cell organelle pH, hyperactivated motility, cytoplasmic cAMP concentration and tail-associated protein tyrosine phosphorylation were investigated, to identify if and how procaine affected equine sperm capacitation. Finally, we tested the fertilizing capability of stallion sperm after procaine preincubation.
Materials and methods
Chemicals and reagents
Alexa Fluor 488-conjugated goat anti-mouse antibody, Hoechst 33342 and 2',7'-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein-acetoxymethyl (BCECF-acetoxymethyl) (AM) ester were obtained from Molecular Probes (Ghent, Belgium). Monoclonal 4G10 Platinum anti-phosphotyrosine mouse antibodies were purchased from Millipore (Overijse, Belgium). Fatty acid-free bovine serum albumin (BSA) (A9418; cell culture tested), Triton X-100, and all other chemicals not otherwise listed were obtained from Sigma-Aldrich (Bornem, Belgium).
Semen collection and preparation
Stallion semen was collected from three adult stallions of proven fertility using a Colorado model artificial vagina (Animal Reproduction Systems; Chino, CA, USA) at the equine reproduction clinics at Ghent and Utrecht Universities. The raw ejaculate was filtered through gauze to remove the gel fraction and any debris, before visual evaluation of sperm motility by light microscopy (200×) on a heated stage at 37 °C; if the motility was acceptable (>65% motile), the semen was immediately transported to the laboratory for further processing. One milliliter of fresh semen with a concentration of 100–300 × 106 spermatozoa/ml was separated through a 45/90% Percoll gradient [29, 30]. Next, the sperm pellet was washed once with noncapacitating medium (NCM) (100 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 5.5 mM glucose, 22 mM 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES), 2.4 mM sodium lactate and 1.0 mM pyruvic acid; pH = 7.4 and 280–300 mOsm; [31]). Per experiment three replicates were performed using one ejaculate from three different stallions (n = 3).
Ejaculates from three boars with proven fertility were collected at AIM Varkens KI Nederland (Vaassen, The Netherlands; a commercial enterprise producing semen doses for commercial pig artificial insemination). Freshly ejaculated sperm was filtered through gauze to remove the gelatinous material and then diluted, washed, and stored in Beltsville Thawing Solution, as described previously [32]. All buffers and other solutions used were iso-osmotic (285–300 mOsm) and kept at room temperature unless stated otherwise. Upon arrival in the laboratory, boar semen was processed in a similar way to stallion semen, with the exception that 1 ml semen was separated through a different density gradient, i.e., 35/70% Percoll [33].
Animal experiments
The study was approved by the Ethics Committee of the Faculty of Veterinary Medicine of Ghent University (EC2013/175 and EC2013/176). The Institutional Animal care and Use Committee of Utrecht University approved with this study.
Sperm capacitation/hyperactivation
In order to provide conditions supportive of sperm capacitation, NCM was modified by replacing sodium lactate with 2.4 mM calcium lactate and adding 25 mM NaHCO3 and 7 mg/ml BSA (pH = 7.4; 280–300 mOsm/kg; osmolality was adjusted by graduated reduction of the NaCl to compensate for the added 25 mM NaHCO3); this medium was pre-equilibrated for at least 2 h in a humidified atmosphere containing 5% CO2 at 38.5 °C and is further referred to as CM (adapted from McPartlin et al. [31]). The washed sperm pellet was diluted to a concentration of 10 × 106 spermatozoa/ml with CM. Hyperactivated motility was induced by resuspending the spermatozoa in (1) CM supplemented with 2.5 mM procaine hydrochloride (Sigma-Aldrich, Bornem, Belgium) at a final concentration of 1 × 106 spermatozoa/ml [8]. To prepare concentrations of up to 5 mM procaine, a stock of 50 mM procaine hydrochloride dissolved in CM was pre-equilibrated for at least 2 h in a humidified atmosphere containing 5% CO2 at 38.5 °C (to restore the pH to the physiological range; 7.2–7.4). Subsequently, 2.5 and 5 mM procaine-containing CM were prepared by diluting the stock solution with the required amount of equilibrated CM. The pH of all incubation media was adjusted to 7.2–7.4. Procaine concentrations higher than 5 mM (10, 25 or 50 mM) were made without stock preparation. To obtain medium osmolality between 280–300 mOsm/kg in these high procaine concentration capacitating media, NaCl concentration was reduced accordingly. The latter media were subsequently pre-equilibrated overnight in a humidified atmosphere containing 5% CO2 at 38.5 °C to restore the pH to 7.2–7.4.
To investigate the effect of the Ca2+ in CM on hyperactived motility, calcium lactate was replaced by sodium lactate, and 2 mM of the Ca2+ chelator ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) was added.
CM without NaCl was prepared by replacing 100 mM NaCl with 200 mM D-mannitol while Na+-free CM was made by replacing the 100 mM NaCl with 200 mM D-mannitol and 25 mM NaHCO3 with KHCO3. CM plus NH4Cl was prepared by replacing the 25 mM NaCl with 25 mM NH4Cl. CM at pH 7.9 was prepared by increasing NaHCO3 to 100 mM and reducing NaCl accordingly, to maintain the osmolality between 280–300 mOsm/kg. All media were pre-equilibrated for at least 2 h in a humidified atmosphere containing 5% CO2 at 38.5 °C before the start of incubation.
Oocyte maturation
Ovaries were collected from slaughtered mares (Euro Meat Group, Moeskroen, Belgium). Within 4 h after slaughter, all follicles larger than 5 mm were aspirated using a 16 gauge needle attached to a vacuum pump (~100 mm Hg), scraped with the bevel of the aspirating needle, and flushed with Dulbecco’s phosphate buffered saline (DPBS) containing 25 IU/ml heparin. A maximum of 30 cumulus-oocyte complexes (COCs) were transferred to 500 μl Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 (DMEM-F12)-based maturation medium [34] and placed in an incubator at 38.2 °C in a humidified atmosphere of 5% CO2-in-air for 28 h. After maturation, COCs were partially or completely denuded by gentle pipetting in 0.05% bovine hyaluronidase diluted in HEPES buffered DMEM-F12 medium. Degenerated oocytes were excluded from subsequent experiments, whereas all nondegenerated oocytes were used for gamete co-incubation with the assumption that an extruded polar body was present (this could not be confirmed in partially cumulus-enclosed oocytes).
In vitro fertilization/gamete co-incubation
Equine gamete co-incubation was performed with some minor modifications in the various capacitating media described above [31]. As previously indicated, sperm was suspended at 1 × 106 spermatozoa/ml in each specific CM (medium pH = 7.2–7.4; previously adjusted by incubation in an atmosphere containing 5% CO2). About 100 μl droplets of these sperm suspensions were pipetted into Petri dishes and covered with 5% CO2-equilibrated mineral oil. Five partially denuded mature oocytes were then transferred to each medium droplet, and the Petri dishes were incubated at 38.2 °C in 5% CO2 in humidified air. After 18 h of co-incubation, partially denuded oocytes were fully denuded by gentle pipetting in 0.05% bovine hyaluronidase in HEPES buffered DMEM/F12. Subsequently, oocytes were checked for sperm penetration or cultured for an additional 6 h to assess oocyte nuclear configuration and second polar body formation or for 2.5 days in groups of five oocytes per 5 μl droplet of DMEM-F12 with 10% fetal calf serum, at 38.5 °C in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2. After IVF, oocytes were fixed at different developmental stages (zygote, 2-cell, 4–8 cell, 8–16 cell) to assess nuclear configuration, second polar body formation, and developmental stage. To control for direct effects of procaine, oocytes were incubated in similar media in the absence of sperm.
Deoxyribonucleic acid configuration and embryonic development
Oocytes and presumptive zygotes or early embryos at various developmental stages were fixed in 4% paraformaldehyde in DPBS at room temperature for 1 h. The fixative was then removed by washing the oocytes twice in wash medium (DPBS containing 0.5% BSA). Next, the fixed oocytes were incubated in a 3.2 μM Hoechst 33342 solution in wash medium for 10 min at room temperature. The oocytes were then washed four times in wash medium and mounted on siliconized glass slides (Marienfeld, Germany) using 1,4-diazabicyclo[2,2,2]octane as antifade and sealed with nail polish. Excessive pressure from the cover slip was prevented by placing a few droplets of Vaseline on the microscope slides prior to mounting. Starting from the time of incubation in Hoechst 33342, oocytes were shielded from the light to prevent fading. Mounted slides were kept at 4 °C in the dark until evaluation. Oocyte degeneration was indicated by irregular morphology of the oocyte. The Hoechst dye stained the deoxyribonucleic acid (DNA) of both the oocyte and the spermatozoa, and can thus be used for detecting possible fertilization, i.e., whether both the female and male pronuclei (PN) and the second polar body (containing condensed DNA) are all present. After 2.5 d in culture, the ability of equine oocytes/zygotes to undergo nuclear duplication and cell cleavage was assessed, with condensed DNA fragments indicative of nuclear degradation visualized using the Hoechst dye.
Sperm motility assessment
To assess the effect on sperm motility of the different capacitating strategies based respectively on procaine-pre-incubation, extracellular Ca2+, Na+ influx inhibition, or weak base activity to increase cytoplasmic pH, motility parameters for sperm in suspension were assessed using a computer-assisted sperm analyzer (CASA: Hamilton-Thorne motility analyzer Ceros version 12.3d; Hamilton-Thorne Research, Beverly, MA, USA). Under defined capacitating conditions, BSA was replaced with 0.02% polyvinyl alcohol to avoid the marked sperm agglutination noted for stallion sperm after centrifugation in BSA-containing medium [11]. For each analysis, 10 μl of sperm solution diluted in different CM was mounted on a prewarmed glass slide (Marienfeld, Lauda-Königshofen, Germany) and maintained at 37 °C using a Tokai Hit thermoplate. Five randomly selected microscopic fields in the center of the slide were scanned four times each, generating 20 scans for each sample. The mean of these five scans per sample was used for statistical analysis. The settings of the CASA-software HTR 12.3 for analyzing motility parameters of stallion sperm were based on those described by Loomis and Graham [35] and Hoogewijs et al. [36]. To evaluate the effect of Na+ omission, or of weak base activity to raise the cytoplasmic pH, the percentages of motile and progressively motile sperm were assessed. To assess the effect of chelating extracellular Ca2+, omitting Na+, or weak base activity on sperm hypermotility, two parameters were used, namely, amplitude of lateral head displacement (ALH, in μm; i.e., the mean width of head oscillations) and curvilinear velocity (VCL, in μm/s; i.e., the average velocity of a sperm along its actual, two-dimensional curvilinear trajectory). An increase in ALH and VCL was taken to indicate induction of hyperactivated motility [8, 10, 11].
Motility patterns of stallion sperm incubated in CM + 2.5 mM procaine, Ca2+-depleted CM + 2.5 mM procaine and CM + 25 mM NH4Cl were imaged using a Moticam 10+ camera (Motic Deutschland GmbH, Wetzlar, Germany) attached to an Olympus BX40 microscope (Olympus Nederland B.V., Leiderdorp, The Netherlands) and equipped with a phase contract objective 20× (Olympus Nederland B.V., Leiderdorp, The Netherlands). Images were acquired using the Motic Images Plus 3.0 ML program (Motic Deutschland GmbH, Wetzlar, Germany).
Cytoplasmic and cell organelle pH measurements
Percoll-separated sperm was washed twice using NCM and stained with 5 μM of the cytoplasmic pH-sensitive dye BCECF-AM [10, 11] or with 5 μM of the cell organelle pH-sensitive marker Lysosensor green DND-189 [28] in NCM, by incubation at 38.5 °C for 30 min. The extracellular dye was then removed by washing the sperm twice in NCM (400 g, 5 min). The washed BCECF-AM loaded sperm (30 × 106 sperm/ml) was added to NCM, CM, CM without NaCl, CM without Na+, CM plus 25 mM NH4Cl, CM at pH 7.9, and CM plus 2.5, 5, 10, 25, or 50 mM procaine. Subsequently, the BCECF signal was measured in 200 μl aliquots of sperm suspension loaded into a 96-well microplate reader (CLARIOstar, BMG LABTECH, Ortenberg, Germany) and excited at 440 and 490 nm. The cytoplasmic pH was proportional to the ratio of fluorescence at 440/490 (expressed in AU). Baseline fluorescence was recorded for each well and set to ratio = 0. A similar approach was used to measure the fluorescence intensity of Lysosensor green DND-189-loaded sperm (30 × 106 sperm/ml) incubated in NCM, CM, CM plus 25 mM NH4Cl, CM at pH 7.9, and CM plus 2.5 or 10 mM procaine. Sperm samples were excited at 443 nm. Readings were taken at 0, 10, 30, and 60 min after the treatments have been added, since a rapid procaine-induced weak base activity in stallion sperm has been reported previously [11].
Cytoplasmic cAMP measurements
Since a rise in cAMP is considered to be an early capacitation response [37], stallion sperm (5 × 106 sperm/ml) was incubated for 15 min and 1 h in NCM, CM, CM without NaCl, CM without Na+, CM plus 25 mM NH4Cl, CM at pH 7.9, CM plus 1 mM caffeine, and CM plus 2.5, 5, 10, 25, or 50 mM procaine. Boar sperm samples incubated in NCM, CM, and CM plus 1 mM caffeine media for 15 min and 1 h were included for comparison. After 15 min and 1 h incubations, sperm samples were centrifuged for 2 min at 1200×g. The sperm pellet was resuspended in 200 μl 0.1 mM HCl, vortexed twice for 2 s and incubated for 20 min at room temperature. Subsequently, sperm samples were centrifuged at 5000×g for 5 min, and all supernatant samples were stored at −80 °C until further analysis [37]. Using a direct cAMP Enzyme Immunoassay Kit (Sigma-Aldrich, Zwijndrecht, The Netherlands), cAMP levels in acetylated sperm supernatant samples were measured by following the manufacturer’s instructions. A standard curve was run for each assay (Figure 10A), and the unknown cAMP concentrations were obtained by logistic curve fitting (as recommended by the manufacturer).
Figure 10.
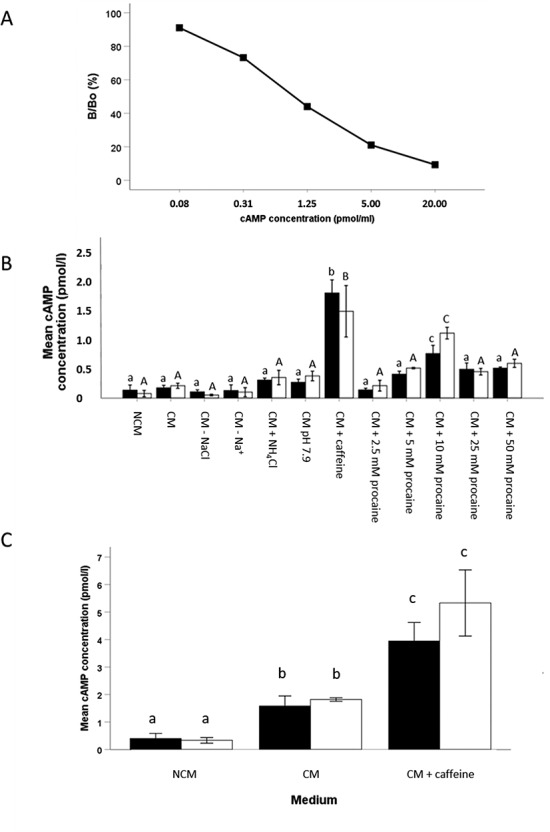
A standard curve was generated for the detection of cAMP levels in stallion sperm samples with a good linearity of detection in the range of 0.08—20 pmol/ml. (B) Mean intracellular cAMP levels in stallion spermatozoa incubated in NCM, CM, CM—NaCl, CM—Na+, CM + 25 mM NH4Cl, CM at pH 7.9, CM + 1 mM caffeine, CM + 2.5–5–10–25–50 mM procaine, for 15 min (black bars) and 1 h (white bars). A procaine concentration-dependent effect on cAMP levels was observed. The highest cAMP level was measured in stallion spermatozoa exposed to caffeine. (C) Boar sperm suspensions incubated in NCM, CM, and CM + 1 mM caffeine. In general, cAMP levels were much higher in boar than stallion sperm after incubation in CM and CM + 1 mM caffeine at 15 min and 1 h. Data represent mean (±SD) cAMP concentration of a suspension of 5 × 106 spermatozoa/ml over three replicates using three different stallions. Within time-point 15 min, values that differ significantly (P < 0.05) are indicated by different small letters. Within time-point 1 h, values that differ (P < 0.05) are indicated by different capitals.
Quantification of tail-associated protein tyrosine phosphorylation
Assessment of protein tyrosine phosphorylation, which is considered a hallmark of late stage capacitation, was performed as described by Leemans et al. [24]. Briefly, spermatozoa were incubated for 4 h in NCM, CM, CM without NaCl, CM without Na+, CM plus 25 mM NH4Cl, CM at pH 7.9, CM plus 1 mM caffeine, and CM plus 2.5, 5, 10, 25, or 50 mM procaine media under atmospheric conditions (5% CO2 or air). The incubated sperm suspensions were subsequently washed twice and fixed in 4% paraformaldehyde in DPBS at room temperature for 15 min. The fixative was removed by three centrifugation steps using DPBS (600 g for 5 min). The washed spermatozoa were subsequently incubated in 0.1% Triton X-100 in DPBS for 10 min at room temperature to ensure complete permeabilization of the membranes. The permeabilized spermatozoa were then incubated in blocking buffer (DPBS containing 1% BSA) for 10 min at room temperature. Next, the spermatozoa were incubated overnight at 4 °C in buffer containing 0.1% BSA and the mouse monoclonal 4G10 Platinum IgG2b protein anti-phosphotyrosine antibody (diluted 1:500). After incubation, unbound antibody was removed by washing the spermatozoa twice with 1 mL DPBS containing 0.1% BSA (600 g for 5 min). The spermatozoa were then stained with a monoclonal goat anti-mouse antibody conjugated to Alexa Fluor 488 (Invitrogen, Molecular Probes, Ghent, Belgium) for 1 h at room temperature. After immunolabeling, the nonbound antibody conjugates were removed by washing three times with DPBS containing 0.1% BSA, and once using DPBS (600 g for 5 min). The immunolabeled spermatozoa were mounted on glass slides under a cover slip and sealed with nail polish. The proportion of spermatozoa with green fluorescent tails among the total sperm population (with Hoechst 33342 fluorescent heads) was determined by randomly scoring 200 spermatozoa. Samples were examined using a Leica DMR microscope equipped with a mercury lamp and appropriate filters, at a magnification of 400×.
Microscopic imaging
Oocyte cleavage, possible second polar body formation, and DNA configuration were examined using an inverted Nikon A1R confocal microscope (Nikon Instruments, Paris, France), mounted on a Nikon Ti body, and with a 60×/1.4 Plan Apo oil immersion lens. Hoechst was excited using a 405 nm diode laser, and a 488 nm Argon laser was used for simultaneous DIC imaging. Images were acquired using Nikon Elements Software and a pinhole setting of 1 Airy unit (AU) and constant acquisition settings (laser power, gain and offset, and scan speed). Digital optical sections were collected across an axial range that spanned the oocyte, at a step size of 1 μm.
Sperm stained for tail-associated protein tyrosine phosphorylation and a DNA counter stain were detected by means of fluorescence microscopy (400×) using a Leica DMR microscope equipped with excitation filters of 360–590 nm and a 100 W mercury lamp. Alexa Fluor 488-conjugated goat anti-mouse antibody and Hoechst 33342 were excited using 495 nm and 343 nm lasers, respectively. Emission spectra of the dyes were then filtered at 519 nm and 483 nm. These emission spectra were detected by blue (LP 425 nm) and green (LP 515 nm) filters corresponding to the emission peaks of the dyes. Images were acquired using the Image Database program (Leica, Van Hopplynus N.V., Brussel, Belgium).
Statistical analysis
All experiments were performed at least three times, and sperm cells for a given comparison were derived from independent ejaculates from each of the three Warmblood stallions. Before analysis, normality of the variables was checked using the Shapiro-Wilk and Kolmogorov-Smirnov tests (P < 0.05). The effect of different capacitating conditions, i.e., procaine-preincubation, extracellular Ca2+ removal, Na+ influx inhibition, and weak base activity, on sperm motility parameters, cytoplasmic pH, cell organelle pH, cAMP, and tail-associated protein tyrosine phosphorylation was assessed by analysis of variance (ANOVA). Overall differences were identified using repeated measures ANOVA with Greenhouse-Geisser and Bonferroni correction, as implemented in the general linear model. Scheffé post-hoc tests were performed for pairwise comparisons. Differences were considered significant if P < 0.05.
The effect of different conditions on embryo development and DNA configuration was analyzed by binary logistic regression for binomially distributed data. Where differences existed, further comparisons between groups were performed by chi-square analysis (χ2 fit tests). All experiments were repeated three times. Differences were considered significant if P < 0.05. All analyses were performed using SPSS version 20 for Windows (SPSS IBM, Brussels, Belgium).
Results
Procaine-induced hyperactivated motility is lost when procaine is washed out
After 30 min, sperm in CM supplemented with procaine demonstrated significantly higher ALH and VCL (ALH: 7.4 ± 0.1 μm and VCL: 173 ± 3 μm/s) than sperm incubated in standard CM (ALH: 3.7 ± 0.3 μm and VCL: 69 ± 1 μm/s) (P < 0.01). However, 30 min after removing procaine by washing, the ALH and VCL values of stallion sperm previously exposed to procaine had returned to pre-exposure levels (ALH: 3.4 ± 0.3 μm and VCL: 72 ± 2 μm/s). Similar observations were made at 2, 4, and 6 h which leads us to conclude that stallion spermatozoa only display hyperactivated motility when directly exposed to procaine (Figure 1). After 2 h incubation, ALH and VCL values decreased in all conditions (Figure 1). These data suggest that the removal of procaine before introducing the sperm to the fertilization medium to avoid the detrimental effects of procaine on equine oocytes during gamete co-incubation did not support hyperactivated sperm motility.
Figure 1.
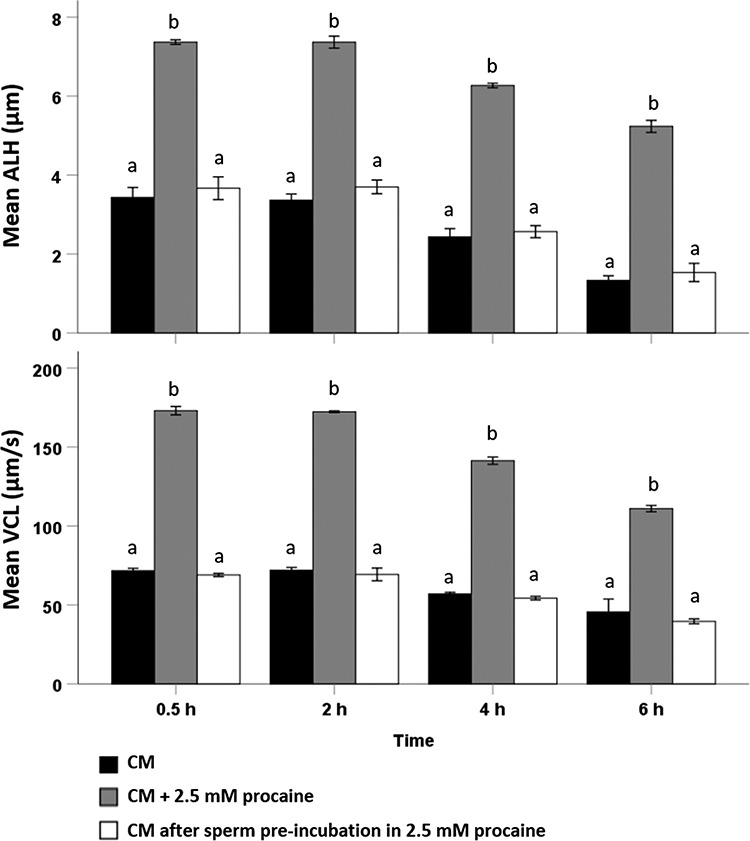
Motility patterns indicative of hyperactivated motility were assessed by CASA in stallion sperm suspensions incubated for 0.5, 2, 4, and 6 h in CM (black bars), CM + 2.5 mM procaine (grey bars), and CM after sperm being washed following pre-incubation in CM +2.5 mM procaine (white bars) (pH = 7.4) (n = 3 samples in each group; three replicates). Parameters of interest included (A) ALH and (B) VCL. Hyperactivated motility in sperm suspensions was triggered by direct exposure to 2.5 mM procaine, whereas washing to remove the procaine resulted in a complete loss of hyperactivated motility. For both ALH and VCL, values that differ significantly (P < 0.05) are indicated by different small letters.
Procaine-induced hyperactivated motility is independent of extracellular Ca2+
At 0.5 h, there were no significant differences in ALH and VCL between Ca2+-depleted (ALH: 7.5 ± 0.3 μm and VCL: 167 ± 6 μm/s) and Ca2+-containing (ALH: 7.5 ± 0.4 μm and VCL: 167 ± 8 μm/s) procaine-supplemented CM, at pH = 7.4 (Figure 2). Similar observations were made at 2, 4, and 6 h incubation time points indicating that procaine-induced hyperactivated motility is independent of an extracellular Ca2+ source (Figure 2). After 2 h incubation, ALH and VCL values decreased in both Ca2+-depleted and Ca2+-containing, procaine-supplemented CM (Figure 2). A similar trend was observed when sperm suspensions were incubated under identical conditions at pH 7.9 (Figure 2). Video clips of stallion spermatozoa showing hyperactivated motility in 2.5 mM procaine CM and Ca2+-depleted 2.5 mM procaine CM + 2 mM EGTA, respectively, are attached as Supplementary Files 1 and 2. These data suggest that hyperactivated motility in stallion sperm is independent of extracellular Ca2+ influx through pH-gated CATSPER channels.
Figure 2.
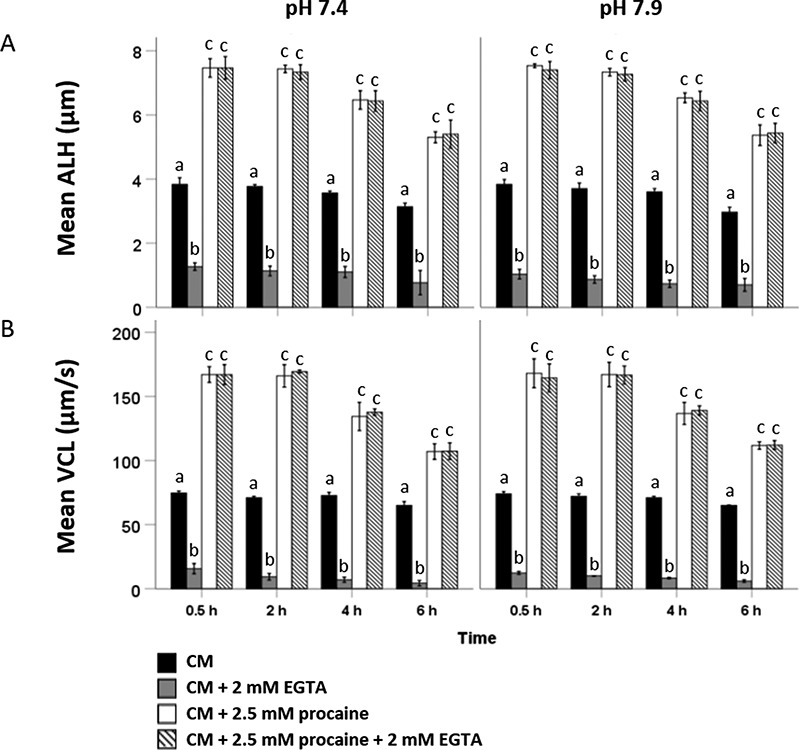
Motility patterns indicative of hyperactivated motility were assessed by CASA in stallion sperm incubated for 0.5, 2, 4, and 6 h in CM (black bars), CM + 2 mM EGTA (grey bars), CM + 2.5 mM procaine (white bars), and CM + 2.5 mM procaine +2 mM EGTA (striped bars) at pH = 7.4 and pH = 7.9 (n = 3 samples in each group; three replicates). Parameters of interest were (A) ALH and (B) VCL. Hyperactivated motility of spermatozoa induced by 2.5 mM procaine is independent of extracellular Ca2+ and pH. For both ALH and VCL, values that differ (P < 0.05) are indicated by different small letters.
Procaine-induced hyperactivated motility is not blocked by inhibiting Na+ influx
Percentages of motile spermatozoa did not differ significantly between four tested media (CM, CM + 2.5 mM procaine, CM lacking NaCl or all sources of Na+) after 5, 15, and 30 min and 1, 2, 4, or 6 h of incubation. After 2 h of incubation, percentages of motile spermatozoa decreased in all conditions tested (Figure 3A).
Figure 3.
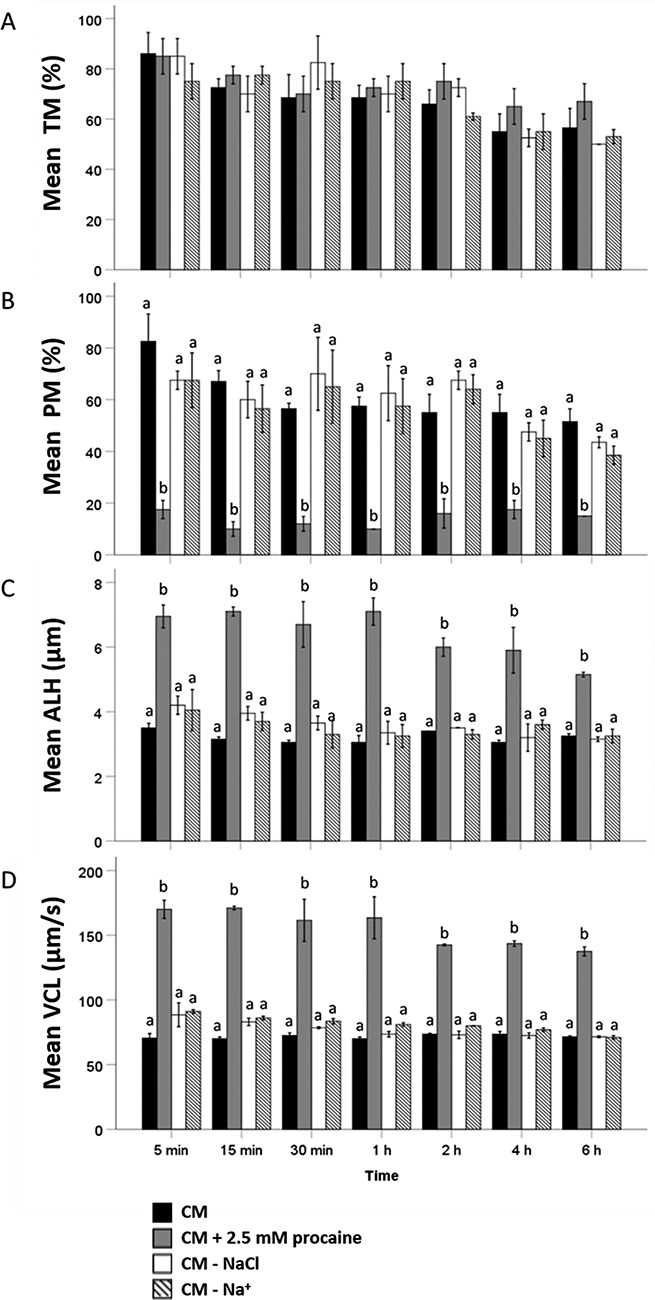
Evaluation of different motility parameters ((A) percentage of total motile or TM; (B) percentage of progressively motile or PM; (C) ALH; and (D) VCL) during 6 h incubation of stallion spermatozoa in (1) CM, (2) CM + 2.5 mM procaine, (3) CM lacking NaCl, and (4) CM lacking Na+ (pH = 7.4) (n = 4 samples in each group; three replicates). Hyperactivated motility in sperm suspensions was triggered by direct exposure to 2.5 mM procaine, whereas the medium with reduced or no Na+ did not have this effect. For percentage of progressively motile, ALH and VCL, values that differ (P < 0.05) are indicated by different small letters.
Significantly lower percentages of progressively motile spermatozoa were observed after 5 min incubation in procaine-supplemented CM (18 ± 4%; P < 0.01) compared to standard CM (83 ± 11%; P < 0.01), CM without NaCl (68 ± 5%; P < 0.01), and CM without Na+ (69 ± 12%; P < 0.01) (Figure 3B); there were no significant differences between the other three media. Similar observations were made at all other incubation time points (Figure 3B). After 2 h incubation, percentages of progressively motile spermatozoa decreased in CM without NaCl and CM without Na+ (Figure 3B).
ALH and VCL values indicative of hyperactivated motility showed the opposite trend. At 5 min incubation, significantly higher ALH and VCL values were observed in CM with 2.5 mM procaine (ALH: 6.9 ± 0.2 μm; VCL: 170 ± 7 μm/s) than in standard CM (ALH: 3.5 ± 0.1 μm; VCL: 71 ± 4 μm/s; P < 0.01; as previously shown [8, 10, 11]), CM without NaCl (ALH: 4.1 ± 0.2 μm; VCL: 89 ± 9 μm/s; P < 0.01), or CM without Na+ (ALH: 3.9 ± 0.4 μm; VCL: 90 ± 2 μm/s; P < 0.01) (Figure 3C and D). No significant differences in ALH and VCL values were observed between the latter three media. A similar pattern was observed at all other incubation time points. After 2 h incubation, ALH and VCL values decreased in CM plus 2.5 mM procaine (Figure 3C and D). These data indicate that procaine-induction of hyperactivated motility is not mediated by inhibition of Na+ influx.
Procaine-induced hyperactivated motility is associated with weak base activity
Percentage of motile spermatozoa (total motility) did not differ significantly between four tested media (CM, CM + 2.5 mM procaine, CM + 25 mM NH4Cl, and CM at pH 7.9) up to 6 h. After 2 h incubation, the proportion of motile sperm had decreased in all four tested conditions (Figure 4A).
Figure 4.
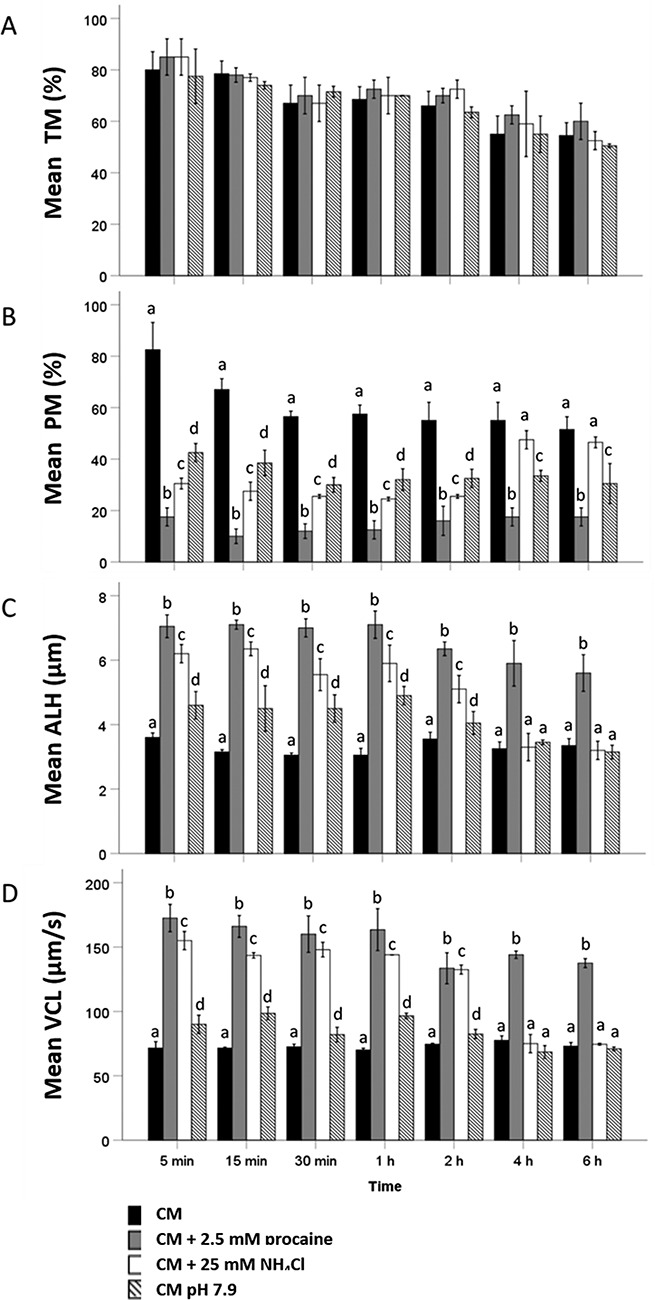
Evaluation of different motility parameters ((A) percentage of total motile or TM; (B) percentage of progressively motile or PM; (C) ALH; and (D) VCL) during 6 h incubation of stallion spermatozoa in (1) CM, (2) CM + 2.5 mM procaine, (3) CM + 25 mM NH4Cl, and (4) CM pH 7.9 (n = 3 samples in each group; three replicates). Hyperactivated motility in sperm suspensions was triggered by exposure to 5 mM procaine and 25 mM NH4Cl and to a lesser extent by CM at pH 7.9. For percentage of progressively motile, ALH and VCL, values that differ (P < 0.05) are indicated by different small letters. Repeated measure ANOVA with Greenhouse-Heisser and Bonferroni correction Scheffé post hoc tests were performed for pairwise comparison.
At 5 min, the percentage of progressively motile spermatozoa differed significantly between the four media tested (Figure 4B). The lowest percentage of progressively motile sperm was observed in the presence of procaine (18 ± 4%) whereas the highest was seen in standard CM (83 ± 11%). In comparison to procaine exposure, slightly but significantly higher percentages of progressively motile spermatozoa were observed for sperm suspensions incubated with NH4Cl (31 ± 2%) while CM at pH 7.9 (43 ± 4%) yielded intermediate percentages of progressive motility (Figure 4B). Similar observations were made at all incubation time points up to 2 h. By 4 h incubation, no difference in percentage of progressively motile spermatozoa was evident between CM (55 ± 7%) and NH4Cl-containing media (48 ± 4%) although a lower incidence of progressive motility was still evident in procaine-supplemented CM (18 ± 4%). CM at pH 7.9 (34 ± 2%) still showed intermediate percentages of progressively motile spermatozoa. Similar measurements were obtained at 6 h (Figure 4B). Parameters indicating hyperactivated motility showed the opposite trend. After 5 min incubation, peak ALH and VCL values were observed in procaine-supplemented medium (ALH: 7.1 ± 0.4 μm; VCL: 173 ± 11 μm/s) and lowest values were observed in CM (ALH: 3.6 ± 0.1 μm; VCL: 72 ± 5 μm/s). Slightly but significantly lower different ALH and VCL values than in the presence of procaine were observed for NH4Cl-supplemented CM (ALH: 6.2 ± 0.3 μm; VCL: 160 ± 22 μm/s), and intermediate values were observed in CM at pH 7.9 (ALH: 4.6 ± 0.4 μm; VCL: 73 ± 11 μm/s) (Figure 4C and D). A similar pattern was observed through the first 2 h of incubation. At 4 h incubation, significantly higher ALH and VCL values were observed in the presence of procaine (ALH: 5.9 ± 0.7 μm; VCL: 144 ± 3 μm/s) than in the three other incubation conditions (CM: ALH: 3.3 ± 0.2 μm, VCL: 75 ± 3 μm/s; NH4Cl supplemented CM: ALH: 3.3 ± 0.4 μm, VCL: 75 ± 7 μm/s; CM at pH 7.9: ALH: 3.5 ± 0.1 μm, VCL: 69 ± 5 μm/s) which did not differ from each other. Similar findings were apparent at 6 h (Figure 4C and D). A video clip of stallion spermatozoa demonstrating hyperactivated motility in 25 mM NH4Cl-supplemented CM is attached as Supplementary File 3.
Given the known effect of NH4Cl on cytoplasmic pH [38] and hyperactivated motility [11, 38], these data suggest that procaine-induced hyperactivated motility is associated with the weak base property of procaine, which presumably elevates the cytoplasmic pH of stallion spermatozoa.
Procaine and NH4Cl both trigger oocyte cleavage
After 2.5 days of culture, we observed a degenerative effect of 2.5 mM procaine, 25 mM NH4Cl, and pH 7.9 on partially cumulus-denuded oocytes (PD). In capacitating conditions, 14 ± 3% PD degenerated; in 2.5 mM procaine capacitating conditions, 27 ± 3% PD degenerated; in NaCl-free conditions, 17 ± 2% PD degenerated; in Na+ free-conditions, 16 ± 2% PD degenerated; in NH4Cl supplemented conditions, 35 ± 2% PD degenerated; and in pH 7.9 conditions, 52 ± 11% PD degenerated. Oocytes exposed to CM at pH 7.9 showed the highest incidence of degeneration, whereas significantly lower incidences were observed in the presence of procaine or NH4Cl. This suggests that the intra- and extracellular pH changes induced by procaine, NH4Cl, and pH 7.9 are toxic to equine oocytes (Figure 5).
Figure 5.
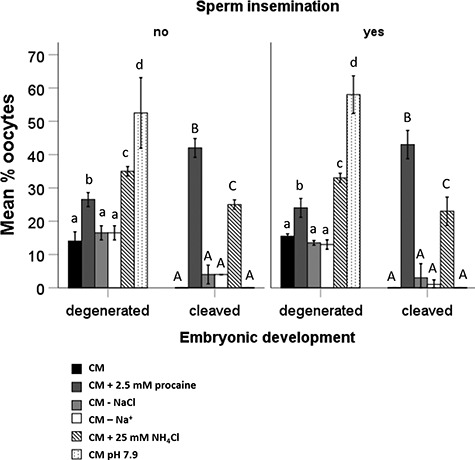
Percentage of PD that either degenerated or cleaved after 2.5 days in culture following incubation with or without spermatozoa in CM, CM + 2.5 mM procaine, CM—NaCl, CM—Na+, CM + 25 mM NH4Cl, and CM at pH 7.9. CM at pH 7.9 had a severe degenerative effect on oocytes. In the presence of 2.5 mM procaine and 25 mM NH4Cl, oocytes cleaved up to the 8-cell stage. Data represent mean (±SD) percentages of oocytes after incubation in CM (black bars), CM + 2.5 mM procaine (dark grey bars), CM—NaCl (light grey bars), CM—Na+ (white bars), CM + 25 mM NH4Cl (hatched bars), and CM pH 7.9 (dotted bars); n = 20 oocytes in each group, three replicates. Values that differ significantly (P < 0.05) are indicated by different small letters for degenerated oocytes or capitals for cleavage.
In agreement with a previous study [10], in the presence of 2.5 mM procaine, 42 ± 3% of oocytes appeared to cleave; similarly, in the presence of NH4Cl, 25 ± 2% of oocytes underwent apparent cleavage. No oocyte cleavage was observed in the medium from which either NaCl or all sources of Na+ were omitted or at pH 7.9. However, none of the oocytes that cleaved in the presence of procaine or NH4Cl developed beyond the 8–16 cell stage and none reached the blastocyst stage. Beyond 5–6 days of incubation, any 8–16 cell stage embryos derived from either treatment began to degenerate (Figures 5 and 6). Moreover, none of these parameters differed significantly between oocytes incubated in the presence or absence of spermatozoa, indicating that procaine and NH4Cl-induced oocyte cytokinesis was sperm-independent (P > 0.12 for all comparisons; Figure 5).
Figure 6.
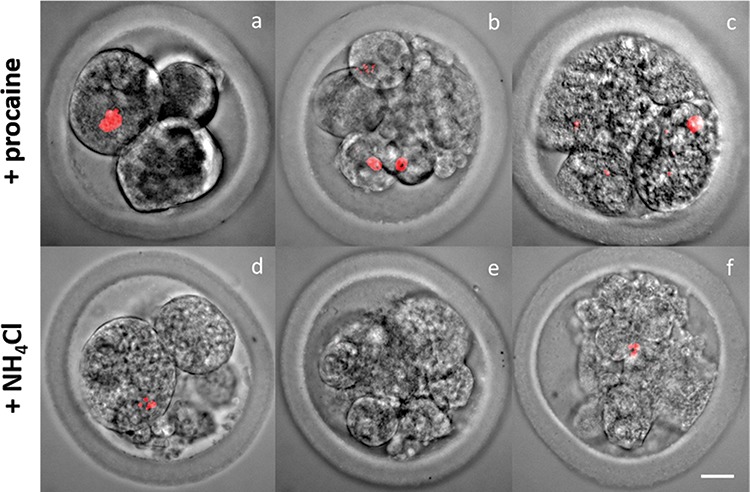
Combined lightmicroscopic and DNA-fluorescence confocal micrographs of embryo developmental stages (DNA configuration in red) (A and D: 2–4 cell; B and E: 8-cell; C and F: 16-cell) after oocytes were incubated for 18 h in CM + 2.5 mM procaine and CM + 25 mM NH4Cl, and subsequently cultured in a DMEM/F12 plus 10% FBS based medium. Oocytes that cleaved in conditions containing 2.5 mM procaine and 25 mM NH4Cl never developed further than the 8–16 cell stage. (original magnification, 400×: Bar = 20 μm).
In short, exposure to procaine or NH4Cl induced sperm-independent cleavage of horse oocytes, whereas inhibition of Na+ influx or exposure to a high pH medium did not.
Procaine and NH4Cl-induced cleavage are accompanied by DNA fragmentation
After 24 h incubation, the majority of the oocytes in CM (83 ± 4%), CM without NaCl (71 ± 4%), and CM without Na+ (78 ± 4%) displayed a normal metaphase spindle, suggesting that inhibiting Na+ influx into equine oocytes does not affect DNA configuration. By contrast, the majority of oocytes exposed to procaine (75 ± 1%) or NH4Cl (53 ± 4%) displayed DNA fragmentation (Figure 6). Similarly, equine oocytes incubated in pH 7.9 CM (89 ± 5%) also exhibited severe DNA degeneration (Figure 7).
Figure 7.
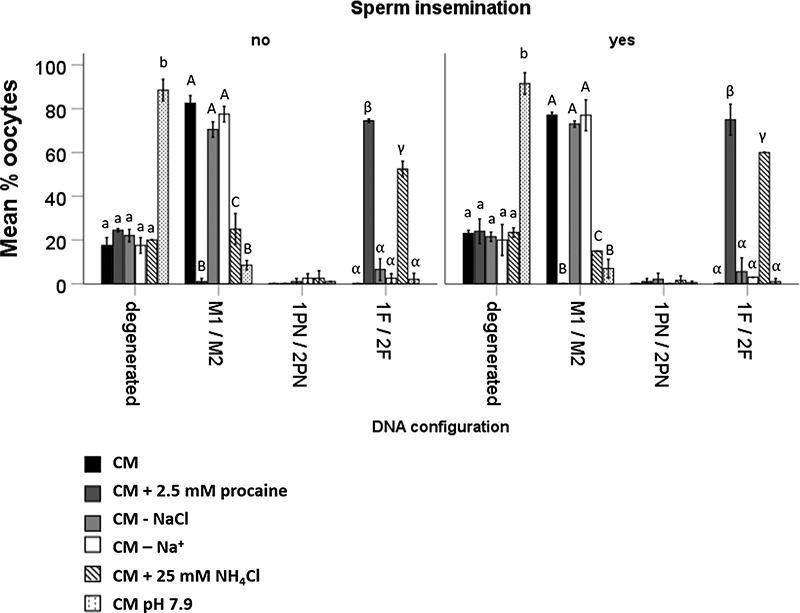
Percentages of PD that showed (1) degeneration, (2) meiosis I stage (MI) or meiosis II stage (MII), (3) 1 PN or 2PN, and (4) 1 DNA fragment (1F) or 2 DNA fragments (2F) after 18 h incubation in CM, CM + 2.5 mM procaine, CM—NaCl, CM—Na+, CM + 25 mM NH4Cl, and CM pH 7.9. In general, oocytes exposed to procaine or NH4Cl rarely formed pronuclei, but instead exhibited condensed DNA fragments. Moreover, high medium pH exerted a degenerative effect on equine oocytes. Data represent mean (±SD) percentages of oocytes after incubation in CM, CM + 2.5 mM procaine, CM lacking NaCl, CM lacking Na+, CM + 25 mM NH4Cl, and CM at pH 7.9; n = 10 oocytes in each group, three replicates. Values that differ significantly (P < 0.05) within degenerated oocytes are indicated by small letters. Values that differ significantly between meiosis I and II oocytes are indicated by capital letters (P < 0.05). Values that differ significantly between oocytes containing DNA fragments are indicated by Greek letters (P < 0.05).
The formation of normal-looking (pro) nuclei (1/2 pronucleus (PN)) was a rare finding in any of the conditions tested (less than 6 ± 5%; Figure 7). Moreover, completion of the second meiotic division, as evidenced by the formation of a second polar body, was not seen in any of the oocytes, suggesting that resumption of metaphase plate of meiotic division II (MII) was not initiated by any of the tested conditions.
After procaine or NH4Cl exposure, very few of the daughter cells in apparently cleaved oocytes contained DNA at all, while others displayed condensed, fragmented pieces of DNA (Figure 7). Effects on oocyte nuclear DNA was independent of the presence or absence of spermatozoa in fertilizing medium (P > 0.09 for all comparisons; Figures 6 and 7).
In short, exposure to procaine or NH4Cl induced DNA fragmentation in equine oocytes which underwent cytokinesis, whereas inhibition of Na+ influx did not (Figure 7).
Procaine elevates cytoplasmic pH in a concentration-dependent way primarily by alkalinizing acidic sperm cell organelles
The BCECF-AM ratio at 0 min in stallion sperm suspensions incubated in NCM was assigned as the baseline (3.10 ± 0.14). After 10 min incubation, no significant increase in BCECF-AM ratio was observed for sperm cells in NCM (2.95 ± 0.07), CM (3.10 ± 0.42), CM without NaCl (3.00 ± 0.28), or CM without Na+ (3.05 ± 0.07) (Figure 8A). In contrast, procaine induced a rapid, concentration-dependent increase in the BCECF-AM ratio during the first 10 min of incubation (after 10 min in 2.5 mM procaine: 3.75 ± 0.07; in 5 mM procaine: 4.55 ± 0.07; in 10 mM procaine: 5.60 ± 0.14; in 25 mM procaine: 11.55 ± 0.07; and in 50 mM procaine: 17.50 ± 0.00) that was sustained for the entire 60 min incubation (Figure 8A). A slight but significant increase in BCECF-AM ratio during the first 10 min was also observed for sperm cells suspended in CM plus 25 mM NH4Cl (3.85 ± 0.07) and CM at pH 7.9 (4.10 ± 0.14) and was also maintained for the rest of the incubation (Figure 8A). Overall, it appears that weak bases, such as procaine and NH4Cl, elevate the cytoplasmic pH in stallion sperm in a concentration-dependent fashion.
Figure 8.
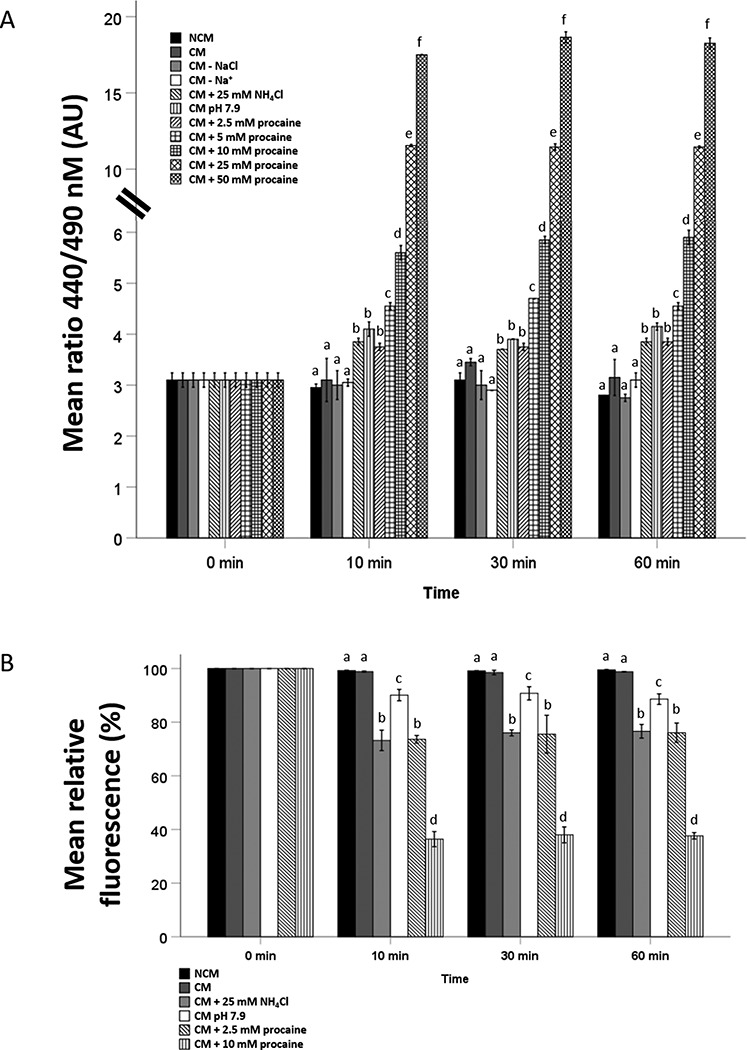
(A) Intracellular pH was assessed at 0, 10, 30, and 60 min in stallion sperm suspensions (30 × 106 sp/ml) exposed to NCM, CM, CM—NaCl, CM—Na+, CM + 25 mM NH4Cl, and CM pH 7.9, CM + 2.5–5–10-25-50 mM procaine using the ratiometric dye BCECF-AM. Increasing procaine concentration was associated with an increase in intracellular pH. Values are mean (±SD) BCECF-AM ratios of sperm in suspensions exposed to different capacitating conditions (three replicates using three different stallions). Within each time point, values that differ significantly (P < 0.05) are indicated by different small letters. (B) Acidic sperm cell organelle pH was assessed at 0, 10, 30, and 60 min in stallion sperm suspensions (30 × 106 sp/ml) exposed to NCM, CM, CM + 25 mM NH4Cl, CM pH 7.9; and CM + 2.5 and 10 mM procaine using Lysosensor Green DND-189 dye. Increasing procaine concentration was associated with a decrease in pH of acidic sperm cell organelles. Values are mean (±SD) relative fluorescence of Lysosensor Green DND-189 of sperm in suspensions exposed to different capacitating conditions (three replicates using three different stallions). Within each time point, values that differ significantly (P < 0.05) are indicated by different small letters.
Next, baseline Lysosensor Green DND-189 fluorescence was recorded in NCM conditions at 0 min and set as 100. After 10 min incubation, no significant decrease in Lysosensor Green DND-189 fluorescence was observed in sperm cells suspended in NCM (99.72 ± 0.09) or CM (98.95 ± 0.12) (Figure 8B). In contrast, NH4Cl and procaine induced a rapid, concentration-dependent decrease in Lysosensor Green DND-189 signal intensity during the first 10 min of incubation (25 mM NH4Cl: 79.55 ± 0.27; 2.5 mM procaine: 72.55 ± 0.27; and 10 mM procaine: 36.65 ± 0.44) that was sustained for the entire 60 min incubation (Figure 8B). A slight but significant decrease in Lysosensor Green DND-189 signal during the first 10 min was also observed for sperm cells suspended in CM at pH 7.9 (89.12 ± 0.32); this was also maintained for the rest of the incubation (Figure 8B). Overall, weak bases such as procaine and NH4Cl increase the pH of acidic cell organelles in stallion sperm in a concentration-dependent fashion whereas high medium pH is much less potent in raising pH of this compartment.
Hyperactivated motility can be reliably induced by 2.5–10 mM procaine
High total motility percentages were observed for sperm cells incubated in 0, 2.5, 5, or 10 mM procaine-containing CM (0 mM: 85 ± 6%; 2.5 mM: 81 ± 6%; 5 mM: 77 ± 4%; 10 mM: 80 ± 2%). Higher procaine concentrations reduced percentages of (total) sperm motility (25: 50 ± 5%; 50 mM: 15 ± 5%) suggesting a procaine concentration-dependent toxic effect on stallion spermatozoa (Figure 9A).
Figure 9.
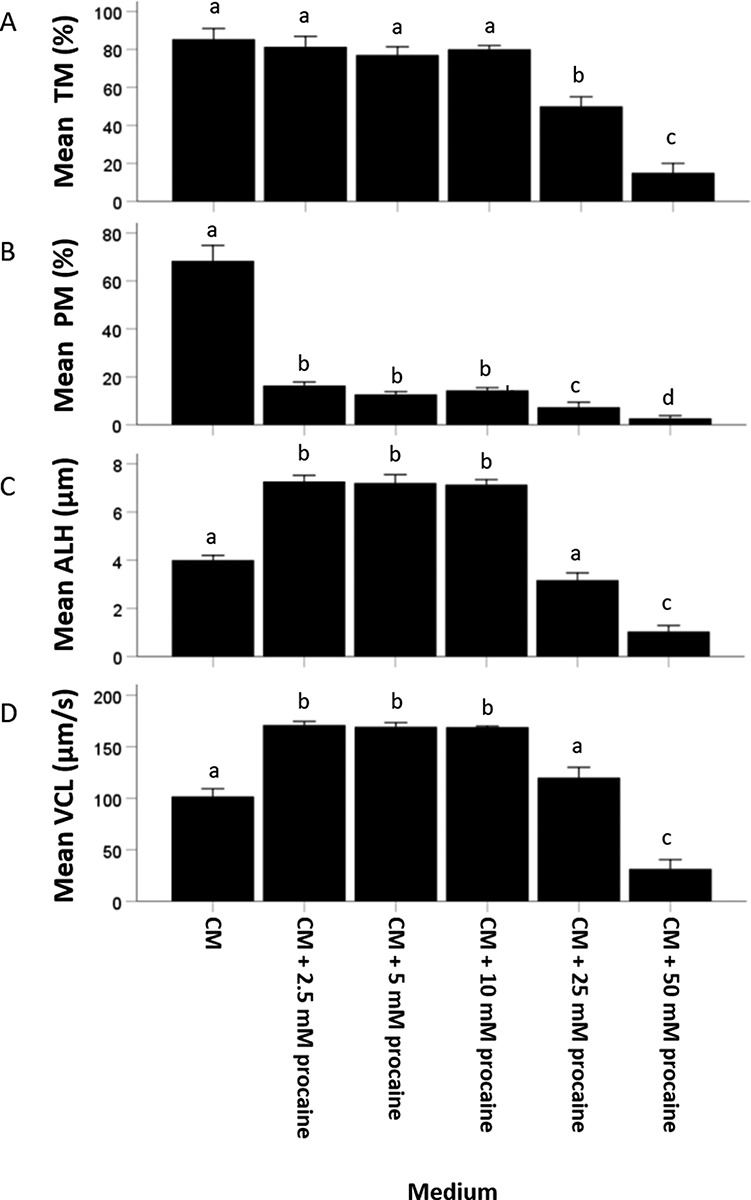
Evaluation of different motility parameters ((A) percentage of total motile or TM; (B) percentage ofprogressively motile or PM; (C) ALH; and (D) VCL) at 30 min incubation of stallion sperm suspended in (1) CM, (2) CM + 2.5 mM procaine, (3) CM + 5 mM procaine, (4) CM + 10 mM procaine, (5) CM + 25 mM procaine, and (6) CM + 50 mM procaine. Hyperactivated motility in sperm suspensions could be reliably induced over a 2.5–10 mM procaine concentration range. A sperm toxic effect of procaine was observed at 25 and 50 mM procaine. For percentage of total motile, percentage of progressively motile, ALH, and VCL, values that differ (P < 0.05) are indicated by different small letters.
Stallion sperm incubated in CM without procaine showed high percentages of progressive motility (68 ± 7%). Supplementation with procaine markedly reduced percentage of progressively motile sperm at all concentrations tested (2.5 mM: 16 ± 2%; 5 mM: 13 ± 1%; 10 mM: 14 ± 1%; 25 mM: 7 ± 2%; 50 mM: 3 ± 1%) (Figure 9B).
Maximum ALH and VCL values, indicating hyperactivated motility, were measured when sperm were incubated in 2.5, 5, and 10 mM procaine-supplemented CM (2.5 mM: ALH = 7.3 ± 0.3 μm and VCL = 171 ± 4 μm/s; 5 mM: ALH = 7.2 ± 0.3 μm and VCL = 169 ± 4 μm/s; 10 mM: ALH = 7.1 ± 0.2 μm and VCL = 169 ± 1 μm/s). Much lower ALH and VCL values were observed for sperm cells incubated in CM without procaine (0 mM: ALH = 4.0 ± 0.2 μm and VCL = 102 ± 8 μm/s) or with higher procaine concentrations (25 mM: ALH = 3.2 ± 0.3 μm and VCL = 120 ± 10 μm/s; 50 mM: ALH = 1.0 ± 0.3 μm and VCL = 31 ± 9 μm/s) (Figure 9C and D).
Overall, hyperactivated motility was reliably induced by procaine within the 2.5–10 mM concentration range, whereas higher procaine concentrations were toxic.
Procaine raises cytoplasmic cAMP levels in a concentration-dependent fashion
At 15 min incubation, no significant differences in cAMP levels were measured between sperm suspensions incubated in NCM (0.14 ± 0.09 pM), CM (0.18 ± 0.04 pM), CM without NaCl (0.11 ± 0.03 pM), CM without Na+ (0.13 ± 0.10 pM), CM with 25 mM NH4Cl (0.31 ± 0.03 pM), CM at pH 7.9 (0.27 ± 0.05 pM), and CM with either 2.5, 5, 25, or 50 mM procaine (2.5 mM: 0.14 ± 0.03 pM; 5 mM: 0.41 ± 0.05 pM; 25 mM: 0.50 ± 0.11 pM; 50 mM: 0.52 ± 0.20 pM) (Figure 10B). Significantly higher cAMP concentrations were observed in 10 mM procaine-containing CM (0.77 ± 0.14 pM), while maximum cAMP concentrations were measured for control sperm suspensions incubated in CM plus 1 mM caffeine (1.82 ± 0.22 pM) (Figure 10B). A trend was apparent for a procaine concentration-dependent effect on cAMP concentrations in stallion spermatozoa.
Interestingly, at 15 min incubation, boar sperm demonstrated significantly higher cAMP levels in CM (1.58 ± 0.37 pM) and CM plus 1 mM caffeine (3.95 ± 0.67 pM) than the respective stallion sperm suspensions (CM: 0.18 ± 0.04 pM; CM plus 1 mM caffeine: 1.82 ± 0.22 pM), whereas no significant difference was observed in cAMP levels between boar (0.40 ± 0.19 pM) and stallion (0.14 ± 0.09 pM) sperm suspensions in NCM. Interestingly, cAMP concentrations in boar spermatozoa incubated in CM (1.82 ± 0.22 pM) were similar to those in stallion spermatozoa incubated in CM plus 1 mM caffeine (1.58 ± 0.37 pM) (Figure 10C). For all medium conditions, no significant difference in cAMP concentrations was observed between sperm suspensions incubated for 15 min and 1 h (Figure 10B and 10C). These data indicate that cytoplasmic cAMP in boar sperm incubated in CM is upregulated to a much higher degree than in stallion sperm.
Procaine induces sperm tail-associated protein tyrosine phosphorylation in a concentration-dependent mode
Minimal tail-associated protein tyrosine phosphorylation was observed in NCM (4 ± 2%), CM without NaCl (6 ± 2%), CM without Na+ (5 ± 1%), NH4Cl-supplemented CM (5 ± 2%), and CM with 25 or 50 mM procaine (25 mM: 6 ± 1%; 50 mM: 4 ± 1%). In agreement with other reports [9, 23], maximal percentages of spermatozoa with tail-associated protein tyrosine phosphorylation were observed at pH 7.9 (71 ± 3%). Slightly lower but not different percentages of sperm with protein tyrosine phosphorylation were observed in CM containing 10 mM procaine (53 ± 8%) or caffeine (51 ± 10%). Intermediate tail-associated protein tyrosine phosphorylation incidences were noted after incubation of stallion spermatozoa in both CM (18 ± 4%) and CM plus 2.5 mM procaine (28 ± 4%) (Figure 11). In this experiment, a clear effect of increased medium pH and caffeine on tail-associated protein tyrosine phosphorylation was confirmed, whereas inhibiting Na+ influx did not promote this capacitation hall mark. In addition, a procaine concentration-dependent effect on tail-associated protein tyrosine phosphorylation was observed at up to 10 mM procaine.
Figure 11.
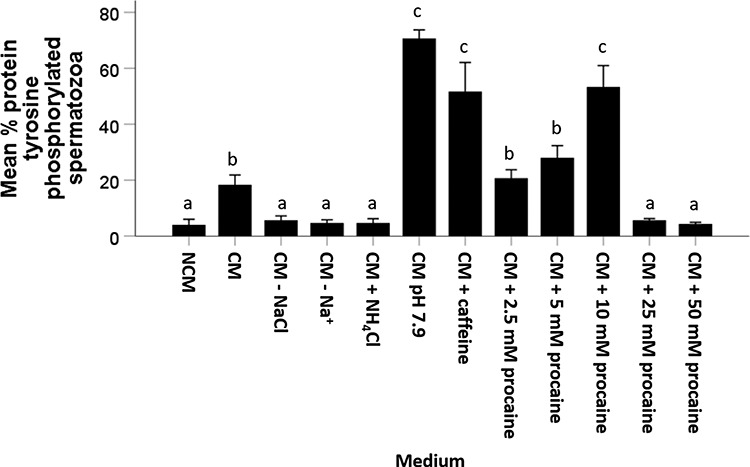
Percentage of sperm with tail-associated protein tyrosine phosphorylation after incubation for 4 h in NCM, CM, CM—NaCl, CM—Na+, CM + 25 mM NH4Cl, CM at pH 7.9, CM + 1 mM caffeine, and CM + 2.5–5–10–25–50 mM procaine. Stallion sperm tail-associated protein tyrosine phosphorylation was induced by 10 mM procaine CM, medium pH 7.9, and caffeine CM. Values that differ significantly (P < 0.05) are indicated by different small letters.
Stallion sperm preincubated in 10 mM procaine capacitating medium do not fertilize equine oocytes in vitro
Oocytes co-incubated in CM with stallion spermatozoa washed after preincubation for 30 min and 4 h in CM, CM + 2.5 mM procaine, and CM + 10 mM procaine did not show oocyte cleavage or cytokinesis at any time point. After sperm had been preincubated for 30 min in CM: 23 ± 6% PD degenerated and 77 ± 5% of parthenogenic division (PD) showed a metaphase plates of meiotic division 1I or II (Mi/MII), in CM + 2.5 mM procaine: 19 ± 8% PD degenerated and 81 ± 5% PD were in MI/MII; and in CM + 10 mM procaine: 27 ± 9% PD degenerated and 73 ± 4% PD were in MI/MII. After 24 h gamete co-incubation, none of the oocytes in any of the groups demonstrated the formation of 2 PN (0 ± 0%) or the presence of a second polar body (0 ± 0%) as evidence of completion of the second meiotic division. Moreover, no differences were observed in any tested group between 0.5 and 4 h sperm preincubation with procaine. These data show that the capacitation-related sperm characteristics induced by 10 mM procaine are not sufficient to support IVF in the horse.
Discussion
The principal aim of this study was to better understand how procaine induces hyperactivated motility in stallion spermatozoa and whether procaine also triggers other capacitation-related characteristics. Procaine induction of hyperactivated motility of stallion spermatozoa was associated with its weak base activity, which elevated cytoplasmic and cell organelle pH. For procaine, a concentration-dependent effect on hypermotility, cytoplasmic and organelle pH, cAMP levels, and tail-associated protein tyrosine phosphorylation was demonstrated. However, whereas hyperactivated motility was induced by 2.5–10 mM procaine, only 10 mM procaine was able to stimulate a significant increase in cAMP content and tail-associated tyrosine phosphorylation. Unfortunately, sperm preincubation in 10 mM procaine CM did not support IVF. Surprisingly, cAMP concentrations were more than 10× lower in stallion sperm than boar sperm suspensions incubated under identical capacitating conditions, which may indicate that current in vitro capacitation media still fail to trigger a fertilization-competent subpopulation of stallion sperm.
We discovered that procaine is only able to elicit hyperactivated sperm motility when it is in direct contact with the sperm. The effect vanished immediately after procaine was washed out of the incubation medium. As discussed by Szatkowski [39], cells can have an open or a closed intracellular buffer system. In a closed buffer system, the total intracellular buffer concentration ([BH+] + [B]) is constant, whereas in an open system, only the concentration of the uncharged species ([B]) is constant within the cell. Procaine hydrochloride can only freely pass through cell membranes in its uncharged form [39, 40], which suggests that procaine in a sperm cell acts as an open buffered system. If so, immediately after removal from the procaine-containing CM, the concentration of uncharged procaine hydrochloride is higher in the sperm cells than in the medium. This facilitates a shift in uncharged procaine hydrochloride to the medium. A drop in intracellular procaine concentration occurs, followed by a drop in cytoplasmic pH. This indicates that a washing step to remove procaine and avoid its toxic effects on the oocyte will result in loss of the procaine-induced sperm hypermotility during gamete co-incubation.
We showed that procaine, a voltage-gated Na+ channel blocker, did not induce hyperactivated motility by inhibiting Na+ influx into the sperm cytosol. In contrast, incubation of hamster sperm cells in low Na+ media did induce hyperactivated motility [21], and it has been suggested that extracellular Na+ suppresses hyperactivation by lowering cytoplasmic Ca2+ in the flagellum via the action of a Na+/Ca2+ exchanger. In this respect, Na+-dependent Ca2+ extrusion has also been demonstrated in mouse spermatozoa [41]. In addition, large amounts of ATP are necessary to facilitate Na+ efflux from the sperm cytosol during stallion sperm incubation [42]. We initially hypothesized that procaine would block ATP-driven Na+ channels/pumps in order to (1) preserve ATP for the support of hyperactivated motility or (2) block Na+/Ca2+ exchangers. However, omitting Na+ from the medium did not influence stallion sperm motility parameters, suggesting that the Na+ effects on hyperactivated motility of hamster spermatozoa do not occur in stallion sperm.
The action of procaine on hyperactivated motility in stallion spermatozoa was independent of extracellular Ca2+ at pH 7.4 and 7.9. Loux et al. [11] similarly observed that procaine-induced hyperactivated motility in stallion spermatozoa was independent of Ca2+ at pH 7.25. These observations contrast with the general concept that onset and maintenance of hyperactivated motility are associated with increased Ca2+ levels in the sperm tail cytosol [13, 43]. Cytoplasmic Ca2+ has been reported to derive from extracellular Ca2+ influx through pH-gated CatSper channels augmented by release from intracellular Ca2+ stores [44–46]. In bull spermatozoa, procaine-induced hyperactivated motility requires extracellular Ca2+ [47]. Based on our results, we postulate that weak bases, e.g., procaine and NH4Cl, directly facilitate Ca2+ release from intracellular stores into the cytoplasm. In support of this hypothesis, procaine was not able to induce hyperactivated motility in demembranated stallion sperm, demonstrating that procaine does not act on the axoneme but instead on membrane or cytoplasmic components [48].
Moreover, we observed a procaine concentration-dependent increase in cytoplasmic pH in both stallion spermatozoa and equine oocytes [10]. Interestingly, 2.5 mM procaine, 25 mM NH4Cl, and alkaline conditions (pH 7.9) all induced a similar rise in cytoplasmic pH in stallion sperm (Figure 8). Loux et al. [11] also found that incubation of stallion spermatozoa in 5 mM procaine supplemented CM was associated with an increase in cytoplasmic pH. Similarly, in a previous study, we observed a procaine concentration-dependent rise in cytoplasmic pH in equine oocytes [10], while Begg et al. [49] reported that exposing sea urchin eggs to either procaine or NH4Cl resulted in a rapid but reversible increase in cytoplasmic pH. It follows that cytoplasmic pH may trigger both sperm hyperactivated motility and oocyte cytokinesis. Clearly, procaine is a more potent inducer of hyperactivated motility in stallion spermatozoa than NH4Cl. This may be because procaine induces a larger cytoplasmic and/or cell organelle pH shift than NH4Cl [39]. Procaine has a much higher lipid solubility than NH4Cl, and this may enhance the ability of uncharged procaine to cross the relevant cell membranes to very stably buffer the cytoplasmic pH [50], whereas NH4Cl probably makes use of hydrophilic channels to cross the membrane, which may have consequences for cytoplasmic and/or acidic cell organelle pH stability. In comparison to procaine and NH4Cl exposure, raising the extracellular pH to 7.9 only had a weak effect on hyperactivated motility.
Interestingly, we showed that procaine- and NH4Cl-induced cytoplasmic pH rises are associated with a concentration-dependent increase in the pH of sperm cell organelles whereas CM at pH 7.9 was less potent in altering sperm organelle pH. Intracellular organelles are known to be able to accumulate weak bases because of their very low internal pH [51–53]. This raises the possibility of a local action of weak bases on intracellular Ca2+ stores in stallion spermatozoa. A similar mechanism has been demonstrated in various somatic cell types: yeast cells, S2 cells (Drosophila), type II pneumocytes (rat), HeLa cells (human), MCDK cells (dog), and RAW 264.7 cells (mouse) [26, 27]. Recently, these findings were confirmed in murine and human sperm. Chávez et al. [28] demonstrated that the acrosome is a Ca2+-containing acidic cell organelle and that acrosomal alkalization by various weak bases triggers Ca2+release and the acrosome reaction. We would like to stress that the necessity of a rise in cytoplasmic Ca2+ during the induction of hyperactivated motility in procaine-exposed stallion spermatozoa has never been demonstrated, and may therefore not be relevant. In this respect, Loux et al. [48] showed that increasing Ca2+ levels did not induce hyperactivated motility in demembranated stallion spermatozoa. On the other hand, a Ca2+-mediated effect of procaine cannot be excluded. NH4Cl-induced hypermotility in bull sperm triggered a cytoplasmic pH rise followed by increased cytosolic Ca2+ levels [38]. Importantly, there is some discussion ongoing about the accuracy of assessing cytoplasmic Ca2+ changes in response to procaine using fluorescent Ca2+ probes, since dye quenching by procaine has been reported [11].
Interestingly and very similar to the effects of procaine [10], NH4Cl induced cytokinesis of equine oocytes followed by further aberrant cleavage divisions up to the 8–16 cell stage, independent of the presence of sperm. During cytoplasmic cleavage, condensed DNA fragments formed and segregated across some of the daughter cells. These effects resembled those of 2.5 mM procaine, albeit that the percentage of oocytes undergoing cytokinesis and DNA fragmentation were lower for NH4Cl exposure. Initially, the similarity to the effects of procaine leads us to believe that the NH4Cl effect on equine oocytes is, like that of procaine, not elicited by a rise in cytosolic Ca2+ [10]. Similar observations have been reported in sea urchin eggs [54, 55], in which procaine failed to trigger either extracellular Ca2+ influx [54] or release from intracellular Ca2+ stores [55]. Furthermore, in pig [16, 17] and cattle [18, 19], the oocyte-activation-related intracellular Ca2+ rise could be inhibited by injecting procaine. Even low concentrations (≤200 μM) of procaine were able to block the ryanodine receptors regulating the Ca2+ channels in the cytoplasmic Ca2+ stores. However, as mentioned above, procaine quenches fluorescent Ca2+ probes [11], which makes it hard to elucidate the relationship between procaine and cytosolic Ca2+ levels. In contrast to procaine and NH4Cl conditions, raising the extracellular pH to 7.9 severely compromised oocyte viability without triggering cytokinesis. Moreover, the replacement of NaCl or all Na+ ions in CM did not affect equine oocyte viability, cleavage, or DNA configuration, indicating that the effects of procaine and NH4Cl are not primarily an effect of modifying Na+ transport across the oolemma.
We observed that procaine-supplemented CM induced a dose-dependent increase in stallion sperm cytoplasmic cAMP with a peak at 10 mM procaine, whereas 25 mM NH4Cl did not. Moreover, 10 mM procaine induced tail-associated protein tyrosine phosphorylation whereas concentrations ≤ 5 mM [8, 10] or >10 mM procaine, and 25 mM NH4Cl, did not. Overall, these findings suggest that the weak base activity of 10 mM procaine results in a specific cytoplasmic pH rise that activates the sAC/cAMP/protein kinase A pathway and the related tyrosine kinases. Incubation in 25 mM NH4Cl, and all other tested procaine concentrations, caused a suboptimal cytoplasmic pH which was not able to adequately activate sAC to generate enough cAMP to support the protein kinase pathway. In contrast, alkaline-CM (pH 7.9) induced a massive protein tyrosine phosphorylation response in the sperm tail (as described previously [9, 10, 23]) without raising cytoplasmic cAMP concentrations. Fernando-Gonzalez et al. [22, 23] reported that a raised pH induces a switch from Ca-calmodulin phosphatase activity to Ca/calmodulin-dependent protein kinase activity. Importantly, we observed that cAMP levels in stallion sperm were significantly lower than in boar sperm incubated in identical CM. These observations may explain in part why stallion spermatozoa preincubated in 10 mM procaine CM were not able to fertilize equine oocytes in vitro. Overall, it appears that none of the tested capacitating conditions were sufficient to induce full capacitation in stallion spermatozoa and/or that the sAC/cAMP/protein kinase A pathway is less important in stallion sperm capacitation than in other species, such as the pig.
Interestingly, the effect of procaine on equine oocytes and spermatozoa is concentration-dependent. Hyperactivated motility in stallion spermatozoa is induced over a wide concentration range (2.5–10 mM procaine), although high percentages of cAMP-dependent, tail-associated protein tyrosine phosphorylated spermatozoa were only observed at a concentration of 10 mM procaine. We therefore propose that procaine-induced hyperactivated motility is induced by a broader cytoplasmic and/or cell organelle pH range than is required to support the sAC/cAMP/protein kinase A pathway. On the other hand, maximal cleavage rates in equine oocytes were observed at a much lower procaine concentration (2.5 mM), whereas >5 mM procaine resulted in degeneration of equine oocytes. In addition, 2.5 mM procaine proved to be very damaging to the oocyte’s DNA. It is likely that equine oocytes are able to accumulate much more of the charged species of a weak base than stallion spermatozoa because the cell volume, and therefore the number of acidic organelles, is much lower in a spermatozoon than an oocyte. It follows that exposing equine oocytes to a lower procaine concentration will generate a similar cytoplasmic/cell organelle pH as exposure of a stallion sperm to a higher procaine concentration.
In conclusion, we have shown that procaine, besides being a potent inducer of hyperactivated motility, is also a trigger of cAMP-dependent tail-associated protein tyrosine phosphorylation in stallion spermatozoa. However, the two capacitation events appear to be induced independently of each other. Hyperactivated motility was induced by a 2.5–10 mM procaine range, whereas cAMP-dependent tail-associated protein tyrosine phosphorylation was only induced at 10 mM. The concentration-dependent actions of procaine on sperm hypermotility, cytoplasmic cAMP concentrations, and protein tyrosine phosphorylation in the sperm tail appear, at least in part, to be associated with its weak base activity which induces a cytoplasmic and sperm cell organelle pH increase. Moreover, the weak base activity of procaine causes unfertilized oocytes to cleave into up to 16 “cells” although apparent cell division is accompanied by severe DNA fragmentation [10]. Overall, we conclude that precise regulation of cytoplasmic and/or cell organelle pH in equine spermatozoa and oocytes seems to be crucial for both sperm capacitation and oocyte activation. Studying how cytoplasmic and cell organelle pH triggers these signaling pathways under physiological conditions may help us understand why they are not properly activated under IVF conditions.
Supplementary Material
Acknowledgements
The authors wish to thank Petra Van Damme for her excellent technical assistance. Fresh stallion semen was kindly provided by the equine reproduction clinics at Ghent and Utrecht Universities.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Palmer E, Bezard J, Magistrini M, Duchamp G. In vitro fertilization in the horse. A retrospective study. J Reprod Fertil Suppl 1991; 44:375–384. [PubMed] [Google Scholar]
- 2. Bézard J, Magistrini M, Battut I, Duchamp G, Palmer E. In vitro fertilization in the Mare. Rec Med Vet 1992; 168:993–1003. [Google Scholar]
- 3. Dell'Aquila ME, Cho YS, Minoia P, Traina V, Fusco S, Lacalandra GM, Maritato F. Intracytoplasmic sperm injection (ICSI) versus conventional IVF on abottoir-derived and in vitro-matured equine oocytes. Theriogenology 1997; 47:1139–1156. [DOI] [PubMed] [Google Scholar]
- 4. Dell'Aquila ME, Cho YS, Minoia P, Traina V, Lacalandra GM, Maritato F. Effects of follicular fluid supplementation of in-vitro maturation medium on the fertilization and development of equine oocytes after in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod 1997; 12:2766–2772. [DOI] [PubMed] [Google Scholar]
- 5. Roasa LM, Choi YH, Love CC, Romo S, Varner DD, Hinrichs K. Ejaculate and type of freezing extender affect rates of fertilization of horse oocytes in vitro. Theriogenology 2007; 68:560–566. [DOI] [PubMed] [Google Scholar]
- 6. Leemans B, Gadella BM, Stout TA, De Schauwer C, Nelis H, Hoogewijs M, Van Soom A. Why doesn't conventional IVF work in the horse? The equine oviduct as a microenvironment for capacitation/fertilization. Reproduction 2016; 152:233–245. [DOI] [PubMed] [Google Scholar]
- 7. Leemans B, Stout TAE, De Schauwer C, Heras S, Nelis H, Hoogewijs M, Van Soom A, Gadella BM. Reproduction 2019.Feb 1. pii: REP–18–0541.R1. doi: 10.1530/REP–18–0541. [Epub ahead of print] Review. 30721132. [DOI] [PubMed] [Google Scholar]
- 8. McPartlin LA, Suarez SS, Czaya CA, Hinrichs K, Bedford-Guaus SJ. Hyperactivation of stallion sperm is required for successful in vitro fertilization of equine oocytes. Biol Reprod 2009; 81:199–206. [DOI] [PubMed] [Google Scholar]
- 9. Leemans B, Gadella BM, Stout TA, Nelis H, Hoogewijs M, Van Soom A. An alkaline follicular fluid fraction induces capacitation and limited release of oviduct epithelium-bound stallion sperm. Reproduction 2015; 150:193–208. [DOI] [PubMed] [Google Scholar]
- 10. Leemans B, Gadella BM, Stout TA, Heras S, Smits K, Ferrer-Buitrago M, Claes E, Heindryckx B, De Vos WH, Nelis H, Hoogewijs M, Van Soom A. Procaine induces cytokinesis in horse oocytes via a pH dependent mechanism. Biol Reprod 2015; 93:23. [DOI] [PubMed] [Google Scholar]
- 11. Loux SC, Crawford KR, Ing NH, Gonzalez-Fernandez L, Macias-Garcia B, Love CC, Varner DD, Velez IC, Choi YH, Hinrichs K. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol Reprod 2013; 89:123. [DOI] [PubMed] [Google Scholar]
- 12. Ambruosi B, Accogli G, Douet C, Canepa S, Pascal G, Monget P, Moros Nicolas C, Holmskov U, Mollenhauer J, Robbe-Masselot C, Vidal O, Desantis S, et al. . Deleted in malignant brain tumor 1 is secreted in the oviduct and involved in the mechanism of fertilization in equine and porcine species. Reproduction 2013; 146:119–133. [DOI] [PubMed] [Google Scholar]
- 13. Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update 2008; 14:647–657. [DOI] [PubMed] [Google Scholar]
- 14. Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 2012; 74:453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrer-Buitrago M, Bonte D, De Sutter P, Leybaert L, Heindryckx B. Single Ca(2+) transients vs oscillatory Ca(2+) signaling for assisted oocyte activation: limitations and benefits. Reproduction 2018; 155:105–119. [DOI] [PubMed] [Google Scholar]
- 16. Mattioli M, Barboni B, Gioia L, Loi P. Cold-induced calcium elevation triggers DNA fragmentation in immature pig oocytes. Mol Reprod Dev 2003; 65:289–297. [DOI] [PubMed] [Google Scholar]
- 17. Petr J, Rajmon R, Lanska V, Sedmikova M, Jilek F. Nitric oxide-dependent activation of pig oocytes: role of calcium. Mol Cell Endocrinol 2005; 242:16–22. [DOI] [PubMed] [Google Scholar]
- 18. Yue C, White KL, Reed WA, Bunch TD. The existence of inositol 1,4,5-trisphosphate and ryanodine receptors in mature bovine oocytes. Development 1995; 121:2645–2654. [DOI] [PubMed] [Google Scholar]
- 19. Viets LN, Campbell KD, White KL. Pathways involved in RGD-mediated calcium transients in mature bovine oocytes. Cloning Stem Cells 2001; 3:105–113. [DOI] [PubMed] [Google Scholar]
- 20. Cahalan MD. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J 1978; 23:285–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takei GL, Fujinoki M. Regulation of hamster sperm hyperactivation by extracellular Na. Reproduction 2016; 151:589–603. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez-Fernandez L, Macias-Garcia B, Loux SC, Varner DD, Hinrichs K. Focal adhesion kinases and calcium/calmodulin-dependent protein kinases regulate protein tyrosine phosphorylation in stallion sperm. Biol Reprod 2013; 88:138. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez-Fernandez L, Macias-Garcia B, Velez IC, Varner DD, Hinrichs K. Calcium-calmodulin and pH regulate protein tyrosine phosphorylation in stallion sperm. Reproduction 2012; 144:411–422. [DOI] [PubMed] [Google Scholar]
- 24. Leemans B, Gadella BM, Sostaric E, Nelis H, Stout TA, Hoogewijs M, Van Soom A. Oviduct binding and elevated environmental ph induce protein tyrosine phosphorylation in stallion spermatozoa. Biol Reprod 2014; 91:13. [DOI] [PubMed] [Google Scholar]
- 25. Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006; 439:737–740. [DOI] [PubMed] [Google Scholar]
- 26. Fois G, Hobi N, Felder E, Ziegler A, Miklavc P, Walther P, Radermacher P, Haller T, Dietl P. A new role for an old drug: ambroxol triggers lysosomal exocytosis via pH-dependent Ca(2)(+) release from acidic Ca(2)(+) stores. Cell Calcium 2015; 58:628–637. [DOI] [PubMed] [Google Scholar]
- 27. Ostrowski PP, Fairn GD, Grinstein S, Johnson DE. Cresyl violet: a superior fluorescent lysosomal marker. Traffic 2016; 17:1313–1321. [DOI] [PubMed] [Google Scholar]
- 28. Chavez JC, De la JL, Jose O, Torres P, Nishigaki T, Trevino CL, Darszon A. Acrosomal alkalization triggers Ca(2+) release and acrosome reaction in mammalian spermatozoa. J Cell Physiol 2018; 233:4735–4747. [DOI] [PubMed] [Google Scholar]
- 29. Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38:1171–1180. [DOI] [PubMed] [Google Scholar]
- 30. Tremoleda JL, Stout TAE, Lagutina I, Lazzari G, Bevers MM, Colenbrander B, Galli C. Effects of in vitro production on horse embryo morphology, cytoskeletal characteristics, and blastocyst capsule formation. Biol Reprod 2003; 69:1895–1906. [DOI] [PubMed] [Google Scholar]
- 31. McPartlin LA, Littell J, Mark E, Nelson JL, Travis AJ, Bedford-Guaus SJ. A defined medium supports changes consistent with capacitation in stallion sperm, as evidenced by increases in protein tyrosine phosphorylation and high rates of acrosomal exocytosis. Theriogenology 2008; 69:639–650. [DOI] [PubMed] [Google Scholar]
- 32. Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, Colenbrander B, Gadella BM. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci 2001; 114:3543–3555. [DOI] [PubMed] [Google Scholar]
- 33. Boerke A, Brouwers JF, Olkkonen VM, van de Lest CH, Sostaric E, Schoevers EJ, Helms JB, Gadella BM. Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization. Biol Reprod 2013; 88:21. [DOI] [PubMed] [Google Scholar]
- 34. Galli C, Colleoni S, Duchi R, Lagutina I, Lazzari G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim Reprod Sci 2007; 98:39–55. [DOI] [PubMed] [Google Scholar]
- 35. Loomis PR, Graham JK. Commercial semen freezing: individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim Reprod Sci 2008; 105:119–128. [DOI] [PubMed] [Google Scholar]
- 36. Hoogewijs M, Rijsselaere T, De Vliegher S, Vanhaesebrouck E, De Schauwer C, Govaere J, Thys M, Hoflack G, Van Soom A et al. . Influence of different centrifugation protocols on equine semen preservation. Theriogenology 2010; 74:118–126. [DOI] [PubMed] [Google Scholar]
- 37. Wertheimer EV, Salicioni AM, Liu W, Trevino CL, Chavez J, Hernandez-Gonzalez EO, Darszon A, Visconti PE. Chloride is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem 2008; 283:35539–35550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol Reprod 2007; 76:660–665. [DOI] [PubMed] [Google Scholar]
- 39. Szatkowski MS. The effect of extracellular weak acids and bases on the intracellular buffering power of snail neurones. J Physiol 1989; 409:103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Winkler MM, Grainger JL. Mechanism of action of NH4Cl and other weak bases in the activation of sea urchin eggs. Nature 1978; 273:536–538. [DOI] [PubMed] [Google Scholar]
- 41. Wennemuth G, Carlson AE, Harper AJ, Babcock DF. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development 2003; 130:1317–1326. [DOI] [PubMed] [Google Scholar]
- 42. Gibb Z, Lambourne SR, Quadrelli J, Smith ND, Aitken RJ. L-carnitine and pyruvate are prosurvival factors during the storage of stallion spermatozoa at room temperature. Biol Reprod 2015; 93:104. [DOI] [PubMed] [Google Scholar]
- 43. Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci USA 1993; 90:4660–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod 2001; 65:1606–1615. [DOI] [PubMed] [Google Scholar]
- 45. Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol 2007; 303:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod 2003; 68:1590–1596. [DOI] [PubMed] [Google Scholar]
- 47. Marquez B, Suarez SS. Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol Reprod 2004; 70:1626–1633. [DOI] [PubMed] [Google Scholar]
- 48. Loux SC, Macias-Garcia B, Gonzalez-Fernandez L, Canesin HD, Varner DD, Hinrichs K. Regulation of axonemal motility in demembranated equine sperm. Biol Reprod 2014; 91:152. [DOI] [PubMed] [Google Scholar]
- 49. Begg DA, Wong GK, Hoyle DH, Baltz JM. Stimulation of cortical actin polymerization in the sea urchin egg cortex by NH4Cl, procaine and urethane: elevation of cytoplasmic pH is not the common mechanism of action. Cell Motil Cytoskeleton 1996; 35:210–224. [DOI] [PubMed] [Google Scholar]
- 50. Westhues M, Fritsch R. Animal Anaesthesia. Philadelphia: Lippincott; 1964. [Google Scholar]
- 51. Johnson RG, Carlson NJ, Scarpa A. deltapH and catecholamine distribution in isolated chromaffin granules. J Biol Chem 1978; 253:1512–1521. [PubMed] [Google Scholar]
- 52. Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol 1981; 90:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 1981; 90:665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zucker RS, Steinhardt RA, Winkler MM. Intracellular calcium release and the mechanisms of parthenogenetic activation of the sea urchin egg. Dev Biol 1978; 65:285–295. [DOI] [PubMed] [Google Scholar]
- 55. Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature 1994; 367:607–614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


