Abstract
Hypothalamic–pituitary–adrenal (HPA) axis dysfunction has long been implicated in the pathophysiology of depression, and HPA axis‐based compounds have served as potential new therapeutic targets, but with no success. This review details sex differences from animal and human studies in the function of HPA axis elements (glucocorticoids, corticotropin releasing factor, and vasopressin) and related compounds tested as candidate antidepressants. We propose that sex differences contribute to the failure of novel HPA axis‐based drugs in clinical trials. Compounds studied preclinically in males were tested in clinical trials that recruited more, if not exclusively, women, and did not control, but rather adjusted, for potential sex differences. Indeed, clinical trials of antidepressants are usually not stratified by sex or other important factors, although preclinical and epidemiological data support such stratification. In conclusion, we suggest that clinical testing of HPA axis‐related compounds creates an opportunity for targeted, personalized antidepressant treatments based on sex.
LINKED ARTICLES
This article is part of a themed section on The Importance of Sex Differences in Pharmacology Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.21/issuetoc
Abbreviations
- ACTH
adrenocorticotropic hormone
- AV
arginine vasopressin
- CRF
corticotropin releasing factor
- HPA
hypothalamic–pituitary–adrenal
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
1. INTRODUCTION
The hypothalamic–pituitary–adrenal (HPA) axis is crucially involved in the stress response, and numerous studies consistently point towards a causal link between dysregulation of the HPA and the appearance of psychopathology (Gold & Chrousos, 1999; Selye, 1936). In particular, the aetiology of depression is thought to stem from the disruption of classic responses to stress including but not limited to alterations in glucocorticoid levels, sensitivity of glucocorticoid receptors, and resistance to the negative feedback (Herman, 2013). This corticosteroid hypothesis of major depression rose to prominence in the 1990s (Holsboer, 2003). Early studies established that during depression, plasma http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2868 levels are often elevated, but they normalize following clinical remission (Gibbons, 1964). In atypical depression, cortisol levels are often lower (Gold & Chrousos, 1999), and in older adults, cortisol levels may be either higher or lower (Bremmer et al., 2007). The persisting dysregulation of the HPA axis, despite treatment, strongly predicts the risk of relapse (Schule, 2007). Finally, antidepressants were discovered to normalize changes in the HPA axis, adding credence to its causal role in the aetiology of depression (Thakore, Barnes, Joyce, Medbak, & Dinan, 1997). Thus, a large body of literature has highlighted the elements of the HPA axis as potential new targets for the treatment of mood disorders, as available antidepressants have a late therapeutic onset and are not effective in a significant percentage of patients (Holsboer, 2003). However, to date, no antidepressant has been marketed that acts directly on the HPA axis. As researchers, we continue to test this hypothesis and learn more about the role that this system plays in the dysregulation of the stress response and the development of depression or other psychoneuroendocrinological conditions. Why? Given the notable failures of HPA axis‐based drugs, what is our hope in continuing to support research that explores these mechanisms?
In this review, we focus on depression and suggest that sex differences and the response of the HPA axis to stress may influence the success or failure of HPA axis‐based drugs. As women are twice as likely to suffer from depression than men, and it has been demonstrated that there are sex differences in depression and the response to antidepressants (Kokras & Dalla, 2017), we will examine how the unforeseen influence of sex contributes to the good, the bad, and the ugly truth about HPA axis‐targeted treatments for depression. Specifically, we suggest that the mismatch between the sex of the animals used in preclinical studies and the sex of patients recruited in clinical trials has contributed to the failure of these compounds. This discrepancy is probably attributed to the fact that, since 1993, the National Institutes of Health has encouraged the inclusion of more women in clinical trials (Merkatz, Temple et al., 1993), whereas a similar recommendation to include females in preclinical research was only issued just recently (Clayton & Collins, 2014). By raising awareness of this matter, we argue for a better policy regarding the sex of the animals and humans in preclinical and clinical studies, an arrangement that will potentially facilitate the discovery of novel treatments.
2. THE HPA AXIS
2.1. Glucocorticoid regulation of the HPA axis
The regulation of the HPA axis relies on the negative feedback of the corticosteroids, mainly cortisol (in primates) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2869 (in most rodents; Figure 1, adapted from Bangasser & Valentino, 2014). In response to a threat, these hormones increase energy through glucose metabolism, lipolysis, and proteolysis and they suppress growth, reproduction, and the immune system (McEwen & Gianaros, 2011). Upon exposure to stress, hypophysiotrophic neurons in the paraventricular nucleus (PVN) of the hypothalamus release http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=912 (CRF) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2168 (AVP), which stimulate the pituitary via http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=212 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=367 receptors, respectively, to secrete http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3633 (ACTH) into the blood. In response to ACTH, the cortex of the adrenal glands produces glucocorticoids, which penetrate the blood–brain barrier and exert their actions in the brain through the high affinity http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=626 (MRs, Type I) and the low affinity http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=625 (GR, Type II). Both receptors influence neural activity by controlling the synthesis of neurotransmitters, neuromodulators, their receptors, and their actions (McEwen, 1987). There is some recent evidence that GRs can initiate rapid non‐genomic signalling from the membrane (Rainville et al., 2017), but the best known function of GRs and MRs is their ability to act as nuclear transcription factors. When bound to their ligand, they undergo a conformational change and translocate to the nucleus where they act as a transcription factor (Groeneweg, Karst, de Kloet, & Joels, 2012). The GR is ubiquitously expressed on cells throughout the body and the brain, with highest expression occurring in the PVN of the hypothalamus and subregions of the hippocampus (Joels, Karst, & Sarabdjitsingh, 2018; Panagiotakopoulos & Neigh, 2014). GRs preferentially bind glucocorticoids when circulating glucocorticoid levels are high and provide negative feedback at the limbic, hypothalamic, and pituitary level (Groeneweg et al., 2012; Joels et al., 2018). MRs which are most highly expressed in the hippocampus have a stronger affinity for glucocorticoids and bind them under basal conditions (Joels et al., 2018). Overall, GRs mediate the stress‐induced changes in the cortisol/corticosterone levels, whereas the MRs are thought to mediate tonic influences of cortisol or corticosterone. However, it was recently found that a decrease in MRs and an altered GR/MR ratio are also associated with depression (Klok et al., 2011; Wu et al., 2013).
Figure 1.
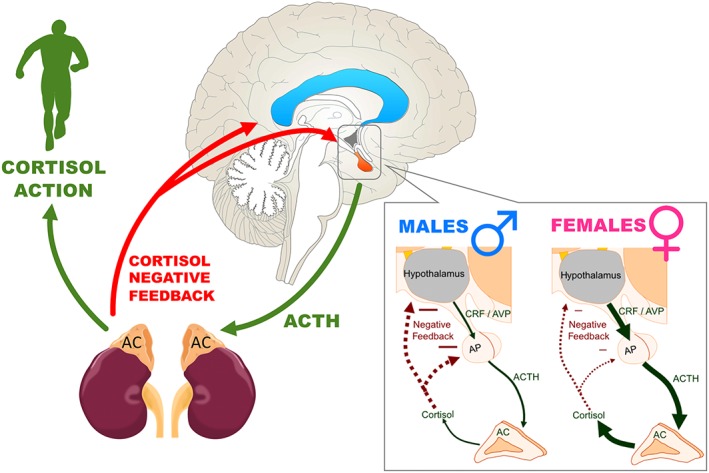
Schematic representing the hypothalamus–pituitary–adrenal (HPA) axis, its activation that results from cortisol's action, as well as cortisol's negative feedback in the brain. On the right, sex differences in the HPA axis response to stress are presented (based on rodent studies). Compared to males (left panel), females (right panel) have greater stress‐induced release of CRF, AVP, ACTH, and cortisol, which could be related to an increased sensitivity of their CRF receptors. Negative feedback (shown with the red dotted arrows) is lower in females and this could result in further enhanced release of stress hormones by the adrenal cortex (AC). Figure adapted from Bangasser and Valentino (2014). ACTH, adrenocorticotropic hormone; AP, anterior pituitary; AVP, arginine vasopressin; CRF, corticotropin releasing factor
2.2. Neuropeptide regulation of the HPA axis
Two neuropeptides, CRF and AVP, synthesized in the parvocellular division of the PVN, and their receptor systems complement the regulation of the HPA axis. CRF activates the HPA axis via the release of ACTH but it is also associated with the behavioural response to stress (Heinrichs & Koob, 2004). Studies indicate that CRF may be hyper‐secreted from both hypothalamic and extra‐hypothalamic neurons in depression (Todorovic, Jahn, Tezval, Hippel, & Spiess, 2005). Similarly, CRF receptors can be found in both hypothalamic and extra‐hypothalamic neurons and are classified in two subtypes, CRF1 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=213. AVP also has ACTH‐releasing properties when administered in humans and in combination with CRF has a synergistic effect resulting in a much greater ACTH response, with both peptides required for maximal stimulation of the HPA axis (Favrod‐Coune et al., 1993). The central vasopressin system is anatomically and functionally separated from the periphery by the blood–brain barrier and regulates behavioural CNS‐mediated responses, including learning and memory, and social behaviours (Ermisch, Brust, Kretzschmar, & Ruhle, 1993). The distribution of the vasopressin receptors in the brain, classified in http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=366, V1B (also named V3), and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=368, is more wide‐spread in comparison to CRF receptors but substantially less than the GR. Circulating glucocorticoids exert a negative feedback effect on the expression of CRF and AVP in parvocellular PVN neurons and the AVP gene, in particular, is targeted by this suppression (Kovacs, Foldes, & Sawchenko, 2000). The mRNA levels of both CRF and the CRF1 receptors are reduced by elevated glucocorticoid levels whereas the mRNA levels of the V1B receptor and subsequent coupling to PLC can be stimulated by glucocorticoids. This effect, in turn, may contribute to the refractoriness of AVP‐stimulated ACTH secretion to glucocorticoid feedback (Aguilera & Rabadan‐Diehl, 2000). Therefore, regulation of the HPA axis by parvocellular AVP is likely critical for sustaining HPA axis activity during chronic stress (Herman & Tasker, 2016), although chronic stress still induces high adrenocortical hormone levels in the absence of AVP (Zelena et al., 2004) and it is probable that whether AVP or CRF is more resistant to glucocorticoid feedback is situation‐ and stressor‐specific.
3. SEX DIFFERENCES IN THE HPA AXIS
There are sex differences in the HPA axis itself, which result in sex‐dependent responses to stress, and sex steroids are thought to be important modulators of the sex‐differentiated stress response (Patchev & Almeida, 1998; Young, 1995). The interaction of gonadal steroid hormones with the HPA axis has been discussed recently in an excellent review (Handa & Weiser, 2014), so it is not described herein.
3.1. Human studies
It has been shown that women with depression have higher cortisol levels than non‐depressed women, whereas this is not always the case in men (Young & Korszun, 2009). Moreover, following stressful events, the HPA axis of depressed patients was less reactive in comparison to healthy controls, but interestingly, this blunted HPA response was less evident in depressed women (Peeters, Nicholson, & Berkhof, 2003). As reviewed extensively by Young and Korszun, gonadal hormones and the circadian rhythm play an important role in modulating the HPA axis in depressed women (Young & Korszun, 2009). One way to examine HPA axis dysregulation in depression is with the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2768 suppression test, a procedure in which the synthetic glucocorticoid dexamethasone is administered and thereafter, changes in cortisol levels are monitored. If the negative feedback of the HPA axis is intact, cortisol levels decline (called suppression) following dexamethasone administration, whereas if there is a dysregulated HPA axis feedback, then the cortisol levels are not suppressed. A further refinement of this procedure is the combined DEX/CRF test, in which dexamethasone (DEX) is administered the day before sampling, and on the testing day, a CRF infusion is performed. In healthy humans, this CRF infusion is not expected to cause an increase in cortisol as the HPA axis should be suppressed by the dexamethasone administration the day before. In the presence of a dysregulated HPA axis negative feedback, the CRF infusion results in a spike of cortisol, despite the previous dexamethasone administration. These tests are useful, because only 30% of depressed patients present clear hypercortisolaemia, whereas as many as 66% of depressed patients display non‐suppression. Moreover, these tests present significant sex differences given that as low as 25% of premenopausal women may present non‐suppression of the HPA axis (Young, Carlson, & Brown, 2001). Indeed, in one study, only 44% of premenopausal women showed non‐suppression following dexamethasone, in comparison to 81% of postmenopausal women, with baseline cortisol and menopausal status explaining 65% of the variance (Young et al., 1993). These sex differences in the frequency of the HPA axis non‐suppression may have significantly impacted the success of clinical trials of HPA axis‐related compounds (Figure 2). Baseline cortisol, female sex, age, and menopausal status, as well as circadian rhythms were found to significantly affect the sensitivity of the negative feedback mechanism of the HPA axis. It is, therefore, concluded that in depressed patients there is a more reliable cortisol hypersecretion in male patients, and the dysregulated negative feedback of the HPA axis in response to glucocorticoids is also more reliably observed in male patients (Young, 1998; Young et al., 1993; Young et al., 2001).
Figure 2.
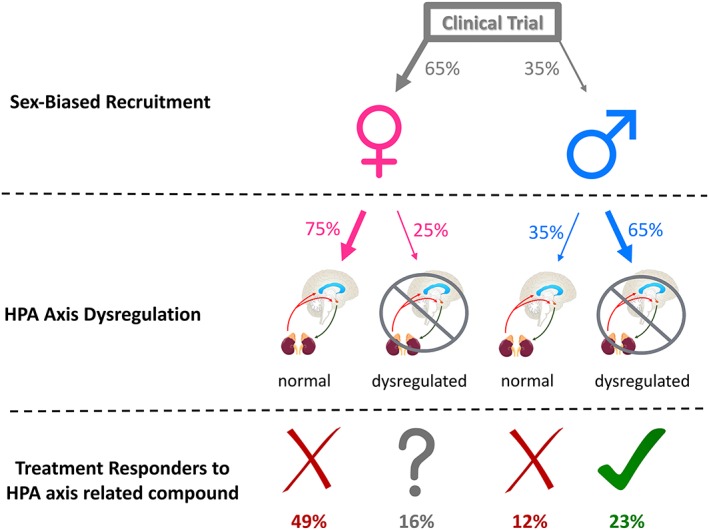
A theoretical model predicting the chance of success of an HPA axis‐related compound in a randomized clinical trial. It is assumed that recruitment will consist of roughly 65% women (e.g., Step 1 of the STAR*D trial and see also Figure 3), and approximately one out of four women, whereas two out of three men will display an HPA axis dysregulation as evidenced by the dexamethasone suppression test or the combined dexamethasone/CRF suppression test (see Young et al., 2001). Based on current evidence reviewed herein, the response of depressed women with HPA axis dysregulation to an HPA axis‐related compound is questionable; therefore, only two out 10 patients recruited would benefit from an HPA axis‐related compound and the clinical trial would fail
3.2. Rodent studies
In rodents, females have higher basal corticosterone levels than males (Kokras et al., 2012), but not all studies find this sex difference, as corticosterone levels seem to depend on strain, age, time of sampling, housing conditions, diet, and gonadal hormone levels (Bangasser & Valentino, 2014; Dalla et al., 2005; Kokras et al., 2018). Moreover, sex differences in globulin corticosteroid binding have been reported in rats and this could influence the amount of unbound, active corticosterone that reaches the brain in each sex (Gala & Westphal, 1966). Interestingly, it seems that globulin corticosteroid binding is positively regulated by oestrogens and negatively by stress (Oyola & Handa, 2017).
Moreover, exposure to stress causes a greater increase in CRF, ACTH, and corticosterone in female than in male rats (Rivier, 1999; Viau, Bingham, Davis, Lee, & Wong, 2005; Figure 1). However, when female rats were adrenalectomized and substituted with stable corticosterone levels, their behavioural response to the stressful forced swim test procedure was not altered, suggesting that stress‐induced HPA axis activation does not play an important role in the female behavioural response to stressful stimuli (Kokras et al., 2012). Similarly, adrenalectomy did not prevent the detrimental effects of acute stress (30 min of tail shock in a restrainer tube) on learning in female rats, whereas it abolished the stress‐induced enhancement in male conditioning (Wood, Beylin, & Shors, 2001). Following a single bout of prolonged stress (combination of 2‐hr tube‐restraint stress, a physiological stressor, group forced swim stress, and a brief exposure to diethyl ether), female rats, but not males, were found to be unaffected when the dexamethasone suppression test was applied (Pooley et al., 2018). Overall, these findngs suggest that although HPA axis activation in response to acute stress is more pronounced in female rats than males, this activation is not directly associated with the acute behavioural stress response in female rats.
Nevertheless, depression has mainly been associated with exposure to chronic unpredictable stress, but chronic stress studies in female rats are less common (Kokras & Dalla, 2014). In one study using chronic unpredictable stress, female sham‐operated (i.e., ovaries intact) stressed rats did not exhibit a dysregulated response to stress following the dexamethasone suppression test, but ovariectomized females did (Mahmoud, Wainwright, Chaiton, Lieblich, & Galea, 2016). Moreover, the HPA axis dysregulation in ovariectomized female rats was not reversed by antidepressant treatment (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=203 5 mg·kg−1; Mahmoud et al., 2016). Although a discussion of all the studies on sex differences in the stress response is beyond the scope of this review, there is preclinical evidence that stress effects in males are more closely linked to the HPA axis function, whereas the female stress response seems to be less dependent on the HPA axis activation and dysregulation following experimental stress procedures.
4. SEX DIFFERENCES IN THE GR/MR SYSTEM
There are notable sex differences in the expression and function of glucocorticoid and mineralocorticoid receptors that affect glucocorticoid sensitivity (Panagiotakopoulos & Neigh, 2014; Patchev & Almeida, 1998). These effects are both region‐ and cell type‐specific, which further complicates the efficacy of treatments that act as simple agonist/antagonists.
4.1. Human studies
To date, no studies have examined sex differences in MR expression in human post‐mortem brain tissue or anywhere in the body within the context of mood disorders. However, there is some indication that sex differences in GR expression exist, at least in depressed subjects. A study of GR expression in the human amygdala found increases in patients with major depression but not bipolar depression (Wang et al., 2014). Further stratification of the samples by sex determined that only women with major depressive disorder differed significantly from controls. A higher expression of GR was also found in the post‐mortem hippocampus of elderly women with depression compared to age‐matched men (Wang, Joels, Swaab, & Lucassen, 2012). In healthy humans, there were sex differences in GR expression located within leukocytes, which varied with the type of white blood cell measured (Loi et al., 2017). Overall, men had a higher expression of GRs than women in white blood cells suggesting that sex differences in the brain and periphery did not match. Together, these data indicate sex differences in GR and MR expression/function in the brain and body and represent a possible pitfall for the development of treatments for mood disorders that act by regulating the HPA axis.
4.2. Rodent studies
In rats, the use of a single prolonged stress protocol, which models post‐traumatic stress disorder, oppositely regulated GR number in the PVN and the hippocampus of males and females (Pooley et al., 2018). Stress up‐regulated GR expression in the PVN of male rats but down‐regulated it in the hippocampus, whereas the opposite occurred in stressed females. Male and female rats responded differently to the trauma behaviourally, and as mentioned previously, females failed to show dexamethasone suppression following exposure to a subsequent acute restraint stress. These effects were largely independent of circulating levels of gonadal hormones (Pooley et al., 2018). Studies using chronic mild stress have also reported regional sex differences including a down‐regulation of both GRs and MRs only occurring in the hypothalamus of female rats (Lu et al., 2015). Additionally, the sex of an animal impacts the translocation of the GR to the nucleus (Bourke et al., 2013) through an interaction with gonadal hormones (Sheng et al., 2003). Transgenic studies in mice further support the premise that there are sex‐based differences for the involvement of GRs in stress sensitivity that are region‐ and cell type‐specific. GR knockout in the forebrain increased depression‐like behaviour in male but not in female mice (Solomon et al., 2012), whereas GR knockout on noradrenergic neurons induced depression‐like behaviour only in female mice and blocked subsequent effects of stress in males (Chmielarz et al., 2013). GR knockout in the PVN, however, had no effect on stress‐associated behaviour in either sex but induced opposite effects on circulating levels of ACTH following acute restraint stress (Solomon et al., 2015). Transgenic studies in MR also support the idea that effects are sex and region specific. Forebrain‐specific knockout of MR led to a generalization of fear response across types of cues and inability to extinguish fear learning in females but not in male mice (Ter Horst, Carobrez, van der Mark, de Kloet, & Oitzl, 2012). Overall it seems that GR or MR knockout results in region and cell type specific effects on behavior that differ by sex.
5. GR/MR MODULATORS AS POTENTIAL ANTIDEPRESSANTS
5.1. Mifepristone
The interest in using GR antagonists as a treatment for psychiatric illness stemmed from research on a treatment of symptoms for Cushing's disease. Notably, case studies from the 1980s and 90s demonstrated that treatment with the synthetic steroid http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2805, which competitively binds GRs and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=627, resolved psychosis, depression, and suicidal ideation in male and female Cushing's patients (Nieman et al., 1985; van der Lely, Foeken, van der Mast, & Lamberts, 1991).
In rodent models, mifepristone had antidepressant‐like effects in males across a multitude of different stress paradigms and behavioural endpoints (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, 2007; Wu et al., 2007). The only mention of behavioural reversal in females was a study on maternal separation stress that used mixed litters (Aisa et al., 2007). A more recent study failed to reverse the effects of maternal deprivation in females using mifepristone (Loi et al., 2017). No other studies have examined behavioural effects of mifepristone treatment in females. Other endpoints have been examined in females, such as blocking the forced swim‐induced apoptosis of adult‐born new neurons in the hippocampus (Llorens‐Martin & Trejo, 2011).
While rodent models have primarily demonstrated effects of mifepristone in males, in humans the data are mixed. Early studies, with extremely low numbers of patients, suggested that perimenopausal women responded best to the drug (Murphy, Filipini, & Ghadirian, 1993). However, larger scale studies (~200 patients, ~50% female) had mixed results depending upon which response criteria were used (DeBattista et al., 2006) or failed to show a difference from placebo on decreasing symptoms in depressed patients (433 patients/60% female; Blasey, Block, Belanoff, & Roe, 2011). Interestingly, age may be a factor in detecting a positive response of the drug. In the 2006 study, the mean age of subjects was ~40 ± 10. The largest group of patients tested were aged 35–64 (68%) with an additional 29% of patients listed as below 34 years of age. The 2011 study did not provide data on the age breakdown, but the average age of patients was ~45 ± 11. In the 2011 study, there was a significantly higher rate of efficacy on their response criterion in patients with high plasma levels of mifepristone (above 1,660 ng·ml−1), indicating that individual differences in drug metabolism may contribute to successful responding (Blasey et al., 2011). However, in this study, age was not examined as a factor. Additional studies have also failed to reveal significant effects of mifepristone on depression rating scales in patients with bipolar depression (60 patients/600 mg·day−1/50% female, average age 48± 9 years), although it did improve spatial performance (Watson et al., 2012). It should be noted that none of the studies have directly compared responses in women to men or examined the menopausal status of women even though mifepristone preferentially binds progesterone receptors and only occupies GRs at higher doses. Age and sex are likely contributing factors to the success or failure of these clinical trials, particularly in females. The above studies did not take into consideration that the drug might be acting by influencing http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2377 or its metabolites, which are implicated in post‐partum depression (Osborne et al., 2017) and in other psychiatric diseases involving psychotic symptoms, such as bipolar disorder and schizophrenia (Sun, Walker, Dean, van den Buuse, & Gogos, 2016).
5.2. Spironolactone and fludrocortisone
There is some indication that targeting the MR is a potential treatment for depression. In male mice, treatment with the MR antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2875 reversed the effects of chronic corticosterone exposure on behaviour in the forced swim test and novel object recognition test (Wu et al., 2013). There is also evidence that MR antagonists have greater effects on cortisol levels in patients with some forms of depression. Studies using the MR antagonist spironolactone in healthy controls found that multiple doses increased cortisol secretion (Young, Lopez, Murphy‐Weinberg, Watson, & Akil, 1998). There are conflicting reports in patients with depression, one study in patients who already had elevated levels of cortisol only found increased levels after treatment in healthy controls (Hinkelmann et al., 2016), whereas another reported greater increases in cortisol secretion in depressed patients compared to healthy controls (Young, Lopez, Murphy‐Weinberg, Watson, & Akil, 2003).
Additionally, patients with depression have greater cognitive empathy than healthy controls and treatment with spironolactone reduced cognitive empathy to levels reported in controls (Wingenfeld et al., 2016). However, others have reported no effect of spironolactone on cortisol levels in patients with treatment‐resistant depression but increases in cortisol levels in healthy controls (Juruena et al., 2013). Initially, it was reported that no effect of spironolactone was found in women suffering from premenstrual syndrome (Burnet, Radden, Easterbrook, & McKinnon, 1991), but a subsequent longer term study did report significant decreases in negative mood related symptoms after six treatment cycles (Wang, Hammarback, Lindhe, & Backstrom, 1995). Importantly, spironolactone has well‐known anti‐androgen effects (Menard, Stripp, & Gillette, 1974) that could influence the response of male and female patients with depression.
A study comparing the response of patients (25 males and 24 females) with psychotic depression, non‐psychotic depression, and healthy controls to the synthetic mineralocorticoid http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2873 found that after treatment, only patients with psychotic depression differed in their cortisol response from healthy controls (Lembke et al., 2013). Specifically, in the patients with psychotic depression (eight males and six females) cortisol levels were suppressed less following dexamethasone administration, which also lasted for less time in comparison to the other groups. Co‐treatment with fludrocortisone during http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7547 treatment of patients with depression was demonstrated to shorten the period to remission in responders, whereas spironolactone had no effect (Otte et al., 2010). Given that spironolactone and fludrocortisone have opposite effects on MRs but are both reported to have antidepressant potential, it is possible that MRs have an inverted “u”‐shaped function in relation to depression. Alternatively, the drugs may exert their effects via different epigenetic or plasticity‐associated mechanisms, which may account for the fact that both an increase and a decrease in MR activity provides antidepressant responses. Therefore, more research should be conducted on the effects of modulating MR activity on mood.
6. SEX DIFFERENCES IN THE CRF SYSTEM
6.1. Human studies
CRF has long been implicated in the pathophysiology of depression. Indeed, some depressed patients had high levels of CRF in their CSF and these were normalized when patients respond to treatment, suggesting that these altered CRF levels contributed to symptomology (Banki, Karmacsi, Bissette, & Nemeroff, 1992; Heuser et al., 1998). In the CNS, patients had high levels of CRF in the PVN and in monoaminergic nuclei, including the locus coeruleus and raphe (Austin, Janosky, & Murphy, 2003; Bissette, Klimek, Pan, Stockmeier, & Ordway, 2003). Although this evidence clearly points to CRF dysregulation in depression, whether there are sex differences in this dysregulation remains unknown because these previous studies either only used one sex (e.g., Austin et al., 2003; Heuser et al., 1998) or the results were not compared between the sexes. In healthy humans, peripheral administration of CRF caused an increased ACTH response in women compared to men (Gallucci et al., 1993). This result suggests that greater CRF‐induced HPA axis activation in women is a potential mechanism contributing to the higher female risk for stress‐associated mood disorders. In addition to activating the HPA axis, CRF acts centrally, often at extra‐hypothalamic sites, to alter cognition, mood, and arousal (for review, see Bangasser, Eck, Telenson, & Salvatore, 2018).
6.2. Rodent studies
In rodents, sex differences have been found in every aspect of CRF function from the inputs that regulate CRF neurons to CRF's postsynaptic efficacy (for review, see Bangasser & Wiersielis, 2018). In the PVN, there are several reports that female rats had greater CRF expression than males (Duncko, Kiss, Skultetyova, Rusnak, & Jezova, 2001; Viau et al., 2005), although this difference was not found in every rat study or with the Crh‐IRES‐Cre::Ai14 (tdTomato) reporter mouse (Sterrenburg et al., 2012; Walker, Cornish, Lawrence, & Campbell, 2018). CRF‐binding protein, which binds free CRF to reduce its bioavailability, was higher in the pituitary of female than male mice (Speert, McClennen, & Seasholtz, 2002), perhaps to compensate for greater hypothalamic CRF production in females. In rodents, there are also well‐documented sex differences in CRF receptors. For example, there was increased CRF1 receptor binding in the amygdala, nucleus accumbens, and cortex of adult female rats but increased CRF2 receptor binding in the amygdala, bed nucleus of the stria terminalis, and hypothalamus in adult male rats (Weathington, Hamki, & Cooke, 2014). These changes likely reflect differences in receptor number, but CRF receptors can also be differentially distributed on distinct cell types in males versus females. In the CA1 region of the hippocampus, CRF receptors colocalized more with opioid http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=317‐containing dendrites in female rats, while in the dorsal raphe, the CRF1 receptor was more prominent on parvalbumin‐containing http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067 neurons in male mice (Howerton et al., 2014; Williams, Akama, Knudsen, McEwen, & Milner, 2011). Sex differences in CRF1 receptors in the locus coeruleus rendered neurons of females more sensitive to CRF than in the locus coeruleus neurons of males (Curtis, Bethea, & Valentino, 2006). This increased sensitivity of female locus coeruleus to CRF was linked to a greater coupling of the CRF1 receptors to Gs in female compared to male rats and increased activation of the cAMP‐PKA signalling cascade (Bangasser et al., 2010). An additional sex difference in the locus coeruleus has been found for CRF1 receptor internalization, or the trafficking of the receptor from the plasma membrane to the cytosol where it can no longer be activated. Both acute stress (15 min of swim stress) in rats and CRF hypersecretion, as modelled by CRF overexpressing mice, induced CRF1 receptor internalization in males, but these manipulations did not cause internalization in females (Bangasser et al., 2010; Bangasser et al., 2013). This sex difference in internalization was associated with greater β‐arrestin binding to the CRF1 receptor in males, which was consistent with β‐arrestin's role in trafficking receptors (Bangasser et al., 2010). Receptor internalization is thought to be compensatory, allowing for the cellular adaptation to excess CRF release. In fact, locus coeruleus neurons of CRF‐overexpressing male mice fired at a rate similar to wild type levels, despite their production of excess CRF (Bangasser et al., 2013). Locus coeruleus neurons of CRF‐overexpressing female mice, in comparison, fired three times faster than wild type controls. These sex differences in CRF1 receptor signalling and trafficking in the locus coeruleus would bias females towards greater arousal in response to stress. As hyperarousal can contribute to some symptoms in depressed patients, such as agitation, restlessness, a lack of concentration, and sleep disturbance, sex differences in CRF modulation of the locus coeruleus‐arousal system may bias females towards certain features of depression.
The findings of sex differences in the CRF system have important clinical implications. There has been an effort to develop CRF1 receptor antagonists for the treatment of depression and other stress‐related disorders in humans. This effort is based on the preclinical data that CRF1 antagonists consistently reduce depressive‐like behaviour; however, these preclinical studies were almost exclusively conducted in male rodents (Chaki et al., 2004; Deak et al., 1999; Mansbach, Brooks, & Chen, 1997; Schulz et al., 1996; Zorrilla, Valdez, Nozulak, Koob, & Markou, 2002). The efficacy of such antagonists in female rodents has not been a major focus of study. Howerton et al. (2014) did compare the effect of CRF1 antagonist administration into the dorsal raphe in male versus female rats and found that it only reduced anxiety in males. The dorsal raphe is just one target of CRF regulation, but this result does highlight how CRF1 antagonists are not equally effective in both sexes. Moreover, the aforementioned data revealed that, in the locus coeruleus, the CRF1 receptor binds proteins differently in males versus females (Bangasser et al., 2010) suggesting a sex difference in the conformation of the receptor that could alter how CRF1 antagonists bind to the receptor causing differential effects in males versus females. These studies highlight the need for the inclusion of female rodents in preclinical evaluations of CRF1 receptor pharmacotherapies.
7. CRF ANTAGONISTS AS POTENTIAL ANTIDEPRESSANTS
Based on the strong preclinical data in males to support CRF1 antagonist efficacy, the pharmaceutical industry developed many small molecule CRF1 antagonists (Murrough & Charney, 2017; Figure 3). The first CRF1 antagonist to enter clinical testing was http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3520 (formerly NBI‐30775). R121919 was tested in a small uncontrolled clinical study in both sexes (11 men and nine women), and some effects were found in reducing depression, but its development was not continued, due to concerns about potential hepatotoxicity (Künzel et al., 2003; Zobel et al., 2000). The bulk of its preclinical testing was done exclusively in male experimental animals (Keck et al., 2001; Keck, Welt, Müller, Landgraf, & Holsboer, 2003; Lancel, Müller‐Preuss, Wigger, Landgraf, & Holsboer, 2002). For example, in male HAB rats, R121919 was shown to be effective in attenuating stress‐induced (exposure to elevated plus maze) hormonal and behavioural changes and in particular, it reversed the decreased testosterone levels either through a central (pituitary) or direct testicular mode of action (Keck et al., 2003). However, a preclinical study, which included female rats was conducted much later than when the clinical development project evolved (Gutman et al., 2008). Interestingly, in this study, R121919 did not exert clear antidepressant effects, as it had opposite results (either enhanced struggling or enhanced floating) in male and female rats bred for low and high activity in a modified swim test respectively (Gutman et al., 2008). Another study using oestrogen‐primed female rats showed that a CRF1 antagonist (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3489, CP‐156,181) reversed or attenuated restraint stress‐induced hormonal abnormalities (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1159, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5049, corticosterone, and progesterone), pointing to the importance of accounting for hormonal effects in studies with HPA axis‐based drugs (Traslavina & Franci, 2011).
Figure 3.
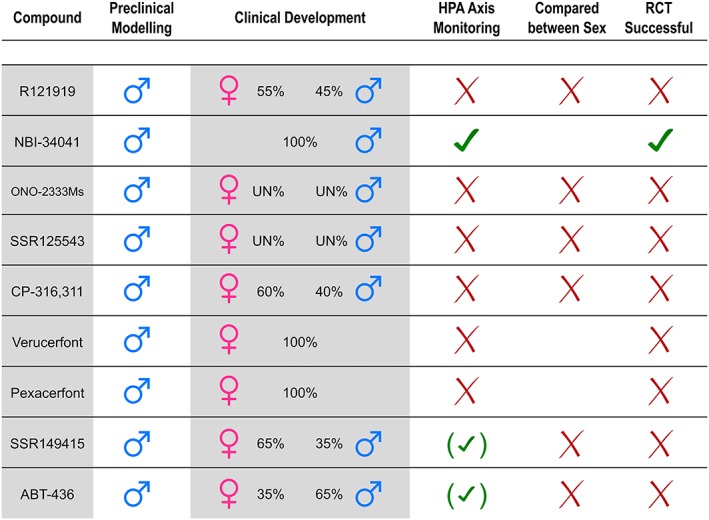
Summary of compounds that have entered into formal clinical testing (Phase II/III randomized control trials) as potential antidepressants. “Preclinical modelling” refers to animal models for depression used to screen for antidepressant potential in preclinical studies of each compound. Percentages of men and women included in clinical testing were calculated and pooled in the case of more than one published study for each compound. “HPA axis monitoring refers” to whether the clinical trial protocol included a functional estimation of the HPA axis of patients with depression. Signs in parentheses means that only some of the pooled studies performed an estimation. “Compared between Sex” refers to whether statistical provisions were made to stratify (not control/adjust) for sex differences. In some cases, only one sex was tested in the clinical development, so we do know the outcome for the sex tested, but the outcome for the other sex remains unknown. “RCT Successful” refers to whether one or more of the clinical trials showed a positive antidepressant response for at least one dose of the compound of interest. Note that no compound has gained regulatory approval, as yet. UN, unknown. RCT, randomized controlled trial
Following those promising results, several other CRF1 antagonists were entered into clinical development programmes (Figure 3). NBI‐34041/SB723620 entered into a Phase I clinical study in 24 healthy males only. Interestingly, the preclinical in vitro testing of the inhibition of ACTH by NBI‐34041 was performed in cells originating from female rats but further in vivo preclinical testing was performed in male rats only. The compound attenuated the behavioural stress response and related neuroendocrine indices in humans and rodents, but the development did not continue (Ising et al., 2007). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=clinical&ligandId=10376 was tested in a Phase II clinical study for recurrent depression and lacked efficacy in a population of 278 men and women (NCT00514865). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=3533 was not effective in reducing depressive symptoms in 580 patients, again of both sexes (NCT01034995). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=clinical&ligandId=10377 was prematurely terminated as it lacked efficacy in 118 men and women with depression (Binneman et al., 2008). Another CRF1 antagonist, verucerfont, lacked efficacy in a clinical trial of 150 depressed women, and no men (NCT00733980), despite the fact that the original behavioural testing was done in male animals (Fabio et al., 2008). Similarly, another CRF1 antagonist, pexacerfont, was unsuccessfully tested in 270 women with depression but was not tested at all in men (NCT00135421).
It should be noted that most preclinical studies of CRF1 antagonists were performed in male animals and many clinical studies recruited predominantly or exclusively women. There is clearly a need to include female rodents in drug development prior to moving novel compounds into clinical trials. Moreover, none of the clinical studies that included patients of both sexes performed an analysis by sex and in some of those studies, an antidepressant, such as a selective 5‐HT (serotonin) reuptake inhibitor (SSRI), was included as an additional treatment arm for comparison purposes—which statistically implied that a CRF1 antagonist should be at least as effective in both sexes as an SSRI in monotherapy.
8. SEX DIFFERENCES IN THE AVP SYSTEM
8.1. Human studies
The AVP system has also been implicated in depression: depressed patients had higher plasma levels of AVP than controls (van Londen et al., 1997), and in a small study of nine depressed patients, fluoxetine treatment resulted in a significant decrease of AVP in CSF (De Bellis, Gold, Geracioti, Listwak, & Kling, 1993). AVP peripheral levels were increased in depressed patients, although interestingly, this finding was restricted to the subgroup of depressed patients with prominent anxiety symptoms (De Winter et al., 2003). Moreover, an analysis of post‐mortem tissue revealed more AVP and V1A receptor expression in the PVN of patients with depression relative to those without (Purba, Hoogendijk, Hofman, & Swaab, 1996; Wang, Kamphuis, Huitinga, Zhou, & Swaab, 2008). Unfortunately, these studies did not compare data by sex. In healthy subjects, sex has been examined, and plasma AVP levels were positively correlated with distress in a pair‐bonded relationship in men, but not in women (Taylor, Saphire‐Bernstein, & Seeman, 2010) and recently it was shown that V1A antagonists attenuated amygdala activation in response to aversive stimuli only in male subjects (Lee et al., 2013).
8.2. Rodent studies
In response to stress, hypothalamic AVP expression is also preferentially altered in males. Specifically, chronic variable mild stress increased AVP in the PVN of male, but not in female mice (Karisetty, Khandelwal, Kumar, & Chakravarty, 2017). However, the effects of stress on hypothalamic AVP can vary. In Californian mice, in which both sexes exhibit territorial aggression, social defeat stress immediately activated AVP neurons in the PVN of both sexes (Steinman et al., 2015). However, 4–9 weeks after stressor cessation, there was a decrease in AVP in the PVN but this was only observed in males (Steinman et al., 2015). Thus, although the direction of AVP regulation may change based on species, time course, and/or stress manipulation, the sex that was most affected (i.e., males) remains consistent, and this was further replicated in AVP‐deficient Brattleboro rats (Fodor et al., 2016). There are also well‐documented sex differences in the extra‐hypothalamic AVP system. Compared to female rats, there were more AVP neurons in the medial amygdala and bed nucleus of the stria terminalis of males and these neurons sent denser projections to several locations, including the septum (De Vries & Panzica, 2006). These anatomical sex differences were established by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2858 (Han & De Vries, 2003). There are also sex differences in V1A receptor binding, such that male rats had higher binding densities than females in eight out of 21 forebrain regions (Dumais & Veenema, 2016). However, such sex differences in V1A receptors are not reported in all species, and sex differences in V1B receptor expression have not been assessed due to a lack of good tools (Dumais & Veenema, 2016). Functionally, extra‐hypothalamic AVP is thought to primarily mediate social behaviour (e.g., pair bonding, social recognition, and aggression; Donaldson & Young, 2008; Veenema, Beiderbeck, Lukas, & Neumann, 2010), which is altered in depression (Weightman, Air, & Baune, 2014). Consistent with the sex differences in hypothalamic AVP, extra‐hypothalamic AVP typically drives changes in social behaviour more in male than in female animals (Dumais & Veenema, 2016). As an example, partner preference was facilitated by AVP in male, but not female prairie voles (Cushing, Martin, Young, & Carter, 2001). Based on these preclinical findings, it is thought that social deficits in depression are more likely to be driven by AVP in males than in females.
9. AVP ANTAGONISTS AS POTENTIAL ANTIDEPRESSANTS
Based on the role of AVP in the stress system, several AVP antagonists were tested as potential antidepressants (Figure 3). V1A antagonists are currently being studied for the treatment of anxiety disorders, post‐traumatic stress disorder, and aggression (NCT02733614, NCT03036397, and NCT02055638). It is unclear if these studies monitor the effect of V1 antagonists on gonadal hormonal levels, as a V1A antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2223 has also been found to prevent alterations in testosterone serum levels in male rats exposed to repeated restraint stress (Gray, Innala, & Viau, 2012). With regard to V1B antagonists, they were studied as potential antidepressants and, therefore, they are the focus of this section.
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2202 is a non‐peptide V1B antagonist, which showed promising results in preclinical behavioural testing in male rodents (Griebel, Stemmelin, Gal, & Soubrié, 2005), but at that time, it was not tested preclinically in female rodents. Interestingly, recently, it was shown that SSR149415 reversed behavioral abnormalities, as well as reduced testosterone levels and gonadal atrophy in male mice exposed to social defeat stress (Wang et al., 2017). SSR149415 was tested in a clinical development programme as a candidate antidepressant, and as reviewed later (Griebel, Beeské, & Stahl, 2012), all clinical trials failed to show a beneficial effect for SSR149415. Interestingly, those clinical trials included patients of both sexes, but the reproductive status of these patients was not mentioned. Moreover, there was seldom any plan for an analysis by sex and analysis taking into consideration the HPA axis dysregulation (possibly using an HPA axis functional test like dexamethasone suppression test or DEX/CRF test). Another V1B antagonist that entered into clinical development was ABT‐436. This antagonist had shown efficacy in preclinical testing using male, but not female, rodents (Van Gaalen et al., 2008). Clinical testing of ABT‐436 also included patients of both sexes with no provisions about potential sex differences or sex‐dependent drug effects and ABT‐436 was not effective in reducing depression symptoms (Katz, Locke, Greco, Liu, & Tracy, 2017). Another Phase II clinical trial, which was prematurely terminated, with just 19 recruited patients out of 216 planned (NCT01741142) also planned to include patients with depression of both sexes, but no results have been published since its termination.
10. OVERALL DISCUSSION
We summarized evidence regarding profound sex differences in several key components of the HPA axis, as demonstrated from preclinical experimental studies in rodents. Limited data from studies on humans also support the existence of significant sex differences in the regulation of the HPA axis (Young, 1998). These sex differences appear to extend to the GR, MR, CRF, and AVP ligands that were tested as candidate antidepressants. However, due to the inertia of preclinical research, most of the evidence for the HPA axis and its response to the administration of various ligands still comes from studies in male rodents (Beery & Zucker, 2011). This creates a problem in translating experimental data to human therapeutics because the preclinical data generated are not necessarily valid for both sexes or, specifically, for psychiatric diseases, such as depression, which present sex differences (Kokras & Dalla, 2017). Moreover, compounds preclinically tested in male experimental animals enter in clinical trials that tend to recruit more, if not exclusively, women than men. Thankfully, regarding the first problem of generating preclinical data from male animals, the recent National Institutes of Health initiative about justifying the selection of sex in preclinical studies is expected to address the issue in the coming years. However, the problem of correctly translating preclinical research remains unresolved. Since the National Institutes of Health guideline about the inclusion of women in clinical trials (Merkatz, Temple et al., 1993), no other formal initiative has been taken regarding the sex‐aware methodology in clinical trials.
Indeed, regarding clinical trials of HPA‐related ligands as potential antidepressants, a number of other reasons, such as target selection, design, and analysis of trials, may have contributed to the failure of novel neuropsychiatric compounds (reviewed in Bespalov et al., 2016). Specifically, for HPA axis‐based compounds, other possible reasons for the observed failures could be related to drug design, as well as pharmacological parameters of new molecules. For example, for CRF antagonists, possible reasons for failure have been reviewed recently in an excellent manuscript dedicated to the influential work of the late Dr. A. Markou (Spierling & Zorrilla, 2017). These include pharmacokinetic issues, such as very high lipophilicity and enhanced toxicity of early CRF antagonists. Later, slower receptor dissociation rates were identified as imperative for optimum pharmacological action of CRF antagonists, but when verucerfont, which had these promising properties and positive animal data, was tested in clinical trials, it failed again (Spierling & Zorrilla, 2017). Other issues regarding CRF antagonists include differences in receptor subtypes between different species (e.g., CRF2 subtypes in humans) and percentage of receptor occupancy. Therefore, it was suggested that ideal candidates should be small molecules, non‐selective antagonists at both CRF subtypes, with inverse agonist activity, or with modulatory activity to CRF‐binding protein or to receptor activity modifying protein 2, as well (Gingell et al., 2016; Weston et al., 2016; Wootten, Christopoulos, & Sexton, 2013). However, although all these properties have been identified by the academic community and the industry, to date, no HPA‐related compound has been successful as an antidepressant. Specifically, for CRF antagonists, revised hypotheses have been proposed, but most of them remain to be tested (Spierling & Zorrilla, 2017). Moreover, these HPA axis‐based compounds are tested for the treatment of other psychiatric diseases, including schizophrenia, or as cognitive enhancers, whereas CRF‐specific antagonists are tested for stress‐induced eating or alcohol abuse and AVP antagonists are tested against aggression (Epstein et al., 2016; Soria et al., 2018). Their effects could be exerted either by their main pharmacodynamic actions or by off‐target effects that also need to be carefully evaluated before entering clinical trials.
Nevertheless, even if ideal molecules are discovered, preclinical models are fully validated and human techniques are refined to the degree that adequate dosing and receptor occupancy in the brain is achieved, again it is imperative to design clinical trials, in such a way that they are valid and predictive (Figure 4). In this context, it cannot go unnoticed that there is a stark mismatch of the chosen sex between preclinical and clinical studies. As reviewed herein, preclinical studies have been conducted mainly in males, whereas in clinical trials, recruitment involved patients of both sexes and those trials lacked the appropriate design and statistical power for a proper analysis by sex (see text above and Figure 3). Moreover, in some cases, relevant clinical trials have exclusively recruited women, despite preclinical evidence coming from male animals, but they did not account for reproductive status or assess changes in gonadal hormonal levels. Several preclinical studies demonstrate that pharmaceutical manipulations of the HPA axis result in sex‐dependent responses and may induce changes in gonadal hormones. A slow growing body of preclinical evidence suggests that the male behavioural response is more affected by HPA axis manipulations than the female. This confirms earlier human observations, generated by the pioneering work of E.A. Young, on the more reliable cortisol hypersecretion from male depressed patients and on the modest frequency of hypercortisolaemia and low rates of dexamethasone non‐suppression in female depressed patients (Young, 1998; Young et al., 1993; Young et al., 2001). Additionally, the possibility that the important pharmacokinetic and pharmacodynamics sex differences that exist could also influence drug response in clinical trials should be taken into consideration (Kokras & Dalla, 2017). Taking all these arguments into consideration, we conclude in this review that another valid and quite probable reason for the failure of the development of HPA‐related antidepressants is the possibility that those candidate antidepressants might be more effective or suitable for men than for women, and this effectiveness may very well also be modulated by a sex × age (including menopausal status) interaction (Van Cauter, Leproult, & Kupfer, 1996).
Figure 4.

Panel (a) shows that if a novel treatment is tested in preclinical studies in male rodents (in blue) and it proves to be effective, but then in clinical studies, it is tested only/mainly in women, it is possible that it will fail. On the other hand, Panel (b) illustrates that if a novel therapy is tested in preclinical studies in both male and female rodents (in blue and orange) and sex differences are assessed, then there are two possible approaches: If sex differences are identified and male animals differ from female animals in the drug response, then clinical trials should be stratified by sex. If males and females do not differ, then both men and women should be included in the study, in order to have equal representation of both sexes. If clinical trials are designed and performed correctly, based on solid translational methodology of preclinical studies, then they have an enhanced probability to show a successful outcome
Another important consideration is that most, if not all, animal models of depression simulate or induce a dysfunctional HPA axis and drugs are tested in this altered HPA axis milieu (Kokras & Dalla, 2014). In fact, many animal models claim to successfully mimic the HPA axis dysregulation, “as observed in depressed patients.” A potential problem is that patients with depression do not necessarily present such HPA axis dysregulation. As noted, chronic dysregulation of the HPA axis is observed only in a subset of depressed patients (van Londen et al., 1997; Young et al., 2001). When data are compared between sex, the frequency of HPA axis dysregulation is lower in women, and preclinical data typically support this sex difference following stress or antidepressant treatment (Kokras et al., 2012; Kokras, Sotiropoulos, Pitychoutis, Almeida, & Papadopoulou‐Daifoti, 2011). It is evident that when developing an HPA axis‐related antidepressant, preclinical results generated in a model with a dysregulated/overactive HPA axis in male rodents will probably not apply to human depressed patients many of which do not present an HPA axis dysregulation. However, to date, relevant clinical trials have underestimated these issues.
Progress also has been hampered on translating preclinical findings into useful clinical treatments because of an incomplete assessment of why compounds fail. Typically, when there is a discrepancy between results derived from preclinical research and from clinical trials, the validity of animal models is questioned. However, it is not clear why it is expected that preclinical findings based on only male animals tested in behavioural assays designed to induce HPA axis dysregulation should be replicated clinically in both men and women with unknown HPA axis status. In this traditional approach, it appears that the validity of animal models is not the major problem, but rather the issues lie in poor optimization of the translational process from preclinical to clinical studies. Therefore, failed clinical trials do not necessarily invalidate animal models but generate important questions about the translational process. Obviously, other issues such as the lack of data robustness, data generalizability, and target engagement when designing and evaluating preclinical studies should also be carefully taken into consideration (Bespalov et al., 2016). However, the academic community, as well as the industry, should take advantage of preclinical knowledge and thoroughly evaluate and interpret preclinical experiments before implementing clinical trials (Kokras & Dalla, 2017).
Clinical trials of HPA axis‐related compounds follow diagnostic criteria as set by the Diagnostic and Statistical Manual of Mental Disorders and do not make distinctions between male and female depressed patients with and without HPA axis dysregulation. The selection of patients in clinical trials has been repeatedly addressed previously (Zimmerman, Chelminski, & Posternak, 2005), as well as the heterogeneity of the syndromal diagnosis of depression (Goldberg, 2011). In relation to this, in most clinical trials there is a lack of patient stratification both in terms of sex, as mentioned before (Ferretti, Women's, & Galea, 2018), but also in terms of HPA axis or reproductive status. However, this is an egg/chicken issue: there is no need to stratify the population of patients with depression based on sex and HPA axis or reproductive status, as there are no available treatments that specifically target those subpopulations. On the other hand, if there was a licensed antidepressant drug more effective in men or women (pre‐ or post‐menopausal), with or without HPA axis dysregulation, then it would be clinically and diagnostically meaningful to identify such subgroups and that would also be reflected in future revisions of the diagnostic criteria. However, for this to take place, clinical trials must take into account the sex, reproductive status, related co‐morbidities, as well as the HPA axis dysregulation, and formally screen for efficacy in those subgroups.
Currently, the pharmaceutical industry is focused on clinical development programmes aiming at a monotherapy for depression with a universally effective novel blockbuster. As such, CRF/AVP formal clinical development aimed at discovering an SSRI analogue that would be effective for more than 65% of depressed patients, irrespective of their sex or other factors. Thus, we are still searching for a drug acting as a “silver bullet” for the treatment of depression. However, based on current knowledge, reviewed earlier herein, it is probably not reasonable to expect HPA axis druggable targets to reveal an antidepressant treatment for every patient with depression. It is possible for example that an HPA axis‐related compound, which would not be effective for every depressed patient, would be effective only in male depressed patients with impaired negative feedback of the HPA axis. Also, unexplored is the possibility of augmenting current monoaminergic treatments with HPA‐related compounds in those patients who present a dysregulated HPA axis while receiving monoaminergic antidepressant treatment.
11. CONCLUSION
In this review, it is suggested that in the search for a “silver bullet”—a new antidepressant treatment that will be effective for every patient—we may have lost, in the clinical testing of HPA axis‐related compounds, an opportunity for either augmentation of available antidepressant treatments or for a targeted‐personalized antidepressant treatment based on sex and the state of the HPA axis. Moreover, based on the data reviewed herein, we question the translational process from preclinical experiments performed in male only animals to clinical trials involving the opposite sex or both sexes.
11.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2017), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Cidlowski et al., 2017).
CONFLICT OF INTEREST
N.K. and C.D. have received honoraria and financial support from Janssen‐Cilag and Elpen S.A. None of those is relevant to this study.
ACKNOWLEDGEMENTS
This work was supported by the National Science Foundation USA (Grant NSF CAREER IOS‐1552416 to D.A.B.), NKUA‐ELKE1650, and a Brain and Behavior foundation NARSAD young investigator award to G.E.H. C.D., G.E.H., and D.A.B. would like to thank Dr Tracey Shors for inspiring us to study sex differences and for her continued mentorship and friendship. C.D. and N.K. would also like to thank Dr O.F.X. Almeida for his continued mentorship and friendship.
Kokras N, Hodes GE, Bangasser DA, Dalla C. Sex differences in the hypothalamic–pituitary–adrenal axis: An obstacle to antidepressant drug development?. Br J Pharmacol. 2019;176:4090–4106. 10.1111/bph.14710
REFERENCES
- Aguilera, G. , & Rabadan‐Diehl, C. (2000). Vasopressinergic regulation of the hypothalamic‐pituitary‐adrenal axis: Implications for stress adaptation. Regulatory Peptides, 96, 23–29. 10.1016/S0167-0115(00)00196-8 [DOI] [PubMed] [Google Scholar]
- Aisa, B. , Tordera, R. , Lasheras, B. , Del Rio, J. , & Ramirez, M. J. (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology, 32, 256–266. 10.1016/j.psyneuen.2006.12.013 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … Sharman, J. L. (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Southan, C. (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, M. C. , Janosky, J. E. , & Murphy, H. A. (2003). Increased corticotropin‐releasing hormone immunoreactivity in monoamine‐containing pontine nuclei of depressed suicide men. Molecular Psychiatry, 8, 324–332. 10.1038/sj.mp.4001250 [DOI] [PubMed] [Google Scholar]
- Bangasser, D. A. , Curtis, A. , Reyes, B. A. , Bethea, T. T. , Parastatidis, I. , Ischiropoulos, H. , … Valentino, R. J. (2010). Sex differences in corticotropin‐releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress‐related psychopathology. Molecular Psychiatry, 15(877), 896–904. 10.1038/mp.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser, D. A. , Eck, S. R. , Telenson, A. M. , & Salvatore, M. (2018). Sex differences in stress regulation of arousal and cognition. Physiology & Behavior, 187, 42–50. 10.1016/j.physbeh.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser, D. A. , Reyes, B. A. , Piel, D. , Garachh, V. , Zhang, X. Y. , Plona, Z. M. , … Valentino, R. J. (2013). Increased vulnerability of the brain norepinephrine system of females to corticotropin‐releasing factor overexpression. Molecular Psychiatry, 18, 166–173. 10.1038/mp.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser, D. A. , & Valentino, R. J. (2014). Sex differences in stress‐related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology, 35, 303–319. 10.1016/j.yfrne.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser, D. A. , & Wiersielis, K. R. (2018). Sex differences in stress responses: A critical role for corticotropin‐releasing factor. Hormones, 17, 5–13. 10.1007/s42000-018-0002-z [DOI] [PubMed] [Google Scholar]
- Banki, C. M. , Karmacsi, L. , Bissette, G. , & Nemeroff, C. B. (1992). CSF corticotropin‐releasing hormone and somatostatin in major depression: Response to antidepressant treatment and relapse. European Neuropsychopharmacology, 2, 107–113. 10.1016/0924-977X(92)90019-5 [DOI] [PubMed] [Google Scholar]
- Beery, A. K. , & Zucker, I. (2011). Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews, 35, 565–572. 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov, A. , Steckler, T. , Altevogt, B. , Koustova, E. , Skolnick, P. , Deaver, D. , … Witkin, J. (2016). Failed trials for central nervous system disorders do not necessarily invalidate preclinical models and drug targets. Nature Reviews Drug Discovery, 15, 516 10.1038/nrd.2016.88 [DOI] [PubMed] [Google Scholar]
- Binneman, B. , Feltner, D. , Kolluri, S. , Shi, Y. , Qiu, R. , & Stiger, T. (2008). A 6‐week randomized, placebo‐controlled trial of CP‐316,311 (a selective CRH1 antagonist) in the treatment of major depression. The American Journal of Psychiatry, 165, 617–620. 10.1176/appi.ajp.2008.07071199 [DOI] [PubMed] [Google Scholar]
- Bissette, G. , Klimek, V. , Pan, J. , Stockmeier, C. , & Ordway, G. (2003). Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology, 28, 1328–1335. 10.1038/sj.npp.1300191 [DOI] [PubMed] [Google Scholar]
- Blasey, C. M. , Block, T. S. , Belanoff, J. K. , & Roe, R. L. (2011). Efficacy and safety of mifepristone for the treatment of psychotic depression. Journal of Clinical Psychopharmacology, 31, 436–440. 10.1097/JCP.0b013e3182239191 [DOI] [PubMed] [Google Scholar]
- Bourke, C. H. , Raees, M. Q. , Malviya, S. , Bradburn, C. A. , Binder, E. B. , & Neigh, G. N. (2013). Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex‐dependent manner. Psychoneuroendocrinology, 38, 84–93. 10.1016/j.psyneuen.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer, M. A. , Deeg, D. J. H. , Beekman, A. T. F. , Penninx, B. W. J. H. , Lips, P. , & Hoogendijk, W. J. G. (2007). Major depression in late life is associated with both hypo‐and hypercortisolemia. Biological Psychiatry, 62, 479–486. 10.1016/j.biopsych.2006.11.033 [DOI] [PubMed] [Google Scholar]
- Burnet, R. B. , Radden, H. S. , Easterbrook, E. G. , & McKinnon, R. A. (1991). Premenstrual syndrome and spironolactone. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 31, 366–368. 10.1111/j.1479-828X.1991.tb02824.x [DOI] [PubMed] [Google Scholar]
- Chaki, S. , Nakazato, A. , Kennis, L. , Nakamura, M. , Mackie, C. , Sugiura, M. , … Steckler, T. (2004). Anxiolytic‐ and antidepressant‐like profile of a new CRF1 receptor antagonist, R278995/CRA0450. European Journal of Pharmacology, 485, 145–158. [DOI] [PubMed] [Google Scholar]
- Chmielarz, P. , Kusmierczyk, J. , Parlato, R. , Schutz, G. , Nalepa, I. , & Kreiner, G. (2013). Inactivation of glucocorticoid receptor in noradrenergic system influences anxiety‐ and depressive‐like behavior in mice. PLoS ONE, 8, e72632 10.1371/journal.pone.0072632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, J. A. , & Collins, F. S. (2014). Policy: NIH to balance sex in cell and animal studies. Nature, 509, 282–283. 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, A. L. , Bethea, T. , & Valentino, R. J. (2006). Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin‐releasing factor. Neuropsychopharmacology, 31, 544–554. 10.1038/sj.npp.1300875 [DOI] [PubMed] [Google Scholar]
- Cushing, B. S. , Martin, J. O. , Young, L. J. , & Carter, C. S. (2001). The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Hormones and Behavior, 39, 48–58. 10.1006/hbeh.2000.1633 [DOI] [PubMed] [Google Scholar]
- Dalla, C. , Antoniou, K. , Drossopoulou, G. , Xagoraris, M. , Kokras, N. , Sfikakis, A. , & Papadopoulou‐Daifoti, Z. (2005). Chronic mild stress impact: Are females more vulnerable? Neuroscience, 135, 703–714. 10.1016/j.neuroscience.2005.06.068 [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D. , Gold, P. W. , Geracioti, T. D. , Listwak, S. J. , & Kling, M. A. (1993). Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin‐releasing hormone and arginine vasopressin in patients with major depression. The American Journal of Psychiatry, 150(4), 656–657. [DOI] [PubMed] [Google Scholar]
- De Vries, G. J. , & Panzica, G. C. (2006). Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience, 138, 947–955. 10.1016/j.neuroscience.2005.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter, R. F. P. , van Hemert, A. M. , DeRijk, R. H. , Zwinderman, K. H. , Frankhuijzen‐Sierevogel, A. C. , Wiegant, V. M. , & Goekoop, J. G. (2003). Anxious–retarded depression: Relation with plasma vasopressin and cortisol. Neuropsychopharmacology, 28, 140–147. 10.1038/sj.npp.1300002 [DOI] [PubMed] [Google Scholar]
- Deak, T. , Nguyen, K. T. , Ehrlich, A. L. , Watkins, L. R. , Spencer, R. L. , Maier, S. F. , … Gold, P. W. (1999). The impact of the nonpeptide corticotropin‐releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress**This research was supported by NIMH Grant MH‐50479 and the Undergraduate Research Opportunities Program at the University of Colorado at Boulder. Endocrinology, 140, 79–86. 10.1210/endo.140.1.6415 [DOI] [PubMed] [Google Scholar]
- DeBattista, C. , Belanoff, J. , Glass, S. , Khan, A. , Horne, R. L. , Blasey, C. , … Alva, G. (2006). Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biological Psychiatry, 60, 1343–1349. 10.1016/j.biopsych.2006.05.034 [DOI] [PubMed] [Google Scholar]
- Donaldson, Z. R. , & Young, L. J. (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322, 900–904. 10.1126/science.1158668 [DOI] [PubMed] [Google Scholar]
- Dumais, K. M. , & Veenema, A. H. (2016). Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex‐specific regulation of social behavior. Frontiers in Neuroendocrinology, 40, 1–23. 10.1016/j.yfrne.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncko, R. , Kiss, A. , Skultetyova, I. , Rusnak, M. , & Jezova, D. (2001). Corticotropin‐releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology, 26, 77–89. 10.1016/S0306-4530(00)00040-8 [DOI] [PubMed] [Google Scholar]
- Epstein, D. H. , Kennedy, A. P. , Furnari, M. , Heilig, M. , Shaham, Y. , Phillips, K. A. , & Preston, K. L. (2016). Effect of the CRF1‐receptor antagonist pexacerfont on stress‐induced eating and food craving. Psychopharmacology, 233, 3921–3932. 10.1007/s00213-016-4424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermisch, A. , Brust, P. , Kretzschmar, R. , & Ruhle, H. J. (1993). Peptides and blood‐brain barrier transport. Physiological Reviews, 73, 489–527. 10.1152/physrev.1993.73.3.489 [DOI] [PubMed] [Google Scholar]
- Fabio, R. D. , St‐Denis, Y. , Sabbatini, F. M. , Andreotti, D. , Arban, R. , Bernasconi, G. , … Castiglioni, E. (2008). Synthesis and pharmacological characterization of novel druglike corticotropin‐releasing factor 1 antagonists. Journal of Medicinal Chemistry, 51, 7370–7379. 10.1021/jm800744m [DOI] [PubMed] [Google Scholar]
- Favrod‐Coune, C. , Raux‐Demay, M. C. , Proeschel, M. F. , Bertagna, X. , Girard, F. , & Luton, J. P. (1993). Potentiation of the classic ovine corticotrophin releasing hormone stimulation test by the combined administration of small doses of lysine vasopressin. Clinical Endocrinology, 38, 405–410. 10.1111/j.1365-2265.1993.tb00522.x [DOI] [PubMed] [Google Scholar]
- Ferretti, M. T. , Women's, B. P. , & Galea, L. A. M. (2018). Improving pharmacological treatment in brain and mental health disorders: The need for gender and sex analyses. Frontiers in Neuroendocrinology, 50, 1–2. 10.1016/j.yfrne.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Fodor, A. , Kovács, K. B. , Balázsfi, D. , Klausz, B. , Pintér, O. , Demeter, K. , … Nadal, R. (2016). Depressive‐and anxiety‐like behaviors and stress‐related neuronal activation in vasopressin‐deficient female Brattleboro rats. Physiology & Behavior, 158, 100–111. 10.1016/j.physbeh.2016.02.041 [DOI] [PubMed] [Google Scholar]
- Gala, R. R. , & Westphal, U. (1966). Further studies on the corticosteroid‐binding globulin in the rat: Proposed endocrine control. Endocrinology, 79, 67–76. 10.1210/endo-79-1-67 [DOI] [PubMed] [Google Scholar]
- Gallucci, W. T. , Baum, A. , Laue, L. , Rabin, D. S. , Chrousos, G. P. , Gold, P. W. , & Kling, M. A. (1993). Sex differences in sensitivity of the hypothalamic‐pituitary‐adrenal axis. Health Psychology, 12, 420–425. 10.1037/0278-6133.12.5.420 [DOI] [PubMed] [Google Scholar]
- Gibbons, J. L. (1964). Cortisol secretion rate in depressive illness. Archives of General Psychiatry, 10, 572–575. 10.1001/archpsyc.1964.01720240026004 [DOI] [PubMed] [Google Scholar]
- Gingell, J. J. , Simms, J. , Barwell, J. , Poyner, D. R. , Watkins, H. A. , Pioszak, A. A. , … Hay, D. L. (2016). An allosteric role for receptor activity‐modifying proteins in defining GPCR pharmacology. Cell Discovery, 2, 16012 10.1038/celldisc.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, P. W. , & Chrousos, G. P. (1999). The endocrinology of melancholic and atypical depression: Relation to neurocircuitry and somatic consequences. Proceedings of the Association of American Physicians, 111, 22–34. 10.1046/j.1525-1381.1999.09423.x [DOI] [PubMed] [Google Scholar]
- Goldberg, D. (2011). The heterogeneity of “major depression”. World Psychiatry, 10, 226–228. 10.1002/j.2051-5545.2011.tb00061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, M. , Innala, L. , & Viau, V. (2012). Central vasopressin V1A receptor blockade impedes hypothalamic‐pituitary‐adrenal habituation to repeated restraint stress exposure in adult male rats. Neuropsychopharmacology, 37, 2712–2719. 10.1038/npp.2012.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel, G. , Beeské, S. , & Stahl, S. M. (2012). The vasopressin V1b receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: Results from 4 randomized, double‐blind, placebo‐controlled studiese. Journal of Clinical Psychiatry, 73, 1403–1411. 10.4088/JCP.12m07804 [DOI] [PubMed] [Google Scholar]
- Griebel, G. , Stemmelin, J. , Gal, C. S.‐L. , & Soubrié, P. (2005). Non‐peptide vasopressin V1b receptor antagonists as potential drugs for the treatment of stress‐related disorders. Current Pharmaceutical Design, 11, 1549–1559. 10.2174/1381612053764797 [DOI] [PubMed] [Google Scholar]
- Groeneweg, F. L. , Karst, H. , de Kloet, E. R. , & Joels, M. (2012). Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology, 350, 299–309. 10.1016/j.mce.2011.06.020 [DOI] [PubMed] [Google Scholar]
- Gutman, D. A. , Coyer, M. J. , Boss‐Williams, K. A. , Owens, M. J. , Nemeroff, C. B. , & Weiss, J. M. (2008). Behavioral effects of the CRF1 receptor antagonist R121919 in rats selectively bred for high and low activity in the swim test. Psychoneuroendocrinology, 33, 1093–1101. 10.1016/j.psyneuen.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Han, T. M. , & De Vries, G. J. (2003). Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. Journal of Neurobiology, 54, 502–510. 10.1002/neu.10157 [DOI] [PubMed] [Google Scholar]
- Handa, R. J. , & Weiser, M. J. (2014). Gonadal steroid hormones and the hypothalamo‐pituitary‐adrenal axis. Frontiers in Neuroendocrinology, 35, 197–220. 10.1016/j.yfrne.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Anderton, S. (2017). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs, S. C. , & Koob, G. F. (2004). Corticotropin‐releasing factor in brain: A role in activation, arousal, and affect regulation. The Journal of Pharmacology and Experimental Therapeutics, 311, 427–440. 10.1124/jpet.103.052092 [DOI] [PubMed] [Google Scholar]
- Herman, J. P. (2013). Neural control of chronic stress adaptation. Frontiers in Behavioral Neuroscience, 7, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, J. P. , & Tasker, J. G. (2016). Paraventricular hypothalamic mechanisms of chronic stress adaptation. Frontiers in Endocrinology (Lausanne), 7, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser, I. , Bissette, G. , Dettling, M. , Schweiger, U. , Gotthardt, U. , Schmider, J. , … Holsboer, F. (1998). Cerebrospinal fluid concentrations of corticotropin‐releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: Response to amitriptyline treatment. Depression and Anxiety, 8, 71–79. [DOI] [PubMed] [Google Scholar]
- Hinkelmann, K. , Hellmann‐Regen, J. , Wingenfeld, K. , Kuehl, L. K. , Mews, M. , Fleischer, J. , … Otte, C. (2016). Mineralocorticoid receptor function in depressed patients and healthy individuals. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 71, 183–188. 10.1016/j.pnpbp.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Holsboer, F. (2003). High‐quality antidepressant discovery by understanding stress hormone physiology. Annals of the new York Academy of Sciences, 1007, 394–404. [DOI] [PubMed] [Google Scholar]
- Howerton, A. R. , Roland, A. V. , Fluharty, J. M. , Marshall, A. , Chen, A. , Daniels, D. , … Bale, T. L. (2014). Sex differences in corticotropin‐releasing factor receptor‐1 action within the dorsal raphe nucleus in stress responsivity. Biological Psychiatry, 75, 873–883. 10.1016/j.biopsych.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising, M. , Zimmermann, U. S. , Künzel, H. E. , Uhr, M. , Foster, A. C. , Learned‐Coughlin, S. M. , … Grigoriadis, D. E. (2007). High‐affinity CRF 1 receptor antagonist NBI‐34041: Preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology, 32, 1941–1949. 10.1038/sj.npp.1301328 [DOI] [PubMed] [Google Scholar]
- Joels, M. , Karst, H. , & Sarabdjitsingh, R. A. (2018). The stressed brain of humans and rodents. Acta Physiologica (Oxford, England), 223, e13066 10.1111/apha.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena, M. F. , Pariante, C. M. , Papadopoulos, A. S. , Poon, L. , Lightman, S. , & Cleare, A. J. (2013). The role of mineralocorticoid receptor function in treatment‐resistant depression. Journal of Psychopharmacology, 27, 1169–1179. 10.1177/0269881113499205 [DOI] [PubMed] [Google Scholar]
- Karisetty, B. C. , Khandelwal, N. , Kumar, A. , & Chakravarty, S. (2017). Sex difference in mouse hypothalamic transcriptome profile in stress‐induced depression model. Biochemical and Biophysical Research Communications, 486, 1122–1128. 10.1016/j.bbrc.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Katz, D. A. , Locke, C. , Greco, N. , Liu, W. , & Tracy, K. A. (2017). Hypothalamic‐pituitary‐adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one‐week randomized phase 1b trial. Brain and Behavior: A Cognitive Neuroscience Perspective, 7, e00628 10.1002/brb3.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck, M. E. , Welt, T. , Müller, M. B. , Landgraf, R. , & Holsboer, F. (2003). The high‐affinity non‐peptide CRH1 receptor antagonist R121919 attenuates stress‐induced alterations in plasma oxytocin, prolactin, and testosterone secretion in rats. Pharmacopsychiatry, 36, 27–31. 10.1055/s-2003-38092 [DOI] [PubMed] [Google Scholar]
- Keck, M. E. , Welt, T. , Wigger, A. , Renner, U. , Engelmann, M. , Holsboer, F. , & Landgraf, R. (2001). The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. European Journal of Neuroscience, 13, 373–380. 10.1046/j.0953-816X.2000.01383.x [DOI] [PubMed] [Google Scholar]
- Klok, M. D. , Alt, S. R. , Irurzun Lafitte, A. J. , Turner, J. D. , Lakke, E. A. , Huitinga, I. , … Derijk, R. H. (2011). Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. Journal of Psychiatric Research, 45, 871–878. 10.1016/j.jpsychires.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Kokras, N. , & Dalla, C. (2014). Sex differences in animal models of psychiatric disorders. British Journal of Pharmacology, 171, 4595–4619. 10.1111/bph.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras, N. , & Dalla, C. (2017). Preclinical sex differences in depression and antidepressant response: Implications for clinical research. Journal of Neuroscience Research, 95, 731–736. 10.1002/jnr.23861 [DOI] [PubMed] [Google Scholar]
- Kokras, N. , Dalla, C. , Sideris, A. C. , Dendi, A. , Mikail, H. G. , Antoniou, K. , & Papadopoulou‐Daifoti, Z. (2012). Behavioral sexual dimorphism in models of anxiety and depression due to changes in HPA axis activity. Neuropharmacology, 62, 436–445. 10.1016/j.neuropharm.2011.08.025 [DOI] [PubMed] [Google Scholar]
- Kokras, N. , Pastromas, N. , Papasava, D. , de Bournonville, C. , Cornil, C. A. , & Dalla, C. (2018). Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology, 87, 93–107. 10.1016/j.psyneuen.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Kokras, N. , Sotiropoulos, I. , Pitychoutis, P. M. , Almeida, O. F. , & Papadopoulou‐Daifoti, Z. (2011). Citalopram‐mediated anxiolysis and differing neurobiological responses in both sexes of a genetic model of depression. Neuroscience, 194, 62–71. 10.1016/j.neuroscience.2011.07.077 [DOI] [PubMed] [Google Scholar]
- Kovacs, K. J. , Foldes, A. , & Sawchenko, P. E. (2000). Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. The Journal of Neuroscience, 20, 3843–3852. 10.1523/JNEUROSCI.20-10-03843.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzel, H. E. , Zobel, A. W. , Nickel, T. , Ackl, N. , Uhr, M. , Sonntag, A. , … Holsboer, F. (2003). Treatment of depression with the CRH‐1‐receptor antagonist R121919: Endocrine changes and side effects. Journal of Psychiatric Research, 37, 525–533. 10.1016/S0022-3956(03)00070-0 [DOI] [PubMed] [Google Scholar]
- Lancel, M. , Müller‐Preuss, P. , Wigger, A. , Landgraf, R. , & Holsboer, F. (2002). The CRH1 receptor antagonist R121919 attenuates stress‐elicited sleep disturbances in rats, particularly in those with high innate anxiety. Journal of Psychiatric Research, 36, 197–208. 10.1016/S0022-3956(02)00009-2 [DOI] [PubMed] [Google Scholar]
- Lee, R. J. , Coccaro, E. F. , Cremers, H. , McCarron, R. , Lu, S. F. , Brownstein, M. J. , & Simon, N. G. (2013). A novel V1a receptor antagonist blocks vasopressin‐induced changes in the CNS response to emotional stimuli: An fMRI study. Frontiers in Systems Neuroscience, 7, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembke, A. , Gomez, R. , Tenakoon, L. , Keller, J. , Cohen, G. , Williams, G. H. , … Schatzberg, A. F. (2013). The mineralocorticoid receptor agonist, fludrocortisone, differentially inhibits pituitary‐adrenal activity in humans with psychotic major depression. Psychoneuroendocrinology, 38, 115–121. 10.1016/j.psyneuen.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens‐Martin, M. , & Trejo, J. L. (2011). Mifepristone prevents stress‐induced apoptosis in newborn neurons and increases AMPA receptor expression in the dentate gyrus of C57/BL6 mice. PLoS ONE, 6, e28376 10.1371/journal.pone.0028376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi, M. , Mossink, J. C. , Meerhoff, G. F. , Den Blaauwen, J. L. , Lucassen, P. J. , & Joels, M. (2017). Effects of early‐life stress on cognitive function and hippocampal structure in female rodents. Neuroscience, 342, 101–119. 10.1016/j.neuroscience.2015.08.024 [DOI] [PubMed] [Google Scholar]
- Lu, J. , Wu, X. Y. , Zhu, Q. B. , Li, J. , Shi, L. G. , Wu, J. L. , … Bao, A. M. (2015). Sex differences in the stress response in SD rats. Behavioural Brain Research, 284, 231–237. 10.1016/j.bbr.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Mahmoud, R. , Wainwright, S. R. , Chaiton, J. A. , Lieblich, S. E. , & Galea, L. A. M. (2016). Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle‐aged female rats. Neuropharmacology, 107, 278–293. 10.1016/j.neuropharm.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Mansbach, R. S. , Brooks, E. N. , & Chen, Y. L. (1997). Antidepressant‐like effects of CP‐154,526, a selective CRF1 receptor antagonist. European Journal of Pharmacology, 323, 21–26. 10.1016/S0014-2999(97)00025-3 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (1987). Glucocorticoid‐biogenic amine interactions in relation to mood and behavior. Biochemical Pharmacology, 36, 1755–1763. 10.1016/0006-2952(87)90234-6 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. , & Gianaros, P. J. (2011). Stress‐and allostasis‐induced brain plasticity. Annual Review of Medicine, 62, 431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard, R. H. , Stripp, B. , & Gillette, J. R. (1974). Spironolactone and testicular cytochrome P‐450: Decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology, 94, 1628–1636. 10.1210/endo-94-6-1628 [DOI] [PubMed] [Google Scholar]
- Merkatz, R. B. , Temple, R. , Sobel, S. , Feiden, K. , Kessler, D. A. , & Working Group on Women in Clinical T (1993). Women in clinical trials of new drugs—A change in food and drug administration policy. New England Journal of Medicine, 329, 292–296. [DOI] [PubMed] [Google Scholar]
- Murphy, B. E. , Filipini, D. , & Ghadirian, A. M. (1993). Possible use of glucocorticoid receptor antagonists in the treatment of major depression: Preliminary results using RU 486. Journal of Psychiatry & Neuroscience, 18, 209–213. [PMC free article] [PubMed] [Google Scholar]
- Murrough, J. W. , & Charney, D. S. (2017). Corticotropin‐releasing factor type 1 receptor antagonists for stress‐related disorders: Time to call it quits? Biological Psychiatry, 82, 858–860. 10.1016/j.biopsych.2017.10.012 [DOI] [PubMed] [Google Scholar]
- Nieman, L. K. , Chrousos, G. P. , Kellner, C. , Spitz, I. M. , Nisula, B. C. , Cutler, G. B. , … Loriaux, D. L. (1985). Successful treatment of Cushing's syndrome with the glucocorticoid antagonist RU 486. The Journal of Clinical Endocrinology and Metabolism, 61, 536–540. 10.1210/jcem-61-3-536 [DOI] [PubMed] [Google Scholar]
- Osborne, L. M. , Gispen, F. , Sanyal, A. , Yenokyan, G. , Meilman, S. , & Payne, J. L. (2017). Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology, 79, 116–121. 10.1016/j.psyneuen.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte, C. , Hinkelmann, K. , Moritz, S. , Yassouridis, A. , Jahn, H. , Wiedemann, K. , & Kellner, M. (2010). Modulation of the mineralocorticoid receptor as add‐on treatment in depression: A randomized, double‐blind, placebo‐controlled proof‐of‐concept study. Journal of Psychiatric Research, 44, 339–346. 10.1016/j.jpsychires.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Oyola, M. G. , & Handa, R. J. (2017). Hypothalamic‐pituitary‐adrenal and hypothalamic‐pituitary‐gonadal axes: Sex differences in regulation of stress responsivity. Stress, 20, 476–494. 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakopoulos, L. , & Neigh, G. N. (2014). Development of the HPA axis: Where and when do sex differences manifest? Frontiers in Neuroendocrinology, 35, 285–302. 10.1016/j.yfrne.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Patchev, V. K. , & Almeida, L. O. F. (1998). Gender specificity in the neural regulation of the response to stress: New leads from classical paradigms. Molecular Neurobiology, 16, 63–77. 10.1007/BF02740603 [DOI] [PubMed] [Google Scholar]
- Peeters, F. , Nicholson, N. A. , & Berkhof, J. (2003). Cortisol responses to daily events in major depressive disorder. Psychosomatic Medicine, 65, 836–841. 10.1097/01.PSY.0000088594.17747.2E [DOI] [PubMed] [Google Scholar]
- Pooley, A. E. , Benjamin, R. C. , Sreedhar, S. , Eagle, A. L. , Robison, A. J. , Mazei‐Robison, M. S. , … Jordan, C. L. (2018). Sex differences in the traumatic stress response: The role of adult gonadal hormones. Biology of Sex Differences, 9, 32 10.1186/s13293-018-0192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba, J. S. , Hoogendijk, W. G. , Hofman, M. A. , & Swaab, D. F. (1996). Increased number of vasopressin‐ and oxytocin‐expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Archives of General Psychiatry, 53, 137–143. 10.1001/archpsyc.1996.01830020055007 [DOI] [PubMed] [Google Scholar]
- Rainville, J. R. , Weiss, G. L. , Evanson, N. , Herman, J. P. , Vasudevan, N. , & Tasker, J. G. (2017). Membrane‐initiated nuclear trafficking of the glucocorticoid receptor in hypothalamic neurons. Steroids, 142, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier, C. (1999). Gender, sex steroids, corticotropin‐releasing factor, nitric oxide, and the HPA response to stress. Pharmacology Biochemistry and Behavior, 64, 737–751. 10.1016/S0091-3057(99)00148-3 [DOI] [PubMed] [Google Scholar]
- Schule, C. (2007). Neuroendocrinological mechanisms of actions of antidepressant drugs. Journal of Neuroendocrinology, 19, 213–226. 10.1111/j.1365-2826.2006.01516.x [DOI] [PubMed] [Google Scholar]
- Schulz, D. W. , Mansbach, R. S. , Sprouse, J. , Braselton, J. P. , Collins, J. , Corman, M. , … Heym, J. (1996). CP‐154,526: A potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proceedings of the National Academy of Sciences of the United States of America, 93, 10477–10482. 10.1073/pnas.93.19.10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye, H. (1936). A syndrome produced by diverse nocuous agents. Nature, 138, 32 10.1038/138032a0 [DOI] [PubMed] [Google Scholar]
- Sheng, Z. , Yanai, A. , Fujinaga, R. , Kawano, J. , Tanaka, M. , Watanabe, Y. , & Shinoda, K. (2003). Gonadal and adrenal effects on the glucocorticoid receptor in the rat hippocampus, with special reference to regulation by estrogen from an immunohistochemical view‐point. Neuroscience Research, 46, 205–218. 10.1016/S0168-0102(03)00056-7 [DOI] [PubMed] [Google Scholar]
- Solomon, M. B. , Furay, A. R. , Jones, K. , Packard, A. E. , Packard, B. A. , Wulsin, A. C. , & Herman, J. P. (2012). Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression‐like behavior in males but not females. Neuroscience, 203, 135–143. 10.1016/j.neuroscience.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, M. B. , Loftspring, M. , de Kloet, A. D. , Ghosal, S. , Jankord, R. , Flak, J. N. , … Herman, J. P. (2015). Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology, 156, 2843–2853. 10.1210/en.2015-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria, V. , Gonzalez‐Rodriguez, A. , Huerta‐Ramos, E. , Usall, J. , Cobo, J. , Bioque, M. , … Labad, J. (2018). Targeting hypothalamic‐pituitary‐adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: A systematic review and narrative synthesis. Psychoneuroendocrinology, 93, 8–19. 10.1016/j.psyneuen.2018.04.012 [DOI] [PubMed] [Google Scholar]
- Speert, D. B. , McClennen, S. J. , & Seasholtz, A. F. (2002). Sexually dimorphic expression of corticotropin‐releasing hormone‐binding protein in the mouse pituitary. Endocrinology, 143, 4730–4741. 10.1210/en.2002-220556 [DOI] [PubMed] [Google Scholar]
- Spierling, S. R. , & Zorrilla, E. P. (2017). Don't stress about CRF: Assessing the translational failures of CRF 1 antagonists. Psychopharmacology, 234, 1467–1481. 10.1007/s00213-017-4556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman, M. Q. , Laredo, S. A. , Lopez, E. M. , Manning, C. E. , Hao, R. C. , Doig, I. E. , … Trainor, B. C. (2015). Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology, 51, 122–134. 10.1016/j.psyneuen.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg, L. , Gaszner, B. , Boerrigter, J. , Santbergen, L. , Bramini, M. , Roubos, E. W. , … Kozicz, T. (2012). Sex‐dependent and differential responses to acute restraint stress of corticotropin‐releasing factor‐producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. Journal of Neuroscience Research, 90, 179–192. 10.1002/jnr.22737 [DOI] [PubMed] [Google Scholar]
- Sun, J. , Walker, A. J. , Dean, B. , van den Buuse, M. , & Gogos, A. (2016). Progesterone: The neglected hormone in schizophrenia? A focus on progesterone‐dopamine interactions. Psychoneuroendocrinology, 74, 126–140. 10.1016/j.psyneuen.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Taylor, S. E. , Saphire‐Bernstein, S. , & Seeman, T. E. (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair‐bond relationships? Psychological Science, 21, 3–7. 10.1177/0956797609356507 [DOI] [PubMed] [Google Scholar]
- Ter Horst, J. P. , Carobrez, A. P. , van der Mark, M. H. , de Kloet, E. R. , & Oitzl, M. S. (2012). Sex differences in fear memory and extinction of mice with forebrain‐specific disruption of the mineralocorticoid receptor. The European Journal of Neuroscience, 36, 3096–3102. 10.1111/j.1460-9568.2012.08237.x [DOI] [PubMed] [Google Scholar]
- Thakore, J. H. , Barnes, C. , Joyce, J. , Medbak, S. , & Dinan, T. G. (1997). Effects of antidepressant treatment on corticotropin‐induced cortisol responses in patients with melancholic depression. Psychiatry Research, 73, 27–32. 10.1016/S0165-1781(97)00106-6 [DOI] [PubMed] [Google Scholar]
- Todorovic, C. , Jahn, O. , Tezval, H. , Hippel, C. , & Spiess, J. (2005). The role of CRF receptors in anxiety and depression: Implications of the novel CRF1 agonist cortagine. Neuroscience and Biobehavioral Reviews, 29, 1323–1333. 10.1016/j.neubiorev.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Traslavina, G. A. , & Franci, C. R. (2011). The CRH‐R(1) receptor mediates luteinizing hormone, prolactin, corticosterone and progesterone secretion induced by restraint stress in estrogen‐primed rats. Brain Research, 1421, 11–19. 10.1016/j.brainres.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Van Cauter, E. , Leproult, R. , & Kupfer, D. J. (1996). Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. The Journal of Clinical Endocrinology & Metabolism, 81, 2468–2473. 10.1210/jcem.81.7.8675562 [DOI] [PubMed] [Google Scholar]
- van der Lely, A. J. , Foeken, K. , van der Mast, R. C. , & Lamberts, S. W. (1991). Rapid reversal of acute psychosis in the Cushing syndrome with the cortisol‐receptor antagonist mifepristone (RU 486). Annals of Internal Medicine, 114, 143–144. 10.7326/0003-4819-114-2-143 [DOI] [PubMed] [Google Scholar]
- Van Gaalen, M. M. , Basso, A. M. , Bespalov, A. Y. , Rueter, L. E. , Oost, T. , Breuer, M. , … Sullivan, J. P. (2008). Antidepressant‐and anxiolytic‐like effects of the vasopressin V1b receptor antagonists ABT‐436 and ABT‐558. Proceedings of Society for Neuroscience, Washington DC, USA Abstract 560.
- van Londen, L. , Goekoop, J. G. , van Kempen, G. M. J. , Frankhuijzen‐Sierevogel, A. C. , Wiegant, V. M. , van der Velde, E. A. , & De Wied, D. (1997). Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology, 17, 284–292. 10.1016/S0893-133X(97)00054-7 [DOI] [PubMed] [Google Scholar]
- Veenema, A. H. , Beiderbeck, D. I. , Lukas, M. , & Neumann, I. D. (2010). Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Hormones and Behavior, 58, 273–281. 10.1016/j.yhbeh.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Viau, V. , Bingham, B. , Davis, J. , Lee, P. , & Wong, M. (2005). Gender and puberty interact on the stress‐induced activation of parvocellular neurosecretory neurons and corticotropin‐releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology, 146, 137–146. 10.1210/en.2004-0846 [DOI] [PubMed] [Google Scholar]
- Walker, L. C. , Cornish, L. C. , Lawrence, A. J. , & Campbell, E. J. (2018). The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH‐IRES‐Cre mouse: A validation study. Neuropharmacology. 10.1016/j.neuropharm.2018.09.037 [DOI] [PubMed] [Google Scholar]
- Wang, B. , Zhou, J. , Zhuang, Y. Y. , Wang, L. L. , Pu, J. X. , Huang, Y. H. , … Lv, J. X. (2017). The non‐peptide vasopressin V1b receptor antagonist, SSR149415, ameliorates spermatogenesis function in a mouse model of chronic social defeat stress. Journal of Cellular Biochemistry, 118, 3891–3898. 10.1002/jcb.26040 [DOI] [PubMed] [Google Scholar]
- Wang, M. , Hammarback, S. , Lindhe, B. A. , & Backstrom, T. (1995). Treatment of premenstrual syndrome by spironolactone: A double‐blind, placebo‐controlled study. Acta Obstetricia et Gynecologica Scandinavica, 74, 803–808. 10.3109/00016349509021201 [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Joels, M. , Swaab, D. F. , & Lucassen, P. J. (2012). Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology, 62, 527–533. 10.1016/j.neuropharm.2011.09.014 [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Verweij, E. W. , Krugers, H. J. , Joels, M. , Swaab, D. F. , & Lucassen, P. J. (2014). Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Structure & Function, 219, 1615–1626. 10.1007/s00429-013-0589-4 [DOI] [PubMed] [Google Scholar]
- Wang, S. S. , Kamphuis, W. , Huitinga, I. , Zhou, J. N. , & Swaab, D. F. (2008). Gene expression analysis in the human hypothalamus in depression by laser microdissection and real‐time PCR: The presence of multiple receptor imbalances. Molecular Psychiatry, 13, 786–799. 10.1038/mp.2008.38 [DOI] [PubMed] [Google Scholar]
- Watson, S. , Gallagher, P. , Porter, R. J. , Smith, M. S. , Herron, L. J. , Bulmer, S. , … Ferrier, I. N. (2012). A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biological Psychiatry, 72, 943–949. 10.1016/j.biopsych.2012.05.029 [DOI] [PubMed] [Google Scholar]
- Weathington, J. M. , Hamki, A. , & Cooke, B. M. (2014). Sex‐ and region‐specific pubertal maturation of the corticotropin‐releasing factor receptor system in the rat. The Journal of Comparative Neurology, 522, 1284–1298. [DOI] [PubMed] [Google Scholar]
- Weightman, M. J. , Air, T. M. , & Baune, B. T. (2014). A review of the role of social cognition in major depressive disorder. Frontiers in Psychiatry, 5 10.3389/fpsyt.2014.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston, C. , Winfield, I. , Harris, M. , Hodgson, R. , Shah, A. , Dowell, S. J. , … Poyner, D. R. (2016). Receptor activity modifying protein‐directed G protein signaling specificity for the calcitonin gene‐related peptide family of receptors. Journal of Biological Chemistry, jbc–M116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, T. J. , Akama, K. T. , Knudsen, M. G. , McEwen, B. S. , & Milner, T. A. (2011). Ovarian hormones influence corticotropin releasing factor receptor colocalization with δ opioid receptors in CA1 pyramidal cell dendrites. Experimental Neurology, 230, 186–196. 10.1016/j.expneurol.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld, K. , Kuehl, L. K. , Dziobek, I. , Roepke, S. , Otte, C. , & Hinkelmann, K. (2016). Effects of mineralocorticoid receptor blockade on empathy in patients with major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience, 16, 902–910. 10.3758/s13415-016-0441-4 [DOI] [PubMed] [Google Scholar]
- Wood, G. E. , Beylin, A. V. , & Shors, T. J. (2001). The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience, 115, 175–187. 10.1037/0735-7044.115.1.175 [DOI] [PubMed] [Google Scholar]
- Wootten, D. , Christopoulos, A. , & Sexton, P. M. (2013). Emerging paradigms in GPCR allostery: Implications for drug discovery. Nature Reviews Drug Discovery, 12, 630–644. 10.1038/nrd4052 [DOI] [PubMed] [Google Scholar]
- Wu, L. M. , Han, H. , Wang, Q. N. , Hou, H. L. , Tong, H. , Yan, X. B. , & Zhou, J. N. (2007). Mifepristone repairs region‐dependent alteration of synapsin I in hippocampus in rat model of depression. Neuropsychopharmacology, 32, 2500–2510. 10.1038/sj.npp.1301386 [DOI] [PubMed] [Google Scholar]
- Wu, T. C. , Chen, H. T. , Chang, H. Y. , Yang, C. Y. , Hsiao, M. C. , Cheng, M. L. , & Chen, J. C. (2013). Mineralocorticoid receptor antagonist spironolactone prevents chronic corticosterone induced depression‐like behavior. Psychoneuroendocrinology, 38, 871–883. 10.1016/j.psyneuen.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Young, E. , & Korszun, A. (2009). Sex, trauma, stress hormones and depression. Molecular Psychiatry, 15, 23. [DOI] [PubMed] [Google Scholar]
- Young, E. A. (1995). The role of gonadal steroids in hypothalamic‐pituitary‐adrenal axis regulation. Critical Reviews in Neurobiology, 9, 371–381. [PubMed] [Google Scholar]
- Young, E. A. (1998). Sex differences and the HPA axis: Implications for psychiatric disease. The Journal of Gender‐Specific Medicine: JGSM: The Official Journal of the Partnership for Women's Health at Columbia, 1, 21–27. [PubMed] [Google Scholar]
- Young, E. A. , Carlson, N. E. , & Brown, M. B. (2001). Twenty‐four‐hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology, 25, 267–276. 10.1016/S0893-133X(00)00236-0 [DOI] [PubMed] [Google Scholar]
- Young, E. A. , Kotun, J. , Haskett, R. F. , Grunhaus, L. , Greden, J. F. , Watson, S. J. , & Akil, H. (1993). Dissociation between pituitary and adrenal suppression to dexamethasone in depression. Archives of General Psychiatry, 50, 395–403. 10.1001/archpsyc.1993.01820170073010 [DOI] [PubMed] [Google Scholar]
- Young, E. A. , Lopez, J. F. , Murphy‐Weinberg, V. , Watson, S. J. , & Akil, H. (1998). The role of mineralocorticoid receptors in hypothalamic‐pituitary‐adrenal axis regulation in humans. The Journal of Clinical Endocrinology and Metabolism, 83, 3339–3345. [DOI] [PubMed] [Google Scholar]
- Young, E. A. , Lopez, J. F. , Murphy‐Weinberg, V. , Watson, S. J. , & Akil, H. (2003). Mineralocorticoid receptor function in major depression. Archives of General Psychiatry, 60, 24–28. 10.1001/archpsyc.60.1.24 [DOI] [PubMed] [Google Scholar]
- Zelena, D. , Földes, A. , Mergl, Z. , Barna, I. , Kovács, K. J. , & Makara, G. B. (2004). Effects of repeated restraint stress on hypothalamo‐pituitary‐adrenocortical function in vasopressin deficient Brattleboro rats. Brain Research Bulletin, 63, 521–530. 10.1016/j.brainresbull.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Zimmerman, M. , Chelminski, I. , & Posternak, M. A. (2005). Generalizability of antidepressant efficacy trials: Differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. American Journal of Psychiatry, 162, 1370–1372. 10.1176/appi.ajp.162.7.1370 [DOI] [PubMed] [Google Scholar]
- Zobel, A. W. , Nickel, T. , Kunzel, H. E. , Ackl, N. , Sonntag, A. , Ising, M. , & Holsboer, F. (2000). Effects of the high‐affinity corticotropin‐releasing hormone receptor 1 antagonist R121919 in major depression: The first 20 patients treated. Journal of Psychiatric Research, 34, 171–181. 10.1016/S0022-3956(00)00016-9 [DOI] [PubMed] [Google Scholar]
- Zorrilla, E. P. , Valdez, G. R. , Nozulak, J. , Koob, G. F. , & Markou, A. (2002). Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety‐like behavior and motor activation in the rat. Brain Research, 952, 188–199. 10.1016/S0006-8993(02)03189-X [DOI] [PubMed] [Google Scholar]


