Abstract
The incidence of dementia, most commonly caused by cerebrovascular and neurodegenerative diseases, continues to grow as our population ages. Alzheimer disease (AD) and vascular cognitive impairment (VCI) are responsible for more than 80% of all cases of dementia. There are few effective, long‐term treatments for AD and VCI‐related conditions (e.g., stroke and cerebral amyloid angiopathy (CAA)). This review focuses on AD (as the most common “neurodegenerative” cause of dementia), CAA (as an “emerging” cause of dementia), and stroke (as the most common cause of “vascular” dementia). We will discuss the available literature on the pharmacological therapies that demonstrate sex differences, which refer to any combination of structural, chromosomal, gonadal, or hormonal differences between males and females. We will emphasize the importance of considering sex as a biological variable in the design of preclinical and clinical studies that investigate underlying pathologies or response to pharmacological interventions in dementia.
LINKED ARTICLES
This article is part of a themed section on The Importance of Sex Differences in Pharmacology Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.21/issuetoc
Abbreviations
- AD
Alzheimer disease
- ADAS‐Cog11
Alzheimer's Disease Assessment Scale‐Cognitive Subscale
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- APOE ε4
ε4 allele of the apolipoprotein E
- Aβ
amyloid‐β
- CAA
cerebral amyloid angiopathy
- ChEIs
cholinesterase inhibitors
- CI
confidence interval
- CIMT
carotid intimal‐medial thickness
- CVD
cardiovascular disease
- HMG‐CoA
3‐hydroxy‐3‐methyl‐glutaryl‐CoA
- ICH
intracerebral haemorrhage
- MCI
mild cognitive impairment
- MHT
menopausal hormone therapy
- MMSE
Mini‐Mental State Examination
- MoCA
Montreal Cognitive Assessment
- OR
odds ratio
- PD
Parkinson disease
- UA
uric acid
- VaD
vascular dementia
- VCI
vascular cognitive impairment
1. INTRODUCTION
The incidence of cerebrovascular and neurodegenerative diseases continues to grow as our population ages. Dementia is one of the most prevalent disorders of ageing and a major contributor to health care cost in the United States. Although the list of “Neurocognitive disorders” in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition that cause dementia is extensive, Alzheimer's disease (AD) and vascular dementia (VaD) are respectively responsible for approximately 60% and 20% of all dementia cases in the world (Rizzi, Rosset, & Roriz‐Cruz, 2014). This review will focus on AD, as the most common “non‐vascular” cause of dementia, cerebral amyloid angiopathy (CAA), as an “emerging” cause of dementia linking AD and stroke, and stroke, as the most common cause of “vascular” dementia. We will also discuss the available literature on the pharmacological therapies for dementia that demonstrate sex differences. Throughout this review, we wish to emphasize the importance of considering sex as a biological variable in the design of preclinical and clinical studies that investigate dementia‐related pathologies or pharmacological responses.
In biomedical research, “sex” refers to any combination of chromosomal, gonadal, or hormonal differences between males and females. In contrast, “gender” refers to any combination of cultural, psychosocial, or belief‐based behavioural differences between men and women. Both sex and gender have been implicated in underlying reasons for differences between women and men in the overall prevalence and incidence of dementia and stroke (Rizzi et al., 2014). In addition to lack of sex‐specific reporting, which will be exemplified throughout this review, analysis of the data is complicated by the fact that all scales and screening methods used for assessing severity of dementia and responses to pharmacological treatments are primarily based on collateral, patient‐, family member‐, or caregiver‐derived information. Many assessment scales have been refined over the years with the purpose of minimizing subjectivity while maximizing objectivity. A few commonly used assessment scales are briefly discussed below, following by the sections on sex differences in pathology and pharmacology of dementia induced by AD, CAA, and stroke.
2. COMMON CAUSES OF DEMENTIA
Most patients with dementia have features of both neurodegeneration and cerebrovascular disease that contributes to their cognitive impairment, known as mixed dementia (Bennett, 2001). AD and VaD can be difficult to distinguish because of overlap in their clinical presentation, risk factors, and pathophysiology. Dementia solely caused by focal stroke is uncommon. Pioneering work by Hachinski et al. (1975) classifies cognitive decline secondary to vascular events as being more abrupt, stepwise, fluctuating, and associated with focal neurological deficit and cerebrovascular risk factors. Vascular cognitive impairment (VCI) is a relatively recent term in the field that refers to the contribution of cerebrovascular pathology to cognitive impairment. The group of conditions that lead to VCI are heterogeneous and continually updated. Recent comprehensive reviews of VCI‐related conditions are available in the literature (van der Flier et al., 2018).
In addition to AD and VaD, several other conditions can lead to dementia. There is a significant lack of sex‐specific reporting in the epidemiology of most of these conditions. Importantly, sex differences are found in most of the conditions for which sex‐specific epidemiological data are available. In Parkinson's disease (PD), a study of 296 PD patients of all ages found that number of men with PD‐related dementia was twice as high as number of women with PD‐related dementia (0.10 vs. 0.05 when % prevalence was calculated; Savica, Grossardt, Rocca, & Bower, 2018). In Huntington's disease, a CAG triplet repeat pathology, with an autosomal dominant inheritance, prevalence data show no sex differences (Ghosh & Tabrizi, 2018). Interestingly, mechanistic studies of triplet repeat expansion and contraction in Huntington's disease pathogenesis have suggested that sex of the parent plays a role in the size of the inherited CAG repeat. One study found that large expansions (>7 CAG repeats larger than the parent's gene) occur almost exclusively through paternal transmission (0.96%; P ≤ 10−7), while offspring of affected mothers are more likely to show no change (P ≤ .01) or contractions in CAG size (P ≤ .002; Kremer et al., 1995). In frontotemporal dementia, the reports vary with geographical location, with a 14:3 ratio reported for males to females with frontotemporal dementia in Cambridgeshire, UK, but a 1:3 ratio for males to females in Italy (Onyike & Diehl‐Schmid, 2013). Sex differences are also seen in dementia patients with underlying endocrine diseases. Lower risk of thyroid‐related dementia was found in women with higher levels of TSH (hazard ratio [HR] of 0.67, 95% confidence interval [CI] [0.54, 0.82]; Chaker et al., 2016). Higher http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1785 levels among dementia patients were seen in women with lower cognitive Z scores (P ≤ .05; Kim et al., 2017). In dementia patients with underlying CNS vasculitis or normal‐pressure hydrocephalus, sex‐specific epidemiological reporting is not readily available. Sex differences in the available epidemiological data on the most common conditions that cause dementia are summarized in Table 1.
Table 1.
Epidemiological differences between men and women in common causes of dementia
| Category | Specific pathology | Available epidemiological data on sex differences | Reference |
|---|---|---|---|
| Neurodegenerative diseases | Alzheimer's dementia (1st; includes CAA) | 3.3 million in women vs. 1.9 million in men (prevalence) | (Hebert, Weuve, Scherr, & Evans, 2013) |
| Parkinson's dementia | 0.05% in women vs. 0.10% in men (prevalence %) | (Savica et al., 2018) | |
| Huntington's dementia | Similar between sexes (prevalence) | (Ghosh & Tabrizi, 2018) | |
| Frontotemporal dementia | Variable prevalence reporting (area dependent) | (Onyike & Diehl‐Schmid, 2013) | |
| Endocrine diseases | Thyroid‐related dementia | Lower risk of dementia in women with higher levels of TSH, with an HR of 0.67 (95% CI [0.54, 0.82]) | (Chaker et al., 2016) |
| Parathyroid‐related dementia | Higher PTH more likely to be women with lower cognitive Z scores (P ≤ .05) | (Kim et al., 2017) | |
| Cerebrovascular/structural | Vascular cognitive impairment (2nd; includes CAA) | Some studies report a higher incidence of VCI in men than in women | (Gorelick et al., 2011) |
| Ischaemic stroke | 3.2% in women vs. 2.5% in men (prevalence) | (Writing Group et al., 2010) | |
| Neuro‐vasculitis | — | (Younger, 2016) | |
| Normal‐pressure hydrocephalus | — | (Martin‐Laez, Caballero‐Arzapalo, Lopez‐Menendez, Arango‐Lasprilla, & Vazquez‐Barquero, 2015) |
3. COMMONLY USED SEVERITY SCALES IN DEMENTIA
3.1. Mini‐Mental State Examination
The Mini‐Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) is the most commonly administered psychometric screening assessment of cognitive functioning. The MMSE is used to screen patients for cognitive impairment, to track changes in cognitive functioning over time, and to assess the effects of therapeutic agents on cognitive function. The MMSE is an approximately 10‐min interview to assess orientation, memory, attention, calculation, language, and visual construction abilities of the patient. A score of 23/24 out of 30 is considered clinically significant (generally defined as social or occupational functional impairments detectible by a trained clinician) with reported sensitivity of 66% and specificity of 99% (O'Bryant et al., 2008).
A meta‐analysis of neurocognitive data from 15 studies (n = 828 men; 1,238 women) by Irvine, Laws, Gale, and Kondel (2012) concluded that men modestly but significantly outperform women in five of the cognitive domains of semantic, non‐semantic, verbal, visuospatial, and memory function. Post‐mortem examination of 1,453 persons who participated in either the Religious Orders Study or the Rush Memory and Aging Project reported a lower mean of MMSE scores for women than men (20.3/30 vs. 21.7/30, P < .008; Oveisgharan et al., 2018). However, composite test scores that measure global cognitive function including MMSE did not significantly predict the male cognitive advantage in any cognitive domain (Irvine et al., 2012), suggesting that such composite cognitive scores may lack sufficient specificity for investigating sex differences in dementia.
3.2. Alzheimer's Disease Assessment Scale‐Cognitive Subscale 11
The Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog 11) is a 70‐point scale designed to assess severity of cognitive impairment, and it is commonly used in mild cognitive impairment (MCI) and AD trials. The ADAS‐Cog is composed of 11 tasks that assess learning and memory, language production and comprehension, constructional and ideational praxis, and orientation. Higher scores indicate worse performance, as it is scored based on number of errors (Hua et al., 2010). Due to the length of ADAS‐Cog test of about 40 min when performed by a trained interviewer, the clinical utility of this scale is limited but it remains the leading assessment tool in dementia drug trials. A 2018 study examining the effect of sex on cognitive changes using the Alzheimer's Disease Neuroimaging Initiative (ADNI) database concluded that the mean worsening in ADAS‐Cog11 score was significantly greater in females than in males among AD patients (Sohn et al., 2018).
3.3. Clinical Dementia Rating, Clinical Dementia Rating‐sum of boxes, and Montreal Cognitive Assessment
The Clinical Dementia Rating‐sum of boxes, with a range from 0 to 18, is the sum of the ratings for the six domains of the Clinical Dementia Rating global dementia rating scale. It provides a quantitative assessment of cognitive and functional impairments based on a semi‐structured interview of the subject and informant. Higher scores indicate greater impairment (Williams, Storandt, Roe, & Morris, 2013). Montreal Cognitive Assessment (MoCA) is a short assessment of attention, concentration, calculation, and orientation, which can be done by any clinician with minimal training (Nasreddine et al., 2005). A MoCA score of 25 or lower out of 30 is considered clinically significant.
4. SEX DIFFERENCES IN THE MOST COMMON DEMENTIA PATHOLOGIES
4.1. Background on AD, CAA, and stroke
AD is a neurodegenerative cause of dementia and is characterized by extracellular aggregation of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4865 stacks of pleated β‐sheets (“plaques”) and intracellular filamentous aggregation of the insoluble microtubule‐associated protein, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9275 (“neurofibrillary tangles”). CAA is an emerging cause of dementia and its pathology has been linked to both AD and stroke. CAA pathogenesis is similar to AD in that both pathologies are caused by pathological Aβ accumulations. In AD, Aβ deposition occurs primarily in the brain parenchyma while in CAA, Aβ preferentially deposits into the basement membrane of the cerebral vasculature, which causes recurrent haemorrhagic stroke. CAA leads to lobar intracerebral haemorrhages (ICH), cortical microhaemorrhages, and white matter damage and leads to progressive cognitive decline and dementia in the elderly. Stroke is a leading cause of death worldwide and a major cause of long‐term disability. In stroke, the interruption of blood supply to a specific vascular territory leads to rapid neuronal death, parenchymal injury, and a host of subsequent neurological problems including dementia.
Unfortunately, there are few effective treatments for AD or CAA, and stroke treatment is limited to acute reperfusion therapy (endovascular or intravenous thrombolytic therapies), which leaves many stroke patients ineligible for any treatment at all. Epidemiological studies consistently show differences in men and women suffering from these debilitating conditions (Bushnell et al., 2018). Therefore, understanding the role of sex as a biological variable is critically important for development of novel pharmacological therapies for the treatment of dementia caused by AD, CAA, stroke, or other cerebrovascular conditions. Within the scope of this review, we will present the available evidence of sex differences in the most common pathologies that cause dementia and response to available treatments for those conditions. We will emphasize the important, and often correctable, factors that continue to negatively impact the current state of our understanding of sex‐dependent responses to these therapies.
4.2. Sex differences in epidemiology of AD
While men may have a higher risk of MCI, women are disproportionally affected by AD; 3.3 million out of the 5.2 million people age 65 and older with AD in the United States are women (Hebert et al., 2013). The epidemiological data are consistent with a higher prevalence of AD in women; however, incidence data examining differences between men and women remain somewhat inconsistent. The majority of studies show a similar incidence of dementia in men and women in the age range of 70–79. But incidence rates are higher in women than men after about 80 years of age, probably confounded by longer life expectancy seen in women and sociocultural detection biases (Roberts et al., 2014). To explain these epidemiological differences, evidence has emerged on the variability of clinical presentation among dementia patients secondary to social isolation, socio‐economic status, access to care, and several other gender‐specific factors (see Section 6). However, the latest understanding of the accumulated evidence supports that, in addition to gender factors, sex is an important contributor to the dimorphic rate of progression to AD in men and women (Lin et al., 2015). These findings suggest that there is an interaction between sex and age as two independent but related biological variables in AD pathology.
4.3. Sex differences in epidemiology of stroke
Stroke is a leading cause of morbidity and mortality worldwide and it disproportionally affects women. There are currently over 500,000 more women stroke survivors in the United States than men, and elderly women bear the brunt of stroke‐related disability. Stroke is the third leading cause of death in women in the United States but has fallen to the fifth leading cause of death in men (Writing Group Members et al., 2016). There are more than 3.8 million women and 3 million men living in the United States today as stroke survivors (Writing Group Members et al., 2016). These statistics are expected to increase as our society continues to age, emphasizing the urgent need for improved understanding of sex‐specific mechanisms that can lead to effective therapeutic options for stroke patients.
4.4. Sex differences in VCI
VCI is the second most common cause of dementia but most patients with “VaD” have a combination of other contributing factors such as AD (van der Flier et al., 2018). VCI classification is most often on the basis of prior history of stroke without neuroimaging evidence of cerebrovascular disease. The risk of post‐stroke dementia has decreased over the past four decades, most likely because of improved treatment of acute stroke and better management of vascular risk factors (Satizabal et al., 2016). The classification of VCI on the basis of prior stroke may inflate the reported estimates of the vascular contribution to dementia. On the other hand, if individuals with mixed dementia and individuals with white matter hyperintensities on MRI are included under the VCI classification, then vascular conditions contribute to a higher percentage of overall dementia cases (Toledo et al., 2013). Specific diagnostic criteria for VCI and improved characterization of vascular contribution to dementia are continually updated (van der Flier et al., 2018).
A meta‐analysis of European studies found that sex differences in prevalence of VaD reversed their trend with advanced age such that VaD was more prevalent in men before age 79, but more prevalent in women after age 85 (Lobo et al., 2000). This finding suggests that caution should be taken when interpreting the prevalence data due to possible survival bias and sex‐ and gender‐specific vascular risk factors that may explain epidemiological sexual dimorphisms. Reviews of sex differences in vascular risk factors with potential contribution to VCI are available (Gannon, Robison, Custozzo, & Zuloaga, 2018).
To date, few pathological studies have addressed sex difference in AD and contribution of cerebrovascular disease to dementia. This may seem contradictory, as there are clear sex‐specific, age‐related increases in incidence of cerebrovascular disease, most commonly stroke. Women are disproportionally affected by stroke at older ages and are significantly more likely to suffer from cognitive decline following stroke (Bushnell et al., 2014). This observation may be confounded by age or the higher rate of large cardioembolic strokes seen in females, secondary to atrial fibrillation than males with this condition (Rienstra, McManus, & Benjamin, 2012).
Post‐mortem examination of 1,453 persons who participated in either the Religious Orders Study or the Rush Memory and Aging Project showed that women had higher levels on a global measure of AD pathology (P < .001), Tau tangle density (P < .001), and Aβ load (P ≤ .056; Oveisgharan et al., 2018). Compared to men, women included in this study had more severe arteriosclerosis (odds ratio [OR] = 1.28, 95% CI [1.04, 1.58], P ≤ .018) but were less likely to have gross infarcts (OR = 0.78, 95% CI [0.61, 0.98], P < .037; Oveisgharan et al., 2018). In addition to potential confounders, uncertainties over diagnostic criteria for VCI and lack of generally accepted protocols for post‐partum neuropathological assessment of suspected VCI make data analysis even more challenging (Skrobot et al., 2016). With the advancement in non‐invasive and minimally invasive techniques for clinical evaluation of patients with cerebrovascular disease, new assessment tools also emerge that can facilitate future studies of sex differences in VCI.
Carotid intimal‐medial thickness (CIMT), vascular reactivity, and arterial stiffness are emerging as markers of arterial ageing and may serve as risk markers for VCI. Increasing evidence that assessment of white matter microstructure by diffusion‐tensor imaging in patients with AD, CAA, and stroke may be able to differentiate between these diseases and controls, but to date, minimal studies have examined sex differences using these techniques (Auriel et al., 2014). Several clinical and neuro‐radiological features have been linked to VCI including CAA, large vessel infarcts, lacunar infarcts, microinfarcts, myelin loss, arteriolosclerosis, and enlarged perivascular spaces, as recently summarized (Skrobot et al., 2016). Although to date, there is only negligible epidemiological data demonstrating sex differences in VCI, there are clear sex differences in many of the underlying processes that contribute to VaD including CAA and stroke, as discussed briefly below.
4.5. Sex differences in CAA
CAA is an increasingly recognized dementia subtype that has been linked to both AD and stroke. CAA pathogenesis is similar to AD in that Aβ protein plaque deposition is a central event. In CAA, the toxic Aβ preferentially deposits into the basement membrane of the cerebral vasculature. CAA‐associated vasculopathy leads to lobar ICH, cortical microhaemorrhage, cortical superficial siderosis, and ischaemic change in the white matter. Recent advancements in neuroimaging‐based diagnostic techniques such as gradient‐echo T2 MRI and amyloid‐binding PET ligands (e.g., Pittsburgh compound B, “PiB PET”) allow for detection of CAA pathology without a need for brain biopsy (Reijmer et al., 2015). To date, few studies have focused on sex differences in CAA.
A population‐based neuroimaging study using specified cut‐off points for amyloid PET, Tau PET, and cortical thickness by MRI of individuals aged 50–89 years stratified participants into eight groups with respect to normal or abnormal levels of cerebral Aβ, Tau protein, and neurodegeneration. When examining sex differences, the group with the greatest proportion of men was normal‐Aβ/normal‐Tau/abnormal‐neurodegeneration (57%, 95% CI [37, 77]) from age 65 to 75 years and the group with the greatest proportion of women was abnormal‐Aβ/normal‐Tau/normal‐neurodegeneration (78%, 95% CI [64, 93]; Jack et al., 2017). However, the analysis showed that the overall effect of sex on the prevalence of all eight groups was small when using cortical thickness as a measure, but more pronounced when using adjusted hippocampal volume, suggesting that different sex‐specific and age‐specific pathological processes may contribute to overall prevalence of dementia.
A recent study examining the roles of ε4 allele of the apolipoprotein E (APOE ε4) and sex on CAA among AD patients concluded that both factors differentially influenced the presence and severity of CAA in AD. An association of APOE ε4 carrier status with a higher overall CAA score was seen using an autopsy‐based histological scoring of CAA severity in 428 confirmed AD cases, which was consistent with earlier studies in AD patients with APOE ε4 allele. However, the effect of sex on CAA pathology was not consistent with results seen in AD studies. After correcting for age, Braak neurofibrillary tangle stage, and Thal amyloid phase, the overall CAA scores were higher in males than in females (Shinohara et al., 2016). This suggests factors that contribute to sex differences in CAA may be different from those that contribute to sex differences in AD.
In an observational study of 82 CAA‐associated ICH patients, serum uric acid (UA) levels, an endogenous neuroprotective molecule involved in sex differences, were significantly lower in those with clinical diagnosis of “possible” CAA when compared to healthy controls. Furthermore, the serum UA levels of those diagnosed with “probable” CAA were significantly lower than those with “possible” CAA (Hu et al., 2014), suggesting that UA may be a molecular mediator in sex differences in CAA severity. Future studies that focus on sex differences may reveal new genetic, metabolic, or environmental risk factors that could aid in early detection or disease‐modifying pharmacological interventions for CAA.
4.6. Sex differences in animal models of dementia
Ageing wild‐type mice do not develop AD, CAA, or most other age‐related disorders seen in humans, thus transgenic mice models are often used in the laboratory. Sex differences have been reported in several animal models of dementia. The Tg‐2576 transgenic model is one of the most widely used AD animal models expressing human APP695 containing the double mutation K670N/M671L under the control of hamster http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6244 (Hsiao et al., 1996). This model develops cognitive impairment beginning around 8 months persisting up to 16 months. Preclinical studies of sex differences showed that female Tg‐2576 mice develop a more prominent cognitive impairment (wrong choice total) compared to their male littermates at 12, 14, and 16 months. Furthermore, female Tg‐2576 mice exhibit increased impairment on memory function testing, when compared to male mice with the same total amount of Aβ in prefrontal cortex.
Some animal models of CAA also exhibit sexual dimorphism. For example, transgenic mice carrying human APOE ε4 allele and familial AD gene, known as EFAD mice, show excess of CAA in females by the age of 7 months, a pattern opposite to that of seen in humans with CAA (Finch & Shams, 2016). This observation of opposite pattern of sex differences between animal models and human data warrants careful evaluation of sex differences in animal models of CAA and AD prior to accepting them as “sufficiently accurate” for the purposes of preclinical studies and the basis for designing randomized clinical trials. Reviews of animal models of dementia and model‐specific sex differences are available (Yang et al., 2018).
4.7. Possible mechanisms of sex differences in AD and stroke
Emerging data suggest that the interplay of multiple biological factors contributes to the mechanisms of sex differences in AD pathogenesis and progression. As noted in a recent comprehensive review (Fisher, Bennett, & Dong, 2018), the most likely AD risk factors that interact with biological sex include (a) differences in chromosomal and gonadal hormones, (b) differences in neuro‐inflammation, immunity, and microglia activation, (c) differences in brain structure, and (d) exposure to psychosocial stressors (see Figures 1 and 2). Some, but not all, aspects of sex differences in AD are generalizable to other causes of dementia including CAA and stroke. Mechanisms involved in sex differences in stroke include (a) differences in chromosomal and gonadal hormones, (b) intrinsic changes in innate immune cells and their response to ischaemic injury, (c) cellular mechanisms (reviewed in Chauhan, Moser, & McCullough, 2017), (d) coagulation cascade (reviewed in Roy‐O'Reilly & McCullough, 2014), and (e) other immunomodulatory mechanisms. Different sets of underlying mechanisms of sex differences in neurodegenerative and neurovascular pathologies are summarized in Figure 1. This review will focus on some, but not all, of these potential underlying mechanisms of sex differences.
Figure 1.

Mechanisms of sex differences in neurodegenerative and neurovascular pathologies. Reference key: 1(Corder et al., 2004), 2(Chauhan et al., 2017), 3(Selvamani, Sathyan, Miranda, & Sohrabji, 2012; Kaidonis, Rao, Ouyang, & Stary, 2018), 4(Sheinerman et al., 2017), 5(Villa et al., 2018), 6(Sarlus & Heneka, 2017), 7(Rhodes, O'Toole, Wright, Czambel, & Rubin, 2001), 8(Wickens, Bangasser, & Briand, 2018), 9(Beeri et al., 2009), 10(Maki, 2013), 11(Paoletti et al., 2004), 12(Lee et al., 2017), 13(Scacchi, Gambina, Broggio, & Corbo, 2014), 14(Roy‐O'Reilly & McCullough, 2014), 15(Miller et al., 2017; Bushnell et al., 2018), 16(Emdin et al., 2016), 17(Selvamani et al., 2012), 18(Giedd, Raznahan, Mills, & Lenroot, 2012), 19(Giedd et al., 2012), and 20(Giedd et al., 2012)
Figure 2.
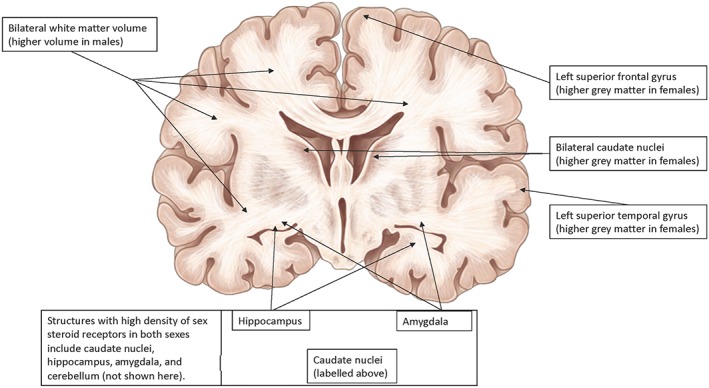
A diagram of a coronal view of the human brain, labelling those anatomical areas with sex differences. Females on average have a higher percentage of grey matter in the caudate nuclei, left superior temporal gyrus, and left superior frontal gyrus while in males, a more rapid increase in white matter volume is seen, which contributes to the overall 10% larger brain volume in men than in women. Areas with high density of sex steroid receptors in both sexes are the caudate nuclei, amygdala, hippocampus, and cerebellum (not shown here)
4.8. Genetic sex differences in AD
Among the numerous genes and single‐nucleotide polymorphisms reported to be associated with increased risk of AD, the strongest known genetic risk factor for late‐onset disease is APOE ε4 gene on chromosome 19. Pathological examination of amyloid plaques and neurofibrillary tangles in a large autopsy study found the highest amyloid burden among female carriers of APOE ε4 allele when compared to male APOE ε4 carriers (Corder et al., 2004). A 2018 study examining the effect of sex on cognitive changes using the ADNI database concluded that among subjects with MCI due to AD, the mean worsening in ADAS‐Cog11 score was significantly greater in females (11.58 ± 14) than in males (6.87 ± 11, P ≤ .006; Sohn et al., 2018). This study also found that among APOE ε4 carriers with MCI, females declined significantly faster than males even though there was no significant difference in APOE ε4 carrier status between males and females. Lastly, females showed greater cognitive decline than males when sex differences were examined in subjects with a higher Tau/Aβ42 ratio, referred to as a “high likelihood of MCI due to AD” group. Although the Tau/Aβ42 ratio cut‐off has been pathologically validated (Shaw et al., 2009) and used as a surrogate to identify biomarker positive MCI subjects, there is no perfect in vivo method to identify MCI due to AD. One major reason for this is that the diagnostic utility of the Tau/Aβ42 ratio in CSF may differ by setting and laboratory (Ritchie et al., 2017). APOE ε4 studies have shed some light on possible genetic factors that contribute to sex differences in AD but additional variables are also involved. In a 2015 study of 398 MCI subjects (141 females, 257 males) in the ADNI‐1 database, analysis showed that women with MCI have greater longitudinal rates of cognitive and functional progression than men, after adjusting for the APOE ε4 effect (Lin et al., 2015). This suggests that APOE ε4 effect does not fully explain the observed sex differences in AD patients.
4.9. Sex differences in glial cells in stroke and AD
Accumulating evidence supports that sex differences are present in microglia and astrocytes. Microglia, the innate immune cell of the brain, exhibits sexual dimorphism both in culture and when transplanted into the brain of the opposite sex (Lenz & McCarthy, 2015). Villa et al. in a recent animal study found that microglia isolated from female adult brain played a neuroprotective role in ischaemic stroke recovery when transplanted into a male adult brain previously depleted of endogenous microglia. Results showed that female microglia reduced ischaemic damage in male mice (Villa et al., 2018). RNA sequencing data of a total of 95 genes involved in microglial inflammatory response have found that 79% of these inflammatory genes are more highly expressed in males, 7% are more highly expressed in females, and 14% are expressed equally between sexes (Villa et al., 2018). In addition to these intrinsic differences contributing to sexual dimorphism in microglia, the immune function of microglia is modulated by sex hormones. Microglia express http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=620 and β (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=621). Activation of ERα after oestrogen treatment significantly inhibits the inflammatory response of microglia, induced by LPS, as measured by mRNA levels of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1633 (Vegeto et al., 2001). Sex differences in the immunological response to ischaemic injury (Spychala, Honarpisheh, & McCullough, 2017) are increasingly recognized and will be an area of considerable interest in the future. Sex differences in astrocytes have been recently reviewed elsewhere (Chowen, Argente‐Arizón, Freire‐Regatillo, & Argente, 2018). The role of microglia and astrocytes in AD pathogenesis has been reviewed elsewhere (Frost & Li, 2017).
4.10. Sex hormones and risk of AD
Effect of oestrogen on lifetime risk of AD has received both research and media attention. Despite high public interest and evidence from research, the majority of clinical studies to date fail to capture critically relevant components of patient reproductive history. These components include oral contraceptive use, number of pregnancies, duration of breastfeeding, regularity of menstrual cycles, post‐partum anovulation, abortions, and miscarriages. The common effect of these important factors is their influence on the cumulative exposure to oestrogen, which can be a major confounder in the clinical studies, if not properly controlled and randomized. In addition, common pregnancy‐related complications such as a history of pre‐eclampsia/eclampsia, which are known to influence dementia and vascular risk, are often not considered (Basit, Wohlfahrt, & Boyd, 2018).
Oestrogens have a wide range of beneficial effects in the brain and other tissues (Pike, 2017). Consistent with these beneficial effects, some but not all studies suggest that reductions of oestrogen are associated with increased risk of AD in postmenopausal women. Studies showing that higher number of pregnancies, which result in overall decrease in lifetime oestrogen exposure, increase women's risk of dementia (Beeri et al., 2009). Other studies showed that longer exposure to endogenous oestrogens (as indicated by number of reproductive years) does not reduce the risk of dementia in women who undergo natural menopause (Geerlings et al., 2001). These finding suggested that reduction in endogenous oestrogen exposure may increase risk of AD while longer oestrogen exposure may not have a protective effect on AD risk. Consistent with suggested detrimental effects of reduced oestrogens before menopause, studies showed that surgically induced menopause (e.g., oophorectomy and/or hysterectomy), when performed prior to natural menopause, significantly increases the risk of dementia (Bove et al., 2014; Rocca, Grossardt, & Shuster, 2011). Surgical menopause performed after natural menopause does not increase the risk of dementia (Imtiaz et al., 2014). In women with AD and mean ages greater than 80, levels of oestrogens (specifically, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2818) in the brain are significantly reduced when compared to age‐matched women without AD (Rosario, Chang, Head, Stanczyk, & Pike, 2011). There is no significant difference in brain levels of oestrogen between men with and without AD (Rosario et al., 2011). A 2013 cohort study of AD risk in 89 elderly women used detailed reproductive and medical history of the subjects to estimate the number of months with oestrogen exposure and number of months with menstrual cycles. The results showed that for every additional month of oestrogen exposure, women had a 0.5% decrease in risk of AD (Fox, Berzuini, & Knapp, 2013). Collectively, these findings suggest a correlation of low oestrogen and higher risk of AD in women. However, large clinical trials investigating effects of oestrogen‐based menopausal hormone therapy (MHT) on risk of dementia have yielded inconsistent results.
Early studies indicated a protective role of oestrogen by observing that oestrogen therapy significantly reduced risk of AD (Zandi et al., 2002). However, the Women's Health Initiative found that oestrogen therapy was associated with increased, rather than the anticipated decreased, risk of MCI and dementia in postmenopausal women older than 65 (Shumaker et al., 2004). These findings have brought forth the idea of “window of opportunity” for MHT (Maki, 2013), suggesting that effects of oestrogen on risk of dementia are age dependent and a “critical period” along women's reproductive timeline (probably around the time of menopause) may exist for beneficial effects of oestrogen in reducing the risk of dementia. Early versus Late Intervention Trial with Estradiol (Hodis et al., 2015) and Kronos Early Estrogen Prevention Study (KEEPS; Wharton, Gleason, Miller, & Asthana, 2013) are two recent clinical trials that aimed to evaluate the optimized timeline for MHT. In a KEEPS continuation study of the effects of MHT on Aβ deposition, results of PiB PET imaging performed in 68 recently postmenopausal women (age: 52–65) showed that transdermal 17‐β estradiol therapy was associated with reduced Aβ deposition, particularly in APOE ε4 carriers (Kantarci et al., 2016). In another imaging‐based study of KEEPS participants, the investigators found that white matter hyperintensities continue to increase after discontinuation of oral conjugated equine oestrogen (Kantarci et al., 2018). These findings suggest that molecular biomarkers and structural changes associated with cognition can be influenced by MHT. However, findings from KEEPS show that in recently postmenopausal women, MHT does not alter cognition, when assessed by MMSE and other clinical cognitive testing (Gleason et al., 2015).
A small observational neuroimaging study of 42 individuals aged 40–60 years investigating sex differences in the endocrine ageing process and development of AD showed that postmenopausal and symptomatic perimenopausal groups had increased AD biomarkers, including hypometabolism, increased Aβ deposition, and reduced grey and white matter volumes in AD‐vulnerable regions (P < .001), when compared to asymptomatic perimenopausal women and to men (Mosconi et al., 2017). Additionally, Aβ deposition, assessed by PiB binding detected by PET imaging, was higher in postmenopausal APOE ε4 carriers than all other groups of women and men (Mosconi et al., 2017). In keeping with previous studies, the neuroimaging findings support that the preclinical AD phase coincides with the endocrine transition of perimenopause, suggesting the optimal window of opportunity for oestrogen‐based therapeutic intervention in women is early in the endocrine ageing process (Mosconi et al., 2017). The evidence for use of MHT for the prevention of chronic conditions in postmenopausal women including dementia and stroke have been systematically reviewed elsewhere (Gartlehner et al., 2017).
Animal studies investigating the underlying mechanisms of oestrogen in AD pathology have demonstrated that depletion of sex steroids by ovariectomy (OVX) significantly increase soluble Aβ levels in the brain of wild‐type rodents (Jayaraman et al., 2012). In transgenic mouse models of AD, OVX accelerates Aβ pathology and worsens the cognitive phenotype (Levin‐Allerhand, Lominska, Wang, & Smith, 2002). Conversely, 17β‐estradiol treatment in OVX AD transgenic mice significantly lower Aβ accumulation when compared to OVX mice treated with placebo (Carroll et al., 2007). Importantly, these findings seem to be strain specific as some transgenic models did not show similar effects of 17β‐estradiol on Aβ (Golub et al., 2008). Interestingly, the protective effects of oestrogen in non‐transgenic and transgenic models of AD may diminish with ageing (Palm et al., 2014), possibly due to regulatory effects of progesterone (Carroll, Rosario, Villamagna, & Pike, 2010), suggesting that beneficial effects of oestrogen in AD pathology may be limited to a critical reproductive period.
In contrast to women, the age‐related loss of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2858 in men significantly increases the risk of AD (Hogervorst, Combrinck, & Smith, 2003). Longitudinal study of 574 men followed for a mean of 19 years showed that low endogenous serum total testosterone may appear as early as 10 years prior to the clinical diagnosis of dementia (Moffat et al., 2004), suggesting testosterone depletion is an early event in AD pathogenesis in men. In addition to serum levels, brain levels of testosterone are also significantly reduced in both early and late stages of AD pathology in men (Rosario et al., 2011). A study in prostate cancer patients found that risk of AD was significantly higher in patients who received androgen‐deprivation therapy (Nead et al., 2016). A study of sex‐specific association of serum sex hormones and cerebral Aβ (assessed by PiB‐PET) showed that in females, higher free testosterone serum levels were associated with lower cerebral Aβ and in males, free testosterone was positively correlated with hippocampal volume and cognitive status (Lee et al., 2017). These findings suggest that testosterone may inhibit early phases of Aβ accumulation in females and delay hippocampal neurodegeneration in males.
Animal studies in male rodents also suggest a protective role for testosterone in development of AD. For example, depletion of endogenous testosterone by orchiectomy in male rats increases soluble Aβ and this effect is reversed by non‐aromatizable androgen http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2856 (Ramsden et al., 2003). Notably, this study also showed that 17β‐estradiol did not reverse the effects of testosterone depletion, indicating a sex‐specific role of androgens, but not oestrogens, in regulation of Aβ‐related pathology in male rats. Age‐related reproductive events and hormonal changes may seem biologically less complex in males when compared to females. Nonetheless, considering age and sex in male‐only studies remains as critically important as in female‐only or mixed‐sex studies. Future clinical and preclinical studies investigating the role of sex hormones in age‐related pathologies such as dementia and stroke must account for the interplay of age and sex as two independent biological variables.
4.11. Sex hormones and risk of stroke
It is well known that young and premenopausal women have a lower risk of cardiovascular disease (CVD) compared to men; however, this protection does not persist in older patients. Female risk climbs after menopause and surpasses that of men by 80 years of age (Benjamin et al., 2018). This suggests a protective role of sex hormones in women. However, randomized prospective clinical trials found that exogenous oestrogen‐based hormone therapy leads to an increased risk of CVD and stroke (Manson et al., 2003). The reasons for this discrepancy remain unclear but could be related to the age of the subjects, pre‐existing CVD, timing and dosage of therapy, the specific type of hormone used, differential effects on specific oestrogen receptor subtypes, and thrombotic/coagulation effects, most of which have been reviewed elsewhere (Rosano, Vitale, & Fini, 2009; Roy‐O'Reilly & McCullough, 2014). Women started on oestrogen‐based hormone therapy around the time of menopause have slower progression of atherosclerosis as measured by CIMT compared to placebo‐treated women (Hodis et al., 2016). Women enrolled in these trials were at low risk for stroke due to their relatively young age (50s); thus, whether the observed CIMT reduction will translate to a lower stroke risk later in life remains to be seen. In addition to previously emphasized need for more recruitment of women, it is important to gather the critically relevant components of reproductive history when designing clinical trials. Any factor that can influence the cumulative oestrogen exposure or sex‐specific vascular history such as pre‐eclampsia (Moatti, Gupta, Yadava, & Thamban, 2014) can potentially be a confounder in clinical trials, if not properly controlled and randomized. The underlying mechanisms of oestrogens and oestrogen receptors in stroke pathology have been reviewed elsewhere (Hara et al., 2016).
As in women, animal models show that the timing of oestrogen administration is critical to its neuroprotective outcome. We have found that a proinflammatory milieu develops with age in female animals, which modulates the effects of oestrogen in a negative way (Liu, Benashski, Xu, Siegel, & McCullough, 2012). Female mice that were supplemented with estradiol at 15 months (i.e., middle age) and subsequently subjected to stroke at 20 months of age had less ischaemic damage. However, if replacement was initiated at 18 months, after a prolonged period of gonadal senescence, and mice were subjected to stroke, estradiol worsened injury. Interestingly, males benefited from treatment regardless of when it was initiated, suggesting an interplay of sex hormones and chromosomal sex effects (XX vs. XY; Bushnell et al., 2018).
4.12. Sex differences in neuroprotection in stroke
One of the endogenous neuroprotective molecules linked to stroke outcome is http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4731. UA is a byproduct of purine metabolism and a potent antioxidant. As a surrogate marker of oxidative stress, UA has been linked to cardiovascular, cerebrovascular, and neurodegenerative disease. Serum levels of UA are not different between the sexes during childhood but are significantly higher in adult men than in adult women. Although UA levels continue to decline in adulthood, this trend reverses in women after menopause when an increase in UA is observed (Nyrnes et al., 2014). Thus, any analysis needs to take these differences in serum UA levels into consideration prior to making inferences about the association of UA with disease. In 2016, a large meta‐analysis of 30 cohort studies showed that women had an excess risk of CDV and death associated with atrial fibrillation compared to men (Emdin et al., 2016). To investigate the specific risk factors that explain the higher risk of atrial fibrillation in women, a recent analysis of 43 established and novel cardiovascular risk factors found multiple factors including serum UA levels are more strongly associated with risk of atrial fibrillation in women even after correction for age and sex‐related differences in serum UA levels with an adjusted HR of 1.27 (95% CI [1.14, 1.41]) for women (Rienstra et al., 2012). These findings indicate that age‐related changes in serum UA levels should be considered as a sex‐specific cardiovascular and cerebrovascular risk factor.
4.13. Structural sex differences linked to neurodegenerative and neurovascular diseases
Longitudinal studies of sex‐dependent brain changes with normal ageing show that parenchymal atrophy and enlargement of the ventricles may contribute to cognitive decline. These changes are greater in women than in men, when comparing the annual 3D tensor based MRI brain atrophy rates (Hua et al., 2010). To delineate the effects of sex and age on AD pathology, sex‐dependent “normal ageing” process should be considered first. Specific anatomical features since early brain development until old age are sexually dimorphic. For example, females on average have a higher percentage of grey matter in several brain regions including the caudate nuclei, left superior temporal gyrus, and left superior frontal gyrus while in males, a more rapid increase in white matter volume is seen, which contributes to the overall 10% larger brain volume in men than in women (Giedd et al., 2012). Figure 2 illustrates the anatomical location of these brain structures with demonstrated sex differences. Current literature lacks systematic studies evaluating sex differences in cognitive function, metabolic changes, and structural brain changes (i.e., white matter tract integrity and vascular collateral formation) that occur with normal ageing. Identifying neuro‐radiologically detectible structural differences that increase susceptibility to dementia could lead to earlier detection of dementia and cerebrovascular disease, and potentially more efficacious treatment.
The exact explanation for these structural sex differences is not fully understood. Existing data show a relatively high density of sex steroid receptors in the caudate nuclei, amygdala, hippocampus, and cerebellum (Garces et al., 2008). In addition to different receptor densities throughout the brain, specific areas such as cortical grey matter have genetically variant http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=628 (Giedd et al., 2012). Both different receptor densities and genetic variations of the sex steroid receptors make these brain regions particularly responsive to circulating sex hormones throughout the lifespan. Along with structural and genetic differences, there are brain connectivity differences between sexes. For instance, women typically show a higher blood flow and connectivity in the parietal association cortex whereas men typically show a similar pattern in visual and motor cortices (Biswal et al., 2010). These findings along with proposed hypothesis that the localization of amyloid burden may be related to the level of connectivity of different brain regions (Buckner et al., 2005) suggest a clear need to investigate neuroanatomical sex differences in AD and other amyloid‐related pathologies.
5. SEX DIFFERENCES IN PHARMACOLOGICAL THERAPIES FOR AD, CAA, AND STROKE
5.1. Background on pharmacological sex differences
The recognition that both pharmacological responses and the adverse side effects depend on a variety of factors including sex is not a recent observation. There are well‐known physiological differences that partly explain sex‐based differences in drug activity or adverse effect profiles. Pharmacokinetics may be significantly different in women due to lower body weight, slower gastrointestinal enzymatic activity and motility, and lower GFRs. Women have higher sensitivity to β‐blockers, anaesthetics, opioids, selective serotonin reuptake inhibitors, and first generation antipsychotics (Whitley & Lindsey, 2009). Conventionally, most of these factors are addressed via dose adjustments in the clinical setting. In general, women have more frequent and severe drug adverse reactions. For instance, QT prolongation after treatment with antipsychotics and antiarrhythmic medications were shown to be both age and hormone dependent (Kurokawa & Furukawa, 2013). In a large 2009 study of adverse drug reactions in hospitalized heart failure patients in Italy, the association of heart failure with higher adverse drug reactions was seen in women (OR 1.58; 95% CI [1.22, 2.05]) but not in men (OR 0.99; 95% CI [0.74, 1.34]; Catananti et al., 2009).
Our understanding of pharmacological sex differences remains limited due to several important, yet correctible, factors including inadequate recruitment of women into clinical trials and the overwhelming under‐reporting of sex‐specific drug responses. Some experts have asserted that the lack of sex‐specific reporting may contribute to low recruitment rates of women in Phase 3 clinical trials (Nowogrodzki, 2017).
5.2. Lack of sex‐specific reporting in neuropharmacological studies
The evidence for the importance of sex as a biological variable in response to therapies is accruing in cardiovascular/cerebrovascular diseases and sleep disorders (Franconi & Campesi, 2014), but a significant knowledge gap exists in regard to neurodegenerative diseases. Despite emerging evidence suggesting the need for sex‐stratified reporting of data in the clinical trials, such data remain significantly under‐reported for drugs used in the treatment of AD. During the 2002 to 2012 observation period, 413 AD trials were performed: 124 Phase 1 trials, 206 Phase 2 trials, and 83 Phase 3 trials (Cummings, Morstorf, & Zhong, 2014). A 2017 systematic review of 33 randomized clinical trials of cholinesterase inhibitors (ChEIs), the cornerstone of pharmacological treatment in symptomatic dementia patients, found that “sex” was not included in the title, introduction, limitations, or conclusion section of any trial. Three out of 33 trials had the word sex in the abstract but only as a demographic characteristic and not as a biological variable (Mehta et al., 2017).
A limited number of reported preclinical findings for sex differences in ChEI and other neuropharmacological therapies for AD, CAA, and stroke are discussed in the following sections to illustrate the need to evaluate the potential knowledge gaps in the field of pharmacological sex differences.
5.3. Sex differences in pharmacological treatments of AD
5.3.1. Cholinesterase inhibitors
ChEIs are the primary pharmacological treatment in symptomatic dementia patients. In animals, sex differences have been reported in most cholinergic markers including http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2465, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2480, ACh receptor distribution, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=294 concentrations (Rhodes et al., 2001). Other animal studies suggested that testosterone influences the entry of ChEIs into the CNS (Wang & Weinstock, 2001). In addition, most FDA‐approved ChEIs are metabolized by the hepatic http://www.guidetopharmacology.org/GRAC/ReceptorFamiliesForward?type=ENZYME&familyId=242#node242 enzymes, regulation of which is influenced by sex and age (Tomita et al., 2006). Despite consistent reports of sex differences in preclinical studies since early 2000s, the data from human clinical trials have remained inconsistent or, at best, inconclusive. One study of 15 clinical trials prior to 2003 found only weak evidence for higher adverse effects of ChEI in women with body weight identified as a possible confounder (Piotrovsky, Van Peer, Van Osselaer, Armstrong, & Aerssens, 2003). Notably, most of these 15 trials had significantly lower recruitment of women. There is enough preclinical evidence that sex differences contribute to the bioavailability and therapeutic effects of ChEIs. Therefore, recruitment thresholds of both men and women should be considered in the design and conduction phases of clinical trials, rather than an afterthought followed by retrospective analysis. Table 2 summarizes the available data on sex differences from animal studies and clinical trials for the approved AD drugs and statins.
Table 2.
Pharmacological sex differences in therapies for dementia
| Drugs | MoA | Major side effects | Availability of preclinical data (animal models) | Evidence for sex differences in efficacy | Evidence for sex differences in adverse effects | References |
|---|---|---|---|---|---|---|
| Donepezil | ChEI | Nausea, vomiting, loss of appetite, and increased frequency of bowel movements | Possible testosterone‐dependent differences and cholinergic signalling differences | Lack of sex‐specific reporting | Lack of sex‐specific reporting | (Giacobini & Pepeu, 2018) |
| Rivastigmine | ChEI | Nausea, vomiting, loss of appetite, and increased frequency of bowel movements | Lack of sex‐specific reporting | Lack of sex‐specific reporting | (Birks & Grimley Evans, 2015) | |
| Galantamine | ChEI | Nausea, vomiting, loss of appetite, and increased frequency of bowel movements | Lack of sex‐specific reporting | Lack of sex‐specific reporting | (Richarz, Gaudig, Rettig, & Schauble, 2014) | |
| Memantine | NMDA receptor antagonist | Headache, constipation, confusion, and dizziness | NMDA receptor sex differences (mostly in NMDA receptor antagonist or knock‐down experiments) | Lack of sex‐specific reporting | Lack of sex‐specific reporting | (Van Kempen et al., 2015) |
| Statins | HMG‐CoA reductase inhibitor | Myopathy, rhabdomyolysis, and elevated liver enzymes | Our search did not yield significant animal data | Lack of sex‐specific reporting | Lack of sex‐specific reporting | (Plakogiannis & Arif, 2016) |
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6599 is a selective, reversible ChEI approved for treatment of mild to severe AD as well as off‐label use in other types of dementia. Unlike its predecessors, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6687 (metabolized by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1319), donepezil (metabolized by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337) is not associated with hepatotoxicity; however, there is an increased risk of QT prolongation and Torsade de Pointes (Howes, 2014). Efficacy of donepezil in Alzheimer's dementia is similar to other approved ChEI such as tacrine and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6602 based on the Clinical Global Impression of Change scale and ADAS‐Cog (Feldman et al., 2001). Preclinical studies of cholinergic pathways suggest that the efficacy and adverse effects of donepezil may not be the same between men and women. Buccafusco et al. found that in Rhesus monkeys, aged males that were treated with donepezil for 5 weeks used different neural substrates and/or used different brain regions than aged females (Buccafusco, Jackson, Stone, & Terry, 2003), suggesting possible sex differences in neurochemical pathways targeted by donepezil and, probably, other ChEIs. To date, however, the few human studies of donepezil with sex‐specific reporting show inconsistent results. Two randomized clinical trials in 2000 and 2001 reported no differences between men and women in the cognitive and functional effectiveness of donepezil compared to placebo (Winblad et al., 2001). On the other hand, a 2012 study of 184 (60–95 years old) AD patients receiving donepezil or rivastigmine showed that women were more sensitive to ChEI therapy (donepezil or rivastigmine) and had less cognitive decline than men when assessed with the MMSE at multiple post‐treatment time points (Scacchi et al., 2014). After examining two oestrogen receptor α (ESR1) gene intronic polymorphisms (PvuII, rs2234693; XbaI, rs9340799), it was found that patients who carried at least one copy of P and X alleles (indicating the absence of a cutting site for PvuII and XbaI) had significantly less cognitive decline than the non‐carriers (Scacchi et al., 2014). This suggests that ESR1 polymorphisms may be another contributor, in addition to APOE ε4, to interindividual variability in clinical manifestation and response to ChEIs therapies in AD patients. There are ongoing clinical trials in efficacy of donepezil in VaD and PD dementia; and it is unknown if sex was considered in the design of these trials.
Rivastigmine (metabolized by sulfate conjugation) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6693 (metabolized by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1329) are ChEIs used for treatment of mild to moderate AD, mild to moderate PD, as well as an off‐label use in treatment of Lewy body dementia. Primary adverse effects of rivastigmine and galantamine include nausea and vomiting among other cholinergic effects. A study in rats comparing the effects of rivastigmine on enzyme activity and cognitive impairment showed significantly greater inhibitory effect on ChE in female brains in the cerebral cortex, striatum, and hippocampus (Wang, Bejar, & Weinstock, 2000). In a pharmacokinetic study of ChEIs in rats, mice, dogs, and rabbits, plasma levels of galantamine were lower in female rats and mice (Monbaliu et al., 2003). These preclinical findings suggest that bioavailability or efficacy of rivastigmine and galantamine may be different between the sexes. Our PubMed search for sex differences in safety or efficacy of these ChEIs did not yield any reported human data.
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4253 (an amantadine derivative) is a non‐competitive http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=455 antagonist and is approved for the treatment of moderate‐to‐severe AD by the FDA. Its mechanism of action differs from ChEIs because it acts on glutamatergic neurotransmission, rather than augmentation of cholinergic function. Memantine undergoes partial hepatic metabolism and about half of the orally administered drug is excreted unchanged by the kidneys. Aβ accumulation seen in AD can block the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=163 (Domingues, Almeida, da Cruz e Silva, Oliveira, & Rego, 2007), which can lead to glutamate‐mediated excitotoxicity and neurodegeneration. Dampening http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369 transmission is the underlying mechanism for the beneficial effects of memantine in AD patients. Studies of sex differences in glutamate system (reviewed here Wickens et al., 2018) have shown women have higher levels of glutamate compared to men in specific brain regions including the striatum, cerebellum, sensorimotor cortex, and anterior cingulate cortex (Zahr et al., 2013). In contrast to brain levels of glutamate, studies examining blood have found higher glutamate concentrations in men compared to women (Zlotnik et al., 2011). These findings suggest that the interaction of memantine and the glutamate system may exhibit sex differences. However, our PubMed search for sex differences in safety or efficacy of memantine did not yield any reported human data. Systemic reviews of memantine monotherapy in AD can be found elsewhere (Matsunaga, Kishi, & Iwata, 2015).
Statins are http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2718‐lowering agents that act as competitive inhibitors of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=639. Statins occupy a portion of the binding site of HMG‐CoA and block cholesterol access to the active site on this enzyme, which is the rate‐limiting step in cholesterol synthesis. Several studies have evaluated the effects of statin therapy on AD risk. In an examination of the association between statin exposure and AD incidence among Medicare beneficiaries during 2006 and 2012, investigators found that specific statin molecules, sex, and race/ethnicity contribute to a reduction in risk of AD (Zissimopoulos, Barthold, Brinton, & Joyce, 2017). For instance, when stratified by sex and race/ethnicity, results indicated that http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2953 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2954 were associated with reduced AD risk for white women only (HR, 0.82, 95% CI [0.70, 0.95] and HR, 0.81, 95% CI [0.67, 0.98], respectively) and high statin exposure (determined by at least 50th percentile of days of filled prescriptions in a given year for at least 2 years during the study period) was not associated with a lower AD risk among Black men. These findings suggest that in addition to sex and age, other factors such as race/ethnicity and specific statin molecule prescribed to each patient may contribute to AD risk.
5.4. Sex differences in pharmacological treatments of CAA
Acute management of CAA‐related ICH is similar to spontaneous ICH (Hemphill et al., 2015). Due to recurrent nature of CAA‐related ICH, specific guidelines for anticoagulation and antiplatelet therapies in CAA patients are an active area of clinical investigation and debate (Banerjee et al., 2017). Many observational studies of ICH patients do not distinguish between CAA‐related and non‐CAA‐related ICH, which makes it difficult to determine whether the conclusions about the use of anticoagulation therapy are applicable to treatment of CAA patients.
5.5. Sex differences in anticoagulation therapy
Sex differences are seen in anticoagulation therapy. In a meta‐analysis of five randomized clinical trials, women treated with warfarin therapy had a greater risk of stroke or systemic thromboembolism than men (OR, 1.279; 95% CI [1.111, 1.473]; P ≤ .001). An analysis of data from four randomized trials evaluating the novel oral anticoagulants (OACs; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6390, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6380, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6388) found that stroke and systemic thromboembolic rates were similar in men and women (OR, 1.146; 95% CI [0.97, 1.354]; P ≤ .109; Pancholy et al., 2014). Notably, an analysis of 4,060 patients with atrial fibrillation from the data from the Atrial Fibrillation Follow‐up Investigation of Rhythm Management trial showed that women spent more time below the therapeutic range than men (Sullivan, Zhang, Zamba, Lip, & Olshansky, 2012). This may explain the greater risk of stroke in women compared to men when treated with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6853 but not when treated with the novel OACs. Reviews of sex differences in endogenous coagulation pathways are available (Roy‐O'Reilly & McCullough, 2014). Sex and gender differences in anticoagulation use, ICH prevention, and stroke management remains a clinical concern.
5.6. Sex differences in stroke therapies
Biological sex may not only influence functional outcome after stroke but may also influence how patients respond to treatment. A meta‐analysis study of effects of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4139 on composite cardiovascular risks showed a risk reduction for both men and women. However, this effect was primarily due to reduced risk of stroke in women while in men, this effect was due to reduced risk of myocardial infarction (Berger et al., 2006). A 2005 study of randomized trials from the United States, Europe, and Australia (n = 2,178) found that women benefited more from treatment with http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2392 than men (Kent, Price, Ringleb, Hill, & Selker, 2005). Possible contributors to these finding are sex differences in coagulation and fibrinolysis as well as a higher prevalence of cardioembolic fibrin‐rich occlusions in women (Roy‐O'Reilly & McCullough, 2014). Reviews of sex differences in response to thrombolytic, endovascular, and aspirin treatments in ischaemic stroke are available (Loikas et al., 2017). In addition to sex‐specific response to FDA‐approved treatments, the role of endogenous and exogenous neuroprotective molecules in response to stroke treatment and cardiovascular risk management are active areas of preclinical and clinical research.
5.7. Sex differences in neuroprotection
Serum UA is also an independent risk factor for the presence of silent brain infarction in women but not in men in patients within the highest quartile of UA levels (Heo & Lee, 2010). As an abundant endogenous antioxidant capable of reducing cellular oxidative stress, UA may also play a role in neurodegenerative diseases. Accelerated cognitive decline has been reported in patients with lower serum UA levels (Irizarry et al., 2009). In a 2017 study of sex‐specific associations of baseline UA with cognition with ageing, investigators found that higher levels of UA at baseline were associated with reduced cognitive declines in men but were not associated with change in cognition over time in women (Kueider et al., 2017). In acute ischaemic stroke, consistent with reduced endogenous antioxidant capacity in women secondary to lower concentration of serum UA, the URICOICTUS phase IIb/III Trial (Chamorro et al., 2014) found that women had a 42.3% excellent outcome (defined as a modified Rankin Score of 0–1 at 90 days, or a score of 2 if premorbid score was 2) with exogenous UA therapy and 20.5% with placebo while men had a 36% excellent outcome with UA therapy and 36.5% with placebo (Llull et al., 2015). The results showed a significant interaction between sex and neuroprotective effect of exogenous UA after stroke. Understanding the effects of UA in cognition and cerebrovascular disease requires further studies in appropriate experimental models. Additionally, investigating whether such effects are age‐related and/or sex‐dependent warrants careful experimental designs in which both age and sex are considered as biological variables with proper corresponding controls and statistical power analysis.
Minocycline is a clinically well‐tolerated tetracycline that showed a sex‐dependent neuroprotective effects in several preclinical studies and one small clinical trial in ischaemic stroke patients. This may be due to the drug's ability to inhibit http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=883. PARP activation plays a major role in http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=734‐independent cell death following ischaemia by depleting NAD(+) and enhancing mitochondrial permeability transition. Early studies showed that loss of PARP is protective in males whereas it led to unexpected exacerbation of ischaemic injury in females (McCullough, Zeng, Blizzard, Debchoudhury, & Hurn, 2005). In 2009, using a middle cerebral artery occlusion model, Li and McCullough showed that minocycline significantly reduced infarct volume and improved behavioural outcomes in male but not female mice (Yuan et al., 2009). This was unrelated to female sex steroid hormones, as minocycline also did not reduce injury in ovariectomized female mice (Li, Oh, & Lau, 2006). The sex‐specific effects of minocycline may be model‐specific as a later study demonstrated efficacy in both sexes in an embolic stroke model (Elewa, Hilali, Hess, Machado, & Fagan, 2006). A later open‐label evaluator‐blinded clinical study showed that among acute stroke patient receiving oral minocycline, male patients benefited from treatment, whereas women did not (Amiri‐Nikpour, Nazarbaghi, Hamdi‐Holasou, & Rezaei, 2015). However, the trial was small and was not powered to detect sex differences. As there was some suggestion of efficacy, a sex‐specific trial that enrolls only men could be of considerable interest. Furthermore, the PARP‐inhibitory effects of minocycline may be disease‐specific and studies of sex‐specific effects of minocycline in multiple sclerosis and breast cancer are ongoing.
5.8. Sex differences in response to statin therapies in stroke
Statins are the most widely used lipid‐lowering agents and are essential in prevention and treatment of CVD. Statins primarily act by competitively inhibiting HMG‐CoA reductase in the cholesterol biosynthesis pathway, which leads to reduced low‐density lipoproteins. In addition to this primary mechanism of action, statins exert their beneficial effects by modulating atherosclerosis progression and the associated inflammatory response via post‐transcriptional modification of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1249. Studies of sex differences in safety and efficacy of statins in several diseases have been debated. For instance, use of statins in patients at risk of ICH has been controversial since the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial in 4,731 patients with recent stroke or transient ischaemic attack found an increased risk of recurrent ICH with high‐dose atorvastatin (HR, 1.68, 95% CI [1.09, 2.58]; Goldstein et al., 2008). However, several subsequent studies found no increase in risk of ICH with sex, suggesting that this earlier finding may have been a “statistical fluke,” that is, a rare event without suggestion of an underlying correlation or causation (Quinn et al., 2017). A large meta‐analysis of six clinical trials (including Stroke Prevention by Aggressive Reduction in Cholesterol Levels) evaluating the effect of atorvastatin on lipid lowering, cardiovascular events, and adverse events in women compared with men concluded that the response of women to atorvastatin was similar to that of men (Hsue et al., 2015). In general, data for statin use in ICH patients remain conflicting (Banerjee et al., 2017) with near complete lack of sex‐specific reporting to date. Another study of 8,372 Australian women born between 1921 and 1926, alive as of January 2003, free of diabetes, and with statin exposure between 2002 and 2013 showed that statin exposure was associated with a higher risk of new‐onset diabetes with a number needed to harm of 131 (95% CI [62, 1,079]) for 5 years of statin exposure. The observed risk increase was dose dependent with the HR of 1.17 (95% CI [0.84, 1.65]) for the lowest dose to 1.51 (95% CI [1.14, 1.99]) for the highest dose of statin included in the study (Jones, Tett, Peeters, Mishra, & Dobson, 2017). This finding suggests that elderly women on statin therapy should be regularly monitored for blood glucose levels to ensure early detection and management of potential new onset diabetes secondary to high‐dose statin exposure. These studies indicate that reports of sex differences in efficacy and side effects of statins have been inconsistent. A possible explanation for the contradictory meta‐analysis findings may be the small representation of women in clinical trials. Further studies are necessary to determine harms and benefits of statin therapy in patients based on specific stroke pathology, sex, and age.
5.9. The “Zolpidem Lesson”
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4348 is a selective non‐benzodiazepine agonist of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=404 A receptor and is used for treatment of insomnia. Women metabolize zolpidem significantly more slowly than men do. Unfortunately, it took several years and many reports of adverse events before dosing recommendations were changed in 2013 to a lower recommended dose in women. Recent studies using immunochemistry and whole cell patch clamp recording of tonic currents have shown that zolpidem modulated the activity of its target receptor in an age‐ and sex‐dependent manner (Chudomel, Hasson, Bojar, Moshe, & Galanopoulou, 2015). Zolpidem serves as an example where the initially neglected sex‐dependent response to a potent pharmacological agent triggered a regulatory change by the FDA.
6. GENDER DIFFERENCES
Cultural and psychosocial factors play a role in biomedical research and clinical practice in a variety of contexts. Most preclinical studies are performed in male animals based on the assumption that females are intrinsically more variable than males due to hormonal variations, an assumption that has been questioned (Franconi, Rosano, & Campesi, 2015). In addition, in vitro studies often do not specify the sex of cells examined, suggesting that bias may also be present in the preclinical setting. In the context of clinical studies, gender factors can contribute to treatment adherence, access to care, living situation, and socio‐economic status. Even the gender of the health care provider and care‐givers can contribute to differential treatment in a wide range of diseases, especially in illnesses with predominant psychiatric components such as dementia.
Social isolation has been associated with negative outcomes in neurological disorders, especially in the older population. According to a 2018 study, the risk of developing dementia in patients with MCI was increased by 50% if patients lived alone, compared with living with someone (Grande et al., 2018). Notably, selected participants who were living alone were more likely to be women and older. This is consistent with recent trends seen in United States census data indicating that many more women over the age of 80 live alone, probably because they often outlive their spouses and other family members. According to the latest 5‐year community survey data from the US Census Bureau, between 2012 and 2016, the statistics of “widowed females” versus “widowed males” was 9% versus 2.6%. Commonly overlooked factors that contribute to lower rates of recruitment and participation of women in trials include physical immobility, lack of personal transportation, cognitive impairment with inability to give consent or to adequately comply with the study guidelines, and the overall lack of social support.
A few recent analyses of large patient databases have highlighted another possible gender‐based factor, which may be a culturally sensitive concept to investigate and address. An analysis of the PINNACLE National Cardiovascular Data Registry from 2008 to 2014 showed that among patients with atrial fibillation, women were significantly less likely to receive OACs at all levels of the CHA2DS2‐VASc score. Despite rapidly increasing use of non‐vitamin K OACs, women had persistently lower rates of OAC use compared to men over time, suggesting the clinical guidelines may be applied differently in women and men (Thompson et al., 2017). Studies are needed to determine whether gender‐based clinical practices are associated with different clinical outcomes, and if so, amplified awareness efforts followed by actions are needed to eliminate such practices.
Mitigation of the gender‐specific factors will be financially taxing on the expensive clinical trials, but the long‐term cost of overlooking clinically significant biological differences in future drug development studies will surpass the incremental cost of providing the practical means for enrolment of more women. Despite the fact that these gender differences may be difficult to model in a laboratory setting, carefully designed experiments on the effects of social isolation on extent of ischaemic injury and post‐stroke recovery have been successfully evaluated (O'Keefe et al., 2014). Therefore, in addition to sex differences discussed in this review, gender differences (illustrated in Figure 3) and their role in pathology, progression, and pharmacological response to therapy in dementia must be considered as an additional variable in the design of new preclinical and clinical studies.
Figure 3.
Gender factors that may contribute to differences seen between men and women with dementia. These factors may also contribute to the low rates of recruitment of women in clinical studies
7. CONCLUSION
Sex and gender are two separate but intertwined variables in medicine. Although it is difficult to isolate the two concepts, both sex and gender affect the aetiology, symptomology, diagnostics, response to treatment, and outcomes from most diseases. Despite this knowledge, biomedical research and reporting on the impact of sex and gender in health and disease remain insufficient. Identification and understanding of the underlying role of sex and gender as two biological variables will be critical to the development of pharmacological therapies to improve the treatment of men and women suffering from dementia, VCI, and stroke, as well as other neurological conditions. Proper experimental models of each disease are necessary due to the diversity in pathophysiology and clinical presentation of these conditions. The importance of well‐designed bench and clinical studies becomes more apparent when one considers that both sex and gender differences may be disease specific and may vary significantly with age. Inadequate recruitment of women in clinical trials and the overwhelming under‐reporting of sex‐specific drug responses and adverse effects in preclinical studies as well as clinical trials continue to serve as major limitations to successful drug development. Sex and gender differences may be disease specific, drug specific, age specific, or any combination of these; and future studies must be designed to either conclusively detect such differences or rule them out. Clinically, the inescapable but reducible level of subjectivity of patient‐derived reporting complicates analysis of the existing data. Of urgent importance is propagating the awareness of potential sex differences in existing clinical guidelines, or their implementation, which need to be closely investigated and eliminated, if found. Our collective efforts in understanding how male and female biology contribute to differences in the diagnosis and treatment of AD, CAA, and stroke, as well as sex‐dependent responses to pharmacological interventions, have far‐reaching consequences that are relevant to many areas of neurobiology and medicine.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017; Alexander, Peters et al., 2017).
ACKNOWLEDGEMENTS
Dr Louise D. McCullough is supported by NIH Grant R37 NSO96593 (National Institute of Neurological Disorders and Stroke) and Grant RF1 AG058463 (National Institute on Aging).
Honarpisheh P, McCullough LD. Sex as a biological variable in the pathology and pharmacology of neurodegenerative and neurovascular diseases. Br J Pharmacol. 2019;176:4173–4192. 10.1111/bph.14675
REFERENCES
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri‐Nikpour, M. R. , Nazarbaghi, S. , Hamdi‐Holasou, M. , & Rezaei, Y. (2015). An open‐label evaluator‐blinded clinical study of minocycline neuroprotection in ischemic stroke: Gender‐dependent effect. Acta Neurologica Scandinavica, 131, 45–50. [DOI] [PubMed] [Google Scholar]
- Auriel, E. , Edlow, B. L. , Reijmer, Y. D. , Fotiadis, P. , Ramirez‐Martinez, S. , Ni, J. , … Greenberg, S. M. (2014). Microinfarct disruption of white matter structure: A longitudinal diffusion tensor analysis. Neurology, 83, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, G. , Carare, R. , Cordonnier, C. , Greenberg, S. M. , Schneider, J. A. , Smith, E. E. , … Werring, D. J. (2017). The increasing impact of cerebral amyloid angiopathy: Essential new insights for clinical practice. Journal of Neurology, Neurosurgery, and Psychiatry, 88, 982–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit, S. , Wohlfahrt, J. , & Boyd, H. A. (2018). Pre‐eclampsia and risk of dementia later in life: Nationwide cohort study. BMJ, 363, k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri, M. S. , Rapp, M. , Schmeidler, J. , Reichenberg, A. , Purohit, D. P. , Perl, D. P. , … Silverman, J. M. (2009). Number of children is associated with neuropathology of Alzheimer's disease in women. Neurobiology of Aging, 30, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, E. J. , Virani, S. S. , Callaway, C. W. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2018). Heart disease and stroke statistics‐2018 update: A report from the American Heart Association. Circulation, 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- Bennett, D. (2001). Public health importance of vascular dementia and Alzheimer's disease with cerebrovascular disease. International Journal of Clinical Practice Supplement, (120), 41–48. [PubMed] [Google Scholar]
- Berger, J. S. , Roncaglioni, M. C. , Avanzini, F. , Pangrazzi, I. , Tognoni, G. , & Brown, D. L. (2006). Aspirin for the primary prevention of cardiovascular events in women and men: A sex‐specific meta‐analysis of randomized controlled trials. JAMA, 295, 306–313. [DOI] [PubMed] [Google Scholar]
- Birks, J. S. , & Grimley Evans, J. (2015). Rivastigmine for Alzheimer's disease. Cochrane Database of Systematic Reviews, (4), CD001191. [DOI] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes, M. , Zuo, X. N. , Gohel, S. , Kelly, C. , Smith, S. M. , … Milham, M. P. (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America, 107, 4734–4739. 10.1073/pnas.0911855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove, R. , Secor, E. , Chibnik, L. B. , Barnes, L. L. , Schneider, J. A. , Bennett, D. A. , & De Jager, P. L. (2014). Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology, 82, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco, J. J. , Jackson, W. J. , Stone, J. D. , & Terry, A. V. (2003). Sex dimorphisms in the cognitive‐enhancing action of the Alzheimer's drug donepezil in aged Rhesus monkeys. Neuropharmacology, 44, 381–389. [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , Snyder, A. Z. , Shannon, B. J. , LaRossa, G. , Sachs, R. , Fotenos, A. F. , … Mintun, M. A. (2005). Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience, 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell, C. D. , Chaturvedi, S. , Gage, K. R. , Herson, P. S. , Hurn, P. D. , Jimenez, M. C. , … Rundek, T. (2018). Sex differences in stroke: Challenges and opportunities. Journal of Cerebral Blood Flow & Metabolism, 38(12), 2179–2191. 10.1177/0271678X18793324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell, C. D. , Reeves, M. J. , Zhao, X. , Pan, W. , Prvu‐Bettger, J. , Zimmer, L. , … Peterson, E. (2014). Sex differences in quality of life after ischemic stroke. Neurology, 82, 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, J. C. , Rosario, E. R. , Chang, L. , Stanczyk, F. Z. , Oddo, S. , LaFerla, F. M. , & Pike, C. J. (2007). Progesterone and estrogen regulate Alzheimer‐like neuropathology in female 3xTg‐AD mice. The Journal of Neuroscience, 27, 13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, J. C. , Rosario, E. R. , Villamagna, A. , & Pike, C. J. (2010). Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer‐like pathology in female 3xTransgenic‐Alzheimer's disease mice. Endocrinology, 151, 2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catananti, C. , Liperoti, R. , Settanni, S. , Lattanzio, F. , Bernabei, R. , Fialova, D. , … Onder, G. (2009). Heart failure and adverse drug reactions among hospitalized older adults. Clinical Pharmacology and Therapeutics, 86, 307–310. [DOI] [PubMed] [Google Scholar]
- Chaker, L. , Wolters, F. J. , Bos, D. , Korevaar, T. I. , Hofman, A. , van der Lugt, A. , … Ikram, M. A. (2016). Thyroid function and the risk of dementia: The Rotterdam Study. Neurology, 87, 1688–1695. [DOI] [PubMed] [Google Scholar]
- Chamorro, A. , Amaro, S. , Castellanos, M. , Segura, T. , Arenillas, J. , Marti‐Fabregas, J. , … URICO‐ICTUS Investigators (2014). Safety and efficacy of uric acid in patients with acute stroke (URICO‐ICTUS): A randomised, double‐blind phase 2b/3 trial. Lancet Neurology, 13, 453–460. [DOI] [PubMed] [Google Scholar]
- Chauhan, A. , Moser, H. , & McCullough, L. D. (2017). Sex differences in ischaemic stroke: Potential cellular mechanisms. Clinical Science, 131, 533–552. [DOI] [PubMed] [Google Scholar]
- Chowen, J. A. , Argente‐Arizón, P. , Freire‐Regatillo, A. , & Argente, J. (2018). Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Frontiers in Neuroendocrinology, 48, 3–12. [DOI] [PubMed] [Google Scholar]
- Chudomel, O. , Hasson, H. , Bojar, M. , Moshe, S. L. , & Galanopoulou, A. S. (2015). Age‐ and sex‐related characteristics of tonic GABA currents in the rat substantia nigra pars reticulata. Neurochemical Research, 40, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder, E. H. , Ghebremedhin, E. , Taylor, M. G. , Thal, D. R. , Ohm, T. G. , & Braak, H. (2004). The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: Modification by age, sex, and APOE polymorphism. Annals of the new York Academy of Sciences, 1019, 24–28. 10.1196/annals.1297.005 [DOI] [PubMed] [Google Scholar]
- Cummings, J. L. , Morstorf, T. , & Zhong, K. (2014). Alzheimer's disease drug‐development pipeline: Few candidates, frequent failures. Alzheimer's Research & Therapy, 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues, A. , Almeida, S. , da Cruz e Silva, E. F. , Oliveira, C. R. , & Rego, A. C. (2007). Toxicity of β‐amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N‐methyl‐D‐aspartate receptor subunits. Neurochemistry International, 50, 872–880. [DOI] [PubMed] [Google Scholar]
- Elewa, H. F. , Hilali, H. , Hess, D. C. , Machado, L. S. , & Fagan, S. C. (2006). Minocycline for short‐term neuroprotection. Pharmacotherapy, 26, 515–521. 10.1592/phco.26.4.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin, C. A. , Wong, C. X. , Hsiao, A. J. , Altman, D. G. , Peters, S. A. , Woodward, M. , & Odutayo, A. A. (2016). Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta‐analysis of cohort studies. BMJ, 532, h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, H. , Gauthier, S. , Hecker, J. , Vellas, B. , Subbiah, P. , Whalen, E. , & Donepezil MSAD Study Investigators Group (2001). A 24‐week, randomized, double‐blind study of donepezil in moderate to severe Alzheimer's disease. Neurology, 57, 613–620. 10.1212/WNL.57.4.613 [DOI] [PubMed] [Google Scholar]
- Finch, C. E. , & Shams, S. (2016). Apolipoprotein E and sex bias in cerebrovascular aging of men and mice. Trends in Neurosciences, 39, 625–637. 10.1016/j.tins.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D. W. , Bennett, D. A. , & Dong, H. (2018). Sexual dimorphism in predisposition to Alzheimer's disease. Neurobiology of Aging, 70, 308–324. 10.1016/j.neurobiolaging.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). ‘Mini‐mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fox, M. , Berzuini, C. , & Knapp, L. A. (2013). Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology, 38, 2973–2982. [DOI] [PubMed] [Google Scholar]
- Franconi, F. , & Campesi, I. (2014). Sex and gender influences on pharmacological response: An overview. Expert Review of Clinical Pharmacology, 7, 469–485. [DOI] [PubMed] [Google Scholar]
- Franconi, F. , Rosano, G. , & Campesi, I. (2015). Need for gender‐specific pre‐analytical testing: The dark side of the moon in laboratory testing. International Journal of Cardiology, 179, 514–535. [DOI] [PubMed] [Google Scholar]
- Frost, G. R. , & Li, Y.‐M. (2017). The role of astrocytes in amyloid production and Alzheimer's disease. Open Biology, 7(12). pii: 170228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon, O. J. , Robison, L. S. , Custozzo, A. J. , & Zuloaga, K. L. (2018). Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochemistry International. 10.1016/j.neuint.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Garces, C. , de Oya, I. , Lopez‐Simon, L. , Cano, B. , Schoppen, S. , Gil, A. , & de Oya, M. (2008). Hormone levels in 12‐ to 15‐year‐old boys and girls in Spain and their relationship with anthropometric variables. Clinical Biochemistry, 41, 621–624. 10.1016/j.clinbiochem.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Gartlehner, G. , Patel, S. V. , Viswanathan, M. , Feltner, C. , Weber, R. P. , Lee, R. , … Lohr, K. (2017). No title. In Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women: An Evidence Review for the U.S. Preventive Services Task Force. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29589880. [PubMed] [Google Scholar]
- Geerlings, M. I. , Ruitenberg, A. , Witteman, J. C. , van Swieten, J. C. , Hofman, A. , van Duijn, C. M. , … Launer, L. J. (2001). Reproductive period and risk of dementia in postmenopausal women. JAMA, 285, 1475–1481. 10.1001/jama.285.11.1475 [DOI] [PubMed] [Google Scholar]
- Ghosh, R. , & Tabrizi, S. J. (2018). Huntington disease. Handbook of Clinical Neurology, 147, 255–278. [DOI] [PubMed] [Google Scholar]
- Giacobini, E. , & Pepeu, G. C. (2018). Sex and gender differences in the brain cholinergic system and in the response to therapy of Alzheimer disease with cholinesterase inhibitors. Current Alzheimer Research, 15(11), 1077–1084. 10.2174/1567205015666180613111504 [DOI] [PubMed] [Google Scholar]
- Giedd, J. N. , Raznahan, A. , Mills, K. L. , & Lenroot, R. K. (2012). Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biology of Sex Differences, 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, C. E. , Dowling, N. M. , Wharton, W. , Manson, J. E. , Miller, V. M. , Atwood, C. S. , … Asthana, S. (2015). Effects of hormone therapy on cognition and mood in recently postmenopausal women: Findings from the randomized, controlled KEEPS‐cognitive and affective study. PLoS Medicine, 12, e1001833. discussion e1001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, L. B. , Amarenco, P. , Szarek, M. , Callahan, A. 3rd , Hennerici, M. , Sillesen, H. , … SPARCL Investigators (2008). Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology, 70, 2364–2370. [DOI] [PubMed] [Google Scholar]
- Golub, M. S. , Germann, S. L. , Mercer, M. , Gordon, M. N. , Morgan, D. G. , Mayer, L. P. , & Hoyer, P. B. (2008). Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiology of Aging, 29, 1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick, P. B. , Scuteri, A. , Black, S. E. , Decarli, C. , Greenberg, S. M. , Iadecola, C. , … American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia (2011). Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande, G. , Vetrano, D. L. , Cova, I. , Pomati, S. , Mattavelli, D. , Maggiore, L. , … Rizzuto, D. (2018). Living alone and dementia incidence: A clinical‐based study in people with mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology, 31(3), 107–113. 10.1177/0891988718774425 [DOI] [PubMed] [Google Scholar]
- Hachinski, V. C. , Iliff, L. D. , Zilhka, E. , Du Boulay, G. H. , McAllister, V. L. , Marshall, J. , … Symon, L. (1975). Cerebral blood flow in dementia. Archives of Neurology, 32, 632–637. 10.1001/archneur.1975.00490510088009 [DOI] [PubMed] [Google Scholar]
- Hara, Y. , Yuk, F. , Puri, R. , Janssen, W. G. , Rapp, P. R. , & Morrison, J. H. (2016). Estrogen restores multisynaptic boutons in the dorsolateral prefrontal cortex while promoting working memory in aged rhesus monkeys. The Journal of Neuroscience, 36, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, L. E. , Weuve, J. , Scherr, P. A. , & Evans, D. A. (2013). Alzheimer disease in the United States (2010‐2050) estimated using the 2010 census. Neurology, 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill, J. C. 3rd , Greenberg, S. M. , Anderson, C. S. , Becker, K. , Bendok, B. R. , Cushman, M. , … American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology (2015). Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 46, 2032–2060. [DOI] [PubMed] [Google Scholar]
- Heo, S. H. , & Lee, S. H. (2010). High levels of serum uric acid are associated with silent brain infarction. Journal of the Neurological Sciences, 297, 6–10. [DOI] [PubMed] [Google Scholar]
- Hodis, H. N. , Mack, W. J. , Henderson, V. W. , Shoupe, D. , Budoff, M. J. , Hwang‐Levine, J. , … for the ELITE Research Group (2016). Vascular effects of early versus late postmenopausal treatment with estradiol. The New England Journal of Medicine, 374, 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis, H. N. , Mack, W. J. , Shoupe, D. , Azen, S. P. , Stanczyk, F. Z. , Hwang‐Levine, J. , … Henderson, V. W. (2015). Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with estradiol testing the menopausal hormone timing hypothesis. Menopause, 22, 391–401. 10.1097/GME.0000000000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst, E. , Combrinck, M. , & Smith, A. D. (2003). Testosterone and gonadotropin levels in men with dementia. Neuro Enocrinology Letters, 24, 203–208. [PubMed] [Google Scholar]
- Howes, L. G. (2014). Cardiovascular effects of drugs used to treat Alzheimer's disease. Drug Safety, 37, 391–395. 10.1007/s40264-014-0161-z [DOI] [PubMed] [Google Scholar]
- Hsiao, K. , Chapman, P. , Nilsen, S. , Eckman, C. , Harigaya, Y. , Younkin, S. , … Cole, G. (1996). Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science, 274, 99–102. 10.1126/science.274.5284.99 [DOI] [PubMed] [Google Scholar]
- Hsue, P. Y. , Bittner, V. A. , Betteridge, J. , Fayyad, R. , Laskey, R. , Wenger, N. K. , & Waters, D. D. (2015). Impact of female sex on lipid lowering, clinical outcomes, and adverse effects in atorvastatin trials. The American Journal of Cardiology, 115, 447–453. [DOI] [PubMed] [Google Scholar]
- Hu, Q. , Liu, A. , Huang, M. , Cheng, L. , Kang, H. , Xu, F. , … Zhu, S. (2014). Lower serum uric acid levels in cerebral amyloid angiopathy: A pilot study. Neurological Sciences, 35, 1035–1039. [DOI] [PubMed] [Google Scholar]
- Hua, X. , Hibar, D. P. , Lee, S. , Toga, A. W. , Jack, C. R. Jr. , Weiner, M. W. , … Alzheimer's Disease Neuroimaging Initiative (2010). Sex and age differences in atrophic rates: An ADNI study with n=1368 MRI scans. Neurobiology of Aging, 31, 1463–1480. 10.1016/j.neurobiolaging.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz, B. , Tuppurainen, M. , Tiihonen, M. , Kivipelto, M. , Soininen, H. , Hartikainen, S. , & Tolppanen, A. M. (2014). Oophorectomy, hysterectomy, and risk of Alzheimer's disease: A nationwide case‐control study. Journal of Alzheimer's Disease, 42, 575–581. [DOI] [PubMed] [Google Scholar]
- Irizarry, M. C. , Raman, R. , Schwarzschild, M. A. , Becerra, L. M. , Thomas, R. G. , Peterson, R. C. , … Aisen, P. S. (2009). Plasma urate and progression of mild cognitive impairment. Neurodegenerative Diseases, 6, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine, K. , Laws, K. R. , Gale, T. M. , & Kondel, T. K. (2012). Greater cognitive deterioration in women than men with Alzheimer's disease: A meta analysis. Journal of Clinical and Experimental Neuropsychology, 34, 989–998. [DOI] [PubMed] [Google Scholar]
- Jack, C. R. Jr. , Wiste, H. J. , Weigand, S. D. , Therneau, T. M. , Knopman, D. S. , Lowe, V. , … Petersen, R. C. (2017). Age‐specific and sex‐specific prevalence of cerebral β‐amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50‐95 years: A cross‐sectional study. Lancet Neurology, 16, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, A. , Carroll, J. C. , Morgan, T. E. , Lin, S. , Zhao, L. , Arimoto, J. M. , … Pike, C. J. (2012). 17β‐estradiol and progesterone regulate expression of β‐amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology, 153, 5467–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M. , Tett, S. , Peeters, G. M. E. E. , Mishra, G. D. , & Dobson, A. (2017). New‐onset diabetes after statin exposure in elderly women: The Australian longitudinal study on women's health. Drugs & Aging, 34, 203–209. [DOI] [PubMed] [Google Scholar]
- Kaidonis, G. , Rao, A. N. , Ouyang, Y. B. , & Stary, C. M. (2018). Elucidating sex differences in response to cerebral ischemia: immunoregulatory mechanisms and the role of microRNAs. Prog Neurobiol. 10.1016/j.pneurobio.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Kantarci, K. , Lowe, V. J. , Lesnick, T. G. , Tosakulwong, N. , Bailey, K. R. , Fields, J. A. , … Miller, V. M. (2016). Early postmenopausal transdermal 17β‐estradiol therapy and amyloid‐β deposition. Journal of Alzheimer's Disease, 53, 547–556. 10.3233/JAD-160258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci, K. , Tosakulwong, N. , Lesnick, T. G. , Zuk, S. M. , Lowe, V. J. , Fields, J. A. , … Miller, V. M. (2018). Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology, 90, e1404–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, D. M. , Price, L. L. , Ringleb, P. , Hill, M. D. , & Selker, H. P. (2005). Sex‐based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: A pooled analysis of randomized clinical trials. Stroke, 36, 62–65. 10.1161/01.STR.0000150515.15576.29 [DOI] [PubMed] [Google Scholar]
- Kim, S. M. , Zhao, D. , Schneider, A. L. C. , Korada, S. K. , Lutsey, P. L. , Guallar, E. , … Michos, E. D. (2017). Association of parathyroid hormone with 20‐year cognitive decline: The ARIC study. Neurology, 89, 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, B. , Almqvist, E. , Theilmann, J. , Spence, N. , Telenius, H. , Goldberg, Y. P. , & Hayden, M. R. (1995). Sex‐dependent mechanisms for expansions and contractions of the CAG repeat on affected Huntington disease chromosomes. American Journal of Human Genetics, 57, 343–350. [PMC free article] [PubMed] [Google Scholar]
- Kueider, A. M. , An, Y. , Tanaka, T. , Kitner‐Triolo, M. H. , Studenski, S. , Ferrucci, L. , & Thambisetty, M. (2017). Sex‐dependent associations of serum uric acid with brain function during aging. Journal of Alzheimer's Disease, 60, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa, J. , & Furukawa, T. (2013). Non‐genomic action of sex steroid hormones and cardiac repolarization. Biological & Pharmaceutical Bulletin, 36, 8–12. [DOI] [PubMed] [Google Scholar]
- Lee, J. H. , Byun, M. S. , Yi, D. , Choe, Y. M. , Choi, H. J. , Baek, H. , … KBASE Research Group (2017). Sex‐specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiology of Aging, 58, 34–40. 10.1016/j.neurobiolaging.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Lenz, K. M. , & McCarthy, M. M. (2015). A starring role for microglia in brain sex differences. The Neuroscientist, 21, 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin‐Allerhand, J. A. , Lominska, C. E. , Wang, J. , & Smith, J. D. (2002). 17α‐estradiol and 17β‐estradiol treatments are effective in lowering cerebral amyloid‐β levels in AβPPSWE transgenic mice. Journal of Alzheimer's Disease, 4, 449–457. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Oh, H. J. , & Lau, Y. F. (2006). The poly (ADP‐ribose) polymerase 1 interacts with Sry and modulates its biological functions. Molecular and Cellular Endocrinology, 257–258, 35–46. [DOI] [PubMed] [Google Scholar]
- Lin, K. A. , Choudhury, K. R. , Rathakrishnan, B. G. , Marks, D. M. , Petrella, J. R. , Doraiswamy, P. M. , & Alzheimer's Disease Neuroimaging Initiative (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y), 1, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Benashski, S. E. , Xu, Y. , Siegel, M. , & McCullough, L. D. (2012). Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. Journal of Neuroendocrinology, 24, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llull, L. , Laredo, C. , Renu, A. , Perez, B. , Vila, E. , Obach, V. , … Chamorro, Á. (2015). Uric acid therapy improves clinical outcome in women with acute ischemic stroke. Stroke, 46, 2162–2167. [DOI] [PubMed] [Google Scholar]
- Lobo, A. , Launer, L. J. , Fratiglioni, L. , Andersen, K. , Di Carlo, A. , Breteler, M. M. , … Hofman, A. (2000). Prevalence of dementia and major subtypes in Europe: A collaborative study of population‐based cohorts. Neurologic diseases in the elderly research group. Neurology, 54, S4–S9. [PubMed] [Google Scholar]
- Loikas, D. , Forslund, T. , Wettermark, B. , Schenck‐Gustafsson, K. , Hjemdahl, P. , & von Euler, M. (2017). Sex and gender differences in thromboprophylactic treatment of patients with atrial fibrillation after the introduction of non‐vitamin K oral anticoagulants. The American Journal of Cardiology, 120, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Maki, P. M. (2013). Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause, 20, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson, J. E. , Hsia, J. , Johnson, K. C. , Rossouw, J. E. , Assaf, A. R. , Lasser, N. L. , … Women's Health Initiative Investigators (2003). Estrogen plus progestin and the risk of coronary heart disease. The New England Journal of Medicine, 349, 523–534. [DOI] [PubMed] [Google Scholar]
- Martin‐Laez, R. , Caballero‐Arzapalo, H. , Lopez‐Menendez, L. A. , Arango‐Lasprilla, J. C. , & Vazquez‐Barquero, A. (2015). Epidemiology of idiopathic normal pressure hydrocephalus: A systematic review of the literature. World Neurosurgery, 84, 2002–2009. 10.1016/j.wneu.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Matsunaga, S. , Kishi, T. , & Iwata, N. (2015). Memantine monotherapy for Alzheimer's disease: A systematic review and meta‐analysis. PLoS ONE, 10, e0123289 10.1371/journal.pone.0123289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough, L. D. , Zeng, Z. , Blizzard, K. K. , Debchoudhury, I. , & Hurn, P. D. (2005). Ischemic nitric oxide and poly (ADP‐ribose) polymerase‐1 in cerebral ischemia: Male toxicity, female protection. Journal of Cerebral Blood Flow and Metabolism, 25, 502–512. [DOI] [PubMed] [Google Scholar]
- Mehta, N. , Rodrigues, C. , Lamba, M. , Wu, W. , Bronskill, S. E. , Herrmann, N. , … Rochon, P. A. (2017). Systematic review of sex‐specific reporting of data: Cholinesterase inhibitor example. Journal of the American Geriatrics Society, 65, 2213–2219. [DOI] [PubMed] [Google Scholar]
- Miller, L. R. , Marks, C. , Becker, J. B. , Hurn, P. D. , Chen, W. J. , Woodruff, T. , … Clayton, J. A. (2017). Considering sex as a biological variable in preclinical research. FASEB J, 31(1), 29–34. 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti, Z. , Gupta, M. , Yadava, R. , & Thamban, S. (2014). A review of stroke and pregnancy: Incidence, management and prevention. European Journal of Obstetrics & Gynecology and Reproductive Biology, 181, 20–27. [DOI] [PubMed] [Google Scholar]
- Moffat, S. D. , Zonderman, A. B. , Metter, E. J. , Kawas, C. , Blackman, M. R. , Harman, S. M. , & Resnick, S. M. (2004). Free testosterone and risk for Alzheimer disease in older men. Neurology, 62, 188–193. [DOI] [PubMed] [Google Scholar]
- Monbaliu, J. , Verhaeghe, T. , Willems, B. , Bode, W. , Lavrijsen, K. , & Meuldermans, W. (2003). Pharmacokinetics of galantamine, a cholinesterase inhibitor, in several animal species. Arzneimittel‐Forschung, 53, 486–495. [DOI] [PubMed] [Google Scholar]
- Mosconi, L. , Berti, V. , Quinn, C. , McHugh, P. , Petrongolo, G. , Varsavsky, I. , … Brinton, R. D. (2017). Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology, 89, 1382–1390. 10.1212/WNL.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , … Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Nead, K. T. , Gaskin, G. , Chester, C. , Swisher‐McClure, S. , Dudley, J. T. , Leeper, N. J. , & Shah, N. H. (2016). Androgen deprivation therapy and future Alzheimer's disease risk. Journal of Clinical Oncology, 34, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowogrodzki, A. (2017). Clinical research: Inequality in medicine. Nature, 550, S18–S19. [DOI] [PubMed] [Google Scholar]
- Nyrnes, A. , Toft, I. , Njolstad, I. , Mathiesen, E. B. , Wilsgaard, T. , Hansen, J. B. , & Løchen, M. L. (2014). Uric acid is associated with future atrial fibrillation: An 11‐year follow‐up of 6308 men and women—The Tromso study. Europace, 16, 320–326. [DOI] [PubMed] [Google Scholar]
- O'Bryant, S. E. , Humphreys, J. D. , Smith, G. E. , Ivnik, R. J. , Graff‐Radford, N. R. , Petersen, R. C. , & Lucas, J. A. (2008). Detecting dementia with the mini‐mental state examination in highly educated individuals. Archives of Neurology, 65, 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe, L. M. , Doran, S. J. , Mwilambwe‐Tshilobo, L. , Conti, L. H. , Venna, V. R. , & McCullough, L. D. (2014). Social isolation after stroke leads to depressive‐like behavior and decreased BDNF levels in mice. Behavioural Brain Research, 260, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyike, C. U. , & Diehl‐Schmid, J. (2013). The epidemiology of frontotemporal dementia. International Review of Psychiatry, 25, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisgharan, S. , Arvanitakis, Z. , Yu, L. , Farfel, J. , Schneider, J. A. , & Bennett, D. A. (2018). Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathologica, 136, 887–900. 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm, R. , Chang, J. , Blair, J. , Garcia‐Mesa, Y. , Lee, H.‐G. , Castellani, R. J. , … Casadesus, G. (2014). Down‐regulation of serum gonadotropins but not estrogen replacement improves cognition in aged‐ovariectomized 3xTg AD female mice. Journal of Neurochemistry, 130, 115–125. 10.1111/jnc.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholy, S. B. , Sharma, P. S. , Pancholy, D. S. , Patel, T. M. , Callans, D. J. , & Marchlinski, F. E. (2014). Meta‐analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. The American Journal of Cardiology, 113, 485–490. 10.1016/j.amjcard.2013.10.035 [DOI] [PubMed] [Google Scholar]
- Paoletti, A. M. , Congia, S. , Lello, S. , Tedde, D. , Orrù, M. , Pistis, M. , … Melis, G. B. (2004). Low androgenization index in elderly women and elderly men with Alzheimer's disease. Neurology, 62(2), 301–303. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14745075 [DOI] [PubMed] [Google Scholar]
- Pike, C. J. (2017). Sex and the development of Alzheimer's disease. Journal of Neuroscience Research, 95, 671–680. 10.1002/jnr.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrovsky, V. , Van Peer, A. , Van Osselaer, N. , Armstrong, M. , & Aerssens, J. (2003). Galantamine population pharmacokinetics in patients with Alzheimer's disease: Modeling and simulations. Journal of Clinical Pharmacology, 43, 514–523. 10.1177/0091270003251853 [DOI] [PubMed] [Google Scholar]
- Plakogiannis, R. , & Arif, S. A. (2016). Women versus men: Is there equal benefit and safety from statins? Current Atherosclerosis Reports, 18, 6. [DOI] [PubMed] [Google Scholar]
- Quinn, K. L. , Macdonald, E. M. , Mamdani, M. M. , Diong, C. , Juurlink, D. N. , & Canadian Drug Safety and Effectiveness Research Network (CDSERN) . (2017). Lipophilic statins and the risk of intracranial hemorrhage following ischemic stroke: A population‐based study. Drug Safety, 40, 887–893. [DOI] [PubMed] [Google Scholar]
- Ramsden, M. , Nyborg, A. C. , Murphy, M. P. , Chang, L. , Stanczyk, F. Z. , Golde, T. E. , & Pike, C. J. (2003). Androgens modulate β‐amyloid levels in male rat brain. Journal of Neurochemistry, 87, 1052–1055. [DOI] [PubMed] [Google Scholar]
- Reijmer, Y. D. , Fotiadis, P. , Martinez‐Ramirez, S. , Salat, D. H. , Schultz, A. , Shoamanesh, A. , … Greenberg, S. M. (2015). Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain, 138, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, M. E. , O'Toole, S. M. , Wright, S. L. , Czambel, R. K. , & Rubin, R. T. (2001). Sexual diergism in rat hypothalamic‐pituitary‐adrenal axis responses to cholinergic stimulation and antagonism. Brain Research Bulletin, 54, 101–113. [DOI] [PubMed] [Google Scholar]
- Richarz, U. , Gaudig, M. , Rettig, K. , & Schauble, B. (2014). Galantamine treatment in outpatients with mild Alzheimer's disease. Acta Neurologica Scandinavica, 129, 382–392. [DOI] [PubMed] [Google Scholar]
- Rienstra, M. , McManus, D. D. , & Benjamin, E. J. (2012). Novel risk factors for atrial fibrillation: Useful for risk prediction and clinical decision making? Circulation, 125, e941–e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, C. , Smailagic, N. , Noel‐Storr, A. H. , Ukoumunne, O. , Ladds, E. C. , & Martin, S. (2017). CSF τ and the CSF τ/Aβ ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews, 3, CD010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi, L. , Rosset, I. , & Roriz‐Cruz, M. (2014). Global epidemiology of dementia: Alzheimer's and vascular types. BioMed Research International, 2014, 908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. O. , Knopman, D. S. , Mielke, M. M. , Cha, R. H. , Pankratz, V. S. , Christianson, T. J. , … Petersen, R. C. (2014). Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology, 82, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca, W. A. , Grossardt, B. R. , & Shuster, L. T. (2011). Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Research, 1379, 188–198. 10.1016/j.brainres.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano, G. M. , Vitale, C. , & Fini, M. (2009). Cardiovascular aspects of menopausal hormone replacement therapy. Climacteric, 12(Suppl 1), 41–46. [DOI] [PubMed] [Google Scholar]
- Rosario, E. R. , Chang, L. , Head, E. H. , Stanczyk, F. Z. , & Pike, C. J. (2011). Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiology of Aging, 32, 604–613. 10.1016/j.neurobiolaging.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy‐O'Reilly, M. , & McCullough, L. D. (2014). Sex differences in stroke: The contribution of coagulation. Experimental Neurology, 259, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlus, H. , & Heneka, M. T. (2017). Microglia in Alzheimer's disease. The Journal of Clinical Investigation, 127(9), 3240–3249. 10.1172/JCI90606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal, C. L. , Beiser, A. S. , Chouraki, V. , Chene, G. , Dufouil, C. , & Seshadri, S. (2016). Incidence of dementia over three decades in the Framingham heart study. The New England Journal of Medicine, 374, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica, R. , Grossardt, B. R. , Rocca, W. A. , & Bower, J. H. (2018). Parkinson disease with and without dementia: A prevalence study and future projections. Movement Disorders, 33, 537–543. 10.1002/mds.27277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacchi, R. , Gambina, G. , Broggio, E. , & Corbo, R. M. (2014). Sex and ESR1 genotype may influence the response to treatment with donepezil and rivastigmine in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry, 29, 610–615. [DOI] [PubMed] [Google Scholar]
- Selvamani, A. , Sathyan, P. , Miranda, R. C. , & Sohrabji, F. (2012). An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One, 7(2), e32662 10.1371/journal.pone.0032662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, L. M. , Vanderstichele, H. , Knapik‐Czajka, M. , Clark, C. M. , Aisen, P. S. , Petersen, R. C. , … Alzheimer's Disease Neuroimaging Initiative (2009). Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of Neurology, 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman, K. S. , Toledo, J. B. , Tsivinsky, V. G. , Irwin, D. , Grossman, M. , Weintraub, D. , … Umansky, S. R. (2017). Circulating brain‐enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimer's Research & Therapy, 9(1), 89 10.1186/s13195-017-0316-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara, M. , Murray, M. E. , Frank, R. D. , Shinohara, M. , DeTure, M. , Yamazaki, Y. , … Kanekiyo, T. (2016). Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer's disease. Acta Neuropathologica, 132, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker, S. A. , Legault, C. , Kuller, L. , Rapp, S. R. , Thal, L. , Lane, D. S. , … Women's Health Initiative Memory Study . (2004). Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA, 291, 2947–2958. 10.1001/jama.291.24.2947 [DOI] [PubMed] [Google Scholar]
- Skrobot, O. A. , Attems, J. , Esiri, M. , Hortobagyi, T. , Ironside, J. W. , Kalaria, R. N. , … Love, S. (2016). Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain, 139, 2957–2969. 10.1093/brain/aww214 [DOI] [PubMed] [Google Scholar]
- Sohn, D. , Shpanskaya, K. , Lucas, J. E. , Petrella, J. R. , Saykin, A. J. , Tanzi, R. E. , … Doraiswamy, P. M. (2018). Sex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer's disease. Scientific Reports, 8, 7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychala, M. S. , Honarpisheh, P. , & McCullough, L. D. (2017). Sex differences in neuroinflammation and neuroprotection in ischemic stroke. Journal of Neuroscience Research, 95, 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, R. M. , Zhang, J. , Zamba, G. , Lip, G. Y. H. , & Olshansky, B. (2012). Relation of gender‐specific risk of ischemic stroke in patients with atrial fibrillation to differences in warfarin anticoagulation control (from AFFIRM). The American Journal of Cardiology, 110, 1799–1802. [DOI] [PubMed] [Google Scholar]
- Thompson, L. E. , Maddox, T. M. , Lei, L. , Grunwald, G. K. , Bradley, S. M. , Peterson, P. N. , … Daugherty, S. L. (2017). Sex differences in the use of oral anticoagulants for atrial fibrillation: A report from the National Cardiovascular Data Registry (NCDR®) PINNACLE Registry. Journal of the American Heart Association, 6 10.1161/JAHA.117.005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo, J. B. , Arnold, S. E. , Raible, K. , Brettschneider, J. , Xie, S. X. , Grossman, M. , … Trojanowski, J. Q. (2013). Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain, 136, 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita, M. , Okuyama, T. , Katsuyama, H. , Hidaka, K. , Otsuki, T. , & Ishikawa, T. (2006). Gene expression in rat lungs during early response to paraquat‐induced oxidative stress. International Journal of Molecular Medicine, 17, 37–44. [PubMed] [Google Scholar]
- van der Flier, W. M. , Skoog, I. , Schneider, J. A. , Pantoni, L. , Mok, V. , Chen, C. L. H. , & Scheltens, P. (2018). Vascular cognitive impairment. Nature Reviews Disease Primers, 4, 18003. [DOI] [PubMed] [Google Scholar]
- Van Kempen, T. A. , Dodos, M. , Woods, C. , Marques‐Lopes, J. , Justice, N. J. , Iadecola, C. , … Milner, T. A. (2015). Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin‐releasing factor type 1 receptor following slow‐pressor angiotensin II hypertension. Neuroscience, 307, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto, E. , Bonincontro, C. , Pollio, G. , Sala, A. , Viappiani, S. , Nardi, F. , … Maggi, A. (2001). Estrogen prevents the lipopolysaccharide‐induced inflammatory response in microglia. The Journal of Neuroscience, 21, 1809–1818. 10.1523/JNEUROSCI.21-06-01809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, A. , Gelosa, P. , Castiglioni, L. , Cimino, M. , Rizzi, N. , Pepe, G. , … Maggi, A. (2018). Sex‐specific features of microglia from adult mice. Cell Reports, 23, 3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. H. , Bejar, C. , & Weinstock, M. (2000). Gender differences in the effect of rivastigmine on brain cholinesterase activity and cognitive function in rats. Neuropharmacology, 39, 497–506. 10.1016/S0028-3908(99)00157-4 [DOI] [PubMed] [Google Scholar]
- Wang, R. H. , & Weinstock, M. (2001). Steroid hormones mediate sex difference in brain levels of tacrine and its hypothermic effect in the rat. Neuropharmacology, 41, 887–894. [DOI] [PubMed] [Google Scholar]
- Wharton, W. , Gleason, C. E. , Miller, V. M. , & Asthana, S. (2013). Rationale and design of the Kronos Early Estrogen Prevention Study (KEEPS) and the KEEPS cognitive and affective sub study (KEEPS Cog). Brain Research, 1514, 12–17. 10.1016/j.brainres.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley, H. , & Lindsey, W. (2009). Sex‐based differences in drug activity. American Family Physician, 80, 1254–1258. [PubMed] [Google Scholar]
- Wickens, M. M. , Bangasser, D. A. , & Briand, L. A. (2018). Sex differences in psychiatric disease: A focus on the glutamate system. Frontiers in Molecular Neuroscience, 11, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. M. , Storandt, M. , Roe, C. M. , & Morris, J. C. (2013). Progression of Alzheimer's disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement, 9, S39–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad, B. , Engedal, K. , Soininen, H. , Verhey, F. , Waldemar, G. , Wimo, A. , … Donepezil Nordic Study Group . (2001). A 1‐year, randomized, placebo‐controlled study of donepezil in patients with mild to moderate AD. Neurology, 57, 489–495. [DOI] [PubMed] [Google Scholar]
- Writing Group Members , Lloyd‐Jones, D. , Adams, R. J. , Brown, T. M. , Carnethon, M. , Dai, S. , … American Heart Association Statistics Committee and Stroke Statistics Subcommittee . (2010). Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation, 121, e46–e215. [DOI] [PubMed] [Google Scholar]
- Writing Group Members , Mozaffarian, D. , Benjamin, E. J. , Go, A. S. , Arnett, D. K. , Blaha, M. J. , … American Heart Association Statistics Committee; Stroke Statistics Subcommittee (2016). Heart disease and stroke statistics‐2016 update: A report from the American Heart Association. Circulation, 133, e38–e360. [DOI] [PubMed] [Google Scholar]
- Yang, J.‐T. , Wang, Z.‐J. , Cai, H.‐Y. , Yuan, L. , Hu, M.‐M. , Wu, M.‐N. , & Qi, J. S. (2018). Sex differences in neuropathology and cognitive behavior in APP/PS1/τ triple‐transgenic mouse model of Alzheimer's disease. Neuroscience Bulletin, 34, 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger, D. S. (2016). Epidemiology of neurovasculitis. Neurologic Clinics, 34, 887–917. [DOI] [PubMed] [Google Scholar]
- Yuan, M. , Siegel, C. , Zeng, Z. , Li, J. , Liu, F. , & McCullough, L. D. (2009). Sex differences in the response to activation of the poly (ADP‐ribose) polymerase pathway after experimental stroke. Experimental Neurology, 217, 210–218. 10.1016/j.expneurol.2009.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr, N. M. , Mayer, D. , Rohlfing, T. , Chanraud, S. , Gu, M. , Sullivan, E. V. , & Pfefferbaum, A. (2013). In vivo glutamate measured with magnetic resonance spectroscopy: Behavioral correlates in aging. Neurobiology of Aging, 34, 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi, P. P. , Carlson, M. C. , Plassman, B. L. , Welsh‐Bohmer, K. A. , Mayer, L. S. , Steffens, D. C. , … Cache County Memory Study Investigators (2002). Hormone replacement therapy and incidence of Alzheimer disease in older women: The Cache county study. JAMA, 288, 2123–2129. [DOI] [PubMed] [Google Scholar]
- Zissimopoulos, J. M. , Barthold, D. , Brinton, R. D. , & Joyce, G. (2017). Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurology, 74, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik, A. , Ohayon, S. , Gruenbaum, B. F. , Gruenbaum, S. E. , Mohar, B. , Boyko, M. , … Teichberg, V. I. (2011). Determination of factors affecting glutamate concentrations in the whole blood of healthy human volunteers. Journal of Neurosurgical Anesthesiology, 23, 45–49. [DOI] [PubMed] [Google Scholar]


