Abstract
Men and women have many differing biological and physiological characteristics. Thus, it is no surprise that the control of metabolic processes and the mechanisms underlying metabolic‐related diseases have sex‐specific components. There is a clear metabolic sexual dimorphism in that up until midlife, men have a far greater likelihood of acquiring cardio‐metabolic disease than women. Following menopause, however, this difference is reduced, suggestive of a protective role of the female sex hormones. Inflammatory processes have been implicated in the pathogenesis of cardio‐metabolic disease with human studies correlating metabolic disease acquisition or risk with levels of various inflammatory markers. Rodent studies employing genetic modifications or novel pharmacological approaches have provided mechanistic insight into the role of these inflammatory mediators. Sex differences impact inflammatory processes and the subsequent biological response. As a consequence, this may affect how inflammation alters metabolic processes between the sexes. Recently, some of our work in the field of inflammatory genes and metabolic control identified a sexual dimorphism in a preclinical model and caused us to question the frequency and scale of such findings in the literature. This review concentrates on inflammatory‐related signalling in relation to obesity, insulin resistance, and type 2 diabetes and highlights the differences observed between males and females. Differences in the activation and signalling of various inflammatory genes and proteins present another reason why studying both male and female patients or animals is important in the context of understanding and finding therapeutics for metabolic‐related disease.
LINKED ARTICLES
This article is part of a themed section on The Importance of Sex Differences in Pharmacology Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.21/issuetoc
Abbreviations
- CD11c
integrin
- DAMPs
damage‐associated molecular patterns
- E1
oestrone
- E2
oestradiol
- ERα
oestrogen receptor α
- ERβ
oestrogen receptor β
- GFAP
glial fibrillary acidic protein promoter driven
- GPER
G‐protein coupled oestrogen receptor
- HCC
hepatocellular carcinoma
- HFD
high‐fat diet
- KO
knockout
- OVX
ovariectomy
- PAMPs
pathogen‐associated molecular patterns
- TLR
toll‐like receptor
- WAT
white adipose tissue
1. INTRODUCTION
Compared with premenopausal women, men have a higher risk of insulin resistance (S. H. Kim & Reaven, 2013; Lee, Ko, Kwak, & Yim, 2016), type 2 diabetes (Kautzky‐Willer, Harreiter, & Pacini, 2016), and cardiovascular disease (Maas & Appelman, 2010). As women progress through menopause, the sex differences in the prevalence of metabolic disease are reduced (Janssen, Powell, Crawford, Lasley, & Sutton‐Tyrrell, 2008; Maas & Appelman, 2010). Even though obesity is more prevalent in women than men (Kanter & Caballero, 2012), a metabolically healthy but obese phenotype seems to be more evident in women (Pajunen et al., 2011). Thus, whereas gluteofemoral fat storage is favoured in premenopausal women, both men and postmenopausal women have increased abdominal fat deposition (Abildgaard et al., 2013; Abildgaard et al., 2018; Karastergiou, Smith, Greenberg, & Fried, 2012; Lovejoy, Champagne, de Jonge, Xie, & Smith, 2008). The distribution of body fat is an important determinant for metabolic health (Karpe & Pinnick, 2015). Abdominal fat deposition contributes substantially to chronic low‐grade inflammation (Schmidt et al., 2015) and is closely related to the development of type 2 diabetes and cardiovascular disease (Snijder et al., 2004; Yusuf et al., 2005), whereas gluteofemoral fat deposition is associated with an improved metabolic profile (Karpe & Pinnick, 2015; Manolopoulos, Karpe, & Frayn, 2010; see depiction in Figure 1 of differences between sexes). Thus, differences in fat deposition are believed to contribute substantially to the divergence in inflammatory mediators between sexes.
Figure 1.
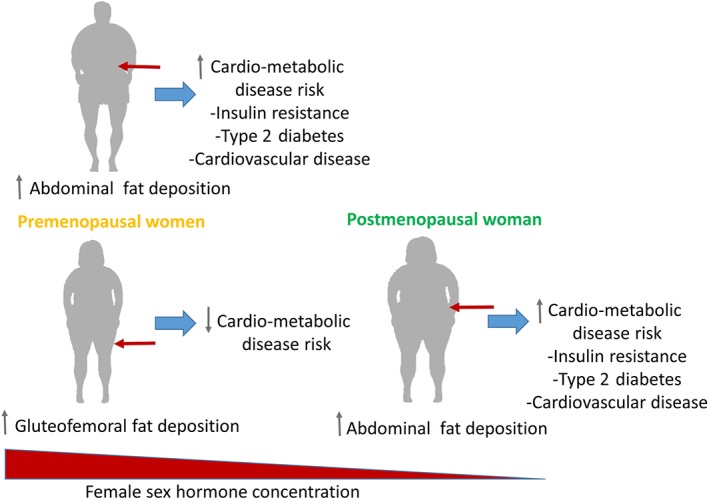
Despite obesity being more prevalent in women than men, prior to menopause, women have a reduced risk of metabolic disease. Following menopause and corresponding to an increase in deposition of abdominal fat and a decrease in the female sex hormones, metabolic disease risk is increased to similar rates as observed in men
The metabolically healthy pear‐shaped body composition seen in premenopausal women is believed to be partly mediated by the female sex hormone, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013. Oestrogen has been shown to increase fat oxidation (Devries, Hamadeh, Graham, & Tarnopolsky, 2005), inhibit lipogenesis (Homma et al., 2000), and improve adipogenic potential in gluteofemoral adipocytes (Cox‐York, Erickson, Pereira, Bessesen, & Van Pelt, 2017), and studies in rodents show that loss of ovarian function leads to a sustained diet‐independent increase in fat mass (Rogers, Perfield, Strissel, Obin, & Greenberg, 2009; Stubbins, Holcomb, Hong, & Nunez, 2012; Stubbins, Najjar, Holcomb, Hong, & Nunez, 2012) that is prevented by oestrogen supplementation (Gorzek et al., 2007; Stubbins, Holcomb, et al., 2012; Stubbins, Najjar, et al., 2012). Furthermore, low serum http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013 (E2) in middle‐aged women is associated with increased visceral fat mass and hepatic lipid deposition (Abildgaard et al., 2018). Interestingly, oophorectomy (the removal of ovaries and therefore the source of oestrogen)‐induced adiposity in mice seems to be highly dependent on decreased physical activity following oophorectomy (Gorzek et al., 2007; Yonezawa et al., 2012)—a finding that has yet to be replicated in humans. Besides improving metabolic function, oestrogen seems to prevent low‐grade inflammation through direct inhibition of leukocyte‐derived cytokine secretion (Harkonen & Vaananen, 2006; Kramer, Kramer, & Guan, 2004; Kramer, Winger, & Kramer, 2007), and plasma levels of pro‐inflammatory cytokines vary throughout the menstrual cycle, showing high levels when oestrogen is low and low levels when oestrogen is high (Bouman, Heineman, & Faas, 2005). Taken together, these studies indicate that oestrogen plays an important role in immunometabolism and could, in part, be responsible for the sex differences in the regulation of inflammatory‐linked mediators.
2. OESTROGEN AND RECEPTOR SIGNALLING
Oestrogen belongs to the family of steroid hormones, and signalling is mediated through two intracellular http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=96 (ERs), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=620 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=621, both belonging to the nuclear receptor family of transcription factors (Heldring et al., 2007). Oestrogen signalling is initiated by binding of oestrogen to the ER and is followed by a cell‐specific transcriptional response depending on the composition of coregulatory proteins (Katzenellenbogen & Katzenellenbogen, 2002). In recent years, a http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=22 (GPER) mediating rapid nongenomic cell signalling has also been identified (Filardo, Quinn, Bland, & Frackelton, 2000). Knockout (KO) of the ERα evokes impaired adipocyte function and profound insulin resistance—findings closely corresponding to oophorectomy (Heine, Taylor, Iwamoto, Lubahn, & Cooke, 2000; Musatov et al., 2007), indicating that ERα likely mediates many of the effects of E2 on metabolism. In contrast, ERβ KO mice show decreased ectopic lipid deposition and improved glucose metabolism (Foryst‐Ludwig et al., 2008), suggesting that the ERα/ERβ ratio in a specific tissue determines the metabolic effects of oestrogen (Barros & Gustafsson, 2011). The specific impact of GPER on adipose tissue metabolism is more controversial (Barton & Prossnitz, 2015; Prossnitz, Arterburn, & Sklar, 2007), but GPER selective agonists might improve pancreatic beta‐cell function and glucose homeostasis (Balhuizen, Kumar, Amisten, Lundquist, & Salehi, 2010; Liu et al., 2013). Different oestrogen derivatives have been shown to have differing affinities to the ER with E2 being the most potent derivative (Kuhl, 2005). In fertile, nonpregnant women, E2 is the dominant oestrogen primarily produced by the ovaries (Simpson, 2003). After menopause, the less potent oestrogen derivative http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2818 is the most abundant, produced from extragonadal sites such as adipose tissue (Hetemaki et al., 2017).
Given the known metabolic gender differences, it is, therefore, important to include both genders in the testing of potential novel therapeutics to treat obesity and diabetes. Indeed, gender differences are observed in relation to a number of current antidiabetic drugs on the market. As reviewed by Franconi and Campesi (2014), insulin, biguanides, sulfonylureas, thiazolidinediones, glucagon‐like peptide‐1 receptor agonists, dipeptidyl peptidase 4 inhibitors, and α‐glucosidase inhibitors all have sex‐specific differences in relation to the effectiveness of the treatment, exposure, or side effects that can occur as a result of treatment. Quite often in preclinical studies of both metabolism and inflammation, only one gender is studied (with this gender most commonly being male). This is a common occurrence so as to reduce potential variance due to hormonal fluctuations in the females. Studying just one sex also keeps costs down, and as more males are utilized in studies, utilizing males in your own studies allows for comparisons between studies more easily. However, this approach may be short sighted and may contribute to missed phenotypes and mechanistic insights.
Recently, our work on genetic alterations in the cytokine http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4983 and its effect on metabolism has produced some further interesting findings in relation to sexual dimorphism. This further highlights the important role of sex in inflammatory‐linked metabolic studies and raises the question as to how prevalent metabolic sexual dimorphism is in metabolic studies of inflammatory‐related cytokines. This review aims to highlight the differences observed between sexes in preclinical genetic models focussing on inflammatory‐related processes.
3. SEX DIFFERENCES IN IMMUNE FUNCTION
Men have a higher prevalence and severity of both viral and bacterial infections than women (Klein, 2000; Roberts, Walker, & Alexander, 2001). In contrast, many autoimmune diseases are more common in women (Whitacre, 2001). Sex differences in immune function are perceived as multifactorial. It is believed that two X chromosomes provide females with a more extensive repertoire of proteins and thus an increased diversity in the immune response compared with males (Fish, 2008). Furthermore, differences in sex hormones are thought to play a causal role. ERs are present on T cells, B cells, macrophages, neutrophils, and NK cells, among others (Fish, 2008), and oestrogen increases the number of regulatory T cells (Arruvito, Sanz, Banham, & Fainboim, 2007), decreases pro‐inflammatory cytokine secretion from leukocytes (Kramer, Kramer et al., 2004), and increases the anti‐inflammatory activity of neutrophils (Garcia‐Duran et al., 1999). Lastly, gender‐related behavioural aspects influence the prevalence of many infectious diseases. Cultural, behavioural, and anatomical differences between the sexes play a prominent role in exposure to pathogens, and whereas women in sub‐Saharan Africa are more than twice as likely to be infected with human immunodeficiency virus type 1 (HIV)‐1 compared with men (Griesbeck, Scully, & Altfeld, 2016), men are more likely to suffer from parasitic infections (Zuk & McKean, 1996). Altogether, these findings highly implicate the importance of considering sex differences in immune‐based responses and disease.
4. SEX DIFFERENCES IN METABOLIC SYNDROME
4.1. Environmental/lifestyle factors
High‐energy diets are a major contributing factor to the growing obesity and diabetes rates worldwide. Rodent models often use high‐fat diets (HFDs) to simulate Western diets and related metabolic conditions. In our hands, when using an HFD intervention to induce obesity and glucose intolerance, we find male mice gain fat mass more rapidly than female mice, but by the end of a 12‐week dietary period, both male and female mice have gained similar amounts of fat mass (Lancaster et al., 2014). Similar findings are observed with glucose tolerance. Male mice are clearly glucose intolerant and have hyperinsulinaemia after 4 weeks of a high‐fat diet, whereas female mice are still protected at that timepoint. It is not until more chronic high‐fat feeding has taken place that glucose intolerance and hyperinsulinaemia are observed in the female mice (Lancaster et al., 2014). Thus, although female mice eventually catch up to their male counterparts in terms of adiposity and glucose intolerance, this is delayed, and there is a period of metabolic protection.
One of the major issues with Westernized lifestyles with an overindulgence in high caloric food, which is high in fat and/or sugar and highly processed, is that it can provide a source of inflammation to metabolic tissues and affect the immune system. Innate immune cells are able to sense and are then activated by pathogens through http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=302, which recognize exogenous pathogen‐associated molecular patterns (PAMPs; e.g., http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5019) and damage‐associated molecular patterns (DAMPs) originating from compromised cells (e.g., http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4731, and heat shock proteins). This, in turn, leads to the activation of innate immune cells (Sun, Ji, Kersten, & Qi, 2012). During the development of metabolic disease, dietary factors could contribute to immune activation from both PAMPs and DAMPs. Over the last decade or so, it has been recognized that alterations to the gut microbiota (dysbiosis) occur with obesity and/or energy‐dense, poorly nutritious diets. Overproduction of LPS in this setting and increased mobility of this and other PAMPs from the gut through the intestinal barrier due to increased gut permeability can increase the exposure of metabolic tissues to inflammatory stimuli and initiate or potentiate inflammatory processes. Nutrient excess can be directly sensed by the pathogen receptors. Saturated long‐chain fatty acids, deleterious lipids such as ceramides, and glucose and cholesterol have all been shown to activate components of these pathways (reviewed in Jin, Henao‐Mejia, & Flavell, 2013). Immune receptors can also be triggered by the production of endogenous DAMPs associated with alterations due to dysregulated metabolism. Such signals may include high‐mobility group box 1 (HMGB1), fetuin, amyloid deposits, hyaluronan, and uric acid, which are increased in metabolically compromised models and individuals and may contribute to the inflammatory milieu (reviewed in Jin et al., 2013).
5. SEX DIFFERENCES IN INFLAMMATORY‐RELATED METABOLIC SYNDROME
Closely linked to environmental risk factors for obesity and type 2 diabetes is the observation of chronic low‐grade inflammation in metabolically compromised patients and animal models. Circulating and tissue‐specific accumulation of pro‐inflammatory cytokines have been investigated as contributing factors to dysfunctional metabolism and arise due to or a combination of genetic predisposition, high caloric intake, and sedentary behaviour.
5.1. Adipose tissue
The adipose tissue is an important organ in the regulation of metabolism via its ability to act as an energy storage sink, removing glucose and lipids from the bloodstream in the postprandial state, and working as an active endocrine gland secreting adipokines. Infiltration of immune cells and activation of inflammatory cascades in adipose tissue has the potential to disrupt the metabolic actions of the adipose tissue beds and contribute to disruption in whole‐body metabolic control (Exley, Hand, O'Shea, & Lynch, 2014). Interestingly, the induction of white adipose tissue (WAT) expression of many inflammatory and immune‐related genes such as F4/80 (macrophage marker), integrin (CD11c), monocyte chemoattractant protein 1, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5073 in response to high‐fat feeding is markedly down‐regulated in the female mice compared with male counterparts (Lancaster et al., 2014). This reduced gene expression of inflammatory cytokines in adipose tissue has been replicated by others (Singer et al., 2015). Accordingly, oophorectomy of female mice has been shown to induce severe adipose tissue inflammation including significant infiltration of macrophages and T‐cells and increased protein levels of TNF‐α and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 (Ludgero‐Correia, Aguila, Mandarim‐de‐Lacerda, & Faria, 2012). This is reflected in general systemic inflammation in the oophorectomized mouse including increased plasma levels of TNF‐α, IL‐6, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4983 and general leukocytosis (Cenci et al., 2003; Ludgero‐Correia et al., 2012; Stubelius, Andersson, Islander, & Carlsten, 2017). Furthermore, LPS injections lead to increased adipose tissue and plasma IL‐6 levels and a more marked fever response in oophorectomized mice compared with SHAM control mice (Iwasa et al., 2014), all in all suggesting that loss of ovarian function mediates an increased inflammatory response.
5.2. Liver
During obesity, lipid accumulation arises in the liver leading to nonalcoholic fatty liver disease. In humans, nonalcoholic fatty liver disease more often affects men, and premenopausal women are equally protected from developing nonalcoholic fatty liver disease (reviewed by Ballestri et al., 2017). It has been suggested that premenopausal females are protected against lipid accumulation due to the partition of fatty acids towards ketone body production rather than very LDL–triacylglycerol. Equally, oophorectomy leads to lipid deposition, and oestrogen treatment after ovariectomy (OVX) protects against fatty liver (Zhu et al., 2013). At the same time, oophorectomy also leads to an up‐regulation of pro‐inflammatory mediators (IκB kinase, IL‐6, and NF‐κB) in the liver, and this is counteracted by oestrogen supplementation (Kireev et al., 2010; Pighon, Gutkowska, Jankowski, Rabasa‐Lhoret, & Lavoie, 2011), suggesting that the loss of ovarian function also increases inflammation in the liver.
5.3. Skeletal muscle
Skeletal muscle is an insulin‐sensitive tissue that plays a vital role in the disposal of glucose in the postprandial state. Women have greater insulin‐stimulated leg glucose uptake than matched men despite having higher intramyocellular triacylglycerol content (Hoeg et al., 2009), whereas increasing lipid levels through lipid infusion results in less insulin resistance of skeletal muscle glucose uptake in women than men (Hoeg et al., 2011). Interestingly, skeletal muscle cells from premenopausal women (high‐oestrogen environment) showed a lower stress response after prolonged in vitro fatty acid (palmitate) treatment compared with skeletal muscle cells from postmenopausal women (low‐oestrogen environment; Abildgaard et al., 2014). Although Torres et al. (2018) recently showed that oestrogen has a direct impact on the mitochondrial membrane viscosity and improves bioenergetic function of the mitochondrion, suggesting that the sex‐mediated differences in lipid tolerability could be partly mediated by oestrogen. Although not as highly studied, like other peripheral insulin‐sensitive tissues, in skeletal muscle inflammation has been linked to insulin resistance. Skeletal muscle can secrete cytokines and other factors and may become inflamed with disrupted metabolism. Likewise, circulating immune cells can infiltrate into the muscle bed and increase inflammatory processes potentially contributing to a decrease in insulin signalling processes. Despite these common findings, for the purposes of this review, we have not been able to identify a paper that has made a side‐by‐side comparison of skeletal muscle inflammatory and immune markers between males and females of any species upon conditions of dietary or genetic obesity or other models of dysregulated metabolism. Chronic low‐grade inflammation may also be important in other metabolic tissues such as the gut, the pancreas, and brain.
6. SEX DIFFERENCES IN PRO‐INFLAMMATORY‐RELATED MEDIATORS
Interleukins are a subgroup of cytokines that are immunomodulating proteins secreted by the immune system in response to stimuli such as infection, trauma, and inflammation. They aid in cell‐to‐cell communication and act in an autocrine, paracrine, and endocrine fashion relaying chemical messengers. Once produced and secreted from their cell of origin, interleukins travel to their target cell(s) and bind(s) to its receptor where they have been shown to play many physiological functions. They are designated numerically (there are 15 in total), and we will now discuss the main interleukins involved in metabolic control and known sex differences in these proteins.
6.1. IL‐1β
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 has been linked to disturbed metabolic homeostasis in both human and animal models. IL‐1β concentrations in adipose tissue are increased in diet‐ and genetically‐induced models of obesity (Stienstra et al., 2010). Deletion of its receptor (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=312 KO) is protective against HFD‐induced insulin resistance (McGillicuddy et al., 2011), whereas administration of recombinant Il‐1β leads to insulin resistance (Wen et al., 2011). Furthermore, reductions in IL‐1β expression are associated with improved insulin sensitivity upon weight loss, whereas elimination of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1770 signalling (the Nlrp3 inflammasome regulates caspase‐1 activation that allows IL‐1β to be released) is beneficial for insulin and glucose tolerance (Vandanmagsar et al., 2011). Deletion of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2042, another pro‐inflammatory signalling mediator that acts via IL‐1 receptor/toll‐like receptors (TLRs), also leads to improved insulin sensitivity (Sun et al., 2017). Apart from a calorie restriction study (Vandanmagsar et al., 2011), all these studies were conducted in male mice only, and there is a scarcity of data in female models that we can identify. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6972 is a recombinant human IL‐1 receptor antagonist that has been shown in trials to be beneficial in patients with type 2 diabetes as it reduces glycated haemoglobin levels (Larsen et al., 2007). Reduced markers of systemic inflammation (C‐reactive protein and IL‐6) were also observed in the patients with this treatment compared with placebo (Larsen et al., 2007). In this trial, both male and female patients were enrolled for the study but with far more men (50 compared with 19). However, there is no breakdown of the treatment effect between sexes to delineate whether this IL‐1 receptor antagonism was equally effective in both sexes. Even if there was, it is likely to be underpowered to determine any real effect due to the lack of female participants compared with male in the cohort.
The monoclonal antibody http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6773, which inhibits IL‐1β, is currently being tested in a large clinical trial named the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study. Despite large reductions in hsCRP and IL‐6, canakinumab did not reduce the incidence of new‐onset diabetes over a median follow‐up period of 3.7 years. Treatment did reduce HbA1c levels compared with placebo during the first 6 to 9 months of the treatment, but no long‐term benefits on HbA1c or fasting plasma glucose were observed (Everett et al., 2018) So although canakinumab reduces inflammatory levels and lowers the rate of cardiovascular events in patients, it does not seem to lead to the prevention of diabetes among patients with prediabetes. The study was adjusted for sex in the analysis, but no results are displayed showing which gender responded either better or worse to this treatment regime.
http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754 (a member of the IL‐1 receptor superfamily) is a pattern recognition receptor and an important component of the immune system in initiating inflammatory signalling via alterations to gene transcription. It plays a critical role in mediating the response to pathogens especially as the receptor for LPS. Metabolic sexual dimorphism is also present in TLR4−/− mice. Despite HFD‐induced inflammatory gene expression being attenuated in TLR4−/− mice in both sexes, whole‐body metabolic phenotypes were different. Although male TLR4−/− mice were not different in comparison with wild‐type (WT) mice in terms of body weight, food intake, or insulin sensitivity in both chow‐fed and HFD‐fed mice, there was a distinct phenotype in female mice (Shi et al., 2006). Female mice were fed chow or a HFD for a period of 39 weeks. No matter which diet was utilized, female TLR4−/− mice were significantly heavier than WT mice, with the TLR4−/− mice on the HFD being almost 10 g heavier by the end of the study. This increase in weight was due solely to an increase in adiposity with lean mass remaining unchanged and was most likely due to an increase in food intake as energy expenditure measured in metabolic chambers was not different.
Despite the typical paradigm that obesity drives insulin resistance, the female TLR4−/− mice were more sensitive to insulin in the insulin tolerance test (Shi et al., 2006). Furthermore, the food intake data suggest TLR4 has a role in the regulation of food intake, specifically in female mice. This finding that the control of food intake is sex‐dependent is unique. Interestingly, similar to this study, differences upon macrophage‐specific deficiency of TLR4 were also only observed in female mice, suggesting a sexual dimorphism in the effects of macrophage TLR4 expression (Coenen et al., 2009). Macrophages have been implicated in the development of both insulin resistance and atherosclerosis. Using a bone marrow transplantation procedure to delete TLR4 from macrophages (Mθ TLR4−/− mice), the authors demonstrated a decrease in macrophage and inflammatory markers in WAT of female chow‐fed Mθ TLR4−/− mice compared with Mθ TLR4+/+ mice but not in male mice. This was also associated with a decreased atherosclerotic lesion area in the females. Despite this, no difference was observed in body composition (Coenen et al., 2009) nor when mice were challenged with an HFD. Thus, under certain metabolic and dietary conditions, but only in female mice, TLR4 may be involved in mediating macrophage accumulation in adipose tissue and vessels.
6.2. IL‐6
IL‐6 is a pro‐inflammatory cytokine that is somewhat paradoxically found elevated in the plasma of obese individuals and linked to the induction of insulin resistance, while at the same time identified as a factor that is released from skeletal muscle and associated with many of the beneficial metabolic aspects of exercise (reviewed in Pal, Febbraio, & Whitham, 2014). Plasma IL‐6 is increased in both rodents and humans with declined ovarian function compared with their fertile counterparts (Cioffi et al., 2002; Stubelius et al., 2017), but the specific role of IL‐6 in relation to ovarian function is not clear. Although traditionally IL‐6 was seen as a factor secreted from immune cells involved in inflammatory processes, the findings that it is released from metabolic organs such as adipose tissue (as an adipokine; Fried, Bunkin, & Greenberg, 1998). and skeletal muscle (myokine; Steensberg et al., 2000) further indicate its potential importance in metabolic control. To elucidate its role and importance, many genetic mouse models of IL‐6 have been produced. Whole‐body deletion of IL‐6 (IL‐6−/− mice) revealed an obesity phenotype and associated insulin resistance (Matthews et al., 2010; Wallenius et al., 2002). In the initial study by Wallenius et al. (2002), both sexes were assessed. An increase in adiposity with IL‐6 deletion was consistently shown in both sexes as well as increased http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5015 levels and leptin insensitivity (leptin resistance) in older mice. IL‐6−/− female mice displayed an altered circulating lipid profile (increased triglyceride and very LDL); however, there was no such difference in the lipid profile of the male IL‐6−/− mice compared with WT. Decreased glucose tolerance was also observed in the female mice; however, equivalent data were not provided for male mice (Wallenius et al., 2002). A later study did indeed indicate that male IL‐6−/− mice displayed insulin resistance when fed a HFD, and concomitant liver inflammation was observed (Matthews et al., 2010). Together, it is likely that IL‐6 is necessary for the maintenance of weight and glucose homeostasis in both sexes, whereas complete loss of IL‐6 plays a role in the regulation of the circulating lipid profile specifically in females.
To determine the impact of tissue‐specific loss of IL‐6 on metabolism, the Hidalgo laboratory has conducted numerous studies utilizing cohorts of floxed IL‐6 mice to investigate the metabolic effect of loss of IL‐6 in a particular tissue/organ. Interestingly, many characteristics of these models occur in a sex‐dependent manner. Deletion of IL‐6 in the CNS specifically in astrocytes (Ast–IL‐6−/− mice, produced by crossing IL‐6 floxed mice with glial fibrillary acidic protein promoter driven (GFAP)–Cre mice) led to an progressive increase in the body weight of the Ast–IL‐6−/− mice compared with floxed control mice after 8 weeks of age but only in male mice (Quintana et al., 2013). This was potentially due to a decrease in the activity levels of the Ast–IL‐6−/− mice. However, as the decrease in activity was seen in both sexes, why the females were protected from weight gain is somewhat of an unsolved mystery. In association with activity, the male floxed control mice had a decrease in exploratory behaviour compared with females as measured by the total number of head dips and time spent head dipping in a hole‐board apparatus test. This defect was completely restored in the Ast–IL‐6−/− male mice, whereas head dipping remained unchanged in the females (Quintana et al., 2013). Interestingly, there are also sex differences observed with transgenic up‐regulation of IL‐6 in astrocytes (GFAP–IL‐6 mice; Hidalgo et al., 2010). Although both sexes were resistant to HFD‐initiated obesity, the female GFAP–IL‐6 mice were much more resistant. This corresponded with a drop in the weight of the visceral adipose tissue depots in the females, whereas there was no such effect in males. In fact, the decrease in weight in the males appeared to have nothing to do at all with the prevention of the accumulation of fat mass but instead was due to a decrease in the size of the liver (Hidalgo et al., 2010). Consequently, both the deletion and up‐regulation of IL‐6 in these cells lead to sex‐specific metabolic phenotypes.
Another tissue‐specific model that has been investigated is the muscle‐specific IL‐6−/− mouse (mIL‐6−/−). In this instance, there were marked differences in the response to the deletion between the sexes with opposing body weights observed (Navia et al., 2014). Although male mice on both a chow control diet and a HFD were protected from weight gain, compared with floxed control mice, the female mice were in fact significantly heavier than their floxed controls (Navia et al., 2014). This was due to an increase in adiposity as the male mIL‐6−/− mice had lower gonadal and subcutaneous WAT mass as well as lower brown adipose tissue mass. In contrast, females displayed higher gonadal and subcutaneous WAT (Navia et al., 2014). As liver and tibialis muscle weights were not different, it is likely that alterations in fat pad weights were driving the observed changes in body weights. Food intake was not different between the sexes, suggesting energy intake was not driving the body composition findings, but in line with the body weight findings, male mIL‐6−/− mice were more active and females less active than floxed controls of each sex in a hole‐board test (Navia et al., 2014). From a glucose control perspective, male mIL‐6−/− mice had decreased blood glucose and insulin levels, whereas females did not have differing glucose or insulin concentrations but did have increased circulating leptin levels (Navia et al., 2014). To assess glucose control, oral glucose tolerance and insulin tolerance tests were performed. In line with the body weight phenotype, male mIL‐6−/− mice tended to have improved glucose excursions when fed a HFD, but these values did not reach statistical significance.
In a follow‐up study, Molinero et al. (2017) demonstrated that a loss of IL‐6 in the muscle results in a lower core body temperature and a higher respiratory exchange ratio in the light phase (the inactive rest phase for a mouse). Using indirect calorimetry, it was found that the female mice but not the male mice had an increased energy expenditure (Molinero et al., 2017), which was somewhat conflicting with the previous finding of an increased body weight and adipose weight in these female mice. Also conflicting was the fact that no differences in physical activity were observed in this analysis, indicating that the previous finding of increased activity on the hole‐board test may indicate increases in exploration rather than activity (Molinero et al., 2017). Nevertheless, these studies provide a clear indication of the important interaction between the expression of genes in the muscle and sex‐specific characteristics and their overall impact on whole‐body metabolism.
The adipose tissue is a vital metabolic organ in that it not only acts as an insulator, an energy storage sink, and a contributor to postprandial glucose disposal but is also an important endocrine organ actively secreting adipokines such as leptin and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3726. Many cytokines including IL‐6 are also adipokines and secreted from the adipose depots throughout the body. Consequently, the role of IL‐6 specifically derived from adipose tissue in contributing to metabolic homeostasis is of interest. To investigate this, Navia and colleagues generated adipose‐specific IL‐6−/− mice by breeding IL‐6 floxed mice with aP2–Cre mice (aP2–IL‐6−/−mice; Ferrer et al., 2014). Once again, in line with the astrocyte and muscle‐specific models, sex differences were observed. The female aP2–IL‐6−/−mice fed a HFD put on less weight than their control mice, but this effect was not observed in the male aP2–IL‐6−/−mice (Ferrer et al., 2014). The difference in weight was likely due to a decrease in the expansion of both the gonadal and subcutaneous WAT regions, although the liver weight was also shown to be lower (Ferrer et al., 2014). A deficiency in adipose IL‐6 also decreased fasting circulating insulin and cholesterol levels specifically in the female mice. Reminiscent of the mIL‐6−/− model, despite the body mass, adipose weight, and plasma insulin findings (in the female mice), no difference in insulin tolerance test and oral glucose tolerance test was observed for either sex (Ferrer et al., 2014). Together, studies into the role of IL‐6 in various tissues demonstrate a robust sex effect on numerous metabolic parameters.
Hepatocellular carcinoma (HCC), the most common form of liver cancer, is closely linked to compromised metabolic homeostasis and obesity. Interestingly, this cancer occurs more frequently in men, reported to be diagnosed at 3–5 times the rate in males as it is in females (Bosch et al., 2004). This sexual dimorphism also holds true in animal models of this condition (Maeda, Kamata, Luo, Leffert, & Karin, 2005). The development of HCC follows a timeline whereby initially hepatic steatosis develops before the later activation of inflammatory pathways in the liver, resulting in an environment conducive to cancer growth. Experimental evidence suggests that IL‐6 is a critical factor in both the initiation of the cancer and the sexual dimorphism. Administration of the chemical carcinogen, diethylnitrosamine (which leads to HCC development), increases IL‐6 levels to a higher degree in males than in females, whereas loss of IL‐6 neutralizes the gender disparity in HCC development (Naugler et al., 2007). Experiments designed to test the female sex hormone hypothesis by treating male mice with oestrogen were successful in reducing IL‐6 levels and suppressing liver injury (Naugler et al., 2007). Obesity caused by genetic alterations or dietary means also has the capacity to promote hepatic inflammation and drive tumourigenesis, a process that involves the induction of IL‐6 and the activation of the transcription factor linked to cancer development, STAT3 (Park et al., 2010).
6.3. IL‐18
IL‐18, a member of the IL‐1 family, is a cytokine that has been linked to alterations in metabolic homeostasis. Clinically, increased levels of IL‐18 correlate with metabolic syndrome traits such as body mass index, waist circumference, plasma triglyceride and fasting glucose, and insulin concentrations in both men and women (Hung, McQuillan, Chapman, Thompson, & Beilby, 2005), suggesting this cytokine is involved in the pathogenesis of the metabolic syndrome. However, genetic manipulation of IL‐18 in animal models has revealed the opposite. Whole‐body deletion of IL‐18 (IL‐18−/− mice) or mice who have had their http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1737 knocked out (IL‐18r−/−), in fact, develop hyperphagia, obesity, and insulin resistance (Netea et al., 2006). Furthermore, mice transgenic for IL‐18 binding protein, a natural antagonist of IL‐18 receptors, also develop insulin resistance (Netea et al., 2006). It has been demonstrated that raising IL‐18 levels may be metabolically protective; recombinant IL‐18 administered intracerebrally or intraperitoneally inhibited food intake and reversed hyperglycaemia (Netea et al., 2006; Zorrilla et al., 2007; Zorrilla & Conti, 2014). IL‐18 receptor α knock‐out (KO) was chosen to genetically manipulate IL‐18 signalling, as IL‐18 receptor α chain is responsible for the extracellular binding of IL‐18. Although mice deficient in the IL‐18 receptor β chain exist; metabolic studies have not been performed. To our knowledge, KO of both receptors does not exist.
Work from our laboratory has also substantiated these findings and provided further mechanistic insight. Treating myotubes in cell culture or skeletal muscle strips with IL‐18 activated the critical metabolic regulator http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540 (AMPK) and increased rates of fat oxidation, whereas in vivo electroporation of IL‐18 into skeletal muscle to increase its expression also activated AMPK and markers of mitochondrial metabolism (Lindegaard et al., 2013). Other work we were involved with found that mice lacking the nucleotide oligomerization domain‐like receptor protein 1 inflammasome have an impaired capacity to produce IL‐18 and as a consequence phenocopy mice lacking IL‐18, with obesity and lipid accumulation was observed (Murphy et al., 2016). Conversely, mice with an activating mutation in http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1768, and therefore increased IL‐18, have decreased adiposity and metabolic dysfunction (Murphy et al., 2016).
In liver, administration of IL‐18 to hepatocytes in vitro reduces the expression of PEPCK, and basal endogenous glucose production is increased in IL‐18−/− mice in association with increased gluconeogenesis (PEPCK mRNA; Netea et al., 2006). Therefore, it has been suggested that IL‐18 is involved in controlling basal hepatic glucose production (Netea et al., 2006). In contrast, we did not find any differences in genes involved in gluconeogenesis (PEPCK or glucose‐6‐phosphat dehydrogenases mRNA) nor in intrahepatic triacylglycerol concentration when comparing IL‐18R−/− with control mice irrespective of diet (Lindegaard et al., 2013), a finding that was reproduced by Pazos et al. (2015). Furthermore, even though the IL‐18R−/− mice on a HFD are insulin‐resistant relative to control mice, there was no evidence of impaired insulin signalling or increased lipogenesis (mRNA expression of key fatty acid synthesis transcription factors/enzyme sterol regulatory element binding protein‐1c) in the liver. Given that neither hepatic lipid deposition nor the expression of key enzymes involved in regulating hepatic glucose production is different when comparing IL‐18R−/− with control mice, it is unlikely that changes in liver insulin sensitivity are responsible for the reduced whole‐body insulin sensitivity observed in IL‐18R−/− male mice. Although studies are still needed to directly measure insulin sensitivity in the liver.
While this preclinical body of work has implicated IL‐18 as an important component of metabolic regulation, a caveat from these studies was that they were performed entirely on male mice. Research into IL‐18 in other experimental settings has indicated the potential presence of sex divergence in physiological responses to IL‐18. Injection of LPS results in significantly higher plasma levels of IL‐18 in male mice (Aoyama, Kotani, & Usami, 2009). Given this, and the known differences between the metabolism of males and females (described above), we set out to investigate whether we could repeat our findings in female mice. We studied female mice with a global deletion of the α isoform of the IL‐18 receptor (IL‐18R−/−) and their littermate WT control mice. Three studies were performed: (a) animals fed a HFD for 16 weeks to simulate our previous studies in males; (b) animals were fed a chow diet and were aged 72 weeks to simulate a mouse equivalent of post menopause (at this age, female mice start to display some similar characteristics to postmenopausal women); and (c) animals (3 weeks old) randomized to either bilateral OVX or control surgery (SHAM) and followed for 16 weeks to simulate depletion of sex hormones. In contrast to male mice, female IL‐18R−/− mice gained less weight and under chow conditions tended to be more sensitive to insulin than their WT littermates (Lindegaard, Abildgaard, Heywood, Pedersen, & Febbraio, 2018). However, with ageing IL‐18R−/− mice showed increases in both visceral and subcutaneous fat depots, and glucose intolerance was now evident. Although performing OVX did not affect body weight in IL‐18R−/− mice, it did exacerbate glucose intolerance and impaired liver insulin signalling processes when compared with SHAM control mice (Abildgaard et al., 2018). From these investigations, we conclude that female IL‐18R−/− mice only present with a similar metabolic phenotype as reported in male IL‐18R−/− mice if the conditions are such that their oestrogen levels are reduced, such as with ageing or after undergoing OVX.
6.4. TNF‐α
TNF‐α has been proposed to be an important cytokine in the pathogenesis of obesity and insulin resistance and is strongly linked to pro‐inflammatory activation. In a study of 104 patients, the levels of circulating TNF‐α were found to be raised in male but not female patients with type 2 diabetes (Pfeiffer et al., 1997), and postmenopausal women show increased TNF‐α levels compared with their premenopausal counterparts (Malutan, Dan, Nicolae, & Carmen, 2014). Animal studies have also identified sex differences regarding this cytokine. TNF‐α mRNA levels in adipose tissue from genetically obese ob/ob male mice (obese due to hyperphagia) were threefold higher compared with female ob/ob mice (Neels, Pandey, Hotamisligil, & Samad, 2006). Despite this, in this instance, there was no statistically significant difference in plasma TNF‐α levels between the sexes. To further investigate the TNF‐α pathway, the researchers investigated the receptors for TNF‐α by genetically deleting them. Deletion of the TNF receptors http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1870 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1871 led to an 85% reduction in TNF‐α mRNA levels in the adipose tissue of male mice (Neels et al., 2006). Conversely, loss of the TNF receptors did not affect adipose tissue gene expression in female ob/ob mice, suggesting that endogenous TNF‐α signalling is a regulator of adipose tissue TNF‐α expression levels in male but not female mice (Neels et al., 2006). Further differences were observed upon administration of TNF‐α to the mice. Although this treatment increased TNF‐α mRNA levels in both sexes, the response was blunted in the females by twofold (Neels et al., 2006). Overall, these data indicate a greater control of TNF‐α expression and signalling in the female mice. In accordance with this, oestrogen supplementation has been shown to blunt increases in TNF‐α in response to artificially induced endotoxaemia in rodents (Hassouna, Obaia, Marzouk, Rateb, & Haidara, 2014), further suggesting that this female advantage in TNF‐α control could be mediated by oestrogen.
6.5. Leptin
Leptin is a circulating adipokine produced by adipose tissue. Leptin acts through binding to the long form of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=307#1712, which leads to many physiological functions including the regulation of appetite and food consumption as well as effects on metabolic rate, bone mass, reproductive system, and insulin secretion. Along with these effects, leptin also is regarded as a pro‐inflammatory factor and participates in innate immunity (Abella et al., 2017). Serum leptin levels are sexually dimorphic in humans (Considine et al., 1996) and rodents (Landt et al., 1998); however, these sexual dimorphisms are reversed between species with women having higher levels than men, whereas male rodents have higher leptin levels than their female counterparts. The gender difference in humans is likely to be due to both a higher proportion of adipose tissue and increased production rate of leptin per unit mass of adipose tissue in women (Gutniak et al., 2001). One potential mechanism leading to this sexual dimorphism is the influence of steroid hormones on the synthesis of transcripts that encode for leptin. Experiments in adipocyte‐like cells (3T3‐L1 murine adipocytes) have demonstrated that dihydrotestosterone reduces leptin transcript levels and as a consequence the amount of leptin found intracellularly and also the leptin that is secreted from the cell. In contrast, 17β‐oestradiol treatment significantly increases the abundance of transcripts encoding leptin and the amount of secreted leptin. Incubating cells with oestrogen and androgen receptor antagonists had opposite effects on transcript abundance to steroid treatments (Jenks, Fairfield, Johnson, Morrison, & Muday, 2017). Thus, although this remains to be determined, sex hormones may play a regulatory role in leptin's effects and secretion. Given leptins' emerging link to inflammatory and immune processes, this adipokine may be yet another determinant of inflammatory‐linked metabolic control and be involved in sex‐specific physiological effects.
7. SEX DIFFERENCES IN ANTI‐INFLAMMATORY‐RELATED MEDIATORS
7.1. IL‐10
In contrast with the previously described pro‐inflammatory cytokines, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975 is a cytokine with potent anti‐inflammatory properties. A lower circulating IL‐10 concentration is associated with being overweight or obese in young adolescents (Chang et al., 2013). In healthy adults, there is a significant positive association between circulating IL‐10 levels and whole‐body insulin sensitivity (Straczkowski, Kowalska, Nikolajuk, Krukowska, & Gorska, 2005), and low IL‐10 production capacity is associated with high plasma glucose, high HbA1c levels, type 2 diabetes, and dyslipidaemia (van Exel et al., 2002). Genetic alterations in IL‐10 (IL‐10KO), IL‐10 receptor KO (IL‐10RKO), and IL‐10 transgenic (IL‐10Tg) mice have been generated (Cintra et al., 2008; Dagdeviren et al., 2016; Hong et al., 2009; Kowalski et al., 2011) along with studies by which IL‐10 is administered as a treatment regime (Kim et al., 2004). Many, but not all, of these models have identified a protective role of IL‐10 against metabolic disease; however, from our analysis, we cannot identify any study involving IL‐10 modulation that has been conducted in female mice, and therefore, the question remains as to whether or not it is of importance in female metabolic processes. Furthermore, both human and rodent studies find no changes in plasma IL‐10 levels with declining ovarian function (Cioffi et al., 2002; Ohtani et al., 2007), indicating that female sex hormones are less likely to have a direct impact on systemic IL‐10 levels.
7.2. Adiponectin
Adiponectin is an adipocyte‐derived secretory protein with profound anti‐inflammatory and insulin‐sensitizing functions. High plasma adiponectin levels are believed to reflect healthy adipose tissue and closely reflect improved metabolic flexibility (Scherer, 2016; Turer & Scherer, 2012; Wang & Scherer, 2016). Adiponectin secretion from the adipose tissue is decreased with increasing central adiposity, whereas lower extremity adiposity is associated with higher concentrations of adiponectin (Arita et al., 1999; Turer et al., 2012). The anti‐inflammatory effects of adiponectin are primarily driven through systemic M2 polarization of macrophages resulting in a decreased IL‐6 and TNF‐α release together with an increased secretion of IL‐10 (Ajuwon & Spurlock, 2005; Kumada et al., 2004; Ohashi et al., 2010).
Several human studies have shown that women have higher levels of circulating adiponectin compared with men, independent of differences in fat mass and insulin sensitivity (Saltevo, Kautiainen, & Vanhala, 2009; Song et al., 2014). Interestingly, male mice fed a HFD showed decreased adiponectin expression in the adipose tissue, compared with chow‐fed male mice, whereas there were no differences in adiponectin expression between chow‐ and HFD‐fed female mice, despite similar increases in visceral fat mass across sexes (Nickelson et al., 2012). This is in accordance with the general perception of healthier adipose tissue and better metabolic flexibility in females compared with males (Karastergiou et al., 2012; Lundsgaard & Kiens, 2014). A study in adolescents showed that as testosterone increased in pubertal boys, plasma adiponectin levels decreased, whereas plasma adiponectin in pubertal girls remained constant (Bottner et al., 2004). Additional studies report stable plasma adiponectin levels during the menstrual cycle (Hall, White, & O'Sullivan, 2009; Kleiblova, Springer, & Haluzik, 2006; Wyskida et al., 2017), altogether suggesting that androgens but not oestrogens could regulate plasma adiponectin levels.
8. SUMMARY
Overall, this review highlights the possibility that metabolic response or control can be altered in a sex‐dependent manner. Specifically, we have identified and described numerous instances in preclinical models whereby alterations in genes and proteins linked to inflammatory pathways lead to improved or disrupted metabolic control (see Figure 2 for a summary). However, whether phenotypes are observed, or the degree to which they are observed, can often be sex‐dependent. While traditionally all sex differences in physiology were suggested to be due to different regulation by sex hormones, these differences could also be attributed to sex‐specific behaviours or genetic and epigenetic effects caused by the inheritance and unequal dosage of genes located on the X and Y chromosomes (Ratnu, Emami, & Bredy, 2017). Investigations of these underlying factors will continue to be an area of interest in the metabolic field over the years to come. What is clear is that this review underscores the importance of examining both sexes in metabolic studies. Recently, the Reue laboratory published a template encompassing considerations for the experimental design of preclinical studies regarding sex differences in metabolism (Mauvais‐Jarvis, Arnold, & Reue, 2017). Adoption of such approaches by laboratories will ensure research is undertaken in a way that takes into account these differences and could lead to novel therapeutic targets and potentially even sex‐specific treatments of metabolic disease in the future.
Figure 2.
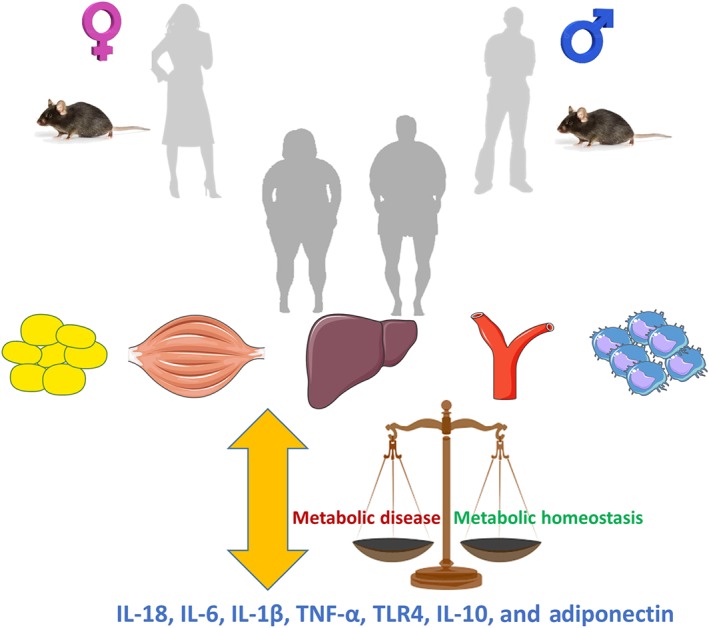
Differences in the effects of inflammatory processes potentially contribute to gender‐specific metabolic control. Mechanistic preclinical studies using a variety of genetic interventions have provided numerous examples of how the male and female metabolic response differs when inflammatory mediators are increased or decreased. Furthermore, many models have not been tested in females limiting knowledge in this area
8.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017a,b).
CONFLICT OF INTEREST
M.A.F. is Chief Scientific Officer and shareholder of N‐Gene Research Laboratories, Inc. All other authors have none to declare.
ACKNOWLEDGEMENTS
We thank the support of the Operational Infrastructure Support Scheme of the Victorian State Government. M.A.F. is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia (NHMRC App1116936). The Centre for Physical Activity Research (CFAS) is supported by a grant from the TrygFonden. CFAS is a member of DD2—the Danish Centre for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, Grant 09‐067009 and 09‐075724). D.C.H. is supported by the Shine on Foundation.
Henstridge DC, Abildgaard J, Lindegaard B, Febbraio MA. Metabolic control and sex: A focus on inflammatory‐linked mediators. Br J Pharmacol. 2019;176:4193–4207. 10.1111/bph.14642
REFERENCES
- Abella, V. , Scotece, M. , Conde, J. , Pino, J. , Gonzalez‐Gay, M. A. , Gomez‐Reino, J. J. , … Gualillo, O. (2017). Leptin in the interplay of inflammation, metabolism and immune system disorders. Nature Reviews Rheumatology, 13(2), 100–109. 10.1038/nrrheum.2016.209 [DOI] [PubMed] [Google Scholar]
- Abildgaard, J. , Danielsen, E. R. , Dorph, E. , Thomsen, C. , Juul, A. , Ewertsen, C. , … Lindegaard, B. (2018). Ectopic lipid deposition is associated with insulin resistance in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism, 103, 3394–3404. 10.1210/jc.2018-00554 [DOI] [PubMed] [Google Scholar]
- Abildgaard, J. , Henstridge, D. C. , Pedersen, A. T. , Langley, K. G. , Scheele, C. , Pedersen, B. K. , & Lindegaard, B. (2014). In vitro palmitate treatment of myotubes from postmenopausal women leads to ceramide accumulation, inflammation and affected insulin signaling. PLoS ONE, 9(7), e101555 10.1371/journal.pone.0101555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abildgaard, J. , Pedersen, A. T. , Green, C. J. , Harder‐Lauridsen, N. M. , Solomon, T. P. , Thomsen, C. , … Lindegaard, B. (2013). Menopause is associated with decreased whole body fat oxidation during exercise. American Journal of Physiology. Endocrinology and Metabolism, 304(11), E1227–E1236. 10.1152/ajpendo.00492.2012 [DOI] [PubMed] [Google Scholar]
- Ajuwon, K. M. , & Spurlock, M. E. (2005). Adiponectin inhibits LPS‐induced NF‐κB activation and IL‐6 production and increases PPARγ2 expression in adipocytes. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 288(5), R1220–R1225. 10.1152/ajpregu.00397.2004 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(S1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174(S1), S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174(S1), S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama, M. , Kotani, J. , & Usami, M. (2009). Gender difference in granulocyte dynamics and apoptosis and the role of IL‐18 during endotoxin‐induced systemic inflammation. Shock, 32(4), 401–409. 10.1097/SHK.0b013e31819c358a [DOI] [PubMed] [Google Scholar]
- Arita, Y. , Kihara, S. , Ouchi, N. , Takahashi, M. , Maeda, K. , Miyagawa, J. , … Matsuzawa, Y. (1999). Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications, 257(1), 79–83. 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- Arruvito, L. , Sanz, M. , Banham, A. H. , & Fainboim, L. (2007). Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: Implications for human reproduction. Journal of Immunology, 178(4), 2572–2578. 10.4049/jimmunol.178.4.2572 [DOI] [PubMed] [Google Scholar]
- Balhuizen, A. , Kumar, R. , Amisten, S. , Lundquist, I. , & Salehi, A. (2010). Activation of G protein‐coupled receptor 30 modulates hormone secretion and counteracts cytokine‐induced apoptosis in pancreatic islets of female mice. Molecular and Cellular Endocrinology, 320(1–2), 16–24. 10.1016/j.mce.2010.01.030 [DOI] [PubMed] [Google Scholar]
- Ballestri, S. , Nascimbeni, F. , Baldelli, E. , Marrazzo, A. , Romagnoli, D. , & Lonardo, A. (2017). NAFLD as a sexual dimorphic disease: Role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Advances in Therapy, 34(6), 1291–1326. 10.1007/s12325-017-0556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, R. P. , & Gustafsson, J. A. (2011). Estrogen receptors and the metabolic network. Cell Metabolism, 14(3), 289–299. 10.1016/j.cmet.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Barton, M. , & Prossnitz, E. R. (2015). Emerging roles of GPER in diabetes and atherosclerosis. Trends in Endocrinology and Metabolism, 26(4), 185–192. 10.1016/j.tem.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, J. , Thabut, D. , Bendtsen, F. , D'Amico, G. , Albillos, A. , Gonzalez Abraldes, J. , … European Study Group on rFVIIa in UGI Haemorrhage . (2004). Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: A randomized, double‐blind trial. Gastroenterology, 127(4), 1123–1130. 10.1053/j.gastro.2004.07.015 [DOI] [PubMed] [Google Scholar]
- Bottner, A. , Kratzsch, J. , Muller, G. , Kapellen, T. M. , Bluher, S. , Keller, E. , … Kiess, W. (2004). Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. The Journal of Clinical Endocrinology and Metabolism, 89(8), 4053–4061. 10.1210/jc.2004-0303 [DOI] [PubMed] [Google Scholar]
- Bouman, A. , Heineman, M. J. , & Faas, M. M. (2005). Sex hormones and the immune response in humans. Human Reproduction Update, 11(4), 411–423. 10.1093/humupd/dmi008 [DOI] [PubMed] [Google Scholar]
- Cenci, S. , Toraldo, G. , Weitzmann, M. N. , Roggia, C. , Gao, Y. , Qian, W. P. , … Pacifici, R. (2003). Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN‐γ‐induced class II transactivator. Proceedings of the National Academy of Sciences of the United States of America, 100(18), 10405–10410. 10.1073/pnas.1533207100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. S. , Chang, C. C. , Chien, E. , Lin, S. S. , Cheng‐Shiuan, T. , Bai, C. H. , & Chao, K. C. (2013). Association between interleukin 1β and interleukin 10 concentrations: A cross‐sectional study in young adolescents in Taiwan. BMC Pediatrics, 13, 123 10.1186/1471-2431-13-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra, D. E. , Pauli, J. R. , Araujo, E. P. , Moraes, J. C. , de Souza, C. T. , Milanski, M. , … Velloso, L. A. (2008). Interleukin‐10 is a protective factor against diet‐induced insulin resistance in liver. Journal of Hepatology, 48(4), 628–637. 10.1016/j.jhep.2007.12.017 [DOI] [PubMed] [Google Scholar]
- Cioffi, M. , Esposito, K. , Vietri, M. T. , Gazzerro, P. , D'Auria, A. , Ardovino, I. , … Molinari, A. M. (2002). Cytokine pattern in postmenopause. Maturitas, 41(3), 187–192. 10.1016/S0378-5122(01)00286-9 [DOI] [PubMed] [Google Scholar]
- Coenen, K. R. , Gruen, M. L. , Lee‐Young, R. S. , Puglisi, M. J. , Wasserman, D. H. , & Hasty, A. H. (2009). Impact of macrophage toll‐like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia, 52(2), 318–328. 10.1007/s00125-008-1221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine, R. V. , Sinha, M. K. , Heiman, M. L. , Kriauciunas, A. , Stephens, T. W. , Nyce, M. R. , … Caro, J. F. (1996). Serum immunoreactive‐leptin concentrations in normal‐weight and obese humans. The New England Journal of Medicine, 334(5), 292–295. 10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- Cox‐York, K. A. , Erickson, C. B. , Pereira, R. I. , Bessesen, D. H. , & Van Pelt, R. E. (2017). Region‐specific effects of oestradiol on adipose‐derived stem cell differentiation in post‐menopausal women. Journal of Cellular and Molecular Medicine, 21(4), 677–684. 10.1111/jcmm.13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdeviren, S. , Jung, D. Y. , Lee, E. , Friedline, R. H. , Noh, H. L. , Kim, J. H. , … Kim, J. K. (2016). Altered interleukin‐10 signaling in skeletal muscle regulates obesity‐mediated inflammation and insulin resistance. Molecular and Cellular Biology, 36(23), 2956–2966. 10.1128/MCB.00181-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries, M. C. , Hamadeh, M. J. , Graham, T. E. , & Tarnopolsky, M. A. (2005). 17β‐Estradiol supplementation decreases glucose rate of appearance and disappearance with no effect on glycogen utilization during moderate intensity exercise in men. The Journal of Clinical Endocrinology and Metabolism, 90(11), 6218–6225. 10.1210/jc.2005-0926 [DOI] [PubMed] [Google Scholar]
- Everett, B. M. , Donath, M. Y. , Pradhan, A. D. , Thuren, T. , Pais, P. , Nicolau, J. C. , … Ridker, P. M. (2018). Anti‐inflammatory therapy with canakinumab for the prevention and management of diabetes. Journal of the American College of Cardiology, 71(21), 2392–2401. 10.1016/j.jacc.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Exley, M. A. , Hand, L. , O'Shea, D. , & Lynch, L. (2014). Interplay between the immune system and adipose tissue in obesity. The Journal of Endocrinology, 223(2), R41–R48. 10.1530/JOE-13-0516 [DOI] [PubMed] [Google Scholar]
- Ferrer, B. , Navia, B. , Giralt, M. , Comes, G. , Carrasco, J. , Molinero, A. , … Hidalgo, J. (2014). Muscle‐specific interleukin‐6 deletion influences body weight and body fat in a sex‐dependent manner. Brain, Behavior, and Immunity, 40, 121–130. 10.1016/j.bbi.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Filardo, E. J. , Quinn, J. A. , Bland, K. I. , & Frackelton, A. R. Jr. (2000). Estrogen‐induced activation of Erk‐1 and Erk‐2 requires the G protein‐coupled receptor homolog, GPR30, and occurs via trans‐activation of the epidermal growth factor receptor through release of HB‐EGF. Molecular Endocrinology, 14(10), 1649–1660. 10.1210/mend.14.10.0532 [DOI] [PubMed] [Google Scholar]
- Fish, E. N. (2008). The X‐files in immunity: Sex‐based differences predispose immune responses. Nature Reviews. Immunology, 8(9), 737–744. 10.1038/nri2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst‐Ludwig, A. , Clemenz, M. , Hohmann, S. , Hartge, M. , Sprang, C. , Frost, N. , … Kintscher, U. (2008). Metabolic actions of estrogen receptor beta (ERβ) are mediated by a negative cross‐talk with PPARγ. PLoS Genetics, 4(6), e1000108 10.1371/journal.pgen.1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi, F. , & Campesi, I. (2014). Sex and gender influences on pharmacological response: An overview. Expert Review of Clinical Pharmacology, 7(4), 469–485. 10.1586/17512433.2014.922866 [DOI] [PubMed] [Google Scholar]
- Fried, S. K. , Bunkin, D. A. , & Greenberg, A. S. (1998). Omental and subcutaneous adipose tissues of obese subjects release interleukin‐6: Depot difference and regulation by glucocorticoid. The Journal of Clinical Endocrinology and Metabolism, 83(3), 847–850. 10.1210/jcem.83.3.4660 [DOI] [PubMed] [Google Scholar]
- Garcia‐Duran, M. , de Frutos, T. , Diaz‐Recasens, J. , Garcia‐Galvez, G. , Jimenez, A. , Monton, M. , … López‐Farré, A. (1999). Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circulation Research, 85(11), 1020–1026. 10.1161/01.RES.85.11.1020 [DOI] [PubMed] [Google Scholar]
- Gorzek, J. F. , Hendrickson, K. C. , Forstner, J. P. , Rixen, J. L. , Moran, A. L. , & Lowe, D. A. (2007). Estradiol and tamoxifen reverse ovariectomy‐induced physical inactivity in mice. Medicine and Science in Sports and Exercise, 39(2), 248–256. 10.1249/01.mss.0000241649.15006.b8 [DOI] [PubMed] [Google Scholar]
- Griesbeck, M. , Scully, E. , & Altfeld, M. (2016). Sex and gender differences in HIV‐1 infection. Clinical Science (London, England), 130(16), 1435–1451. 10.1042/CS20160112 [DOI] [PubMed] [Google Scholar]
- Gutniak, M. K. , Svartberg, J. , Hellstrom, P. M. , Holst, J. J. , Adner, N. , & Ahren, B. (2001). Antidiabetogenic action of glucagon‐like peptide‐1 related to administration relative to meal intake in subjects with type 2 diabetes. Journal of Internal Medicine, 250(1), 81–87. 10.1046/j.1365-2796.2001.00862.x [DOI] [PubMed] [Google Scholar]
- Hall, N. , White, C. , & O'Sullivan, A. J. (2009). The relationship between adiponectin, progesterone, and temperature across the menstrual cycle. Journal of Endocrinological Investigation, 32(3), 279–283. 10.1007/BF03346467 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkonen, P. L. , & Vaananen, H. K. (2006). Monocyte‐macrophage system as a target for estrogen and selective estrogen receptor modulators. Annals of the New York Academy of Sciences, 1089, 218–227. 10.1196/annals.1386.045 [DOI] [PubMed] [Google Scholar]
- Hassouna, A. , Obaia, E. , Marzouk, S. , Rateb, M. , & Haidara, M. (2014). The role of sex hormones in induced‐systemic inflammation in female albino rats. Acta Physiologica Hungarica, 101(1), 112–127. 10.1556/APhysiol.101.2014.1.12 [DOI] [PubMed] [Google Scholar]
- Heine, P. A. , Taylor, J. A. , Iwamoto, G. A. , Lubahn, D. B. , & Cooke, P. S. (2000). Increased adipose tissue in male and female estrogen receptor‐α knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 97(23), 12729–12734. 10.1073/pnas.97.23.12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring, N. , Pike, A. , Andersson, S. , Matthews, J. , Cheng, G. , Hartman, J. , … Gustafsson, J. Å. (2007). Estrogen receptors: How do they signal and what are their targets. Physiological Reviews, 87(3), 905–931. 10.1152/physrev.00026.2006 [DOI] [PubMed] [Google Scholar]
- Hetemaki, N. , Savolainen‐Peltonen, H. , Tikkanen, M. J. , Wang, F. , Paatela, H. , Hamalainen, E. , … Mikkola, T. S. (2017). Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism, 102(12), 4588–4595. 10.1210/jc.2017-01474 [DOI] [PubMed] [Google Scholar]
- Hidalgo, J. , Florit, S. , Giralt, M. , Ferrer, B. , Keller, C. , & Pilegaard, H. (2010). Transgenic mice with astrocyte‐targeted production of interleukin‐6 are resistant to high‐fat diet‐induced increases in body weight and body fat. Brain, Behavior, and Immunity, 24(1), 119–126. 10.1016/j.bbi.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Hoeg, L. , Roepstorff, C. , Thiele, M. , Richter, E. A. , Wojtaszewski, J. F. , & Kiens, B. (2009). Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. Journal of Applied Physiology (Bethesda, MD: 1985), 107(3), 824–831. 10.1152/japplphysiol.91382.2008 [DOI] [PubMed] [Google Scholar]
- Høoeg, L. D. , Sjøberg, K. A. , Jeppesen, J. , Jensen, T. E. , Frosig, C. , Birk, J. B. , … Kiens, B. (2011). Lipid‐induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes, 60(1), 64–73. 10.2337/db10-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma, H. , Kurachi, H. , Nishio, Y. , Takeda, T. , Yamamoto, T. , Adachi, K. , … Murata, Y. (2000). Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. The Journal of Biological Chemistry, 275(15), 11404–11411. 10.1074/jbc.275.15.11404 [DOI] [PubMed] [Google Scholar]
- Hong, E. G. , Ko, H. J. , Cho, Y. R. , Kim, H. J. , Ma, Z. , Yu, T. Y. , … Kim, J. K. (2009). Interleukin‐10 prevents diet‐induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes, 58(11), 2525–2535. 10.2337/db08-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, J. , McQuillan, B. M. , Chapman, C. M. , Thompson, P. L. , & Beilby, J. P. (2005). Elevated interleukin‐18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(6), 1268–1273. 10.1161/01.ATV.0000163843.70369.12 [DOI] [PubMed] [Google Scholar]
- Iwasa, T. , Matsuzaki, T. , Kinouchi, R. , Gereltsetseg, G. , Murakami, M. , Munkhzaya, M. , … Irahara, M. (2014). Changes in central and peripheral inflammatory responses to lipopolysaccharide in ovariectomized female rats. Cytokine, 65(1), 65–73. 10.1016/j.cyto.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Janssen, I. , Powell, L. H. , Crawford, S. , Lasley, B. , & Sutton‐Tyrrell, K. (2008). Menopause and the metabolic syndrome: The Study of Women's Health Across the Nation. Archives of Internal Medicine, 168(14), 1568–1575. 10.1001/archinte.168.14.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks, M. Z. , Fairfield, H. E. , Johnson, E. C. , Morrison, R. F. , & Muday, G. K. (2017). Sex steroid hormones regulate leptin transcript accumulation and protein secretion in 3T3‐L1 cells. Scientific Reports, 7(1), 8232 10.1038/s41598-017-07473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, C. , Henao‐Mejia, J. , & Flavell, R. A. (2013). Innate immune receptors: Key regulators of metabolic disease progression. Cell Metabolism, 17(6), 873–882. 10.1016/j.cmet.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Kanter, R. , & Caballero, B. (2012). Global gender disparities in obesity: A review. Advances in Nutrition, 3(4), 491–498. 10.3945/an.112.002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou, K. , Smith, S. R. , Greenberg, A. S. , & Fried, S. K. (2012). Sex differences in human adipose tissues—The biology of pear shape. Biology of Sex Differences, 3(1), 13 10.1186/2042-6410-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe, F. , & Pinnick, K. E. (2015). Biology of upper‐body and lower‐body adipose tissue—Link to whole‐body phenotypes. Nature Reviews. Endocrinology, 11(2), 90–100. 10.1038/nrendo.2014.185 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen, B. S. , & Katzenellenbogen, J. A. (2002). Biomedicine. Defining the “S” in SERMs. Science, 295(5564), 2380–2381. 10.1126/science.1070442 [DOI] [PubMed] [Google Scholar]
- Kautzky‐Willer, A. , Harreiter, J. , & Pacini, G. (2016). Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocrine Reviews, 37(3), 278–316. 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Higashimori, T. , Park, S. Y. , Choi, H. , Dong, J. , Kim, Y. J. , … Kim, J. K. (2004). Differential effects of interleukin‐6 and ‐10 on skeletal muscle and liver insulin action in vivo. Diabetes, 53(4), 1060–1067. 10.2337/diabetes.53.4.1060 [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , & Reaven, G. (2013). Sex differences in insulin resistance and cardiovascular disease risk. The Journal of Clinical Endocrinology and Metabolism, 98(11), E1716–E1721. 10.1210/jc.2013-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireev, R. A. , Tresguerres, A. C. , Garcia, C. , Borras, C. , Ariznavarreta, C. , Vara, E. , … Tresguerres, J. A. (2010). Hormonal regulation of pro‐inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats. Biogerontology, 11(2), 229–243. 10.1007/s10522-009-9242-2 [DOI] [PubMed] [Google Scholar]
- Kleiblova, P. , Springer, D. , & Haluzik, M. (2006). The influence of hormonal changes during menstrual cycle on serum adiponectin concentrations in healthy women. Physiological Research, 55(6), 661–666. [DOI] [PubMed] [Google Scholar]
- Klein, S. L. (2000). The effects of hormones on sex differences in infection: From genes to behavior. Neuroscience and Biobehavioral Reviews, 24(6), 627–638. 10.1016/S0149-7634(00)00027-0 [DOI] [PubMed] [Google Scholar]
- Kowalski, G. M. , Nicholls, H. T. , Risis, S. , Watson, N. K. , Kanellakis, P. , Bruce, C. R. , … Febbraio, M. A. (2011). Deficiency of haematopoietic‐cell‐derived IL‐10 does not exacerbate high‐fat‐diet‐induced inflammation or insulin resistance in mice. Diabetologia, 54(4), 888–899. 10.1007/s00125-010-2020-5 [DOI] [PubMed] [Google Scholar]
- Kramer, P. R. , Kramer, S. F. , & Guan, G. (2004). 17β‐Estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte‐derived macrophages. Arthritis and Rheumatism, 50(6), 1967–1975. 10.1002/art.20309 [DOI] [PubMed] [Google Scholar]
- Kramer, P. R. , Winger, V. , & Kramer, S. F. (2007). 17β‐Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes. Molecular and Cellular Endocrinology, 279(1–2), 16–25. 10.1016/j.mce.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl, H. (2005). Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric, 8(Suppl 1), 3–63. 10.1080/13697130500148875 [DOI] [PubMed] [Google Scholar]
- Kumada, M. , Kihara, S. , Ouchi, N. , Kobayashi, H. , Okamoto, Y. , Ohashi, K. , … Matsuzawa, Y. (2004). Adiponectin specifically increased tissue inhibitor of metalloproteinase‐1 through interleukin‐10 expression in human macrophages. Circulation, 109(17), 2046–2049. 10.1161/01.CIR.0000127953.98131.ED [DOI] [PubMed] [Google Scholar]
- Lancaster, G. I. , Kraakman, M. J. , Kammoun, H. L. , Langley, K. G. , Estevez, E. , Banerjee, A. , … Gerondakis, S. (2014). The dual‐specificity phosphatase 2 (DUSP2) does not regulate obesity‐associated inflammation or insulin resistance in mice. PLoS ONE, 9(11), e111524 10.1371/journal.pone.0111524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt, M. , Gingerich, R. L. , Havel, P. J. , Mueller, W. M. , Schoner, B. , Hale, J. E. , & Heiman, M. L. (1998). Radioimmunoassay of rat leptin: Sexual dimorphism reversed from humans. Clinical Chemistry, 44(3), 565–570. [PubMed] [Google Scholar]
- Larsen, C. M. , Faulenbach, M. , Vaag, A. , Vølund, A. , Ehses, J. A. , Seifert, B. , … Donath, M. Y. (2007). Interleukin‐1‐receptor antagonist in type 2 diabetes mellitus. The New England Journal of Medicine, 356(15), 1517–1526. 10.1056/NEJMoa065213 [DOI] [PubMed] [Google Scholar]
- Lee, S. , Ko, Y. , Kwak, C. , & Yim, E. S. (2016). Gender differences in metabolic syndrome components among the Korean 66‐year‐old population with metabolic syndrome. BMC Geriatrics, 16, 27 10.1186/s12877-016-0202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard, B. , Abildgaard, J. , Heywood, S. E. , Pedersen, B. K. , & Febbraio, M. A. (2018). Female sex hormones are necessary for the metabolic effects mediated by loss of interleukin 18 signaling. Molecular Metabolism, 12, 89–97. 10.1016/j.molmet.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard, B. , Matthews, V. B. , Brandt, C. , Hojman, P. , Allen, T. L. , Estevez, E. , … Febbraio, M. A. (2013). Interleukin‐18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes, 62(9), 3064–3074. 10.2337/db12-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Kilic, G. , Meyers, M. S. , Navarro, G. , Wang, Y. , Oberholzer, J. , & Mauvais‐Jarvis, F. (2013). Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia, 56(2), 370–381. 10.1007/s00125-012-2764-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy, J. C. , Champagne, C. M. , de Jonge, L. , Xie, H. , & Smith, S. R. (2008). Increased visceral fat and decreased energy expenditure during the menopausal transition. International Journal of Obesity, 32(6), 949–958. 10.1038/ijo.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludgero‐Correia, A. Jr. , Aguila, M. B. , Mandarim‐de‐Lacerda, C. A. , & Faria, T. S. (2012). Effects of high‐fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition, 28(3), 316–323. 10.1016/j.nut.2011.07.014 [DOI] [PubMed] [Google Scholar]
- Lundsgaard, A. M. , & Kiens, B. (2014). Gender differences in skeletal muscle substrate metabolism—Molecular mechanisms and insulin sensitivity. Frontiers in Endocrinology (Lausanne), 5, 195 10.3389/fendo.2014.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, A. H. , & Appelman, Y. E. (2010). Gender differences in coronary heart disease. Netherlands Heart Journal, 18(12), 598–602. 10.1007/s12471-010-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, S. , Kamata, H. , Luo, J. L. , Leffert, H. , & Karin, M. (2005). IKKβ couples hepatocyte death to cytokine‐driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell, 121(7), 977–990. 10.1016/j.cell.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Malutan, A. M. , Dan, M. , Nicolae, C. , & Carmen, M. (2014). Proinflammatory and anti‐inflammatory cytokine changes related to menopause. Prz Menopauzalny, 13(3), 162–168. 10.5114/pm.2014.43818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos, K. N. , Karpe, F. , & Frayn, K. N. (2010). Gluteofemoral body fat as a determinant of metabolic health. International Journal of Obesity, 34(6), 949–959. 10.1038/ijo.2009.286 [DOI] [PubMed] [Google Scholar]
- Matthews, V. B. , Allen, T. L. , Risis, S. , Chan, M. H. , Henstridge, D. C. , Watson, N. , … Febbraio, M. A. (2010). Interleukin‐6‐deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia, 53(11), 2431–2441. 10.1007/s00125-010-1865-y [DOI] [PubMed] [Google Scholar]
- Mauvais‐Jarvis, F. , Arnold, A. P. , & Reue, K. (2017). A guide for the design of pre‐clinical studies on sex differences in metabolism. Cell Metabolism, 25(6), 1216–1230. 10.1016/j.cmet.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy, F. C. , Harford, K. A. , Reynolds, C. M. , Oliver, E. , Claessens, M. , Mills, K. H. , & Roche, H. M. (2011). Lack of interleukin‐1 receptor I (IL‐1RI) protects mice from high‐fat diet‐induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes, 60(6), 1688–1698. 10.2337/db10-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinero, A. , Fernandez‐Perez, A. , Mogas, A. , Giralt, M. , Comes, G. , Fernandez‐Gayol, O. , … Hidalgo, J. (2017). Role of muscle IL‐6 in gender‐specific metabolism in mice. PLoS ONE, 12(3), e0173675 10.1371/journal.pone.0173675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, A. J. , Kraakman, M. J. , Kammoun, H. L. , Dragoljevic, D. , Lee, M. K. , Lawlor, K. E. , … Masters, S. L. (2016). IL‐18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metabolism, 23(1), 155–164. 10.1016/j.cmet.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Musatov, S. , Chen, W. , Pfaff, D. W. , Mobbs, C. V. , Yang, X. J. , Clegg, D. J. , … Ogawa, S. (2007). Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America, 104(7), 2501–2506. 10.1073/pnas.0610787104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler, W. E. , Sakurai, T. , Kim, S. , Maeda, S. , Kim, K. , Elsharkawy, A. M. , & Karin, M. (2007). Gender disparity in liver cancer due to sex differences in MyD88‐dependent IL‐6 production. Science, 317(5834), 121–124. 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- Navia, B. , Ferrer, B. , Giralt, M. , Comes, G. , Carrasco, J. , Molinero, A. , … Hidalgo, J. (2014). Interleukin‐6 deletion in mice driven by aP2–Cre–ERT2 prevents against high‐fat diet‐induced gain weight and adiposity in female mice. Acta Physiologica (Oxford, England), 211(4), 585–596. 10.1111/apha.12328 [DOI] [PubMed] [Google Scholar]
- Neels, J. G. , Pandey, M. , Hotamisligil, G. S. , & Samad, F. (2006). Autoamplification of tumor necrosis factor‐α: A potential mechanism for the maintenance of elevated tumor necrosis factor‐α in male but not female obese mice. The American Journal of Pathology, 168(2), 435–444. 10.2353/ajpath.2006.050699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea, M. G. , Joosten, L. A. , Lewis, E. , Jensen, D. R. , Voshol, P. J. , Kullberg, B. J. , … van der Meer, J. W. (2006). Deficiency of interleukin‐18 in mice leads to hyperphagia, obesity and insulin resistance. Nature Medicine, 12(6), 650–656. 10.1038/nm1415 [DOI] [PubMed] [Google Scholar]
- Nickelson, K. J. , Stromsdorfer, K. L. , Pickering, R. T. , Liu, T. W. , Ortinau, L. C. , Keating, A. F. , Perfield J. W. (2012). A comparison of inflammatory and oxidative stress markers in adipose tissue from weight‐matched obese male and female mice. Experimental Diabetes Research, 2012, 859395 10.1155/2012/859395, 1, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, K. , Parker, J. L. , Ouchi, N. , Higuchi, A. , Vita, J. A. , Gokce, N. , … Walsh, K. (2010). Adiponectin promotes macrophage polarization toward an anti‐inflammatory phenotype. The Journal of Biological Chemistry, 285(9), 6153–6160. 10.1074/jbc.M109.088708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani, M. , Garcia, A. , Rogers, A. B. , Ge, Z. , Taylor, N. S. , Xu, S. , … Fox, J. G. (2007). Protective role of 17β‐estradiol against the development of Helicobacter pylori‐induced gastric cancer in INS‐GAS mice. Carcinogenesis, 28(12), 2597–2604. 10.1093/carcin/bgm150 [DOI] [PubMed] [Google Scholar]
- Pajunen, P. , Kotronen, A. , Korpi‐Hyövälti, E. , Keinanen‐Kiukaanniemi, S. , Oksa, H. , Niskanen, L. , … Peltonen, M. (2011). Metabolically healthy and unhealthy obesity phenotypes in the general population: The FIN‐D2D Survey. BMC Public Health, 11, 754 10.1186/1471-2458-11-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, M. , Febbraio, M. A. , & Whitham, M. (2014). From cytokine to myokine: The emerging role of interleukin‐6 in metabolic regulation. Immunology and Cell Biology, 92(4), 331–339. 10.1038/icb.2014.16 [DOI] [PubMed] [Google Scholar]
- Park, E. J. , Lee, J. H. , Yu, G. Y. , He, G. , Ali, S. R. , Holzer, R. G. , … Karin, M. (2010). Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL‐6 and TNF expression. Cell, 140(2), 197–208. 10.1016/j.cell.2009.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos, P. , Lima, L. , Tovar, S. , Gonzalez‐Touceda, D. , Dieguez, C. , & Garcia, M. C. (2015). Divergent responses to thermogenic stimuli in BAT and subcutaneous adipose tissue from interleukin 18 and interleukin 18 receptor 1‐deficient mice. Scientific Reports, 5, 17977 10.1038/srep17977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, A. , Janott, J. , Möhlig, M. , Ristow, M. , Rochlitz, H. , Busch, K. , … Schifferdecker, E. (1997). Circulating tumor necrosis factor α is elevated in male but not in female patients with type II diabetes mellitus. Hormone and Metabolic Research, 29(3), 111–114. 10.1055/s-2007-979001 [DOI] [PubMed] [Google Scholar]
- Pighon, A. , Gutkowska, J. , Jankowski, M. , Rabasa‐Lhoret, R. , & Lavoie, J. M. (2011). Exercise training in ovariectomized rats stimulates estrogenic‐like effects on expression of genes involved in lipid accumulation and subclinical inflammation in liver. Metabolism, 60(5), 629–639. 10.1016/j.metabol.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Prossnitz, E. R. , Arterburn, J. B. , & Sklar, L. A. (2007). GPR30: A G protein‐coupled receptor for estrogen. Molecular and Cellular Endocrinology, 265‐266, 138–142. 10.1016/j.mce.2006.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana, A. , Erta, M. , Ferrer, B. , Comes, G. , Giralt, M. , & Hidalgo, J. (2013). Astrocyte‐specific deficiency of interleukin‐6 and its receptor reveal specific roles in survival, body weight and behavior. Brain, Behavior, and Immunity, 27(1), 162–173. 10.1016/j.bbi.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Ratnu, V. S. , Emami, M. R. , & Bredy, T. W. (2017). Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. Journal of Neuroscience Research, 95(1–2), 301–310. 10.1002/jnr.23886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, C. W. , Walker, W. , & Alexander, J. (2001). Sex‐associated hormones and immunity to protozoan parasites. Clinical Microbiology Reviews, 14(3), 476–488. 10.1128/CMR.14.3.476-488.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, N. H. , Perfield, J. W. 2nd , Strissel, K. J. , Obin, M. S. , & Greenberg, A. S. (2009). Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy‐induced obesity. Endocrinology, 150(5), 2161–2168. 10.1210/en.2008-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltevo, J. , Kautiainen, H. , & Vanhala, M. (2009). Gender differences in adiponectin and low‐grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gender Medicine, 6(3), 463–470. 10.1016/j.genm.2009.09.006 [DOI] [PubMed] [Google Scholar]
- Scherer, P. E. (2016). The multifaceted roles of adipose tissue‐therapeutic targets for diabetes and beyond: The 2015 Banting Lecture. Diabetes, 65(6), 1452–1461. 10.2337/db16-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, F. M. , Weschenfelder, J. , Sander, C. , Minkwitz, J. , Thormann, J. , Chittka, T. , … Himmerich, H. (2015). Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE, 10(3), e0121971 10.1371/journal.pone.0121971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, H. , Kokoeva, M. V. , Inouye, K. , Tzameli, I. , Yin, H. , & Flier, J. S. (2006). TLR4 links innate immunity and fatty acid‐induced insulin resistance. The Journal of Clinical Investigation, 116(11), 3015–3025. 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, E. R. (2003). Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology, 86(3–5), 225–230. 10.1016/S0960-0760(03)00360-1 [DOI] [PubMed] [Google Scholar]
- Singer, K. , Maley, N. , Mergian, T. , DelProposto, J. , Cho, K. W. , Zamarron, B. F. , … Lumeng, C. N. (2015). Differences in hematopoietic stem cells contribute to sexually dimorphic inflammatory responses to high fat diet‐induced obesity. The Journal of Biological Chemistry, 290(21), 13250–13262. 10.1074/jbc.M114.634568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder, M. B. , Zimmet, P. Z. , Visser, M. , Dekker, J. M. , Seidell, J. C. , & Shaw, J. E. (2004). Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: The AusDiab Study. International Journal of Obesity and Related Metabolic Disorders, 28(3), 402–409. 10.1038/sj.ijo.0802567 [DOI] [PubMed] [Google Scholar]
- Song, H. J. , Oh, S. , Quan, S. , Ryu, O. H. , Jeong, J. Y. , Hong, K. S. , & Kim, D. H. (2014). Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatrics, 14, 8 10.1186/1471-2318-14-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg, A. , van Hall, G. , Osada, T. , Sacchetti, M. , Saltin, B. , & Klarlund Pedersen, B. (2000). Production of interleukin‐6 in contracting human skeletal muscles can account for the exercise‐induced increase in plasma interleukin‐6. The Journal of Physiology, 529(Pt 1), 237–242. 10.1111/j.1469-7793.2000.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra, R. , Joosten, L. A. , Koenen, T. , van Tits, B. , van Diepen, J. A. , van den Berg, S. A. , … Netea, M. G. (2010). The inflammasome‐mediated caspase‐1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metabolism, 12(6), 593–605. 10.1016/j.cmet.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straczkowski, M. , Kowalska, I. , Nikolajuk, A. , Krukowska, A. , & Gorska, M. (2005). Plasma interleukin‐10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care, 28(8), 2036–2037. 10.2337/diacare.28.8.2036 [DOI] [PubMed] [Google Scholar]
- Stubbins, R. E. , Holcomb, V. B. , Hong, J. , & Nunez, N. P. (2012). Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. European Journal of Nutrition, 51(7), 861–870. 10.1007/s00394-011-0266-4 [DOI] [PubMed] [Google Scholar]
- Stubbins, R. E. , Najjar, K. , Holcomb, V. B. , Hong, J. , & Nunez, N. P. (2012). Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes, Obesity & Metabolism, 14(1), 58–66. 10.1111/j.1463-1326.2011.01488.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubelius, A. , Andersson, A. , Islander, U. , & Carlsten, H. (2017). Ovarian hormones in innate inflammation. Immunobiology, 222(8–9), 878–883. 10.1016/j.imbio.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Sun, S. , Ji, Y. , Kersten, S. , & Qi, L. (2012). Mechanisms of inflammatory responses in obese adipose tissue. Annual Review of Nutrition, 32, 261–286. 10.1146/annurev-nutr-071811-150623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. J. , Kim, S. P. , Zhang, D. , Sun, H. , Cao, Q. , Lu, X. , … Quon, M. J. (2017). Deletion of interleukin 1 receptor‐associated kinase 1 (Irak1) improves glucose tolerance primarily by increasing insulin sensitivity in skeletal muscle. The Journal of Biological Chemistry, 292(29), 12339–12350. 10.1074/jbc.M117.779108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M. J. , Kew, K. A. , Ryan, T. E. , Pennington, E. R. , Lin, C. T. , Buddo, K. A. , … Neufer, P. D. (2018). 17β‐Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metabolism, 27(1), 167–179 e167. 10.1016/j.cmet.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer, A. T. , Browning, J. D. , Ayers, C. R. , Das, S. R. , Khera, A. , Vega, G. L. , … Scherer, P. E. (2012). Adiponectin as an independent predictor of the presence and degree of hepatic steatosis in the Dallas Heart Study. The Journal of Clinical Endocrinology and Metabolism, 97(6), E982–E986. 10.1210/jc.2011-3305 [DOI] [PubMed] [Google Scholar]
- Turer, A. T. , & Scherer, P. E. (2012). Adiponectin: Mechanistic insights and clinical implications. Diabetologia, 55(9), 2319–2326. 10.1007/s00125-012-2598-x [DOI] [PubMed] [Google Scholar]
- van Exel, E. , Gussekloo, J. , de Craen, A. J. , Frolich, M. , Bootsma‐Van Der Wiel, A. , Westendorp, R. G. , & Leiden 85 Plus Study . (2002). Low production capacity of interleukin‐10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85‐Plus Study. Diabetes, 51(4), 1088–1092. 10.2337/diabetes.51.4.1088 [DOI] [PubMed] [Google Scholar]
- Vandanmagsar, B. , Youm, Y. H. , Ravussin, A. , Galgani, J. E. , Stadler, K. , Mynatt, R. L. , … Dixit, V. D. (2011). The NLRP3 inflammasome instigates obesity‐induced inflammation and insulin resistance. Nature Medicine, 17(2), 179–188. 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius, V. , Wallenius, K. , Ahren, B. , Rudling, M. , Carlsten, H. , Dickson, S. L. , … Jansson, J. O. (2002). Interleukin‐6‐deficient mice develop mature‐onset obesity. Nature Medicine, 8(1), 75–79. 10.1038/nm0102-75 [DOI] [PubMed] [Google Scholar]
- Wang, Z. V. , & Scherer, P. E. (2016). Adiponectin, the past two decades. Journal of Molecular Cell Biology, 8(2), 93–100. 10.1093/jmcb/mjw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, H. , Gris, D. , Lei, Y. , Jha, S. , Zhang, L. , Huang, M. T. , … Ting, J. P. (2011). Fatty acid‐induced NLRP3–ASC inflammasome activation interferes with insulin signaling. Nature Immunology, 12(5), 408–415. 10.1038/ni.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre, C. C. (2001). Sex differences in autoimmune disease. Nature Immunology, 2(9), 777–780. 10.1038/ni0901-777 [DOI] [PubMed] [Google Scholar]
- Wyskida, K. , Franik, G. , Wikarek, T. , Owczarek, A. , Delroba, A. , Chudek, J. , … Olszanecka‐Glinianowicz, M. (2017). The levels of adipokines in relation to hormonal changes during the menstrual cycle in young, normal‐weight women. Endocrine Connections, 6(8), 892–900. 10.1530/EC-17-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa, R. , Wada, T. , Matsumoto, N. , Morita, M. , Sawakawa, K. , Ishii, Y. , … Sasaoka, T. (2012). Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high‐fat diet. American Journal of Physiology. Endocrinology and Metabolism, 303(4), E445–E456. 10.1152/ajpendo.00638.2011 [DOI] [PubMed] [Google Scholar]
- Yusuf, S. , Hawken, S. , Ounpuu, S. , Bautista, L. , Franzosi, M. G. , Commerford, P. , … INTERHEART Study Investigators . (2005). Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case–control study. Lancet, 366(9497), 1640–1649. 10.1016/S0140-6736(05)67663-5 [DOI] [PubMed] [Google Scholar]
- Zhu, L. , Brown, W. C. , Cai, Q. , Krust, A. , Chambon, P. , McGuinness, O. P. , & Stafford, J. M. (2013). Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway‐selective insulin resistance. Diabetes, 62(2), 424–434. 10.2337/db11-1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla, E. P. , & Conti, B. (2014). Interleukin‐18 null mutation increases weight and food intake and reduces energy expenditure and lipid substrate utilization in high‐fat diet fed mice. Brain, Behavior, and Immunity, 37, 45–53. 10.1016/j.bbi.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla, E. P. , Sanchez‐Alavez, M. , Sugama, S. , Brennan, M. , Fernandez, R. , Bartfai, T. , & Conti, B. (2007). Interleukin‐18 controls energy homeostasis by suppressing appetite and feed efficiency. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11097–11102. 10.1073/pnas.0611523104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk, M. , & McKean, K. A. (1996). Sex differences in parasite infections: Patterns and processes. International Journal for Parasitology, 26(10), 1009–1023. 10.1016/S0020-7519(96)80001-4 [DOI] [PubMed] [Google Scholar]


