Abstract
A 32-year-old man started building a wooden desk atop Mount Fuji at an altitude of 3,776 m. Over the course of the second day, he developed lassitude and cough and experienced a headache that night; however, he continued to work. He was transported to our hospital with an altered level of consciousness. On arrival, chest radiography revealed increased opacities in both lungs, and magnetic resonance imaging (MRI) revealed a high-intensity signal in the splenium on diffusion-weighted imaging. He received mechanical ventilation following tracheal intubation. His respiratory function improved, and he was extubated on the fourth hospital day. Physical examination showed no motor weakness, and although he responded to verbal commands, he was unable to speak and was unresponsive to visual stimulation. On the seventh hospital day, head MRI showed improvement in the lesion in the splenium, although other signal changes were observed in the body of the corpus callosum. His verbal responsiveness and voice volume improved on a daily basis. Two months after the incident, he continued to experience mild recent memory disturbance. The patient described in this case report showed delayed signal changes in the body of the corpus callosum, possibly secondary to the onset of microbleed-induced edema.
Keywords: brain, lung, edema, aphasia, hypoxia
Introduction
High altitudes are challenging for the human body owing to the progressive reduction in the barometric pressure and subsequent reduction in the partial pressure of oxygen in such environments, which leads to a series of physiological responses that enable individuals to tolerate hypoxia and ensure adequate supply of oxygen to the tissues1). Most of these adaptations are observed at heights ≥2,000 m above sea level. Individuals are at risk of developing any of the following forms of acute altitude illness: (1) acute mountain sickness (AMS), a syndrome of nonspecific symptoms including headache, lassitude, dizziness, and nausea, (2) high-altitude cerebral edema (HACE), a potentially fatal illness characterized by ataxia and/or decreased consciousness and, (3) high-altitude pulmonary edema (HAPE), a noncardiogenic form of pulmonary edema resulting from excessive hypoxic pulmonary vasoconstriction, which can be fatal2).
We report a case of mountain sickness with lung edema and motor aphasia with delayed signal changes in the corpus callosum on magnetic resonance imaging (MRI).
Patient
A 32-year-old man started building a wooden desk atop Mount Fuji, the highest mountain in Japan (3,776 m above sea level). He reached the summit of Mount Fuji over a period of 3 hours using an off-road vehicle from 1,400 m above sea level. This was the first time he had traveled to the summit of the mountain. He denied a significant medical history. Over the course of the second day at the summit of Mount Fuji, he developed lassitude and cough and experienced a headache that night; however, he continued to work. Based on the history obtained from those who accompanied him, his condition was poor from days 2 to 5. He was transported to our hospital by a mountain rescue ambulance and a physician-staffed helicopter with an altered level of consciousness. Emergency medical services provided 3 L min-1 of oxygen because pulse oximetry revealed an oxygen saturation level <90% at 14:45 hours. On arrival at our hospital at 15:36 hours, his Glasgow Coma Scale was 10 (E3: eye opening in response to verbal commands, V2: incomprehensible sounds in response to stimulation, M5: localized movements in response to pain stimulation) with restless confusion. His blood pressure was 104/70 mmHg, his heart rate was 76 beats/min, and his oxygen saturation determined by pulse oximetry was 99% on 10 L min-1 of oxygen. He did not receive carbon dioxide (CO2) re-breathing treatment.3) Chest auscultation revealed crackles in bilateral lung fields. Electrocardiography revealed non-specific findings, and cardiac ultrasonography revealed normal wall motion. Chest radiography revealed an increased opacity in the left dominant lung field (Figure 1). Arterial blood gas analysis revealed the following findings with <10 L min-1 of oxygen being administered to the patient: pH 7.48, partial pressure of CO2 30.5 mmHg, partial pressure of oxygen (PO2) 120 mmHg, serum bicarbonate 22.7 mmol/L, and serum lactate 1.3 mmol/L. Chest computed tomography (CT) revealed ground-glass opacities in both lungs, suggesting pulmonary edema (Figure 2). Head CT was normal; however, MRI revealed a high-intensity signal in the splenium on diffusion-weighted imaging (DWI) (Figure 3). T2*-weighted imaging (T2*WI) revealed an area of relatively low intensity in the corpus callosum (Figure 4). Blood tests showed the following results: white blood cell count 13,500 cells/mm3 (reference range 3,600–8,900), total serum protein 6.4 g/dL (reference range 6.5–8.5), serum albumin 3.6 g/dL (reference range 4–5.2), serum creatinine 1.06 mg/dL (reference range 0.5–0.8), and serum C-reactive protein 0.8 mg/dL (reference range <0.3). Levels of fibrinogen degradation products or cardiac enzymes (troponin T and creatine kinase-MB) were not elevated. Following tracheal intubation, mechanical ventilation was initiated for HAPE, and he was administered 26 mg of dexamethasone for HACE to prevent suffocation secondary to difficulty associated with sputum buildup4). Severe hypoxia (PaO2/fraction of the inspired oxygen [P/F] ratio=nearly 120) and a restless state necessitated the administration of sedatives and mechanical ventilation with positive end-expiratory pressure. His respiratory function improved on the second hospital day (P/F ratio=400), and he was extubated on the fourth hospital day. Prolonged ventilator support was maintained to protect his airway until we observed an improvement in his altered level of consciousness. On physical examination, he showed no motor weakness, and although he was responsive to verbal commands, he was unable to speak and was unresponsive to visual stimuli. On the fifth hospital day, he was able to speak a word at a time with a weak voice. On the seventh hospital day, MRI of the brain showed improvement in the lesion of the splenium; however, other signal changes were observed in the body of the corpus callosum (Figure 5). CT performed on the same day showed normalization of changes observed in the bilateral lung fields. His verbal responsiveness and voice volume improved on a daily basis. He was transported to a local medical facility near his home for rehabilitation. He was living independently 2 months after the incident; however, he continued to experience mild recent memory disturbance.
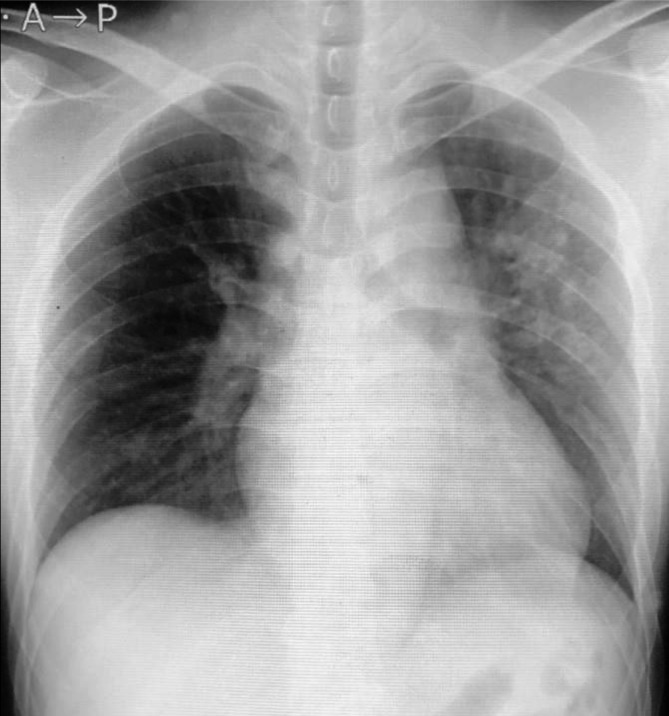
Figure 1.
Chest radiograph obtained on arrival showing an increased opacity in the left dominant lung field.
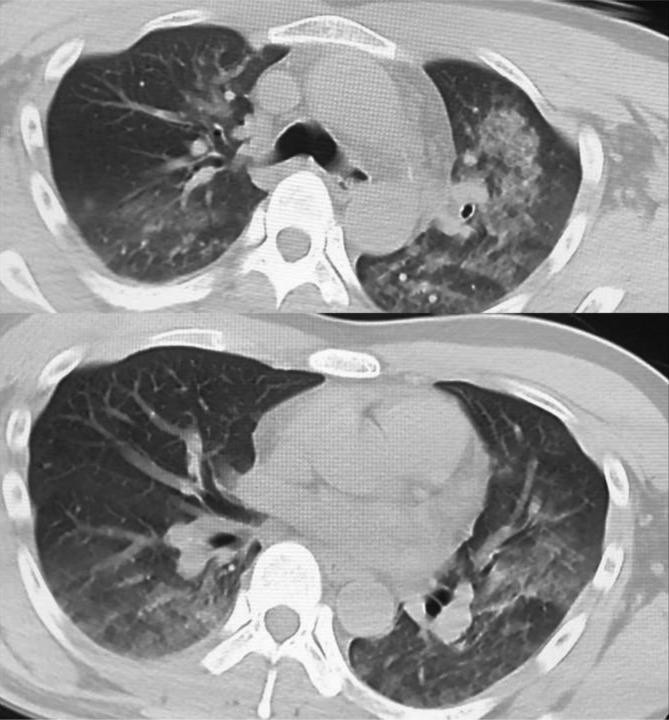
Figure 2.
Chest computed tomography (CT) scan obtained on arrival showing a ground-glass appearance in the bilateral lung fields, suggesting lung edema.
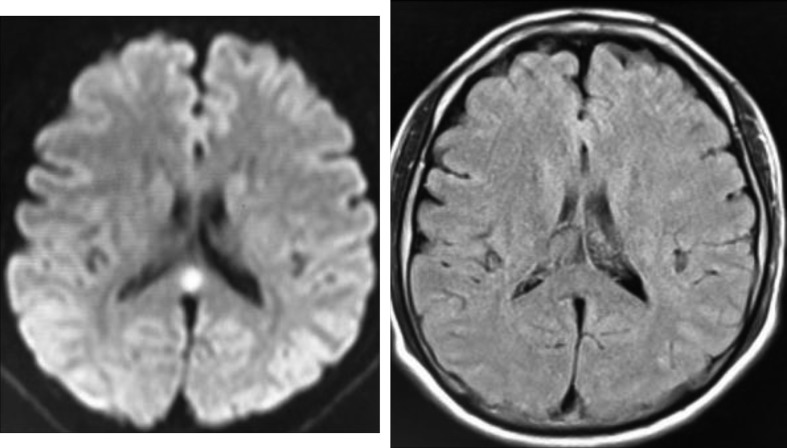
Figure 3.
Brain magnetic resonance imaging (MRI) scan obtained on arrival showing a high-intensity signal in the splenium on a diffusion-weighted image. However, fluid-attenuated inversion recovery image shows negative findings.
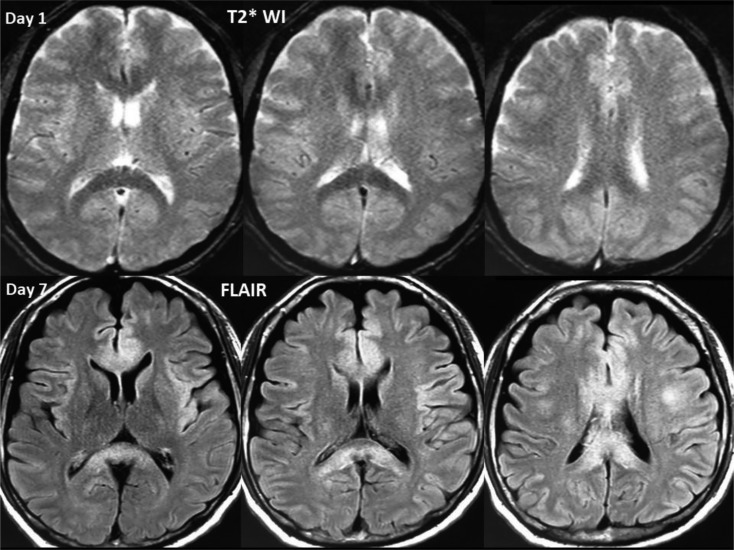
Figure 4.
T2*-weighted image (T2*WI) obtained on arrival and fluid-attenuated inversion recovery (FLAIR) image obtained on day 7. T2*WI image shows an area of relatively low intensity in the corpus callosum. This area shows a high-intensity signal on FLAIR image obtained on day 7, suggesting delayed microbleed-induced edema.
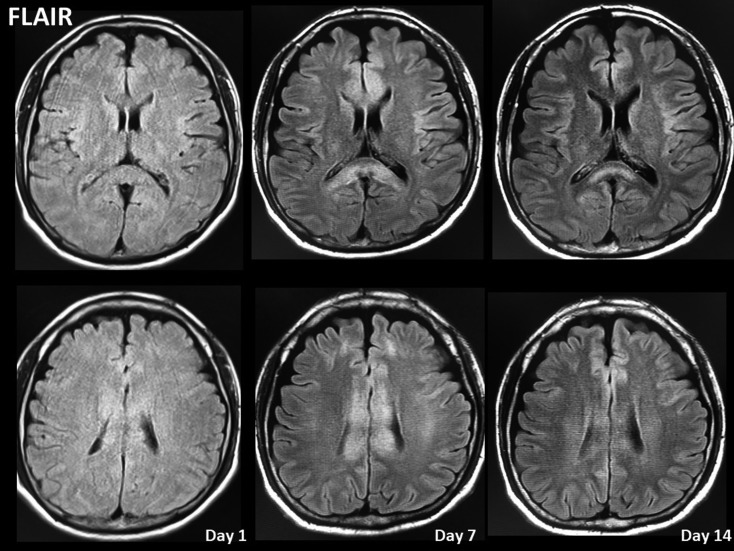
Figure 5.
Time course of magnetic resonance imaging (MRI) scans obtained in the present case. MRI scan showing delayed corpus callosum lesions on fluid-attenuated inversion recovery image despite the absence of high-intensity signal in this area on diffusion-weighted image (DWI) obtained on arrival.
Discussion
The patient described in this case report showed AMS following HAPE and HACE. This case is unique with respect to the time course of development of neurological symptoms and MRI findings. Peculiar speech abnormalities have been described in patients with corpus callosum disturbances5). However, to date, delayed MRI lesions have not been reported in cases of HACE.
A previous study reported HACE in 9 consecutive patients (men aged 18 to 35 years) after being evacuated from high-altitude locations. Notably, 8 of the 9 men developed concomitant pulmonary edema6). T2WI showed an intense signal in a few areas of the white matter, particularly in the splenium of the corpus callosum, and no gray matter abnormalities were identified in 7 of the senile patients. Control subjects showed no such abnormalities. All patients recovered completely; in the 4 patients in whom follow-up MRI data were available, we observed complete resolution of changes. Thus, it can be inferred that MRI changes are not so rare among patients with high altitude-induced neurological disturbances. The patient described in this case report showed delayed lesions in the body of the corpus callosum. Initially, even DWI failed to detect signal changes in the body of the corpus callosum.
The delay in lesion development could perhaps be attributed to vasospasm. Vasospasm of the anterior and/or posterior callosal artery can cause delayed ischemic lesions after subarachnoid bleeding7). However, this patient did not show such lesions. Another possibility is that the delayed lesion represented a HACE-induced microbleed (MH)8,9,10). Significant MH was observed after reoxygenation in an animal model of hypoxic cerebral edema11). The increased oxidative stress associated with rapid oxygenation may have contributed, at least in part, to these findings. In the present case, T2*WI on arrival showed a relatively low-intensity signal in the corpus callosum compared with deep white matter signals. Subsequent fluid-attenuated inversion recovery images revealed delayed signal changes. Notably, MH-induced edema might have additionally contributed to the increase in the MRI lesions that occurred during recovery in the present case.
T2*WI performed on day 1 in the present case revealed a “honeycomb-like” texture throughout large areas of the brain. This appearance and distribution of the larger “holes” are findings compatible with MH observed in patients with HACE9). Notably, MH persists as a footprint of HACE. Therefore, additional high-resolution susceptibility-weighted MRI after recovery may clearly reveal MH9) and resolve the uncertainty associated with T2*WI findings. Unfortunately, we were unable to perform such imaging because the patient was transferred to a distant local hospital.
Conclusion
The patient described in this case report showed unique delayed signal changes in the body of the corpus callosum, possibly secondary to MH-induced edema.
Author Contributions
All authors have made substantial contributions to the study and endorse the data and conclusions.
Financial/Material Support
This manuscript received financial support from the Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities, 2015-2019. (The constitution of a total researching system for comprehensive disaster and medical management, corresponding to wide-scale disasters.)
Informed Consent
The patient’s informed consent was obtained for publication of this data.
Conflict of interest
We do not have any conflict of interest to declare.
References
- 1.Cogo A. The lung at high altitude. Multidiscip Respir Med 2011; 6: 14–15. doi: 10.1186/2049-6958-6-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev 2017; 26: 1–14. doi: 10.1183/16000617.0096-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey TC, Raichle ME, Winterborn MH. Effect of carbon dioxide in acute mountain sickness: a rediscovery. Lancet 1988; 2: 639–641. doi: 10.1016/S0140-6736(88)90465-5 [DOI] [PubMed] [Google Scholar]
- 4.Bärtsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med 2013; 368: 2294–2302. doi: 10.1056/NEJMcp1214870 [DOI] [PubMed] [Google Scholar]
- 5.Saba S, Blum S. Aphasia due to isolated infarction of the corpus callosum. BMJ Case Rep 2014; 2014: bcr2014204316. doi: 10.1136/bcr-2014-204316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackett PH, Yarnell PR, Hill R. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA 1998; 280: 1920–1925. doi: 10.1001/jama.280.22.1920 [DOI] [PubMed] [Google Scholar]
- 7.Dinc N, Lescher S, Quick-Weller J. Outcome, prognostic factors, and follow-up results after subarachnoid hemorrhage from pericallosal artery aneurysms. World Neurosurg 2017; 99: 566–571. doi: 10.1016/j.wneu.2016.12.079 [DOI] [PubMed] [Google Scholar]
- 8.Pichler Hefti J, Hoigné-Perret P, Kottke R. Extensive microhemorrhages of the cerebellar peduncles after high-altitude cerebral edema. High Alt Med Biol 2017; 18: 182–184. doi: 10.1089/ham.2016.0103 [DOI] [PubMed] [Google Scholar]
- 9.Schommer K, Kallenberg K, Lutz K. Hemosiderin deposition in the brain as footprint of high-altitude cerebral edema. Neurology 2013; 81: 1776–1779. doi: 10.1212/01.wnl.0000435563.84986.78 [DOI] [PubMed] [Google Scholar]
- 10.Kallenberg K, Dehnert C, Dörfler A. Microhemorrhages in nonfatal high-altitude cerebral edema. J Cereb Blood Flow Metab 2008; 28: 1635–1642. doi: 10.1038/jcbfm.2008.55 [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann A, Kunze R, Helluy X. High-field MRI reveals a drastic increase of hypoxia-induced microhemorrhages upon tissue reoxygenation in the mouse brain with strong predominance in the olfactory bulb. PLoS One 2016; 11: e0148441. doi: 10.1371/journal.pone.0148441 [DOI] [PMC free article] [PubMed] [Google Scholar]