Abstract
Purpose
We aimed to find the possible key targets of Yougui pill and Buzhong Yiqi decoction for the treatment of sexual dysfunction.
Materials and Methods
The composition of Yougui pill combined with Buzhong Yiqi decoction was obtained, and its effective components of medicine were screened using ADME; the component target proteins were predicted and screened based on the TCMSP and BATMAN databases. Target proteins were cross-validated using the CTD database. We performed gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses for target proteins using the Cytoscape plugin ClueGO + CluePedia and the R package clusterProfiler, respectively. Subsequently, protein-protein interaction (PPI) analyses were conducted using the STRING database. Finally, a pharmacological network was constructed.
Results
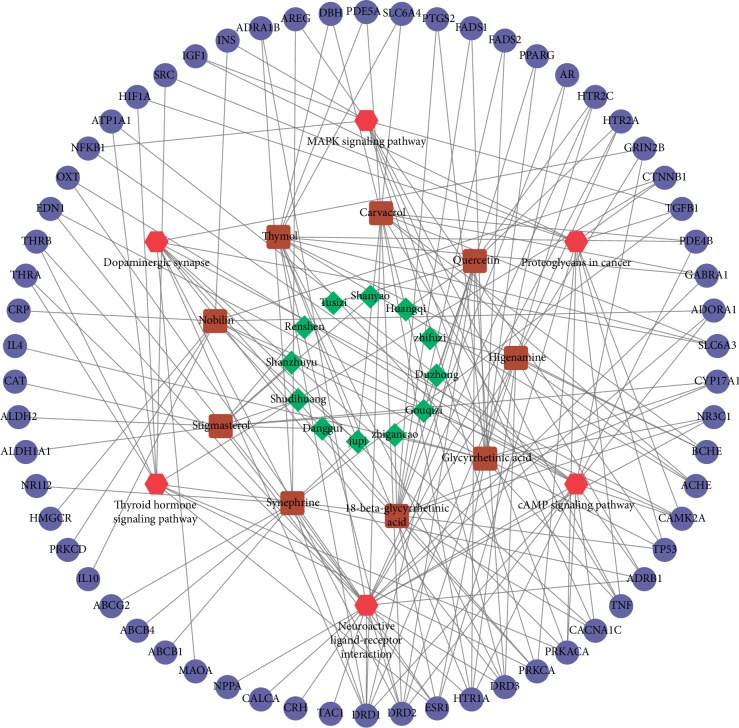
The pharmacological network contained 89 nodes and 176 relation pairs. Among these nodes, there were 12 for herbal medicines (orange peel, licorice, Eucommia, Aconite, Astragalus, Chinese wolfberry, yam, dodder seed, ginseng, Cornus officinalis, Rehmannia, and Angelica), 9 for chemical components (18-beta-glycyrrhetinic acid, carvacrol, glycyrrhetinic acid, higenamine, nobilin, quercetin, stigmasterol, synephrine, and thymol), 62 for target proteins (e.g., NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53), and 6 for pathways (MAPK signaling pathway, proteoglycans in cancer, dopaminergic synapse, thyroid hormone signaling pathway, cAMP signaling pathway, and neuroactive ligand-receptor interaction).
Conclusion
NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53 may be important targets for the key active elements in the decoction combining Yougui pill and Buzhong Yiqi. Furthermore, these target proteins are relevant to the treatment of sexual dysfunction, probably via pathways associated with cancer and signal transduction.
1. Introduction
Sexual dysfunction is difficulty experienced by a couple or an individual during any stage of a normal sexual activity [1]. It is reported that the sexual dysfunction included orgasm disorders, arousal disorders, and interest or desire disorders [2]. The two most common male sexual dysfunctions are premature ejaculation and erectile dysfunction [3]. Erectile dysfunction is defined as a persistent inability to attain or maintain an erection sufficient to permit satisfactory sexual performance [4]. Premature ejaculation is ejaculation earlier than desired and with minimal stimulation [5]. In both men and women, factors including diabetes mellitus, age, general health, cardiovascular disease, other chronic health problems, and urinary tract infections play significant roles in the development of sexual dysfunction [2]. About 5% to 20% of men suffer from moderate to severe erectile dysfunction [6], and the prevalence rate of premature ejaculation is 20% to 30% [7]. Sexual dysfunction has severe adverse effects on patient's quality of life, such as marital discord, loss of self-esteem and self-confidence, anxiety, and poor self-image [8], and therefore, effective clinical treatments for sexual dysfunction are necessary.
Yougui pill and Buzhong Yiqi are important traditional Chinese medicines (TCMs). Yougui pill decoction consists of Rehmannia root, dogwood fruit, yam rhizome, Chinese wolfberry, antler glue, dodder seed, Eucommia bark, Angelica, cinnamon bark, and monkshood root. Buzhong Yiqi decoction is a tonic for the middle Jiao and replenishes and raises qi [9, 10]. It is composed of Astragalus membranaceus, Zhigancao, Codonopsis pilosula, largehead atractylodes rhizome, Angelica sinensis, rattletop, Radix bupleuri, and dried tangerine or orange peel. Previous studies reveal that the combination decoction of Yougui pill and Buzhong Yiqi can be used to effectively treat sexual dysfunction [11–13]. However, the molecular mechanism has remained unclear until now.
Network pharmacology is an integrated multidisciplinary concept, and it provides a novel network mode encompassing “multiple targets, multiple effects, complex diseases” based on polypharmacology and systems biology [14]. Gui-Biao et al. have proposed network pharmacology as a new approach to the research of Chinese herbal medicine [15]. The herbal formulas of TCM are multitarget and multicomponent therapeutics; consequently, network pharmacology methods are well suited to fundamental studies of the combination rules for multiple ingredients in these prescriptions [16]. The interactions between relevant targets and active compounds are also better understood by the use of these methods [17]. In the present study, firstly, the composition of Yougui pill combined with Buzhong Yiqi decoction was obtained. The effective components of medicine were screened, and component target proteins were predicted and screened. Then, target proteins were cross-validated, and the gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses for target proteins were conducted. After that, protein-protein interaction (PPI) and pharmacological networks were constructed. We aimed to find the possible key targets of Yougui pill and Buzhong Yiqi decoction for the treatment of sexual dysfunction.
2. Materials and Methods
2.1. Composition of Traditional Chinese Medicine Preparation
The composition of the combined Yougui pill and Buzhong Yiqi decoction was as follows: Rehmannia, 24 g; yam, 30 g; Cornus officinalis, 15 g; Chinese wolfberry, 9 g; dodder, 12 g; deer horn gum, 12 g; Eucommia, 12 g; Cinnamomum cassia, 6 g; Angelica, 9 g; Aconite, 6 g; Astragalus membranaceus, 18 g; licorice, 9 g; ginseng, 6 g; orange peel, 6 g; Morinda, 15 g; Atractylodes, 9 g; and Xianling spleen, 15 g.
The TCMSP database (http://lsp.nwu.edu.cn/browse.php?qc=herbs) [18] was used to obtain composition information for these ingredients, including number of components, molecule name, molecular weight, fatty water partition coefficient, hydrogen bond donor acceptor number, oral bioavailability (OB), intestinal epithelial permeability, blood-brain barrier (BBB), drug-likeness (DL), and drug half-life (HL).
In addition, TCMID database (http://183.129.215.33/tcmid/search/) was used to search relevant information of that was not included in the TCMSP database.
2.2. Screening of Effective Components of Medicine
Screening for possible small drug molecules within the combined decoction was performed, based on absorption, distribution, metabolism, and excretion (ADME) parameters [19, 20] from the TCMSP database, where available, and from the TCMID database if not. The ADME parameters used were OB, DL, and drug HL, which were all predicted values. The recommended screening and categorization thresholds as used for TCMSP were as follows: OB: ≥30%; DL ≥ 0.18; BBB: <−0.3 is nonpenetrating (BBB-), from −0.3 to + 0.3 represents moderate penetrating (BBB±), and >0.3 represents strong penetrating (BBB+); HL : drug half-life ≤ 4 hours: the fast-elimination group, between 4 and 8 hours are the midelimination group, and ≥8 hours are the slow-elimination group.
Screening of medicine components was based on conventional parameters, and the threshold values were OB ≥ 40% and DL ≥ 0.2.
When there was no small molecule information of herbal chemistry in the TCMSP database, the information in the TCMID database was applied.
2.3. Prediction and Screening of Component Target Proteins
The TCMSP and BATMAN (http://bionet.ncpsb.org/batman-tcm/) [21] databases were used to predict the target proteins for our small molecule candidates. BATMAN uses similarity-based methods to predict the potential targets of traditional Chinese medicine components. For our study, we obtained targets from the DrugBank, KEGG, and TTD databases, and BATMAN ranked these target proteins according to interactions between potential targets and similarity of known targets, based on likelihood scores from high to low. The top 50 target proteins with scores >20 were selected.
2.4. Cross-Validation of Target Proteins
The Mount Desert Island Biological Laboratory publishes its Comparative Toxicogenomics Database (CTD) (http://ctdbase.org/, updated 2018) [22] to promote understanding of how environmental risks affect human health. In brief, it provides information on chemical-gene/protein interactions and chemical-disease and gene-disease relationships, to help those investigating mechanisms of diseases due to environmental factors.
In order to search the genes related to sexual dysfunction in the CTD database, the “sexual dysfunction” was used as key words, and ranking was conducted based on the inference score [23, 24]. Target proteins with scores >40, which were also target proteins predicted in the previous step, were identified in order to narrow the range of target proteins. Inference scores were calculated using the logarithmic transformation products of two common neighborhood statistics and then used to evaluate the functional correlation between proteins in protein-protein interaction (PPI) networks.
2.5. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analyses
GO functional analysis was performed using the Cytoscape plugins ClueGO + CluePedia [25]. The relevant biological process was selected, and the threshold value was set at P.adjust ≤0.01. Due to the large network, global option GO levels 1–4 were selected, and a functional network was constructed using Cytoscape. The GO tree, being a directed acyclic graph, is a complex structure. To accommodate this, the ClueGO plug-in algorithm enables division of GO items into several levels. When using data without a hierarchy (KEGG, BioCarta), its level is specified as −1. At the first GO levels (1–3), GO data is very common, including many related genes, providing general biological information. GO levels 9–14 operate with specific terms, which have few related genes but a large amount of information, with a higher research value. The kappa coefficient is used for consistency testing or the measurement of classification accuracy; it is calculated based on a confusion matrix [26]. In the ClueGO plugin, the kappa coefficient shows the relationship between GO terms based on overlapping genes. The higher the kappa coefficient, the stronger the term association strength.
KEGG pathway enrichment analysis for target proteins was conducted based on the R package clusterProfiler, with a threshold value of P ≤ 0.01.
2.6. Protein-Protein Interaction (PPI) Analysis
PPI analysis was performed using the STRING database (version: 10.0, http://www.string-db.org/) [27]. A required confidence level (combined score) > 0.9 was selected as the threshold value, and the relevant tsv format files were downloaded. Cytoscape software was applied to construct the network.
2.7. Construction of Pharmacological Network
Based on the herbal medicine-component-target proteins-pathway data generated by previous steps, a pharmacological network was constructed using Cytoscape. The network showed the pathway regulation mechanisms of related target proteins found for important components in the decoction.
3. Results
3.1. Composition and Component Screening
The number of chemical constituents identified for each herbal medicine ingredient was as follows: Rehmannia, 76; yam, 71; Cornus officinalis, 226; Chinese wolfberry, 118; dodder, 29; deer horn gum, 2; Eucommia, 147; Cinnamomum cassia, 100; Angelica, 125; Aconite, 5; Astragalus, 87; licorice, 17; ginseng, 190; orange peel, 109; Morinda, 174; Atractylodes, 55; and Xianling spleen, 4. After screening, herbal components of significance were identified (Table 1).
Table 1.
Significant herbal components after screening.
| Ingredients | Before (number) | After (number) | Source |
|---|---|---|---|
| Rehmanniae radix preparata | 76 | 1 | TCMSP |
| Rhizoma dioscoreae | 71 | 9 | TCMSP |
| Cornus officinalis | 226 | 8 | TCMSP |
| Lycii fructus | 188 | 21 | TCMSP |
| Semen cuscutae | 29 | 7 | TCMSP |
| Pulvis cornu cervi | 2 | 2 | TCMID |
| Eucommia cortex | 147 | 20 | TCMSP |
| Cinnamomum cassia | 100 | 0 | TCMSP |
| Angelica sinensis radix | 125 | 1 | TCMSP |
| Radix Aconiti lateralis praeparata | 5 | 5 | TCMID |
| Hedysarum multijugum Maxim | 87 | 15 | TCMSP |
| Radix Glycyrrhizae preparata | 17 | 17 | TCMID |
| Panax ginseng | 190 | 13 | TCMSP |
| Citrus reticulata | 109 | 109 | TCMID |
| Morinda officinalis radix | 174 | 12 | TCMSP |
| Atractylodes macrocephala Koidz | 55 | 4 | TCMSP |
| Herba epimedii | 4 | 4 | TCMID |
| Total | 1605 | 248 |
3.2. Prediction and Screening of Component Target Proteins and Cross-Validation
A total of 15 herbal medicine ingredients and 110 important components with target records were obtained after completion of predictions using the BATMAN online tool. The predicted targets of the first 50 for each component and the list of targets with scores >20 were obtained. In total, we obtained 13 herbal medicine ingredients and 64 important components with 1747 targets (containing repetition, see Supplementary ).
These targets were compared with our results for genes relevant to sexual dysfunction with an inference score >40 in the CTD database. We obtained 73 intersection-related targets (Figure 1; Supplementary ).
Figure 1.
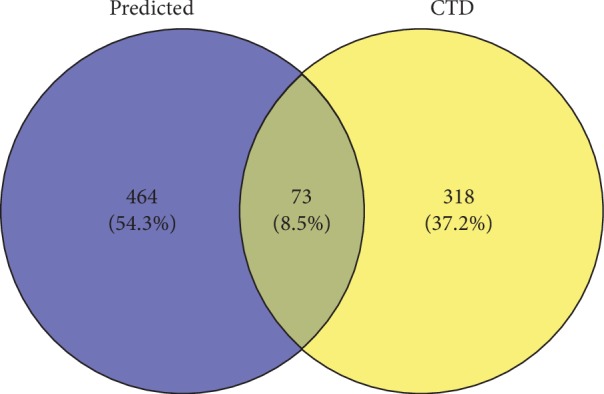
Venn diagrams for cross-validation targets.
3.3. GO and KEGG Pathway Analyses
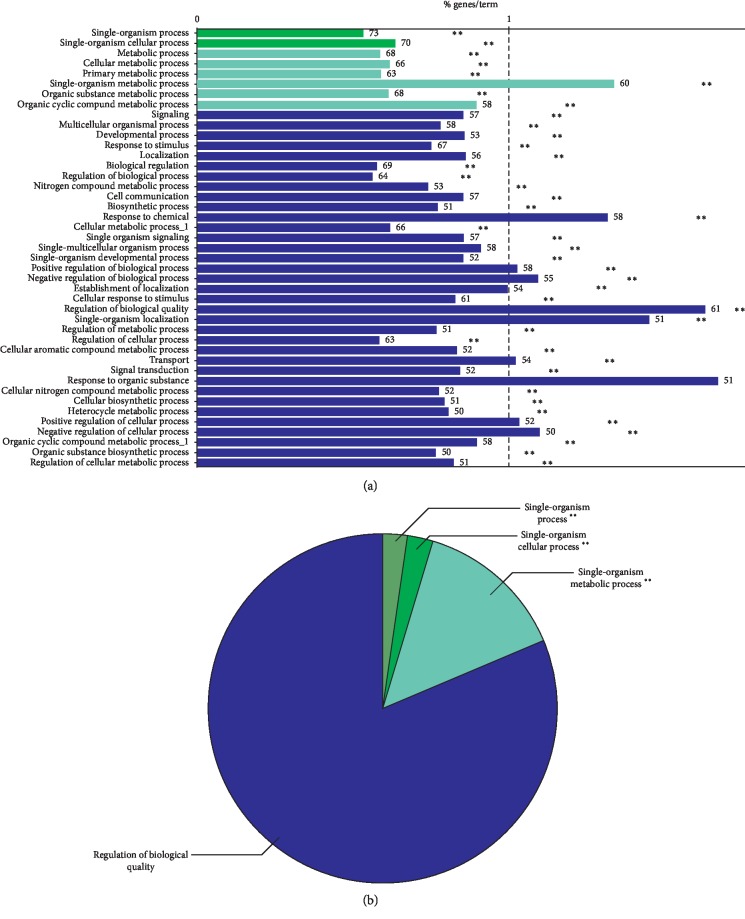
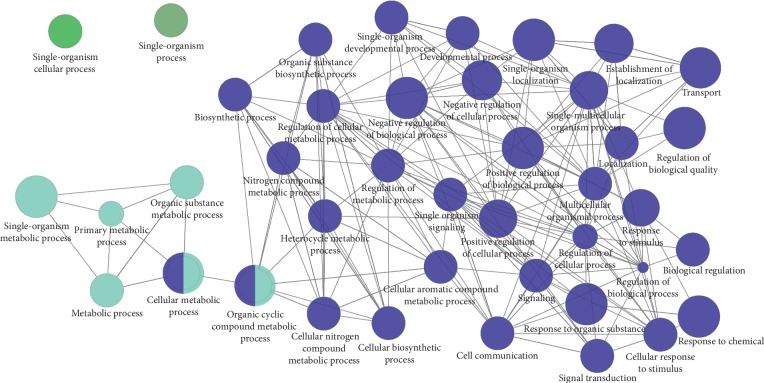
GO functional analysis showed that a total of 43 GO function items (2 repeats) were enriched (Figure 2(a)), and that the GO function was divided into 4 categories based on the kappa coefficient (Figure 2(b)). Moreover, the GO functional network indicated that the 73 target proteins were enriched in 43 biological processes and classified as either single-organism, single-organism cellular, or single-organism metabolic processes, or regulation of biological quality (Figure 3).
Figure 2.
The results of gene ontology (GO) functional analyses: (a) histogram; (b) pie chart. ∗∗ Produced by the analysis software and show no significance.
Figure 3.
Gene ontology (GO) functional network for 73 target proteins. Nodes: GO terms; bigger nodes indicated smaller P values; the two-point line represents the correlation between functions, and the larger the kappa coefficient, the thicker the line.
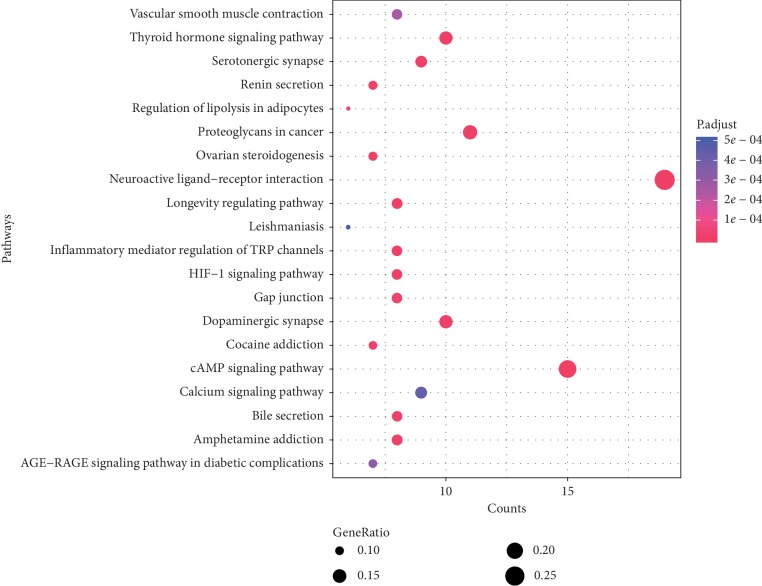
After pathway analysis using clusterProfiler, a total of 66 pathways were obtained. The top 20 significantly enriched pathways, such as the cAMP signaling pathway, neuroactive ligand-receptor interaction, and proteoglycans in cancer, are illustrated in Figure 4.
Figure 4.
The top 20 pathways. The longitudinal axis: the pathway name; the transverse axis: the number of enriched genes; the size of the dot represents the proportion of the number of enriched genes to the total number of genes, and the larger the proportion is, the larger the dot is; the redder the dot color, the more significant the P value.
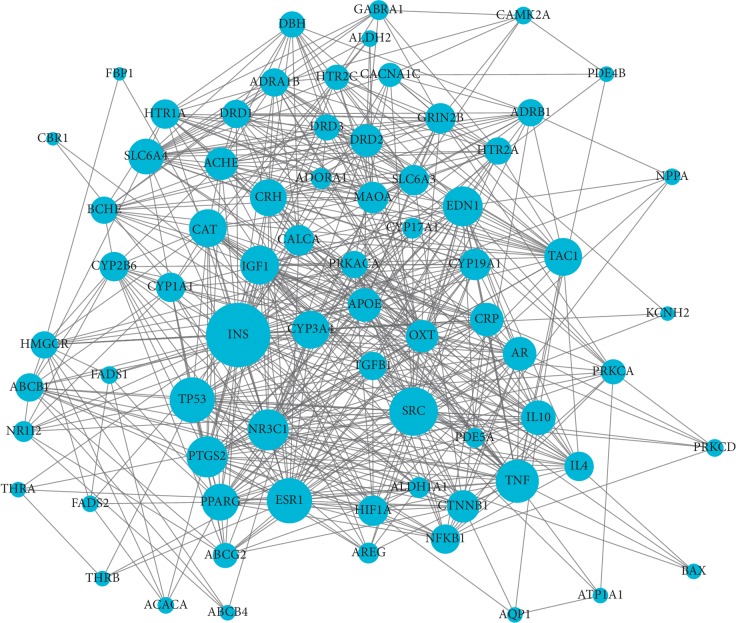
3.4. PPI Analysis
As shown in Figure 5, 73 proteins and 573 relation pairs were found in the PPI network. The top 10 proteins with higher degrees (Supplementary ) were INS (degree = 50), SRC (degree = 35), TP53 (degree = 32), ESR1 (degree = 32), TNF (degree = 30), PTGS2 (degree = 28), NR3C1 (degree = 28), EDN1 (degree = 27), IGF1 (degree = 26), and CAT (degree = 25); these are potential hub proteins.
Figure 5.
The protein-protein interaction (PPI) network. Dots: target proteins; lines: interactions between proteins; the size of the dot represents the correlation degree with other proteins, and the stronger the correlation degree is, the greater the dot is.
3.5. Construction of Pharmacological Network
Data were refined by retaining only small molecules with a degree ≥10 and pathways with enriched targets ≥10 and by removing nodes without corresponding herbal medicine-component-target proteins-pathways. The processed data were used to construct a pharmacological network. As shown in Figure 6, the network included 89 nodes and 176 relation pairs. Among these nodes, there were 12 herbal medicine nodes (orange peel, licorice, Eucommia, Aconite, Astragalus, Chinese wolfberry, yam, dodder seed, ginseng, Cornus officinalis, Rehmannia, and Angelica), 9 chemical component nodes (18-beta-glycyrrhetinic acid, carvacrol, glycyrrhetinic acid, higenamine, nobilin, quercetin, stigmasterol, synephrine, and thymol), 62 target protein nodes (e.g., NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53), and 6 pathway nodes (MAPK signaling pathway, proteoglycans in cancer, dopaminergic synapse, thyroid hormone signaling pathway, cAMP signaling pathway, and neuroactive ligand-receptor interaction).
Figure 6.
The pharmacological network. Green rhombus: herbal medicine; brown square: components; blue dots: targets; red hexagon: pathways.
4. Discussion
TCM does not match the chemical drug paradigm of a single component with a single target. It may have a weak effect on a single target, but it can inhibit an entire pathological process through network interaction, playing a unique therapeutic role in maintaining the balance of the body [28]. In this study, following network pharmacology analyses, we identified 12 key herbs (orange peel, licorice, Eucommia, Aconite, Astragalus, Chinese wolfberry, yam, dodder seed, ginseng, Cornus officinalis, Rehmannia, and Angelica) which corresponded to 9 key components (18-beta-glycyrrhetinic acid, carvacrol, glycyrrhetinic acid, higenamine, nobilin, quercetin, stigmasterol, synephrine, and thymol). These 9 key components corresponded in turn to 62 target protein nodes, among which 7 nodes (NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53) belonged to hub proteins of the PPI network. Furthermore, the network included 6 pathways: the MAPK signaling pathway, proteoglycans in cancer, dopaminergic synapse activity, the thyroid hormone signaling pathway, the cAMP signaling pathway, and neuroactive ligand-receptor interaction.
The literature was reviewed for relevant activity of the 9 key components. In streptozotocin-induced diabetic rats, quercetin can ameliorate erectile dysfunction [29]. In the process of rat penis erection, quercetin can restore some function of the NO-cGMP pathway [30]. There are no previous studies directly reporting associations between the other 8 key components and sexual dysfunction. However, these 8 components are associated with inflammation. Kao et al. reported that 18-beta-glycyrrhetinic acid and glycyrrhizic acid inhibit inflammation via activation of the glucocorticoid receptor and PI3K/Akt/GSK3 beta-signaling [31]. Fachini-Queiroz et al. suggested that carvacrol and thymol had anti-inflammatory effects [32]. In rat brains with ischemic damage, use of higenamine can reduce inflammation [33]. De Mieri et al. indicated that germacranolide nobilin 1 also had anti-inflammatory effects [34]. In a guinea pig model of ovalbumin-induced asthma, stigmasterol regulated allergic airway inflammation [35]. Berghe et al. reported that synephrine derivatives could be regarded as anti-inflammatory agents [36]. Furthermore, inflammation plays a significant role in erectile dysfunction [37]. Thus, these 9 components are directly relevant components of the Yougui pill and Buzhong Yiqi decoction used to treat sexual dysfunction.
In the current study, NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53 were important target protein nodes for our 9 key components. NR3C1 has been reported as a significant downregulator of inflammation [38]. Several studies suggest that CAT plays significant roles in inflammation [39, 40]. Ham et al. indicated that gain-of-function mutations of TP53 could promote inflammation in glioblastoma [41]; low-grade inflammation plays an important role in the pathogenesis of vasculogenic erectile dysfunction [37]. Vignozzi et al. indicated that the damage done to the developing penis by hyperestrogenism involved estrogen receptors (of which ESR1 is one) [42]; estrogen could also mediate erectile dysfunction induced by metabolic syndrome. Schramek and Waldhauser [43] showed that prostaglandin E1 injection was effective for erectile dysfunction; PTGS2 may have a role here. In patients with erectile dysfunction, TNF-α levels in serum are increased; TNF-α levels are inversely related to sexual performance [44]. Insulin resistance is an independent predictor of erectile dysfunction and reducing insulin resistance may be useful for preventing erectile dysfunction [45]. In the diabetic rat, insulin treatment may operate to recover erectile function by restoring expression of a sex hormone receptor [46]. Therefore, NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53 may be important target proteins for the decoction of Yougui pill combined with Buzhong Yiqi as used to treat sexual dysfunction.
In addition, our data revealed that the 6 pathways (MAPK signaling pathway, proteoglycans in cancer, dopaminergic synapse, thyroid hormone signaling pathway, cAMP signaling pathway, and neuroactive ligand-receptor interaction) may be candidate key pathways involved in the progression of sexual dysfunction. The MAPK signaling pathway plays a role in some human disorders including neurodegenerative diseases and cancers [47]. Sexual dysfunction is a potential complication of some cancer therapy [48]. Steers indicated that signal transduction molecules might be effective methods for enhancing erectile function [49]. Central dopamine plays key roles in the control of sexual function [50]. We infer that the target proteins of our key components may be relevant to sexual dysfunction via pathways associated with cancer (MAPK signaling pathway and proteoglycans in cancer) and signal transduction (dopaminergic synapse, thyroid hormone signaling pathway, cAMP signaling pathway, and neuroactive ligand-receptor interaction).
In conclusion, NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53 may be important target proteins for the key components of the decoction containing Yougui pill and Buzhong Yiqi. Furthermore, the nine key chemical components identified in this study targeted proteins including NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53, affecting the MAPK signaling pathway, proteoglycans in cancer, dopaminergic synapse activity, the thyroid hormone signaling pathway, the cAMP signaling pathway, and neuroactive ligand-receptor interaction. These pathways are potentially highly relevant to sexual dysfunction. However, these relationships will need to be substantiated by further research.
Acknowledgments
This study was funded by Shanghai Key Medical Specialty (Program) (Grant no. ZK2019A03). The Scientific Research Project was funded by The Fifth People's Hospital of Shanghai, Fudan University (Grant no. 2019WYZT02).
Data Availability
All the data supporting the results reported in the article can be found within the manuscript.
Additional Points
Highlights. (1) NR3C1, ESR1, PTGS2, CAT, TNF, INS, and TP53 may be important target proteins. (2) Nine chemical components were obtained from Yougui pill and Buzhong Yiqi decoction. (3) Six pathways were critical for mechanisms of Yougui pill and Buzhong Yiqi decoction.
Disclosure
Yangyun Wang and Wandong Yu should be regarded as the co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Supplementary Table 1: the 73 intersection-related targets. Supplementary Table 2: the top 10 proteins with higher degrees. Supplementary file 1: 13 herbal medicine and 64 important components with 1747 targets.
References
- 1.James D. Abnormal psychology. AJN the American Journal of Nursing. 2014;48(4620):p. 219. [Google Scholar]
- 2.McCabe M. P., Sharlip I. D., Lewis R., et al. Risk factors for sexual dysfunction among women and men: a consensus statement from the Fourth International Consultation on Sexual Medicine 2015. The Journal of Sexual Medicine. 2016;13(2):153–167. doi: 10.1016/j.jsxm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Hatzimouratidis K., Amar E., Eardley I., et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. European Urology. 2010;57(5):804–814. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Basson R., Wierman M. E., Van Lankveld J., Brotto L. Reports: summary of the recommendations on sexual dysfunctions in women. The Journal of Sexual Medicine. 2010;7(1):314–326. doi: 10.1111/j.1743-6109.2009.01617.x. [DOI] [PubMed] [Google Scholar]
- 5.Rowland D., Mcmahon C. G., Abdo C., et al. Disorders of orgasm and ejaculation in men. Journal of Sexual Medicine. 2010;1(1):58–65. doi: 10.1111/j.1743-6109.2010.01782.x. [DOI] [PubMed] [Google Scholar]
- 6.Feldman H. A., Goldstein I., Hatzichristou D. G., Krane R. J., Mckinlay J. B. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. Journal of Urology. 1994;151(1):54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 7.Porst H., Montorsi F., Rosen R. C., Gaynor L., Grupe S., Alexander J. The premature ejaculation prevalence and attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. European Urology. 2007;51(3):816–824. doi: 10.1016/j.eururo.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein F. O., Shirani S., Wuerth D., Finkelstein S. H. Therapy insight: sexual dysfunction in patients with chronic kidney disease. Nature Clinical Practice Nephrology. 2007;3(4):200–207. doi: 10.1038/ncpneph0438. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Shergis J. L., Wu L., et al. A systematic review and meta-analysis of the herbal formula Buzhong Yiqi Tang for stable chronic obstructive pulmonary disease. Complementary Therapies in Medicine. 2016;29:94–108. doi: 10.1016/j.ctim.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Li E.-q., Zhao A.-b., Cao K.-j., Chen X.-y., Dai H.-y., Wu X.-l. Effects of liuwei dihuang decoction, Buzhong Yiqi decoction and compound danshen decoction on the marrow-suppressed mice. Chinese Journal of Experimental Traditional Medical Formulae. 2010;5 [Google Scholar]
- 11.Hongzhu L. Treatment of Impotence from Qi Deficiency and its Clinical Study. Guangzhou, China: Traditional Chinese Medicine University of Guangzhou; 2016. [Google Scholar]
- 12.Jianjun W. Treatment of 50 cases of male sexual dysfunction with Guipi decoction and Yougui Pill. The Latest Medical Information Abstracts in the World. 2015;13:146. [Google Scholar]
- 13.Jinxiang Z. Observation on the curative effect of Yougui Pill in the treatment of penile erectile dysfunction. Proceedings of the National Conference on Male Medicine of Integrated Traditional Chinese and Western Medicine; 2005. [Google Scholar]
- 14.Pan J.-H. New paradigm for drug discovery based on network pharmacology. Chinese Journal of New Drugs and Clinical Remedies. 2009;28:721–726. [Google Scholar]
- 15.Gui-Biao Z., Qing-Ya L., Qi-Long C., Shi-Bing S. Network pharmacology: a new approach for Chinese herbal medicine research. Evidence-Based Complementray and Alternative Medicine. 2013;2013(8):9. doi: 10.1155/2013/621423.621423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Zhang B., Jiang D., Wei Y., Zhang N. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae. BMC Bioinformatics. 2010;11(11) doi: 10.1186/1471-2105-11-s11-s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F., Guochun L., Yang Y., Shi L., Xu L., Yin L. A network pharmacology approach to determine active ingredients and rationality of herb combinations of modified-Simiaowan for treatment of gout. Journal of Ethnopharmacology. 2015;168:1–16. doi: 10.1016/j.jep.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Ru J., Peng L., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1) doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madden J. C. Challenges and Advances in Computational Chemistry and Physics. Berlin, Germany: Springer; 2010. In silico approaches for predicting Adme properties. [DOI] [Google Scholar]
- 20.Ertl P., Rohde B., Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. Journal of Medicinal Chemistry. 2000;43(20):3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z., Guo F., Wang Y., et al. BATMAN-TCM: a bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine. Scientific Reports. 2016;6(1):21146. doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allan Peter D., King B. L., Susan M., et al. The comparative toxicogenomics database: update 2011. Nucleic Acids Research. 2013;41(1):D1104–D1114. doi: 10.1093/nar/gkq813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barabási A.-L., Albert R. Emergence of scaling in random networks. Science. 1999;286(5439):509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- 24.Hua L., Shoudan L. Local network topology in human protein interaction data predicts functional association. PLos One. 2009;4(7):e6410. doi: 10.1371/journal.pone.0006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindea G., Mlecnik B., Hackl H., et al. ClueGO: a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da W. H., Sherman B. T., Tan Q., et al. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biology. 2007;8(9):R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damian S., Andrea F., Stefan W., et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Research. 2015;43(D1):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B., Wang X., Li S. An integrative platform of TCM network pharmacology and its application on a herbal formula, qing-Luo-yin. Evidence-Based Complementary and Alternative Medicine. 2013;2013:12. doi: 10.1155/2013/456747.456747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Wang Y., Yang Z., et al. Antioxidant treatment with quercetin ameliorates erectile dysfunction in streptozotocin-induced diabetic rats. Journal of Bioscience and Bioengineering. 2011;112(3):215–218. doi: 10.1016/j.jbiosc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Huang C., Liu S., et al. Effects of quercetin on intracavernous pressure and expression of nitrogen synthase isoforms in arterial erectile dysfunction rat model. International Journal of Clinical & Experimental Medicine. 2015;8(5):p. 7599. [PMC free article] [PubMed] [Google Scholar]
- 31.Kao T.-C., Shyu M.-H., Yen G.-C. Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. Journal of Agricultural and Food Chemistry. 2010;58(15):8623–8629. doi: 10.1021/jf101841r. [DOI] [PubMed] [Google Scholar]
- 32.Fachini-Queiroz F. C., Kummer R., Estevao-Silva C. F., et al. Effects of thymol and carvacrol, constituents of thymus vulgaris L. essential oil, on the inflammatory response. Evidence-Based Complementary and Alternative Medicine. 2012;2012:10. doi: 10.1155/2012/657026.657026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha Y. M., Kim M. Y., Park M. K., et al. Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. Apoptosis. 2012;17(5):463–474. doi: 10.1007/s10495-011-0688-8. [DOI] [PubMed] [Google Scholar]
- 34.De Mieri M., Kaiser M., Brun R., Thormann U., Imanidis G., Hamburger M. Anti-trypanosomal cadinanes synthesized by transannular cyclization of the natural sesquiterpene lactone nobilin. Bioorganic & Medicinal Chemistry. 2015;23(7):1521–1529. doi: 10.1016/j.bmc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Antwi A. O., Obiri D. D., Osafo N. Stigmasterol modulates allergic airway inflammation in Guinea pig model of ovalbumin-induced asthma. Mediators of Inflammation. 2017;2017:11. doi: 10.1155/2017/2953930.2953930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berghe W. V., De Bosscher K., Van Calenbergh S., Haegeman G., Lacey C. J. Synephrine derivatives useful as anti-inflammatory agents. 2011. Google Patents CA-2634359A1.
- 37.Vlachopoulos C., Rokkas K., Ioakeimidis N., Stefanadis C. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. European Urology. 2007;52(6):1590–1600. doi: 10.1016/j.eururo.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Farrell A. K., Slatcher R. B., Tobin E. T., et al. Socioeconomic status, family negative emotional climate, and anti-inflammatory gene expression among youth with asthma. Psychoneuroendocrinology. 2018;91:62–67. doi: 10.1016/j.psyneuen.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi X., Shi Z., Huang H., et al. Ability of recombinant human catalase to suppress inflammation of the murine lung induced by influenza A. Inflammation. 2014;37(3):809–817. doi: 10.1007/s10753-013-9800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howard M. D., Greineder C. F., Hood E. D., Muzykantov V. R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. Journal of Controlled Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ham S. W., Jeon H.-Y., Jin X., et al. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death & Differentiation. 2019;26(3):409–425. doi: 10.1038/s41418-018-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vignozzi L., Filippi S., Comeglio P., et al. Estrogen mediates metabolic syndrome-induced erectile dysfunction: a study in the rabbit. The Journal of Sexual Medicine. 2014;11(12):2890–2902. doi: 10.1111/jsm.12695. [DOI] [PubMed] [Google Scholar]
- 43.Schramek P., Waldhauser M. Dose-dependent effect and side-effect of prostaglandin E1 in erectile dysfunction. British Journal of Clinical Pharmacology. 1989;28(5):567–571. doi: 10.1111/j.1365-2125.1989.tb03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlachopoulos C., Aznaouridis K., Ioakeimidis N., et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. European Heart Journal. 2006;27(22):2640–2648. doi: 10.1093/eurheartj/ehl341. [DOI] [PubMed] [Google Scholar]
- 45.Russo G. I., Cimino S., Fragalà E., et al. Insulin resistance is an independent predictor of severe lower urinary tract symptoms and of erectile dysfunction: results from a cross-sectional study. The Journal of Sexual Medicine. 2014;11(8):2074–2082. doi: 10.1111/jsm.12587. [DOI] [PubMed] [Google Scholar]
- 46.Shirai M., Yamanaka M., Shiina H., et al. Androgen, estrogen, and progesterone receptor gene regulation during diabetic erectile dysfunction and insulin treatment. Urology. 2004;64(6):1244–1249. doi: 10.1016/j.urology.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 47.Kim E. K., Choi E. J. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Miles C., Candy B., Jones L., Williams R., Tookman A., King M. Interventions for sexual dysfunction following treatments for cancer. Cochrane Database of Systematic Reviews. 2007;17(4) doi: 10.1002/14651858.CD005540.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Steers W. D. Pharmacologic treatment of erectile dysfunction. Reviews in Urology. 2002;4(3):S17. [PMC free article] [PubMed] [Google Scholar]
- 50.Giuliano F., Allard J. Dopamine and male sexual function. European Urology. 2001;40(6):601–608. doi: 10.1159/000049844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: the 73 intersection-related targets. Supplementary Table 2: the top 10 proteins with higher degrees. Supplementary file 1: 13 herbal medicine and 64 important components with 1747 targets.
Data Availability Statement
All the data supporting the results reported in the article can be found within the manuscript.