Abstract
Aims
Postural orthostatic tachycardia syndrome (POTS) is a disorder of unknown aetiology characterized by orthostatic intolerance and tachycardia with diverse other symptoms, including neurocognitive deficits. Cerebral oximetry non-invasively measures cerebral tissue saturation (SctO2) and has been shown to be informative in syncope evaluation. We aimed to assess SctO2 in POTS patients and those with normal response to orthostatic provocation, relative to haemodynamic parameters and symptoms.
Methods and results
Thirty-four patients with POTS (29.1 ± 9.5 years; 26 females) and 34 age-/sex-matched controls with normal head-up tilt tests (HUTs) were included. SctO2 at rest and during HUT were compared between POTS and controls. The relation between SctO2, systolic blood pressure (SBP), and heart rate (HR) during HUT was linearly assessed. SctO2 values were related to dizziness or syncope during HUT. The minimum SctO2-value during HUT was lower (65.4 ± 5.6 vs. 68.2 ± 4.2%, P = 0.023) and changes in SctO2 from supine to minimum HUT value were more pronounced in POTS patients (−5.7 ± 2.9% vs. −4.3 ± 2.1%, P = 0.028). Decrease in SBP from supine to minimum HUT value (P = 0.004) and increase in HR from supine to HUT value at 3 min (P = 0.022) correlated with more pronounced SctO2 decrease in POTS but not controls. SctO2 did not predict syncope or dizziness during HUT.
Conclusion
Postural orthostatic tachycardia syndrome patients have lower cerebral tissue saturation during orthostatic provocation compared with those subjects having normal haemodynamic response to tilt. Orthostatic decrease in cerebral saturation only weakly correlates with HR increase and does not predict vasovagal reflex in POTS. Other hitherto unknown factors may affect cerebral tissue saturation in POTS.
Keywords: Postural orthostatic tachycardia syndrome , Cerebral oximetry , Head-up tilt , Orthostatic intolerance , Haemodynamic monitoring
What’s new?
Cerebral oximetry has been shown to be clinically informative in the evaluation of unknown syncope. In this study, we show that cerebral oximetry is feasible also in patients with postural orthostatic tachycardia syndrome (POTS).
Postural orthostatic tachycardia syndrome patients have lower cerebral tissue saturation during orthostatic provocation compared with those subjects having normal haemodynamic response to head-up tilt.
Orthostatic decrease in cerebral saturation only weakly correlates with heart rate increase and does not predict vasovagal reflex in POTS.
One or more hitherto unknown factors may be responsible for the lower cerebral tissue saturation that can be observed in POTS patients.
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a disorder of unknown origin characterized by orthostatic intolerance and increased heart rate (HR) of ≥30 b.p.m. during orthostasis in the absence of orthostatic hypotension.1 In addition to the orthostatic intolerance and tachycardia, patients with POTS experience several debilitating symptoms including light-headedness, nausea, blurred vision, fatigue, mental confusion (‘brain-fog’), chest pain, and gastrointestinal problems. Syncope may occur although pre-syncopal symptoms are more common. Several potential underlying mechanisms have been suggested for POTS including autonomic denervation, hypovolaemia, hyperadrenergic stimulation, and autoantibodies against adrenergic receptors.2,3
Light-headedness and neurocognitive deficits are among the most disabling symptoms experienced by POTS patients and have been assumed to be a result of cerebral hypoperfusion, despite maintenance of normal blood pressure.4 Head-up tilt test (HUT) has been used for more than half a century to study the physiological adaptations to changes in body position.5 Previous studies have analysed cerebral circulation during active standing or HUT in POTS patients, but findings have been inconsistent.6–10 Cerebral oximetry is a method that can be used to study cerebral circulation by measuring the cerebral tissue oxygen saturation (SctO2), which may, therefore, be regarded as assessing the cerebral consequences of a dysfunctional orthostatic response.11
A POTS is increasingly recognized. Not only is there individual suffering but also high societal costs.12 As many of the incapacitating POTS symptoms are hard to explain and may not be tachycardia related,3,12 cerebral oximetry may aid their understanding. We, thus, aimed to compare SctO2 between POTS and controls with normal haemodynamic response to drug-free tilt and to assess the relation between SctO2, haemodynamic parameters, and POTS symptoms.
Methods
Study population
The current study was based on the Syncope Study of unselected Population in Malmo (SYSTEMA) cohort, currently consisting of ∼2200 patients with syncope and orthostatic intolerance who were evaluated at Skåne University Hospital, Malmö, Sweden during 2008–18. The SYSTEMA cohort has previously been described in detail.13 In short, all patients underwent cardiovascular autonomic testing, including head-up tilt test with continuous haemodynamic monitoring (detailed below) and additional tests, including ambulatory electrocardiogram or 24-h blood pressure monitoring when clinically indicated. From 2013, cerebral oximetry has been performed in 354 patients of this cohort. Of these 354 patients, 34 patients were diagnosed with POTS according to Heart Rhythm Society criteria3 and all were adjudicated by a senior cardiologist with special expertise in POTS (A.F.). A one-to-one age-/sex-matched control group of 34 patients with normal HUT result (excluding nitroglycerine provocation) were also included from among the 354 patients undergoing cerebral oximetry. Patients with spontaneous vasovagal syncope (VVS) during HUT, with orthostatic hypotension, coronary artery disease, diabetes mellitus, or stroke were excluded from controls. A flowchart describing selection of the study subjects is shown in Figure 1. All patients gave written informed consent. The study was approved by the regional ethical review board in Lund (DNR 08/82, DNR 2015/224 and 2017/295) and all procedures were performed in accordance with the Helsinki Declaration.
Figure 1.
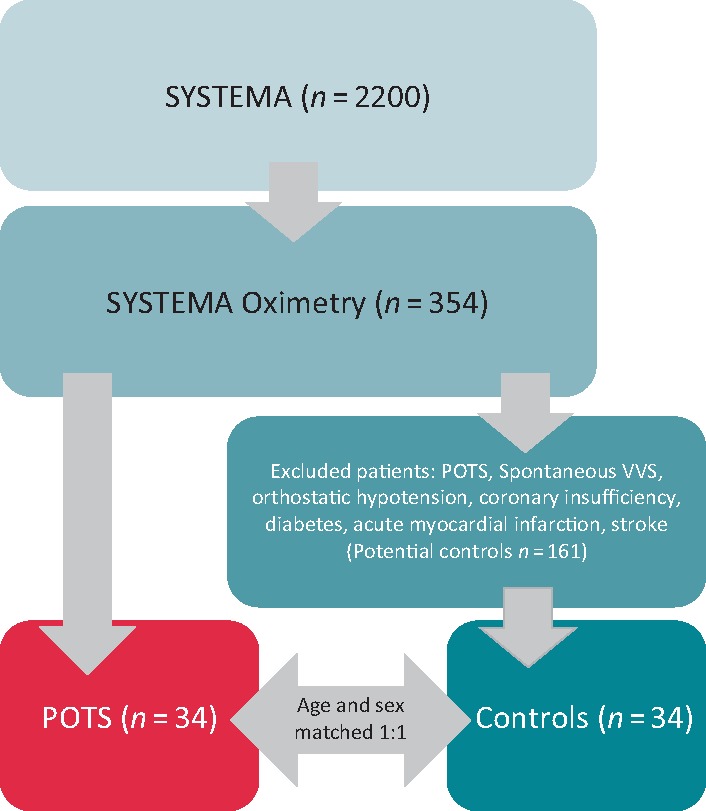
Flowchart, subject selection. The current study was based on a study cohort named SYSTEMA, including 2074 patients. Cerebral oximetry was performed on 354 of the cohort, from whom the POTS and control groups were selected. Thirty-four POTS patients were selected for the current study. A control group of 34 age- and sex-matched patients with negative head-up tilt were also included from SYSTEMA. POTS, postural orthostatic tachycardia syndrome; SctO2, cerebral tissue oxygen saturation; SYSTEMA, Syncope Study of unselected population in Malmo; VVS, vasovagal syncope.
Head-up tilt test
All patients were asked to abstain from their regular medications on the day of the examination. HUT tests were performed as previously described.14 After a supine rest of 15 min, patients were tilted head-up to 60°–70°. If syncope or pre-syncope with typical prodromal symptoms occurred, the test was considered positive and the patient was immediately tilted back to supine. Arterial blood pressure was continuously recorded using a photoplethysmographic device (Nexfin, BMEYE, Amsterdam, The Netherlands or Finapres Nova, Finapres Medical Systems, PH Enschede, The Netherlands), together with peripheral oxygen saturation (SPO2) and electrocardiogram. In this study, only the first 20 min of measurements were included as, afterwards, sublingual nitroglycerine was routinely administered and was, therefore, considered incompatible with the study aims.
Cerebral oximetry
Cerebral oximetry was measured with near-infrared spectroscopy (NIRS). Near-infrared spectroscopy is a non-invasive method yielding mixed blood oxygen saturation levels in cerebral tissue by determining the ratio of oxygenated haemoglobin to total haemoglobin at microvascular level. It reflects a proportional mix of arterial and venous blood in the outer regions of the frontal hemispheres. Normal range of SctO2 is stated to be 60–80%.15 SctO2 was assessed using the Fore-Sight absolute cerebral oximeter (CAS Medical Systems Inc., Branford, CT, USA), which was used in a previous study in the SYSTEMA cohort.14 As previously described,14 the Fore-Sight monitor has two sensors for bilateral monitoring and projects four precise wavelengths (690, 780, 805, and 850 nm) into the brain. Having multiple wavelengths permits compensation for scattering losses and interference from other light absorbers such as fluid and melanin. Absolute cerebral oximetry and haemodynamic parameters were measured simultaneously in the same file time synchronized.
Definition of included variables and statistical analyses
SctO2 values were collected in supine position, and at 1, 3, and 10 min of head-up tilt. The minimum SctO2 during the test (SctO2 min) was defined as the minimum saturation value at any time (prior to reflex activation) during the 20 min of passive HUT. SctO2 delta was calculated as the difference between SctO2 in supine position and minimum, 1, 3, and 10 min of HUT, respectively. SctO2 at the defined time points was compared between POTS and controls using Student’s independent samples t-test. SctO2 levels were also compared according to sex. Apart from the continuous variables, the proportion of patients with SctO2 <65%, indicating the lower limit of normal in subjects with normal HUT,14 was compared between POTS and controls, using Pearson’s χ2 test. Linear regression was applied to study the association between SctO2 and haemodynamics [systolic blood pressure (SBP) and HR] at the defined time points. Finally, SctO2 values at the different time points were compared according to occurrence of syncope/no syncope, spontaneous VVS/no spontaneous VVS, and dizziness/no dizziness, in the POTS group, using Student’s t-test. Data were analysed using SPSS software version 25 (SPSS, Chicago, IL, USA). A P-value of <0.05 was considered significant for all tests.
Results
Patient characteristics and HUT results
Mean age in POTS and control groups was 29.1 ± 9.5 years and 29.4 ± 9.0, respectively. Twenty-six patients were female and eight were male in each group. Twenty-seven POTS patients (79.5%) and 33 controls (97.1%) reported history of syncope (P = 0.024), whereas a history of orthostatic intolerance was reported by 29 POTS patients (85.3%) and 28 controls (82.4%), respectively (P = 0.742). One control was investigated due to recurrent pre-syncope but reported no episodes of manifest syncope. The clinical characteristics of POTS and controls are shown in Table 1.
Table 1.
Baseline characteristics, SctO2, and haemodynamic parameters in POTS and controls during head-up tilt test
| POTS (n = 34) | Control (n = 34) | P-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 29.1 ± 9.5 | 29.4 ± 9.0 | 0.898 |
| Proportion of women (%) | 76.5 | 76.5 | 1.000 |
| Resting HR (b.p.m.) | 79.6 ± 17.6 | 69.5 ± 12.5 | 0.010 |
| Resting SBP (mmHg) | 124.0 ± 17.3 | 126.8 ± 12.7 | 0.450 |
| History of syncope (%) | 79.4 | 97.1 | 0.024 |
| History of OI (%) | 85.3 | 82.4 | 0.742 |
| SctO2 | |||
| Supine (pp) | 71.1 ± 4.6 | 72.5 ± 3.4 | 0.150 |
| 1 min (pp) | 68.4 ± 5.9 | 70.4 ± 4.2 | 0.111 |
| 3 min (pp) | 67.8 ± 6.0a | 69.7 ± 4.2a | 0.139 |
| 10 min (pp) | 67.8 ± 5.5b | 69.4 ± 4.3a | 0.193 |
| Minimum (pp) | 65.4 ± 5.6 | 68.2 ± 4.2 | 0.023 |
| Delta (pp) | 5.7 ± 2.9 | 4.3 ± 2.1 | 0.028 |
| <0.65 1 min (%) | 29.4 | 8.8 | 0.031 |
| <0.65 3 min (%) | 30.3a | 9.1a | 0.030 |
| <0.65 10 min (%) | 21.2b | 9.1a | 0.170 |
| <0.65 minimum (%) | 35.3 | 23.5 | 0.287 |
| HR (b.p.m.) | |||
| Supine | 79.3 ± 17.7 | 69.5 ± 12.5 | 0.010 |
| 1 min | 105.1 ± 20.0 | 81.6 ± 15.5 | <0.001 |
| 3 min | 108.7 ± 16.3a | 82.2 ± 13.3a | <0.001 |
| 10 min | 108.7 ± 15.8b | 82.8 ± 13.2a | <0.001 |
| Max | 123.3 ± 20.2 | 86.4 ± 14.2 | <0.001 |
| SBP (mmHg) | |||
| Supine | 124.0 ±17.3 | 126.8 ± 12.7 | 0.450 |
| 1 min | 127.5 ± 20.5 | 133.6 ± 15.0 | 0.164 |
| 3 min | 125.4 ± 22.0a | 130.6 ± 15.5a | 0.270 |
| 10 min | 124.9 ± 21.5b | 124.9 ± 13.8a | 0.986 |
| Minimum | 115.6 ± 22.2 | 118.7 ± 11.7 | 0.479 |
| SPO2 (pp) | |||
| Supine | 99.0 ± 1.0 | 99.1 ± 1.0 | 0.781 |
| 1 min | 99.3 ± 1.0 | 99.0 ± 0.8 | 0.497 |
| 3 min | 99.2 ± 0.8 | 98.6 ± 1.2 | 0.137 |
| 10 min | 98.4 ± 1.3 | 97.8 ± 1.5 | 0.413 |
| Minimum | 97.5 ± 1.1 | 97.1 ± 1.2 | 0.467 |
SPO2 had been performed only on a minority of subjects: POTS: SPO2 supine, n = 15; SPO2 1 min, n = 15; SPO2 3 min, n = 16; SPO2 10 min, n = 13; SPO2 minimum, n = 15. Controls: SPO2 supine, n = 8; SPO2 1 min, n = 8; SPO2 3 min, n = 8; SPO2 10 min n = 7; SPO2 minimum, n = 8.
b.p.m., beats per minute; HR, heart rate; OI, orthostatic intolerance; POTS, postural orthostatic tachycardia syndrome; pp, percentage points; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation; SPO2, peripheral saturation.
Missing values due to syncope <10 min or inadequate SctO2 signal quality = 1.
Missing values due to syncope <10 min or inadequate SctO2 signal quality = 4.
Seven POTS patients experienced VVS during passive HUT and an additional eight POTS patients had VVS after nitroglycerine administration. By the study design, no control subject had VVS during passive HUT, however, after nitroglycerine administration 23 controls had VVS.
Changes in cerebral tissue oxygenation during head-up tilt test
Whereas no difference in SctO2 in the supine position was observed, the minimum SctO2 values, prior to vasovagal reflex activation, during HUT were lower among POTS patients compared with controls (P = 0.023). Accordingly, the difference between baseline SctO2 and minimum SctO2 (SctO2 delta min) was more pronounced in POTS compared with controls (P = 0.028). There were no significant differences in SctO2 values between POTS and controls at 1, 3, and 10 min of head-up tilt, respectively. However, the proportion of patients with SctO2 below 65% at 1 and 3 min was higher among POTS patients than controls (Table 1).
Among the POTS patients, SctO2 was lower in women compared with men at all-time points except for 10 min of head-up tilt. The difference was most pronounced at 3 min of head-up tilt (66.7% vs. 71.3%; P = 0.009). On the contrary, there were no differences in SctO2 in the control group (Supplementary material online, Table S1).
Changes in cerebral tissue oxygenation in relation to haemodynamic parameters during HUT
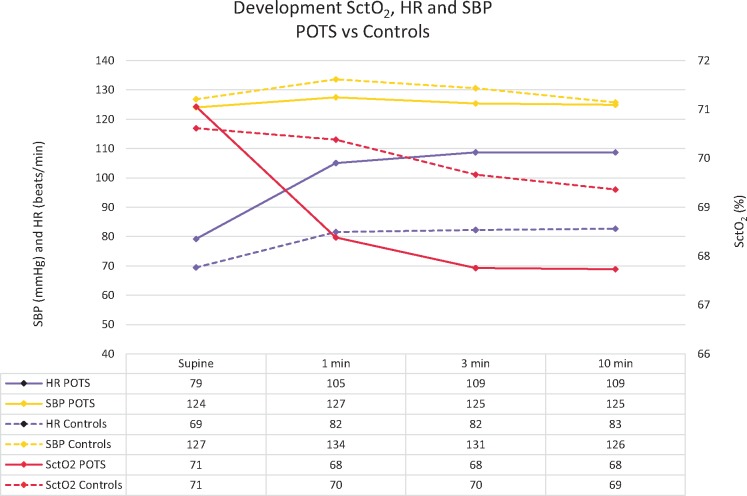
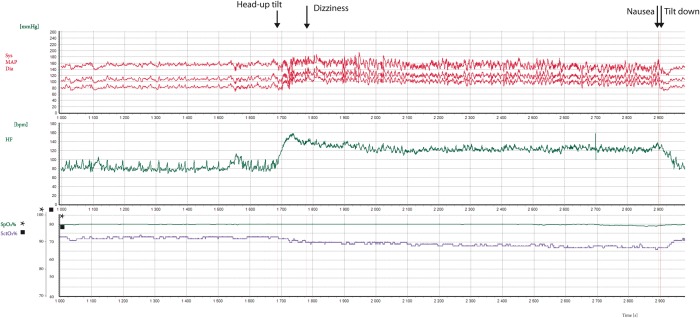
There were no associations between SBP or HR and SctO2 in the supine position or during HUT at 1, 3, or 10 min or at SctO2 min. The change in SctO2 in POTS patients during HUT was associated with the delta-SBP with more SBP decrease in parallel with a greater reduction in SctO2 from supine. There was also a correlation between delta-SctO2 in POTS patients and delta-HR at 3 min (baseline to 3 min), for which a greater increase in HR was associated with a greater decrease in SctO2 (Table 2). SctO2 was not correlated with SBP or HR in controls (Table 3). The changes in SctO2, SBP, and HR supine and during HUT in POTS and controls are summarized in Figure 2. Figure 3 shows an example of a POTS patient, in whom change in SctO2 is the reciprocal of change in HR during HUT.
Table 2.
The association of haemodynamic factors with SctO2 during head-up tilt test in the POTS group (n = 34)
| Dependent variables | Independent variables | B (% points) | P-value (age adjusted) |
|---|---|---|---|
| SctO2 supine | SBP supine | 0.0384 | 0.410 (0.337) |
| SctO2 1 min | SBP 1 min | 0.0657 | 0.191 (0.117) |
| SctO2 3 min | SBP 3 min | 0.0674a | 0.166 (0.095) |
| SctO2 10 min | SBP 10 min | 0.0285b | 0.561 (0.411) |
| SctO2 min | SBP min | 0.0603 | 0.176 (0.107) |
| SctO2 delta min | SBP delta min | 0.1170 | 0.004 (0.003) |
| SctO2 supine | HR supine | 0.0144 | 0.753 (0.722) |
| SctO2 1 min | HR 1 min | −0.0035 | 0.946 (0.958) |
| SctO2 3 min | HR 3 min | −0.0275a | 0.678 (0.694) |
| SctO2 10 min | HR 10 min | 0.0341b | 0.610 (0.645) |
| SctO2 min | HR max | 0.0305 | 0.623 (0.536) |
| SctO2 delta min | HR delta max | 0.0308 | 0.802 (0.809) |
| SctO2 delta 1 min | HR delta 1 min | 0.0360 | 0.221 (0.223) |
| SctO2 delta 3 min | HR delta 3 min | 0.0809a | 0.022 (0.022) |
| SctO2 delta 10 min | HR delta 10 min | −0.0166b | 0.726 (0.687) |
Units for SctO2 percentage points; SBP mmHg; HR b.p.m. SctO2 delta and SBP delta min were calculated as decreases (positive direction), whereas HR delta was calculated as an increase from supine position.
HR, heart rate; POTS, postural orthostatic tachycardia syndrome; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation.
Missing values = 1
Missing values = 4.
Table 3.
The association of haemodynamic factors with SctO2 during head-up tilt test in the control group (n = 34)
| Dependent variables | Independent variables | B (% points) | P-value (age adjusted) |
|---|---|---|---|
| SctO2 supine | SBP supine | 0.0137 | 0.771 (0.320) |
| SctO2 1 min | SBP 1 min | 0.0610 | 0.217 (0.094) |
| SctO2 3 min | SBP 3 min | 0.0525a | 0.280 (0.056) |
| SctO2 10 min | SBP 10 min | 0.0231a | 0.696 (0.225) |
| SctO2 min | SBP min | −0.0090 | 0.888 (0.822) |
| SctO2 delta min | SBP delta min | 0.0175 | 0.667 (0.649) |
| SctO2 supine | HR supine | −0.0475 | 0.316 (0.313) |
| SctO2 1 min | HR 1 min | −0.0257 | 0.594 (0.527) |
| SctO2 3 min | HR 3 min | −0.0060a | 0.916 (0.867) |
| SctO2 10 min | HR 10 min | −0.0002a | 0.997 (0.879) |
| SctO2 min | HR max | −0.0101 | 0.846 (0.761) |
| SctO2 delta min | HR delta max | 0.0322 | 0.404 (0.465) |
| SctO2 delta 1 min | HR delta 1 min | −0.0118 | 0.748 (0.678) |
| SctO2 delta 3 min | HR delta 3 min | 0.0074a | 0.899 (0.986) |
| SctO2 delta 10 min | HR delta 10 min | −0.0057a | 0.902 (0.766) |
Units for SctO2 percentage points; SBP mmHg; HR b.p.m. SctO2 delta and SBP delta min were calculated as decreases (positive direction), whereas HR delta was calculated as an increase from supine position.
HR, heart rate; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation.
Missing values = 1.
Figure 2.
Mean SctO2, SBP, and HR at rest and during HUT in POTS and controls. HR, heart rate; HUT, head-up tilt test; POTS, postural orthostatic tachycardia syndrome; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation.
Figure 3.
Example of a POTS patient during head-up tilt displaying changes in HR, blood pressure, and SctO2. Note the inverse correlation between HR and SctO2 in this POTS patient during head-up tilt. HR, heart rate; POTS, postural orthostatic tachycardia syndrome; SctO2, cerebral tissue oxygen saturation.
Changes in cerebral tissue oxygenation according to syncope or reported dizziness
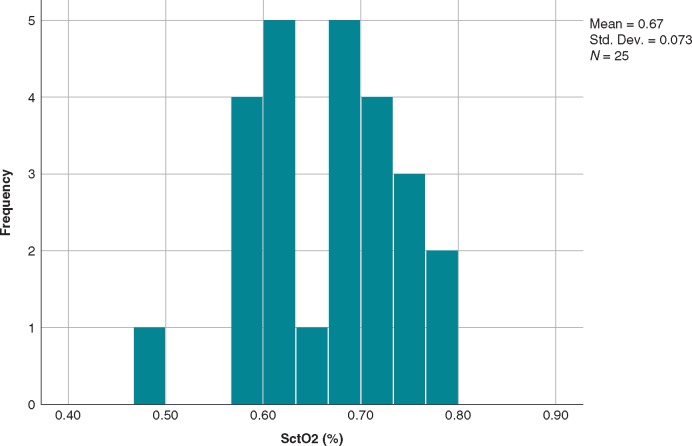
There were no differences in SctO2 between POTS patients who experienced syncope at any time during HUT (n = 15; 44%) and patients who did not (n = 19; 56%), these data include the post-nitroglycerine phase outcome, syncope, or no syncope, where appropriate (Table 4). Moreover, SctO2 did not differ in POTS patients during head-up tilt in terms of spontaneous VVS (n = 7; 21%) and no spontaneous VVS (n = 27; 79%) (Table 4). The SctO2 levels during syncope in the seven patients with spontaneous VVS ranged from 48% to 63%. Finally, there was no difference in SctO2 levels between the 25 (74%) POTS patients who reported dizziness during HUT and patients who did not (Table 4). The median SctO2 value when POTS patients-reported dizziness was 69% (min 48%, max 78%). No clear cut-off point was seen when patients-reported dizziness and the corresponding SctO2 at the time (Figure 4).
Table 4.
SctO2 in POTS patients during head-up tilt test according to syncope/no syncope, spontaneous vasovagal syncope/no spontaneous vasovagal syncope, and dizziness/no dizziness during the test
| Syncope (n = 15) | No syncope (n = 19) | P-value | |
|---|---|---|---|
| SctO2 supine | 71.3 ± 6.2 | 70.9 ± 2.8 | 0.817 |
| SctO2 1 min | 69.1 ± 7.5 | 67.8 ± 4.3 | 0.516 |
| SctO2 3 min | 68.5 ± 7.4 | 67.1 ± 4.6b | 0.506 |
| SctO2 10 min | 69.3 ± 6.0a | 66.7 ± 5.2b | 0.227 |
| SctO2 min | 65.7 ± 6.7 | 65.2 ± 4.7 | 0.772 |
| SctO2 delta | 5.6 ± 2.6 | 5.7 ± 3.2 | 0.843 |
|
| |||
| Spontaneous VVS (n = 7) | No spontaneous VVS (n = 27) | P-value | |
|
| |||
| SctO2 supine | 70.6 ± 4.7 | 71.2 ± 4.6 | 0.756 |
| SctO2 1 min | 69.1 ± 5.4 | 68.2 ± 6.1 | 0.707 |
| SctO2 3 min | 68.6 ± 6.3 | 67.5 ± 6.0b | 0.692 |
| SctO2 10 min | 70.0 ± 4.1a | 67.4 ± 5.7b | 0.389 |
| SctO2 min | 65.1 ± 5.4 | 65.5 ± 5.8 | 0.890 |
| SctO2 delta | 5.4 ± 3.4 | 5.7 ± 2.9 | 0.828 |
|
| |||
| Dizziness (n = 25) | No dizziness (n = 9) | P-value | |
|
| |||
| SctO2 supine | 71.1 ± 5.3 | 71.0 ± 1.5 | 0.946 |
| SctO2 1 min | 68.2 ± 6.7 | 68.9 ± 2.6 | 0.668 |
| SctO2 3 min | 67.8 ± 6.7 | 67.8 ± 3.2b | 0.995 |
| SctO2 10 min | 68.0 ± 6.1c | 66.7 ± 3.1c | 0.588 |
| SctO2 min | 65.4 ± 6.3 | 65.4 ± 3.4 | 0.984 |
| SctO2 delta | 5.7 ± 2.7 | 5.6 ± 3.6 | 0.914 |
Units for SctO2 percentage points.
POTS, postural orthostatic tachycardia syndrome; SctO2, cerebral tissue oxygen saturation; VVS, vasovagal syncope.
Missing data points: n = 3.
Missing data points: n = 1.
Missing data points: n = 2.
Figure 4.
SctO2 in POTS patients during head-up tilt test at the time of self-reported dizziness. SctO2 and Dizziness (defined as the cerebral tissue saturation at the time when patients-reported dizziness during head-up tilt). POTS, postural orthostatic tachycardia syndrome; SctO2, cerebral tissue oxygen saturation.
Discussion
We report that POTS patients have lower cerebral tissue oxygen saturation (SctO2) during head-up tilt (HUT) compared with patients with normal response to HUT, and that change in SctO2 from baseline to minimum values is more pronounced in POTS patients. We have also observed that orthostatic intolerance symptoms are not directly related to lower upright SctO2 values, especially in relation to orthostatic dizziness and reflex syncope susceptibility.
Previous studies have measured changes in cerebral blood flow (CBF) velocity in POTS, for which the findings have been inconsistent, showing both higher and lower CBF velocity in POTS patients compared with controls.6,8–10,16 Heterogeneity of POTS patients and HUT protocols may explain the inconsistent findings.
Cerebral tissue oximetry is determined by the oxygen content of blood, tissue diffusivity of oxygen, and cerebral metabolic rate of oxygen (CMRO2). Since the factors affecting blood oxygen content and cerebral metabolic rate are relatively stable over short periods of time, SctO2 might usefully complement CBF assessment.11 A previous study showed decrease in cerebral oxygenated haemoglobin during HUT but no significant decrease in CBF velocity in POTS patients compared with controls.6 Thus, another, as yet unexplained, contributing factor reducing cerebral oxygen saturation may be present in POTS.
While accounting for cerebral autoregulation, it is reasonable to assume that haemodynamic factors may at least partially influence SctO2, particularly in pathological conditions where cerebral autoregulatory mechanisms may be dysfunctional. However, the increase in HR from supine to the value recorded after 3 min head-up tilt was only weakly related to a more pronounced decrease in SctO2 in POTS patients and there were no correlations between HR and SctO2 at other time points. Furthermore, a more pronounced decrease in SBP during HUT related to a more pronounced decrease in SctO2, whereas there was no such relation in controls, despite similar blood pressure values and changes. In all, the absence of a consistent relation between SctO2 and haemodynamic parameters point out that SctO2 may be partly dependent on factors not directly related to haemodynamic factors. In accordance with the observation of a lower oxygenated cerebral haemoglobin during HUT in POTS compared with controls despite similar CBF,6 our current results further support the hypothesis that lower SctO2 in POTS patients during HUT may be explained by one or more hitherto unknown factors.
Light-headedness and neurocognitive deficits (brain-fog) are among the most disabling symptoms experienced by POTS patients and these symptoms do not necessarily correlate with haemodynamic disturbances. Dizziness and syncope are both symptoms of cerebral hypoperfusion and based on physiological reasoning one might assume that SctO2 is at least partly responsible. However, we did not observe a significant relationship between SctO2 and reported dizziness during HUT. Moreover, we did not find any differences in SctO2 between POTS patients who experienced syncope during HUT and those who did not. This is in contrast to our previous study on patients with unexplained syncope,14 which showed a steeper decrease in SctO2 from baseline in patients with syncope during HUT compared with patients without syncope (measured one minute prior to reflex activation or the end of tilt, respectively). On the other hand, SctO2 levels during syncope were similar between that study14 and our current study.
The normal range for SctO2 has been reported to be 60–80%.15 On the other hand, previous data from the same cohort as that from which the current study subjects were selected indicated that subjects with normal response to HUT all had SctO2 levels ≥65%.14 Accordingly, we applied a stricter cut-off point at SctO2 <65% as ‘abnormal’ in the present study. The threshold in SctO2 below which dizziness and other symptoms may be expected is likely to be subject to considerable variability, depending on their pathogenesis. Of note, SctO2 levels in the POTS group were lower in women, whereas no sex differences in SctO2 levels could be observed in the control group. Interestingly, in patients with syncope or orthostatic intolerance but other diagnoses than POTS, SctO2 levels are in general slightly higher among women than men.14 Whether or not the sex differences in SctO2 levels may be related to the pathophysiology and the female predominance of POTS remains to be explored.
Symptoms thought to be attributable to cerebral hypoperfusion could also occur in POTS patients in whom cerebral perfusion and SctO2 are entirely normal. The role of psychological aspects of symptoms in POTS has been evaluated. POTS patients have been shown to have heightened somatic vigilance and catastrophic cognitions, which may predispose them to increased disability.17 POTS patients, however, do not have higher incidence of depressive or anxiety disorders than the general population according to another study.18 The pathophysiology of postural tachycardia in POTS is not a response to increased anxiety, but rather a response to venous pooling in order to maintain mean arterial pressure.19
The lower SctO2 found during HUT, in POTS, in this study raises the question whether it is a consequence or a partial cause of POTS symptoms. The differences in SctO2 seen in this study are relatively small and of unknown clinical significance but the possible benefit of raising SctO2 cannot be excluded. Similarly, increase in cerebral perfusion may also offer benefit.
POTS is likely to be a heterogeneous disease. Several overlapping POTS subtypes have been described to understand mechanisms of POTS, including hyperadrenergic and hypovolaemic types.2,3 Further studies taking into consideration these possible POTS phenotypes in relation to SctO2 during tilt, would be valuable in the search for clues of the underlying pathophysiology.
Limitations
Our study has a number of important limitations. Even though the control group consisted of patients with normal response to orthostatic provocation, they were all patients with a history of syncope and/or orthostatic intolerance. However, even if we had taken people with no history of syncope or orthostatic intolerance some would have experienced syncope. Previous data indicates that up to 13% of normal subjects experience syncope during passive HUT20 and the number is likely to be higher after nitroglycerine provocation. On the other hand, the fact that these ‘normals’ sustained no syncope on the index tilt indicates that on that day they had normal haemodynamics and no syncope, also there was no evidence of POTS in any of them. As an additional limitation, the number of participants in this single-centre study is small and our results should be validated in independent and larger populations. Further, we were unable to determine if the lower cerebral tissue oxygenation during HUT was cause or consequence of the disease.
Finally, NIRS was measured from the forebrain with a penetration depth of 2.5 cm. Thus, cerebral tissue oxygenation in deeper regions of the brain was lacking.
Conclusions
We have demonstrated that monitoring of cerebral oximetry is feasible in POTS patients during head-up tilt testing. POTS patients have lower cerebral tissue oxygen saturation during orthostatic provocation compared with patients with normal haemodynamic response, however, the decrease in cerebral saturation only weakly correlates with HR increase and is not predictive of vasovagal reflex. One or more hitherto unknown factors may be responsible for the lower cerebral tissue saturation observed in POTS patients during orthostatic provocation.
Supplementary Material
Acknowledgements
The authors thank the staff of the Tilt Laboratory of the Department of Clinical Physiology and Nuclear Medicine and personnel from the Syncope Unit, Department of Cardiology at Skåne University Hospital, Malmö, Sweden. They also thank Erik Hellered for the design of Figure 2.
Funding
This study was supported by grants from the Swedish Heart and Lung Foundation, The Swedish Heart and Lung Association, The Medical Faculty of Lund University, ALF funds, Skåne University Hospital Funds, The Crafoord Foundation, Ernhold Lundströms Research Foundation, Region Skåne, Hulda and Conrad Mossfelt Foundation, and Anna-Lisa and Sven Eric Lundgrens Foundation for Medical Research.
Conflict of interest: A.F. reports personal fees from Medtronic Inc. and Cardiome Corp., and patent royalties from Thermo Fisher Scientific outside the submitted work. R.S. reports consultancy for Medtronic Inc., Member of Speakers' Bureau Abbott Labs and Stockholder in AstraZeneca PLC, Edwards LifeSciences, and Boston Scientific Inc. V.H. reports previous educational congress grant from Boston Scientific Inc. outside the submitted work. E.B. has been employed by AstraZeneca after completion of this study.
References
- 1. Schondorf R, Low PA.. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993;43:132–7. [DOI] [PubMed] [Google Scholar]
- 2. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019;285:352–66. [DOI] [PubMed] [Google Scholar]
- 3. Sheldon RS, Grubb BP, Olshansky B, Shen W-K, Calkins H, Brignole M. et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm Society 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 2012;87:1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benditt DG, Adkisson WO, Sutton R.. 66—head-up tilt table testing In Zipes DP, Jalife J, (eds). Cardiac Electrophysiology: From Cell to Bedside. 6th ed. Philadelphia: W.B. Saunders; 2014. p637–48. [Google Scholar]
- 6. Medow MS, Kothari ML, Goetz AM, O'Donnell-Smith MB, Terilli C, Stewart JM.. Decreasing cerebral oxygen consumption during upright tilt in vasovagal syncope. Physiol Rep 2017;5:e13286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Endo A, Fujita Y, Fuchigami T, Takahashi S, Mugishima H, Skatani K.. Changes in cerebral blood oxygenation induced by active standing test in children with POTS and NMS. Adv Exp Med Biol 2014;812:253–61. [DOI] [PubMed] [Google Scholar]
- 8. Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM.. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 2009;297:H664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin KJ, Kim SE, Park KM, Park J, Ha SY, Kim SE. et al. Cerebral hemodynamics in orthostatic intolerance with normal head-up tilt test. Acta Neurol Scand 2016;134:108–15. [DOI] [PubMed] [Google Scholar]
- 10. Schondorf R, Benoit J, Stein R.. Cerebral autoregulation is preserved in postural tachycardia syndrome. J Appl Physiol 2005;99:828–35. [DOI] [PubMed] [Google Scholar]
- 11. Steppan J, Hogue CW.. Cerebral and tissue oximetry. Best Pract Res Clin Anaesthesiol 2014;28:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garland EM, Celedonio JE, Raj SR.. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015;15:60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fedorowski A, Burri P, Struck J, Juul-Moller S, Melander O.. Novel cardiovascular biomarkers in unexplained syncopal attacks: the SYSTEMA cohort. J Intern Med 2013;273:359–67. [DOI] [PubMed] [Google Scholar]
- 14. Bachus E, Holm H, Hamrefors V, Melander O, Sutton R, Magnusson M. et al. Monitoring of cerebral oximetry during head-up tilt test in adults with history of syncope and orthostatic intolerance. Europace 2018;20:1535–42. [DOI] [PubMed] [Google Scholar]
- 15. Scott JP, Hoffman GM.. Near-infrared spectroscopy: exposing the dark (venous) side of the circulation. Paediatr Anaesth 2014;24:74–88. [DOI] [PubMed] [Google Scholar]
- 16. Jacob G, Atkinson D, Jordan J, Shannon JR, Furlan R, Black BK. et al. Effects of standing on cerebrovascular resistance in patients with idiopathic orthostatic intolerance. Am J Med 1999;106:59–64. [DOI] [PubMed] [Google Scholar]
- 17. Benrud-Larson LM, Sandroni P, Haythornthwaite JA, Rummans TA, Low PA.. Correlates of functional disability in patients with postural tachycardia syndrome: preliminary cross-sectional findings. Health Psychol 2003;22:643–8. [DOI] [PubMed] [Google Scholar]
- 18. Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I. et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry 2009;80:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masuki S, Eisenach JH, Johnson CP, Dietz NM, Benrud-Larson LM, Schrage WG. et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol 2007;102:896–903. [DOI] [PubMed] [Google Scholar]
- 20. Petersen ME, Williams TR, Gordon C, Chamberlain-Webber R, Sutton R.. The normal response to prolonged passive head up tilt testing. Heart 2000;84:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.