Molecular movement through plasmodesmata, which connect plant cells to each other, is dynamically regulated by light and the circadian clock to promote intercellular transport during the day.
Abstract
Plasmodesmata (PD) are essential for plant development, but little is known about their regulation. Several studies have linked PD transport to chloroplast-centered signaling networks, but the physiological significance of this connection remains unclear. Here, we show that PD transport is strongly regulated by light and the circadian clock. Light promotes PD transport during the day, but light is not sufficient to increase rates of PD transport at night, suggesting a circadian gating mechanism. Silencing expression of the core circadian clock gene, LHY/CCA1, allows light to strongly promote PD transport during subjective night, confirming that the canonical plant circadian clock controls the PD transport light response. We conclude that PD transport is dynamically regulated during the day/night cycle. Due to the many roles of PD in plant biology, this discovery has strong implications for plant development, physiology, and pathogenesis.
Plasmodesmata (PD) are nanoscopic, membrane-bound tunnels that connect the cytosol of neighboring plant cells, transporting small molecules, proteins as large as 80 kD (Kim et al., 2005; Paultre et al., 2016), small RNAs, and viruses (Sager and Lee, 2014; Brunkard and Zambryski, 2017). The rate of PD transport between cells changes during the course of plant development (Roberts et al., 1997, 2001; Oparka et al., 1999; Crawford and Zambryski, 2001; Stadler et al., 2005) and in response to stress (Faulkner et al., 2013; Caillaud et al., 2014; Cui and Lee, 2016; Lim et al., 2016; Brunkard and Zambryski, 2017). Despite decades of intense research, however, very little is known about the molecular mechanisms that regulate PD transport. The only clearly established mechanism to dynamically regulate PD transport is callose deposition: under various stress conditions and specific developmental contexts, callose (β-1,3-glucans) is synthesized and accumulates in the cell walls around PD, blocking transport through the PD (Lee et al., 2011; Benitez-Alfonso et al., 2013; Lim et al., 2016). Callose deposition can be reversed by β-1,3-glucanases, enzymes that degrade callose surrounding PD and thus restore transport through PD (Zavaliev et al., 2011).
To identify molecular pathways that regulate PD transport, we and others have conducted forward genetic screens. These screens to identify factors controlling transport through PD have repeatedly revealed that chloroplasts influence PD transport (Provencher et al., 2001; Benitez-Alfonso et al., 2009; Burch-Smith and Zambryski, 2010, 2012; Burch-Smith et al., 2011; Stonebloom et al., 2012; Brunkard et al., 2013; Carlotto et al., 2016; Bobik et al., 2019). This has led to a paradigm shift, focusing less on the role of structural changes directly at PD and more on how cellular physiology influences the function of PD. Although many groups have now demonstrated that chloroplast function and PD transport are tightly connected, the biological significance of this relationship between chloroplasts and PD remains unresolved.
Given the connection between PD transport and chloroplast physiology, we hypothesized that PD transport might be sensitive to light. A pioneering study in maize (Zea mays) seedlings demonstrated that PD transport decreases during deetiolation, when dark-grown seedlings are first exposed to light and chloroplast biogenesis is initiated (Epel and Erlanger, 1991). PD transport also decreases at the midtorpedo stage of Arabidopsis (Arabidopsis thaliana) embryogenesis, which is when embryos initiate chloroplast biogenesis. PD transport does not decrease at this stage of embryogenesis in mutants defective in chloroplast biogenesis, including ise1, ise2, clpr2, and uL15c (Burch-Smith et al., 2011; Carlotto et al., 2016; Bobik et al., 2019). Therefore, at least in some developmental and physiological contexts, changes in PD transport are coordinated with chloroplast biogenesis.
Beyond these studies indicating that light-dependent chloroplast biogenesis influences PD transport, little is known about how light influences plant cell-cell transport. One report suggested that PD transport can increase when starvation is induced by detaching a leaf and transferring it to complete darkness (Liarzi and Epel, 2005), but whether PD transport was impacted by light signaling or starvation stresses in this experimental system was not resolved. An ultrastructural study of several C4 grass species found that PD frequency in the cell wall increases when seedlings are grown under higher light intensities (Sowiński et al., 2007). Here, we combine genetic and physiological approaches to show that PD transport is dynamically regulated by light and the circadian clock throughout the diurnal cycle.
RESULTS
PD Transport Rates Are Higher during the Day
As a preliminary experiment, we performed a simple qualitative assay to monitor PD transport in Arabidopsis during the day or night using fluorescent tracers. Arabidopsis seedlings were germinated on plates containing Murashige and Skoog medium and grown in a 12-h-light/12-h-dark light cycle. Three hours after dawn or dusk, a fluorescent tracer was applied to seedlings by cutting the plant just below the base of the shoot and applying a small volume (∼10 μL) of fluorescent tracer to the remaining root portion (Supplemental Fig. S1A). Seedlings were kept on plates at high relative humidity throughout the experiment to limit transpiration and 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) transport via xylem vessels. After 10 min, the cotyledons of seedlings were visualized. Low-molecular-mass (∼500 D) HPTS (also known as pyranine) moved rapidly and extensively through the ground tissue via PD from the cut surface of the root into the hypocotyl and then into the cotyledons during the day (Supplemental Fig. S1B) but not at night (Supplemental Fig. S1C). This result implied that PD transport is higher during the day than at night.
To better quantify the differences in PD transport between the day and the night, we used a GFP movement assay in Nicotiana benthamiana leaves. Plants were grown in a 12-h-light/12-h-dark light cycle under otherwise constant conditions. Agrobacterium tumefaciens carrying 35SPRO:GFP T-DNA was infiltrated into leaves with very low inoculum (OD600nm = 10−4, less than 100 cells total infiltrated per leaf) at either dusk or dawn, after which GFP movement from distinct, individual transformed cells in the leaves was visualized 36 to 60 h post infiltration. GFP expression typically becomes stable ∼24 h after infiltration (sufficient time for T-DNA transfer and transcription) and remains stable for 72 to 96 h, at which time host RNA-silencing mechanisms begin to reduce transgene expression (Voinnet et al., 2015). The Cauliflower mosaic virus 35S promoter is widely used for strong and consistent gene expression and is not regulated by diurnal cues or the circadian clock (Suárez-López et al., 2001). Across all experiments (except for the photoperiod experiment, described in more detail below and in "Materials and Methods"), the fourth expanded leaf from the top of a 5-week-old plant was agroinfiltrated. For consistency, only similar plants and leaves of nearly identical size and age were used for these assays, and GFP movement was only visualized in the proximal 25% of the agroinfiltrated leaf. Each transformed cell is reported as an independent sample (n), the experiments were conducted using at least eight to ten separate plants, and each experiment was repeated at least three times to ensure reproducibility. Sampling details are described in “Materials and Methods” and in Supplemental Table S1. Results of this movement assay are presented as the number of cells GFP has spread to from the transformed cell (examples in Fig. 1B). Compared with most other approaches, this method is minimally invasive, since N. benthamiana lacks the Brassicaceae-specific A. tumefaciens immunity receptor EF-Tu RECEPTOR (EFR), leaves are infiltrated with only a few dozen A. tumefaciens cells (which are far outnumbered by endogenous phyllobacteria), the agroinfiltration does not require significant pressure or wounding in N. benthamiana leaves, and no foreign molecules are injected into cells by pressure or wounding; instead, individual transformed leaf cells produce the fluorescent tracer GFP, permitting noninvasive imaging. Moreover, unlike many fluorescent dyes, which can be sequestered in vacuoles or exported to the apoplast, GFP remains symplastic, freely moving through cytosol and nucleosol but not trafficking across membranes, and thus only moves between cells via PD (Crawford and Zambryski, 2001).
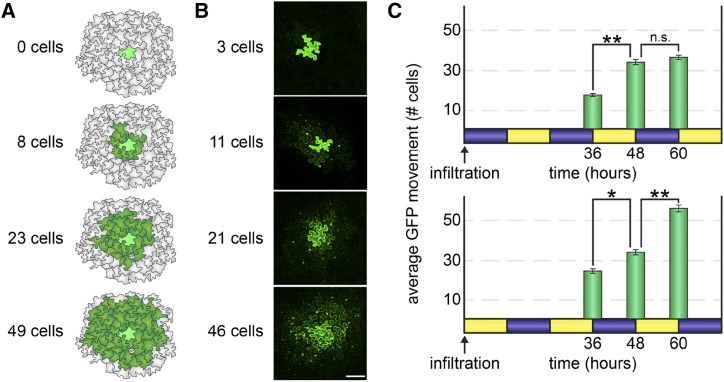
Figure 1.
PD transport is higher during the day than at night. A, To measure rates of PD transport, we used a quantitative GFP movement assay. A very low inoculum of A. tumefaciens cells (less than 100 bacterial cells) was gently infiltrated by syringe into N. benthamiana leaves so that a handful of individual epidermal cells were transformed to express monomeric GFP. GFP spread to neighboring epidermal cells was then quantified 48 h after infiltration (or as indicated in each experiment). Illustrated examples of GFP movement are shown here. Movement is scored by counting the number of neighboring cells to which GFP has moved (dark green) from the transformed cell (bright green). B, Representative confocal microscopy images of the GFP movement assay. GFP fluorescence is brightest in the nuclei in cells neighboring the transformed cell. Bar = 100 μm. C, N. benthamiana leaves were agroinfiltrated to express GFP at either dusk (top) or dawn (bottom) in 5-week-old plants grown under 12-h-light (yellow)/12-h-dark (blue) cycles. GFP movement from the transformed cell was then assayed 36, 48, or 60 h later. In leaves infiltrated at dusk (top), GFP movement significantly increased during the second day but did not change during the third night. In leaves infiltrated at dawn (bottom), GFP movement somewhat increased during the second night but dramatically increased during the third day. *, P < 10−3 and **, P < 10−5; n.s., not significant.
Whether leaves were infiltrated at dawn or dusk, GFP moved the same distance after 48 h (to 33 ± 1 cells, n ≥ 106 transformed cells, P = 0.26; Fig. 1C), demonstrating that the time of infiltration had no impact on the observed rates of PD transport. When observed after 36 h, however, GFP moved significantly more in leaves that have experienced two days and one night than in leaves that have experienced one day and two nights (to 25 ± 1 cells [dawn infiltration] versus to 17 ± 1 cells [dusk infiltration], n ≥ 117 transformed cells, P < 10−7; Fig. 1C). Similarly, when observed after 60 h, GFP also moved significantly more in leaves that have experienced three days and two nights than in leaves that have experienced two days and three nights (to 54 ± 1 cells [dawn infiltration] versus to 35 ± 1 cells [dusk infiltration], n ≥ 102 transformed cells, P < 10−10; Fig. 1C). Thus, GFP moves more in plants that have experienced two days (daytime light periods) than in leaves that have experienced one day, and GFP moves the most in leaves that have experienced three days, demonstrating that the rate of PD transport is higher during the day than at night.
Remarkably, an additional night has little impact on GFP movement, suggesting that PD transport at night is relatively limited. GFP movement is only slightly lower in leaves 36 h after infiltration at dawn (i.e. leaves that experienced two days and one night) than in leaves 48 h after infiltration (i.e. leaves that experienced two days and two nights; GFP moved to 25 ± 1 cells 36 h after infiltration at dawn versus to 33 ± 1 cells 48 h after infiltration, n ≥ 106 transformed cells, P < 10−3; Fig. 1C, bottom). PD transport was not significantly different in leaves observed 60 h after infiltration at dusk (i.e. leaves that experienced two days and three nights) than in leaves observed 48 h after infiltration at dusk (60 h: GFP moved to 35 ± 1 cells versus 48 h: GFP moved to 33 ± 1 cells, n ≥ 102 transformed cells, P = 0.2; Fig. 1C, bottom) or 48 h after infiltration at dawn (n ≥ 111 transformed cells, P = 0.91; Fig. 1C, bottom). These results support our preliminary hypothesis that PD transport is, in fact, dramatically lower at night than during the day.
Diurnal Changes in PD Transport Are Not Due to Callose Deposition
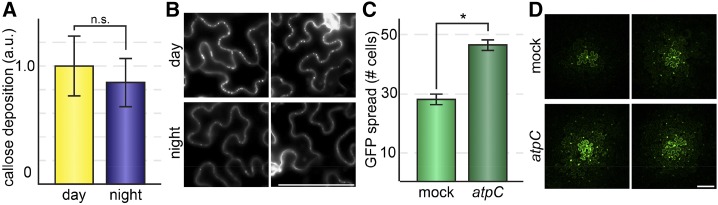
PD transport can be restricted during stress responses and cellular differentiation by deposition of a polysaccharide, namely callose, in the plant cell wall surrounding PD. We therefore investigated whether callose levels around PD are higher at night than during the day, which could explain the decreased rate of PD transport at night. We infiltrated leaves with Aniline Blue using state-of-the-art methods (Cui and Lee, 2016) to stain callose in leaves 3 h after dawn or 3 h after dusk and then used confocal laser-scanning fluorescence microscopy followed by quantitative image analysis to measure callose levels at PD in leaf cell walls. For these experiments, each sample is the average fluorescence intensity of Aniline Blue at PD in four separate fields of view in a leaf, with comparable numbers of PD included in each field of view, such that the relative callose levels are based on measurements of fluorescence from hundreds of individual PD. Callose levels at PD are somewhat lower at night than during the day (1 ± 0.25 arbitrary units of fluorescence, day; 0.86 ± 0.2 arbitrary units of fluorescence, night; n = 8 plants, P = 0.08; Fig. 2). Therefore, the difference in PD transport is likely not due to a reversible, nocturnal deposition of callose at PD.
Figure 2.
A and B, PD callose deposition is not significantly different between day and night. Fluorescence intensity of Aniline Blue-stained leaves was assayed using confocal scanning laser microscopy in the leaf epidermis of N. benthamiana collected 3 h after dawn or 3 h after dusk. Callose levels were slightly lower at night than during the day (n = 8 leaves, P = 0.08). a.u., Arbitrary units; ns, not significant. Bar = 100 μm. C, Reducing ATP availability by silencing AtpC with VIGS significantly increased the rate of GFP transport (n ≥ 21 transformed cells; *, P = 0.003), demonstrating that light-dependent chloroplast ATP synthesis and PD transport do not positively correlate. D, Representative images of the GFP movement assay in mock (TRV::GUS) and atpC (TRV::AtpC) plants. Bar = 100 μm.
Another simple explanation for the change in PD transport could be reduced ATP availability at night, for example, if PD transport is largely driven by cytosolic convection powered by ATP-dependent cytoskeletal activity (Pickard, 2003). Inhibition of chloroplast and/or mitochondrial activity (and thus inhibition of ATP generation), however, can cause either increased or decreased transport (Benitez-Alfonso et al., 2009; Stonebloom et al., 2012), suggesting that this simple model is not a sufficient explanation. Here, using virus-induced gene silencing (VIGS), we tested whether silencing the expression of AtpC, which encodes a subunit of the chloroplast ATP synthase, influences PD transport. We chose this gene because silencing AtpC has been shown to promote the intercellular spread of Tobacco mosaic virus (Bhat et al., 2013). We observed increased PD transport after silencing AtpC, from 28 ± 2 cells in mock (TRV::GUS) plants to 47 ± 2 cells in TRV::AtpC-knockdown leaves (n ≥ 21 transformed cells, P < 0.003; Fig. 2, C and D). These results support the hypothesis that higher ATP levels do not correlate with increased PD transport and hint that increased spread of Tobacco mosaic virus in TRV::AtpC-knockdown plants could be a consequence of increased PD transport.
PD Transport Is Regulated by the Plant Circadian Clock
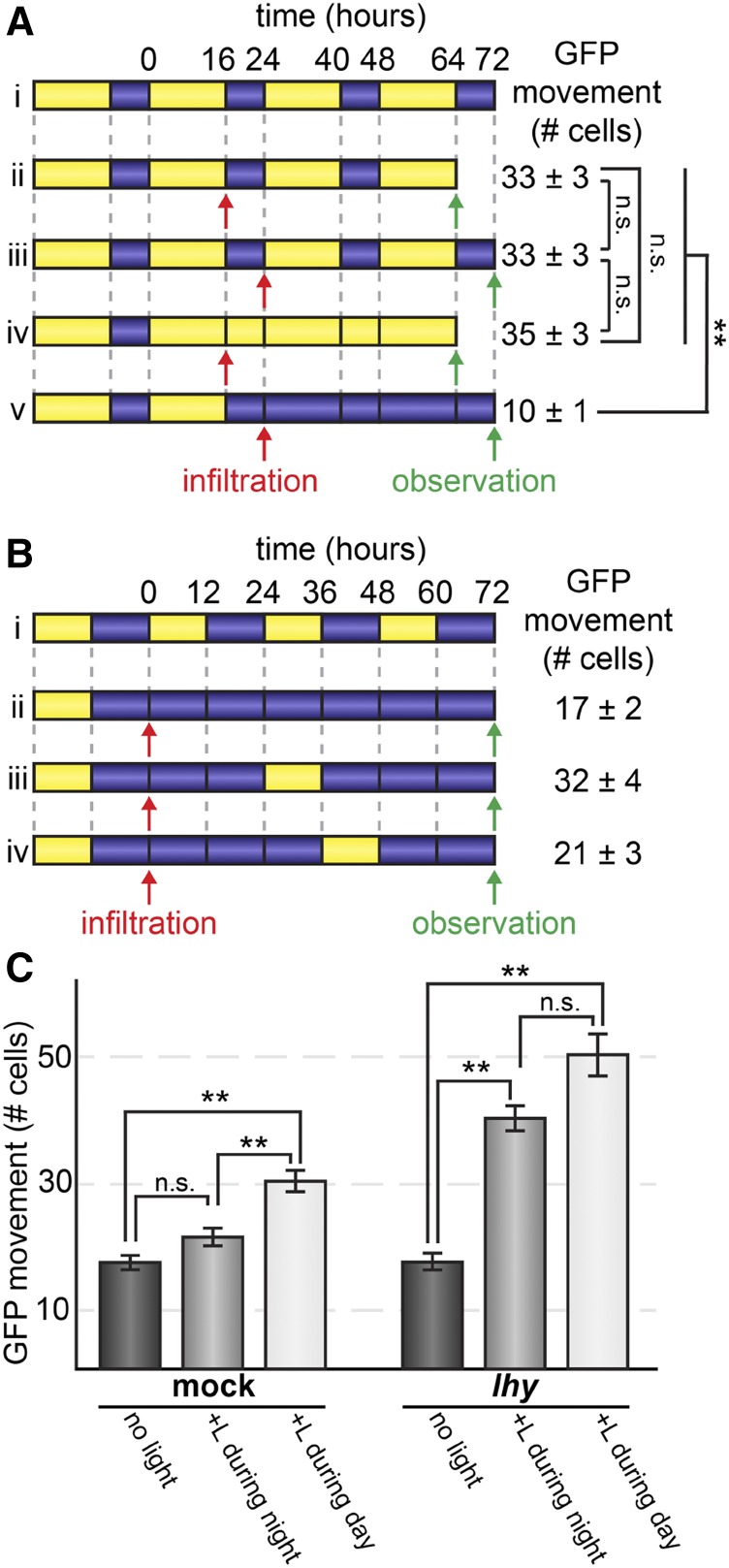
High rates of PD transport during the day could be caused directly by light signaling or could be mediated by light-entrained signaling pathways controlled by the circadian clock (Harmer, 2009). To distinguish between these possibilities, we first tested whether transferring N. benthamiana plants grown in 16-h-light/8-h-dark cycles to either constant light or constant dark impacted PD transport. We conducted these first experiments using 16-h-light/8-h-dark cycles (Fig. 3A) and then also tested 12-h-light/12-h-dark cycles (described below; Fig. 3, B and C), with comparable results. After 48 h, there was no significant difference in GFP movement between plants under constant light or under continued day/night cycles (16-h-light/8-h-dark cycle), with GFP moving 35 ± 3 cells under constant light versus 33 ± 3 cells under cycling light/dark conditions (n ≥ 60 transformed cells, P = 0.35; Fig. 3A). GFP movement was severely lower in plants transferred to constant dark, however, moving an average of only 10 ± 1 cells (n ≥ 60 transformed cells, P < 10−23; Fig. 3A). Light is therefore necessary for the higher rate of PD transport during the day, but constant light is insufficient to significantly promote PD transport.
Figure 3.
PD transport is regulated by the circadian clock. Light treatment is represented by yellow, dark and treatment is represented by blue. Line i of both A and B shows the day/night cycles that would be experienced by plants if they were not transferred to new light regimes. A, After growing in 16-h-light/8-h-dark cycles, N. benthamiana leaves were agroinfiltrated at dawn or dusk (as indicated by red infiltration arrows) to express GFP. GFP movement was then assayed after 48 h (as indicated by green observation arrows) of continued light/dark cycles (lines ii and iii), or 48 h of constant light starting at the end of the day (line iv), or constant darkness starting at the end of the night (line v). Constant light did not affect PD transport, but constant darkness significantly decreased PD transport (**, P < 10−23; ns, not significant). B, PD transport was assayed in mock-treated plants (TRV::GUS) that had been growing in 12-h-light/12-h-dark cycles and then transferred to constant dark conditions; GFP movement was assayed 72 h after agroinfiltration. Subjective days and nights are shown in line i. Treatment with 12 h of light during the second subjective day after agroinfiltration (line iii) strongly increased PD transport compared with PD transport in constant dark (line ii), but treatment with 12 h of light during the second subjective night (line iv) had no significant effect on PD transport. C, Leaves of mock-treated plants (left) or TRV::LHY knockdowns (right) under 12-h-light/12-h-dark photoperiod conditions were agroinfiltrated to express GFP at subjective dawn. Plants were transferred to the dark and either maintained in complete darkness for 72 h (dark gray bars) or exposed to 12 h of light during the second subjective night (light gray bars) or 12 h of light during the second subjective day (white bars). Mock-treated plants distinguished between light applied during subjective night or subjective day, significantly increasing PD transport only after exposure to light during the day. TRV::LHY knockdowns did not distinguish between subjective night or subjective day, significantly increasing PD transport after exposure to light during either time period. **, P < 10−7; ns, not significant.
In plants, the circadian clock serves primarily to anticipate regular environmental changes and to gate responses to irregular or fluctuating stimuli (Harmer, 2009). For example, the circadian clock prevents the induction of photosynthesis-associated nuclear genes by brief bursts of light at night, preventing wasteful protein synthesis (Kay and Millar, 1992; Anderson et al., 1997). Gating can be observed by entraining plants under regular day/night cycles, moving them into constant conditions, and then applying a stimulus (e.g. light) at either subjective day or subjective night; ungated responses will be observed in both cases, but gated responses will differ depending on when the stimulus is applied. We next tested whether a stimulus gating mechanism at night could explain the insufficiency of constant light to increase the rates of PD transport described above (Fig. 3A). N. benthamiana plants were grown under 12-h-light/12-h-dark photoperiods and then transferred to constant darkness for 72 h. One group of plants was then exposed to 12 h of light only during the second subjective day (+L during day), whereas a second group of plants was exposed to 12 h of light only during the second subjective night (+L during night), and a third group of plants was maintained in constant darkness (no light). As expected, PD transport was low in constant darkness (21 ± 4 cells). PD transport only slightly increased when 12 h of light was applied during the second subjective night (to 21 ± 3 cells, n ≥ 59 transformed cells, P = 0.03) but was much higher when light was applied during the second subjective day (to 32 ± 4 cells, n ≥ 70 transformed cells, P < 10−5; Fig. 3, B and C).
To confirm whether the circadian clock is responsible for gating PD transport at night, we took a genetic approach and used VIGS to knock down the expression of the core circadian clock gene, LATE ELONGATED HYPOCOTYL (LHY). Reverse transcription quantitative PCR (RT-qPCR) analysis confirmed that VIGS reduced LHY expression to 2.8% of mock-infected LHY transcript levels in leaves collected 1 h after subjective dawn, when wild-type LHY expression peaks. LHY and its recently evolved paralog, CCA1, are required to maintain circadian rhythms in Arabidopsis; similarly, silencing LHY (there are no distinct CCA1 orthologs in Solanaceae) abolishes circadian rhythms in Nicotiana spp. (Yon et al., 2016). We conducted this experiment side by side with the gating experiment described above, which involved mock infection with the VIGS vectors. As expected, PD transport was as low in constant darkness in TRV::LHY-knockdown leaves as in mock leaves (17 ± 1 cells in mock versus 16 ± 1 cells in TRV::LHY, n ≥ 70 transformed cells, P = 0.75; Fig. 3C). Unlike the mock treatment leaves, however, TRV::LHY-knockdown leaves significantly increased PD transport whether light was applied during the day or the night (to 50 ± 2 cells when applied during the subjective day, n ≥ 31 transformed cells, P < 10−7, or to 40 ± 1 cells when applied during the subjective night, n ≥ 71 transformed cells, P < 10−9; Fig. 3C). Thus, gating by the LHY-dependent circadian clock is required to prevent light from inducing higher rates of PD transport at night. These results demonstrate that PD transport is under tight regulation by both light and the circadian clock to promote intercellular trafficking during the day and limit movement between cells at night.
Diurnal PD Transport Rates Decrease with Leaf Age But Are Not Correlated with Photoperiod
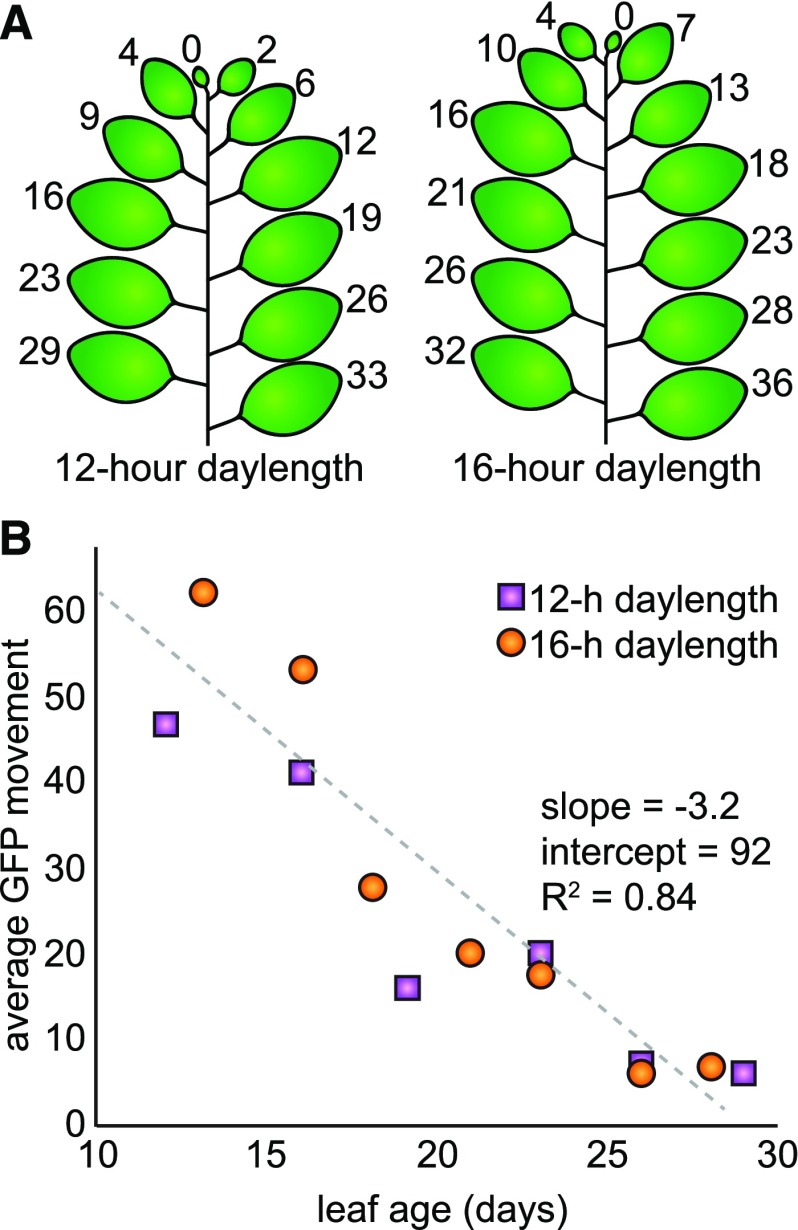
We next tested whether PD transport is lower in plants grown with shorter daylengths, since the above results show that PD transport is positively regulated by light. Daylength can impact the rate of leaf emergence, however, such that it is difficult to know a priori which leaf stage(s) to compare. Instead of selecting a single leaf stage, therefore, we assayed PD transport in several leaves of mature plants grown under different photoperiods. We grew N. benthamiana plants under 12- or 16-h daylengths, recording the dates of leaf emergence throughout their development. Six weeks after germination, we simultaneously assayed PD transport with the GFP movement assay in all leaves between 10 and 30 d old. This experiment was replicated three times, and the dates of leaf emergence did not change across replicates (but were different between plants grown under 12- or 16-h daylengths, as shown; Fig. 4). PD transport, as measured by the quantitative GFP movement assay, decreases in an approximately linear pattern with respect to leaf age, regardless of photoperiod conditions (Fig. 4). Remarkably, there is no significant difference in the rate of PD transport of plants grown under 12- or 16-h photoperiods, as tested by an analysis of covariance (ANCOVA) with respect to leaf age (n ≥ 59 cells, ANCOVA P = 0.27, homogeneity of regressions P = 0.25). Thus, the rate of PD transport is indistinguishable between plants that have developed under either 12- or 16-h photoperiods when comparing leaves of the same age.
Figure 4.
A, Leaf emergence was recorded every day for N. benthamiana plants grown under 12- or 16-h daylengths. The age of each leaf (in number of days) in the mature plant at the time of the GFP movement assay (6 weeks after germination) is shown. Cotyledons are not included in this diagram because they senesced within the first 6 weeks of growth. B, Average GFP movement in leaves of different ages is shown for plants grown with 12-h daylengths (purple boxes) or 16-h daylengths (orange boxes). GFP movement was assayed 48 h after agroinfiltration with a low inoculum of A. tumefaciens that transformed cells to express GFP. PD transport declines in the same linear relationship with leaf age in both sets of plants (n ≥ 59 transformed cells, ANCOVA P = 0.27, homogeneity of regressions P = 0.25). The linear relationship is depicted with a gray dashed line with slope −3.2 and y intercept 92.
DISCUSSION
Here, we have shown that the rate of molecular transport through PD changes during the diurnal cycle. GFP and fluorescent tracers move more rapidly between plant cells during the day than at night. The higher rates of PD transport during the day are light dependent. Light is not sufficient to increase the rate of PD transport at night, however, because of regulation by the circadian clock. Thus, multiple regulatory mechanisms dynamically control PD transport during the course of the diurnal cycle.
A previous report argued that light down-regulates PD transport in leaves (Liarzi and Epel, 2005) rather than promoting PD transport, as we report here. These earlier studies were based on very different physiological conditions, however. One study showed that PD transport is higher in young sink leaves after they are detached from plants grown under 16-h-light/8-h-dark photoperiods and transferred to nutrient-free media in constant darkness (Liarzi and Epel, 2005). Transferring individual leaves to constant darkness, however, induces starvation responses, whereas transferring entire plants to constant darkness does not (Weaver and Amasino, 2001). Thus, we assert that the opposite results obtained in the previous report are likely due to complications from detaching the leaf, whereas, in our experiments, all conditions were kept constant except for the light environment.
Our results measuring PD transport in leaves of multiple ages on the same plant demonstrate that PD transport within the leaf epidermis gradually changes as leaves age, rather than abruptly shifting from high PD transport to low PD transport during the sink-to-source transition. As the plant shoot develops, leaves transition from heterotrophic sinks for carbohydrates, nitrogen, and other resources to photoautotrophic sources of carbohydrates for the rest of the plant (Masclaux et al., 2000). The sink-to-source physiological transition is supported by the restriction of PD transport in source leaves, which can then actively load sugars into the phloem via directional sugar transporters at the plasma membrane without allowing the sugars to move back into the leaf through PD (Turgeon, 1989). In this way, restriction of PD transport permits the formation of a steep carbohydrate concentration gradient in the phloem, shifting the water potential to promote rapid transport of molecules through the phloem and driving vascular plant growth and development. Previous studies often used qualitative measurements to define the sink-to-source transition in PD transport (specifically, fluorescent constructs that either do or do not move from the phloem into the ground tissue [Roberts et al., 1997; Imlau et al., 1999]) and emphasized a bimodal sink-to-source transition within leaves: PD transport is restricted first in the distal region of the leaves, and then this restriction moves proximally until PD transport is low throughout the entire leaf (Oparka et al., 1999). The data presented here demonstrate instead that PD transport decreases gradually and quantitatively as a leaf ages.
Although PD transport rates are higher during the day, we observed no significant effect of daylength on PD transport in plants grown under 12- or 16-h photoperiods. One hypothesis to address this result is that PD transport primarily occurs during only the early hours of the day, not the late afternoon. This is supported by our results comparing mock (TRV::GUS) versus TRV::LHY plants (Fig. 3C): when plants were transferred to darkness for 72 h but treated with 12 h of light during the subjective day, GFP movement was greater in TRV::LHY knockdowns (50 ± 4 cells) than in mock-treated plants (33 ± 3 cells). The most straightforward explanation for this difference is that the circadian clock gates PD transport during a portion of the day. Indeed, many light-dependent diurnal processes are gated by the circadian clock during the day. For example, photosynthetic rates are typically highest during the morning and significantly lower in the late afternoon, when the benefits of photosynthesizing more sugars are outweighed by the costs associated with photosynthesis (e.g. water loss via stomata exacerbated by higher temperatures; Parry et al., 1993). It is possible that PD transport rates, like photosynthetic rates, are also higher during the first hours of the day and then decrease under regulation by the circadian clock. This hypothesis would also explain why the reduction of daylength from a 16-h to a 12-h photoperiod would not strongly impact PD transport, because the duration of daylight at the end of the day is reduced and most PD transport would primarily occur during the first few hours after dawn.
CONCLUSION
As a working model, we propose that light promotes PD transport during the day and that darkness and the circadian clock repress PD transport at night and possibly also during part of the day. This regulation is likely independent of the well-established callose deposition mechanism that can restrict PD transport. The potential physiological implications of this work are clear: cell-cell transport and signaling is strongly modulated by the time of day. Ongoing efforts to dissect the biology controlled by PD transport in plants, including hormone transport and regulatory networks (Benitez-Alfonso et al., 2013; Besnard et al., 2014; Han et al., 2014; Lim et al., 2016), host-pathogen interactions (Khang et al., 2010; Wang et al., 2013; Caillaud et al., 2014), and protein and small RNA trafficking (Gallagher et al., 2014; Brunkard and Zambryski, 2017), will be informed by the discovery that PD transport is tightly and dynamically regulated by the circadian clock.
MATERIALS AND METHODS
Plant Growth Conditions
Nicotiana benthamiana accession Nb-1 plants were grown as described (Brunkard et al., 2015) at 22°C to 24°C on light carts under ∼100 μE m−2 s−1 photosynthetically active radiation using Sylvania Gro-Lux Wide Spectrum bulbs with either 12- or 16-h daylengths, as indicated in the text. Plants were transferred to complete darkness but otherwise kept in constant conditions, where noted in the text. For the photoperiod experiment, plants were photographed every day after germination in order to accurately record leaf ages.
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 plants were grown at 22°C to 24°C in growth chambers under ∼100 μE m−2 s−1 photosynthetically active radiation using Sylvania Gro-Lux Wide Spectrum bulbs with 12-h daylengths. Seeds were surface sterilized, stratified in the dark at 4°C for 2 d, and sown on 0.5× Murashige and Skoog medium (Caisson Labs) with 0.8% (w/v) agar and pH adjusted to 5.5. Seedlings were kept on plates to maintain high relative humidity throughout all experiments.
Agroinfiltrations
Agrobacterium tumefaciens strain GV3101 was grown overnight in lysogeny broth medium at 28°C, 250 rpm, with kanamycin, gentamicin, and rifampicin (each at 50 µg mL−1). Cultures were resuspended in infiltration medium (10 mm MgCl2, 10 mm MES, and 200 µm acetosyringone, pH 5.6, adjusted with KOH) to OD600nm = 10−4 for GFP movement assays or OD600nm = 1 for VIGS, as previously described (Brunkard et al., 2015).
Assaying PD Transport with GFP Transformation in Single Leaves
Except for the photoperiod experiment (described in detail in the "Assaying PD Transport with GFP Transformation in Mulitple Leaves" section), all movement assays were conducted using the fourth expanded leaf of 5-week-old plants. GFP movement assays were conducted as previously described (Brunkard et al., 2015; Fig. 1), observing GFP movement in only the proximal 25% of the leaf. For each experiment, two, three, or four plants were assayed for each condition per replicate (observing GFP movement from up to 30 individual transformed cells selected randomly from one leaf on each plant), and the entire experiment was replicated three times, so that each experiment was conducted in at least eight to 10 plants. Movement is scored by counting the number of neighboring cells to which GFP has moved from the original, transformed cell.
Assaying PD Transport with GFP Transformation in Multiple Leaves
For the photoperiod experiment, GFP movement assays were performed as previously described (Brunkard et al., 2015), with the difference that A. tumefaciens carrying the 35SPRO:GFP T-DNA binary vector was infiltrated into every leaf between 10 and 30 d old of the plant in 6-week-old plants. GFP movement was observed in all leaves in the proximal 25% region of the leaf using three biological replicates. Leaves beyond 30 d old were not included because GFP was generally unable to move beyond the transformed cell, and thus the GFP movement assay was not sufficiently sensitive to assay changes in PD transport in these leaves. Leaves less than 10 d old were not included because GFP spread so far (to as many as 100 cells) that it became impractical to consistently and accurately distinguish spreading GFP foci.
Assaying PD Transport with Fluorescent Tracers
HPTS (Sigma-Aldrich) was dissolved in 0.5× Murashige and Skoog medium (Caisson Labs) at 1 mg mL−1. Ten microliters of HPTS was applied to the proximal root remaining immediately after cutting off the distal root near the root-shoot connection in 4-d-old Arabidopsis seedlings. Seedlings were kept on plates throughout the experiment to maintain high relative humidity, which we found prevented HPTS from traveling apoplastically (via xylem vessels). Cotyledons were then observed approximately 10 min after application of the dye. At least three individual seedlings were assayed per experiment, and the experiment was repeated three times, with representative images shown.
VIGS
Silencing triggers were cloned as previously described (Brunkard et al., 2015). Briefly, RNA was isolated from N. benthamiana Nb-1 with the Spectrum Plant Total RNA kit (Sigma-Aldrich) with on-column DNase I digestion (New England Biolabs). cDNA was synthesized from RNA using random hexamers and SuperScript III reverse transcriptase (Fisher Scientific). Silencing triggers were amplified with Phusion DNA polymerase (New England Biolabs), digested with XbaI and XhoI (New England Biolabs) alongside digestions of pYL156 (Liu et al., 2002), and ligated with Promega T4 DNA ligase (Fisher Scientific). Ligations were transformed into XL1-Blue Escherichia coli, miniprepped (Bioneer), and Sanger sequenced to confirm insertion sequences. The AtpC trigger was cloned using the same sequence as previously reported (Bhat et al., 2013), with oligonucleotides 5′-gactctagaTTCCTAACCATAACTCATCAGG-3′ and 5′-gatctcgagAAAACATCATCAGCAATGG-3′ (XbaI and XhoI restriction sites were introduced by PCR with these oligonucleotides and are indicated in lowercase).
Two young leaves were infiltrated with equal inocula of A. tumefaciens carrying binary vectors encoding the two Tobacco rattle virus VIGS constructs (TRV1 and TRV2-trigger; Liu et al., 2002). A TRV2-GUS trigger was used as a negative control, because GUS does not have any sequence similarity to endogenous transcripts in N. benthamiana (Stonebloom et al., 2009). A TRV2-NbPDS (PHYTOENE DESATURASE) trigger was used as a positive control for silencing, because pds knockdowns exhibit strong photobleaching phenotypes that can be monitored visually (Stonebloom et al., 2009). Silencing efficiency RT-qPCR analysis and GFP movement assays were conducted 14 d after infiltration.
RT-qPCR
N. benthamiana upper leaves, comparable to the leaves used for the GFP movement assay, were collected 2 weeks post infection with either TRV::LHY or TRV::GUS, a mock control (Stonebloom et al., 2009). Tissue from three replicate plants was collected 1 h after dawn, when LHY is strongly expressed in wild-type plants. RNA was isolated with the Spectrum Plant Total RNA (Sigma-Aldrich) kit with on-column DNase I digestion (New England Biolabs). cDNA was synthesized from RNA using oligo(dT)18 and SuperScript III reverse transcriptase (Fisher Scientific). qPCR was performed in parallel for all samples using a validated reference gene (EF1α) for N. benthamiana VIGS experiments (Liu et al., 2012) and primers specific to LHY: 5′-TAGCTGGAGATGCTGGGAAT-3′ and 5′-TGAAAAGAGCCTGGAATGCT-3′.
Assaying PD Callose Deposition with Aniline Blue
Leaves were infiltrated with sterile 0.01% (w/v) Aniline Blue (Sigma-Aldrich) in 10 mm K3PO4 (pH 12; Zavaliev and Epel, 2015) and left to stain for 1 h in the dark (Cui and Lee, 2016). Leaves were then mounted on slides, and the abaxial epidermis was imaged with a Zeiss 710 confocal scanning laser microscope equipped with a W Plan-Apochromat 40x/1.0 DIC M27 objective. Four fields of view from the proximal 25% of the leaf were imaged from each leaf. Each PD was identified manually in ImageJ, and Aniline Blue fluorescence intensity per PD was recorded. Average callose levels per PD from each leaf were considered independent samples. Callose levels per PD were then averaged across all leaves sampled. One leaf from four plants was assayed for each condition per experiment, and the experiment was repeated twice.
Microscopy
GFP was observed in the epidermis of N. benthamiana leaves using a Zeiss 710 confocal scanning laser microscope. Identical settings (such as laser strength and gain) were used for all GFP experiments. HPTS and Aniline Blue staining were also observed with a Zeiss 710 confocal scanning laser microscope. For quantification, Aniline Blue staining was observed with a W Plan-Apochromat 40x/1.0 DIC M27 objective, and PD fluorescence intensity in images was analyzed using ImageJ. FITC-Dextran unloading in roots was observed with epifluorescence illumination using a Zeiss AxioImager M2.
Quantification and Statistical Analysis
Each cell transformed to express GFP was considered an independent sample for the movement assay. Movement assay results are presented as the average movement of GFP and se. Differences in GFP movement between two given conditions were compared using unpaired heteroscedastic Student’s t tests in Excel, with P < 0.01 considered significantly different.
Independent samples of callose levels (as described above) were compared using unpaired heteroscedastic Student’s t tests in Excel, with P < 0.01 considered significantly different.
An ANCOVA (Fig. 4B) was conducted using Excel, with P < 0.01 considered significantly different.
Accession Numbers
The sequence of NbLHY can be found in the SolGenomics data libraries (https://solgenomics.net) under accession number Niben101Scf02026g01002.1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. PD transport is higher during the day than at night.
Supplemental Table S1. Detailed sampling information for GFP movement assays.
ACKNOWLEDGMENTS
We thank Steven Ruzin and Denise Schichnes at the College of Natural Resources Biological Imaging Facility (University of California, Berkeley) and De Wood and Tina Williams at the U.S. Department of Agriculture Western Regional Research Center for microscopy support. We thank Anne M. Runkel (University of California, Berkeley) for generously providing assistance with conducting experiments. We thank Claire Bendix (University of California, Berkeley) for constructive comments on experimental design and the article.
Footnotes
This work was supported by the National Institutes of Health (grant 5-DP5-OD023072 and a graduate research fellowship to J.O.B.).
Articles can be viewed without a subscription.
References
- Anderson SL, Somers DE, Millar AJ, Hanson K, Chory J, Kay SA (1997) Attenuation of phytochrome A and B signaling pathways by the Arabidopsis circadian clock. Plant Cell 9: 1727–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D (2009) Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA 106: 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Faulkner C, Pendle A, Miyashima S, Helariutta Y, Maule A (2013) Symplastic intercellular connectivity regulates lateral root patterning. Dev Cell 26: 136–147 [DOI] [PubMed] [Google Scholar]
- Besnard F, Refahi Y, Morin V, Marteaux B, Brunoud G, Chambrier P, Rozier F, Mirabet V, Legrand J, Lainé S, et al. (2014) Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 505: 417–421 [DOI] [PubMed] [Google Scholar]
- Bhat S, Folimonova SY, Cole AB, Ballard KD, Lei Z, Watson BS, Sumner LW, Nelson RS (2013) Influence of host chloroplast proteins on Tobacco mosaic virus accumulation and intercellular movement. Plant Physiol 161: 134–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik K, Fernandez JC, Hardin SR, Ernest B, Ganusova EE, Staton ME, Burch-Smith TM (2019) The essential chloroplast ribosomal protein uL15c interacts with the chloroplast RNA helicase ISE2 and affects intercellular trafficking through plasmodesmata. New Phytol 221: 850–865 [DOI] [PubMed] [Google Scholar]
- Brunkard JO, Burch-Smith TM, Runkel AM, Zambryski P (2015) Investigating plasmodesmata genetics with virus-induced gene silencing and an Agrobacterium-mediated GFP movement assay. Methods Mol Biol 1217: 185–198 [DOI] [PubMed] [Google Scholar]
- Brunkard JO, Runkel AM, Zambryski PC (2013) Plasmodesmata dynamics are coordinated by intracellular signaling pathways. Curr Opin Plant Biol 16: 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JO, Zambryski PC (2017) Plasmodesmata enable multicellularity: New insights into their evolution, biogenesis, and functions in development and immunity. Curr Opin Plant Biol 35: 76–83 [DOI] [PubMed] [Google Scholar]
- Burch-Smith TM, Brunkard JO, Choi YG, Zambryski PC (2011) Organelle-nucleus cross-talk regulates plant intercellular communication via plasmodesmata. Proc Natl Acad Sci USA 108: E1451–E1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Zambryski PC (2010) Loss of INCREASED SIZE EXCLUSION LIMIT (ISE)1 or ISE2 increases the formation of secondary plasmodesmata. Curr Biol 20: 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Zambryski PC (2012) Plasmodesmata paradigm shift: Regulation from without versus within. Annu Rev Plant Biol 63: 239–260 [DOI] [PubMed] [Google Scholar]
- Caillaud MC, Wirthmueller L, Sklenar J, Findlay K, Piquerez SJM, Jones AME, Robatzek S, Jones JDG, Faulkner C (2014) The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. PLoS Pathog 10: e1004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotto N, Wirth S, Furman N, Ferreyra Solari N, Ariel F, Crespi M, Kobayashi K (2016) The chloroplastic DEVH-box helicase ISE2 involved in plasmodesmata regulation is required for group II intron splicing. Plant Cell Environ 39: 165–173 [DOI] [PubMed] [Google Scholar]
- Crawford KM, Zambryski PC (2001) Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol 125: 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Lee JY (2016) Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nat Plants 2: 16034. [DOI] [PubMed] [Google Scholar]
- Epel BL, Erlanger MA (1991) Light regulates symplastic communication in etiolated corn seedlings. Physiol Plant 83: 149–153 [Google Scholar]
- Faulkner C, Petutschnig E, Benitez-Alfonso Y, Beck M, Robatzek S, Lipka V, Maule AJ (2013) LYM2-dependent chitin perception limits molecular flux via plasmodesmata. Proc Natl Acad Sci USA 110: 9166–9170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KL, Sozzani R, Lee CM (2014) Intercellular protein movement: Deciphering the language of development. Annu Rev Cell Dev Biol 30: 207–233 [DOI] [PubMed] [Google Scholar]
- Han X, Hyun TK, Zhang M, Kumar R, Koh EJ, Kang BH, Lucas WJ, Kim JY (2014) Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev Cell 28: 132–146 [DOI] [PubMed] [Google Scholar]
- Harmer SL. (2009) The circadian system in higher plants. Annu Rev Plant Biol 60: 357–377 [DOI] [PubMed] [Google Scholar]
- Imlau A, Truernit E, Sauer N (1999) Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SA, Millar AJ (1992) Circadian-regulated cab gene transcription in higher plants In Young MW, ed, Molecular Genetics of Biological Rhythms. Marcel Dekker, New York, pp 73–89 [Google Scholar]
- Khang CH, Berruyer R, Giraldo MC, Kankanala P, Park SY, Czymmek K, Kang S, Valent B (2010) Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22: 1388–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Cho E, Crawford K, Hempel FD, Zambryski PC (2005) Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc Natl Acad Sci USA 102: 2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Wang X, Cui W, Sager R, Modla S, Czymmek K, Zybaliov B, van Wijk K, Zhang C, Lu H, et al. (2011) A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23: 3353–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liarzi O, Epel BL (2005) Development of a quantitative tool for measuring changes in the coefficient of conductivity of plasmodesmata induced by developmental, biotic, and abiotic signals. Protoplasma 225: 67–76 [DOI] [PubMed] [Google Scholar]
- Lim GH, Shine MB, de Lorenzo L, Yu K, Cui W, Navarre D, Hunt AG, Lee JY, Kachroo A, Kachroo P (2016) Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe 19: 541–549 [DOI] [PubMed] [Google Scholar]
- Liu D, Shi L, Han C, Yu J, Li D, Zhang Y (2012) Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 7: e46451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30: 415–429 [DOI] [PubMed] [Google Scholar]
- Masclaux C, Valadier MH, Brugière N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211: 510–518 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97: 743–754 [DOI] [PubMed] [Google Scholar]
- Parry MAJ, Delgado E, Vadell J, Keys AJ, Lawlor DW, Medrano H (1993) Water stress and the diurnal activity of ribulose-1,5-bisphosphate carboxylase in field grown Nicotiana tabacum genotypes selected for survival at low CO2 concentrations. Plant Physiol Biochem 31: 113–120 [Google Scholar]
- Paultre DSG, Gustin MP, Molnar A, Oparka KJ (2016) Lost in transit: Long-distance trafficking and phloem unloading of protein signals in Arabidopsis homografts. Plant Cell 28: 2016–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard WF. (2003) The role of cytoplasmic streaming in symplastic transport. Plant Cell Environ 26: 1–15 [Google Scholar]
- Provencher LM, Miao L, Sinha N, Lucas WJ (2001) Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13: 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AG, Cruz SS, Roberts IM, Prior D, Turgeon R, Oparka KJ (1997) Phloem unloading in sink leaves of Nicotiana benthamiana: Comparison of a fluorescent solute with a fluorescent virus. Plant Cell 9: 1381–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts IM, Boevink P, Roberts AG, Sauer N, Reichel C, Oparka KJ (2001) Dynamic changes in the frequency and architecture of plasmodesmata during the sink-source transition in tobacco leaves. Protoplasma 218: 31–44 [DOI] [PubMed] [Google Scholar]
- Sager R, Lee JY (2014) Plasmodesmata in integrated cell signalling: Insights from development and environmental signals and stresses. J Exp Bot 65: 6337–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowiński P, Bilska A, Barańska K, Fronk J, Kobus P (2007) Plasmodesmata density in vascular bundles in leaves of C4 grasses grown at different light conditions in respect to photosynthesis and photosynthate export efficiency. Environ Exp Bot 61: 74–84 [Google Scholar]
- Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N (2005) Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J 41: 319–331 [DOI] [PubMed] [Google Scholar]
- Stonebloom S, Brunkard JO, Cheung AC, Jiang K, Feldman L, Zambryski P (2012) Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiol 158: 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebloom S, Burch-Smith T, Kim I, Meinke D, Mindrinos M, Zambryski P (2009) Loss of the plant DEAD-box protein ISE1 leads to defective mitochondria and increased cell-to-cell transport via plasmodesmata. Proc Natl Acad Sci USA 106: 17229–17234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120 [DOI] [PubMed] [Google Scholar]
- Turgeon R. (1989) The sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol 40: 119–138 [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D (2015) Retraction: An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 84: 846. [DOI] [PubMed] [Google Scholar]
- Wang X, Sager R, Cui W, Zhang C, Lu H, Lee JY (2013) Salicylic acid regulates Plasmodesmata closure during innate immune responses in Arabidopsis. Plant Cell 25: 2315–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM (2001) Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiol 127: 876–886 [PMC free article] [PubMed] [Google Scholar]
- Yon F, Joo Y, Cortés Llorca L, Rothe E, Baldwin IT, Kim SG (2016) Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers. New Phytol 209: 1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliev R, Epel BL (2015) Imaging callose at plasmodesmata using aniline blue: Quantitative confocal microscopy. Methods Mol Biol 1217: 105–119 [DOI] [PubMed] [Google Scholar]
- Zavaliev R, Ueki S, Epel BL, Citovsky V (2011) Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 248: 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]