Abstract
Muscular hypotonia is considered as one of the rarest forms of initial onset signs of TBM, in addition to aphasia and hyponatremia, the awareness of those rare onset signs, a well‐conducted diagnostic approach and early treatment can improve the outcome.
Keywords: hyponatremia, muscular hypotonia, mycobacterium Tuberculosis, Tuberculosis meningitis
Muscular hypotonia is considered as one of the rarest forms of initial onset signs of TBM, in addition to aphasia and hyponatremia, the awareness of those rare onset signs, a well‐conducted diagnostic approach and early treatment can improve the outcome.
1. INTRODUCTION
Tuberculosis (TB) is a major global health problem. One of its deadliest forms is Tuberculosis meningitis (TBM), which mortality is higher in children and HIV coinfection.1, 2 It remains a highly underdiagnosed disease, so early recognition is crucial. The clinical outcome depends upon the time of therapy initiation. The onset symptoms are widely various, and one of the rarest symptoms are muscular hypotonia and hyponatremia. Every clinician should be aware of these symptoms in order to suspect TBM and treat it efficiently.
The aim of this case presentation is to report an uncommon presentation of meningitis TB presenting with muscular hypotonia in an HIV‐negative patient and its outcome.
2. CASE REPORT
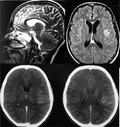
A 24‐year‐old man, with no past medical history or TB contact history, presented to the emergency room with aphasia and a neurological disorder. The patient reported an intermittent fever during the past 3 weeks with headaches and cough. No night sweats, fatigue, or respiratory signs (chest tightness or difficulty in breathing) were noted, and a 3‐kg weight loss was reported. Physical examination revealed an altered conscious with a Glasgow Coma Scale score of 10 out of 15, aphasia, stiff neck, and hemiparesis. These signs were associated with muscular hypotonia with no induced pathologic reflex. Heart rate was at 135 bpm with a regular sinus rhythm, and no abnormal heart murmurs were noted. Lung auscultation was clear. There were no palpable superficial lymph nodes. Due to the neurological disorder, the patient was intubated and sedated then admitted to the intensive care unit. Blood examinations showed an hemoglobin level of 12.6 g/dL, a white blood cell count of 29 840 mm3/mL, C‐reactive protein rate was 172 mg/mL, and low sodium level. Human immunodeficiency virus detection was negative. The chest X‐ray (Figure 1) revealed generalized symmetrical interstitial infiltrates. Chest CT‐scan showed basal consolidation in both lung fields with pleural effusion (Figure 1). An abdominal ultrasonography was done, but it was without abnormalities. Brain CT‐scan was initially performed and showed subarachnoid hemorrhage over the left fronto‐parietal region and on the right parietal region. Cerebrospinal fluid (CSF) analysis showed 80 cells mm3/mL with lymphocytes predominance (80%), a low glucose level (16.4 mg/dL), with a central glucose level of 103 mg/dL (ratio 0.15), and an elevated proteins level (219 mg/dL). Meningoencephalitis was suspected and a brain MRI was performed. The MRI showed leptomeningeal enhancement on the left fronto‐parietal region (Figure 2). First‐line antituberculosis drugs (Isoniazid, rifampicin, pyrazinamide, and ethambutol) associated with dexamethasone (0.4 mg/kg/day) were started. Gene Xpert MTB/Rifampicin was positive for MTB both in CSF and distal protected aspirate so the diagnosis of TBM was retained. During hospitalization, the patient developed a Ventilator Associated Pneumonia with a septic shock due to a multiresistant Pseudomonas aeruginosa treated by Imipenem and Amikacin. On the 15th day of hospitalization, a tracheotomy was performed after a failed extubation attempt due to the still present muscular hypotonia. After 1‐month of anti‐TB treatment with HRZE, weaning from mechanical ventilation was done successfully. And, the patient still presented the muscular hypotonia, which was responsible for a persisting right ptosis and a swallowing dysfunction (the diagnosis of tracheoesophageal fistula was eliminated), and finally, the patient was discharged.
Figure 1.
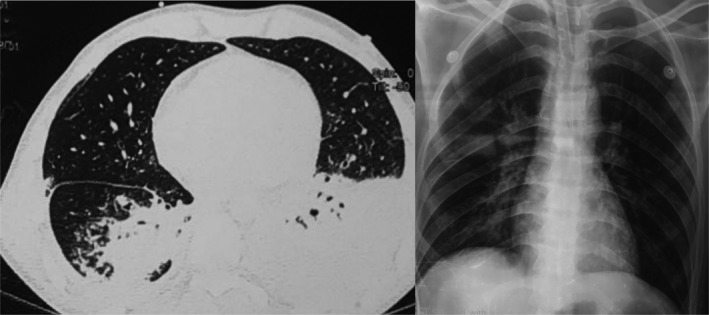
Chest X‐ray and CT scan showing basal consolidation in both lung fields with pleural effusion
Figure 2.
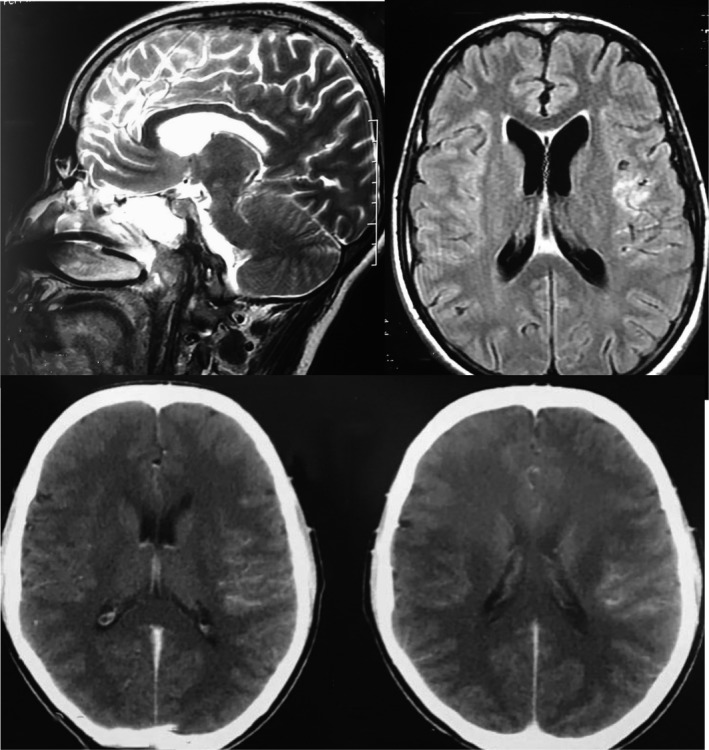
Contrast‐enhanced magnetic resonance image of brain showing meningeal enhacement
3. DISCUSSION
Tuberculosis remains a worldwide chronic infectious disease. It is caused by the Mycobacterium tuberculosis. Various extrapulmonary manifestations were described. Tuberculosis meningitis accounts for 1% of all new TB reported cases annually, and this form is considered as the most serious one with a high‐mortality rate.3 Tuberculosis meningitis is a result of hematogenous spread of Mycobacterium, and patients with HIV‐infection or any other immunodeficiency diseases are most likely to suffer from it. Other risk factors exists as well as including diabetes mellitus, alcoholism, use of immunosuppressive drugs, and corticosteroid treatment.2, 4
As for the clinical signs, they are nonspecific therefore hard to distinguish from other bacterial meningitis. Usual bacterial Meningitis symptoms such as headaches, fever, and stiff neck are typical but they could be absent especially in early stages. And their persistence, for several days to several months, is considered highly predictive of TBM.5 In our case, the patient presented with an advanced clinical stage characterized by cranial nerve (CN) palsies (most commonly seen are CN III, VI, and VII), hemiparesis in addition to Glasgow Coma Scale score of 10 or less.6, 7 Although, in 50% of cases, an active or a past history of pulmonary TB is objectified by chest X‐ray.7
When TBM is suspected, lumbar puncture should be repeated in order to increase the diagnostic yield. Biochemical cerebrospinal fluid (CSF) analysis shows that the percentage of lymphocytes is one of the strongest predictors of TBM.8, 9 Protein levels could be elevated in addition to the CSF glucose/blood glucose ratio <0.5. The diagnosis often remains problematic and difficult. Microscopic detection of Mycobacterium and culture is with low sensitivity and requires a long time then Polymerase chain reaction (PCR) has been used with a specificity of 100%.10, 11
In 40% of patients with TBM, hyponatremia (plasma sodium level < 135 mmol/L) was present. This hyponatremia can be explained by cerebral salt wasting (CSW), which includes low sodium levels, natriuresis, and volume contraction caused by brain injury as main characteristics, as well as the syndrome of inappropriate antidiuretic hormone (SIADH). However, distinguishing between SIADH and CSW is difficult and critical.12
Due to the nonspecific clinical presentation of TBM, neuroimaging findings are important for diagnosis. However, MRI proved more accurate than brain CT‐scan. And therefore, it is considerate as the primary neuroimaging diagnosis modality in TBM. The most common finding in the brain MRI is meningeal enhancement, hydrocephalus, basal exudates, infarcts (occur as a result of vasculitis), and tuberculomas.13
According to the British infection society guidelines,14 chemotherapy for TBM should follow the model of pulmonary tuberculosis: An intensive phase of treatment followed by a continuation one. Isoniazid with its good CSF penetration and potent bactericidal activity and rifampicin are the main first‐line agents in the pharmacological treatment.15 However, there are no controlled trials data guiding the choice of the fourth drug but the total length of treatment varies from six to 12 months. As for the adjunctive corticosteroid treatment of TBM, it is proved to improve the outcome in HIV‐negative patients but its benefit remains uncertain in HIV‐infected ones.16
4. CONCLUSION
Tuberculosis infection of the CNS accounts for about 5% of all cases of TB and is highly underdiagnosed because it can be presented by a broad range of typical and atypical signs. Although, muscular hypotonia is considered as one of the rarest forms of initial onset signs of TBM, in addition to aphasia and hyponatremia, the awareness of those rare onset signs, a well‐conducted diagnostic approach and early treatment can improve the outcome of this infection.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Dr Mohamed Aziz Daghmouri, Dr Charni Imen, Dr Selma Ghedira, and Dr Mohamed Houissa: contributed to initial diagnostics and treatment of the patient. Dr Lotfi Rebai, Dr Cheima Ghouibi, and Dr Mohamed Aziz Daghmouri: contributed to case report manuscript.
Daghmouri MA, Cherni I, Rebai L, Ghouibi C, Ghedira S, Houissa M. Muscular hypotonia as an onset manifestation of Tuberculosis meningitis in an HIV‐negative patient. Clin Case Rep. 2019;7:2177–2180. 10.1002/ccr3.2474
REFERENCES
- 1. Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta‐analysis. Lancet Infect Dis. 2014;14(10):947‐957. [DOI] [PubMed] [Google Scholar]
- 2. Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS ONE. 2011;6(5):e20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chin JH. Tuberculous meningitis. Neurol Clin Pract. 2014;4(3):199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cecchini D, Ambrosioni J, Brezzo C, et al. Tuberculous meningitis in HIV‐infected patients: drug susceptibility and clinical outcome. AIDS Lond Engl. 2007;21(3):373‐374. [DOI] [PubMed] [Google Scholar]
- 5. Katrak SM, Shembalkar PK, Bijwe SR, Bhandarkar LD. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J Neurol Sci. 2000;181(1‐2):118‐126. [DOI] [PubMed] [Google Scholar]
- 6. Christensen A‐SH, Andersen AB, Thomsen VO, Andersen PH, Johansen IS. Tuberculous meningitis in Denmark: a review of 50 cases. BMC Infect Dis. 2011;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005;4(3):160‐170. [DOI] [PubMed] [Google Scholar]
- 8. Moghtaderi A, Alavi‐Naini R, Izadi S, Cuevas LE. Diagnostic risk factors to differentiate tuberculous and acute bacterial meningitis. Scand J Infect Dis. 2009;41(3):188‐194. [DOI] [PubMed] [Google Scholar]
- 9. Youssef FG, Afifi SA, Azab AM, et al. Differentiation of tuberculous meningitis from acute bacterial meningitis using simple clinical and laboratory parameters. Diagn Microbiol Infect Dis. 2006;55(4):275‐278. [DOI] [PubMed] [Google Scholar]
- 10. Pai M, Flores LL, Pai N, Hubbard A, Riley LW, Colford JM. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta‐analysis. Lancet Infect Dis. 2003;3(10):633‐643. [DOI] [PubMed] [Google Scholar]
- 11. Shirani K, Talaei Z, Yaran M, Ataei B, Mehrabi‐Koushki A, Khorvash F. Diagnosed tuberculous meningitis using cerebrospinal fluid polymerase chain reaction in patients hospitalized with the diagnosis of meningitis in referral hospitals in Isfahan. J Res Med Sci Off J Isfahan Univ Med Sci. 2015;20(3):224‐227. [PMC free article] [PubMed] [Google Scholar]
- 12. Davis A, Meintjes G, Wilkinson RJ. Treatment of Tuberculous meningitis and its complications in adults. Curr Treat Options Neurol. 2018;20(3):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raut T, Garg RK, Jain A, et al. Hydrocephalus in tuberculous meningitis: Incidence, its predictive factors and impact on the prognosis. J Infect. 2013;66(4):330‐337. [DOI] [PubMed] [Google Scholar]
- 14. Thwaites G, Fisher M, Hemingway C, et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167‐187. [DOI] [PubMed] [Google Scholar]
- 15. Pouplin T, Bang ND, Toi PV, et al. Naïve‐pooled pharmacokinetic analysis of pyrazinamide, isoniazid and rifampicin in plasma and cerebrospinal fluid of Vietnamese children with tuberculous meningitis. BMC Infect Dis. 2016;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4:1‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]


