Abstract
Background
Heart failure (HF) is a chronic disease that affects over 1% of Canadians and at least 26 million people worldwide. With the continued rise in disease prevalence and an aging population, HF-related costs are expected to create a significant economic burden. Many mobile health (mHealth) apps have been developed to help support patients’ self-care in the home setting, but it is unclear if they are suited to the needs or capabilities of older adults.
Objective
This study aimed to identify HF apps and evaluate whether they met the criteria for optimal HF self-care.
Methods
We conducted a systematic search of all apps available exclusively for HF self-care across Google Play and the App Store. We then evaluated the apps according to a list of 25 major functions pivotal to promoting HF self-care for older adults.
Results
A total of 74 apps for HF self-care were identified, but only 21 apps were listed as being both HF and self-care specific. None of the apps had all 25 of the listed features for an adequate HF self-care app, and only 41% (31/74) apps had the key weight management feature present. HF Storylines received the highest functionality score (18/25, 72%).
Conclusions
Our findings suggest that currently available apps are not adequate for use by older adults with HF. This highlights the need for mHealth apps to refine their development process so that user needs and capabilities are identified during the design stage to ensure the usability of the app.
Keywords: mHealth, heart failure, self-care, mobile phone
Introduction
Heart failure (HF) is the most important cardiovascular condition leading to hospitalization and rehospitalization in older adults, and it has a significant economic burden [1]. Despite an overall decline in HF hospitalization rates, readmission rates remain high [2,3]. A systematic review found that HF readmissions can be reduced if patients with HF adopt self-care (hazard ratio [HR] 0.80; 95% CI 0.71-0.89) [3,4]. Specifically, weight monitoring has been identified as a pivotal component of HF self-care as weight gain has been independently associated with a poor postdischarge prognosis, considering it is the last common step before worsening of clinical outcomes (HR per kg increase 1.16; 95% CI 1.09-1.23; P<.001) [5]. However, many older adults find daily weight monitoring and adjusting diuretics to be challenging [6-8]. In addition to managing comorbid conditions, many older adults with HF exhibit mild cognitive impairment and poor medication adherence, both of which are associated with reduced ability to self-care [9-11]. To adequately promote HF self-care, strategies should be targeted to the patient’s cognitive capabilities, learning needs, and literacy and numeracy levels [11].
Mobile health (mHealth) apps have been developed to support patients with self-care [12,13]. Unfortunately, although the initial uptake of mHealth apps looked promising, the majority of individuals have stopped using them because of reasons such as a loss of interest, manual data entry burden, and hidden costs [14,15]. Older adults do not commonly use mHealth apps because of the perception that they are not suited to their needs or capabilities [16]. This may explain the shortcomings of previous programs that have failed to promote self-care and utilize the opportunity to help decrease HF-related hospitalizations, deaths, and costs to health care systems [9-12].
Previous studies have reviewed current apps for HF self-care and found that there are limited number of apps available to support disease management [12,17]. Nevertheless, these studies were unable to effectively evaluate app quality because of their lack of disease specificity within the rating scale design [18,19]. For example, the commonly used Mobile Application Rating Scale (MARS) was able to provide an overall assessment of the quality of apps with respect to engagement, functionality, aesthetics, information, and subjective opinion, but it does not evaluate the usability or effectiveness of the app features specific for the disease population [12,15,18,19]. Therefore, generic health apps (eg, WebMD) receive higher app quality scores using the MARS even if they do not have crucial app features (eg, weight management) for proper self-care [17]. The lack of disease specificity can be attributed to the absence of a reference architecture to guide the scale’s development. Other chronic disease app rating scales or checklists have been developed using similar constructs as the MARS, leaving them to face the same shortcomings with their app evaluations [20,21]. This highlights the need to further evaluate the adequacy of the current HF self-care apps available.
To address this gap, we conducted a systematic search of all the apps currently available exclusively for HF self-care. We used Chindalo et al’s peer-reviewed mHealth app reference architecture to define the app design requirements [19]. Contrary to other rating scales, this architecture allows us to combine the evaluative components related to the aesthetics, usability, and HF self-care to effectively evaluate whether the current HF apps are meeting the end user’s self-care needs and capabilities [19]. The objective of this study was to determine the number of HF apps available and evaluate whether they met the criteria to promote HF self-care.
Methods
Search Strategy
We conducted an extensive search across Google Play and the App Store to identify all available apps for HF self-care. The search was facilitated with the use of following key terms: HF management, HF manager, HF self-care, HF, and HF tracker. Apps were included in the review if they (1) were HF specific and (2) contained a self-care component (ie, medication, symptom management, reminder system, and behavior tracking). Apps were excluded if they were intended for use in a conference, for education, or for reference purposes.
App Adequacy Assessment
In accordance with Chindalo et al’s reference architecture, we developed a list of 25 major functions that would promote HF self-care for older adults [19,22-24] (Table 1). These features were identified according to HF self-care and patient-engagement guidelines [19] as well as the expertise from our clinician authors (CD and KK). Before the start of the study, a design session was conducted where CD (cardiologist/HF specialist) and KK (family physician/clinical information technology architect) created individual lists for potential app features. A second design session was conducted with CD, KK, and SW to finalize the list of app functions as well as the specific functions required for app adequacy. All 25 major functions were not deemed required to adequately promote HF self-care but were more beneficial if included. App adequacy was determined if the following standard disease management features were included: (1) diagnosis, (2) weight, (3) behavior tracking, (4) self-care, and (5) notifications. These 5 features were chosen based on their ability to capture factors related to HF management protocol, personalized care for older adults, and health promotion [25,26].
Table 1.
List of app features required for an adequate heart failure self-care app.
| # | App feature | App descriptors |
| 1 | Prescribed | Physician prescribed for treatment; pharmacist recommendation |
| 2 | Diagnosisa | Patient predetermined diagnosis included (acute heart failure) |
| 3 | Patient demographics | Age; sex or gender; location |
| 4 | Patient sociocultural | Literacy; numeracy; socioeconomic status; culture/ethnicity; parental history |
| 5 | Patient symptoms | Shortness of breath; dizziness; orthopnea; leg edema or general swelling; paroxysmal nocturnal dyspnea |
| 6 | Patient behaviors | Smoking; exercise; fitness/movement; salt intake |
| 7 | Patient physiological observations | Heart rate; blood pressure; elevated jugular venous pressure; chest crackles; heart murmurs |
| 8 | Weighta | Management; monitoring; tracker |
| 9 | Comorbidities | Presence of other diseases (eg, diabetes and hypertension) |
| 10 | Drug list | List of medications |
| 11 | Laboratory results | Hemoglobin and hematocrit; creatinine and estimated glomerular filtration rate; brain natriuretic peptide; thyroid stimulating hormone; lipid profile |
| 12 | Diagnostic testing | Electrocardiogram; chest x-ray; echocardiogram |
| 13 | Behavior trackinga | Diet; exercise; patient-reported experience; compliance with medications |
| 14 | Education/recommendation | Behaviorally appropriate; culturally appropriate; health literacy appropriate; accredited/credible sources; evidence based |
| 15 | Self-carea | Self-maintenance; self-management: system provides patient with recommendation if clinical condition changes (eg, if weight increases, take extra Lasix); algorithm based or physician guidance; self-confidence |
| 16 | Health system utilization | Reviewed by family doctor; reviewed by nurse clinician, practitioner, or physician assistant; visit to EDb; hospitalization; seen by specialist |
| 17 | Notificationsa | Presence of reminder or notification |
| 18 | Integrations | Integrated into personal health record and electronic medical record; integrated into other health and fitness apps |
| 19 | Social supports | Connect/share results with caregiver or family; contact caregiver or family |
| 20 | Patient reported outcome measure/patient reported experience measure | Patient experience of care; app experience; quality of life; cognitive assessment; patient progress |
| 21 | Incentives to use | Easy access to provider; gamification; social aspect—connect with others |
| 22 | Predictive analytics | Length of stay, acuity of admission, comorbidities, ED visits, (readmissions); hospital admission risk prediction |
| 23 | Outcomes | Visit to family physician, specialist, or ED; hospitalized; death |
| 24 | Safety issues | Risk of falls; worsening kidney function; hyper- or hypokalemia |
| 25 | User interface | Easy to navigate functionality; simple to screen with minimal content on each page; features for visual (font size and color), hearing (audio cues), or general accessibility |
aStandard disease management feature for heart failure.
bED: emergency department.
Within each of the 25 functions, a list of descriptors was developed to help specify the components within the listed function. If the app included 1 of the descriptors, the feature was listed as present (Table 1). For example, the self-care feature consisted of 3 components including self-maintenance, self-management, and self-confidence [6,7]. Self-maintenance includes actions associated with treatment adherence, such as taking medication or following treatment regimens. Self-management includes the recognition of and response to changes in symptoms. Finally, self-confidence refers to the individual’s assurance in implementing necessary decisions during the management process. Self-confidence is not an explicit self-care behavior but has been recognized as an important moderator of self-care effectiveness [6,7,27]. Thus, self-confidence would not be captured in app functionality as clearly as self-maintenance and self-management, but instead could be expressed as a series of patient experience–related questions (Figure 1). Overall, if an app included any 1 of the 3 self-care components, the feature would be counted as being present.
Figure 1.
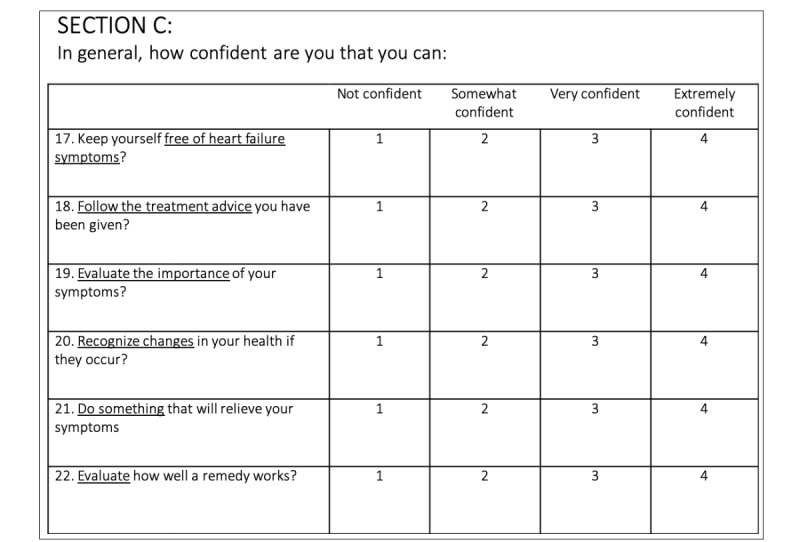
Sample heart failure self-care confidence questions.
App Screening and Evaluation
A total of 2 reviewers (SW and KK) completed a preliminary screening of apps available in both Google Play and the App Store using the key terms mentioned previously. Following the search, apps were reviewed according to their title and summary description (Figure 1). This screening served as a method to separate the bulk of non-HF self-care apps from further evaluation.
Once screening was completed, a calibration session was held among the 8 reviewers (SW, KK, AG, AD, HW, HS, NN, and DL). During the calibration session, each reviewer was asked to evaluate and score the same 5 apps before the start of the session (Figure 1). Reviewers had a Google Sheet created for them with the list of functions for evaluation. During the session, the 5 apps were then reviewed on a summary sheet, and any discrepancies regarding scoring were discussed and resolved. This allowed us to standardize the training among all reviewers and ensure that we had at least an 85% agreement rate when evaluating the remaining apps. Additional information and comments from the calibration session were recorded and added to the final protocol. The evaluation sheet on Google Sheets was also revised for the final app evaluations.
After the calibration session, the remaining apps were assigned for evaluation, where 2 different reviewers evaluated each app. Each evaluation was completed on the revised Google Sheet with the respective app assignments. Once all the app evaluations were completed, the data were combined into a summary sheet for review. Each app score was then reviewed by the 2 assigned reviewers to ensure that they were within the 85% agreement rate. To determine if the scores met the 85% agreement rate, we conducted interrater agreement statistic and reviewed the kappa value. If the apps did not meet the 85% agreement threshold, the 2 reviewers completed an in-person or virtual evaluation session to review the app discrepancies. Both reviewers were required to provide evidence (ie, screenshot or quote) to support the presence of the feature, and a discussion was held until consensus was achieved. All supporting evidence was sent to SW for a final review. Following consensus, a postreview screening was then completed to filter out any apps that were not HF or self-care specific. The remaining apps within the inclusion criteria were analyzed through a descriptive analysis to assess the app search’s findings.
Reviewers evaluated each app based on the description, screenshots, videos, and reviews available on each app store website. Owing to the limited resources and to ensure a consistent method of app evaluation, we did not download the apps. Our rationale for not downloading apps was also based on the premise that users decide to download an app after reviewing it externally [28-31]. Many of the guidelines assisting patients with choosing a health app have urged users to become more meticulous with the apps they install and, in turn, have provided them with a series of questions to consider before downloading [29-31]. For example, in 2 articles, they suggested users consider the following questions before downloading an app: (1) does the app consider your needs (ie, symptoms and disease management), (2) is the app made by a health care system/physician or by a controversial company (ie, pharmaceutical company), (3) does the app have positive reviews (ie, on the Web or by physicians), (4) is the app regularly updated, and (5) are the app features relevant for you (ie, review screenshots and description of features) [29,30]. Currently, about 90% of most information for decision making about app adoption is available in its documentation [29,32,33]. Given the minimal incremental information available from the app itself, we felt that downloading the apps would not significantly change our evaluation.
In accordance with the guidelines for app review before download, reviewers also extracted the following data from each app: number of downloads, date of last update, cost, and developer [29,30]. However, considering that apps from the App Store do not publicly list their number of downloads or date of last update, reviewers omitted the number of downloads criteria for these apps and used the latest version date for the last update.
Reviewer Training
Each reviewer selected was equipped with postsecondary experience in the electronic health or health technology field to allow them to effectively evaluate the respective apps. Reviewers were required to follow the training protocol in accordance with the mHealth design architecture as well as attend the calibration session previously described [19].
Results
Preliminary screening identified a total of 74 apps as HF self-care apps within the combined app store searches (Figure 2). From these 74 apps, none of the apps had all the 25 listed features required to promote HF self-care, and only 32% (24/74) apps had 10 features or more present. Moreover, only 51 out of the 74 apps had a self-care feature present. Instead, the majority of the apps reviewed were used mainly for education purposes (Table 2).
Figure 2.
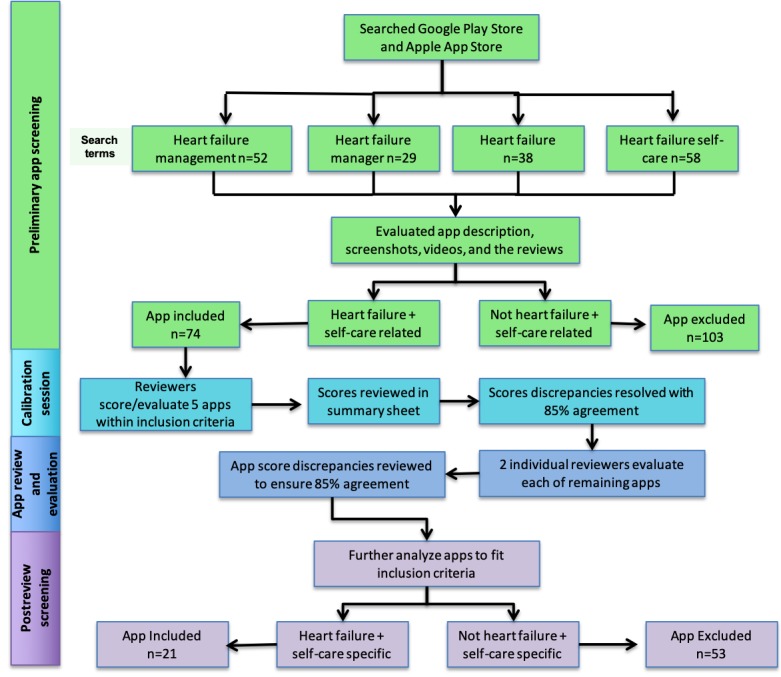
Conceptual study design of the mobile health app review.
Table 2.
Features present in the reviewed heart failure self-care apps (N=74).
| App feature | Apps, n (%) |
| Education/recommendations | 67 (90) |
| Self-care | 51 (68) |
| User interface | 51 (68) |
| Diagnosis | 40 (54) |
| Notifications | 33 (44) |
| Weight | 31 (41) |
| Patient demographics | 28 (37) |
| Patient symptoms | 27 (36) |
| Patient physiological observations | 26 (35) |
| Behavior tracking | 22 (29) |
| Patient behaviors | 19 (25) |
| Drug list | 18 (24) |
| Patient reported outcome measure/patient reported experience measure | 18 (24) |
| Incentives to use | 18 (24) |
| Comorbidities | 9 (12) |
| Lab results | 9 (12) |
| Diagnostic testing | 9 (12) |
| Social supports | 9 (12) |
| Prescribed | 8 (10) |
| Health system utilization | 8 (10) |
| Outcomes | 7 (9) |
| Predictive analytics | 6 (8) |
| Patient socio-cultural | 6 (8) |
| Safety issues | 0 (0) |
Following the postreview screening, 21 apps were listed as both HF and self-care specific. Moreover, 53 apps were excluded for the following reasons: (1) HF specific but not for self-care (n=9), (2) used for self-care but not specifically for HF (n=16), and (3) neither HF nor self-care specific but used for general cardiac education (n=28). As there is an increasing number of apps for entertainment or novelty purposes, from the 53 apps excluded, 12 were shortlisted for this reason. From the 21 HF self-care apps included, more than 50% (12/21, 57%) of the apps had 10 features or more (Table 3; Multimedia Appendix 1). The apps scores ranged from 0 to 18, where HF Storylines achieved the highest score. The most prevalent feature among these apps was diagnosis and user interface (20/21, 95%; Table 3). However, from the included apps, only 1 was found to have been evaluated by patients or investigated in a clinical setting (Medly). All reviewer’s app scores fell within the 85% agreement rate (mean kappa=0.86).
Table 3.
Features present in filtered heart failure self-care apps (N=21).
| App feature | Apps, n (%) |
| Diagnosis | 20 (95) |
| User interface | 20 (95) |
| Self-care | 19 (90) |
| Notifications | 18 (85) |
| Education/recommendations | 17 (80) |
| Weight | 17 (80) |
| Patient demographics | 13 (61) |
| Patient symptoms | 13 (61) |
| Patient physiological observations | 11 (52) |
| Drug-list | 11 (52) |
| Behavior tracking | 10 (47) |
| Incentives to use | 10 (47) |
| Patient behaviors | 9 (42) |
| Integrations | 8 (38) |
| Patient reported outcome measure/patient reported experience measure | 8 (38) |
| Social supports | 5 (23) |
| Health system utilization | 4 (19) |
| Prescribed | 4 (19) |
| Patient sociocultural | 3 (14) |
| Lab results | 3 (14) |
| Comorbidities | 2 (9) |
| Diagnostic testing | 0 (0) |
| Predictive analytics | 0 (0) |
| Outcomes | 0 (0) |
| Safety issues | 0 (0) |
Many apps include features such as patient demographics, patient symptoms, education, self-care, notifications, and, most notably, weight. Unexpectedly, less than 50% (9/21) of the apps included a patient behavior or a behavior tracking feature, both of which are vital for adequate HF self-care (Table 3). Only 11 of the apps included a drug list within the app to keep track of medications or, more specifically, the usage of diuretics. In addition, a limited number of apps included social support (5) or were by prescription (4), and even fewer included a patient sociocultural (3) or comorbidities (2) feature. None of the reviewed apps included diagnostic testing, predictive analytics, outcomes, or safety measures (Table 3).
Table 4 displays a list of the total score of the filtered HF self-care apps with their respective app characteristics. Of the apps available, only 9 listed their number of downloads, and among these apps, the number of downloads varied with the total app scores. Surprisingly, the lowest-scoring app had a higher number of downloads compared with the highest-scoring app. This discrepancy could be linked to more effective marketing strategies, public brand awareness, or a well-regarded national health organization for certain apps [31,32].
Table 4.
Total score of the filtered heart failure self-care apps and their corresponding number of downloads, last updates, and cost (N=21).
| App name | Total app score | Number of downloads | Last updated | Cost (US $) | Developer |
| Heart Failure | 2 | 1000-5000 | December 22, 2014 | 0 | Leon Do |
| HF Coach | 5 | —a | April 29, 2016 | 50 | Etectera Edutainment Inc |
| HF Defender | 7 | 1000-5000 | November 10, 2016 | 0 | Cardio Fortress |
| HF Buddy | 8 | — | June 08, 2016 | 0 | Singapore Health Services |
| HF Monitoring | 8 | — | February 22, 2017 | 0 | Van Phuc Nguyen |
| HF Tracker | 8 | — | October 31, 2014 | 0 | Rebecca Boxer |
| HF Path | 8 | — | January 04, 2017 | 7 | American Heart Association |
| HF Log | 9 | — | February 09, 2016 | 0 | Narnar LLC |
| HF Buddy | 9 | 500-1000 | April 08, 2016 | 0 | — |
| Heart Scribe | 10 | 50-100 | July 30, 2016 | 0 | Rohan Tanjea |
| Heart Failure Health | 11 | — | — | 0 | Self-Care Catalyst Inc |
| Health Plus | 12 | — | May 12, 2016 | 0 | Hany Assaad |
| Heart Lessons | 12 | — | Apr 17, 2017 | 0 | Palo Alto Medical Foundation for Health Care, Research and Education |
| My HF | 12 | 1000-5000 | September 22, 2016 | 0 | Les Laboratoires Servier |
| WOW ME 200mg | 12 | 100-500 | July 24, 2013 | 0 | AtantiCare Regional Medical Center Inc |
| HF Self- Management | 13 | — | August 11,2015 | 0 | — |
| Pulsario | 14 | — | October 07, 2016 | 0 | Cardio Fortress Inc |
| My Heart Mate | 14 | — | November 14, 2016 | 0 | Elevator Entertainment |
| Heart Partner | 16 | — | October 07, 2016 | 0 | Novartis Pharmaceuticals |
| Medly | 17 | — | Ongoing | — | University Health Network |
| HF Storylines | 18 | 500-1000 | March 10, 2017 | 0 | HF Society of America |
aNot available.
With respect to cost, the majority of apps could be downloaded for free; however, 2 apps had an associated cost. For consistency, apps were not downloaded. As a result, we found that the 2 apps with a download cost had relatively lower scores (score of 5=US $50 and score of 8=US $7) and did not list their number of downloads.
Each app’s last update varied from 2013 to 2019; however, most of the recently updated apps received higher scores. One of the most recently updated apps (HF Storylines) obtained the highest total app score of 18.
Discussion
Principal Findings
Self-care is pivotal for HF patients to prevent worsening of HF, yet the majority of current HF apps available are neither HF or self-care specific. To our knowledge, this is the first study to identify and evaluate apps exclusively for HF self-care. We found 21 apps that were both HF and self-care specific. From the 21 apps, few contained key features such as behavior tracking. Apps that included the self-care feature were also listed as only being capable of self-maintenance. Thus, patients would be able to, at most, follow their treatment regimen but would not be able to respond to any changes. Potential features to expand on self-care could include medication titration algorithms to adjust medication doses according to weight fluctuations or the use of telehealth services to connect with a physician to modify their treatment regime [34,35]. Loop diuretics are currently used as the agent of choice for reducing symptoms of HF and controlling weight. Diuretics are traditionally adjusted by physicians. However, with self-directed medication titration becoming more commonly used for chronic disease management, this feature could be an opportunity to improve patient HF self-care in the home setting [34,35].
Our findings suggest that the current available apps are not able to support patients adequately with HF self-care; instead, are in need of further redesign or development. Many developers may have limited resources to accommodate all 25 features in a single app. Therefore, to appropriately engage patients in self-care, apps should at minimum have the following functions: (1) diagnosis, (2) weight, (3) behavior tracking, (4) self-care, and (5) notifications. However, from our systematic search, none of the apps even had these 5 functional features. Not only are these app features key for HF self-care but they can also be easily transferable to other health conditions, such as diabetes or asthma, as it captures the essential components for treatment management. The specifics detailing each feature will differ depending on the condition, but it provides a sufficient baseline category to allow the consumer, researcher, or clinician to incorporate key components for the app evaluation. The consumer, researcher, or clinician may list similar or different components within the 5 features, but with this, they are able to incorporate their perspective within 5 wider categories, while maintaining its relevance for multiple audiences. A prime example of an effective mHealth app is BlueStar from WellDoc Diabetes Management. The BlueStar app is a digital therapeutic for diabetes mellitus type 2 that serves as a virtual coach for patients, providing tailored guidance and facilitating the coordination of diabetes care with their existing care team [36]. BlueStar is a clinically validated tool developed by endocrinologists and clinical diabetes educators and has been evaluated in a clinical trial and reviewed in over 40 publications [37,38]. Patients who used BlueStar showed significant improvements in their diabetes management and reported a high satisfaction when using the app. These improvements are strongly linked to the diabetes expertise leading the development and evaluation of this tool, as their guidance helps ensure that the intervention is aligned with self-management principles integral to patient care [36,37]. Older adults with chronic disease already face many challenges with managing their condition, and the use of technology may further contribute to their difficulties if poorly designed. To ensure apps assist with patient self-care regimens, they should be developed in a manner similar to BlueStar, where specific disease management and patient usability criteria are used to both design and evaluate app effectiveness [37].
One surprising finding from this app search was that the lowest-scoring apps had a relatively higher number of downloads compared with the highest-scoring app (Table 4). These findings ultimately question whether the inclusion of more app features or just the standard features for disease management are more appealing for the end user. Features that patients and consumers view as valuable can vary depending on their self-care abilities. However, many studies have indicated that the primary reason apps fail to maintain user activity is because of the complexity of the app as a whole or the lack of growth in app functionality in accordance with user needs [39,40]. Thus, as effective self-care promotion is the primary goal of app usage, apps would require the inclusion of the standard disease management features, but their presentation should also be modified to accommodate for the challenges older adults face with app use. In previous literature, older adults have indicated that in-app customizable considerations improved the likelihood of their continued usage as they were able to modify their preferences according to their changing needs [20,21]. This could include characteristics such as customizing screen or font sizes and incorporating text to speech, audio cues, or in-app automation features as needed [21,22].
Nevertheless, it is also important to note that the fact that lower-scoring apps had higher downloads can also be attributed to several external factors promoting public app awareness. This includes factors such as effective marketing, links to a national health body, or the use of Web-based search engine optimization [29,32]. From our findings, we found that the app with the highest downloads (My HF) had a moderate score of 12, but it was developed by a privately owned pharmaceutical company that specializes in medication for cardiological conditions (Les Laboratories Servier). Although apps built by health care systems scored the highest, they had much lower download rates (Table 4). With these discrepancies between app scores and the number of downloads, our findings display how higher downloads may not be an appropriate representation for app effectiveness because of its potential ties to a developer with more marketing power.
Limitations
The limitations of our study were as follows: (1) the apps were not downloaded but were reviewed based on app description, screenshots, videos, and reviews from current/past users, and there are good reasons to believe that the quality of the assessment is not severely compromised, as mentioned above; (2) descriptions for review varied in detail and quality (eg, download data not available for Apple iOS apps); (3) we were only able to review the number of downloads but could not quantify active app use; and (4) actual HF patients were not consulted to define the criteria for adequate HF self-care app.
Future Research
Future studies should involve end users to better understand their needs with the design of an app to ensure the uptake and usability of an intervention. Specifically, the use of engagement strategies with HF patients and health care providers would be strongly desirable to ensure the findings of this study are congruent with what is experienced in reality. In our app evaluation, we incorporated app user reviews to assess user perspectives, but this method is limited to the feedback available and the quality of the responses on the Web. This study also did not evaluate the value of the 5 functional criteria for HF self-care compared with the remaining categories. Future studies should aim to understand the relative importance of each criteria in relation to patient outcomes, potentially with the use of focus groups or user testing to develop priority weightings for each function. In addition to this, as the potential rise in scale modification for disease specificity could lead to inappropriate features being selected for app shortlisting, there is a need to also evaluate the priority features in relation to other common chronic conditions (ie, diabetes and asthma). We believe there is value in the inclusion of the 5 minimum features for app evaluation, as it allows for specific components to be embedded within wider priority categories and provides a baseline mode to manage the use of multiple modified scales. However, before any scales can be managed or reviewed, future studies need to confirm the reliability of the 5 features for the management of priority app categories.
Conclusions
In summary, our study was the first to specifically evaluate HF self-care apps according to the criteria essential to promote HF self-care for older adults. We found that there was a lack of usable apps to promote HF self-care for older adults, and this is mainly because of the lack of a patient-centered design. With a rise in the aging population, identifying features pivotal for patient self-care will be crucial to increase their user experience and ensure the longevity of the app’s use.
Acknowledgments
The authors would like to thank Everett McKay for providing his ongoing expertise throughout the design and conduct of this project.
Abbreviations
- HF
heart failure
- HR
hazard ratio
- mHealth
mobile health
- MARS
Mobile Application Rating Scale
Appendix
Total features present in preliminary and postreview screening apps. PROMS: patient reported outcome measure; PREMS: patient reported experience measure.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Heart and Stroke Foundation of Canada. 2017. [2019-09-16]. 2016 Report on the Health of Canadians: The Burden of Heart Failure https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-heart-month/heartandstroke-reportonhealth-2016.ashx?la=en.
- 2.Gandhi S, Chen S, Hong L, Sun K, Gong E, Li C, Yan LL, Schwalm J. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2017 Feb;33(2):219–31. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Cajita MI, Gleason KT, Han H. A systematic review of mhealth-based heart failure interventions. J Cardiovasc Nurs. 2016;31(3):E10–22. doi: 10.1097/JCN.0000000000000305. http://europepmc.org/abstract/MED/26544175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lycholip E, Aamodt IT, Lie I, Šimbelytė T, Puronaitė R, Hillege H, de Vries A, Kraai I, Stromberg A, Jaarsma T, Čelutkienė J. The dynamics of self-care in the course of heart failure management: data from the IN TOUCH study. Patient Prefer Adherence. 2018;12:1113–22. doi: 10.2147/PPA.S162219. doi: 10.2147/PPA.S162219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker GM, Fudim M, Greene SJ, Hernandez AF, O'Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA, Mentz RJ. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF trial. JACC Heart Fail. 2017 Jan;5(1):1–13. doi: 10.1016/j.jchf.2016.09.012. https://linkinghub.elsevier.com/retrieve/pii/S2213-1779(16)30498-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riegel B, Barbaranelli C, Carlson B, Sethares KA, Daus M, Moser DK, Miller J, Osokpo OH, Lee S, Brown S, Vellone E. Psychometric testing of the revised self-care of heart failure index. J Cardiovasc Nurs. 2019;34(2):183–92. doi: 10.1097/JCN.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedlar N, Lainscak M, Mårtensson J, Strömberg A, Jaarsma T, Farkas J. Factors related to self-care behaviours in heart failure: a systematic review of European Heart Failure Self-Care Behaviour Scale studies. Eur J Cardiovasc Nurs. 2017 Apr;16(4):272–82. doi: 10.1177/1474515117691644. [DOI] [PubMed] [Google Scholar]
- 8.Spaling MA, Currie K, Strachan PH, Harkness K, Clark AM. Improving support for heart failure patients: a systematic review to understand patients' perspectives on self-care. J Adv Nurs. 2015 Nov;71(11):2478–89. doi: 10.1111/jan.12712. [DOI] [PubMed] [Google Scholar]
- 9.Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJ, Quinn TJ. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017 Jun;23(6):464–75. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Mlynarska A, Golba KS, Mlynarski R. Capability for self-care of patients with heart failure. Clin Interv Aging. 2018;13:1919–27. doi: 10.2147/CIA.S178393. doi: 10.2147/CIA.S178393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark AM, Spaling M, Harkness K, Spiers J, Strachan PH, Thompson DR, Currie K. Determinants of effective heart failure self-care: a systematic review of patients' and caregivers' perceptions. Heart. 2014 May;100(9):716–21. doi: 10.1136/heartjnl-2013-304852. [DOI] [PubMed] [Google Scholar]
- 12.Riegel B. I forgot: memory and medication adherence in heart failure. Circ Heart Fail. 2016 Dec;9(12):e003642. doi: 10.1161/CIRCHEARTFAILURE.116.003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Pérez B, de la Torre-Díez I, López-Coronado M, Herreros-González J. Mobile apps in cardiology: review. JMIR Mhealth Uhealth. 2013 Jul 24;1(2):e15. doi: 10.2196/mhealth.2737. https://mhealth.jmir.org/2013/2/e15/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Research2Guidance. 2015. [2018-07-15]. mHealth App Developer Economics 2015 https://research2guidance.com/product/mhealth-developer-economics-2015/
- 15.Cajita MI, Hodgson NA, Budhathoki C, Han HR. Intention to use mhealth in older adults with heart failure. J Cardiovasc Nurs. 2017;32(6):E1–7. doi: 10.1097/JCN.0000000000000401. http://europepmc.org/abstract/MED/28248747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hägglund E, Lyngå P, Frie F, Ullman B, Persson H, Melin M, Hagerman I. Patient-centred home-based management of heart failure. Findings from a randomised clinical trial evaluating a tablet computer for self-care, quality of life and effects on knowledge. Scand Cardiovasc J. 2015 Aug;49(4):193–9. doi: 10.3109/14017431.2015.1035319. [DOI] [PubMed] [Google Scholar]
- 17.Creber RM, Maurer MS, Reading M, Hiraldo G, Hickey KT, Iribarren S. Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the mobile application rating scale (MARS) JMIR Mhealth Uhealth. 2016 Jun 14;4(2):e74. doi: 10.2196/mhealth.5882. https://mhealth.jmir.org/2016/2/e74/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, Mani M. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015 Mar 11;3(1):e27. doi: 10.2196/mhealth.3422. https://mhealth.jmir.org/2015/1/e27/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chindalo P, Keshavjee K, Karim A, Brahmbhatt R, Saha N. Health apps by design: a reference architecture for mobile engagement. Int J Handheld Comput Res. 2016;7(2):34–43. doi: 10.4018/IJHCR.2016040103. [DOI] [Google Scholar]
- 20.Anderson K, Burford O, Emmerton L. App chronic disease checklist: protocol to evaluate mobile apps for chronic disease self-management. JMIR Res Protoc. 2016 Nov 4;5(4):e204. doi: 10.2196/resprot.6194. https://www.researchprotocols.org/2016/4/e204/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyatt JC, Thimbleby H, Rastall P, Hoogewerf J, Wooldridge D, Williams J. What makes a good clinical app? Introducing the RCP health informatics unit checklist. Clin Med (Lond) 2015 Dec;15(6):519–21. doi: 10.7861/clinmedicine.15-6-519. http://europepmc.org/abstract/MED/26621937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson K, Burford O, Emmerton L. Mobile health apps to facilitate self-care: a qualitative study of user experiences. PLoS One. 2016;11(5):e0156164. doi: 10.1371/journal.pone.0156164. http://dx.plos.org/10.1371/journal.pone.0156164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hundert AS, Huguet A, McGrath PJ, Stinson JN, Wheaton M. Commercially available mobile phone headache diary apps: a systematic review. JMIR Mhealth Uhealth. 2014 Aug 19;2(3):e36. doi: 10.2196/mhealth.3452. https://mhealth.jmir.org/2014/3/e36/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware P, Ross HJ, Cafazzo JA, Laporte A, Gordon K, Seto E. User-centered adaptation of an existing heart failure telemonitoring program to ensure sustainability and scalability: qualitative study. JMIR Cardio. 2018 Dec 6;2(2):e11466. doi: 10.2196/11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods LS, Duff J, Roehrer E, Walker K, Cummings E. Patients' experiences of using a consumer mhealth app for self-management of heart failure: mixed-methods study. JMIR Hum Factors. 2019 May 2;6(2):e13009. doi: 10.2196/13009. https://humanfactors.jmir.org/2019/2/e13009/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefler LL, Rhoads SJ, Harris M, Funderburg AE, Lubin SA, Martel ID, Faulkner JL, Rooker JL, Bell DK, Marshall H, Beverly CJ. Evaluating the use of mobile health technology in older adults with heart failure: mixed-methods study. JMIR Aging. 2018 Dec 4;1(2):e12178. doi: 10.2196/12178. https://aging.jmir.org/2018/2/e12178/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riegel B, Lee S, Hill J, Daus M, Baah FO, Wald JW, Knafl GJ. Patterns of adherence to diuretics, dietary sodium and fluid intake recommendations in adults with heart failure. Heart Lung. 2019;48(3):179–85. doi: 10.1016/j.hrtlng.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobile Application Development Companies UK. 2016. [2019-09-16]. How Do People Discover And Decide To Download Your App? https://www.overpass.co.uk/people-discover-decide-download-app/
- 29.Aterja A. HuffPost. 2018. [2019-09-16]. How to Choose the Best Health App for You https://www.huffpost.com/entry/how-to-choose-the-best-health-app-for-you_b_5a54d736e4b0ee59d41c0e01.
- 30.Armstrong S. Which app should I use? Br Med J. 2015 Sep 9;351:h4597. doi: 10.1136/bmj.h4597. [DOI] [PubMed] [Google Scholar]
- 31.Visser BJ, Bouman J. There’s a medical app for that. Br Med J. 2012 Apr 18;344:e2162. doi: 10.1136/sbmj.e2162. [DOI] [Google Scholar]
- 32.Tiongson J. Think With Google. 2015. [2019-09-16]. Mobile App Marketing Insights: How Consumers Really Find and Use Your Apps https://www.thinkwithgoogle.com/consumer-insights/mobile-app-marketing-insights/
- 33.Basilico A, Marceglia S, Bonacina S, Pinciroli F. Advising patients on selecting trustful apps for diabetes self-care. Comput Biol Med. 2016 Apr 1;71:86–96. doi: 10.1016/j.compbiomed.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, Peterson PN, Pressler SJ, Schocken DD, Whellan DJ. State of the science. Circulation. 2009 Sep 22;120(12):1141–63. doi: 10.1161/circulationaha.109.192628. [DOI] [PubMed] [Google Scholar]
- 35.Kuljis J, Money AG, Perry M, Barnett J, Young T. Technology-assisted self-testing and management of oral anticoagulation therapy: a qualitative patient-focused study. Scand J Caring Sci. 2017 Sep;31(3):603–17. doi: 10.1111/scs.12374. [DOI] [PubMed] [Google Scholar]
- 36.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008 Jun;10(3):160–8. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 37.Desveaux L, Shaw J, Saragosa M, Soobiah C, Marani H, Hensel J, Agarwal P, Onabajo N, Bhatia RS, Jeffs L. A mobile app to improve self-management of individuals with type 2 diabetes: qualitative realist evaluation. J Med Internet Res. 2018 Mar 16;20(3):e81. doi: 10.2196/jmir.8712. https://www.jmir.org/2018/3/e81/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welldoc Inc. [2017-07-12]. Bluestar Digital Therapeutics Assistant | Welldoc Wellness https://www.welldoc.com/product/
- 39.Mendiola MF, Kalnicki M, Lindenauer S. Valuable features in mobile health apps for patients and consumers: content analysis of apps and user ratings. JMIR Mhealth Uhealth. 2015 May 13;3(2):e40. doi: 10.2196/mhealth.4283. https://mhealth.jmir.org/2015/2/e40/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liew MS, Zhang J, See J, Ong YL. Usability challenges for health and wellness mobile apps: mixed-methods study among mhealth experts and consumers. JMIR Mhealth Uhealth. 2019 Jan 30;7(1):e12160. doi: 10.2196/12160. https://mhealth.jmir.org/2019/1/e12160/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total features present in preliminary and postreview screening apps. PROMS: patient reported outcome measure; PREMS: patient reported experience measure.


