Abstract
Neural crest cells are a transient stem-like cell population that forms in the dorsal neural tube of vertebrate embryos and then migrates to various locations to differentiate into diverse derivatives such as craniofacial bone, cartilage, and the enteric and peripheral nervous systems. The current dogma of neural crest cell development suggests that there is a specific hierarchical gene regulatory network (GRN) that controls the induction, specification, and differentiation of these cells at specific developmental times. Our lab has identified that a marker of differentiated neurons, Tubulin Beta-III (TUBB3), is expressed in premigratory neural crest cells. TUBB3 has previously been identified as a major constituent of microtubules and is required for the proper guidance and maintenance of axons during development. Using the model organism, Callus gallus, we have characterized the spatiotemporal localization of TUBB3 in early stages of development. Here we show TUBB3 is expressed in the developing neural plate, is upregulated in the pre-migratory cranial neural crest prior to cell delamination and migration, and it is maintained or upregulated in neurons in later developmental stages. We believe that TUBB3 likely has a role in early neural crest formation and migration separate from its role in neurogenesis.
Keywords: Tubulin Beta-III, TUBB3, SOX9, SNAI2, Neural crest, Neuron
1. Introduction
Neural crest (NC) cells are a vertebrate-specific, ectodermally derived, cell population that begin as epithelial ectodermal cells and subsequently undergo an epithelial to mesenchymal transition (EMT) and migrate to diverse locations within the developing embryo to create various derivatives (Hutchins et al., 2018; Rogers and Nie, 2018). During neurulation in avian embryos, the neural plate rolls into the neural tube, and NC cells are formed at the dorsal region of the neuroepithelium. Premigratory NC cells are thought to be multipotent progenitor cells that have the ability to give rise to multiple cell types (Kerosuo et al., 2015). NC cells can become neurons, melanocytes, cartilage, glia, and form the bulk of the enteric and peripheral nervous systems among a multitude of other derivatives (Acloque et al., 2008). There is an established gene regulatory network that outlines the molecular pathways that drive the formation and differentiation of NC cells (Simoes-Costa and Bronner, 2015), however, there is still a lack of understanding about NC cell programming. Are neural crest cells fated prior to migration and differentiation, or do they only acquire fates as they interact with their environments? Although our current study cannot provide definitive answers to this question, we have discovered the expression of a marker of terminally differentiated cells, specifically neurons, in premigratory cranial NC cells, which are well-established as multi-potent stem-like cells (Kerosuo et al., 2015).
Ectodermal stem cells become neural progenitors, epidermal progenitors, and neural plate border cells (Plouhinec et al., 2005). Neural plate border cells can then become either NC or sensory placodes (Steventon et al., 2014). The neural plate border is defined by the expression of PAX7 in avian embryos (Groves and LaBonne, 2014; Murdoch et al., 2012) and PAX3 in frog (Maczkowiak et al., 2010; Plouhinec et al., 2014) and zebrafish (Garnett et al., 2012), along with other border markers (MSX1, ZIC1, etc.). The expression of these border markers is paired with the reduction of neural plate markers such as SOX2 and SOX3 (Roellig et al., 2017). A complex cascade of factors is involved in the NC gene regulatory network, and Pax7-positive cells contribute to the cells that become both NC and dorsal central nervous system (CNS) lineages (Murdoch et al., 2012). For these cells to then become definitive NC, Sox9 or Snai2 must be activated (Stuhlmiller and Garcia-Castro, 2012). Definitive NC cells then activate genes that will drive the cells towards migration (Lander et al., 2011; Rogers et al., 2013; Strobl-Mazzulla and Bronner, 2012; Taneyhill et al., 2007; Tien et al., 2015), later activating genetic pathways that drive them towards a specific derivative (i.e., neurons, melanocytes, cartilage, etc.) (Adameyko et al., 2012; Espinosa-Medina et al., 2017; Young and Newgreen, 2001). Questions still remain about when NC cells know what they will become. Pigment cell fate determination has been studied extensively in anamniotes such as zebrafish. Melanocytes and iridophore fates are regulated by the presence or absence of certain transcription factors such as sox10 and mitfa during early development (Petratou et al., 2018). Additional consideration has been given to understanding the suite of transcription factors, their spatiotemporal expression, and the requirement for such proteins in the formation of the NC cell population (Lignell et al., 2017; Simoes-Costa and Bronner, 2016; Simoes-Costa et al., 2014). However, one major theory that has been postulated in the field is that there are proteins only upregulated in the determined or terminally differentiating NC cell derivatives, and class III beta-Tubulin (TUBB3) is one of these proteins.
TUBB3 is an element of microtubules, which are dynamic components of the intracellular cytoskeleton that have been linked to NC cell migration (Francis et al., 2011; Moore et al., 2013). TUBB3 is an established marker of proliferative and terminally differentiated neurons (Lee et al., 1990; Lee and Pixley, 1994; Menezes and Luskin, 1994), and was first characterized in chicken embryos using Western blot and immunohistochemistry (IHC). TUBB3 was identified specifically as a neural-tissue specific tubulin protein with multiple isoforms. Additional characterization identified that it was highly expressed in non-proliferative differentiating cells (Lee et al., 1990). TUBB3 has also been investigated in the sensory and nonsensory regions of the avian inner ear (Molea et al., 1999) as well as the developing olfactory sensory system (Lee and Pixley, 1994; Roskams et al., 1998). However, one main factor that defines TUBB3 expression, is that it resides in cells that are either differentiating into, or have already become neurons.
Here, we have characterized the endogenous spatiotemporal expression of TUBB3 at multiple early stages of avian development (Table 1). We performed IHC at stages coinciding with NC cell induction, specification, EMT, as well as neuronal differentiation, and observed that TUBB3 is expressed prior to neurogenesis in the neural plate and in premigratory cranial NC cells. Using antibodies marking NC progenitors (PAX7), definitive NC cells (SOX9 and SNAI2), and markers of differentiating sensory structures (PAX6, SOX2), we identified that TUBB3 co-localizes with NC markers prior to NC EMT, and is maintained in early and late migrating NC cells. Additionally, there are premigratory NC cell populations that are positive for TUBB3 and NC markers, but also a subset of cells that may individually express NC markers in the absence of TUBB3. Although the focus in this study is to characterize the expression of TUBB3 in progenitor cells, as a positive control, we have additionally confirmed that the same protein is in fact expressed in cranial and spinal neurons and ganglia in later stages of chicken development.
Table 1.
Stages analyzed and number of replicates.
| Developmental relevance | Stage | Number |
|---|---|---|
| Gastrulation/neural induction | HH4 | 10 |
| HH5 | 8 | |
| HH6 | 4 | |
| Neurulation | HH7 | 4 |
| Neural crest specification | HH8 | 18 |
| Neural crest EMT/migration | HH9 | 4 |
| HH10 | 3 | |
| HH11 | 3 | |
| HH12 | 1 | |
| Cranial ganglia formation | HH13 | 3 |
| HH14 | 7 | |
| HH15 | 7 | |
| HH16 | 2 |
2. Materials and methods
2.1. Chicken embryos
Fertilized chicken eggs were obtained from local sources (Sunstate Ranch, CA) and incubated at 37 °C to the desired stages according to the criteria of Hamburger and Hamilton (HH). Use of chicken embryos was approved by the California State University Northridge IACUC protocol: 1516-012a, c.
2.2. Immunohistochemistry
For immunohistochemistry (IHC), chicken embryos were fixed on filter paper in 4% paraformaldehyde (PFA) in phosphate buffer for 15–25 min at room temperature. After fixation, embryos were washed in TBST + Ca2+ with 0.5% Triton X-100 and de-papered. For blocking, embryos were incubated in TBST + Ca2+ with 0.5% Triton X-100 and 10% donkey serum for at least 1 h at room temperature. Primary antibodies were diluted in blocking solution and incubated with embryos for 3 h at room temperature or for 24–48 h at 4° Celsius. After incubation with primary antibodies, whole embryos were washed in TBST + Ca2+, incubated with AlexaFluor secondary antibodies diluted in blocking buffer with DAPI (4′,6-diamidino-2-phenylindole), for 3 h at room temperature or 12 h at 4 °C, washed in TBST + Ca2+, and post-fixed in 4% for 30 min- 1 h in PFA at room temperature. Antibodies used in the study: Mouse α-Neuron-specific beta-III Tubulin (R & D Systems, MAB1195), Mouse α-Pax7 (DSHB), Rabbit α-Sox9 (Millipore, AB5535MI), Mouse α- PAX6 (DSHB, PAX6), Rabbit α-Slug/Snai2 (Cell signaling, 9585T), Rabbit α- SOX2 (Proteintech, 20118-1-AP). Information for all antibodies can be found in the Key Resources Table. After IHC all embryos were imaged in both whole mount and transverse section using a Zeiss Imager M2 with Apotome capability and optical processing software.
Key resources table
| Reagent or resource | Source | Identifier |
|---|---|---|
|
| ||
| Antibodies | ||
| Mouse anti-Tubulin beta III IgG2a | R & D Systems | MAB1195 |
| Mouse anti-Pax7 IgG1 | Developmental Studies Hybridoma Bank |
PAX7 |
| Rabbit anti-Sox9 IgG | Millipore | AB5535MI |
| Rat anti-N-cadherin IgG2a | Developmental Studies Hybridoma Bank |
MNCD2 |
| Mouse anti-Pax6 IgG1 | Developmental Studies Hybridoma Bank |
PAX6 |
| Rabbit anti-Sox2 IgG | Proteintech | 20118-1-AP |
| Rabbit anti-Slug/Snai2 | Cell Signaling | 9585T |
|
| ||
| Morpholino Oligomers | ||
|
| ||
| TUBB3MO | Gene Tools | 5′-TGGATGTGGACGATCTCCCTCAT-3′ |
|
| ||
| Experimental Models: Organisms/Strains | ||
|
| ||
| Gallus Gallus | SunState Farms, CA | |
2.3. Electroporation of antisense morpholino
A translation blocking antisense morpholino to TUBB3 (TUBB3MO) was designed (5′ GCGTTCCCGCTATCCGGCACATGGA-3′) and injected into HH stage 4 embryos unilaterally. Injection of the fluorescein-tagged morpholino (0.75–1 mM plus 0.5–1.5 mg/ml of PCI carrier plasmid DNA (Voiculescu et al., 2008) was performed by air pressure using a glass micropipette targeted to the presumptive neural plate region. HH stage 4 electroporations were conducted on whole chick embryo explants placed ventral side up on filter paper rings. The TUBB3 morpholino and carrier vector were injected on the right side of the embryo. Platinum electrodes were placed vertically across the chick embryos and electroporated with five pulses of 6.3 V in 50 ms at 100-ms intervals.
2.4. Western blot
Embryo lysate was isolated from 20 to 30 manually dissected chicken embryos from stage HH4-12 for Western blot analysis. Lysate was isolated using lysis buffer (50 mM Tris-HCL pH 7.4 with 150 mM NaCl plus 1.0% NP-40 and EDTA-free protease inhibitor (Roche complete, # 11697498001). SDS page was run on precast 8–12% bistris gel (Invitrogen, # NP0321BOX) for 3 h at 48 V, gel was transferred to nitrocellulose at 90 V for 1 h. Nitrocellulose membranes were washed in TBST + Calcium with 0.5% Triton X-100, blocked and incubated with primary antibody (see above) in TBST + Calcium with 0.5% Triton X-10 with 5.0% BSA, incubated in (5%) milk protein in (TBST + Calcium) with secondary antibody, and visualized using ECL kit (GE Healthcare Lifesciences, # RPN2232) and exposed to film (GeneMate, #F-9024-8×10).
3. Results and discussion
3.1. Antibody verification
First, we wanted to verify the stage of expression and that the protein size was correct using Western blot analysis. The predicted size of the Gallus gallus TUBB3 protein is 50.43 kDa (kD). Our antibody detects a protein that runs at approximately 49 kD on a denaturing protein gel (Fig. 1A). Use of different concentrations of the primary antibody in Western blot demonstrated various levels of sensitivity. At 1:1000 dilution, the protein appears to only be expressed in HH7-12 stage embryos, however, at 1:100 dilution, we were able to identify expression at HH4-5, HH7-8, and HH7-12 (Fig. 1A). To verily that our results are consistent with bonafide TUBB3 expression, we performed knockdown experiments using a translation-blocking morpholino to TUBB3 (TUBB3MO). Injection and subsequent electroporation of the TUBB3MO unilaterally into HH4 embryos resulted in the loss of TUBB3 protein expression on one side of the embryo (Fig. 1B–D1). We additionally verified that the primary antibody alone gave no signal by incubating embryos with the primary antibody (Fig. 1E, G) in the presence of DAPI stain (Fig. 1F and G), and without secondary antibody, we saw no expression.
Fig. 1. Verification of TUBB3 antibody.
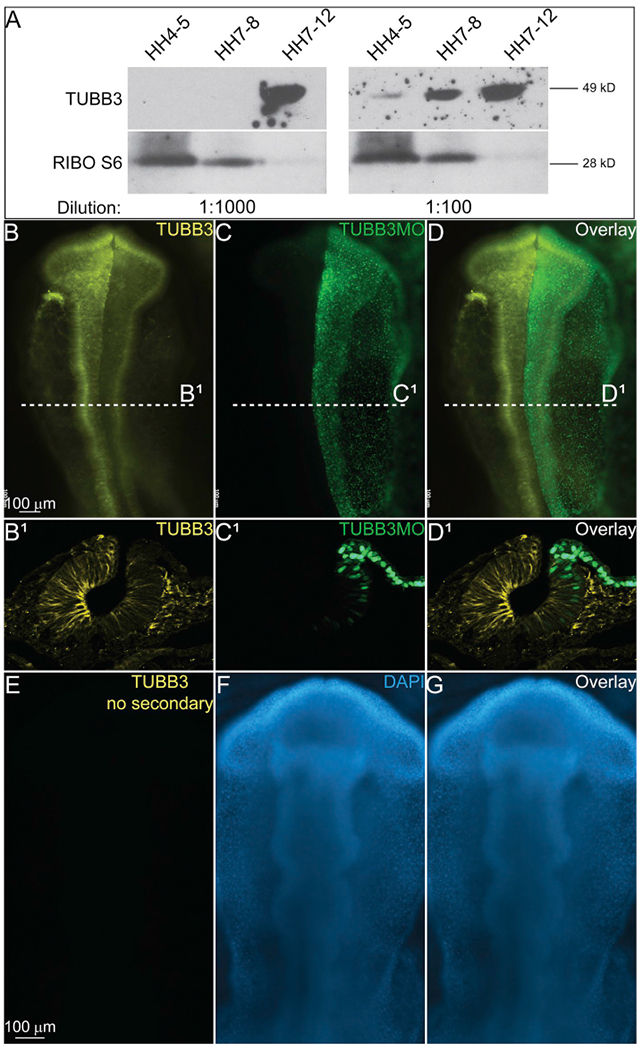
(A) Western blot using antibodies for TUBB3 and Ribosomal S6 proteins. Loading control (Ribo S6) demonstrates amount of protein loaded in each lane, but blots were incubated with two different primary antibody concentrations (as labeled). TUBB3 antibody identified a single band at approximately 49kDa. At 1:1000 concentration, TUBB3 expression is only seen in HH7-12 lane, but at 1:100, primary antibody shows bands in HH4-5, HH7-8, and HH712 lanes. IHC for TUBB3 (yellow, B, B1, D, D1, E) and stain for DAPI (blue, F, G). Embryo in B-D has been injected unilaterally with a TUBB3 translation-blocking FITC-labeled morpholino (green, C, C1, D, D1). Injected area has a reduction in TUBB3 expression demonstrating specificity of antibody. (E–G) Embryo incubated with TUBB3 primary and DAPI stain, but no secondary antibody. (B-D, E-G) Whole mount embryos with anterior to the top and posterior to the bottom. (B1-D1) Transverse sections of embryo from (B–D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Expression of TUBB3 during neurulation
Previous studies established that expression of TUBB3 was not detectable using Western blot or IHC until embryonic day 13–14 in mouse embryos (Menezes and Luskin, 1994) and in stages HH12-14 in chicken embryos (Lee et al., 1990). However, studies from our lab and others have previously demonstrated that antibody penetration can be altered by shorter fixation times and novel identification of protein expression for previously established proteins is possible (Rogers et al., 2013, 2018). In the previous TUBB3 studies, the mouse brains were fixed for 18–24 h and the chicken tissues were fixed for 24 h, but in this study our tissues were fixed for 15–25 min at room temperature. Here we have identified early expression of TUBB3 during neurulation and NC cell formation in chicken embryos.
To determine the stage that TUBB3 protein is first expressed, we tested the antibody on gastrula (Hamburger Hamilton (HH) stage 5–6, Fig. 2A–C) and neurula stage embryos (HH6-7, Fig. 2D–F, D1–F1). TUBB3 was expressed at both stages, but its expression was stronger during neurulation (Fig. 2D, D1, F, F1). We identified that TUBB3 was only expressed in the ectoderm (neural plate (NP), neural plate border (NPB), Fig. 2D1, F1). It was not expressed in the cranial mesenchyme (Meso), which is marked by SNAI2 at this stage (Fig. 2E1, F1). SNAI2, a well-known NC cell marker (Heeg-Truesdell and LaBonne, 2004; Tien et al., 2015; Tribulo et al, 2004; Vernon and LaBonne, 2006), is expressed in the developing mesoderm prior to NC specification (Fig. 2B, E, E1), and TUBB3 expression did not overlap with SNAI2 in these stages. Additionally, we performed IHC using antibodies to clarify the tissues in which TUBB3 was expressed. Paired IHC with antibodies against neural cadherin (NCAD) and the NC progenitor marker, PAX7, demonstrated that at HH7 + /8− (3 somite stage (SS)), TUBB3 overlaps in the neuroepithelium with NCAD (Fig. 2G–J, G1–J1), and appears to be enhanced on the apical side of the forming neural tube (neural groove, NG). It is also co-expressed in the developing putative NC region marked by PAX7 (Fig. 2I, G1–J1). To gain a deeper understanding of the extent of TUBB3 expression in putative NC forming regions, we utilized an HH8 embryo (5 SS), which has three separate stages of NC cell development in one embryo. At HH8, cranial NC cells are definitively determined as they upregulate the expression of bonafide NC markers SNAI2 and SOX9 after neural tube closure (Fig. 2K–M, Fig. 3), the hindbrain regions are specified, and the trunk NC are being induced, but remain NPB cells as the embryo finishes gastrulating posteriorly (Fig. 2K–M). In cranial regions, TUBB3 is expressed in the neural tube as well as in the NC cells (Fig. 2K1–M1). In the more posterior NC territories, TUBB3 is expressed throughout the developing NG/NP regions. TUBB3 also has non-neural ectodermal expression at all axial levels as well as mesodermal expression in more posterior regions (Fig. 2K1, K2). In each stage of development, TUBB3 is expressed in the developing neuroepithelium as well as the presumptive NC regions.
Fig. 2. TUBB3 is expressed in the developing neural plate prior to NC specification.
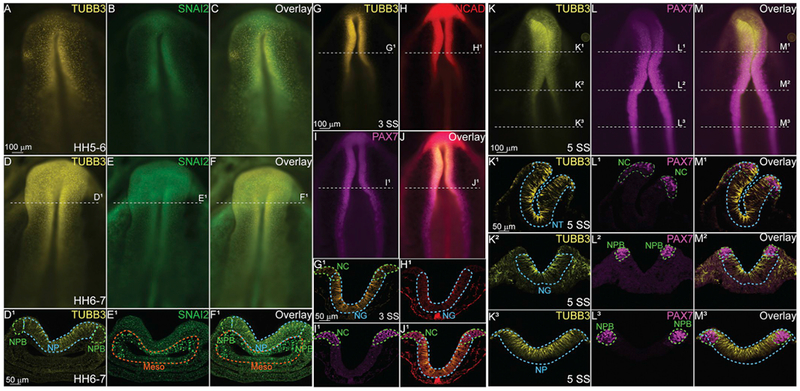
Immunohistochemistry (IHC) using antibodies against TUBB3 (yellow; A, C, D, F, D1, FI, G, G1, J, J1, K, K1–3, M, M1–3.), a neuron marker, SNAI2 (green; B, E, E1, F1), a definitive NC marker, N-cadherin (NCAD) (red; H, J, H1, J1), a cell adhesion molecule, and PAX7 (magenta, I, I1, J1, L, L1–3, M, M1–3), a neural crest progenitor marker. At stage HH5–6 (A–C) and stage 6–7 (D-F1) TUBB3 is expressed by cells in the neural plate (NP) and neural plate border (NPB) prior to the neural tube closure. Cranial mesenchyme/mesoderm is Meso. (G–J) IHC using antibodies against (G) TUBB3, (H) NCAD, (I) PAX7 and (J) Overlay in a 3 SS embryo. (G1-J1) Transverse sections through G-J. IHC for (K, K1–3) TUBB3, (L, L1–3) PAX7, and (M, M1–3) Overlay in a 5 SS embryo. TUBB3 is expressed in the NT (cranial), neural groove (NG, vagal), and NP at these axial levels. (A-F, G-J, K-M) are whole mounts with anterior to the top and posterior to the bottom while (D1-F1, G1-J1, K1-M3) are transverse sections with dorsal to the top and ventral to the bottom. These data suggest that TUBB3 protein may have early roles in addition to later fate determination. Scale bars are as marked. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3. TUBB3 is upregulated in premigratory and migratory NC cells.
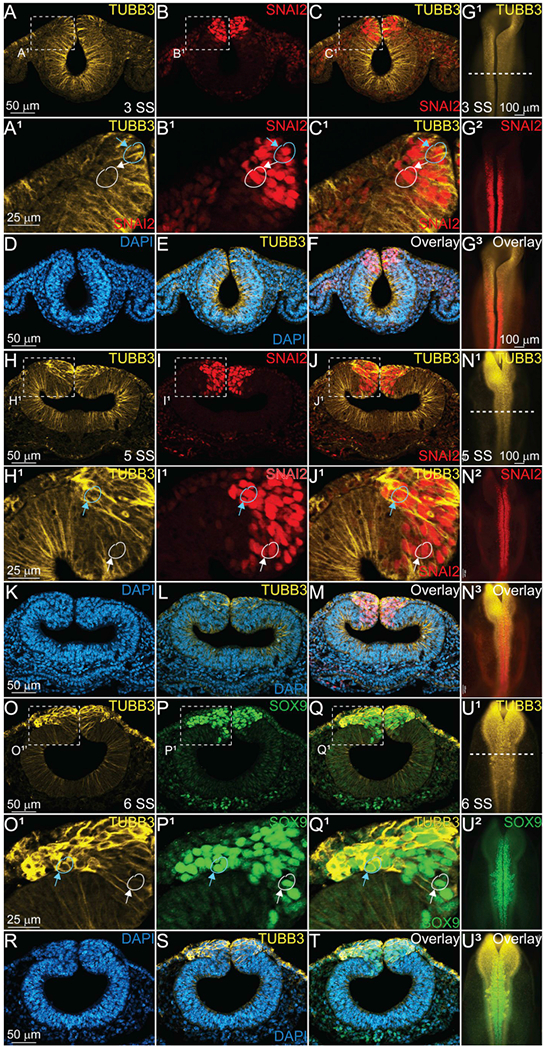
IHC using antibodies against TUBB3 (yellow, A, A1, C, C1, E, F, G1, G3, H, H1, J, J1, L, M, N1, N3, O, O1, Q, Q1, S, T, U1, U3), SNAI2 (red, B, B1, C, C1, F, G2, G3, I, I1, J, J1, M, N2, N3), SOX9 (green, P, P1, Q, Q1, T, U2, U3), and stain for DAPI (blue, D-F, K-M, R-T), in 3 SS (A-G3), 5 SS (H-N3) and 6 SS (O-U3) embryos and sections. (A-C1) transverse sections from 3 SS embryo in (G1–3) demonstrates TUBB3 expression in the neural tube, non-neural ectoderm, and cranial mesenchyme. Blue circle and blue arrow represents cells that are TUBB3+ and SNAI2+ or SOX9 + ; white circle and arrow represents cell that seem to be expressing SNAI2 or SOX9 only without TUBB3. At 5 SS (H, H1, J, J1, L, M, N1, N3), and 6 SS (O, O1, Q, Q1, S, T, U1, U3), TUBB3 expression is enhanced in the dorsal neural tube where definitive cranial NC cells marked by SNAI2 (I, I1, J, J1, M, N2, N3) and SOX9 (P, P1, Q, Q1, T, U2, U3) are assembling for migration. (G1–3, N1–3, U1–3) are whole embryos with anterior to the top and posterior to the bottom, while all other images are transverse sections with dorsal to the top and ventral to the bottom. Scale bars are as marked. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Microtubules function during cell division (Memberg and Hall, 1995), migration (Francis et al, 2011), and structural maintenance and changes (Logan et al, 2018) among other cellular processes, and therefore, the expression of TUBB3 in NP cells during neurulation suggests that the beta-III isoform of the protein functions in one of these aspects at this stage. Since the NP is undergoing drastic morphological changes during neural tube closure, and the cells are also proliferating, the specific role of TUBB3 is difficult to pinpoint. However, the expression of TUBB3 at early stages could be transient, and simply necessary for apical constriction and neural tube closure that is then turned off and upregulated in neurons. To determine if its expression was transient in neurulating neural progenitors, we performed IHC analysis on later stage embryos during/after neural tube closure, and during the NC specification and EMT stages.
3.3. TUBB3 expression is upregulated in premigratory NC cells
To determine whether TUBB3 expression was maintained during early development, we collected embryos as neurulation was completed and in subsequent stages. IHC for TUBB3 in HH7 + /HH8− (3 SS) embryos demonstrated that TUBB3 was still expressed throughout the neural tube (Fig. 3A, A1, C, C1), and although it seemed to be localized to the apical side of the neural tube cells based on comparative expression of type I cadherin proteins (Fig. 2), there was not a difference in expression with regards to dorsal-ventral localization. Additionally, TUBB3 was expressed in the non-neural ectoderm and the cranial mesenchyme at this stage (Fig. 3A, A1, C, C1). At 3 SS, SNAI2 is also expressed (Fig. 3B, C, G2). In this stage of development, SNAI2 was expressed in the premigratory definitive NC cells (Fig. 3B, B1, C, C1), and it overlapped with TUBB3 in a majority of the premigratory NC cells (Fig. 3C1, blue circle, blue arrow). Each of the proteins is co-expressed in a subset of neuroepithelial cells (Fig. 3A–G), but there are cells that appear to only express SNAI2 (Fig. 3B1–C1, white circle, white arrow). However, it is impossible to definitively determine whether there are non-TUBB3-expressing NC cells at this level of resolution, and therefore future experiments are necessary to define these populations more closely. These data suggest the possibility that each of these cell types is developing with distinct programs, or that the expression of TUBB3 could be transient and location specific.
At a slightly later stage, HH8 (5 SS), TUBB3 expression is maintained in the developing neuroepithelium, but at this stage, some premigratory NC cells have upregulated its expression (Fig. 3H, J, H1, J1, N1, N3). At this stage, SNAI2-positive cells appear to be starting to delaminate from the neuroepithelium (Fig. 3I, J, I1, J1, N2), but the delaminating cells are not necessarily the ones that have enhanced TUBB3 expression. There are cells that express both proteins (Fig. 3H1–J1, blue circle, blue arrow), but there are also some that appear SNAI2-only positive cells (Fig. 3H1–J1, white circle, white arrow). It is at this stage, just prior to NC EMT where we begin to see specific upregulation of TUBB3 in NC cells. We hypothesize that its role could be in cell division or in supporting the collective migration of the cells out of the neural tube. Future studies will dissect these possibilities.
As NC cells begin migrating ventrolaterally out of the dorsal neural tube at HH8 + /HH9− (6 SS), TUBB3 expression is upregulated in the newly emigrating cells (Fig. 3O–U3). Comparing its expression to an additional marker of definitive NC cells, SOX9 (Betancur et al., 2009; Liu et al., 2013) (Fig. 3P, Q, P1, Q1, T, U2, U3), it is apparent that the cells at the leading edge of the migratory NC have upregulated TUBB3 compared to the premigratory and migratory cells that are in more medial locations (Fig. 3O–Q1) suggesting that TUBB3 may assist these cells in migrating, but the specific function is unknown. Future studies will identify the possible role of microtubules in these cells; however, TUBB3 may potentially assist in the cell-cell adhesion, protrusion, contraction, or retraction during NC EMT and migration (Etienne-Manneville, 2013).
3.4. TUBB3 expression in neurons and ganglia
To confirm that the TUBB3 we see in early developing embryos is the previously established neuron-specific protein, we performed IHC on later stages of chicken embryos (Table 1). At 15 SS, TUBB3 is maintained in the developing brain (Fig. 4A–A3, D–D3), as well as migratory NC cells (Fig. 4B–B3, D–D3). It is additionally expressed in the developing retinal epithelium (R) marked by PAX6 (Fig. 4A1, C1, D1). At this stage, TUBB3-positive cells are expressed throughout the anterior central nervous system at the midbrain axial level (Fig. 4A2–D2), and TUBB3 is maintained in the ventrally migrating NC cells marked by SOX9 (Fig. 4A2–D2), but it appears absent from the non-neural ectodermal cells marked by PAX6 (Fig. 4A2, C2, D2). In the hindbrain, TUBB3 is expressed strongly throughout the neuroepithelium (Fig. 4A3, D3), and is additionally expressed in a subset of migratory NC cells marked by SOX9 (Fig. 4A3, B3, D3). In contrast to the more anterior axial levels, TUBB3 is not drastically upregulated in the premigratory NC cells (compare with Fig. 3H with Fig. 4A3).
Fig. 4. TUBB3 is maintained in cranial ganglia.
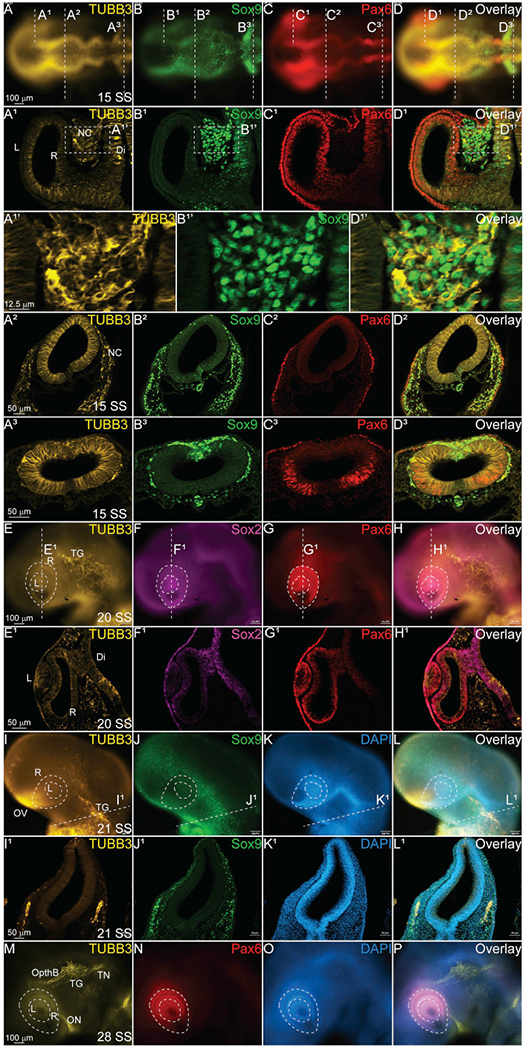
IHC using antibodies against TUBB3 (yellow, A-A3, D-D3, E, E1, H, H1, I, I1, L, L1, M, P), SOX9 (green, B-B3, D-D3, J, J1, L, L1), PAX6 (red, C-C3, G, G1, H, H1, N), and SOX2 (magenta, F, F1, H, H1) and stain for DAPI (blue, K, K1, L, L1, O, P) in multiple stages of chick embryos. (A1-D3) Transverse sections at different axial levels from 15 SS embryo indicated in (A–D) show that TUBB3 co-localizes with SOX9+ migratory cranial NC cells. (A1’, B1’, D1’) Zoom in of regions indicated in dashed boxes from (A1, B1, D1) demonstrating overlap. (E1-H1) Coronal section of 20 SS embryo indicated in (E–H) shows that TUBB3 maintains expression in the brain and developing eye. (I1-L1) Transverse sections from 21 SS embryo indicated in (I–L) show that TUBB3 co-localizes with SOX9 + cells that are condensing to form the TG. (M–P) Verification that TUBB3 is expressed in differentiating cranial neurons. (A–D) Whole mount embryo with anterior to the left and posterior to the right. (E-H, I-L, M-P) Whole mount embryos with anterior to the top/left, posterior down, dorsal to the right. R = retina, L = lens, NC = neural crest, Di = diencephalon, TG = trigeminal ganglia, OV = olfactory vesicle, TN = trigeminal nerve, ON = optic nerve, OpthB = ophthalmic branch. Scale bars are as marked. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At 20 SS, TUBB3 is expressed in the cells are beginning to coalesce in the region that will form the trigeminal ganglia (Fig. 4E, TG), and expression is starting in the developing lens (L) marked by both SOX2 (Fig. 4E–H, E1–H1) and PAX6 (Fig. 4G, G1, H, H1) as well as the retina marked by PAX6 (Fig. 4E1, G1). At 21 SS, the TG begins forming posterior to the developing eye, and as previously reported, TUBB3 is highly expressed along with SOX9 (Fig. 4I–L, 4I1–L1) (Moody et al., 1989). Compared to DAPI (Fig. 4K, K1, O), it is clear that TUBB3 and SOX9 are expressed in discrete tissues. At 28 SS, TUBB3 expression is maintained in the growing TG, the trigeminal nerve (TN), and the ophthalmic branch (OpthB) as well as the optic nerve (ON) (Fig. 4M) which leads directly to the developing eye marked by PAX6 (Fig. 4M, N, P). And it is upregulated in the olfactory vesicle (OV, Fig. 4M).
3.5. Distinct expression of TUBB3 in the developing trunk
Our identification of novel TUBB3 expression in the premigratory and early migrating NC cells in the anterior embryo suggests that TUBB3 may play a role in NC development. To identify if this expression was maintained in NC cells at all axial levels, we analyzed the developing trunk regions in similarly staged chicken embryos. By HH12 (12 SS), NC cells are formed in the anterior trunk region and they express both SOX9 (Fig. 5B, D) and PAX7 (Fig. 5C and D). In whole mount embryos, it is difficult to discern whether TUBB3 co-localizes with the premigratory NC cells (Fig. 5A–D), however, in transverse section, it is clear that TUBB3 has a more traditional expression pattern (Fig. 5A1), and is not upregulated in the NC territory in the dorsal neural tube in stark contrast to the cranial regions (Compare Figs. 3–5A1–D1). In a slightly later HH13 embryo (17–18 SS), TUBB3 appears to be expressed only in cells that are differentiating into neurons (Fig. 5E1,I), and it still does not co-localize with premigratory or migratory SOX9+ or PAX7+ cells in the dorsal spinal cord (Fig. 5F1–H1,I–L). These data suggest that TUBB3 may have a specialized role in cranial NC development that is not conserved in trunk NC cells. Of note, TUBB3 does co-localize with SOX9 + sclerotome cells (Fig. 5I, arrow), but is absent from the PAX7 + dermomyotome cells (Fig. 5C1, D1, asterisk).
Fig. 5. TUBB3 does not co-localize with trunk NC cells.
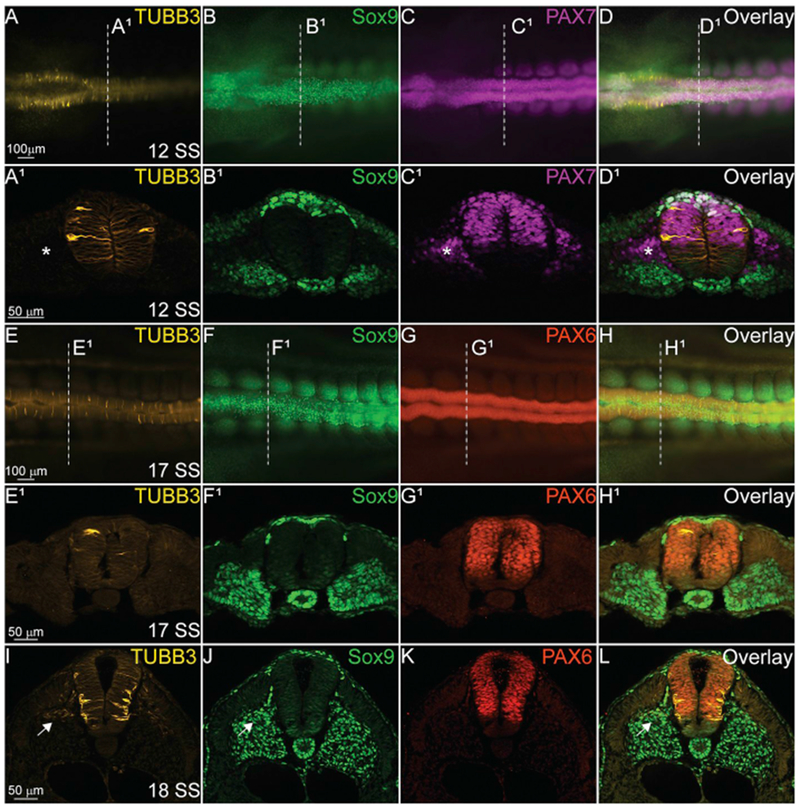
IHC using antibodies against TUBB3 (yellow, A, A1, D, D1, E, E1, H, H1, I, L), SOX9 (green, B, B1, D, D1, F, F1, H, H1, J, L), PAX7 (magenta, C, C1, D, D1), and PAX6 (red, G, G1, H, H1, K, L) in the spinal cord of various staged embryos. TUBB3 is expressed intermittently in the developing spinal cord (A1, E1) at 12 SS and 17 SS, but expression becomes more stereotypic of differentiating spinal neurons at 18 SS (I). (A-D, E-H) Whole mount embryos with anterior to the left and posterior to the right. Dorsal side is facing reader. (A1-D1, E1-H1, I-L) Transverse sections with dorsal to the top and ventral to the bottom. Asterisks indicate TUBB3-/PAX7 + cells and arrows indicate TUBB3+/SOX9+ cells. Scale bars are as marked. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Summary
TUBB3 is expressed in the developing neural plate during neurulation, and is increased in the dorsal neural tube during cranial NC specification (Figs. 1–3). Prior to NC specification, TUBB3 is expressed in the neural plate border, and overlaps with cells that will become both the central nervous and peripheral nervous systems (Figs. 2–5). We identified additional intermittent expression in the non-neural ectoderm and cranial mesenchyme. As cranial NC cells become specified and express definitive NC cell markers such as SNAI2 and SOX9, TUBB3 is upregulated in the dorsal neural tube (Fig. 3H–U3). Its expression appears to be localized to the cells under the most tension like the apical neural tube during neurulation (Fig. 2K1–K3), the two sides of the closing neural tube (Fig. 2H, H1) and the leading edge of the migratory cranial NC cells (Fig. 3O, Q, O1, Q1). In contrast to the cranial neural crest cells which migrate collectively, the trunk expression of TUBB3 did not co-localize with premigratory NC (Fig. 5), and rather, expression was intermittent and in cells that were elongated with medial projecting processes (likely neurons). Our results suggest one of multiple possibilities for the spatial localization of TUBB3 during early avian development. It is possible that TUBB3 is expressed in cranial NC cells as they are specified to form neurons suggesting a very early fate determination mechanism. It is also possible that TUBB3 functions to regulate the cytoskeletal matrix during phases of development that require maintenance of cell structure like collective cell migration. It is also possible that TUBB3 has a unique function in early cranial neural tube and NC cells that is yet to be discovered. Future experiments in our lab will dissect these possibilities.
Acknowledgements
This research was supported by a National Institutes of Health, NICHD grant to CDR (R15HD092170-01). We thank our colleagues from the Rogers Lab at California State University Northridge Department of Biology who provided insight and expertise that greatly assisted the research. We additionally thank CSUN BUILD PODER for funding to JC. The authors declare no competing financial interests.
Funding sources
References
- Acloque H, Wilkinson DG, Nieto MA, 2008. In situ hybridization analysis of chick embryos in whole-mount and tissue sections. Methods Cell Biol. 87, 169–185. [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Furlan A, Zinin N, Aranda S, Kitambi SS, Blanchart A, Favaro R, Nicolis S, Lubke M, Muller T, Birchmeier C, Suter U, Zaitoun I, Takahashi Y, Ernfors P, 2012. Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur PA, Sauka-Spengler T, Bronner-Fraser M, 2009. c-Myb, Ets-1 and Sox9 directly activate a Sox10 core enhancer in delaminating cranial neural crest. Dev. Biol. 331 438–438. [Google Scholar]
- Espinosa-Medina I, Jevans B, Boismoreau F, Chettouh Z, Enomoto H, Muller T, Birchmeier C, Burns AJ, Brunet JF, 2017. Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc. Natl. Acad. Sci. U. S. A. 114, 11980–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, 2013. Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 29, 471–499. [DOI] [PubMed] [Google Scholar]
- Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C, 2011. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS One 6, e26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett AT, Square TA, Medeiros DM, 2012. BMP, Wnt and FGF signals are integrated through evolutionarily conserved enhancers to achieve robust expression of Pax3 and Zic genes at the zebrafish neural plate border. Development 139, 4220–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, LaBonne C, 2014. Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Dev. Biol. 389, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C, 2004. A slug, a fox, a pair of sox: transcriptional responses to neural crest inducing signals. Birth Defects Res. C Embryo Today 72, 124–139. [DOI] [PubMed] [Google Scholar]
- Hutchins EJ, Kunttas E, Piacentino ML, Howard A.G.A.t., Bronner ME, Uribe RA, 2018. Migration and diversification of the vagal neural crest. Dev. Biol. 444 (Suppl. 1), S98–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerosuo L, Nie S, Bajpai R, Bronner ME, 2015. Crestospheres: long-term maintenance of multipotent, premigratory neural crest stem cells. Stem Cell Rep. 5, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander R, Nordin K, LaBonne C, 2011. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sipl. J. Cell Biol. 194, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A, 1990. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil. Cytoskelet. 17, 118–132. [DOI] [PubMed] [Google Scholar]
- Lee VM, Pixley SK, 1994. Age and differentiation-related differences in neuron-specific tubulin immunostaining of olfactory sensory neurons. Brain Res. Dev. Brain Res. 83, 209–215. [DOI] [PubMed] [Google Scholar]
- Lignell A, Kerosuo L, Streichan SJ, Cai L, Bronner ME, 2017. Identification of a neural crest stem cell niche by Spatial Genomic Analysis. Nat. Commun. 8, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JA, Wu MH, Yan CH, Chau BK, So H, Ng A, Chan A, Cheah KS, Briscoe J, Cheung M, 2013. Phosphorylation of Sox9 is required for neural crest delamination and is regulated downstream of BMP and canonical Wnt signaling. Proc. Natl. Acad. Sci. U. S. A. 110, 2882–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CM, Bowen CJ, Menko AS, 2018. Functional role for stable microtubules in lens fiber cell elongation. Exp. Cell Res. 362, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczkowiak F, Mateos S, Wang E, Roche D, Harland R, Monsoro-Burq AH, 2010. The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Dev. Biol. 340, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memberg SP, Hall AK, 1995. Dividing neuron precursors express neuron-specific tubulin. J. Neurobiol. 27, 26–43. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB, 1994. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J. Neurosci. 14, 5399–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molea D, Stone JS, Rubel EW, 1999. Class III beta-tubulin expression in sensory and nonsensory regions of the developing avian inner ear. J. Comp. Neurol. 406, 183–198. [PubMed] [Google Scholar]
- Moody SA, Quigg MS, Frankfurter A, 1989. Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J. Comp. Neurol. 279, 567–580. [DOI] [PubMed] [Google Scholar]
- Moore R, Theveneau E, Pozzi S, Alexandre P, Richardson J, Merks A, Parsons M, Kashef J, Linker C, Mayor R, 2013. Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion. Development 140, 4763–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B, DelConte C, Garcia-Castro MI, 2012. Pax7 lineage contributions to the mammalian neural crest. PLoS One 7, e41089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petratou K, Subkhankulova T, Lister JA, Rocco A, Schwetlick H, Kelsh RN, 2018. A systems biology approach uncovers the core gene regulatory network governing iridophore fate choice from the neural crest. PLoS Genet. 14, e1007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, Leconte L, Sauka-Spengler T, Bovolenta P, Mazan S, Saule S, 2005. Comparative analysis of gnathostome Otx gene expression patterns in the developing eye: implications for the functional evolution of the multigene family. Dev. Biol. 278, 560–575. [DOI] [PubMed] [Google Scholar]
- Plouhinec JL, Roche DD, Pegoraro C, Figueiredo AL, Maczkowiak F, Brunet LJ, Milet C, Vert JP, Pollet N, Harland RM, Monsoro-Burq AH, 2014. Pax3 and Zicl trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Dev. Biol. 386, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roellig D, Tan-Cabugao J, Esaian S, Bronner ME, 2017. Dynamic transcriptional signature and cell fate analysis reveals plasticity of individual neural plate border cells. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Nie S, 2018. Specifying neural crest cells: from chromatin to morphogens and factors in between. Wiley Interdiscip. Rev. Dev. Biol. e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Saxena A, Bronner ME, 2013. Sipl mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J. Cell Biol. 203, 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Sorrells LK, Bronner ME, 2018. A catenin-dependent balance between N-cadherin and E-cadherin controls neuroectodermal cell fate choices. Mech. Dev. 152, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams AJ, Cai X, Ronnett GV, 1998. Expression of neuron-specific beta-III tubulin during olfactory neurogenesis in the embryonic and adult rat. Neuroscience 83, 191–200. [DOI] [PubMed] [Google Scholar]
- Simoes-Costa M, Bronner ME, 2015. Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Bronner ME, 2016. Reprogramming of avian neural crest axial identity and cell fate. Science 352, 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Tan-Cabugao J, Antoshechkin I, Sauka-Spengler T, Bronner ME, 2014. Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Res. 24, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B, Mayor R, Streit A, 2014. Neural crest and placode interaction during the development of the cranial sensory system. Dev. Biol. 389, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl-Mazzulla PH, Bronner ME, 2012. A PHD12-Snail2 repressive complex epigenetically mediates neural crest epithelial-to-mesenchymal transition. J. Cell Biol. 198, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Garcia-Castro MI, 2012. Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 69, 3715–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M, 2007. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134, 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien CL, Jones A, Wang H, Gerigk M, Nozell S, Chang C, 2015. Snail2/Slug co-operates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development 142, 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Sanchez SS, Mayor R, 2004. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msxl is required for the proper development of the neural crest. Dev. Biol. 275, 325–342. [DOI] [PubMed] [Google Scholar]
- Vernon AE, LaBonne C, 2006. Slug stability is dynamically regulated during neural crest development by the F-box protein Ppa. Development 133, 3359–3370. [DOI] [PubMed] [Google Scholar]
- Voiculescu 0, Papanayotou C, Stern CD, 2008. Spatially and temporally controlled electroporation of early chick embryos. Nat. Pro toe. 3, 419–426. [DOI] [PubMed] [Google Scholar]
- Young HM, Newgreen D, 2001. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat. Rec. 262, 1–15. [DOI] [PubMed] [Google Scholar]


