Hypersensitivity reactions (HSRs) to platinum-based chemotherapies have increased as platin use expanded with rising cancer rates. Patients with HSRs to platinum-based chemotherapeutics are often treated with second or third line treatments, leading to inferior outcomes and decreased survival.1
Chemotherapy desensitization allows patients to safely receive first-line therapy despite HSRs,2,3 but are largely performed in inpatient settings.3,4 However, the World Allergy Organization suggests that after an initial successful inpatient desensitization, subsequent desensitizations can be performed in outpatient settings.5 Chemotherapy desensitizations at our institution require an inpatient admission coordinated between multi-disciplinary teams (i.e., pharmacy, oncology, allergy, nursing etc.), and are thus resource and time intensive. To understand if oncology patients could undergo chemotherapy desensitizations as outpatients, we designed and implemented process improvements. The overall goal was to improve operations with decreased use of resources and lower time commitment for oncology patients while maintaining patient safety.
We designed a strategy for transitioning low-risk oncology patients needing desensitization to an outpatient chair. We focused on oxaliplatin desensitizations, as we perform this procedure frequently and have well established protocols.2,6,7 We hypothesized that the outpatient desensitization chair for low-risk desensitizations would decrease time to chemotherapy administration, have comparable safety, and improve patient experience.
In January 2017, we implemented two dedicated outpatient chemotherapy desensitization chairs, managed by trained staff (nurses, Hematology-Oncology nurse practitioners [NPs] and attending physicians, an Allergy-Immunology fellow or NP, with an Allergy/Immunology attending). In this model, nurses were present during the entire desensitization with scheduled vital signs every 15 minutes. After initial evaluation of the patient in person, the Hematology-Oncology and Allergy NP/fellows/attending physicians were available on call during the procedure. Coincident with this operational change, we held educational meetings for all involved in the care team to review patient selection, management of HSRs, and communication.
Inclusion criteria for the outpatient desensitization were: a history of likely type I HSR to oxaliplatin amenable to desensitization (patients with suspected SJS/TEN, DRESS, or other severe type IV HSRs were excluded); low-risk2,6,7 and tolerated at least one inpatient desensitization without a serious HSR (greater than grade I by Common Terminology Criteria for Adverse Events grading)8; age >18 years old; capacity to give informed consent; chemotherapy infusions anticipated to be less than 12 hours (including additional required infusions, such as 5-fluorouracil); and physical ability to sit up in chair for up to 12 hours.
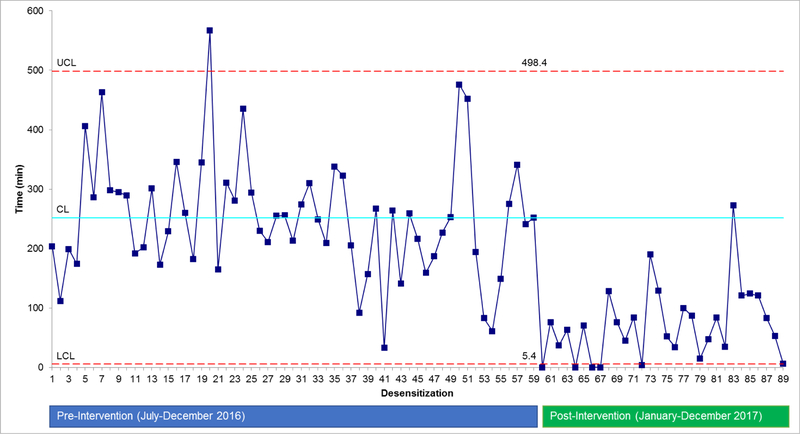
We compared time from patient hospital arrival to desensitization start in the post-intervention period (January 2017-December 2017) to a pre-intervention period (July 2016 -December 2016). A control chart with 3 sigma comparison was used to identify variation.9
To assess safety, electronic health records were reviewed for patient characteristics, severity of initial HSR, and HSRs in the study period. Confidence interval (CI) for frequencies were calculated using exact (i.e., Clopper Pearson) methods. We compared frequency of serious HSR (higher than grade 1) with a Chi-square test, with a two-sided p < 0.05 considered statistically significant.
We assessed patient experience using a survey internally developed with Partners Clinical Process Improvement Leadership Program10 to evaluate the overall experience, wait time, safety, and routine disruption from the patient’s perspective.
In the pre-intervention period, 16 patients completed 59 desensitizations in the inpatient setting. Most were male (62%) with mean age 57 years (range 36–73 years; standard deviation (STD) ± 12 years). In the post-intervention period, 7 patients completed 30 outpatient desensitizations. Most were male (57%) and mean age was 59 years (range 40–78 years; STD ± 15 years).
Compared to the pre-intervention period, the post-intervention period was associated with a 71.8% reduction in the mean time to start of desensitization (71 min vs. 252 min; p<0.0001) (Figure 1).
Figure 1.
Control chart demonstrating the amount of time from oncology patient arrival to start of the oxaliplatin desensitization infusion, comparing pre- and post-intervention periods. Pre-intervention = inpatient desensitization. Post-intervention = use of the outpatient infusion chairs for desensitization. UCL= upper control limit. CL = control limit. LCL=Lower control limit
In the pre-intervention group, there were 18 HSRs among 59 desensitizations (30.5%, 95% CI 19.5% to 44.5%). HSRs were grade 4 HSRs with hypotension or anaphylaxis (n=2), grade 2 HSRs with vomiting or throat/chest tightness (n=7), grade 1B HSR with flushing/pruritus and back pain (n=1), and grade 1A HSRs with flushing or pruritus (n=8).
In post-intervention group, there were seven HSRs among 30 desensitizations (23.3%, 95% CI 9.9% to 42.3%). HSRs were grade 1A HSRs with flushing or pruritus (n=4), and grade 1B HSRs with flushing or pruritus in addition to back pain and/or hypertension (n=3). These patients were managed with antihistamines and/or corticosteroids and tolerated the remainder of the desensitizations without any further reactions.
Comparing the pre- and post- intervention HSRs by grade of HSR, higher grade reactions were more likely to occur in the pre-intervention period (50% vs. 0%; p=0.027).
Patient experience surveys, completed in 10 patients (5 inpatients and 5 outpatient desensitization chair patients), showed improved patient experience, including significantly less disruption of the daily routine for those receiving outpatient desensitizations using a Likert scale (mean 2.0 (STD±1) vs. 3.6 (STD±0.9); p<0.03). Other patient survey findings were non-significant.
This process improvement evaluation demonstrated that chemotherapy outpatient desensitizations for oxaliplatin in low-risk patients was associated more than 3-fold reduction in the mean time to start of desensitization, saving these oncology patients on average more than 3 hours of wait time. Further, HSR frequency was similar and observed HSRs in the outpatient chair in these selected patients were less severe, although the post-intervention group was lower risk than the pre-intervention group. Also, patients reported less disruption, suggesting that this process improvement was also a welcome change from the patient perspective. We also anticipate a cost savings from the healthcare system perspective, which would be due to lower personnel time and higher acuity patients filling the inpatient beds (an opportunity cost).
This study used strict patient selection criteria (including that patients had tolerated at least one inpatient desensitization without a serious HSR), had a small sample size, and reports on the experience of a single center. While we limited patient selection to those receiving oxaliplatin desensitizations, we would expect similar findings with other chemotherapeutics. Future work might expand on this further to identify which patients, if any, can initiate their desensitization infusions in the outpatient infusion chair, avoiding inpatient desensitization all together. Additional analyses are needed to evaluate the healthcare resource impact of shifting desensitization care settings.
Using process improvement tools, we found that chemotherapy outpatient desensitizations can be operationalized and may improve timeliness, efficiency, and satisfaction while maintaining patient safety. Outpatient chemotherapy desensitization programs might increase access to first-line chemotherapies for patients with chemotherapy HSRs.
Supplementary Material
Acknowledgments
Financial disclosure: This work was funded in part by grant T32HL116275 from the National Institutes of Health for Dr. Sara Barmettler. All other authors declare no relevant financial disclosures.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
We have received permission from the Editor-In-Chief for a total of six authors in advance of submission of this manuscript.
References:
- 1.Bruchim I, Jarchowsky-Dolberg O, Fishman A. Advanced (>second) line chemotherapy in the treatment of patients with recurrent epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2013;166(1):94–98. doi: 10.1016/j.ejogrb.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Wang AL, Patil SU, Long AA, Banerji A. Risk-stratification protocol for carboplatin and oxaliplatin hypersensitivity: repeat skin testing to identify drug allergy. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2015;115(5):422–428. doi: 10.1016/j.anai.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 3.Lee C-W, Matulonis UA, Castells MC. Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol. 2005;99(2):393–399. doi: 10.1016/j.ygyno.2005.06.028 [DOI] [PubMed] [Google Scholar]
- 4.Green Robin; Downey Andrea; McCaffrey Kathleen; Fusco Eileen; Muggia Franc. A Risky Business: Implementing a Platinum-Based Desensitization Protocol in the Outpatient Setting. Oncology Nursing Forum. March2006, Vol. 33 Issue 2, P437–438. 2p. [Google Scholar]
- 5.Kowalski ML, Ansotegui I, Aberer W, et al. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: World Allergy Organization Statement. World Allergy Organ J. 2016;9(1):33. doi: 10.1186/s40413-016-0122-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil SU, Long AA, Ling M, et al. A protocol for risk stratification of patients with carboplatin-induced hypersensitivity reactions. J Allergy Clin Immunol. 2012;129(2):443–447. doi: 10.1016/j.jaci.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 7.Wong JT, Ling M, Patil S, Banerji A, Long A. Oxaliplatin Hypersensitivity: Evaluation, Implications of Skin Testing, and Desensitization. J Allergy Clin Immunol Pract. 2014;2(1):40–45. doi: 10.1016/j.jaip.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute: Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Bethesda, MD: National Cancer Institute, 2009. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed March 8, 2019. [Google Scholar]
- 9.SPC Press -- Book: Understanding Statistical Process Control - by Donald J. Wheeler. https://www.spcpress.com/book_understanding_statistical_process_control.php. Accessed September 14, 2018.
- 10.Rao SK, Carballo V, Cummings BM, Millham F, Jacobson JO. Developing an Interdisciplinary, Team-Based Quality Improvement Leadership Training Program for Clinicians: The Partners Clinical Process Improvement Leadership Program. Am J Med Qual Off J Am Coll Med Qual. 2017;32(3):271–277. doi: 10.1177/1062860616648773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.