Abstract
Background.
Minimally invasive lobectomy is associated with decreased morbidity and length of stay. However, there have been few published analyses using recent, population-level data to compare clinical outcomes and cost by surgical approach, inclusive of robotic-assisted thoracoscopic surgery (RATS). The objective of this study was to compare outcomes and hospitalization costs among patients undergoing open, video-assisted thoracoscopic surgery (VATS) and RATS lobectomy.
Methods.
We identified patients who underwent elective lobectomy in the Healthcare Cost and Utilization Project Florida State Inpatient Database (2008 to 2014). Hierarchical logistic and linear regression models were used to compare in-hospital mortality, postoperative complications, prolonged length of stay, 30-day readmissions, and index hospitalization costs among cohorts.
Results.
We identified 15,038 patients, of whom 8501 (56.5%), 4608 (30.7%), and 1929 (12.8%) underwent open, VATS, and RATS lobectomy, respectively. Robotic-assisted lobectomies comprised less than 1% of total lobectomy volume in 2008, and grew to 25% of lobectomy volume by 2014. Both VATS and RATS lobectomies were associated with decreased in-hospital mortality compared with thoracotomy (VATS odds ratio 0.69, 95% confidence interval, 0.50 to 0.94; RATS odds ratio 0.58, 95% confidence interval, 0.35 to 0.96; P = .016). After adjusting for patient age, sex, income, comorbidities, and hospital teaching status, VATS lobectomy was 2% less expensive (P = .007) and robotic-assisted lobectomy was 13% more expensive (P < .001) than the open approach.
Conclusions.
Minimally invasive approaches were associated with improved clinical outcomes compared with open lobectomy. However, only robotic-assisted lobectomy has had rapid growth in utilization. Despite additional cost, RATS lobectomy appears to provide a viable minimally invasive alternative for general thoracic procedures.
Minimally invasive technologies have revolutionized care for many thoracic surgery patients. Thoracoscopic lung resection is associated with decreased postoperative pain, faster recovery, and shorter hospital length of stay (LOS) with similar oncologic outcomes compared with open thoracotomy.1,2 The development of robotic-assisted thoracoscopic surgery (RATS) in the early 2000s introduced enhanced visualization, improved dexterity, accessibility to challenging locations, and superior ergonomics.3–5 However, there are few published studies that used recent population-level data to explore trends in utilization and compare clinical outcomes and costs among RATS, video-assisted thoracoscopic surgery (VATS), and open thoracotomy.
We aimed to examine our primary outcome of trends in utilization of open thoracotomy, VATS, and RATS for elective lobectomy. Additionally, we compared our secondary outcomes of in-hospital mortality, complications, prolonged LOS (14 days or longer), 30-day readmissions, and index hospitalization costs by surgical approach.
Patients and Methods
Data Sources
We utilized the Healthcare Cost and Utilization Project (HCUP) Florida State Inpatient Database (SID) from 2008 to 2014 to perform this retrospective cohort study.6 This SID is maintained by the HCUP, sponsored by the Agency for Healthcare Research and Quality, and captures approximately 90% of all state discharges.5 This database includes principal and secondary diagnoses codes, procedure codes, admission and discharge status, and total hospitalization charges on all patients, regardless of payer type. In addition, a unique identifier is assigned to each patient that can be used to link to additional admissions (including to other hospital facilities) within the same state. That allows for examination of readmissions. This study was exempt from Institutional Review Board approval at Washington University.
Patient Selection
The study included adult patients who underwent elective lobectomy for lung cancer through either open, VATS, or RATS approaches. We utilized International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM), codes to classify lobectomy into either open (code 32.41) or thoracoscopic (VATS) approaches (code 32.49; Supplemental Table 1). In 2007, codes were introduced to indicate robotic-assistance (codes 17.45 or 17.49). To allow time for routine adoption of this code, we started our study in 2008. We identified RATS patients as those who had dual codes of thoracoscopic lobectomy (code 32.49) with robotic-assistance (codes 17.45 or 17.49). We excluded patients who had a prior thoracotomy, who were coded for both open and thoracoscopic approaches during the same hospitalization, or for whom the approach was unknown. We also excluded patients admitted through the emergency department, patients with metastatic disease as defined by Elixhauser classification, and patients who had the ICD-9-CM code V64.42 indicating a conversion to thoracotomy. Conversions were excluded because of undercoding of the initial approach (ie, VATS or RATS). Comparisons of patients who had conversions, patients who had planned thoracotomies, and patients who had thoracoscopy without conversion are listed in Supplemental Tables 2 and 3.
Data Collection and Variable Definitions
We collected information on age, sex, median income by ZIP code, comorbidities, and postoperative complications. Teaching hospital status was abstracted from the American Hospital Association Annual Survey (Health Forum, Chicago, IL). Patient comorbidities were defined using the Elixhauser classification, using ICD-9-CM diagnosis codes from inpatient visits 1 year before the index admission.7 In-hospital major and minor postoperative complications were identified using diagnosis codes (Supplemental Table 1). Major complications included myocardial infarction, acute respiratory insufficiency, pulmonary embolism, hemorrhage, empyema, and septicemia. Additional outcomes included in-hospital mortality, LOS, prolonged LOS (14 days or longer), 30-day readmissions, 90-day readmissions, total index hospitalization costs, and 90-day costs. Total hospitalization charges were used to estimate costs from the hospital payer perspective by applying the facility-specific cost-to-charge ratio, developed by HCUP using data from the Centers for Medicare and Medicaid services.8
Statistical Analysis
Cochran-Armitage tests were used to compare utilization trends of surgical approaches over time. Similar to previously published analyses of hospital procedure volume, we calculated the average annual hospital RATS volume by dividing the total number of RATS performed by a facility by the number of years in which the facility performed RATS.9,10 Continuous variables were compared using Kruskal-Wallis tests, and categoric data were assessed using χ2 tests. All P values were two-tailed and adjusted for multiple comparisons by dividing by the number of tests. Missing covariate data were handled with separate categoric variables in descriptive and inferential analysis.
The association between surgical procedures and the risks for in-hospital mortality, major postoperative complications, prolonged LOS, and 30-day readmission were analyzed using hierarchic logistic regression with clustering at the hospital level using the GLIMMIX procedure in SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC). Linear regression with clustering at the hospital level was performed to analyze the association between surgical procedures and index hospitalization cost. Given the highly skewed and nonnormal distribution (Kolmogorov-Smirnov test for normality: D = 0.221, P < .01), hospitalization costs were log-transformed before inclusion in the linear regression model. Covariates used for adjustment were selected a priori based on clinical relevance and included age, sex, Elixhauser comorbidities, income quartile, and hospital teaching status.
Results
Utilization Trends
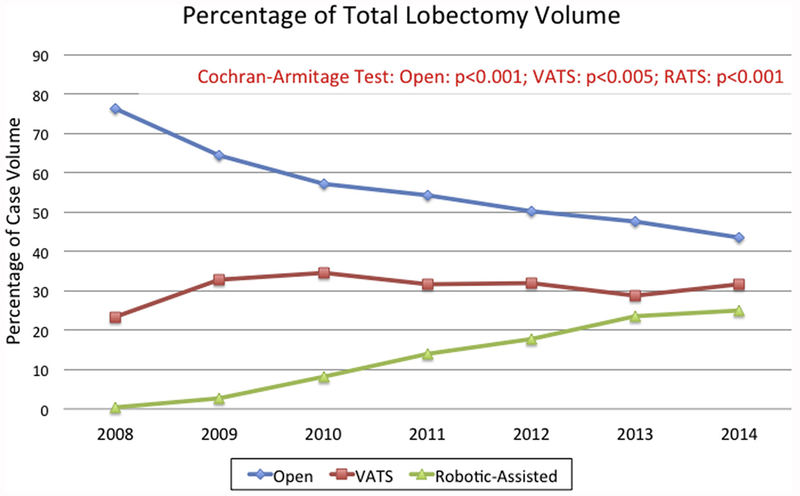
We identified 15,038 patients who met inclusion or exclusion criteria (Supplemental Figure 1), of whom 8501 (56.5%), 4608 (30.7%), and 1929 (12.8%) underwent elective open, VATS, and RATS lobectomy, respectively. There were significant changes in utilization trends of each surgical approach (open, P < .001; VATS, P < .005; RATS, P < .001). In 2008, open, VATS, and RATS approaches made up 76.2%, 23.4%, and less than 1% of annual lobectomy volume (n = 2257), respectively. By 2014, open, VATS, and RATS approaches comprised 43.4%, 31.6%, and 25.0% of annual lobectomy volume (n = 2055), respectively (Figure 1).
Figure 1.
Annual trends in lobectomy volume by approach: open (blue line); video-assisted thoracoscopic surgery (VATS [red line]); and robotic-assisted thoracoscopic surgery (RATS [green line]).
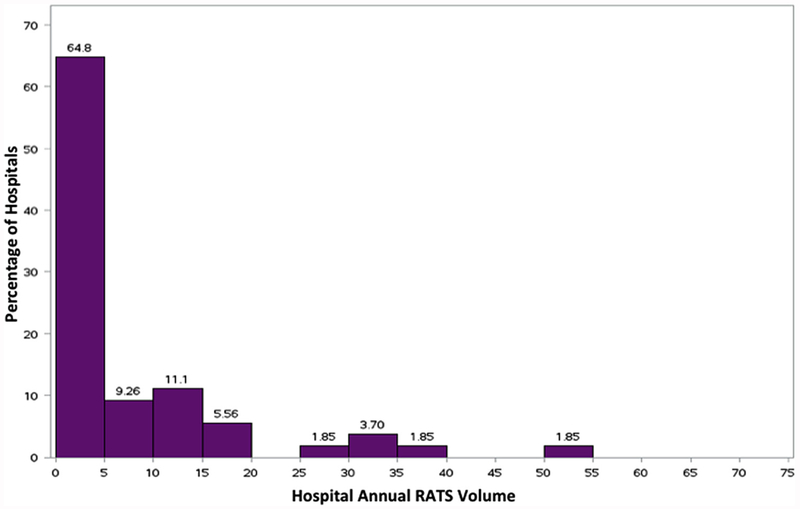
We identified 54 hospitals that performed RATS lobectomy. The numbers of hospitals adopting RATS lobectomy grew markedly over the study period (Table 1). However, the median number of RATS lobectomies performed by hospital annually remained stable. The median annual RATS procedure volume was only 5 cases per year (interquartile range [IQR], 1.5 to 10.75). The distribution of annual procedure volume is illustrated in Figure 2.
Table 1.
Trends in Hospital Adoption of Robotic-Assisted Thoracoscopic Surgery Lobectomy
| Variable | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|
| Robotic hospitals | 2 | 6 | 14 | 23 | 35 | 37 | 38 |
| Total RATS lobectomies | a | 59 | 170 | 301 | 391 | 486 | 513 |
| Median RATS lobectomies per hospital | 4.5 (1-8) | 8.5 (2-17) | 3.5 (2-25) | 5 (2, 18) | 5 (2, 13) | 7 (2, 18) | 6 (2, 20) |
Omitted because less than 11, in compliance with Healthcare Cost and Utilization Project data use agreement.
Values are n or n (interquartile range).
RATS, robotic-assisted thoracoscopic surgery.
Figure 2.
Distribution of hospital annual robotic-assisted thoracoscopic surgery (RATS) lobectomy volume.
Patient and Hospital Characteristics
Patients who received thoracoscopy had lower prevalence of certain comorbidities, including congestive heart failure (P = .009), pulmonary hypertension (P = .001), chronic obstructive pulmonary disease (P = .001), diabetes mellitus (P = .043), obesity (P = .024), and unintended weight loss/malnutrition (P < .001; Table 2). Between VATS and RATS patients, similar prevalence of comorbidities was observed. Of note, RATS surgeries were more likely to be performed in teaching hospitals (P < .001).
Table 2.
Patient Demographics and Hospital Characteristics
| Variables | Open (n = 8501 [56.5]) |
VATS (n = 4608 [30.7]) |
RATS (n = 1929 [12.8]) |
P Value All Cohorts |
P Valuea RATS Vs Open |
P Valuea RATS Vs VATS |
|---|---|---|---|---|---|---|
| Age, y | 67.8 ±10.3 | 68.4±10.1 | 68.8±10.1 | <0.001 | <0.001 | 0.071 |
| Male | 4179 (49.2) | 2057 (44.6) | 840 (43.6) | <0.001 | <0.001 | 0.417 |
| Comorbidity | ||||||
| CHF | 499 (5.9) | 215 (4.7) | 95 (4.9) | .009 | .106 | .653 |
| Valve disease | 575 (6.8) | 287 (6.2) | 141 (7.3) | .243 | .397 | .107 |
| PH | 310 (3.7) | 117 (2.5) | 51 (2.6) | .001 | .032 | .807 |
| PVD | 1068 (12.6) | 466 (10.1) | 195 (10.1) | <.001 | .003 | .996 |
| COPD | 4999 (58.8) | 2439 (52.9) | 953 (59.4) | <.001 | <.001 | .009 |
| Diabetes mellitus | 1856 (21.8) | 920 (20) | 405 (21) | .043 | .420 | .345 |
| Hypertension | 5663 (66.7) | 2998 (65.1) | 1258 (65.2) | .152 | .240 | .905 |
| Renal disease | 622 (7.3) | 309 (6.7) | 121 (6.3) | .174 | .108 | .520 |
| Liver disease | 186 (2.2) | 84 (1.8) | 43 (2.2) | .334 | .911 | .278 |
| Obesity | 942 (11.1) | 444 (9.6) | 218 (11.3) | .024 | .781 | .042 |
| Weight loss | 417 (4.9) | 154 (3.3) | 86 (4.5) | <.001 | .408 | .029 |
| Alcohol abuse | 381 (4.5) | 180 (3.9) | 70 (3.6) | .120 | .096 | .594 |
| Drug use | 119 (1.4) | 47 (1) | 20 (1) | .119 | .209 | .951 |
| Elixhauser CI | 10 (6, 17) | 9 (6, 15) | 8 (6, 14) | <.001 | <.001 | <.001 |
| Median income by ZIP code | <.001 | <.001 | <.001 | |||
| Q < 25% | 2570 (40) | 1092 (23.7) | 547 (28.3) | |||
| Q 25%-50% | 2949 (34.7) | 1638 (35.5) | 585 (30.3) | |||
| Q 51%-75% | 1958 (23) | 1275 (27.7) | 544 (28.2) | |||
| Q > 75% | 891(10.5) | 526 (11.4) | 213 (11) | |||
| Unknown | 133 (1.6) | 77 (1.7) | 40 (2.1) | |||
| Teaching hospital | 4472 (52.6) | 2375 (51.6) | 1321 (68.5) | <.001 | <.001 | <.001 |
P < .025 considered statistically significant to correct for multiple comparisons.
Values are mean ± SD or n (%).
CHF, congestive heart failure; CI, comorbidity index; COPD, chronic obstructive pulmonary disease; PH, pulmonary hypertension; PVD, peripheral vascular disease; Q, quartile; RATS, robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
In-hospital Mortality
Unadjusted in-hospital mortality was significantly different among cohorts, with higher mortality in the open cohort (open, 2.2%; VATS, 1.4%; RATS, 1.1%; P < .001; Table 3). In-hospital mortality was significantly lower in RATS compared with open lobectomy (P < .001), but was similar between RATS and VATS lobectomy. When risk-adjusted, RATS and VATS had a 42% and 31% decreased risk of in-hospital mortality compared with open lobectomy, respectively (RATS odds ratio [OR] 0.58, 95% confidence interval [CI], 0.35 to 0.96); VATS OR 0.69, 95% CI, 0.50 to 0.94; P = .016; Table 4).
Table 3.
Perioperative and Longitudinal Outcomes by Surgical Approach
| Outcome | Open (n = 8501 [56.5]) |
VATS (n = 4608 [30.7]) |
RATS, (n = 1929 [12.8]) |
P Value All Cohorts |
P Valuea RATS vs Open |
P Valuea RATS vs VATS |
|---|---|---|---|---|---|---|
| In-hospital mortality | 185 (2.2) | 66 (1.4) | 22 (1.1) | <.001 | .003 | .350 |
| Any major complication | 1030 (12.1) | 325 (7.1) | 97 (5) | <.001 | <.001 | .607 |
| Myocardial infarction | 73 (0.86) | 27 (0.59) | 14 (0.73) | .225 | .562 | .514 |
| ARI | 572 (6.7) | 161 (3.5) | 28 (1.5) | <.001 | <.001 | <.001 |
| Pulmonary embolism | 57 (0.67) | 23 (0.50) | b | .271 | .198 | .651 |
| Hemorrhage | 152 (1.8) | 58 (1.3) | 26 (1.4) | .047 | .178 | .770 |
| Empyema | 156 (1.8) | 40 (0.87) | 14 (0.73) | <.001 | .001 | .562 |
| Septicemia | 187 (2.2) | 73 (1.6) | 23 (1.2) | .003 | .005 | .230 |
| Any minor complication | 3883 (45.7) | 1934 (50) | 736 (38.2) | <.001 | <.001 | <.001 |
| SVA/AF | 1887 (22.2) | 930 (20.2) | 349 (18) | <.001 | <.001 | .046 |
| Postoperative air leak | 320 (3.8) | 190 (4.1) | 155 (8) | <.001 | <.001 | <.001 |
| Pneumonia | 857 (10.1) | 324 (7) | 100 (5.2) | <.001 | <.001 | .006 |
| Pulmonary edema | 23 (0.27) | b | b | .530 | .622 | .771 |
| Wound complication | 54 (0.64) | b | 11 (0.57) | .005 | .743 | .021 |
| Deep vein thrombosis | 46 (0.54) | 17 (0.37) | b | .022 | .010 | .069 |
| Urinary tract infection | 354 (4.2) | 154 (3.3) | 64 (3.3) | .031 | .087 | .960 |
| Length of stay, d | 7 (5-9) | 5 (4-8) | 4 (3-6) | <.001 | <.001 | <.001 |
| Prolonged LOS, >14 d | 1032 (12.1) | 358 (7.8) | 83 (4.3) | <.001 | <.001 | <.001 |
| 30-day readmission | 961 (11.3) | 502 (10.9) | 187 (9.7) | .122 | .041 | .150 |
| 90-day readmission | 964 (11) | 445 (9.66) | 177 (9.18) | .001 | .001 | .545 |
| Index hospitalization cost, $ | 17,200 (13,638-22,922) |
17,802 (13,998-23,095) |
20,377 (16,442-26,272) |
<.001 | <.001 | <.001 |
| 90-day cost, $ | 18,464 (14,144-26,361) |
18,832 (14,429-25,780) |
21,538 (16,936-29,262) |
<.001 | <.001 | <.001 |
P < .025 considered statistically significant to adjust for multiple comparisons;
Omitted because less than 11, in compliance with Healthcare Cost and Utilization Project data use agreement.
Values are n (%) or median (interquartile range).
ARI, acute respiratory insufficiency; LOS, length of stay; RATS, robotic-assisted thoracoscopic surgery; SVA/AF, supraventricular arrhythmia/atrial fibrillation; VATS, video-assisted thoracoscopic surgery.
Table 4.
Hierarchical Logistic Regression of Perioperative and 30-Day Outcomes
| Covariate | In-hospital Mortality OR (95% CI) |
P Value |
Major Complications OR (95% CI) |
P Value |
Prolonged LOSa OR (95% CI) |
P Value |
30-Day Readmission OR (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|---|
| Age, y | 1.04 (1.02-1.05) | <.001 | 1.00 (0.99-1.01) | 0.974 | 1.01 (1.00-1.02) | 0.003 | 1.01 (1.00-1.02) | 0.002 |
| Sex | <.001 | <.001 | <.001 | .283 | ||||
| Male | … | … | … | … | ||||
| Female | 0.62 (0.47-0.81) | 0.76 (0.67-0.85) | 0.68 (0.60-0.77) | 0.94 (0.85-1.02) | ||||
| Income by ZIP code | .660 | .047 | .731 | .446 | ||||
| Q < 25% | … | … | … | … | ||||
| Q 25%-50% | 1.19 (0.86-1.65) | 1.13 (0.97-1.31) | 0.98 (0.85-1.14) | 0.91 (0.80-1.03) | ||||
| Q 51%-75% | 1.23 (0.86-1.77) | 1.26 (1.07-1.49) | 1.01 (0.86-1.19) | 0.93 (0.81-1.08) | ||||
| Q > 75% | 1.12 (0.69-1.82) | 1.22 (0.98-1.52) | 0.89 (0.71-1.11) | 0.89 (0.75-1.08) | ||||
| Comorbidity | ||||||||
| CHF | 2.87 (2.03-4.05) | <.001 | 2.28 (1.87-2.78) | <.001 | 2.19 (1.80-2.68) | <.001 | 1.48 (1.21-1.81) | .001 |
| Valve disease | 1.31 (0.89-1.92) | .192 | 0.95 (0.77-1.19) | .671 | 1.11 (0.90-1.38) | .339 | 0.97 (0.79-1.19) | .750 |
| PH | 4.72 (3.27-6.80) | <.001 | 4.63 (3.72-5.77) | <.001 | 2.54 (2.00-3.23) | <.001 | 1.19 (0.91-1.55) | .210 |
| PVD | 0.83 (0.58-1.19) | .343 | 1.06 (0.89-1.25) | .526 | 1.14 (0.97-1.34) | .123 | 1.18 (1.01-1.38) | .032 |
| COPD | 1.47 (1.10-1.96) | .009 | 1.56 (1.37-1.78) | <.001 | 2.18 (1.91-2.50) | <.001 | 1.29 (1.16-1.44) | <.001 |
| Diabetes mellitus | 1.25 (0.94-1.68) | .130 | 1.14 (0.99-1.31) | .072 | 0.99 (0.86-1.15) | .922 | 1.08 (0.95-1.22) | .273 |
| Hypertension | 0.88 (0.65-1.20) | .423 | 1.01 (0.88-1.15) | .909 | 0.83 (0.73-0.95) | .006 | 1.20 (1.06-1.36) | .004 |
| Renal disease | 1.63 (1.14-2.32) | .007 | 1.28 (1.05-1.56) | .015 | 1.52 (1.25-1.85) | <.001 | 1.08 (0.89-1.31) | .424 |
| Liver disease | 1.25 (0.60-2.63) | .554 | 0.95 (0.66-1.38) | .797 | 1.63 (1.17-2.27) | .004 | 1.32 (0.95-1.82) | .096 |
| Obesity | 0.81 (0.53-1.24) | .329 | 1.11 (0.93-1.33) | .285 | 0.90 (0.74-1.09) | .286 | 1.07 (0.91-1.27) | .415 |
| Weight loss | 6.96 (5.07-9.55) | <.001 | 4.38 (3.60-5.32) | <.001 | 6.42 (5.33-7.74) | <.001 | 1.35 (1.08-1.40) | .008 |
| Alcohol abuse | 0.98 (0.56-1.74) | .957 | 1.14 (0.88-1.48) | .323 | 1.46 (1.14-1.85) | .002 | 1.13 (0.89-1.45) | .320 |
| Drug use | 0.44 (0.10-1.93) | .277 | 1.27 (0.81-1.99) | .299 | 1.47 (0.97-2.23) | .069 | 1.85 (1.26-2.73) | .002 |
| Hospital teaching status | .972 | .331 | .544 | .258 | ||||
| No AMA affiliation | … | … | … | … | ||||
| AMA affiliation | 0.99 (0.72-1.38) | 0.90 (0.81-1.99) | 0.94 (0.77-1.15) | 1.07 (0.95-1.21) | ||||
| Surgical approach | .016 | <.001 | <.001 | .155 | ||||
| Open | … | … | … | … | ||||
| VATS | 0.69 (0.50-0.94) | 0.62 (0.54-0.73) | 0.66 (0.57-0.76) | 1.01 (0.89-1.14) | ||||
| RATS | 0.58 (0.35-0.96) | 0.43 (0.34-0.56) | 0.38 (0.29-0.49) | 0.85 (0.71-1.01) |
More than 14 days.
AMA, American Medical Association; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio; PH, pulmonary hypertension; PVD, peripheral vascular disease; Q, quartile; RATS, robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
Perioperative Complications
Of the study cohort, 1452 patients (9.7%) had at least one major postoperative complication. Compared with open lobectomy, RATS had a lower incidence of complications such as acute respiratory insufficiency (P < .001), empyema (P = .001), and septicemia (P = .005; Table 3). Robotic-assisted thoracoscopic surgery was associated with a lower incidence of acute respiratory insufficiency compared with VATS (P < .001). Robotic-assisted thoracoscopic surgery and VATS were associated with 57% and 38% decreased adjusted risk of any major postoperative complication developing compared with open lobectomy, respectively (RATS OR 0.43, 95% CI, 0.34 to 0.56); VATS OR 0.62, 95% CI, 0.54 to 0.73; both P < .001; Table 4).
Prolonged Length of Stay
The RATS patients had significantly shorter LOS compared with patients who underwent open lobectomy (4 days, IQR, 1 to 3, vs 7 days, IQR, 5 to 9; P < .001) and compared with patients who underwent VATS lobectomy (4 days, IQR, 1 to 3, vs 5 days, IQR, 4 to 8; P < .001; Table 3). On adjusted analysis, RATS and VATS approaches were associated with 62% and 34% reduced odds of prolonged LOS compared with open lobectomy, respectively (RATS OR 0.38, 95% CI, 0.29 to 0.49); VATS OR 0.66 (95% CI, 0.57 to 0.76; both P < .001; Table 4).
Thirty-Day Readmission
The 30-day readmission rate for the study cohort was 11% (Table 3). There were significantly fewer 30-day readmissions observed in the RATS group compared with the open cohort (P < .001), but readmissions were similar between RATS and VATS patients (P = .150). On adjusted analysis, surgical approach was not associated with 30-day readmission (Table 4).
Hospitalization Costs
Hospitalization costs for patients undergoing RATS lobectomy were significantly more expensive than open lobectomy ($20,377 vs $17200, P < .0001) and VATS lobectomy ($20,377 vs $17802, P < .001; Table 3). These trends were also observed in unadjusted 90-day costs. On linear regression, RATS was 13% more expensive than open lobectomy (OR 1.13, 95% CI, 1.10 to 1.15; Table 5). Meanwhile, VATS was 2% less expensive than open lobectomy (OR 0.98, 95% CI, 0.96 to 0.99).
Table 5.
Hierarchical Linear Regressiona of Inpatient Costs
| Covariate | Estimate | P Value |
|---|---|---|
| Intercept | 15,787.91 (14,893.80-16,725.67) | <.001 |
| Age, y | 1.001 (1.0008-1.002) | <.001 |
| Sex | .006 | |
| Male | … | |
| Female | 0.92 (0.91-0.93) | |
| Income quartile by ZIP Code | ||
| Quartile < 25% | … | … |
| Quartile 25%-50% | 0.98 (0.97-0.99) | .043 |
| Quartile 51%-75% | 0.98 (0.96-0.99) | .041 |
| Quartile > 75% | 0.98 (0.95-0.99) | .037 |
| Comorbidity | ||
| Congestive heart failure | 1.23 (1.19-1.26) | <.001 |
| Valve disease | 1.02 (0.99-1.04) | .194 |
| Pulmonary hypertension | 1.28 (1.24-1.33) | <.001 |
| Peripheral vascular disease | 1.04 (1.02-1.05) | <.001 |
| COPD | 1.12 (1.11-1.14) | <.001 |
| Diabetes mellitus | 1.02 (1.01-1.04) | .003 |
| Hypertension | 0.99 (0.98-1.01) | .272 |
| Renal disease | 1.10 (1.07-1.13) | <.001 |
| Liver disease | 1.07 (1.02-1.12) | .003 |
| Obesity | 1.05 (1.03-1.07) | <.001 |
| Weight loss | 1.59 (1.54-1.64) | <.001 |
| Alcohol abuse | 1.09 (1.06-1.13) | <.001 |
| Drug use | 1.05 (0.99-1.11) | .093 |
| Hospital teaching status | .495 | |
| No AMA affiliation | … | |
| AMA affiliation | 1.01 (0.98-1.05) | |
| Surgical approach | ||
| Open | … | |
| VATS | 0.98 (0.96-0.99) | 0.007 |
| RATS | 1.13 (1.10-1.15) | <.001 |
Linear regression equation given in Supplemental Table 4.
AMA, American Medical Association; COPD, chronic obstructive pulmonary disease; RATS, robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
Comment
We observed significant changes in the utilization of lobectomy approaches over the study period. Open lobectomies declined from 75% of total volume in 2008 to only 43% by 2014. Meanwhile, RATS lobectomies grew to represent 25% of total volume. This growth is striking, especially compared with the more modest growth observed in VATS utilization. Our study extends findings published by nationally representative studies that utilized data from earlier time periods. Paul and associates1 examined the HCUP National Inpatient Sample from 2008 to 2010 to study utilization trends of RATS and VATS lobectomy. Among hospitals that performed minimally invasive lobectomy, the percentage of robotic volume grew from 10% to 24.6%. Meanwhile, VATS volume grew from 36.2 to 42.7%. Kent and colleagues5 examined lung resection approaches using eight HCUP SIDs from 2008 to 2010. Both studies found similar incidence of risk-adjusted in-hospital mortality, LOS, routine discharges. However, the study by Paul and associates1 noted that, RATS patients had 2.64 times increased odds of experiencing an iatrogenic complication (ie, bleeding) compared with VATS patients. Our study builds off these publications with more recent data, which highlight not only the rapid adoption of RATS surgery but also suggest the potential influence of the learning-curve effect on improvement in outcomes.
The learning curve for RATS may be less steep compared with VATS. Studying the learning curve can be challenging, but a few studies have utilized cumulative sum analysis, which monitors outcomes continuously to provide signals of trends toward greater than expected adverse outcomes. Puri and colleagues11 previously used cumulative sum analysis of data from The Society of Thoracic Surgeons General Thoracic Surgery Database (STS GTSDB) to establish VATS proficiency as 50 cases. In an abstract presented at the 2018 Southern Thoracic Surgical Association meeting, Feczko and colleagues12 used cumulative sum analysis of the STS GTSDB to study RATS proficiency in three groups: de novo, open-to-robotic, and VATS-to-robotic surgeons. In terms of major morbidity and mortality, all groups achieved acceptable proficiency by the 20th case. The only outcome where open-to-robotic surgeons lagged behind was operative time, defined as 225 minutes.
We demonstrated that the quality afforded by RATS compared with VATS is comparable for multiple endpoints. Our findings are consistent with retrospective studies that utilized alternative large databases. Louie and colleagues13 queried the STS GTSD to compare VATS and RATS approaches for patients with early stage non-small cell lung cancer. Examining lobectomies from 2009 to 2013, the researchers found similar morbidity, LOS, 30-day mortality, and nodal upstaging between the two approaches. However, they observed that patients who underwent RATS tended to be older and had more comorbid disease. Additional studies have demonstrated that RATS can be performed without compromise to oncologic quality or long-term survival. In a prospective multiinstitutional registry, Yang and colleagues14 examined overall and disease-free survival for clinical stage I non-small cell lung cancer patients by open, VATS, and RATS approach. Using a propensity-matched cohort, the researchers found similar 5-year overall survival by approach. Compared with VATS patients, RATS patients had significantly better 5-year disease-free survival (72.7% vs 65.5%) and more mediastinal node stations sampled (5 vs 3 stations). This finding suggests that RATS may provide adequate if not superior oncologic quality, although that has yet to be studied in a randomized controlled trial.
One barrier to adoption of RATS is the upfront escalation in costs. We found that, on average, RATS was 12% more costly than open lobectomy. From the payer perspective, that can be challenging when attempting to justify the expense for new technologies. Additional studies have attempted to estimate the value of thoracoscopic approaches using cost-effectiveness approaches. Kaur and colleagues15 performed a micro-costing analysis from the payer perspective examining medical and indirect costs accumulated during the in-hospitalization and 30-day postoperative period. They identified that RATS cases were on average $3116 more expensive per case, and this difference was mainly driven by longer intraoperative time. Postoperative outcomes were similar. Previous studies have found potential savings associated with RATS. Nasir and colleagues16 performed a detailed cost comparison of surgical approach, taking into account direct costs, indirect costs, and surgeon professional fees. Average costs were generated for each hospital day. Compared with VATS, patients undergoing RATS incurred an additional $3880, primarily incurred on the first day. However, compared with thoracotomy, RATS was (on average) $3988 less expensive, primarily due to shorter LOS. Reductions in operative time and LOS through improved training and experience and care pathways may be one aspect where RATS can generate cost savings.
Important limitations should be noted. First are the limitations inherent in observational research, including unmeasured confounding and treatment selection bias. Few randomized, controlled trials have compared minimally invasive approaches to open thoracotomy. Unlike our study, these studies did not observe significant differences in postoperative complications or LOS in patients randomly assigned to VATS or open thoracotomy17,18. This finding could suggest that there is unobserved confounding that could lead to the positive effects observed among minimally invasive cohorts in our study. The SID does not contain cancer-specific data including stage, histology, tumor size, or tumor location, which are important factors in selecting surgical approach. However, it is difficult to know whether unobserved confounders were the only explanation for these disparate findings, as these studies often were powered for different outcomes other than morbidity or LOS. An additional limitation is external validity. Given, that this analysis uses the Florida SID, differences in the underlying patient population or surgical practices could potentially limit generalizability of findings to the national setting. Finally, we excluded conversions because of the inability to determine the initial approach and the planned vs unplanned nature in these cases. These exclusions could introduce bias and make outcomes associated with thoracoscopy appear more favorable. However, only 488 patients had a conversion, which comprised only 3% of the study cohort before exclusion.
There are, however, also important strengths of this study to recognize. It is one of the few studies to use recent population level data to characterize recent trends in surgical approach, which captures both clinical and cost data.
In conclusion, we have demonstrated that RATS utilization has risen markedly and is associated with reduced in-hospital mortality, major complication rates, and LOS, compared with open thoracotomy. Compared with VATS, RATS lobectomy is similar on multiple endpoints, but is more costly. Given the clinical outcomes advantages and evidence of its rapid adoption over time, RATS may represent a viable alternative for incorporation of minimally invasive technology into routine practice.
Supplementary Material
Acknowledgments
Melanie Subramanian receives funding support from the National Institutes of Health (NIH) T32 cardiothoracic training grant (T32HL007776-23). The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the NIH and grant number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ).
Abbreviations and Acronyms
- CI
confidence interval
- HCUP
Healthcare Cost and Utilization Project
- ICD-9
International Classification of Diseases, Ninth Revision
- IQR
interquartile range
- LOS
length of stay
- OR
odds ratio
- RATS
robotic-assisted thoracoscopic surgery
- SID
State Inpatient Database
- STS GTSD
The Society of Thoracic Surgeons General Thoracic Surgery Database
- VATS
video-assisted thoracoscopic surgery
Footnotes
Presented at the Fifty-fifth Annual Meeting of The Society of Thoracic Surgeons, San Diego, CA, Jan 26-29, 2019.
The Supplemental Tables and Supplemental Figure can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2019.06.049] on http://www.annalsthoracicsurgery.org.
References
- 1.Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg. 2013;43:813–817. [DOI] [PubMed] [Google Scholar]
- 2.Nwogu CE, D’Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg. 2015;99:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerfolio RJ, Watson C, Minnich DJ, et al. One hundred planned robotic segmentectomies: early results, technical details, and preferred port placement. Ann Thorac Surg. 2016;101:1089–1095 [discussion: 1095-1096]. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: state of the art and perspectives. Lung Cancer. 2016;101:28–34. [DOI] [PubMed] [Google Scholar]
- 5.Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–242 [discussion: 242-244]. [DOI] [PubMed] [Google Scholar]
- 6.Healthcare Cost and Utilization Project (HCUP). HCUP State Inpatient Databases (SID) 2008-2014. Available at: www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed Xxxx, X, XXXX.
- 7.Elixhauser A, Steiner C, Harris DR, et al. Comorbiditymeasures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 8.Healthcare Cost and Utilization Project (HCUP).Cost-to-charge ratio files 2018. Available at: https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. Accessed Xxxx, X, XXXX.
- 9.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725 [discussion: 726]. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 11.Puri V, Gaissert HA, Wormuth DW, et al. Defining proficiency for The Society of Thoracic Surgeons participants performing thoracoscopic lobectomy. Ann Thorac Surg. 2019;107:202–208. [DOI] [PubMed] [Google Scholar]
- 12.Feczko AF, Wang H, Nishimura K, et al. Proficiency of robotic lobectomy based on prior surgical technique in The Society of Thoracic Surgeons General Thoracic Database. Paper presented at: 65th Annual Meeting of the Southern Thoracic Surgical Association Nov 7-10, 2018; Amelia Island, FL. [Google Scholar]
- 13.Louie BE, Wilson JL, Kim S, et al. Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stage I and stage II non-small cell lung cancer using The Society of Thoracic Surgeons Database. Ann Thorac Surg. 2016;102:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HX, Woo KM, Sima CS, et al. Long-term Survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur MN, Xie F, Shiwcharan A, et al. Robotic versus video-assisted thoracoscopic lung resection during early program development. Ann Thorac Surg. 2018;105:1050–1057. [DOI] [PubMed] [Google Scholar]
- 16.Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg. 2014;98:203–208 [discussion: 208-209]. [DOI] [PubMed] [Google Scholar]
- 17.Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–844. [DOI] [PubMed] [Google Scholar]
- 18.Long H, Tan Q, Luo Q, et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg. 2018;105:386–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.