Abstract
Background:
Use of electronic cigarettes (e-cigarettes) has increased exponentially since their appearance on the U.S. market around 2007. To provide preclinical models of vaping that incorporate olfactory cues and chemosensory effects (including flavors) that play a role in human vaping behavior, the feasibility of using a modified e-cigarette device for delivery of aerosolized nicotine was examined in a nicotine discrimination procedure in mice.
Methods:
Adult female and male C57BL/6 mice were trained to discriminate 0.75 mg/kg subcutaneous (s.c.) nicotine from saline. After determination of a s.c. nicotine dose-effect curve, aerosolized freebase nicotine and nicotine-containing tobacco products (i.e., non-flavored and Arctic Blast e-liquids) were evaluated.
Results:
Nicotine (s.c.) dose-dependently substituted in mice of both sexes, although females showed less sensitivity and greater variability. By contrast, aerosolized nicotine, regardless of formulation, produced concentration-dependent increases up to maximum of 46–62% nicotine-associated responding. Brain nicotine concentrations for each sex were similar for s.c. 0.75 mg/kg nicotine and 30 mg/ml freebase nicotine.
Conclusions:
Mice of both sexes readily acquired s.c. nicotine discrimination, but females showed less sensitivity. Further, all three formulations of aerosolized nicotine produced increases in nicotine-like responding in mice of each sex. However, the maximum magnitude of these increases did not engender a similar degree of substitution as s.c. 0.75 mg/kg nicotine, despite similar brain concentrations of nicotine at 30 mg/ml aerosolized nicotine. Additional research is needed for determination of the reason(s); however, results here demonstrate initial feasibility for examination of the discriminative stimulus effects of vaped drugs such as nicotine.
Keywords: discrimination, nicotine, route of administration, sex differences
1.0. Introduction
Electronic cigarettes (e-cigarettes) appeared in the U.S. market around 2007 and have grown in popularity in the ensuing decade. Adult use of e-cigarettes rose from 0.3% in 2010 to 4.5% in 2016, with over half of these users under the age of 35 (Mirbolouk et al., 2018). Similarly, high school student use of e-cigarettes rose from 1.5% to 20.8% from 2011 to 2018 (Cullen et al., 2018). Furthermore, e-cigarettes that allow the user to fill a tank with e-liquid (vs. cig-a-likes pre-filled with nicotine) (Brown and Cheng, 2014; Grana et al., 2014) are increasingly being used to vaporize other substances such as cannabis (Giroud et al., 2015; Morean et al., 2015).
Because inhalation is the most common route of administration of nicotine-containing tobacco products in humans, a recent push to provide more translationally relevant preclinical models has concentrated on development of equipment and methods that allow exposure of freely moving rodents to aerosolized nicotine (George et al., 2010; Lefever et al., 2017a; Ponzoni et al., 2015; Smith et al., 2015). Aerosol delivery has several advantages over injection for assessment of addiction to nicotine-containing tobacco products. For example, inhaled nicotine reaches the brain much faster than subcutaneously (s.c.) injected nicotine (10–20 s for inhalation in humans) (Benowitz et al., 2009), and speed of absorption and distribution is positively correlated with abuse liability (Benowitz, 1990; Henningfield and Keenan, 1993). In addition, aerosol exposure incorporates many of the olfactory cues and chemosensory effects (including flavors) that play a role in human experimentation, initiation, and maintenance of vaping behavior, especially in youth (Goldenson et al., 2016; Hoffman et al., 2016).
Feasibility of using modified e-cigarette devices for delivery of aerosolized nicotine has been demonstrated by showing that acute exposure using this method produced pharmacological effects in mice that are characteristic of injected nicotine, including effects on locomotion, temperature, and antinociception (Lefever et al., 2017a). Further, for nicotine, the observed in vivo effects are accompanied by increases in plasma and brain concentrations of the parent drug and its metabolites (Lefever et al., 2017a). In addition, exposure to aerosolized nicotine has been shown to increase subsequent intravenous nicotine self-administration and to induce dependence with chronic administration in rats (George et al., 2010; Gilpin et al., 2014; Ponzoni et al., 2015). In the present study, we add drug discrimination to the repertoire of behavioral procedures in which the effects of aerosolized nicotine have been investigated.
Drug discrimination is a pharmacologically selective animal model of the subjective effects of psychoactive drugs in humans (Moser et al., 2011; Schuster and Johanson, 1988) and is one of the primary procedures suggested by the Food and Drug Administration for use in abuse liability assessment (Food and Drug Administration, 2010). Previous studies of nicotine discrimination have reported that nicotine has robust discriminative stimulus effects in male mice at training doses ranging from 0.4 to 1.78 mg/kg (Cunningham and McMahon, 2013; Stolerman et al., 1999; Varvel et al., 1999). Here, we trained both male and female C57BL/6 mice to discriminate s.c. nicotine from saline and determined the degree to which aerosolized e-liquids containing nicotine substituted for injected nicotine. The goal of this study was to determine the effects of route of administration (parenteral injection vs. aerosol exposure) and formulation (flavored vs. non-flavored) on the discriminative stimulus effects of nicotine, a drug which is most often abused via smoking or vaping.
2.0. Materials and Methods
2.1. Subjects
Experimentally naïve adult male and female C57BL/6 mice (Envigo, Frederick, MA) were singly housed in polycarbonate mouse cages in a temperature-controlled (20–22°C) colony room with a 12-h light/dark cycle (lights on at 6:00 AM). They were allowed to acclimate to the animal facility for one week prior to testing. All mice were maintained at ~90% of free-feeding body weight with ad libitum access to water. Mice in the drug discrimination experiment (n=8/sex) were trained and tested during the light phase. Separate mice (n=6/sex) were used for determination of nicotine/cotinine levels in plasma and brain and were sacrificed during the light phase. All studies reported in this manuscript were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by our Institutional Animal Care and Use Committee.
2.2. Apparatus
Mice were trained and tested in mouse operant chambers (Coulbourn Instruments, Whitehall, PA) housed within light- and sound-attenuating cubicles. Each chamber was outfitted with a house light, white noise generator, and two nose-poke apertures with stimulus lights over each aperture. A pellet feeder delivered 20 mg food pellets (Bioserv Inc., Frenchtown, NJ) into a pellet trough (with a light) centered between the two apertures. Chamber operations (i.e., illumination of house and stimulus lights, generation of white noise, delivery of food pellets, and recording of nose pokes) were controlled by a computer system (Coulbourn Instruments, Graphic State Software, v 3.03, Whitehall, PA).
Aerosol was delivered from a modified e-cigarette device to mouse-sized chambers, as described previously (Lefever et al., 2017a; Lefever et al., 2017b; Marusich et al., 2016). Briefly, an iStick 30 W Variable Wattage personal vaporizer (ELeaf, Irvine, CA, USA) supplied power (7 W) to a CE5-S tank and bottom dual coil clearomizer (1.8Ω) (Aspire, Kent, WA, USA). Air was pumped through the tank at 1 L/min by an adjustable air pump (Pacific Coast Distributing, Phoenix, AZ) to generate aerosol, which was delivered via Tygon tubing (Fisher Scientific, Pittsburgh, PA, USA) directed by 3-way stopcocks (Grainger, Raleigh, NC, USA) into an EZ-177 Sure-Seal mouse induction anesthesia chamber (10 cm × 10 cm × 10 cm) (E-Z-Anesthesia, Palmer, PA, USA).
2.3. Chemicals
(−)-Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in physiological saline (Patterson Veterinary, Devens, MA) for injection. (−)-Nicotine free base (Sigma-Aldrich) was mixed with a 50:50 propylene glycol (USP) and glycerin (USP) solution (Sigma-Aldrich) for aerosol administration. Commercially available nicotine-containing e-liquids were acquired from Avail Vapor (Richmond, VA). These products used a vehicle of 50:50 blend of propylene glycol and vegetable glycerin. Doses of nicotine for injection are expressed as mg/kg of the base. Nicotine was injected subcutaneously (s.c.) at a volume of 10 mL/kg 10 min before the start of the operant session. Concentrations for aerosol administration are expressed as mg/mL in the e-cigarette tank and may not be representative of the actual amount of drug administered.
Chemicals and reagents for the analysis of e-liquid samples were purchased commercially and included nicotine, anabasine, myosmine (Sigma-Aldrich, St. Louis, MO), cotinine (Toronto Research Chemicals, Toronoto, ON), nornicotine (Ark-Pharm, Arlington Heights, IL), anatabine (Matrix Scientific, Elgin, SC). β-Nicotyrine was supplied internally from RTI International. Internal standards included anabasine-d4, myosmine-d4, nornicotine-d4 (Toronto Research Chemicals), cotinine-d3 (Santa Cruz Biotechnology, Dallas, TX), and nicotine-d3 (Cambridge Isotope Laboratories, Tewksbury, MA). Acetone, propylene glycol (Fisher Scientific, Hampton, NH), and glycerin (Spectrum, New Brunswick, NJ) were used as diluents. Instruments utilized helium (AirGas, Durham, NC) and nitrogen was supplied internally by a house system. An internal standard solution was prepared in acetone containing 472 ng/mL myosmine-d4, 466 ng/mL anabasine-d4, 485 ng/mL cotinine-d3, 948 ng/mL nornicotine-d4, and 500 ng/mL nicotine-d3. Working solutions containing a final injection concentration of nicotine (12–8000 ng/mL), anabasine (60–6000 ng/mL), β-nicotyrine (9–1800 ng/mL), myosmine (15–3600 ng/mL), cotinine (70–8000 ng/mL), nornicotine (20–8000), and anatabine (24–1200 ng/mL) were prepared in acetone.
2.4. Chemical Analysis of E-Liquids
Non-flavored and Arctic Blast nicotine-containing e-liquids were acquired from Avail Vapor (Richmond, VA). These commercially available e-liquids contain tobacco extracts. Concomitant with assessment of these solutions in drug discrimination, we identified and quantified nicotine and non-nicotine alkaloids present in these solutions.
2.4.1. Preparation of Standards and Samples
Standards were prepared for minor alkaloids by combining 100 uL of working solution, 100 uL of 50/50 propylene glycol/glycerin (w/w) that has been diluted 1:10 in acetone, 50 uL of internal standard solution, and 750 uL of neat acetone, for a final solvent composition of 99% acetone and 1% propylene glycol/glycerin composition. Samples were prepared in triplicate by weighing out approximately 100 mg of e-liquid into a 2 mL amber vial and added 9 uL of acetone per mg of E-liquid weighed (e.g., a 99.1 mg aliquot of Arctic Blast had 891.9 uL of acetone added) for an initial 1:10 dilution. For minor alkaloid analysis, 100 uL of sample was then combined with 50 uL of internal standard solution in 850 uL of acetone to match the standard composition described above. Additional dilutions needed to be performed for nicotine sample analysis, 10 µL of the initial 1:10 diluted E-liquid was combined with 990 uL of acetone (1:1000 diluted from initial sample). These samples were then combined with 50 µL of internal standard solution in 850 uL of acetone to match standards of the same composition of 0.01% propylene glycol/glycerin in acetone.
2.4.2. Analysis of E-Liquid Samples
Analysis was performed on an Agilent (Santa Clara, CA) 7890A Gas Chromatographic system coupled to an Agilent 7000 MS triple quadrupole running positive EI at 70 eV and a source temperature of 230 °C. One microliter was injected for chromatographic analysis using a Restek (Bellefonte, PA) Rtx-VMS column (30 m x 0.25 mmID x 1.4 µm) which was held at 70 °C for 2 minutes, increased at 30 °C/min to 190 °C (2-minute hold), then raised at 25 °C/min to 260 °C (5-minute hold) for a total run time of 15.8 minutes. A split ratio of 5:1 was used and a constant flow of 1 mL/min of helium was run through the column. Segmented MRM sequences were set up corresponding to the known elution time of each standard. After a 2-minute solvent delay, all traces were monitored for the first 7 minutes of acquisition time (9-minutes total). Nicotine (162.2>84.1) and nicotine-d3 (165.2>87.1) were monitored from 9–10 minutes. Anabasine (162.2>106.1), anatabine (160.1>54.1), B-nicotyrine (158.1>116.9), nornicotine (148.1>118.8), and myosmine (146.1>91.1) were all monitored from 10–13.5 minutes along with the internal standards anabasine-d4 (166.1>137), anatabine-d4 (164.1>53.9), and myosmine-d4 (150.1>121.1). Cotinine (176.1>98.0) and cotinine-d3 (179.1>101.1) were both monitored from 13.5 minutes to the end of the run. Internal standards were monitored for 50 ms and analytes were monitored for 75 ms. Collision energy was optimized for each compound.
2.5. Drug Discrimination Procedure
Male and female mice (n=8/sex) were trained to respond on one of the two nose-poke apertures in the operant chamber following subcutaneous (s.c.) administration of 0.75 mg/kg nicotine and to respond on the other aperture following s.c. saline administration according to a fixed ratio 10 (FR10) schedule of food reinforcement, under which 10 consecutive responses on the correct (injection-appropriate) lever resulted in delivery of a food pellet. Responses on the incorrect lever reset the ratio requirement on the correct lever. Daily injections were administered on a double alternation sequence of training drug and vehicle/saline (e.g., nicotine, nicotine, saline, saline). Daily 15 min training sessions were held Monday-Friday until the mice reliably consistently met three criteria: (1) the first completed FR10 was on the correct lever, (2) ≥ 80% of the total responding occurred on the correct lever, and (3) response rate must have been ≥ 0.1 responses/s. When these criteria had been met for the most recent training drug and saline sessions and 8 of the 10 most recent sessions, reliable discrimination had been established and stimulus substitution testing began.
Stimulus substitution tests were conducted in place of training sessions, with baseline discrimination training continuing between stimulus substitution test days. During the 15 min stimulus substitution tests, 10 consecutive responses on either aperture delivered reinforcement. If a mouse responded on the other aperture prior to completing 10 responses on an aperture, the ratio requirement on the original aperture was reset. To be eligible for a stimulus substitution test, mice must have completed a training session the previous day in which the three above criteria had been met. In addition, the mouse must have met these same criteria during the most recent training session with the alternate training compound (training drug or saline). After passing stimulus substitution tests for the training drug and saline, substitution dose-response curves were determined for s.c. nicotine injection at 10-min pre-treatment intervals.
Completion of the injected nicotine dose-effect curve was followed by concentration-response curves with aerosolized nicotine-containing products: two commercially purchased e-liquids, non-flavored and Arctic Blast (menthol-flavored), and with freebase nicotine formulated in our laboratory. Exposure to aerosolized e-liquids was achieved by placing mice individually into closed anesthesia chambers and generating aerosol for 10 s. Stopcocks were used to seal the aerosol in the chamber during a 5-min exposure period while the mouse remained in the chamber. The duration of the exposure period was based on preliminary experiments and on previous work with nicotine in the aerosol delivery apparatus (Lefever et al., 2017a). After the exposure period, mice were removed from the anesthesia chambers and placed in the operant chambers immediately after the exposure period for a drug discrimination test session.
For each dose-/concentration-effect curve, doses/concentrations were administered in ascending order, with saline, nicotine, and aerosolized vehicle control tests interspersed between curves. The dose-effect curve for s.c. nicotine was determined first followed by curves for non-flavored, Arctic Blast, and freebase nicotine solutions.
2.6. Determination of Nicotine/Cotinine Concentrations in Plasma and Brain
On the day of dosing/ aerosol exposure, mice were weighed and transferred to the dosing room to acclimate for 1 h. Subsequently, six mice of each sex were dosed with a single s.c. injection of 0.75 mg/kg nicotine; another six mice of each sex were exposed to e-cigarette vapor containing 30 mg/ml freebase nicotine; and the remaining four mice were not dosed but tissues were used for blanks during analysis. Mice given s.c. injections were sacrificed 10 min after dosing, and mice dosed with vapor were sacrificed immediately after dosing commenced. For each route of administration, this timing matches the pre-treatment times used during the drug discrimination study. Injected and vaped drug formulations and dosing procedures were also identical to those used during the drug discrimination study. Upon sacrifice via CO2 exposure, whole brain was harvested and snap frozen. Blood was collected via cardiac puncture and spun down in EDTA vials; plasma was aliquoted off and snap frozen. All samples were labeled and kept on dry ice until placed into a −80 freezer for storage until analysis (~ 10 days later).
2.6.1. Biological Sample Preparation
Plasma calibration standards and quality control samples were prepared using pooled blank plasma. Samples were prepared by spiking 25 µl of plasma with 10 µl of internal standard solution (500 ng/ml nicotine d-3 and continine-d3). Calibration standards were created at 1, 2, 5, 10, 100, 500, and 1000 ng/ml for both analyte and quality control samples were made at 5 and 500 ng/ml for both analytes. Sample extraction was achieved by mixing the sample with 100 µl of methanol at 1200 rpm for 3 min and then centrifuging at 3724 RCF for 10 minutes at 4 °C in a Beckman Coulter Allegra X-15R centrifuge (Pasadena, CA). Fifty µl of supernatant was mixed with 50 µl of water and analyzed by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). Whole brain samples were homogenized in high purity water at 4 µl per mg of brain tissue. Brains were homogenized with stainless steel beads (2.8 mm, OPS Diagnostics, Lebanon, NJ) in a SPEX SamplePrep Geno-Grinder (Metuchen, NJ) at 1750 rpm for 1 min × 2. Pooled blank brain homogenate was used for brain calibration standards and quality control samples. Brain homogenate was processed as described above for plasma.
2.6.2. Analysis of Biological Samples
Sample preparations were analyzed via a HPLC-MS/MS system consisting of an Agilent 1100 (Santa Clara, CA) HPLC system coupled to an API-4000 mass spectrometer with TurboIonSpray source (SCIEX, Framingham, MA). The autosampler temperature was maintained at 10 °C. Chromatography was performed using a Phenomenex Luna Phenyl-Hexyl column (150 × 4.6 mm i.d., 3 µM particle size) and a Phenomenex SecurityGuard AQ C18 (4 × 2 mm) guard column. Five microliters of sample were injected, and the column was maintained at 30 °C. The flow rate was 0.8 mL/min, and the mobile phases consisted of 0.1 % formic acid in water with 5 mM ammonium formate (mobile phase A) and 0.1% formic acid in methanol (mobile phase B). The initial conditions were 20% B and held for 1 min. The linear gradient was increased to 95% B over 3 min and held at 95% B for 0.2 min before returning to initial conditions. MS detection was performed in positive ion mode with an ion source temperature of 500 °C, and an ion spray voltage of 2000 V. Transitions monitored were 163.20 → 84.0 for nicotine, 177.12 → 80.1 for cotinine, 166.10 → 89.1 for nicotine-d3, and 180.20 → 101.0 for cotinine-d3. Analyst software 1.6.2 (SCIEX, Redwood City, CA) was used for data acquisition and analysis.
2.7. Data Analysis
For each drug discrimination session, percentage of total responses on the drug-associated aperture and response rate (responses/s on both apertures) were calculated. ED50 and slope values (and 95% confidence intervals, CI) and Y-intercepts (± SEM) were calculated on the linear part of the drug manipulandum selection log dose-response curve for nicotine using least squares linear regression analysis, followed by F-test to determine whether slopes differed across sex [GraphPad Prism 7.0 (GraphPad Software, San Diego, CA)]. Aperture selection (percentage of nicotine-associated aperture responses) and response-rate data for s.c. nicotine were analyzed using separate split-plot ANOVAs, with sex as the between subject factor and dose as the within subject factor. Because nicotine dose could not be calculated for aerosolized drug (and, therefore, could not be equalized across sex), aperture selection and response rate data for aerosolized were analyzed separately for each sex through the use of two-way concentration X flavor repeated measures ANOVAs. Nicotine and cotinine concentrations in the plasma and brain were analyzed with separate two-way (sex X route of administration) between-subject ANOVAs. For all ANOVAs, Tukey post hoc tests (α=0.05) were used, as appropriate, to determine differences between individual means for main effects and/or interactions. NCSS 11 Statistical Software (2016; NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/ncss) was Statistical Software (2016; NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/ncss) was used for all ANOVAs.
3.0. Results
Although all 16 mice met the initial acquisition criteria, 2 of the female mice did not exhibit characteristic dose-dependent substitution during the subsequent nicotine dose-effect curve determination. These 2 mice responded minimally (< 10%) on the nicotine-associated aperture at the 0.75 mg/kg training dose and did not show substantial responding on this aperture at other doses, despite having met the acquisition criteria earlier. For this reason, data from these 2 female mice were omitted from analysis. The 8 male mice and remaining 6 female mice successfully acquired and stably maintained the nicotine discrimination. Average number of sessions (± S.E.M.) to meet criteria was 32 (± 6.8) and 28 (± 6.1) for male and female mice, respectively. In the s.c. nicotine dose-effect curve, nicotine produced dose-dependent substitution in mice of both sexes, with nicotine doses > 0.25 and 0.5 mg/kg eliciting significant substitution in male and female mice, respectively [Figure 1, panels A and B; sex X dose interaction: F(5,60)=2.56, p<0.05]. The slopes of the linear part of the dose-effect curves for males [103 (95% CI: 68.2–137.7)] and females [81 (95% CI: 22.9–138.4)] were not significantly different [F(1,44)=0.49, p=0.49], nor were the Y-intercepts for males (118 ± 13) and females (91 ± 10) significantly different [F(1,45)=3.2, p=0.08]. ED50 values for nicotine were 0.22 mg/kg (95% CI: 0.16 – 0.29) and 0.31 mg/kg (95% CI: 0.06 – 0.46) for males and females, respectively. Overall response rates were significantly increased by 0.075 and 0.25 mg/kg and decreased by 1 mg/kg nicotine [Figure 1, panels C and D; main effect of dose: F(5,60)=19.27, p<0.05].
Figure 1.
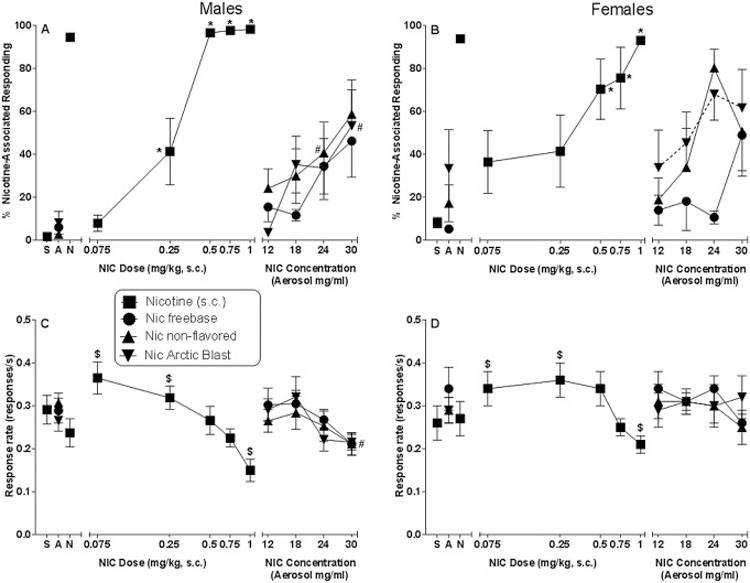
Effects of s.c. nicotine (filled squares) on percentage of responses that occurred on the nicotine-associated aperture in male (panel A) and female (panel B) C57BL/6 mice trained to discriminate 0.75 mg/kg nicotine from saline. Response rates for male (panel C) and female (panel D) mice for each dose are also shown. The right side of each panel shows results of substitution tests with aerosolized freebase nicotine (filled circles) and two commercially available nicotine-containing e-liquids, non-flavored (filled triangles) and Arctic Blast (filled inverted triangles). Each point represents the mean (± SEM) of data for 8 male mice (panels A and C) or 6 female mice (panels B and D). Points at the left side of each panel represent results of control tests with saline (S), aerosol vehicle (A), and s.c. nicotine training dose (N). The saline and nicotine control tests were conducted before initiation of the s.c. nicotine dose-effect curve determination whereas an aerosol vehicle control test was conducted prior to concentration-effect curve determinations for each nicotine-containing solution. Nicotine s.c. dose-effect curve analysis: Asterisk (*) indicates significant interaction of sex X dose (p<0.05) and difference at specified concentration compared to sex-specific vehicle condition. Dollar sign ($) indicates significant main effect of dose (p<0.05) compared to overall vehicle condition across sexes. Aerosolized nicotine concentration-effect curve analysis: Dotted connecting line indicates significant main effect of flavor (p<0.05) compared to freebase nicotine aerosol concentration-effect curve in female mice. Pound sign (#) indicates significant main effect of concentration (p<0.05) compared to overall aerosol condition for all flavors in male mice.
While injected nicotine produced over 93% responding on the nicotine-associated aperture at one or more nicotine doses in mice of both sexes, aerosolized nicotine (at concentrations up to 30 mg/ml) produced maximums of 46–62% responding on the nicotine-associated aperture (Figure 1, right side of panels A and B). In males, nicotine-like responding was significantly elevated overall at concentrations of 24 and 30 mg/ml compared to the aerosol vehicle condition whereas significant increases were not observed in females [main effect of concentration in males: F(4,28)=8.08, p<0.05], albeit lack of significance may have been impacted by higher overall nicotine-aperture responding during exposure to aerosol vehicle in females. Flavor did not appear to influence the degree of substitution in males, as non-flavored, Arctic Blast, and freebase nicotine solutions produced similar concentration-effect curves (Figure 1, panel A). In contrast, females showed significantly greater responding on the nicotine-associated aperture after exposure to Arctic Blast nicotine solution than after exposure to freebase nicotine [Figure 1, panel B; main effect of flavor: F(2,10)=7.02, p<0.05]. This difference was most prominent at lower concentrations, as the concentration-effect curves for nicotine-like responding for each flavor appeared to converge at the 30 mg/ml concentration. Response rates were not affected in females by any of the three nicotine-containing solutions, but overall rate was decreased in males at the 30 mg/ml concentration [Figure 1, panels C and D for males and females, respectively; main effect of concentration for males: F(4,28)=5.27, p<0.05].
Table 1 shows the results of chemical analysis of two concentrations of non-flavored and Arctic Blast e-liquids (and their vehicles). While mean nicotine concentrations were 9–17% greater than the advertised amount for the e-liquids, they were similar across flavor at each concentration In contrast, concentrations of minor alkaloids (e.g., myosmine, anabasine, anatabine, cotinine) varied across the two products, with the percentage of non-nicotinic minor alkaloids averaging 6–7% and 1–2% for non-flavored and Arctic Blast e-liquids, respectively.
Table 1.
Concentrations of nicotine and minor alkaloids in commercially purchased non-flavored and Arctic Blast e-liquids
| Alkaloid1 | Non-Flavored2 | Arctic Blast2 | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1.2 | 1.8 | 0 | 1.2 | 1.8 | ||
| Nicotine mg/ml | 0.59 (0.07) | 13.33 (17.51) | 20.79 (26.00) | 1.74 (0.17) | 13.03 (5.40) | 21.11 (7.91) | |
| % nicotine | 0 (0.001) | 1.33 (0.011) | 2.08 (0.015) | 0 (0.001) | 1.30 (0.038) | 2.11 (0.073) | |
| Anabasine µg/ml | 0 (0) | 9.12 (0.53) | 15.26 (1.27) | 0.16 (0.28) | 0.12 (0.22) | 0.41 (0.68) | |
| Anatabine µg/ml | 0 (0) | 57.00 (0.06) | 86.14 (4.23) | 0 (0) | 1.45 (0.03) | 2.49 (0.68) | |
| Cotinine µg/ml | 0 (0) | 3.12 (0.11) | 4.85 (0.18) | 0.20 (0.34) | 0.63 (0.22) | 1.12 (0.16) | |
| Myosmine µg/ml | 0.07 (0.12) | 17.51 (0.88) | 26.00 (1.14) | 0.17 (0.21) | 5.40 (0.17) | 7.91 (0.08) | |
| β-Nicotyrine µg/ml | n/d | 1.56 (0.16) | 3.40 (1.16) | n/d | 2.00 (0.83) | 3.23 (0.85) | |
| Nornicotine µg/ml | 0 (0) | 14.5 (0.18) | 21.55 (4.61) | 1.14 (1.61) | 6.56 (1.00) | 12.75 (0.72) | |
All values shown as mean (± SEM) for 3 determinations, except for nornicotine (n=2). Note scale differences for alkaloids: nicotine concentrations are expressed in mg/ml and % nicotine; concentrations for all other alkaloids are expressed in µ/ml.
Column headings are the advertised % nicotine contained in each product.
Figure 2 shows results of analysis of plasma and brain samples from male and female mice exposed to injected nicotine (0.75 mg/kg, s.c.) or aerosolized freebase nicotine (30 mg/ml) at the pre-session exposure times used for the discrimination sessions. Whereas concentrations of nicotine in plasma and brain were similar across route of administration, nicotine concentrations were significantly greater in females than males in both plasma [Figure 2, panel A; main effect of sex: F(1,20)=6.05, p<0.05] and brain [Figure 2, panel B; main effect of sex: F(1,20)=5.55, p<0.05]. In contrast, cotinine concentrations were similar across sex, but significantly differed across route of administration in both plasma [Figure 2, panel C; main effect of route of administration: F(1,20)=35.67, p<0.05] and brain [Figure 2, panel D; main effect of route of administration: F(1,20)=35.69, p<0.05], with greater cotinine concentrations observed after aerosol exposure.
Figure 2.
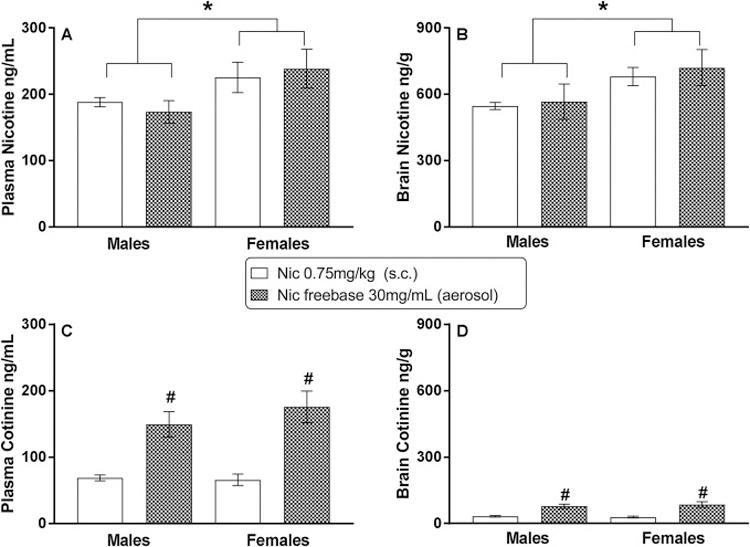
Plasma and brain concentrations of nicotine (panels A and B, respectively) and cotinine (panels C and D, respectively) following s.c. injection of 0.75 mg/kg (training dose) nicotine (unfilled bars) and aerosolized 30 mg/ml freebase nicotine (shaded bars) in adult male and female C57BL/6 mice. Each bar represents the mean (± SEM) of data for 6 mice. * indicates significant (p<0.05) main effect of sex. # indicates significant (p<0.05) main effect of route of administration.
4.0. Discussion
In the present study, s.c. nicotine engendered dose-dependent substitution for the training dose in mice of both sexes. For males, the results are consistent with previous reports of successful acquisition of nicotine discrimination in male rodents (Rosecrans, 1989; Stolerman et al., 1999; Wooters et al., 2009); however, to our knowledge, this study represents the first report of nicotine discrimination in mice of both sexes, albeit a single study has reported nicotine discrimination with a single test dose in male and female rats (Troisi, 2018). In female mice, the stimulus effects of nicotine appeared to be less robust than in males. For example, although over 90% substitution was observed at the 0.75 mg/kg training dose during preceding control tests, this degree of substitution was not attained in all female mice at doses lower than 1 mg/kg during the dose-effect curve determination. Further, responding on the nicotine-associated aperture was more variable in female mice at the lower doses, suggesting that the training dose may have been sub-optimal for females. Interestingly, nicotine concentrations in the brain after injection of the training (0.75 mg/kg) dose were significantly greater in female than male mice (present results), providing added support to the hypothesis that females were less sensitive to its effects. These results are consistent with findings that nicotine’s discriminative stimulus effects are attenuated in women as compared with men (Perkins, 1999). A meta-analysis also revealed that female rats self-administer more i.v. nicotine than males (Flores et al., 2019). Yet, other research showed that female mice are not less sensitive than males to all of nicotine’s pharmacological effects (Damaj, 2001; Isiegas et al., 2009). Together, these results demonstrate that sex differences in nicotine’s pharmacological effects are task-specific, a finding which suggests that sex differences in nicotine pharmacokinetics are probably not responsible.
In contrast with over 93% nicotine-aperture responding observed with s.c. nicotine at one or more doses, maximal substitution of aerosolized freebase nicotine was 46–49% in both sexes. While we aerosolized freebase nicotine concentrations up to 30 mg/ml, a limitation of our method is that determination of the exact nicotine dose that each mouse received is not possible. Hence, the aerosol exposure regimen may not have resulted in sufficient nicotine concentrations entering the brain to engender nicotine-like discriminative stimulus effects. While this hypothesis seemingly receives partial support from the finding that increases in nicotine-like responding after exposure to aerosolized freebase nicotine were concentration-dependent (and hence, conceivably could have been further increased with greater nicotine concentrations), nicotine concentrations in the brains of male and female mice that were exposed to 30 mg/ml nicotine freebase e-liquid via the exposure regimen used in the discrimination experiment were comparable to the nicotine concentrations observed in mice injected s.c. with the 0.75 mg/kg training dose of nicotine. Further, timing of the sacrifice of each group of mice corresponded with the start of the discrimination session, suggesting that these brain nicotine concentrations would have been present during the discrimination session. Based upon these results, greater nicotine-like responding following aerosolized nicotine would have been expected. Given our previous findings showing that aerosolized nicotine within the same concentration range decreased locomotor activity and body temperature in male and female ICR mice (Lefever et al., 2017a), as well as the present findings, this low degree of substitution of aerosolized nicotine for injected nicotine is somewhat puzzling.
One possible modulatory factor that may have affected degree of substitution is route of administration. Whole body exposure to nicotine aerosol produces a complex pharmacokinetic profile, as pulmonary absorption represents only one route through which aerosolized nicotine may be absorbed. Absorption also may occur via oral (e.g., licking aerosol condensation deposits on fur) and percutaneous (e.g., through skin or mucosa) routes, all of which also complicates determination of dose (Cryan et al., 2007). Yet, previous studies have shown that exposure to vapor or smoke generated by heating or burning other drugs (toluene or phencyclidine) results in cross-substitution with the respective injected drug (Shelton and Slavova-Hernandez, 2009; Wessinger et al., 1985). Similarly, we recently reported that synthetic cannabinoids aerosolized using the same system as for the present study substituted almost completely for injected Δ9-tetrahydrocannabinol in male and female C57BL/6 mice (Wiley et al., 2019). However, neither nicotine aerosol nor tobacco smoke has been tested previously in the discrimination paradigm.
Other factors that may have contributed to the unexpected results are the rate of nicotine metabolism or the training dose of nicotine. While a full pharmacokinetic profile of nicotine was beyond the scope of this study, the present results showed that aerosolized freebase nicotine resulted in greater concentrations of cotinine (nicotine’s major metabolite) than injected nicotine in mouse plasma and brain, despite similar concentrations of nicotine. These results suggest that metabolism of nicotine may have been quicker following aerosol exposure and that the resulting increased cotinine may have served to modulate nicotine’s pharmacological effects in the discrimination paradigm (Crooks and Dwoskin, 1997). This hypothesis cannot be eliminated by the present results and will require further research to test. The strength of the stimulus effects of nicotine also depends upon training dose, with the nicotine dose-effect curve shifting rightward as training dose increases (Cunningham and McMahon, 2013). Hence, choice of a lower training dose conceivably could have increased the magnitude of substitution by aerosolized nicotine.
To determine whether non-nicotine constituents of tobacco and/or menthol flavoring would increase substitution, evaluation of commercially purchased nicotine-containing e-liquids also was undertaken. Previous research has shown that several minor alkaloids contained in tobacco share and/or modulate addiction-related effects of nicotine in rodent models, including its reinforcing and interoceptive effects (Caine et al., 2014; Clemens et al., 2009; Costello et al., 2014; Goldberg et al., 1989; Hall et al., 2014). Similarly, menthol has been shown to enhance nicotine’s reinforcing effects in rats (Biswas et al., 2016; Henderson et al., 2017) and increase the duration of its antinociceptive and hypothermic effects in mice (Alsharari et al., 2015), possibly through affecting its pharmacokinetics and/or upregulation of nicotinic acetylcholine receptors or receptor subunits in reward-related brain regions (Abobo et al., 2012; Alsharari et al., 2015; Henderson et al., 2017). Analysis of the e-liquids tested here revealed that, whereas nicotine concentrations were similar to concentrations listed on the labels, concentrations of minor alkaloids (e.g., myosmine, anabasine, anatabine, cotinine) varied across the two products, with the percentage of non-nicotinic minor alkaloids averaging 6–7% and 1–2% for non-flavored and Arctic Blast e-liquids, respectively (Table 1). Further, Arctic Blast contained measurable quantities of menthol whereas the non-flavored e-liquid did not (unpublished data, Brian Thomas). Although neither non-flavored nor Arctic Blast e-liquid produced the same degree of substitution as the s.c. nicotine training dose (0.75 mg/kg), concentration-dependent increases in nicotine-aperture responding (to a maximum of 59%) were observed in male mice. For males, these increases paralleled those seen with freebase nicotine (which did not contain other tobacco alkaloids), whereas modest, but statistically significant, enhancement (shift upward) was produced by Arctic Blast in female mice, a finding that is consistent with greater responsivity of women than men to sensory effects of smoking (e.g., flavors) (Kistler et al., 2017; Perkins et al., 1999; Perkins et al., 2001).
In summary, these results demonstrate for the first time that s.c. nicotine served as a discriminative stimulus in female mice, as has been shown for male rodents in the present study and previously (Cunningham and McMahon, 2013; Rosecrans, 1989; Stolerman et al., 1999; Wooters et al., 2009). In contrast with data from their male counterparts, the stimulus effects of nicotine in female mice showed greater variability at lower doses and were not maximal except at a dose (1 mg/kg) exceeding the 0.75 mg/kg training dose, suggesting that the training dose may have been sub-optimal in females. Lower brain nicotine concentrations in females than males following s.c. injection of 0.75 mg/kg nicotine also are supportive of the hypothesis that females were less sensitive to nicotine’s discriminative stimulus effects. In mice of both sexes, the degree of substitution decreased when route of administration was changed from s.c. injection to aerosolization via a modified e-cigarette device, suggesting that the nicotine-like stimulus effects of aerosolized nicotine were reduced regardless of formulation (i.e., freebase nicotine versus commercial nicotine-containing tobacco e-liquid). While further research is needed for conclusive determination of reason(s) for this reduction, concentration-dependent increases in nicotine-aperture responding observed here with aerosolized nicotine demonstrate initial feasibility of examination of discriminative stimulus effects of vaped drugs such as nicotine.
Highlights.
Discriminative stimulus effects of aerosol and s.c. nicotine were compared in mice.
Female mice were less sensitive to s.c. nicotine’s discriminative stimulus effects.
Aerosolized nicotine increased responding on the nicotine aperture in both sexes.
Maximum substitution for aerosol nicotine was only partial in both sexes.
Brain nicotine concentrations after aerosol exposure and injection were similar.
Acknowledgements
The authors thank Daniel Barrus, Nikita Pulley, and Michelle McCombs for excellent technical assistance. This research was supported by RTI internal research and development funding and by U.S. National Institutes of Health / National Institute on Drug Abuse grants DA-045003, DA-044377, and DA-040460.
Role of Funding Source
This research was funded by RTI internal research and development funding and by U.S. National Institutes of Health / National Institute on Drug Abuse grants DA-045003, DA-044377, and DA-040460. NIH/NIDA did not have any other role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of NIH or NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Abobo CV, Ma J, Liang D, 2012. Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res 14, 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI, 2015. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One 10, e0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, 1990. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. NIDA Res Monogr 99, 12–29. [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P 3rd, 2009. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192, 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas L, Harrison E, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T, Lage J, Liu X, 2016. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl) 233, 3417–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Cheng JM, 2014. Electronic cigarettes: product characterisation and design considerations. Tob Con 23 Suppl 2, ii4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK, 2014. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol 22, 9–22. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M, 2009. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol 12, 1355–1366. [DOI] [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM, 2014. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology 39, 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP, 1997. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol 54, 743–753. [DOI] [PubMed] [Google Scholar]
- Cryan SA, Sivadas N, Garcia-Contreras L, 2007. In vivo animal models for drug delivery across the lung mucosal barrier. Adv Drug Delivery Rev 59, 1133–1151. [DOI] [PubMed] [Google Scholar]
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA, 2018. Notes from the field: Use of electronic cigarettes and any tobacco product among middle and high school students - United States, 2011–2018. MMWR Morb Mortal Wkly Rep 67, 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR, 2013. Multiple nicotine training doses in mice as a basis for differentiating the effects of smoking cessation aids. Psychopharmacology (Berl) 228, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, 2001. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther 296, 132–140. [PubMed] [Google Scholar]
- Flores RJ, Uribe KP, Swalve N, O’Dell LE, 2019. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiol Behav 203, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, 2010. Guidance for industry: Assessment of abuse potential of drugs U.S. Department of Health and Human Services, Silver Spring, MD. [Google Scholar]
- George O, Grieder TE, Cole M, Koob GF, 2010. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacol Biochem Behav 96, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O, 2014. Nicotine vapor inhalation escalates nicotine self-administration. Addict Biol 19, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B, 2015. E-Cigarettes: A review of new trends in cannabis use. Int J Env Res Pub Health 12, 9988–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS, 1989. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 97, 295–302. [DOI] [PubMed] [Google Scholar]
- Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, Samet JM, Leventhal AM, 2016. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug Alcohol Depend 168, 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA, 2014. E-cigarettes: a scientific review. Circulation 129, 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, Rose JE, Levin ED, 2014. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav 120, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA, 2017. Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology 42, 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM, 1993. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol 61, 743–750. [DOI] [PubMed] [Google Scholar]
- Hoffman AC, Salgado RV, Dresler C, Faller RW, Bartlett C, 2016. Flavour preferences in youth versus adults: a review. Tob Con 25, ii32–ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA, 2009. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res 11, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler CE, Crutchfield TM, Sutfin EL, Ranney LM, Berman ML, Zarkin GA, Goldstein AO, 2017. Consumers’ preferences for electronic nicotine delivery system product features: A structured content analysis. Int J Env Res Pub Health 14, E613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Lee YO, Kovach AL, Silinski MA, Marusich JA, Thomas BF, Wiley JL, 2017a. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend 172, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Thomas BF, Barrus DG, Peiper NC, Kevin RC, Wiley JL, 2017b. Vaping synthetic cannabinoids: A novel preclinical model of e-cigarette use in mice. Subst Abuse Res Treat 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL, 2016. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology 55, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbolouk M, Charkhchi P, Kianoush S, Uddin SMI, Orimoloye OA, Jaber R, Bhatnagar A, Benjamin EJ, Hall ME, DeFilippis AP, Maziak W, Nasir K, Blaha MJ, 2018. Prevalence and distribution of e-cigarette use among U.S. adults: Behavioral risk factor surveillance system, 2016. Ann Intern Med 169, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Krishnan-Sarin S, 2015. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics 136, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser P, Wolinsky T, Duxon M, Porsolt RD, 2011. How good are current approaches to nonclinical evaluation of abuse and dependence? J Pharmacol Exp Ther 336, 588–595. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals National Academies Press, Washington, D.C. [Google Scholar]
- Perkins KA, 1999. Nicotine discrimination in men and women. Pharmacol Biochem Behav 64, 295–299. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR, 1999. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1, 301–315. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S, 2001. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res 3, 141–150. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D, 2015. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacology 25, 1775–1786. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, 1989. Nicotine as a discriminative stimulus: A neurobehavioral approach to studying central cholinergic mechanisms. J Subst Abuse 1, 287–300. [PubMed] [Google Scholar]
- Schuster CR, Johanson CE, 1988. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser 4, 161–175. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Slavova-Hernandez G, 2009. Characterization of an inhaled toluene drug discrimination in mice: effect of exposure conditions and route of administration. Pharmacol Biochem Behav 92, 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA, 2015. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PloS One 10, e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR, 1999. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 141, 297–306. [DOI] [PubMed] [Google Scholar]
- Troisi JR 2nd, 2018. The discriminative stimulus effects of nicotine & ethanol with two distinct olfactory contexts in male and female rats. Behav Processes 157, 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD, 1999. Discriminative stimulus (DS) properties of nicotine in the C57BL/6 mouse. Pharmacol Biochem Behav 63, 27–32. [DOI] [PubMed] [Google Scholar]
- Wessinger WD, Martin BR, Balster RL, 1985. Discriminative stimulus properties and brain distribution of phencyclidine in rats following administration by injection and smoke inhalation. Pharmacol Biochem Behav 23, 607–612. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Glass M, Thomas BF, 2019. Do you feel it now? Route of administration and Delta(9)-tetrahydrocannabinol-like discriminative stimulus effects of synthetic cannabinoids in mice. Neurotoxicology 73, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Bevins RA, Bardo MT, 2009. Neuropharmacology of the interoceptive stimulus properties of nicotine. Curr Drug Abuse Rev 2, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]


