Abstract
To assess motor cortex neurophysiology, including the mechanisms of neuroplasticity, transcranial magnetic stimulation (TMS) is typically applied to the motor “hotspot”— the optimal site for inducing a twitch in a given target muscle. It is known that the effects of suprathreshold repetitive TMS (rTMS) spread along functional connections beyond the specific cortical stimulation target, and yet, it is unknown whether the aftereffects of subthreshold intermittent theta-burst stimulation (iTBS), an ultra-high frequency patterned rTMS protocol, extend beyond the targeted muscle.
We investigated whether and to what extent iTBS induces changes in the cortical output to other intrinsic hand muscles with adjacent cortical representation to the target. 16 healthy adults underwent neuronavigated TMS-iTBS targeting the first dorsal interosseus (FDI) hotspot. Proportion of motor evoked potentials (MEPs) at the resting motor threshold (RMT), baseline MEP amplitude, and iTBS-induced changes in MEP amplitude were compared between FDI, abductor pollicis brevis (APB) and abductor digiti minimi (ADM) muscles. MEP amplitudes recorded from the three muscles at RMT and suprathreshold intensities indicated the chosen hotspots were relatively selective for FDI. Nevertheless, iTBS induced significant facilitation of MEPs recorded from both FDI and APB, but not ADM. Surprisingly, the MEP modulation was greater in APB, even when controlling for the baseline MEP amplitude. These results indicate that iTBS modulation of cortico-spinal excitability extends beyond the representation of the targeted muscle. Results have implications both for how iTBS may be used in clinical treatment and for the safety guidelines for the application of iTBS.
Keywords: transcranial magnetic stimulation, theta-burst stimulation, plasticity, specificity
Introduction
Transcranial magnetic stimulation (TMS) is a versatile, noninvasive technique for investigating human brain neurophysiology in vivo. Most of our knowledge of the underlying physiological mechanisms of TMS comes from studying its effects on the motor cortex. Despite decades of research, fundamental questions still remain. A single pulse of suprathreshold TMS delivered over the primary motor cortex (M1) will evoke a descending volley along the cortico-spinal tract reflecting polysynaptic activation via layer-V pyramidal neurons (Di Lazzaro & Rothwell, 2014) . The activity summates in the spinal cord and at the neuromuscular junction, resulting in a compound muscle action potential, termed a motor evoked potential (MEP). The MEP amplitude can be measured via surface electromyography (EMG), providing a measure of cortico-motor excitability (Rossini and Rossi, 1998). Repetitive TMS (rTMS) uses patterned trains of pulses to induce frequency-specific changes in cortical excitability that outlast the stimulation (Pascual-Leone et al., 1994). Applying this approach to M1, the change in cortico-motor excitability (i.e., MEP amplitude) following rTMS can be used to investigate the mechanisms of cortical plasticity (Hallett, 2007; Pascual-Leone et al., 2011).
The first step in nearly any TMS application is to identify the motor hotspot, which corresponds to the region in M1 that consistently elicits a visible twitch of the target muscle and larger MEPs compared to those from non-target, neighboring muscles (Rossini et al., 2015). Identification of the motor hotspot for a given target muscle can be improved by individual MRI-guided neuronavigation (Julkunen et al., 2009). Most TMS neurophysiological studies target one of the following intrinsic hand muscles: first dorsal interosseus (FDI), abductor pollicis brevis (APB), and abductor digiti minimi (ADM). Despite their partially overlapping representation in M1 (Pascual-Leone et al., 1994), hotspots for FDI, APB, and ADM show some orientation specificity (Bashir et al., 2013). That is, one can improve the selectivity of TMS for a given target muscle—lower motor threshold and/or higher MEP amplitude compared to its neighbors—by changing the direction of the induced electrical field relative to precentral gyrus (Laakso et al., 2014).
The focality of rTMS effects in the brain remains unresolved, despite its implications for clinical and basic science applications of rTMS. Early experiments in healthy individuals demonstrated that applying short rTMS trains at suprathreshold intensities resulted in a spread of excitation beyond the target muscle, likely due to the breakdown of surrounding inhibitory cortico-cortical connections, secondary to the rTMS train itself (Pascual-Leone et al., 1994). The effects of rTMS have been shown to spread beyond the cortical stimulation target along functional connections in the brain (Eldaief et al., 2011; van der Werf et al., 2010). In the past 15 years, a form of patterned, high-frequency rTMS, termed theta-burst stimulation (TBS), has emerged as a potential means for inducing longer-lasting plasticity with shorter stimulation durations (Huang et al., 2005). The development of TBS was based on electrophysiological studies of long-term potentiation (LTP) and long-term depression (LTD) in the rodent hippocampus (Diamond et al., 1988). Accordingly, changes in MEP amplitude following intermittent TBS (iTBS) and continuous TBS (cTBS) have been interpreted as reflecting LTP- and LTD-like plasticity, respectively. A number of studies have explored the effects of iTBS and cTBS on antagonistic muscles at rest and under contraction (Fang et al., 2014; Mirdamadi et al., 2016, 2015). Yet, to our knowledge, the focality of iTBS aftereffects in synergistic intrinsic hand muscles has not been explored.
Since the spread of the induced electrical field increases with stimulation intensity (Roth et al., 2007), it is assumed that selectivity of TMS measures obtained from a given muscle (relative to its neighboring muscles) would be greater at subthreshold than at suprathreshold intensities. Based on this assumption, it can be hypothesized that the aftereffects of plasticity-inducing rTMS protocols that use subthreshold intensities, such as iTBS, should be most prominent when measured from the target muscle. To our knowledge, however, this hypothesis has never been explicitly tested. We aimed to address this gap by comparing the aftereffects of iTBS on MEP amplitudes recorded simultaneously from FDI, APB, and ADM when TMS was applied over the FDI hotspot. We predicted that if the target muscle, FDI, was relativity isolated (i.e., there was a higher proportion of responses at threshold and/or greater amplitude of suprathreshold MEPs recorded from FDI at baseline), iTBS would have a greater effect on FDI, compared to non-target muscles, i.e., APB and ADM. In contrast, if iTBS aftereffects are comparable between target and non-target muscles the spread may not be intensity-dependent and other physiological particularities of the motor pathway may play a role (i.e., TMS cortical orientation-specificity of different muscles or relative thresholds of overlapping cortical representations (Mirdamadi et al., 2016).
Methods
Participants
Data collected from healthy participants who served as control subjects in a previous iTBS study (Fried et al., 2016) were analyzed. All participants provided written informed consent prior to enrollment and received monetary compensation upon completion. The study protocols were approved by the Institutional Review Board at Beth Israel Deaconess Medical Center in accordance with the Declaration of Helsinki. The cohort consisted of 16 right-handed adults with a mean (± SD) age of 63 (± 8) years. Full details on eligibility criteria and participant characteristics can be found in a previous report (Fried et al., 2016).
Transcranial magnetic stimulation and electromyography
All study protocols followed the recommended guidelines for the safe application of TMS endorsed by the International Federation of Clinical Neurophysiology (IFCN) (Rossi et al., 2009). A frameless stereotaxic neuronagivation system (Nexstim Ltd., Helsinki, Finland) was used in conjunction with the subject’s own T1-weighted anatomical MRI to identify the hand knob of the left primary motor cortex and ensure consistent targeting throughout the session. Peak-to-peak amplitude of MEPs elicited by single-pulse TMS (spTMS) were recorded by surface electromyography (EMG; Nexstim) of the FDI, APB, and ADM muscles of the right hand using a belly-tendon electrode montage with a common ground on the styloid process of the wrist. Responses from ADM were not available in 2 participants. ADM responses from a third subject were rejected for excessive background noise in the EMG. The eXimia Navigated Brain System v. 3.2 (Nexstim; https://nexstim.com/healthcare-professionals/nbs-system/) was used to integrate TMS, EMG, and neuronavigation.
Full details on TMS procedures have been reported elsewhere (Fried et al., 2016). Briefly, the hotspot for the right FDI was defined as the site that resulted in a visual movement of the FDI muscle and MEPs that were generally larger in FDI than APB or ADM. All neurophysiological measures including resting motor threshold (rMT) and active motor threshold (aMT) as well as spTMS and iTBS intensities were assessed using FDI as the target muscle. Following IFCN guidelines (Rossini et al., 2015), rMT and aMT were determined individually using the Nexstim and MagPro (MagVenture A/S, Farum, Denmark) stimulators, respectively, and used to set the intensity of subsequent stimulation. rMT was defined as the minimum intensity of stimulation (as % of the maximum stimulator output; MSO) necessary to elicit MEPs ≥ 50 µV in at least 5 of 10 trials with the FDI relaxed. aMT was defined as the minimum %MSO necessary to elicit MEPs ≥ 200 µV in at least 5 of 10 trials with the FDI slightly contracted (using live EMG to ensure consistent contraction between ~100–200 µV). A passively-cooled MCF-B65 figure-of-8 coil fitted with a Nexstim tracking array and connected to a MagPro X100 stimulator (MagVenture) was used to assess aMT and to perform iTBS at 80% of aMT. The Nexstim and MagPro coils were handheld tangentially to the scalp and oriented to induce a biphasic (anterior-posterior—posterior-anterior) current in the brain perpendicular to the precentral gyrus (approximately 45° from the midline). Before and after iTBS, single TMS pulses were applied in blocks of 30 pulses at 120% of rMT to assess iTBS-induced changes in cortico-motor reactivity. Figure 1 illustrates the timeline of the study procedures and representative examples of MEPs recorded from the three different hand muscles.
Figure 1.
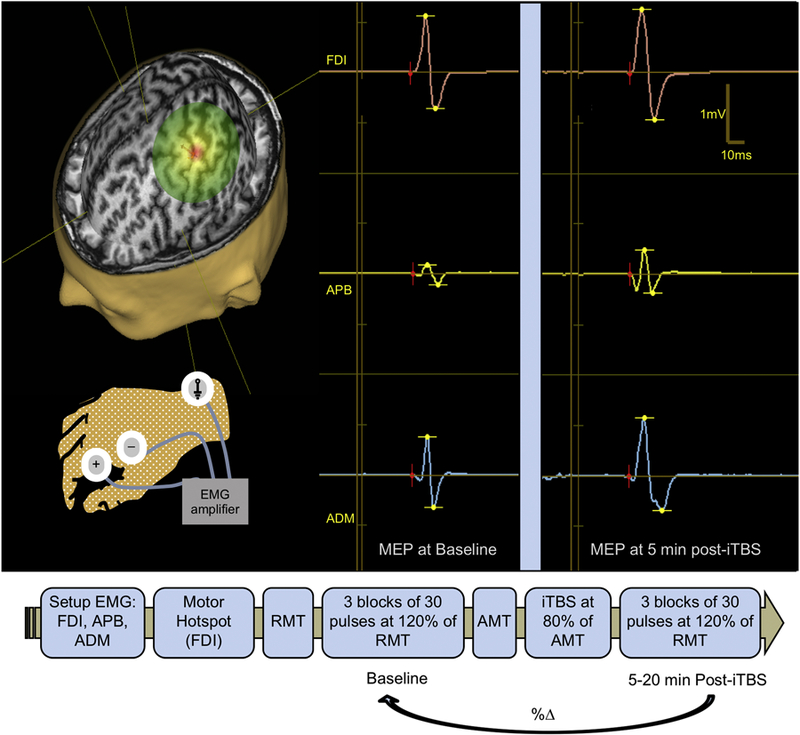
Top. Transcranial magnetic stimulation (TMS) assessments of cortical excitability—average amplitude of motor evoked potentials (MEPs) elicited by single-pulse TMS—and cortical plasticity—the change in excitability induced by intermittent theta-burst stimulation (iTBS). (A) Magnetic Resonance (MR)-guided TMS was applied to the left primary motor cortex ‘‘hand knob” and resulting MEPs were recorded from the right first dorsal interosseous (FDI), abductor pollicis brevis (APB) and abductor digiti minimi (ADM) muscles by surface electromyography (EMG). (B) Example MEP traces before and after iTBS recorded from FDI, APB, and ADM. Bottom. Time-line of the study visit.
Statistical analyses
Statistical analyses were performed using JMP Pro software version 13.0 (SAS Institute, Cary, NC, USA) using two-tailed tests with the α level set to 0.05. Assumptions of linear regression analyses were tested using Shapiro–Wilk and Breusch–Pagan tests. In cases where these assumptions were not met, data were either log10-transformed or non-parametric analyses were conducted, as indicated. Data from all three muscles included: (1) the proportion of MEPs ≥ 50 µV at 100% of rMT for FDI, (2) the average MEP amplitude (mV) from three blocks at baseline, and (3) the average MEP amplitude assessed 5–20 minutes following iTBS (T5, T10, T20). To compare the effect of iTBS across the three muscles, the mean of all post-iTBS MEPs over T5–T20 was calculated and expressed as the percent change from baseline (%ΔMEP). This interval was selected as it represents the period of the maximal iTBS-induced facilitation (Wischnewski and Schutter, 2015).
Data analysis proceeded as follows: (1) To statistically confirm the selectivity of the chosen hotspot for FDI as the target muscle compared to the two non-target muscles (APB and ADM), and to assess its relative specificity at threshold and suprathreshold intensities, the proportion of MEPs ≥ 50 µV at 100% rMT and average baseline MEP amplitudes at 120% rMT were entered as dependent variables into separate random-effects linear-regression models with the main crossed-random effect of Muscle (FDI, APB, ADM). Baseline MEP amplitudes were log10-transformed to meet the assumptions of normality. Planned pairwise comparisons between FDI and the two non-target muscles (APB, ADM) were performed using Student’s t-tests with Holm-Bonferroni adjustment for multiple comparisons. (2) To assess the effect of iTBS on cortico-motor reactivity for each muscle, the average MEP amplitudes at T5–T20 were compared to baseline MEP amplitudes using separate Wilcoxon signed-rank tests. (3) Based on the comparison of MEPs at rMT and baseline, only APB was significantly differentiated from FDI (see Results). Therefore, the maximal effect of iTBS was compared between FDI and APB by entering %ΔMEP values into a random-effects linear model with the main crossed-random effect of Muscle (FDI vs. APB). The ratio of MEP amplitudes at baseline (i.e., FDI/APB) was added as a covariate to assess whether differences in the effect of iTBS could be attributed to differences in MEP amplitude at baseline. For the sake of completeness, a similar comparison of %ΔMEP values between FDI and ADM (with the FDI/ADM ratio as a covariate) was conducted on 13 subjects with available ADM data. However, as differences between ADM and FDI at threshold or suprathreshold intensities (i.e., baseline) did not reach significance, we did not expect the iTBS aftereffects to be different in ADM and FDI. (4) Finally, to explore the relationship between MEP amplitude and %ΔMEP over T5–T20 from the target muscle and the corresponding values from the two non-target muscles, we conducted separate Spearman’s Rho correlations between the MEP amplitudes (mV) from FDI and those from either APB or ADM ratio, as well as between %ΔMEP values from FDI and those from either APB or ADM.
Results
All values represent mean ± standard deviation unless otherwise specified. Across all subjects, rMT was 47.38 ±11.7% of Nexstim maximum stimulator output (MSO), while aMT was 46.63 ±8.6% of MagPro MSO.
At baseline, the analysis of MEP amplitude in all three muscles confirmed the selectivity of the chosen motor hotspot for FDI. The random-effects linear model found a significant main effect of Muscle (F2,25 = 4.44, p = .022, ) on the proportion of MEPs ≥ 50 µV at threshold (Figure 2). Specifically, the proportion of such MEPs from FDI (0.54) was significantly greater than from APB (0.33, p = .013) and numerically, though non-significantly, greater than from ADM (0.42, p = .141).
Figure 2.
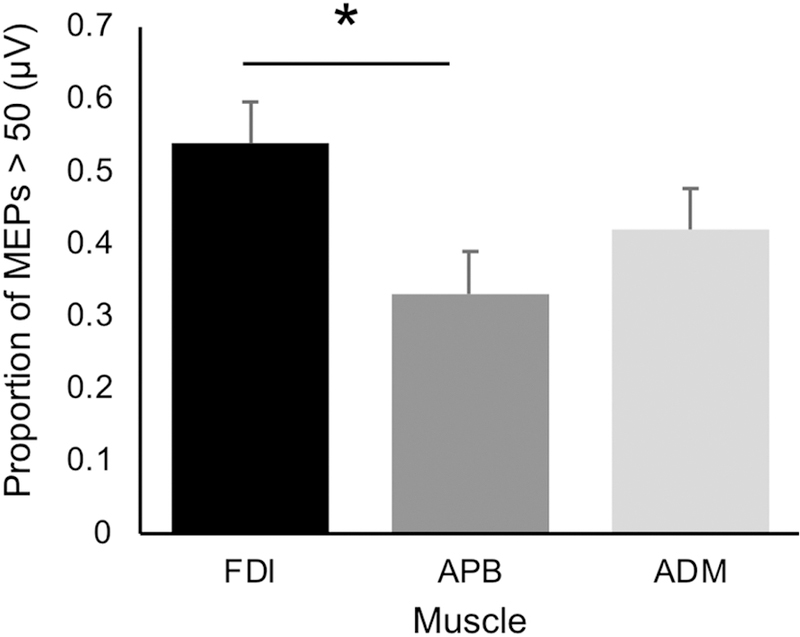
Proportion of MEP responses larger than 50 μV at baseline (the criteria for threshold MEP). Data shown represent the proportion ± standard error in each muscle. This proportion did not reach 50% in either the APB or ADM muscle indicating that the stimulation intensity was below threshold on average for these muscles.
For log10-transformed baseline MEP amplitudes, the random-effects linear model found a significant main effect of Muscle (F2,27 = 4.26, p = .025, ). Planed pairwise comparisons demonstrated baseline MEP amplitudes from FDI were significantly greater than APB (p = .019), but not ADM (p = .393) (Illustrated in Figure 3).
Figure 3.
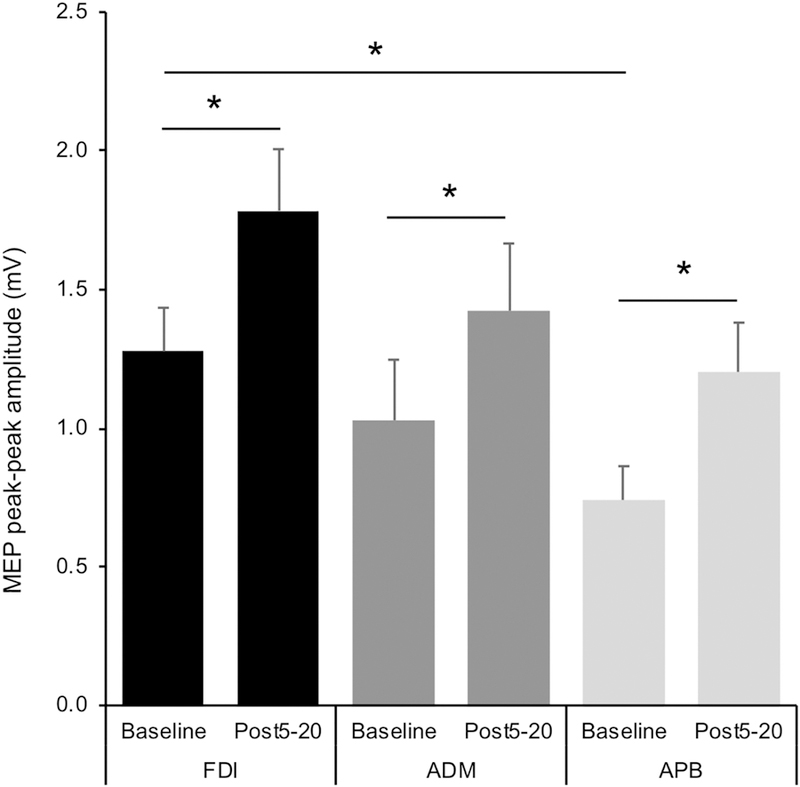
MEP amplitude (mV) at baseline and 5–20 minutes after iTBS in each muscle.Error bars represent the peak-to-peak MEP amplitude (mean ± standard error). Significance bars (below) illustrate differences within each muscle and between baseline values (above).
Regarding the magnitude of iTBS aftereffects in each muscle, the Wilcoxon signed-rank test found iTBS induced a significant facilitation in MEP amplitude in all three muscles (FDI: Z=55.5, p = .002; APB: Z=54.5, p = .003; ADM: Z=42, p = .002) (Figure 3).
The random-effects linear model comparing the effect of iTBS between FDI and APB revealed a significant main effect of Muscle (F1,12 = 12.07, p = .045, ), after controlling for the FDI/APB ratio of baseline MEPs (Figure 4). Specifically, the magnitude of facilitation was greater in APB (84.5 ±81.0%) than FDI (49.3 ±63.8%), while the FDI/APB ratio of MEPs at baseline was not a significant predictor (p = .992). Among 13 participants with available ADM data, and the effect of iTBS on ADM (56.1 ± 58.6%) was not significantly different from FDI (F1,9 = 0.71, p = .715, ), after controlling for the FDI/ADM ratio of MEPs at baseline, which was not a significant predictor (p = .957).
Figure 4.
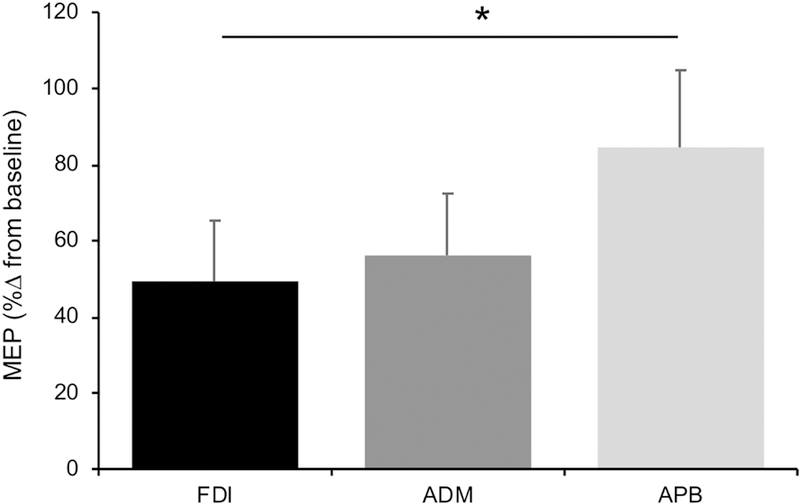
Effects of iTBS compared between muscles. Data shown represent the average (±standard error) %∆ from baseline in MEP amplitude post-iTBS in each muscle.
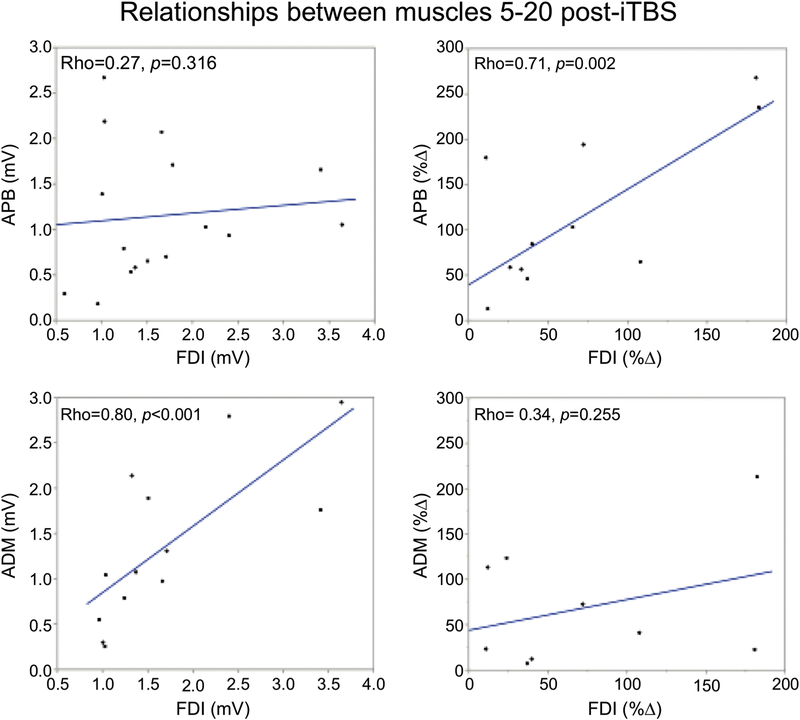
Lastly, Spearman’s Rho correlations found a significant relationship between the MEP amplitudes (mV) over T5–T20 in FDI and ADM (Rho = 0.80, p = <.001) but not between those in FDI and APB (Rho = 0.27, p = .316). In contrast, %ΔMEP over T5–T20 were significantly correlated between FDI and APB (Rho = 0.71, p = .002) but not between FDI and ADM (Rho = 0.34, p = .255) (Figure 5). These results demonstrate a strong association between ADM and FDI in the raw MEP amplitudes, but not the effect of iTBS, while the opposite was observed for APB and FDI.
Figure 5.
Correlations between the target (FDI) and non-target muscles (APB, ADM) in MEP amplitude and %∆ in MEP amplitude (mV). Top row presents FDI vs APB, bottom row represents FDI vs ADM. The left column represents the correlation between MEP amplitude and the right column represents the correlation between %∆.
Discussion
The present study found that standard iTBS applied over the FDI hotspot induced a co-modulation of MEPs in other intrinsic hand muscles (i.e., APB and ADM) with adjacent cortical representations to FDI. While the proportion of MEPs at FDI threshold and the average MEP amplitude at baseline were higher in FDI than APB (confirming the selectivity of the chosen hotspot for FDI), iTBS-induced modulation was unexpectedly greater in APB than in FDI. Importantly, this effect persisted after controlling for the ratio of baseline MEP amplitudes between the two muscles. By contrast, ADM was closer to FDI in terms of both the proportion of MEPs at the FDI threshold and average baseline amplitudes; and while iTBS did induce significant facilitation from ADM, the magnitude of aftereffects was not significantly different from FDI. These results indicated that (1) subthreshold iTBS exerts neuromodulatory effects that extend beyond the targeted region, and (2) the pattern of coactivation from single pulse TMS and spread of iTBS-induced neuromodulation likely reflect important functional and anatomical differences between APB and ADM in terms of their relationship with FDI.
Repetitive TMS protocols, both conventional and patterned (TBS), applied to the motor cortex are thought to modulate MEP amplitudes by increasing the efficacy of excitatory synapses (Huang et al., 2005; Pascual-Leone et al., 1994a). This was highlighted in a previous study (Pascual-Leone et al., 1994a) that found repeated trains of rTMS lead to progressively earlier onset of the spread of excitation, and thus modulation of MEPs was likely the result of increased efficacy of excitatory synapses. In the present work, the modulation of MEPs from APB can be understood given its cortical and peripheral proximity to FDI and the functional overlap between the two muscles (Li et al., 2018). However, the finding that, even after controlling for differences in baseline MEP amplitude, iTBS aftereffects were in fact significantly larger in APB than FDI was unexpected. There are a number of possible explanations for this finding. Several studies have provided insights into the composition of the descending corticospinal volleys induced by TMS over the motor cortex in patients with epidural electrodes surgically implanted into their upper cervical segments (Di Lazzaro et al., 2003; Di Lazzaro et al., 2001). These studies have yielded evidence suggesting spTMS typically activates pyramidal tract neurons (PTNs) indirectly via excitation of pre-synaptic axons, which can be observed as descending volleys of synchronized activity, termed I-waves (Di Lazzaro & Rothwell, 2014). These I-waves have early (I1 wave) as well as late components (late I-waves), and evidence suggests the pulse waveform affects the composition of the TMS-induced corticospinal volleys. In particular, a biphasic pulse waveform with an initial anterior-to-posterior (AP) current induced in the brain (i.e., AP-PA currents) preferentially elicits I1 waves and at higher stimulus intensities can directly activate the PTNs resulting in D-waves (Di Lazzaro et al., 1998). Additionally, motor mapping studies have shown that subtle variations in the orientation of the TMS coil relative to the precentral gyrus can influence the selectivity of responses in muscles that have otherwise partially overlapping representations (Bashir et al., 2013; Wassermann et al., 1994). Considering these two findings together, it is possible that the coil orientation in the present study was optimal for FDI activation at a threshold level and baseline (resulting in D-waves and I1 wave), but sub-optimal for APB, resulting in a larger proportion of late I-waves. While this explanation lacks the explicit confirmation provided by epidural recording of descending volleys, it nonetheless fits our observation that MEPs recorded from APB tended to be smaller than those from FDI. Additionally, in light of the evidence that iTBS has been found to selectively enhance the late I waves without influencing the I1 wave (Lazzaro et al., 2008), we postulate that the greater iTBS response in APB than in FDI could, in part, be due to the fact that suboptimal stimulation of APB at baseline yielded late I-waves that were modulated to a greater extent by iTBS than the I1-/D-waves resulting from the more optimally aligned stimulation of the FDI hotspot.
An alternative explanation can be provided based on the relative propensity of iTBS to recruit intracortical networks of both target and non-target muscles. A number of previous studies have assessed the effects of iTBS (Mirdamadi et al., 2015) and cTBS (Fang et al., 2014; Mirdamadi et al., 2016) on non-target, antagonistic wrist muscles when stimulation is applied to the target muscle. In contrast to our results, these studies found that the magnitude of the effect of TBS on the target muscle was a function of the relative difference between the rMTs of the two muscle (Mirdamadi et al., 2015). We did not assess the rMT of the non-target muscles, but our analysis did demonstrate the selectivity of the chosen hotspot for FDI; stimulation at rMT and baseline resulted in a significantly greater proportion of MEP responses and larger MEP amplitudes from the FDI compared to the APB. In addition, we found that the magnitude of responses in the non-target muscle was not a function of the baseline MEP amplitude. The contrasting results in our study may be related to the synergistic nature of the target and non-target muscles in the present study compared to the antagonist nature of the wrist muscles targeted in the aforementioned studies. Previous studies have suggested that the relative recruitment of different interneuron networks of target and non-target muscles in the stimulated region is responsible for the aftereffects of TBS (Mirdamadi et al., 2016). M1 responses exhibit both convergent and divergent properties as well as horizontal interconnections in control of movement (Schieber, 2001); the same pyramidal neurons can terminate on spinal motor neurons that innervate different muscles (divergence) while contraction of the same muscle can be elicited by stimulating a wide region of M1 (convergence).
The finding that the MEP amplitudes over T5–T20 were correlated between FDI and ADM, but not between FDI and APB, can be attributed to divergent effects arising from the common peripheral innervation of FDI and ADM (by the ulnar nerve), compared to the innervation of the APB by the median nerve. In contrast, the finding that the magnitude of maximal iTBS aftereffects were correlated between FDI and APB, but not between FDI and ADM, is consistent with the notion that iTBS modulates the overlapping representations of synergistic intrinsic hand muscles (Wassermann et al., 1994), and can be attributed to the convergent recruitment of several intracortical networks arising from the greater overlap between cortical representations of FDI and APB compared to those of FDI and ADM.
Activation of corticospinal pathways by TMS is thought to be mediated by activation of both inhibitory and excitatory intracortical interneurons that project onto the large pyramidal tract neurons in layer V of the cortex (Lazzaro et al., 2001). These inhibitory and excitatory inputs typically balance each other out, avoiding the possibility of a horizontal spread of excitation (Pascual-Leone et al., 1994). However, rTMS at higher frequencies can result in the summation of excitatory post-synaptic potentials leading to a net excitation of neighboring pyramidal cells and an eventual horizontal spread of excitation (Pascual-Leone et al., 1994). Indeed, early experiments showed that following conventional rTMS protocols at suprathreshold (110–200%) intensities, a spread of excitation beyond the target muscle is observed (Pascual-Leone et al., 1994). In this study, however, we are concerned with the modulation induced by iTBS during several minutes post-stimulation. Yet the present results have important safety implications for the therapeutic uses of iTBS. With increased likelihood of greater facilitation at higher intensities and frequencies (Rossi et al., 2009), the risk of seizure may be heightened. Further, there is evidence that iTBS can yield persistent physiological effects even in the absence of parallel behavioral changes (Muellbacher et al., 2000). This has led to the recommendation that concurrent EEG recordings be used during the application of iTBS in those with heightened risk of seizure (Rossi et al., 2009). Fortunately, very few seizure events have been reported to date from iTBS when performed following the safety guidelines (Rossi et al., 2009; Rossini et al., 2015). Nonetheless, documented evidence suggesting iTBS-induced modulation of MEPs from non-target, intrinsic hand muscles should be considered in populations with increased risk of seizure. This is especially important if iTBS is applied multiple times per day, which has been shown to lead to varying degrees of either homeostatic (Müller-Dahlhaus and Ziemann, 2015; Murakami et al., 2012) or non-homeostatic metaplasticity (Opie et al., 2017; Tse et al., 2018).
The results of the present study should be interpreted in context of the following limitations: We only measured the aftereffects of iTBS within the intrinsic hand muscles and, therefore, were limited in our ability to assess iTBS aftereffects beyond these three muscles. Additionally, we only measured the effect of iTBS on the three muscles when applied over the FDI hotspot, but not the APB and ADM hotspots, nor did we assess RMT at these other spots. Thus, the present analyses could not assess whether the effects of iTBS on MEPs from APB and ADM were influenced by the suboptimal alignment of the coil relative to the hotspot for the non-target muscles. This may be of interest to future studies. Finally, the age range for the study was 50–75y, representing a span from middle-age to senescence. While age has been associated with increased recruitment of areas of association cortex when performing cognitive tasks (Cabeza, 2002; Cabeza et al., 2002), we are not aware of any evidence of age-related reorganization within intrinsic hand muscles of the motor cortex. A simple linear regression revealed age was not associated with the mean %∆ over the first 20 minutes post-iTBS for any muscle (R’s=−.06 – −.30, p’s= 0.253 – 0.836), thus we do not believe age was a factor within this cohort. Nevertheless, we cannot rule out the possibility that younger individual might show a different pattern of responses across muscles.
We found that the facilitatory effects of iTBS targeting the FDI hotspot were not specific to the MEPs recorded from that muscle and extended to adjacent APB and ADM muscles. MEPs from the APB muscle showed significant facilitation that was greater than MEPs from FDI. Importantly, these differences could not be accounted for by lack of selectivity of the chosen hotspot (for FDI relative to APB) or by differences in baseline MEP amplitude elicited by supra-threshold, single-pulse TMS. These results have implications for the safety of iTBS application in populations with increased risk of seizure and also regarding the selectivity and efficacy of iTBS to modulate cortico-spinal and, potentially, cortico-cortical connections.
Highlights.
iTBS to FDI induces facilitation in non-targeted muscles (APB, ADM) with adjacent cortical representation
iTBS aftereffects were larger in non-targeted APB than FDI.
The pattern of responses in ADM and APB likely reflect distinct anatomical and functional relationships with FDI
Acknowledgement
We thank Erica Seligson, Natasha Atkinson, and Sadhvi Saxena (Beth Israel Deaconess Medical Center) for assistance with data collection and Ann Connor (Beth Israel Deaconess Medical Center) for regulatory oversight and compliance, and for assistance with evaluation of participant health and medical history.
Funding
The original data were obtained and analyzed with support from the National Institutes of Health (NIH R21 NS082870). The contributions of P.J.F. were further supported by the NIH (R21 AG051846). A.P.-L. was supported in part by the Sidney R. Baer Jr. Foundation, the NIH (R01MH100186, R01HD069776, R01NS073601, R21 NS082870, R21 MH099196, R21 NS085491, R21 HD07616), Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758), and the Football Players Health Study at Harvard University.
A.J. was supported by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada (NSERC PDF 454617) and the Canadian Institutes of Health Research (CIHR 41791). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Institutes of Health or the Sidney R. Baer Jr. Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A portion of these findings was presented at the 47th annual meeting of the Society for Neuroscience, Washington D.C., USA, November 11th -15th 2017.
Conflicts of Interest Statement
A.P.-L. serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Constant Therapy, Cognito, and Neosync; and is listed as inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. The authors declare no competing interests.
References
- Bashir S, Perez JM, Horvath JC, Pascual-Leone A, 2013. Differentiation of motor cortical representation of hand muscles by navigated mapping of optimal TMS current directions in healthy subjects. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 30, 390–395. 10.1097/WNP.0b013e31829dda6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17, 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR, 2002. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage 17, 1394–1402. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC, 1998. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol 109, 397–401. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, 2014. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J. Physiol 592, 4115–4128. 10.1113/jphysiol.2014.274316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose GM, 1988. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J. Neurosci. Off. J. Soc. Neurosci 8, 4079–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A, 2011. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci 108, 21229–21234. 10.1073/pnas.1113103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J-H, Huang Y-Z, Hwang I-S, Chen J-JJ, 2014. Selective modulation of motor cortical plasticity during voluntary contraction of the antagonist muscle. Eur. J. Neurosci 39, 2083–2088. 10.1111/ejn.12565 [DOI] [PubMed] [Google Scholar]
- Fried PJ, Schilberg L, Brem A-K, Saxena S, Wong B, Cypess AM, Horton ES, Pascual-Leone A, 2016. Humans with Type-2 Diabetes Show Abnormal Long-Term Potentiation-Like Cortical Plasticity Associated with Verbal Learning Deficits. J. Alzheimers Dis. JAD 55, 89–100. 10.3233/JAD-160505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, 2007. Transcranial magnetic stimulation: a primer. Neuron 55, 187–199. 10.1016/j.neuron.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Julkunen P, Säisänen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, Könönen M, 2009. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage 44, 790–795. 10.1016/j.neuroimage.2008.09.040 [DOI] [PubMed] [Google Scholar]
- Laakso I, Hirata A, Ugawa Y, 2014. Effects of coil orientation on the electric field induced by TMS over the hand motor area. Phys. Med. Biol 59, 203 [DOI] [PubMed] [Google Scholar]
- Lazzaro V, Oliviero A, Mazzone P, Insola A, Pilato F, Saturno E, Accurso A, Tonali P, Rothwell J, 2001. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp. Brain Res 141, 121–127. 10.1007/s002210100863 [DOI] [PubMed] [Google Scholar]
- Lazzaro VD, Oliviero A, Pilato F, Mazzone P, Insola A, Ranieri F, Tonali PA, 2003. Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol. Res 25, 143–150. 10.1179/016164103101201292 [DOI] [PubMed] [Google Scholar]
- Lazzaro VD, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC, 2008. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J. Physiol 586, 3871–3879. 10.1113/jphysiol.2008.152736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Wei N, Cheng M, Hou X, Song J, 2018. Dynamical Coordination of Hand Intrinsic Muscles for Precision Grip in Diabetes Mellitus. Sci. Rep 8, 4365 10.1038/s41598-018-22588-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirdamadi JL, Suzuki LY, Meehan SK, 2016. Motor cortical plasticity in extrinsic hand muscles is determined by the resting thresholds of overlapping representations. Neuroscience 333, 132–139. 10.1016/j.neuroscience.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirdamadi JL, Suzuki LY, Meehan SK, 2015. Agonist contraction during intermittent theta burst stimulation enhances motor cortical plasticity of the wrist flexors. Neurosci. Lett 591, 69–74. 10.1016/j.neulet.2015.02.020 [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M, 2000. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 111, 1002–1007. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus F, Ziemann U, 2015. Metaplasticity in human cortex. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 21, 185–202. 10.1177/1073858414526645 [DOI] [PubMed] [Google Scholar]
- Murakami T, Müller-Dahlhaus F, Lu M-K, Ziemann U, 2012. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J. Physiol 590, 5765–5781. 10.1113/jphysiol.2012.238519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie GM, Vosnakis E, Ridding MC, Ziemann U, Semmler JG, 2017. Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimulat 10, 298–304. 10.1016/j.brs.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Cohen LG, Brasil-Neto JP, Valls-Solé J, Hallett M, 1994a. Differentiation of sensorimotor neuronal structures responsible for induction of motor evoked potentials, attenuation in detection of somatosensory stimuli, and induction of sensation of movement by mapping of optimal current directions. Electroencephalogr. Clin. Neurophysiol 93, 230–236. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A, 2011. Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topogr 24, 302–315. 10.1007/s10548-011-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M, 1994. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117, 847–858. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 120, 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U, 2015. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol 126, 1071–1107. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, 1998. Clinical applications of motor evoked potentials. Electroencephalogr. Clin. Neurophysiol 106, 180–194. [DOI] [PubMed] [Google Scholar]
- Roth Y, Amir A, Levkovitz Y, Zangen A, 2007. Three-dimensional Distribution of the Electric Field Induced in the Brain by Transcranial Magnetic Stimulation Using Figure-8 and Deep H-coils. J. Clin. Neurophysiol 24, 31–38. 10.1097/WNP.0b013e31802fa393 [DOI] [PubMed] [Google Scholar]
- Schieber MH, 2001. Constraints on Somatotopic Organization in the Primary Motor Cortex. J. Neurophysiol 86, 2125–2143. 10.1152/jn.2001.86.5.2125 [DOI] [PubMed] [Google Scholar]
- Tse NY, Goldsworthy MR, Ridding MC, Coxon JP, Fitzgerald PB, Fornito A, Rogasch NC, 2018. The effect of stimulation interval on plasticity following repeated blocks of intermittent theta burst stimulation. Sci. Rep 8 10.1038/s41598-018-26791-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf YD, Sanz-Arigita EJ, Menning S, van den Heuvel OA, 2010. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci 11, 145 10.1186/1471-2202-11-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Hallett M, 1994. Cortical motor representation of the ipsilateral hand and arm. Exp. Brain Res 100, 121–132. 10.1007/BF00227284 [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJLG, 2015. Efficacy and Time Course of Theta Burst Stimulation in Healthy Humans. Brain Stimulat 8, 685–692. 10.1016/j.brs.2015.03.004 [DOI] [PubMed] [Google Scholar]