Abstract
Most of Parkinson’s disease (PD) patients experience gastrointestinal dysfunctions, including gastric hypomotility. The dorsal motor nucleus of the vagus (DMV) modulates the motility of the upper gastrointestinal (GI) tract. Paraquat (P) administration induces Parkinsonism in experimental models, and we have developed recently an environmental model of Parkinsonism in which rats are treated with subthreshold doses of P and lectins (P+L), in both models rats develop reduced gastric motility prodromal to the full extent of motor deficits. The aim of the present study was to examine whether the membrane properties of DMV neurons in these two experimental models of Parkinsonism were altered. Whole cell recordings in slices containing DMV neurons were conducted in male Sprague Dawley rats which received either injections of paraquat (10mg/kg i.p.; 10P), or oral administration of paraquat (1 mg/kg) and lectin (0.05% w/v; P+L). Morphological reconstructions of DMV neurons were conducted at the end of the recordings. The repolarization kinetics of the afterhyperpolarization phase of the action potential was accelerated in 10P neurons vs control, while the phase plot revealed a slower depolarizing slope. At baseline, the amplitude of miniature excitatory postsynaptic currents was increased in P+L neurons. No differences in the morphology of DMV neurons were observed. These data indicate that the membrane and synaptic properties of DMV neurons are altered in rodent models of Parkinsonism, in which neurons of 10P and P+L rats demonstrate an increased excitatory transmission, perhaps in an attempt to counteract the paraquat-induced gastric hypomotility.
Keywords: Electrophysiology, vagus nerve, gastrointestinal tract
INTRODUCTION
Parkinson’s disease (PD), the second most frequent neurodegenerative disorder after Alzheimer’s disease, is characterized by the chronic and progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The lack of dopaminergic inputs causes the striatum to fire irregularly, resulting in an overall dysregulation of basal ganglia activity underlying the cardinal motor symptoms of the disease, i.e. tremor at rest, rigidity and bradikynesia (Przedborski, 2017; Dickson, 2018). In addition to the motor dysfunctions, a variety of non-motor symptoms are associated with PD (Schapira et al., 2017), including gastrointestinal (GI) complications such as esophageal dysfunctions, delayed gastric emptying, and constipation. These GI dysfunctions are prodromal to the emergence of the motor symptoms and the clinical diagnosis of possible PD appearing up to 20 years prior to diagnosis (Cersosimo et al., 2013; Pellegrini et al., 2015; Pellegrini et al., 2016). Indeed Gl symptoms in healthy individuals have been positively correlated with an increased risk of developing PD later in life (Natale et al., 2008; Poewe, 2008; Jost, 2010; Fasano et al., 2015).
The fine modulation of the functions of the upper Gl tract is under the control of preganglionic parasympathetic neurons of the dorsal motor nucleus of the vagus (DMV), (Browning & Travagli, 2014; Travagli & Anselmi, 2016; Bove & Travagli, 2019). DMV neurons are spontaneously active with a firing rate of about 1 pulse per second (p.p.s.) (Travagli et al., 1991). Their spontaneous activity is modulated mainly by GABA, glutamate, and catecholamines inputs arising from the adjacent nucleus tractus solitarius (NTS) (Travagli & Anselmi, 2016; Bove & Travagli, 2019). In addition to NTS inputs, the DMV receives inputs from other higher centers, including a tonic dopaminergic input which originates from the SNpc via the newly described nigro-vagal pathway, which activation increases gastric tone and motility (Anselmi et al., 2017; Anselmi et al., 2018).
Evidence supports a strong correlation between the use of the herbicide paraquat (P) and the incidence of PD (Nandipati & Litvan, 2016). Indeed, in addition to the classical model of PD, i.e. intraperitoneal injections of high doses of P, the newly described model of environmental PD is able to induce the parkinsonian phenotype by replicating a more realistic exposure. Indeed, oral administration of subthreshold doses of the herbicide, combined with the ingestion of lectins, which are highly concentrated in raw vegetables and grains, and has been correlated to a higher risk of developing idiopathic PD (Ho et al., 1989; Anselmi et al, 2018) replicates the cardinal motor symptoms of parkinsonism. Specifically, with both models we showed an impairment of the nigro-vagal pathway resulting in prodromal Gl symptoms, the presence of misfolded α-synuclein, a histological hallmark of PD, in enteric, DMV, and SNpc neurons, as well as motor symptoms of Parkinsonism which were relieved by L-Dopa treatment (Anselmi et al, 2017; Anselmi et al, 2018).
Despite the clear correlation between PD and Gl disorders, little is known about the consequences of the disease on DMV neuronal properties. A recent paper by Lasser-Katz and collaborators has shown that, in a model of α-synuclein over-expression, DMV neurons initiate a “stressless-pacemaking” that prevents the increase in excitability observed in SNpc neurons in the same model (Lasser-Katz et al., 2017). A comprehensive analysis of the biophysical properties of DMV neurons in rats treated with P alone or P+L, however, has not been conducted.
The aim of the present study was to examine whether the membrane properties of DMV neurons in these two experimental models of Parkinsonism were altered.
EXPERIMENTAL PROCEDURES
All procedures were conducted in accordance with the National Institutes for Health guidelines, with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee and according to journal policies and regulations on animal experimentation.
Animals
Male Sprague-Dawley rats (Charles River, Kingston, NY, USA) were housed under a standard 12-hour light/dark cycle at 24°C and had ad libitum access to food and water. After weaning (post-natal day 21) rats were separated into 3 groups: i) naïve animals that received no treatment (control group; CTL; total N=64), ii) 10P group (total N=71), which received i.p. injections of 10mg/kg of paraquat (Sigma-Aldrich, St. Louis, MO), weekly for three consecutive weeks (Anselmi et al., 2017), and iii) P+L group (total N=30), which received 7 days of daily oral administration of a solution containing 1 mg/kg of P and 0.05% (w/v) of lectin from pisum sativum (Sigma-Aldrich)(Anselmi et al., 2018). Rats from group ii were tested 2 days after the final injection of P and rats from group iii were tested 2 weeks after the end of the treatment. Although the previous studies were performed up to 2 (10P; (Anselmi et al., 2017; Anselmi et al., 2018)) and 4 weeks (P+L; (Anselmi et al., 2017; Anselmi et al., 2018)), at the time points used herein, rats already showed the gastrointestinal dysfunction associated with Parkinsonism (Anselmi et al., 2017; Anselmi et al., 2018).
Electrophysiology
Rats were anesthetized with Isoflurane (VEDCO, St. Joseph, MO) in a custom-made anesthetic chamber and a bilateral pneumothorax was performed after abolition of the foot-pinch withdrawal reflex. The brainstem was then removed and placed immediately in chilled (4°C), oxygenated Krebs’ solution (see Solution composition section). Four-six coronal slices (250-300μm thick) spanning the entire rostro-caudal extent of the DVC were cut using a vibratome. The slices were then incubated in oxygenated Krebs’ solution at 25±1°C for at least 90 min prior to recording. Electrophysiological recordings were performed from a single slice held in place by a nylon mesh in a custom-made 500μl perfusion chamber with flowing Krebs’ solution at 32±1°C. A Nikon E600FN was used to locate the DVC in the slice.
Whole cell recordings of DMV neurons were made with patch pipettes (3-5 MΩ resistance) filled with a potassium gluconate solution or a potassium chloride solution (see Solution composition section). Data were sampled every 100μs, filtered at 2 kHz, digitized via a Digidata 1322A interface (Molecular Devices, San Jose, CA), acquired, stored and analyzed on a PC utilizing pCIamp 10 software (Molecular Devices) or with MiniAnalysis 60 (Synaptosoft, Fort Lee, NJ). The junction potential was corrected manually and recordings were accepted only if the series resistance was <20 MΩ.
Electrophysiological properties measured included, in current clamp configuration: (1) duration of the action potential at threshold, (2) amplitude and decay kinetics of the afterhyperpolarization (AHP) phase of the action potential, and (3) frequency of action potential firing, expressed as pulses s−1, in response to 400 ms-long DC pulses (20 to 270 pA in step increments). In voltage clamp configuration: (1) membrane input resistance (measured from the current deflection obtained by stepping the membrane from −70 to −80 mV for 400 ms), (2) amplitude and decay time of the current underlying the AHP evoked by stepping the membrane from −50 to +10 mV for 24 ms, and (3) frequency, amplitude, rise and decay time, and charge transferred of miniature excitatory and inhibitory postsynaptic currents in the presence of tetrodotoxin (TTX; 0.3μM) and bicuculline (10μM) or kynurenic acid (1mM), respectively. To analyze the decay time of the current underlying the evoked AHP, the current was fitted with two exponentially decaying functions to the Chebyshev domain to obtain the decay values for τ1 and τ2:
To characterize and compare action potentials across groups, the dynamic changes of the events were evaluated by performing a phase plot analysis (Jenerick, 1963; Trombin et al., 2011; Fohlmeister, 2015). Briefly, changes of membrane potential as differential of the time (expressed as mV/ms) were plotted against the instantaneous voltage value (mV). The resulting loop graph was utilized to compare the threshold value (Vthresh), peak voltage value (Vpeak), repolarizing voltage value (Vrepol) as well as the rates of depolarization and repolarization calculated from the slopes of the loop graph. The differential equation dV/dt was calculated in pCIamp 10, and the data was plotted and analyzed in SigmaPlot 11.0 (Systat Software, San Jose, CA).
At the end of the experiment, neurons were filled with neurobiotin (2.5% w/v; 0.3nA depolarizing pulse, 600ms duration, every 2 s) for 20 min to permit postfixation reconstruction. Slices were then immersed overnight in Zamboni’s fixative (see Solutions composition), transferred in PBS+0.05% Na Azide, and stored at 4°C until analyzed.
Morphological reconstruction of neurons
The morphological reconstruction of patched neurons was performed as described previously (Browning et al., 1999). Briefly, slices were cleared of fixative in PBS-TX and kept at 4°C until the injected neurobiotin (Vector Labs) was visualized using a cobalt-nickel enhancement of the Avidin D–horseradish peroxidase (Avidin D–HRP). Slices were incubated in Avidin D–HRP solution (see Solution composition section) for 2 h. After rinsing in PBS, and incubation for 15–20 min in Avidin D–HRP and DAB solutions (see Solution composition section), the slice was incubated for an additional 15 min in the presence of 3% H2O2. The slice was then rinsed in PBS, placed on a coverslip, air for τ1 and τ2: dried, cleared in alcohol and xylene, and mounted in Cytoseal™60 (Thermo Scientific, Cheshire, WA).
Three-dimensional reconstructions of individual neurobiotin-labeled neurons, digitized at a final magnification of 600X were made using Neurolucida® software (Microbrightfield, Williston, VT). The morphological features that were assessed include: soma area and diameter, form factor, whether the cell has bipolar or multipolar somata, number of segments (i.e., branching of dendrites), branch order, and extension in the x- and y-axes. Data analysis was performed as described previously (Browning et al., 1999; Martinez-Pena y Valenzuela et al., 2004).
Solutions composition
Krebs’ solution (in mM): 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 10 D-Glucose maintained at 295-300 mOsm, and at pH 7.4 by bubbling with 95% O2 and 5% CO2.
Intracellular potassium gluconate solution (in mM): 128 potassium gluconate, 10 KCI, 0.3 CaCl2, 1 MgCl2, 10 HEPES, 1 EGTA, 2 ATP-Na, 0.25 GTP-Na, adjusted to pH 7.35-7.4 with KOH, with osmolarity 270-285 mOsm.
Intracellular potassium chloride solution (in mM): 140 KCl, 1 CaCl2,1 MgCl2, 10 HEPES, 1 EGTA, 2 ATP-Na, 0.25 GTP-Na, adjusted to pH 7.35-7.4 with KOH, with osmolarity 270-285 mOsm.
Zamboni’s fixative was made with 1.6% (w/v) paraformaldehyde, 19 mM KH2PO4, and 100 mM Na2HPO4•7H20 in 240 ml saturated picric acid-1,600 ml H20, adjusted to pH 7.4 with HCl.
PBS-TX was composed of (in mM): NaCI 115, Na2HPO4 75, KH2PO4 7.5, and 0.3% Triton X-100. Avidin D-HRP solution was made with 0.002% Avidin D-HRP in PBS containing 1% Triton X-100; 0.05% DAB in PBS containing 0.5% gelatin supplemented with 0.025% CoCl2 and 0.02% NiNH4SO4.
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich.
Statistical analysis
Data were analyzed by using one-way ANOVA followed by a correction for multiple comparisons using the post-hoc Tukey’s multiple comparison test, and χ2 square test (Graph Pad Prism, Graph Pad Software Inc., La Jolla, CA) and are reported as mean±SEM. For ANOVA tests, a Barlett test for equal variances was conducted to determine if the variances analyzed were statistically different. When that was the case, the Kruskal-Wallis test was used instead followed by post-hoc Dunn’s multiple comparison test. Data points with a value over two standard deviations from the mean were excluded. In all instances, significance was set at p<0.05. Multiple samples were acquired from individual cells from single animals, implying the acquisition of non-independent data. A minimum of 5 observations from the same cluster data was obtained in order to have a sufficient power in our analysis (Aarts et al., 2014).
RESULTS
Paraquat treatment alters the basic membrane properties and action potential characteristics of DMV neurons
Systemic administration of paraquat (10P group) accelerated significantly the decay kinetics of the action potential afterhyperpolarization phase from 131±68ms in control animals (N=71 cells from 36 animals) to 99±6ms in 10P treated animals (N=80 cells from 37 animals; Kruskal-Wallis H=6.297 d.f.=2, p<0.05). Gastric administration of paraquat and lectins (P+L group) did not alter the decay kinetics of the action potential afterhyperpolarization (146±11ms for P+L; N=64 cells from 25 animals; Kruskal-Wallis H=12.42, d.f.=2, p>0.05).
The amplitude of the action potential AHP, the input resistance (Rinp), and the duration of the action potential measured at firing threshold were similar among the groups (F(2,220)=2.91 for amplitude of AHP, F(2,86)=0.83 for Rinp and F(2,212)=1.76 for duration of action potential, p>0.05 for all).
Data are summarized in figure 1.
Figure 1. Paraquat treatment alters the basic membrane properties and action potential characteristics of DMV neurons.
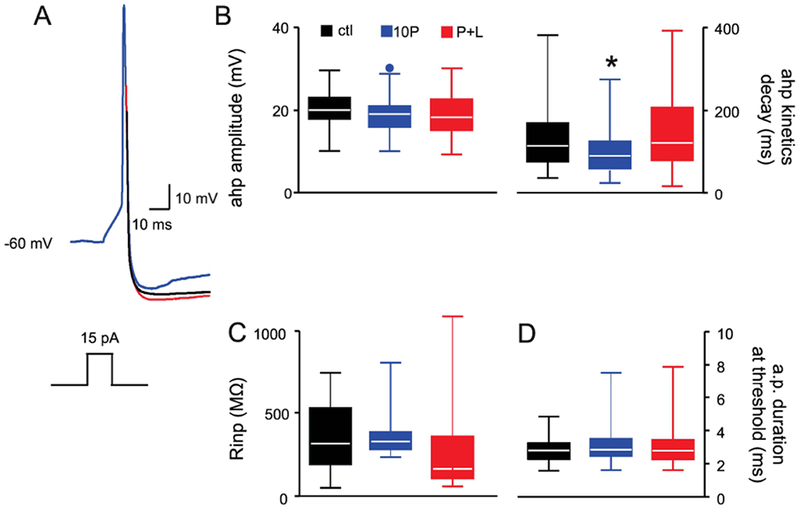
A. Representative traces of evoked action potentials from control (CTL, black), 10P- (blue), and P+L- (red) treated rats. Note that the action potentials have similar amplitude among the groups, but the AHP of DMV neuron from the 10P treated rat has faster kinetics of decay.
Holding potential= −60 mV.
B. Whisker box plot showing the amplitude (left panel) and decay kinetics (right panel) of the afterhyperpolarization phase of the action potential. A significant reduction in the decay kinetics of the afterhyperpolarization phase of the action potential was observed in DMV neurons from 10P treated rats. Dots above the whisker box plot are outliers. Left whisker box plot is a Tukey boxplot, right whisker box plot depicts medians, 25th and 75th quartiles.
Black bar: CTL, N=71 cells from 36 animals; blue bar, 10P, N=80 cells from 37 animals; red bar: P+L, N=64 cells from 25 animals. *p < 0.05 vs control.
C. Whisker box plot showing that the input resistance is unaffected by the different treatments. The plot depicts medians, 25th and 75th quartiles.
Black bar: CTL, N=30 cells from 18 animals; blue bar: 10P, N=26 cells from 13 animals; red bar: P+L, N=30 cells from 13 animals.
D. Whisker box plot showing that the duration of the action potential at threshold value is similar across all groups. The plot depicts medians, 25th and 75th quartiles.
E. Black bar: CTL, N=71 cells from 36 animals; blue bar, 10P, N=80 cells from 37 animals; red bar: P+L, N=64 cells from 25 animals. p > 0.05.
The contribution of calcium-dependent potassium currents that underlies the action potential AHP (Baptista et al., 2005) was also examined in the voltage-clamp configuration. Similarly to that observed in current-clamp configuration, the amplitude of the resulting current was comparable across groups (F(2,142)=0.16; p>0.05). Conversely, the fast component of the kinetic of decay was reduced significantly in all treated groups compared to control (44±3.8ms for controls, N=19 cells from 12 animals; 11±0.9ms for 10P, N=41 cells from 20 animals; 13±0.9ms for P+L, N=23 cells from 11 animals; Kruskal-Wallis H= 6.29, d.f.=2; p<0.05; fig. 2).
Figure 2. Paraquat treatment alters the kinetics of decay of the Ca2+-K+ currents.

A. Representative voltage clamp traces of IK(Ca) recorded from CTL (black), 10P (blue), and P+L (red) neurons after application of a depolarization step from −50 to +10 mV. Traces were normalized for amplitude to provide a better visualization of the changes in the decay kinetics.
B. Whisker box plot showing the IK(ca) amplitude. Dots above the whisker box plot are outliers.
Black bar: CTL, N=57 cells from 25 animals ; blue bar: 10P, N=53 cells from 21 animals; red bar: P+L, N=35 cells from 13 animals, p > 0.05 for all.
C. Whisker box plot showing the two components of the IK(ca) decay kinetics expressed as τ1 (left panel) and τ2 (right panel). A significant reduction in the decay kinetics of the fast component of the IK(ca) was observed in all treatment groups vs control. The plot depicts medians, 25th and 75th quartiles.
Black bar: CTL, N=33-19 cells from 12 animals; blue bar: 10P, N=41 cells from 20 animals; red bar: P+L, N=23 cells from 11 animals. *p < 0.05 vs control.
Data are summarized in figure 2 and Table 1.
Table 1.
Slow decay time constant of the Ca2+-dependent K+ current measured in voltage-clamp configuration.
| Groups | Control | 10P | P+L |
|---|---|---|---|
| τ1 (ms) | 58 ± 3.5 | 57 ± 2.9 | 52 ± 2.5 |
| N (cells/animals) | 33/12 | 41/20 | 23/11 |
To better understand the characteristics of the action potential, a phase plot analysis of the action potential was performed on a subset of neurons from each group. Neurons from control animals exhibit a much steeper depolarization slope compared to the treated groups (5.6±0.39 ms−1 for controls; 3.7±0.4 ms−1 for 10P; 3.6±0.4 msȒ1 for P+L; N=6 cells for all; N=5 animals for CTL and 6 for 10P and P+L; Kruskal-Wallis H=9.58 d.f.=2, p<0.05). No differences were observed in the other phase plot parameters analyzed. Data are summarized in figure 3 and Table 2.
Figure 3. Phase plot analysis of the action potential reveals changes in the depolarizing slope following paraquat treatment.
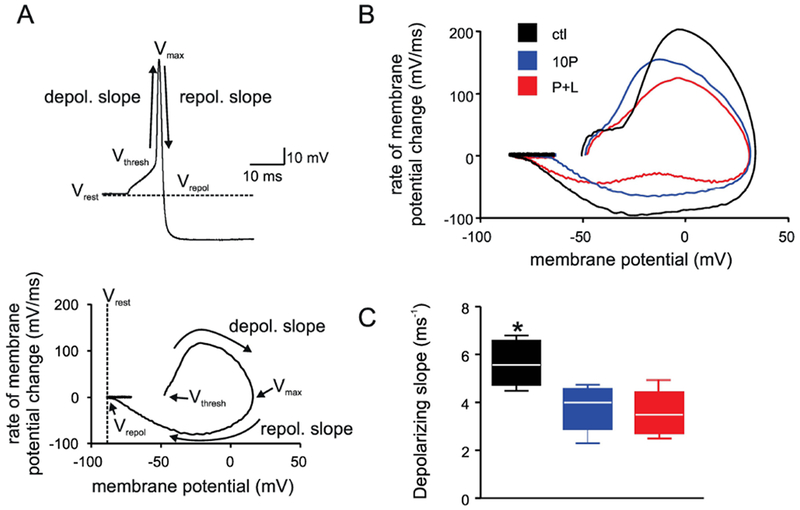
A. Schematic representation of the phase plot analysis. Top: sample action potential showing the voltage at: rest (Vrest), threshold (Vthresh), peak (Vmax), and repolarization (Vrepol), and the slope for the measurement of depolarizing and repolarizing phases. Bottom: sample phase plot calculated from the above action potential, with the corresponding aforementioned parameters.
B. Representative phase plots from control (black), 10P (blue), and P+L (red).
C. Whisker box plot showing that the slope of repolarization is reduced significantly after paraquat treatment.
Black bar: CTL; blue bar: 10P; red bar: P+L; N=6 cells for all; N=5 animals in control, and N=6 animals in 10P and P+L. *p < 0.05 vs 10P and P+L.
Table 2.
Action potential parameters as described by the phase plot analysis.
| Groups | Control | 10P | P+L |
|---|---|---|---|
| Slope of repolarization (ms−1) | −1.4 ± 0.11 | −1.4 ± 0.09 | −1.3 ± 0.07 |
| Vmax (mV) | 29.5 ± 3.4 | 28.3 ± 2.5 | 29.4 ± 2.5 |
| Vrepol (mV) | −87.7 ± 1.1 | −81.1 ± 1.7 | −82.5 ± 1.4 |
| N (cells/animals) | 6/5 | 6/6 | 6/6 |
These data suggest that treatment with high doses of paraquat, i.e. 10P, acts on DMV neurons directly to alter their basic membrane properties by accelerating the kinetics of repolarization of the action potential AHP phase. Conversely, either treatment with high, i.e. 10P, as well as subthreshold, i.e. P+L, doses of paraquat altered both the IK(ca) and the depolarization slope of the action potential phase plot.
Paraquat treatment does not affect the response of DMV neurons to current injection
Since we observed an acceleration in the kinetics of the AHP phase of the action potential following P treatment, the number of action potentials fired in response to injection of direct current pulses of increasing amplitude were examined (400 ms duration; 20–270 pA). When averaging the number of action potentials fired at each current step, no significant differences were observed across groups (N=75 cells from 35 animals for control; N= 87 cells from 32 animals 10P; and N= 52 cells from 25 animals for P+L; F(2,211 )=1.09, p> 0.05). Similarly, when analyzing the frequency of distribution of action potentials in discrete bins, we observed that, when stimulated with 270 pA of direct current, all groups have similar distribution of the number of action potentials fired, although the 10P-treated group shows a trend towards a rightward shift in the frequency (Fig. 4; χ2 (8)= 5.216, p>0.05).
Figure 4. Paraquat treatment does not affect the response to current injection of DMV neurons.
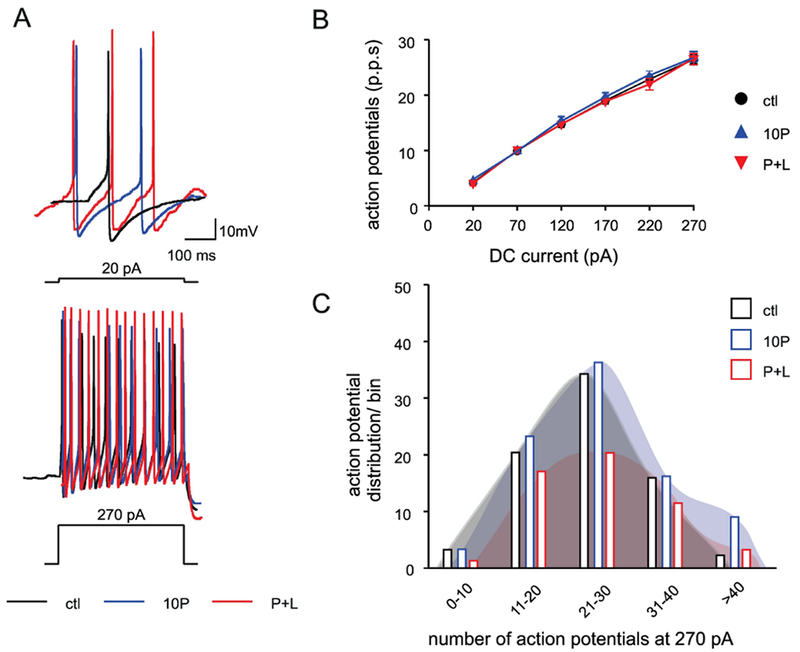
A. Representative traces showing the response after injection of 20 (top trace) and 270 pA (bottom trace) direct current (DC, 400 ms long).
B. Frequency-response curves for DMV neurons from control (black), 10P (blue), and P+L (red).
CTL (black; N=75 cells from 35 animals), 10P (blue N=87 cells from 32 animals), and P+L (red N=52 cells from 25 animals). p>0.05
C. Frequency distribution plot of neurons in the different bins (x axis) showing the maximum number of action potentials fired by DMV neurons at 270 pA.
Black open bars: CTL; blue open bars: 10P; red open bars: P+L. χ2 > 0.05.
Collectively, these data suggest that the changes in the basic properties of the action potential of neurons from animals treated with 10P do not appear to impact the response of DMV neurons to current injection significantly.
Paraquat and lectins administration increase the amplitude of miniature excitatory postsynaptic currents at baseline
Neurons from P+L rats showed a significant increase in the amplitude of miniature excitatory postsynaptic currents (mEPSCs) from 26±1.0 to 33±1.6 pA at baseline in control (N=37 cells from 16 animals) and P+L (N=23 cells from 11 animals), respectively (F(2,97)=7.69, p<0.05). Conversely, the increase in mEPSCs amplitude was not observed in neurons from 10P-treated rats. Likewise, no differences among the groups were observed when frequency, rise and decay time, and area of the mEPSCs were analysed (F(2,97)=0.35, p>0.05).
Data are summarized in figure 5 and Table 3.
Figure 5. The amplitude of mEPSCs is larger in DMV neurons from rats treated with paraquat and lectins.

A. Representative traces showing miniature excitatory post-synaptic current events (mEPSC) recorded from control (CTL, black), 10P- (blue), and P+L (red) treated rats in the presence of 0.3 μM TTX and 10 μM bicuculline.
Holding voltage= −50mV
Whisker box plot showing the amplitude (B), frequency (C), rise (D) and decay (E) times, and area (F) of mEPSCs. Dots outside the whisker box plot are outliers.
Black bars: CTL (N=37 cells from 16 animals); Blue bars: 10P (N=40 cells from 17 animals); Red bars: P+L (N=23 cells from 11 animals). *p<0.05
Table 3.
mEPSCs and mIPSCs characteristics recorded in voltage-clamp configuration.
| Groups | Control | 10P | P+L |
|---|---|---|---|
| mEPSCs frequency (events s−1) | 2 ± 0.3 | 1.7 ± 0.2 | 1.7 ± 0.3 |
| mEPSCs amplitude (pA) | 26.3 ± 1.0 | 27.4 ± 1.2 | 33.3 ± 1.6* (p<0.05 v ctl) |
| mEPSCs rise time (ms) | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.5 ± 0.1 |
| mEPSCs decay time (ms) | 3.1 ± 0.2 | 3.0 ± 0.2 | 2.7 ± 0.1 |
| mEPSCs area (pA/ms) | 82.1 ± 4.8 | 84.2 ± 5 | 95.1 ± 4.8 |
| N (cells/animals) | 37/16 | 40/17 | 23/11 |
| mIPSCs frequency (events s−1) | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0. |
| mIPSCs amplitude (pA) | 58.1 ± 3 | 60 ± 3.8 | 62 ± 3.6 |
| mIPSCs rise time (ms) | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.8 ± 0.1 |
| mIPSCs decay time (ms) | 5.4 ± 0.3 | 4.7 ± 0.2 | 5.5 ± 0.2 |
| mIPSCs area (pA/ms) | 294.1 ± 18.7 | 322 ± 20.5 | 274.5 ± 20.4 |
| N (cells/animals) | 41/16 | 37/10 | 26/10 |
No differences in the amplitude, frequency, rise and decay time, or area under the curve were observed in miniature inhibitory postsynaptic currents. Data are summarized in Table 3.
These data suggest that following oral administration of a combination of paraquat and lectin there is an increase in the baseline amplitude of mEPSCs, suggesting post-synaptic alterations in glutamatergic transmission.
The morphological properties of DMV neurons are unaltered in rodent models of parkinsonism
At the end of the electrophysiological recording, neurons were filled with neurobiotin® to reconstruct neuronal morphology. The morphological reconstruction of DMV neurons did not reveal differences in neuronal size, number of segments, segments length, and branch order across groups. Data are summarized in Table 4.
Table 4.
Morphological characteristics of DMV neurons.
| Groups | Control | 10P | P+L |
|---|---|---|---|
| X axis | 313 ± 21.4 | 274 ± 18.2 | 301 ± 21.4 |
| Y axis | 170 ± 12.1 | 187 ± 11.5 | 195 ± 20.5 |
| Soma area | 267 ± 14.6 | 282 ± 13.6 | 241 ± 16.3 |
| Number of segments | 8.5 ± 0.6 | 8.4 ± 0.4 | 8.1 ± 0.6 |
| Average segment length | 220 ± 15.9 | 260 ± 42 | 222 ± 12 |
| Branch order | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.4 ± 0.2 |
| N (cells/animals) | 25/17 | 45/25 | 21/10 |
Altogether, these data suggest that P treatment does not appear to affect DMV neuronal morphology in rats.
DISCUSSION
In the present study we report that some of the membrane and synaptic properties of DMV neurons are altered in rodent models of Parkinsonism. Specifically, we have shown that (i) following an established model of PD induction, i.e. three weekly i.p. injections of 10mg/kg of paraquat, DMV neurons have faster kinetics of repolarization of the AHP phase; (ii) voltage clamp recording of lKca show faster kinetics of the fast component in the PD models analyzed herein; (iii) the phase plot analysis of the action potential revealed that DMV neurons from both models of PD showed a slower depolarization slope compared to control; and, (iv) DMV neurons from P+L treated rats have a larger amplitude of mEPSCs at baseline.
Our previous studies reported that, at the time points utilized in the present work, rats from both the 10P and the P+L groups display gastric dysfunction already (Anselmi et al., 2017; Anselmi et al., 2018) despite the fact that this relatively short time-span does not allow for a widespread degeneration of SNpc neurons (Nandipati & Litvan, 2016), supporting the idea that membrane alterations, but not neuronal death, may be restricted to the vagal complex rather than also involving the nigro-vagal pathway (Anselmi et al., 2017).
The involvement of the DMV in the pathogenesis of environmental PD has been confirmed by several studies. Immunohistochemical analysis of tissues harvested from PD patients revealed the presence of Lewy Bodies (LB), pathologic aggregates of α-synuclein, in isolated myenteric plexus neurons (Goedert et al., 2013), as well as motor neurons of the DMV (Braak et al., 2003a). This distinct spatial pattern of distribution of LB, which correlates with the appearance of Gl symptoms prior to the development of the motor deficits, was interpreted by Braak and collaborators as a possible triggering factor of PD pathology. Indeed, under this hypothesis, an environmental “unknown pathogen” is absorbed in the ENS and then transported retrogradely via the vagus nerve to the DMV (Braak et al., 2003b; Hawkes et al., 2010; Visanji et al., 2013). Our recent description of a monosynaptic nigro-vagal pathway supports the involvement of the DMV, and provides a direct point of connection between the vagus nerve and the SNpc, the area ultimately affected by neurodegeneration and responsible for the classic motor symptoms associated with PD (Anselmi et al., 2017). Impairment of this pathway has been proven to be responsible in part for the gastric dysfunction observed in a paraquat model of PD (Anselmi et al., 2017).
The importance of the vagus nerve and the DMV in the progression of PD was further confirmed in other animal models (Pan-Montojo et al., 2010; Pan-Montojo & Funk, 2010; Noorian et al., 2012; Holmqvist et al., 2014; Chandra et al., 2017), as well in a novel model of environmental PD (Anselmi et al., 2018) which utilizes oral administration of subthreshold doses of paraquat and lectin, and it is able to recapitulate the histological, motor, and Gl features of PD (Anselmi et al., 2018). Although the involvement of the nigro-vagal pathway and the vagus nerve is supported by the aforementioned studies, what is less understood are the direct effects of environmental toxins on DMV neurons.
The data presented herein suggest that systemic administration of paraquat accelerated the repolarization of the AHP phase of the action potential of DMV neurons. Increasing the rate at which DMV neurons repolarize, however, did not increase their excitatory response to current injection. This evidence supports the “stressless pacemaking theory” observed in a α-synuclein over-expressing mouse model in which DMV neurons are able to engage an antioxidative response to α-synuclein over-expression to avoid disruption of their function (Lasser-Katz et al., 2017). The authors of this study suggested that, unlike SNpc neurons, DMV neurons maintain their physiological autonomous firing rate by reducing the number of functional CaV 1.2 and 2.3 channel complexes, resulting in a reduction of the mean Cav currents. The CaV 1.2 subunit seems to have a role in slowing down pacemaking (Cooper et al., 2015), so the reduction in CaV 1.2 levels observed in this α-synuclein over-expressing model of PD might underlie the results described in the present manuscript.
The importance of Ca2+ influx in DMV neurons is also highlighted by the variations in IK(ca) observed in this manuscript. Indeed, in all the experimental models used in the present study the decay kinetics of this current were faster. IK(ca), specifically the apamin-sensitive small conductance (SK), is an important contributor of the afterhyperpolarization phase of DMV neurons (Sah & McLachlan, 1992; Travagli et al., 1992; Browning et al., 1999). The faster repolarization observed in the 10P treated rats, and the reduction in the kinetics of the IK(ca) observed in all PD models might represent a mechanism by which DMV neurons attempt, although unsuccessfully, to overcome the gastric dysfunctions observed in these two models.
The phase plot analysis of action potential, first described by Jenerick (Jenerick, 1963), was used to uncover subtle differences in the action potential characteristics following PD induction. While the slope of repolarization was not different between the various groups, we observed a reduction in the slope of depolarization following paraquat treatment. This alteration, which indicates that the paraquat-treated neurons require more time to reach the peak of the action potential (Vmax), has been reported in other neuronal types in neurological conditions such as epilepsy (Trombin et al., 2011). Prior studies on hippocampal (Magee & Johnston, 1995; Magee & Carruth, 1999) and neocortical principal neurons (Stuart & Sakmann, 1994) suggested that Na+-dependent dendritic-generated action potentials also exhibit slowing of the depolarization slope, implying that seizure-related action potentials might be generated in the dendrites, rather than the soma. Changes in action potential features are also correlated to an increase in extracellular K+, which is related to the generation of non-somatic (i.e. dendritic) events. Indeed, [K+]0 levels increase during a seizure, and local [K+]0 increase has been shown to cause extrasomatic firing in hippocampal in vitro slices (Avoli et al., 1998). Changes in either Na+ or K+ levels might potentially happen at the DMV synapses, and would consequently increase the excitability of a neuronal population with only subtle changes in the membrane properties of the postsynaptic neurons. This evidence might further support the idea that DMV neurons are resilient to environmental stressors by initiating an anti-oxidative response that is not observed in SNpc neurons of animals exposed to PD-related stressors, i.e. overexpression of α-synuclein (Subramaniam et al., 2014; Bove & Travagli, 2019).
The idea that, in these models, there is a disruption of the delicate microenvironment of the DVC, rather than the intrinsic membrane properties of DMV neurons, would explain some of the changes observed in the present manuscript and, by consequence, have a major role in the gastric dysfunctions reported previously (Anselmi et al., 2017; Anselmi et al., 2018) , and supports the theory that PD is a circuit-based disorder rather than a neuronal-deficit disease (Bove & Travagli, 2019). Indeed, we have shown that, at baseline conditions, neurons of animals treated with P+L have larger mEPSCs. This postsynaptic effect might be explained as a form of activity-dependent homeostasis, a phenomenon related to synaptic plasticity (O’Brien et al., 1998; Turrigiano et al., 1998; Murthy et al., 2001; Han & Stevens, 2009). Generally speaking, homeostatic changes require a long time course to become stable, and strengthen steadily. Increase in mEPSCs amplitude has been observed to be triggered following neuronal silencing, without a concurrent increase in mEPSCs event frequency (O’Brien et al., 1998; Turrigiano et al., 1998; Wierenga et al., 2005). These changes depend on the neuronal population studied however, on the time course of neuronal silencing, and the state of maturation of the neurons (Han & Stevens, 2009). Neuroplasticity in brainstem neurocircuits has been described extensively (Browning & Travagli, 2006; Zsombok et al., 2011; Blake & Smith, 2014; Browning & Travagli, 2014; Travagli & Anselmi, 2016), and we have recently hypothesized that maladaptive neuroplasticity could underlie the unusual gastric biphasic response to DA microinjection in the DVC observed in 10P-treated animals (Bove et al., 2019). Given that the increase in the amplitude of mEPSCs reported in this manuscript has only been observed in our P+L model of environmental PD (Anselmi et al., 2018), the involvement of the sensory component of the vagus nerve cannot be excluded. Further studies focusing on the NTS to DMV synapse are hence necessary. This postsynaptic adaptation could also be part of the attempt of DMV neurons to avoid the gastrointestinal dysfunction observed in PD.
Axotomized and/or damaged nerves show morphological changes and retraction of the dendritic tree (Sumner & Sutherland, 1973; Purves, 1975), and damaged motoneurons show a progressive reduction in postsynaptic EPSP (Mendell & Munson, 1999). Although the vagal neurocircuitry plays a role in PD pathogenesis, our data appear to indicate that, at the time points analyzed in the present study, major morphological changes have not occurred yet.
In summary, in the present study we have shown that the membrane and synaptic properties of DMV neurons are altered in rodent models of parkinsonism. The faster AHP phase observed in neurons of 10P rats, the changes in IK(Ca) observed in all models, and the increased excitatory transmission in P+L rats suggest a futile attempt to counteract the paraquat-induced gastric hypomotility.
Highlights.
Parkinson’s disease patients experience gastrointestinal dysfunctions, including vagally-mediated gastric hypomotility.
Rodent models of Parkinsonism were induced with different doses of paraquat with or without lectin co-administration.
Whole cell recordings of vagal motoneurons showed altered action potential and synaptic parameters in paraquat-treated rats.
No differences in the morphology of vagal motoneurons were observed.
The increased excitation of vagal motoneurons may reflect an attempt to counteract the paraquat-induced gastric hypomotility.
ACKNOWLEDGEMENTS
Funding: This work was supported by NIH grant DK-55530, and a grant from Michael J. Fox Foundation for Parkinson’s Disease to R. A. Travagli.
The authors would like to thank Dr. K.N. Browning for proof-reading and providing critical comments to earlier versions of the manuscript, to Cesare M. and Zoraide Travagli for support and encouragement, and to V. Gentile for his continued support. A special thanks to J. Dylan Weissenkampen, Kim K. Doheny and Fumi Gardner for providing crucial help with some of the statistical analysis.
ABBREVIATIONS
- AHP
Afterhyperpolarization
- CTL
Control
- DMV
Dorsal Motor Nucleus of the Vagus
- GI
Gastrointestinal
- L
Lectin (from P. Sativum)
- L-Dopa
Levo dopa
- mEPSC
Miniature Excitatory Post-Synaptic Currents
- mIPSC
Miniature Inhibitory Post-Synaptic Currents
- P
Paraquat
- PD
Parkinson’s Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarts E, Verhage M, Veenvliet JV, Dolan CV & van der Sluis S (2014) A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci, 17, 491–496. [DOI] [PubMed] [Google Scholar]
- Anselmi L, Bove C, Coleman FH, Le K, Subramanian MP, Venkiteswaran K, Subramanian T & Travagli RA (2018) Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. Naturepj Parkinson’s Disease, 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi L, Toti L, Bove C, Hampton J & Travagli RA (2017) A Nigro-Vagal Pathway Controls Gastric Motility and is Affected in a Rat Model of Parkinsonism. Gastroenterology, 153, 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Methot M & Kawasaki H (1998) GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci, 10, 2714–2722. [DOI] [PubMed] [Google Scholar]
- Baptista V, Zheng ZL, Coleman FH, Rogers RC & Travagli RA (2005) Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Research, 1052, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake CB & Smith BN (2014) cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am J Physiol Regul Integr Comp Physiol, 307, R711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove C, Anselmi L & Travagli RA (2019) Altered gastric tone and motility response to brainstem dopamine in a rat model of parkinsonism. Am J Physiol Gastrointest Liver Physiol, 317(1), G1–G7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove C & Travagli RA (2019) Neurophysiology of the brain stem in Parkinson’s disease. J Neurophysiol, 121, 1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del TK, Rub U, De Vos RA, Jansen Steur EN & Braak E (2003a) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging, 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP & Del Tredici K (2003b) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna), 110, 517–536. [DOI] [PubMed] [Google Scholar]
- Browning KN, Renehan WE & Travagli RA (1999) Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. Journal of Physiology, 517, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN & Travagli RA (2006) Short-term receptor trafficking in the dorsal vagal complex: An overview. Auton. Neurosci, 126-127, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN & Travagli RA (2014) Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol, 4, 1339–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutierrez C, Micheli FE & Benarroch EE (2013) Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J. Neurol, 260, 1332–1338. [DOI] [PubMed] [Google Scholar]
- Chandra R, Hiniker A, Kuo YM, Nussbaum RL & Liddle RA (2017) alpha-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G, Lasser-Katz E, Simchovitz A, Sharon R, Soreq H, Surmeier DJ & Goldberg JA (2015) Functional segregation of voltage-activated calcium channels in motoneurons of the dorsal motor nucleus of the vagus. J Neurophysiol, 114, 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW (2018) Neuropathology of Parkinson disease. Parkinsonism Relat Disord, 46 Suppl 1, S30–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Visanji NP, Liu LW, Lang AE & Pfeiffer RF (2015) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol, 14, 625–639. [DOI] [PubMed] [Google Scholar]
- Fohlmeister JF (2015) Voltage gating by molecular subunits of Na+ and K+ ion channels: higher-dimensional cubic kinetics, rate constants, and temperature. J Neurophysiol, 113, 3759–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Del TK & Braak H (2013) 100 years of Lewy pathology. Nat. Rev. Neurol, 9, 13–24. [DOI] [PubMed] [Google Scholar]
- Han EB & Stevens CF (2009) Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc Natl Acad Sci U S A, 106, 10817–10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K & Braak H (2010) A timeline for Parkinson’s disease. Parkinsonism Relat Disord, 16, 79–84. [DOI] [PubMed] [Google Scholar]
- Ho SC, Woo J & Lee CM (1989) Epidemiologic study of Parkinson’s disease in Hong Kong. Neurology, 39, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R & Li JY (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol, 128, 805–820. [DOI] [PubMed] [Google Scholar]
- Jenerick H (1963) Phase Plane Trajectories of the Muscle Spike Potential. Biophys J, 3, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost WH (2010) Gastrointestinal dysfunction in Parkinson’s Disease. J Neurol. Sci, 289, 69–73. [DOI] [PubMed] [Google Scholar]
- Lasser-Katz E, Simchovitz A, Chiu WH, Oertel WH, Sharon R, Soreq H, Roeper J & Goldberg JA (2017) Mutant alpha-Synuclein Overexpression Induces Stressless Pacemaking in Vagal Motoneurons at Risk in Parkinson’s Disease. J Neurosci, 37, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC & Carruth M (1999) Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol, 82, 1895–1901. [DOI] [PubMed] [Google Scholar]
- Magee JC & Johnston D (1995) Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol, 487, 67–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pena y Valenzuela IM, Browning KN & Travagli RA (2004) Morphological differences between planes of section do not influence the electrophysiological properties of identified rat dorsal motor nucleus of the vagus neurons. Brain Research, 1003, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM & Munson JB (1999) Retrograde effects on synaptic transmission at the Ia/motoneuron connection. J Physiol Paris, 93, 297–304. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF & Zhu Y (2001) Inactivity produces increases in neurotransmitter release and synapse size. Neuron, 32, 673–682. [DOI] [PubMed] [Google Scholar]
- Nandipati S & Litvan I (2016) Environmental Exposures and Parkinson’s Disease. Int J Environ Res Public Health, 13, 881–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale G, Pasquali L, Ruggieri S, Paparelli A & Fornai F (2008) Parkinson’s disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol. Motil, 20, 741–749. [DOI] [PubMed] [Google Scholar]
- Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM & Greene JG (2012) Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol Dis, 48, 9–19. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD & Huganir RL (1998) Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron, 21, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H & Funk RH (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One, 5, e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Montojo FJ & Funk RH (2010) Oral administration of rotenone using a gavage and image analysis of alpha-synuclein inclusions in the enteric nervous system. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini C, Antonioli L, Colucci R, Ballabeni V, Barocelli E, Bernardini N, Blandizzi C & Fornai M (2015) Gastric motor dysfunctions in Parkinson’s disease: Current pre-clinical evidence. Parkinsonism. Relat Disord, 21, 1407–1414. [DOI] [PubMed] [Google Scholar]
- Pellegrini C, Colucci R, Antonioli L, Barocelli E, Ballabeni V, Bernardini N, Blandizzi C, de Jonge WJ & Fornai M (2016) Intestinal dysfunction in Parkinson’s disease: Lessons learned from translational studies and experimental models. Neurogastroenterol. Motil, 28, 1781–1791. [DOI] [PubMed] [Google Scholar]
- Poewe W (2008) Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol, 15 Suppl 1, 14–20. [DOI] [PubMed] [Google Scholar]
- Przedborski S (2017) The two-century journey of Parkinson disease research. Nat Rev Neurosci, 18, 251–259. [DOI] [PubMed] [Google Scholar]
- Purves D (1975) Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol, 252, 429–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P & McLachlan EM (1992) Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J. Neurophysiol, 68, 1834–1841. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR & Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci, 18, 509. [DOI] [PubMed] [Google Scholar]
- Stuart GJ & Sakmann B (1994) Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature, 367, 69–72. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Althof D, Gispert S, Schwenk J, Auburger G, Kulik A, Fakler B & Roeper J (2014) Mutant alpha-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J Neurosci, 34, 13586–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE & Sutherland FI (1973) Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol, 2, 315–328. [DOI] [PubMed] [Google Scholar]
- Travagli RA & Anselmi L (2016) Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol, 13, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD & Vicini S (1991) Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol, 260, G531–G536. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA & Vicini S (1992) Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am. J. Physiol, 263, G508–G517. [DOI] [PubMed] [Google Scholar]
- Trombin F, Gnatkovsky V & de Curtis M (2011) Changes in action potential features during focal seizure discharges in the entorhinal cortex of the in vitro isolated guinea pig brain. J Neurophysiol, 106, 1411–1423. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC & Nelson SB (1998) Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature, 391, 892–896. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Brooks PL, Hazrati LN & Lang AE (2013) The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun, 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K & Turrigiano GG (2005) Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci, 25, 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV & Smith BN (2011) Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci, 31, 14024–14031. [DOI] [PMC free article] [PubMed] [Google Scholar]


