Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are a chemical class widely used in industrial and commercial applications because of their unique physical and chemical properties. Between 2013 and 2016 PFAS were detected in public water systems and private wells in El Paso County, Colorado. The contamination was likely due to aqueous film forming foams used at a nearby Air Force base.
Objective:
To cross-sectionally describe the serum concentrations of PFAS in a highly exposed community, estimate associations with drinking water source, and explore potential demographic and behavioral predictors.
Methods:
In June 2018, serum PFAS concentrations were quantified and questionnaires administered in 213 non-smoking adult (ages 19–93) participants residing in three affected water districts. Eighteen PFAS were quantified and those detected in >50% of participants were analyzed: perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorononanoate (PFNA) and perfluoroheptane sulfonate (PFHpS). Unadjusted associations were estimated between serum PFAS concentrations and several predictors, including water consumption, demographics, personal behaviors and employment. A multiple linear regression model estimated adjusted associations with smoking history.
Results:
Study participants’ median PFHxS serum concentration (14.8 ng/mL) was approximately 12 times as high as the U.S. national average. Median serum concentrations for PFOS, PFOA, PFNA and PFHpS were 9.7 ng/mL, 3.0 ng/mL, 0.4 ng/mL and 0.2 ng/mL, respectively. Determinants of PFHxS serum concentrations were water district of residence, frequency of bottled water consumption, age, race/ethnicity, and smoking history. Determinants of serum concentrations for the other four PFAS evaluated included: water district of residence, bottled water consumption, age, sex, race/ethnicity, smoking history, and firefighter or military employment.
Conclusions:
Determinants of serum concentrations for multiple PFAS, including PFHxS, included water district of residence and frequency of bottled water consumption. Participants’ dominant PFAS exposure route was likely consumption of PFAS-contaminated water, but certain demographic and behavioral characteristics also predicted serum concentrations.
Introduction:
Per- and polyfluoroalkyl substances (PFAS), a class of chemicals with unique physical and chemical properties, are widely used in industrial and commercial applications (Buck et al, 2011). PFAS have been in widespread production and use since the 1950s and have become ubiquitous in human serum in industrialized countries (CDC 2019). Animal studies have demonstrated that PFAS are hepatotoxic, immunotoxic, tumorigenic, and may produce reproductive and developmental toxicity following exposure to pregnant females (Dewitt 2012; Dewitt et al. 2009; Lau 2007). Studies of populations with higher-than-background environmental exposures (mostly via drinking water) and occupational exposures have been essential for comparing the toxic effects of low-to-moderate chronic exposure to PFAS in humans with the effects reported in animal studies(Barry, 2013; Darrow, 2016; Frisbee et al. 2010; Gallo et al. 2012; Winquist 2014). Among the clinical parameters repeatedly associated with PFAS exposure in humans are elevated serum lipids, liver enzymes, and markers of immunologic function (Dalsager et al. 2016; Darrow 2016; Eriksen et al. 2013; Fisher 2013; Frisbee et al. 2010; Fu 2014; Gallo et al. 2012; Gleason 2015; Grandjean 2016; Lin et al. 2010; Steenland 2009). The C8 study reported probable links between PFOA exposure and certain autoimmune diseases and cancers (Barry 2013; Steenland 2013; Vieira 2013), however, studies of chronic cardiovascular, immune system and metabolic disease risks associated with these biomarker alterations have so far been inconclusive (Averina 2019; Karnes 2014; Mattsson et al. 2015; Sun 2018; Sunderland 2019; Winquist 2014).
Previous reports of PFAS water contamination in the U.S. have generally focused on facilities with current or historical manufacturing of PFAS (Emmett et al. 2006; Hansen 2002; MPCA, 2019; Herrick et al. 2017). Another potential source of PFAS in drinking water is the contamination of ground and surface water due to use of aqueous film-forming foams (AFFF) at airports, military installations, and fire-fighting training sites (Anderson 2016; Hu et al. 2016; Weiss et al. 2012). PFAS have been recently detected in hundreds of public water systems more than half of U.S. states (Anderson 2016; Hu et al. 2016; Weiss et al. 2012). While no national enforceable standards have been set for PFAS in drinking water, in 2016, the United States Environmental Protection Agency (USEPA) established health advisory levels (70 ng/L) for two commonly measured PFAS, perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) (USEPA 2016).
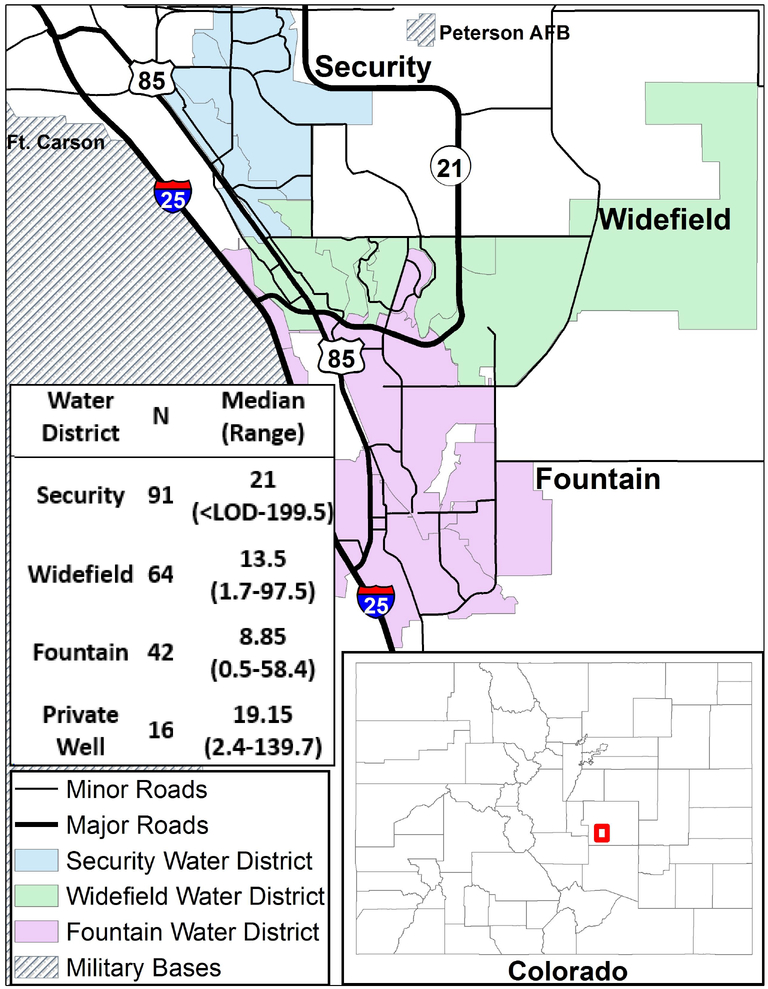
Between 2013 and 2016, PFAS concentrations above the USEPA health advisory level for PFOS and PFOA were detected in public water systems in Fountain, Security and Widefield in El Paso County, Colorado, where the concentration gradient of the AFFF-contaminated plume decreases from north to south along the aquifer, away from Peterson Air Force Base (CDPHE 2018; Finley 2016) (Figure 1). These three water systems, that combined serve approximately 80,000 people, were likely contaminated prior to 2013 and potentially much earlier. The impacted public water systems either installed effective treatment facilities or changed water sources so that most drinking water-related exposure to PFAS in these communities was reduced considerably beginning in August, 2015 (CDPHE 2018; El Paso County Health Dept. 2017). The highest level among the measured PFAS in water was generally for perfluorohexane sulfonate (PFHxS), which is structurally similar to PFOS but does not have a USEPA-established health advisory level. However, in 2018 the Agency for Toxic Substances and Disease Registry (ATSDR) identified an intermediate oral intake minimal risk level for PFHxS of 2×10−5 mg/kg/day (ATSDR 2018), and the Minnesota Department of Health has designated a maximum health based value (HBV), equal to their HBV of PFOS, of 27ng/L in drinking water (ITRC 2019).
Figure 1: Map of study area, with table indicating the range and median serum PFHxS concentrations of PFAS-AWARE participants by water district of residence (ng/mL; N=213).
Abbreviations: N, number of observations; PFHxS, perfluorohexane sulfonate; ng/mL, nanograms per milliliter; < LOD, below limit of detection.
This PFHxS-dominant exposure mixture is unlike the PFOA-dominant exposure profile examined in other cohorts also exposed to PFAS contaminated drinking water, such as the C8 Health Project (C8 Science Panel 2017), and may pose unique health risks to the exposed population. Little is known about the health effects of human exposure to PFAS in areas with drinking water contaminated by AFFF, and no systematic biomonitoring has been done in this Colorado community until the present study: PFAS Assessment of Water and Resident Exposure, hereafter referred to as “PFAS-AWARE”. At present, few studies have been published on populations exposed specifically to AFFF-related PFAS contamination (Daly et al., 2018; Gyllenhammer et al., 2015; Li et al., 2017; Rotander 2015).
The objective of this work is to describe the serum concentrations of PFAS in a highly exposed community, to estimate the associations between serum concentrations of these PFAS and drinking water source, as well as to explore other potential determinants of PFAS serum concentrations, such as participant demographics, dietary and cleaning habits, employment, and smoking history. In addition, smoking history was explored in a multivariable linear regression model to investigate whether the observed associations were robust to covariate adjustment.
Methods:
Study Population
Throughout the first half of 2018, the PFAS-AWARE study enrolled 213 adults whose primary residence was in Fountain, Security or Widefield, Colorado for at least two years during the period of known public water-system contamination, which was August 2012 to August 2015. Participants were recruited via outreach to community groups, newspaper ads, television news stories, and mailings to affected households between January and June 2018. To be eligible, participants were required to live on an impacted private well or public water system, be over 18 years old, not currently pregnant, non-smokers, and willing to provide a blood sample.
All participants attended a clinic at a central location in Security, Colorado in June 2018. Each participant provided a 20-mL non-fasting blood sample to quantify serum PFAS concentrations. At the same study visit, a questionnaire was administered by study personnel to assess potential sources of PFAS exposure as well as to collect demographic information and an abbreviated health history. The study protocol was approved by the Colorado Multiple Institutional Review Board (protocol 17–2182) and all participants provided informed consent prior to study procedures. The analysis of blinded specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subject research.
Exposure Assessment Questionnaire
The questionnaire aimed to assess the contribution to serum PFAS concentrations from drinking PFAS-contaminated water versus other sources of exposure. Variables used to assess exposure to PFAS-impacted water include: water district of residence, bottled water use, workplace water district, and home water source (public system versus private well). Participants also reported on potential non-water sources of PFAS including: frequency of dusting and vacuuming, dietary habits, and potential occupational exposures. Basic demographic information, an abbreviated health history, and a complete list of medications and supplements were also collected. The questionnaire was developed based on previous literature and guidance on evaluating PFAS exposure from multiple sources (ATSDR 2018; Siebenaler et al. 2017). The complete questionnaire is available in the Supplemental Materials.
Quantification of Serum PFAS
Blood was centrifuged within 30 minutes of collection to obtain 0.5 mL aliquots of serum, which were then transferred to 2mL polypropylene cryovials and stored at −80°C prior to shipment to the laboratory. Twenty PFAS were quantified in serum at CDC using HPLC-Turbo Ion Spray ionization tandem mass spectrometry (HPLC-MS/MS) with isotope dilution. The PFAS measured included: perfluorobutane sulfonate (PFBS), PFHxS, perfluoroheptane sulfonate (PFHpS), linear PFOS (n-PFOS), sum of perfluoromethylheptane sulfonate isomers (Sm-PFOS), 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-FOSAA), 2-(N-ethyl perfluorooctane sulfonate l-perfluorooctane sulfonamido) acetate (Et-FOSAA), perfluorobutanoate (PFBA), perfluoropentanoate (PFPeA), perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), linear PFOA (n-PFOA), sum of branched PFOA isomers (Sb-PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA), tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoroproppoxy)-propanoate (HFPO-DA), dodecafluoro-3H-4,8-dioxanoate (NADONA), 9-chlorohexadecafluoro-3-oxanonane-1-sulfonate (9CL-PF) (Table 3 and Supplementary Table 1). One hundred μL aliquots of serum were diluted with formic acid and processed with online solid phase extraction coupled to HPLC-MS/MS; the limit of detection (LOD) was 0.1 ng/mL for all PFAS. Personnel conducting the laboratory analysis were blinded to the identity of the participant providing the specimen. Additional information on laboratory analysis performed at the CDC can be found in Kato et al. 2018.
Table 3.
Summary statistics for selected measured serum PFAS concentrations detected in >80% of study participants (ng/mL; N=213) compared to those measured in NHANES, 2015–20161.
| PFAS | This Study | NHANES, 2015–2016 | |||
|---|---|---|---|---|---|
| Detection frequency1 | Median (IQR) | 90th Percentile | Median | 90th Percentile | |
| PFHxS | 99.5 | 14.8 (7.4–30.9) | 49.7 | 1.20 | 3.40 |
| Total PFOS | 99.5 | 9.7 (5.6–16.7) | 28.1 | 4.80 | 13.2 |
| Total PFOA2 | 99.5 | 3.0 (1.5–5.0) | 7.4 | 1.57 | 3.37 |
| PFNA | 99.5 | 0.4 (0.3–0.6) | 0.8 | 0.60 | 1.40 |
| PFHpS | 82.6 | 0.2 (0.1–0.4) | 0.6 | N/A | N/A |
Abbreviations: PFAS, poly- and perfluoroalkyl substances; N, number of observations; IQR, interquartile range; ng/mL, nanograms per milliliter; PFHxS, perfluorohexane sulfonate; n-PFOS, linear perfluorooctane sulfonate; Sm-PFOS, sum of perfluoromethylheptane sulfonate isomers; Total PFOS, sum of n-PFOS and Sm-PFOS; n-PFOA, linear perfluorooctanoate; Total PFOA, sum of linear and branched perfluorooctanoate; PFNA, perfluorononanoate; PFHpS, perfluoroheptane sulfonate; N/A, not addressed.
Limit of detection (LOD) for all compounds is 0.1 ng/mL.
There were no branched perfluorooctanoates (PFOA) detected in any samples, therefore the “Total PFOA” value is equal to the concentration of linear PFOA.
Statistical Analysis
All study data were input into and managed using REDCap (Research Electronic Data Capture) tools hosted at the University of Colorado Denver (Harris et al. 2009).
Summary statistics including median, interquartile range (IQR), and 90th percentiles were computed for all PFAS measured. Spearman’s Rank test was conducted to evaluate pairwise correlations among PFAS. Total PFOA and Total PFOS were computed as the sum of the linear and branched isomers where Total PFOA=n-PFOA+sb-PFOA and Total PFOS=n-PFOS + Sm-PFOS. We initially analyzed n-PFOS and Sm-PFOS separately and as a sum (Total PFOS) to evaluate if the linear and branched isomers behaved differently from one another. However, findings between the two were generally consistent (supplementary tables 2, 4–7), therefore, in this work we only present the analyses for Total PFOS, or PFOS. No samples had detectable concentrations of Sb-PFOA, therefore, Total PFOA is equal to n-PFOA and in the remainder of this work is referred to as Total PFOA, or PFOA. Additional statistical analyses were only conducted for PFAS with a detection frequency of greater than 50%, i.e., the following five PFAS: PFHxS, Total PFOS, Total PFOA, PFNA and PFHpS. Detection frequency for the other PFAS ranged from 0% to 44.6% (Supplementary Table 1).
Unadjusted associations between each of these five PFAS concentration and each of several behavioral and demographic predictors were analyzed using 1) linear or Tobit (for PFAS with <85% detectable concentration-PFHpS only) regression for natural log-transformed continuous predictors and 2) nonparametric Kruskal-Wallis rank-sum tests to determine differences between groups for categorical predictors. Dunn’s test with Holm-Šidák adjustment for multiple comparisons was conducted as a post-hoc test for the Kruskal-Wallis analysis (Dinno 2015; Holm 1979).
All of the aforementioned PFAS that were included in the analysis, with the exception of PFHpS, were detected in 212 of 213 samples. For the one sample out of 213 with PFAS concentrations below the LOD, one half the LOD (0.05) was substituted (Eastoe 2006) and linear regression was performed for evaluating associations with continuous predictors. For PFHpS, because ~18% of samples had concentrations below the LOD, Tobit regression was performed to account for the left censored data (Lubin 2004). Kruskal-Wallis was used to test for differences in serum concentrations between categories of predictors (Helsel 2005). Additionally, a multiple linear regression model was fit to evaluate if smoking history predicts PFAS concentrations independent of age, sex, race/ethnicity, water district or residence and bottled water consumption. The covariates included in the multiple regression model were those that were either a) likely confounders associated with both smoking and serum PFAS or b) highly associated with just the outcome, serum PFAS concentration. All PFAS serum concentrations were log-normally distributed and were therefore natural-log transformed to better approximate a normal distribution or analyzed using nonparametric statistics.
All statistical analyses were conducted using the statistical software Stata (Version 15, StataCorp, College Station, TX, USA). The significance levels for all statistical analyses were set at p<0.05.
Results:
Study population demographics and behavioral characteristics
The original study population consisted of 220 adults recruited from the Fountain, Security and Widefield, Colorado area. Seven participants were excluded from the analysis because they did not live in the area for at least two years during the period of known contamination (August, 2012 to August, 2015), resulting in 213 individuals being included in this analysis. Table 1 shows the baseline characteristics reported by participants via questionnaire. The population was largely female (62%), age greater than 50 (75.1%), non-Hispanic white (74.6%), never smokers (61.5%), and served by the public water systems (92.5%). About half of the participants reported current employment (55.9%) and about a quarter (23%) reported their 2012–2015 workplace was served by one of the three contaminated drinking water systems. Furthermore, 30.5% reported ever having served in the military and 8.0% reported ever working as a firefighter.
Table 1.
Selected baseline characteristics of study population by water district of residence1 (N=213).
| Characteristics | Total N 213 (%) | Fountain 52 (%) | Security 96 (%) | Widefield 65 (%) |
|---|---|---|---|---|
| Sex | ||||
| Female | 132 (62.0) | 31 (59.6) | 60 (62.5) | 41 (63.1) |
| Male | 81 (38.0) | 21 (40.4) | 36 (37.5) | 24 (36.9) |
| Age (years) | ||||
| 19 to 35 | 17 (8.0) | 5 (9.6) | 10 (10.4) | 2 (3.1) |
| 36 to 50 | 36 (16.9) | 8 (15.4) | 19 (19.8) | 9 (13.8) |
| 51 to 65 | 82 (38.5) | 19 (36.5) | 38 (39.6) | 25 (38.5) |
| > 65 | 78 (36.6) | 20 (38.5) | 29 (30.2) | 29 (44.6) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 159 (74.6) | 45 (86.5) | 70 (72.9) | 44 (67.7) |
| Non-Hispanic Non-White | 27 (12.7) | 4 (7.7) | 12 (12.5) | 11 (16.9) |
| Hispanic | 27 (12.7) | 3 (5.8) | 14 (14.6) | 10 (15.4) |
| BMI (kg/m2) | ||||
| Normal (18.5–24.9) | 48 (22.5) | 21 (40.4) | 16 (16.7) | 11 (16.9) |
| Overweight (25.0–29.9) | 94 (44.1) | 15 (28.9) | 48 (50.0) | 31 (47.7) |
| Obese (> 30.0) | 71 (33.8) | 16 (30.8) | 32 (33.3) | 23 (35.4) |
| Smoking Status | ||||
| Past Smoker | 82 (38.5) | 14 (26.9) | 39 (40.6) | 29 (44.6) |
| Never Smoker | 131 (61.5) | 38 (73.1) | 57 (59.4) | 36 (55.4) |
| Currently employed | ||||
| Yes | 119 (55.9) | 29 (55.8) | 60 (62.5) | 30 (46.2) |
| Military service (ever) | ||||
| Yes | 65 (30.5) | 14 (26.9) | 33 (34.4) | 18 (27.7) |
| Firefighter (ever) | ||||
| Yes | 17 (8.0) | 4 (7.7) | 6(6.3) | 7 (10.8) |
| 2012–2015 workplace served by contaminated water district | ||||
| Yes | 49 (23.0) | 19 (36.5) | 21 (21.9) | 9 (13.9) |
| Source of water at home (years 2012–2015) | ||||
| Public water system | 197 (92.5) | 42 (80.8) | 91 (94.8) | 64 (98.5) |
| Private well | 16 (7.5) | 10 (19.2) | 5 (5.2) | 1 (1.5) |
Town that participant lived in during known exposure period of August 1st, 2012 to August 1st, 2015. If participant lived in multiple towns during this time period the assigned town is the one they lived in the longest.
Table 2 shows self-reported behaviors evaluated as potential predictors of exposure. These factors include bottled water use, vacuuming and dusting frequencies, and frequency of fast food and microwave meal consumption. Some responses were condensed into fewer groups due to small cell sizes. For example, fewer than five individuals each stated that they never vacuumed or that they never dusted, so the “never” and “once a month” answers were combined into one group. More than half of participants reported vacuuming (67.8%), dusting (84.1%), consuming fast food (81.2%) and consuming microwave meals (84.1%) at frequencies of once a week or less, and about half of the participants (48.8%) reported drinking mostly or only tap water.
Table 2.
Selected self-reported behaviors of the study population by water district of residence1 (N=213).
| Behavior | Total 213(%) | Fountain 52 (%) | Security 96 (%) | Widefield 65 (%) |
|---|---|---|---|---|
| Bottled water use (as of date questionnaire was administered)2 | ||||
| Mostly/only tap | 104 (48.8) | 26 (50.0) | 45 (46.9) | 33 (50.8) |
| Half tap/half bottled | 30 (14.1) | 9 (17.3) | 15 (15.6) | 6 (9.2) |
| Mostly/only bottled | 79 (37.0) | 17 (32.7) | 36 (37.5) | 26 (40.0) |
| Vacuuming frequency3 | ||||
| Never/once a month | 37 (17.4) | 13 (25.0) | 15 (15.6) | 9(13.9) |
| Once a week | 107 (50.2) | 27 (51.9) | 50 (52.1) | 30 (46.2) |
| > once a week | 69 (32.4) | 12 (23.1) | 31 (32.3) | 26 (40.0) |
| Dusting frequency3 | ||||
| Never/once a month | 90 (42.3) | 25 (48.1) | 44 (45.8) | 21 (32.3) |
| Once a week | 89 (41.8) | 20 (38.5) | 35 (36.5) | 34 (52.3) |
| > once a week | 33 (15.5) | 6 (11.5) | 17 (17.7) | 10 (15.4) |
| Missing | 1 (0.5) | 1 (1.9) | 0 (0.0) | 0 (0.0) |
| Fast food consumption frequency3 | ||||
| Never | 39 (18.3) | 12 (23.1) | 15 (15.6) | 12 (18.5) |
| Once a month | 65 (30.5) | 19 (36.5) | 31 (32.3) | 15 (23.1) |
| Once a week | 69 (32.4) | 12 (23.1) | 29 (30.2) | 28 (43.1) |
| > once a week | 39 (18.3) | 9 (17.3) | 21 (21.9) | 9 (13.9) |
| Missing | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Microwave meal consumption frequency3 | ||||
| Never | 96 (45.1) | 26 (50.0) | 42 (43.8) | 28 (43.1) |
| Once a month | 66 (31.0) | 17 (32.7) | 29 (30.2) | 20 (30.8) |
| Once a week | 17 (8.0) | 5 (9.6) | 10 (10.4) | 2 (3.1) |
| > once a week | 34 (16.0) | 4 (7.7) | 15 (15.6) | 15 (23.1) |
Town that participant lived in during known exposure period of August 1st, 2012 to August 1st, 2015. If participant lived in multiple towns during this time period the assigned town is the one they lived in the longest.
Participants were asked where they get the water that they currently use for drinking.
For questions of frequency, participants were asked to give their best estimate of their behaviors over the past year.
Serum PFAS concentrations
Table 3 shows summary statistics for the five PFAS included in the analysis. PFHxS, PFOS, PFOA and PFNA were detected in 99.5% of samples, and PFHpS was detected in 82.6%. While we initially analyzed n-PFOS and Sm-PFOS separately and as a sum (Total PFOS) to evaluate if the isomers behaved differently from one another, findings were generally consistent regardless of the way we treated the isomers (supplementary tables 2, 4–7), so only analyses for Total PFOS are presented in the main tables. However, it is worth noting that some of the water-related associations are stronger for n-PFOS than for Sm-PFOS (Supplementary Table 7).
The PFAS quantified at the highest serum concentration in this population was PFHxS. For PFHxS, the median and 90th percentile were 14.8 ng/mL and 49.7 ng/mL, respectively, which are 12 and 15 times as high as the U.S. median (1.2 ng/mL) and 90th percentile (3.40 ng/mL) observed in the 2015–2016 National Health and Nutrition Examination Survey (NHANES) (CDC 2019). Similarly, the PFAS-AWARE PFOS median (9.7 ng/mL) and 90th percentile (28.1 ng/mL) were 2.0 and 2.1 times as high as the NHANES median (4.80 ng/ml) and 90th percentile (13.2 ng/ml). PFOA concentrations were also about twice as high in this study population (median 3.0 ng/mL, 90th percentile 7.4 ng/mL) compared to NHANES (median 1.57 ng/mL, 90th percentile 3.37 ng/mL), while PFNA concentrations were lower (median 0.4 ng/mL, 90th percentile 0.8 ng/mL) than those seen in NHANES (median 0.6 ng/mL, 90th percentile 1.4 ng/mL). No information is available in NHANES for PFHpS. However, in 50 serum samples purchased from a commercial lab in 2016, Kato et al. found a serum PFHpS median of 0.3 ng/mL and 90th percentile of 0.7 ng/mL, concentrations quite similar to the median (0.2 ng/mL) and 90th percentile (0.6 ng/mL) observed in this study population (Kato et al., 2018).
Spearman’s rank correlation analysis (rs, Supplementary Table 2) shows that all compounds are moderately to strongly correlated with one another (p<0.05). The most highly correlated compounds are PFHpS with PFHxS (rs =0.87), PFOS (rs =0.87) and PFOA (rs =0.82), and PFOA with PFHxS (rs =0.85). While still significant, the least correlated are PFNA with PFHxS (rs =0.25), PFHpS (rs =0.42) and PFOA (rs =0.48).
Demographic and behavioral predictors of PFAS
All serum PFAS concentrations were significantly positively associated with age (Table 4). Age was most highly correlated with PFOS (R2=0.19) followed by PFHxS (R2=0.12) and PFOA (R2=0.10) and least strongly correlated with PFNA (R2=0.06). Tobits regression showed that PFHpS correlation was in the same range as PFHxS and PFOA (pseudo R2 of 0.11).
Table 4.
Unadjusted associations between age and select serum PFAS1 (N=213).
| PFAS | Percent Change in PFAS for each 1-year increase in age | ||
|---|---|---|---|
| Coefficient (95% CI) | R2 (adj.) | p-value | |
| PFHxS | 2.63 (1.71, 3.67) | 0.12 | < 0.001 |
| Total PFOS | 2.63 (1.92, 3.46) | 0.19 | < 0.001 |
| Total PFOA1 | 1.82 (1.11, 2.53) | 0.10 | < 0.001 |
| PFNA | 1.11 (0.50, 1.61) | 0.06 | < 0.001 |
| PFHpS2 | 3.15 (2.33, 3.87) | 0.11 | < 0.001 |
Abbreviations: PFAS, poly- and perfluoroalkyl substance; N, number of observations; CI, confidence interval; R2(adj.), adjusted coefficient of determination; PFHxS, perfluorohexane sulfonate; Total PFOS, sum of linear perfluorooctane sulfonate and sum of perfluoromethylheptane sulfonate isomers; PFOA, sum of linear and branched perfluorooctanoate; PFNA, perfluorononanoate; PFHpS, perfluoroheptane sulfonate.
PFAS were natural log-transformed for the analysis, however, results presented above have been back transformed and represent the percent change.
There were no branched perfluorooctanoates (PFOA) detected in any samples, therefore the “Total PFOA” value is equal to the concentration of linear PFOA.
Computed using Tobit regression to account for ~18% left-censored data.
Tables 5 and 6 show the associations between categorical predictors and serum PFAS concentrations. Serum PFAS concentrations varied significantly between groups for water district of residence and bottled water consumption. There was no significant difference in serum concentrations between individuals living on private wells versus those served by a public water system. However, most private well-owners lived in Fountain (62.5%), and Fountain residents had lower PFAS serum concentrations than residents of Widefield or Security. Furthermore, a quarter of private well owners reported drinking mostly or only bottled water, and ~19% reported use of either granular activated carbon or reverse osmosis treatment systems (Supplementary Table 3).
Table 5:
Categorical predictors of select serum PFAS concentrations (ng/mL; N=213).
| Characteristic | PFHxS | Total PFOS | Total PFOA1 | PFNA | PFHpS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | GM | 95% CI | GM | 95% CI | GM | 95% CI | GM | 95% CI | GM | 95% CI | |
| Home water district | |||||||||||
| Fountain | 52 | 9.3 | 7.0, 12.2 | 7.2 | 5.9, 8.8 | 2.4 | 2.0, 2.9 | 0.42 | 0.35, 0.51 | 0.16 | 0.12, 0.21 |
| Security | 96 | 19.1 | 15.0, 24.4 | 10.0 | 8.2, 12.2 | 3.1 | 2.6, 3.7 | 0.42 | 0.37, 0.48 | 0.25 | 0.20, 0.30 |
| Widefield | 65 | 13.7 | 11.0, 17.1 | 11.0 | 9.0, 13.4 | 2.9 | 2.4, 3.6 | 0.46 | 0.40, 0.54 | 0.22 | 0.18, 0.28 |
| p-value | < 0.001 | 0.006 | 0.05 | 0.48 | 0.03 | ||||||
| Source of water at home (years 2012–2015) | |||||||||||
| Public water system | 197 | 14.1 | 12.1, 16.5 | 9.3 | 8.2, 10.5 | 2.8 | 2.5, 3.1 | 0.43 | 0.39, 0.47 | 0.21 | 0.18, 0.24 |
| Private well | 16 | 19.3 | 10.2, 36.8 | 12.8 | 8.7, 18.8 | 3.9 | 2.7, 5.6 | 0.48 | 0.34, 0.67 | 0.31 | 0.18, 0.51 |
| p-value | 0.34 | 0.16 | 0.09 | 0.68 | 0.15 | ||||||
| Bottled water use (as of date questionnaire was administered) | |||||||||||
| Mostly/only tap | 104 | 18.9 | 15.3, 23.3 | 10.7 | 9.2, 12.5 | 3.4 | 3.0, 4.0 | 0.45 | 0.40, 0.51 | 0.25 | 0.21, 0.30 |
| Half tap/half bottled | 30 | 12.4 | 8.6, 17.9 | 9.5 | 7.1, 12.7 | 2.4 | 1.7, 3.3 | 0.49 | 0.37, 0.64 | 0.19 | 0.13, 0.27 |
| Mostly/only bottled | 79 | 10.8 | 8.5, 13.8 | 8.1 | 6.5, 10.1 | 2.4 | 2.2, 2.9 | 0.40 | 0.35, 0.45 | 0.19 | 0.15, 0.23 |
| p-value | < 0.001 | 0.06 | 0.002 | 0.19 | 0.06 | ||||||
| 2012–2015 workplace served by contaminated water district | |||||||||||
| No | 164 | 14.5 | 12.2, 17.2 | 9.8 | 8.5, 11.2 | 2.9 | 2.5, 3.3 | 0.46 | 0.41, 0.51 | 0.22 | 0.19, 0.25 |
| Yes | 49 | 14.0 | 10.4, 18.8 | 8.4 | 6.6, 10.6 | 2.7 | 2.1, 3.4 | 0.36 | 0.31, 0.43 | 0.20 | 0.15, 0.27 |
| p-value | 0.72 | 0.18 | 0.52 | 0.003 | 0.67 | ||||||
| Sex | |||||||||||
| Female | 132 | 14.3 | 12.1, 17.1 | 8.4 | 7.3, 9.6 | 2.8 | 2.5, 3.2 | 0.43 | 0.38, 0.48 | 0.19 | 0.16, 0.23 |
| Male | 81 | 14.7 | 11.2, 19.3 | 11.7 | 9.4, 14.5 | 2.9 | 2.4, 3.6 | 0.44 | 0.39, 0.50 | 0.26 | 0.21, 0.32 |
| p-value | 0.52 | < 0.001 | 0.42 | 0.40 | 0.03 | ||||||
| Race/Ethnicity | |||||||||||
| NH White | 159 | 16.0 | 13.4, 19.0 | 9.9 | 8.6, 11.4 | 3.1 | 2.7, 3.5 | 0.43 | 0.39, 0.47 | 0.23 | 0.20, 0.27 |
| NH Non-White | 27 | 12.3 | 8.1, 18.7 | 9.7 | 7.8, 12.0 | 2.1 | 1.5, 2.8 | 0.47 | 0.37, 0.60 | 0.21 | 0.15, 0.29 |
| Hispanic | 27 | 9.5 | 6.3, 14.3 | 7.3 | 5.1, 10.4 | 2.3 | 1.7, 3.2 | 0.43 | 0.32, 0.57 | 0.15 | 0.10, 0.22 |
| p-value | 0.01 | 0.08 | 0.002 | 0.79 | 0.04 | ||||||
| Smoking status | |||||||||||
| Past smoker | 82 | 20.9 | 17.2, 25.4 | 12.7 | 10.8, 15.0 | 3.5 | 3.1, 4.1 | 0.45 | 0.39, 0.51 | 0.29 | 0.24, 0.35 |
| Never smoker | 131 | 11.5 | 9.4, 14.0 | 7.9 | 6.8, 9.2 | 2.5 | 2.1, 2.9 | 0.43 | 0.38, 0.48 | 0.18 | 0.15, 0.21 |
| p-value | < 0.001 | < 0.001 | 0.002 | 0.34 | < 0.001 | ||||||
| BMI category | |||||||||||
| Normal | 48 | 14.0 | 10.3, 19.0 | 10.0 | 8.1, 12.5 | 3.2 | 2.6, 3.9 | 0.49 | 0.41, 0.59 | 0.21 | 0.16, 0.28 |
| Overweight | 94 | 15.1 | 11.8, 19.4 | 9.7 | 7.9, 11.8 | 2.6 | 2.2, 3.2 | 0.40 | 0.35, 0.45 | 0.23 | 0.19, 0.28 |
| Obese | 71 | 14.0 | 11.1, 17.6 | 9.0 | 7.5, 10.8 | 2.9 | 2.5, 3.5 | 0.45 | 0.39, 0.53 | 0.20 | 0.16, 0.25 |
| p-value | 0.61 | 0.52 | 0.72 | 0.44 | 0.57 | ||||||
| Firefighter (ever) | |||||||||||
| No | 196 | 14.3 | 12.3, 16.8 | 9.2 | 8.1, 10.4 | 2.8 | 2.5, 3.2 | 0.43 | 0.39, 0.47 | 0.21 | 0.19, 0.24 |
| Yes | 17 | 16.0 | 9.9, 25.8 | 14.0 | 10.4, 19.0 | 3.1 | 2.2, 4.3 | 0.47 | 0.38, 0.58 | 0.25 | 0.17, 0.38 |
| p-value | 0.83 | 0.03 | 0.80 | 0.66 | 0.43 | ||||||
| Military (Ever) | |||||||||||
| No | 148 | 14.3 | 11.9, 17.2 | 8.7 | 7.5, 10.0 | 2.9 | 2.5, 3.3 | 0.43 | 0.39, 0.48 | 0.21 | 0.18 0.24 |
| Yes | 65 | 14.9 | 11.5, 19.3 | 11.8 | 9.7, 14.3 | 2.8 | 2.3, 3.4 | 0.44 | 0.38, 0.51 | 0.24 | 0.20, 0.30 |
| p-value | 0.89 | 0.02 | 0.97 | 0.29 | 0.23 | ||||||
| Vacuuming frequency | |||||||||||
| Never/once a month | 37 | 15.1 | 10.6, 21.4 | 9.5 | 7.3, 12.3 | 3.2 | 2.5, 4.1 | 0.43 | 0.37, 0.49 | 0.21 | 0.15, 0.28 |
| Once a week | 107 | 14.5 | 11.6, 18.1 | 9.3 | 7.7, 11.1 | 2.8 | 2.4, 3.3 | 0.44 | 0.38, 0.50 | 0.22 | 0.19, 0.27 |
| > once a week | 69 | 14.1 | 11.1, 18.1 | 9.9 | 8.2, 12.0 | 2.7 | 2.3, 3.3 | 0.43 | 0.38, 0.50 | 0.21 | 0.17, 0.27 |
| p-value | 0.80 | 0.90 | 0.51 | 0.95 | 0.83 | ||||||
| Dusting frequency | |||||||||||
| Never/once a month | 90 | 13.7 | 10.7, 17.6 | 8.3 | 6.8, 10.1 | 2.7 | 2.3, 3.3 | 0.43 | 0.37, 0.49 | 0.20 | 0.16, 0.24 |
| Once a week | 89 | 14.4 | 11.6, 17.9 | 10.7 | 9.0, 12.7 | 2.9 | 2.4, 3.4 | 0.44 | 0.39, 0.50 | 0.23 | 0.19, 0.29 |
| > once a week | 33 | 18.8 | 13.8, 25.6 | 10.9 | 8.5, 13.8 | 3.4 | 2.8, 4.1 | 0.44 | 0.36, 0.54 | 0.24 | 0.19, 0.31 |
| p-value | 0.35 | 0.10 | 0.48 | 0.89 | 0.23 | ||||||
| Fast food consumption frequency | |||||||||||
| Never | 39 | 17.1 | 11.6, 25.2 | 10.2 | 7.9, 13.3 | 3.3 | 2.6, 4.2 | 0.44 | 0.36, 0.53 | 0.24 | 0.17, 0.34 |
| Once a month | 65 | 14.5 | 11.4, 18.5 | 9.6 | 7.8, 11.7 | 2.9 | 2.5, 3.5 | 0.43 | 0.36, 0.50 | 0.22 | 0.18, 0.28 |
| Once a week | 69 | 15.2 | 12.0, 19.2 | 10.4 | 8.5, 12.6 | 2.9 | 2.4, 3.5 | 0.47 | 0.41, 0.55 | 0.22 | 0.18, 0.28 |
| > once a week | 39 | 11.4 | 7.3, 17.7 | 7.7 | 5.4, 10.9 | 2.3 | 1.7, 3.3 | 0.38 | 0.30, 0.48 | 0.18 | 0.14, 0.24 |
| p-value | 0.82 | 0.61 | 0.65 | 0.76 | 0.54 | ||||||
| Microwave meal consumption frequency | |||||||||||
| Never | 96 | 15.4 | 12.4, 19.1 | 9.7 | 8.3, 11.2 | 2.8 | 2.4, 3.3 | 0.44 | 0.39, 0.49 | 0.22 | 0.18, 0.27 |
| Once a month | 66 | 13.6 | 10.6, 17.4 | 10.0 | 8.2, 12.1 | 2.9 | 2.5, 3.5 | 0.45 | 0.39, 0.53 | 0.21 | 0.17, 0.26 |
| Once a week | 17 | 16.5 | 9.9, 27.5 | 8.6 | 5.5, 13.5 | 2.9 | 2.1, 4.2 | 0.37 | 0.28, 0.50 | 0.20 | 0.11, 0.35 |
| > once a week | 34 | 12.9 | 7.9, 20.9 | 8.7 | 5.5, 13.7 | 2.7 | 1.8, 3.9 | 0.41 | 0.31, 0.56 | 0.23 | 0.16, 0.31 |
| p-value | 0.94 | 0.89 | 1.00 | 0.55 | 0.93 | ||||||
Abbreviations: PFAS, poly- and perfluoroalkyl substance; N, number of observations; GM, geometric mean, CI, confidence interval; PFHxS, perfluorohexane sulfonate; Total PFOS, sum of linear perfluorooctane sulfonate and sum of perfluoromethylheptane sulfonate isomers; Total PFOA, sum of linear and branched perfluorooctanoate; PFNA, perfluorononanoate; PFHpS, perfluoroheptane sulfonate; NH, non-Hispanic.
There were no branched perfluorooctanoates (PFOA) detected in any samples, therefore the “Total PFOA” value represents just the linear PFOA.
p-value from Kruskal-Wallis analysis of variance test.
Table 6.
Results of Dunn’s test post hoc analysis with a Holm– Šidák adjustment for serum PFAS (N=213).
| Dunn’s Multiple Comparison Test | PFHxS | Total PFOS | Total PFOA | PFNA | PFHpS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Difference in Rank Sum | p-value | Difference in Rank Sum | p-value | Difference in Rank Sum | p-value | Difference in Rank Sum | p-value | Difference in Rank Sum | p-value | |
| Home water district | ||||||||||
| Fountain vs. Security | −4.26 | < 0.001 | −2.72 | 0.007 | −2.41 | 0.02 | −0.56 | 0.29 | −2.62 | 0.01 |
| Fountain vs. Widefield | −1.87 | 0.03 | −2.96 | 0.005 | −1.71 | 0.09 | −1.20 | 0.31 | −1.62 | 0.10 |
| Security vs Widefield | 2.40 | 0.02 | −0.52 | 0.30 | 0.60 | 0.27 | −0.79 | 0.38 | 0.93 | 0.18 |
| Bottled water use (as of date questionnaire was administered) | ||||||||||
| Mostly/only tap vs. Half tap/half bottled | 2.34 | 0.02 | 0.64 | 0.26 | 2.16 | 0.03 | −0.59 | 0.28 | 1.32 | 0.18 |
| Mostly/only tap vs. mostly/only bottled | 3.66 | < 0.001 | 2.38 | 0.03 | 3.31 | 0.001 | 1.46 | 0.14 | 2.31 | 0.03 |
| Half tap/half bottled vs. mostly/only bottled | 0.29 | 0.39 | 1.04 | 0.28 | 0.22 | 0.41 | 1.59 | 0.16 | 0.34 | 0.37 |
| 2012–2015 workplace served by contaminated water district | ||||||||||
| No vs. Yes | 0.36 | 0.36 | 1.33 | 0.09 | 0.64 | 0.26 | 3.00 | 0.001 | 0.42 | 0.33 |
| Sex | ||||||||||
| Female vs. Male | −0.65 | 0.26 | −3.40 | < 0.001 | −0.81 | 0.21 | −0.84 | 0.20 | −2.20 | 0.01 |
| Race/Ethnicity | ||||||||||
| NH White vs. Hispanic | 2.74 | 0.009 | 2.25 | 0.04 | 2.52 | 0.01 | −0.17 | 0.43 | 2.57 | 0.02 |
| NH White vs. NH Non-White | 1.66 | 0.09 | 0.39 | 0.35 | 2.86 | 0.006 | −0.69 | 0.57 | 0.55 | 0.29 |
| Hispanic vs. NH Non-White | −0.82 | 0.21 | −1.42 | 0.15 | 0.26 | 0.40 | −0.40 | 0.57 | −1.54 | 0.12 |
| Smoking Status | ||||||||||
| Never vs. Former | −4.14 | < 0.001 | −3.94 | < 0.001 | −3.06 | 0.001 | −0.95 | 0.17 | −3.64 | < 0.001 |
| Firefighter (ever) | ||||||||||
| No vs. Yes | −0.22 | 0.41 | −2.21 | 0.01 | −0.25 | 0.40 | −0.45 | 0.33 | −0.78 | 0.22 |
| Military (Ever) | ||||||||||
| No vs. Yes | −0.13 | 0.45 | −2.41 | 0.008 | −0.04 | 0.48 | −1.05 | 0.15 | −1.20 | 0.11 |
Abbreviations: PFAS, poly- and perfluoroalkyl substances; N, number of observations; NH, non-Hispanic; PFHxS, perfluorohexane sulfonate; Total PFOS, sum of linear perfluorooctane sulfonate and sum of perfluoromethylheptane sulfonate isomers; Total PFOA, Sum of linear and branched perfluorooctanoate; PFNA, perfluorononanoate; PFHpS, perfluoroheptane sulfonate
There were no branched perfluorooctanoates (PFOA) detected in any samples, therefore the “Total PFOA” value represents just the linear PFOA.
Evaluation of demographic characteristics showed significant differences in serum PFAS concentrations between groups for sex, race/ethnicity, smoking status and employment as a firefighter or service in the military. No significant differences were observed by BMI category or by frequency of any of the following behaviors: vacuuming, dusting, fast food consumption, and microwave meal consumption.
Categorical Predictors of PFHxS
Serum concentrations of PFHxS were significantly higher in those that lived in the Security water district (Geometric Mean (GM), 19.1 ng/mL) compared to either Fountain (GM, 9.3 ng/mL) or Widefield (GM, 13.7 ng/mL) water district residents. PFHxS concentrations were also significantly higher in those that reported drinking mostly or only tap water (GM, 18.9 ng/mL) compared to individuals who reported drinking either half bottled and half tap (GM, 12.4 ng/mL), or, only or mostly bottled water (GM, 10.8 ng/mL) (Table 6). Serum concentrations of PFHxS also varied significantly by race/ethnicity and smoking status: non-Hispanic whites (GM, 16.0 ng/mL) and former-smokers (GM, 20.9 ng/mL) had higher serum PFHxS concentrations than Hispanics (GM, 9.5 ng/mL) and never-smokers (GM, 11.5 ng/mL), respectively.
Categorical Predictors of PFOS
Residents of the Fountain water district had significantly lower concentrations of PFOS (GM, 7.2 ng/mL) than either Security water district residents (GM, 10.0 ng/mL) or Widefield water district residents (GM, 11.0 ng/mL). PFOS concentrations also differed by sex and smoking history: females (GM, 8.4 ng/mL) and never smokers (GM, 7.9 ng/mL) had significantly lower serum concentrations than males (GM, 11.7 ng/mL) and former smokers (GM, 12.7 ng/mL). Additionally for PFOS, serum concentrations were significantly different by firefighter employment and military service. Individuals who were never a firefighter (GM, 9.2 ng/mL) or had never served in the military (GM, 8.7 ng/mL) had significantly lower concentrations of PFOS than those who had (GM firefighter, 14.0 ng/mL; GM military, 11.8 ng/mL).
Categorical Predictors of PFOA
While serum PFOA concentrations did not vary significantly by water district of residence, they were significantly higher in those reporting drinking only or mostly tap-water (GM, 3.4 ng/mL) compared to those reporting drinking only or mostly bottled water (GM, 2.4 ng/mL). Further, serum PFOA concentrations were significantly higher for non-Hispanic whites (GM, 3.1 ng/mL) compared to other racial/ethnic groups (GM Hispanic, 2.3 ng/mL; GM non-Hispanic non-white, 2.1 ng/mL). Serum PFOA concentrations also differed significantly by smoking history, with former smokers (GM, 3.5 ng/mL) having higher serum concentrations than never smokers (GM, 2.5 ng/mL).
Categorical Predictors of PFHpS
Residents of the Fountain water district had significantly lower concentrations of PFHpS (GM, 0.16 ng/mL) than those living in the Security water district (GM, 0.25 ng/mL). In addition, females (GM, 0.19 ng/mL), Hispanics (GM, 0.15 ng/mL) and never smokers (GM, 0.18 ng/mL) had significantly lower serum PFHpS concentrations than males (GM, 0.26 ng/mL), non-Hispanic whites (GM, 0.23 ng/mL), and former smokers (GM, 0.29 ng/mL), respectively.
Categorical Predictors of PFNA
Serum PFNA concentrations were not significantly different across groups for any predictors evaluated other than location of participant’s 2012–2015 workplace. Individuals reporting a workplace outside of Fountain, Security or Widefield had significantly higher serum concentrations (GM, 0.46 ng/mL) than those reporting a workplace within one of the three PFAS-affected towns (GM, 0.36 ng/mL).
Multivariable Regression Results
Smoking history as a predictor of PFAS serum concentration was examined with a multivariable regression model including the following variables as predictors: age, sex, race/ethnicity, water district of residence and bottled water consumption (Table 7). Smoking history remained a significant predictor of serum concentrations for PFHxS only (β, 32.3 [0.20, 75.1], p-value=0.05).
Table 7:
Model estimates for multivariable regression analysis of smoking status, water district, bottled water use, age, sex and race/ethnicity as predictors of select serum PFAS1 (N=213).
| Variable | PFHxS | Total PFOS | Total PFOA2 | PFNA | PFHpS3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Smoking status4 | 32.3 (0.20, 75.1) | 0.05 | 20.9 (−2.5, 50.7) | 0.08 | 16.2 (−6.8, 43.3) | 0.18 | −4.2 (−20.5, 15.0) | 0.65 | 11.6 (−10.4, 37.7) | 0.34 |
| Home water district5 | ||||||||||
| Security | 116.0 (55.3, 200.0) | < 0.001 | 43.3 (11.6, 85.9) | 0.006 | 35.0 (4.3, 73.3) | 0.02 | 1.0 (−18.9, 25.9) | 0.92 | 66.5 (28.4, 116.0) | <0.001 |
| Widefield | 37.7 (−3.9, 97.4) | 0.08 | 41.9 (6.6, 87.8) | 0.02 | 19.7 (−9.4, 58.4) | 0.21 | 7.3 (−15.6, 36.3) | 0.58 | 25.9 (−5.2, 66.5) | 0.11 |
| Bottled water use (as of date questionnaire was administered)6 | ||||||||||
| Half tap/half bottled | −30.2 (−52.8, 3.2) | 0.07 | −3.9 (−29.5, 31.0) | 0.80 | −27.4 (−46.2, −1.7) | 0.04 | 8.5 (−16.5, 40.5) | 0.53 | −19.7 (−51.1, 9.2) | 0.16 |
| Mostly/only bottled | −37.5 (−52.8, −16.5) | 0.001 | −18.1 (−34.3, 2.8) | 0.09 | −24.4 (−39.3, −6.0) | 0.01 | −11.3 (−26.7, 6.9) | 0.20 | −18.1 (−34.3, 2.9) | 0.09 |
| Age | 2.3 (1.4, 3.4) | < 0.001 | 2.4 (1.7, 3.1) | < 0.001 | 1.6 (0.90, 2.3) | < 0.001 | 1.1 (0.50, 1.7) | 0.001 | 2.9 (2.2, 3.8) | < 0.001 |
| Sex7 | 0.30 (−22.9, 31.0) | 0.98 | 39.1 (12.7, 71.6) | 0.002 | 1.2 (−17.3, 24.6) | 0.91 | 3.5 (−13.1, 23.4) | 0.70 | 35.0 (9.9, 66.5) | 0.005 |
| Race/Ethnicity8 | ||||||||||
| NH-Nonwhite | −18.1 (−45.1, 20.9) | 0.32 | 0.20 (−26.7, 36.3) | 0.99 | −30.9 (−48.8, −5.9) | 0.02 | 12.7 (−13.1, 46.2) | 0.37 | −9.5 (−33.6, 23.4) | 0.53 |
| Hispanic | −28.1 (−51.8, 8.0) | 0.11 | −17.3 (−40.0, −11.3) | 0.23 | −13.9 (−36.9, 18.5) | 0.35 | 7.0 (−18.1, 40.5) | 0.62 | −24.4 (−45.7, 3.9) | 0.08 |
| R2 (adj.) | 0.25 | 0.27 | 0.17 | 0.04 | 0.17 | |||||
Abbreviations: PFAS, poly- and perfluoroalkyl substance; N, number of observations; CI, confidence interval; PFHxS, perfluorohexane sulfonate; Total PFOS, sum of linear perfluorooctane sulfonate and sum of perfluoromethylheptane sulfonate isomers; PFOA, Sum of linear and branched perfluorooctanoate; PFNA, perfluorononanoate; PFHpS, perfluoroheptane sulfonate; NH, Non-Hispanic; R2 (adj.), adjusted coefficient of determination;
PFAS were natural log-transformed for the analysis, however, results presented above have been back transformed and represent the percent change.
There were no branched perfluorooctanoates (PFOA) detected in any samples, therefore the “Total PFOA” value is equal to the concentration of linear PFOA.
Computed using Tobit regression to account for ~18% left-censored data.
Reference group for smoking status is never smoker.
Reference group for water district is Fountain.
Reference group for bottled water consumption is mostly/only tap water.
Reference group for sex is female.
Reference group for race/ethnicity is non-Hispanic white.
Discussion
Overall, participants in this study had higher-than U.S. national background serum concentrations of PFHxS, with the study median approximately twelve times as high as the national median. The population also had relatively high concentrations of PFOS and PFOA, with the study medians approximately twice as high as the national median. PFNA had concentrations slightly lower than those reported in the U.S. general population (CDC 2019). These findings are consistent with the suspected source of contamination (AFFF) as beginning in 1970 AFFF mixtures were typically dominated by sulfonates, including PFHxS, PFHpS and PFOS (Place 2012). Water district of residence and tap water versus bottled water consumption significantly predicted serum concentrations of PFHxS. Water district of residence also predicted PFOS and PFHpS concentrations, and tap vs bottled water use predicted PFOA.
Other predictors of PFHxS serum concentrations in this population are age, race/ethnicity, and smoking history. Additional factors that predicted higher PFOS, PFOA, and PFHpS serum concentrations in this population are age, male sex (PFOS and PFHpS), white non-Hispanic race/ethnicity (PFOA and PFHpS), history of smoking, and former firefighter or military employment (PFOS).
Serum concentrations varied by water district, with a clear trend showing higher concentrations closer to the likely source and lower concentrations further away from Peterson Air Force base (Figure 1). This trend follows the north to south concentration gradient along the aquifer, where Security wells had the highest measured levels of PFAS, followed by Widefield, and then Fountain (CDPHE 2018). To our knowledge, only one other U.S. study, conducted in New Hampshire, has focused on a community exposed specifically to AFFF-contaminated water (Daly et al., 2018). Daly et al. found results in agreement with ours, namely that the primary predictors of serum concentrations for PFHxS, PFOS and PFOA were related to water consumption. Another study conducted in Sweden also focused on an AFFF-impacted community, however, the purpose of that study was primarily to develop half-life estimates rather than to identify predictors of exposure (Li et al, 2017). Development of half-life estimates for this Colorado study population is to be completed following a second round of blood sampling which will take place approximately one year after the initial (June 2018) blood draw.
Participants who reported mostly or only drinking tap water had significantly higher concentrations of PFHxS and PFOA than those who reported mostly or only drinking bottled water. PFHxS is unique in that persons who drank mostly or only tap water had significantly higher concentrations than even individuals who reported drinking half bottled and half tap water, suggesting tap water as the primary source of exposure.
In addition, we found that age was positively associated with serum concentrations of all PFAS evaluated. This is in agreement with some of the current literature (Cho 2015; Zhang 2010; Herrick 2017) although others have not found such an association (Calafat 2007; Ericson 2007; Vassiliadou 2010; Kannan et al. 2004). As the length of time that the aquifer has been contaminated is presently unknown, it is unclear if the association with age is related to the number of years during which individuals drank contaminated water or the lifelong accumulation of PFAS from other sources. AFFF was used at Peterson Air Force base beginning in 1970, so it is probable that the aquifer was contaminated well before the EPA testing took place in 2013 (El Paso County Health Dept. 2017).
Serum concentrations of PFHxS, PFOA and PFHpS were significantly higher in non-Hispanic White participants than their Hispanic and non-Hispanic non-white (PFOA only) counterparts. Similarly, serum concentrations of PFOS were marginally higher in non-Hispanic whites compared to Hispanics. While the current literature on how race and ethnicity may influence serum PFAS concentrations is sparse, our findings that non-Hispanic white participants had higher PFAS serum concentrations than non-Hispanic non-white or Hispanic participants are consistent with the findings of studies evaluating relationships between maternal and child PFAS concentrations (Harris et al. 2017; Sagiv et al. 2015), the effect of pregnancy on serum PFAS concentrations (Jain 2013), as well as an analysis of NHANES data (Calafat 2007).
Several other studies have reported that males have higher concentrations of certain PFAS than females (Calafat 2007; Siebenaler 2017; Harada et al. 2004; Herrick et al. 2017; Ericson 2007; Góralczyk 2015; Vassiliadou 2010). One explanation for this finding may be that females have additional excretion pathways via menstruation, breastfeeding and childbirth (Yang 2016; Wong 2014; Lorber 2015; Liu 2011). Our study found that women had significantly lower concentrations than men of both PFOS and PFHpS, in agreement with some recent literature (Siebenaler 2017; Harada et al. 2004; Ericson 2007; Góralczyk 2015; Vassiliadou 2010). While we did not see statistically significant differences by sex with the other PFAS studied (including PFHxS), across all five PFAS evaluated, females had lower geometric mean serum concentrations than males. Other studies have also found no difference in serum concentrations or excretion rates between males and females for PFHxS (Haug 2009; Zhang 2010) PFOS (Haug 2009; Kannan et al. 2004; Olsen et al. 2004; Zhang 2010), PFOA (Haug 2009; Kannan et al. 2004; Zhang 2010), PFNA and PFHpS (Haug 2009; Zhang 2010).
Except for serum PFNA concentrations, in this study former smokers had significantly higher PFAS serum concentrations compared to never smokers. Multiple regression examining the influence of smoking status on PFAS concentrations independent of age, sex, race/ethnicity, water district of residence and bottled water consumption revealed that a significant relationship with smoking history remained for only PFHxS. This indicates that other factors may have been confounding the association seen between former smokers and serum concentrations of PFOS, PFOA and PFHpS. For example, men are more likely to smoke than women, and men also are more likely to have higher PFAS serum concentrations than women. To our knowledge, few other studies have evaluated smoking history as a predictor of serum PFAS concentrations. A Korean study found significantly higher serum PFAS concentrations in smokers compared to non-smokers (Cho 2015) as did a study using NHANES data for females (Jain 2013). However, another conducted in Wisconsin found no difference in serum concentrations based on smoking status (Christensen 2016) and another in Japan also found null results with regard to the influence of smoking (Harada et al. 2004). At present, there is limited literature on how a history of smoking may affect PFAS serum concentrations.
Individuals who reported ever being employed as a firefighter or ever having served in the military had significantly higher concentrations of PFOS compared to those without these potential occupational exposures. We speculate that these individuals have experienced work-related exposure to PFAS. PFOS is a compound known to be used in both military and firefighting operations (Rotander 2015; Posner 2012). While somewhat surprising that there was no notable difference for these groups in PFHxS serum concentrations, it is possible that limited occupational exposures were obscured by the relatively high non-occupational exposures to PFHxS in this population.
This study did not find behavioral factors, such as cleaning frequency and fast food consumption frequency, to be important predictors of serum PFAS concentrations. By contrast, a recent study conducted in North Carolina (Siebenaler 2017) found that serum PFHxS concentrations were significantly higher in individuals who vacuumed once per month or less versus in those who vacuumed greater than once per month, and in those who ate microwave meals greater than once per month versus those who never ate microwave meals. The reason for the discrepancy may be that the population evaluated by Siebenaler et al. experienced only background exposures from common sources. In contrast, for the highly exposed community in this study, the drinking water source appears to dominate exposure. It is likely that the contribution from other sources, such as food wrappings and dust, is relatively small in comparison to the contribution of drinking water in this population.
A limitation of this analysis is that it does not consider duration of exposure beyond the eligibility criteria of living in one of the affected communities for at least two years during the time period of known contamination (August 2012 to August 2015). In future studies, we plan to collect residential history information that will allow us to differentiate between long-term and short-term residents. In addition, we did not include some other factors related to drinking water exposure, such as filter use and quantity of water consumed per day.
Filter use was excluded from the analysis because many filter types (i.e., refrigerator filters) are ineffective at removing PFAS, and those that are effective (granular activated carbon and reverse osmosis) must be maintained regularly (New Hampshire Dept. of Env. Services 2016). We did not inquire about filter maintenance in our questionnaire. Further, whether or not filters reduced PFAS exposure from the AFFF in drinking water is highly dependent on when it was installed, and many participants could not recall this date with certainty. Filters installed after August 2015 (for example, in response to the announcement of lower EPA health advisories in 2016) likely did not alter the participant’s peak exposure compared to participants without filters in their homes. We also opted exclude the question on amount of water consumed per day due to uncertainly about whether or not this was truly predictive of exposure to contamination. As noted above, participants often had difficulty recalling when they had installed filters in their homes and the type of filters they had, so there was variation in how often the filters were reportedly used. Similarly, it is worth noting that our bottled water variable was defined as current bottled water use rather than bottled water use prior to PFAS mitigation efforts. Participants generally had difficulty recalling exactly when they switched from tap water to bottled water. Therefore, for our analyses we operated under the assumption that current bottled water use is a likely indicator of at least some amount of reduced tap-water consumption during the exposure period.
An additional limitation of this study is we did not collect socio-economic data in our questionnaires. Some previous studies have shown a relationship between serum PFAS levels and indicators of socioeconomic status (Harris et al. 2017; Kato et al. 2014; Sagiv et al. 2015).
One important strength of the PFAS-AWARE study is the variability in water PFAS levels and, consequently, serum concentrations, in the three impacted communities. In a future manuscript we will evaluate the effects of the wide range of PFAS exposure observed for multiple PFAS analytes. Secondly, PFAS-AWARE brings a new data to the broader PFAS literature because the key compound of interest in this study population is PFHxS rather than the more extensively studied PFOS and PFOA. This study is one of few evaluating a population highly exposed to PFHxS originating from AFFF in drinking water. Lastly, measurement of multiple PFAS allows us to better characterize this unique AFFF dominated exposure profile and to distinguish between predictors of PFAS that are likely AFFF-related from those that may primarily result from dietary (e.g. PFNA), residential, and other potential sources.
Conclusions:
In this highly exposed population the most important factors in predicting serum PFAS concentrations were home water district and frequency of bottled water use, as well as age, sex and smoking history. No significant associations were found for behaviors that may indicate routes of exposure other than drinking contaminated water, such as home cleaning frequency and fast food consumption. These findings support our hypothesis that for PFHxS, PFOS, PFOA and PFHpS, the dominant exposure route for individuals in these communities was likely consumption of PFAS-contaminated water.
Understanding the varied sources of exposure as well as potential demographic and behavioral characteristics that may affect PFAS serum concentrations will be beneficial moving forward with remediation of contaminated sites, providing guidance to citizens on how to lower their personal PFAS exposure, and identifying vulnerable populations.
Supplementary Material
Acknowledgements:
This work was supported by the grant titled “Exposure and Health Effects from Poly- and Perfluoroalkyl Substances in Colorado Water” and funded by the National Institute of Environmental Health Sciences (NIEHS) under Grant No. R21ES029394. Additional support was provided by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Any opinion, findings, and conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Declaration: CPH has served and continues to serve as a consulting and/or testifying expert in various matters related to PFAS litigation. The other authors declare they have no actual or potential competing financial interests (KEB, APS, CM, AMC, JLA).
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References:
- Anderson RH, Long GC, Porter RC, Anderson JK. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere. 2016;150:678–685. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2018. Per- and Polyfluoroalkyl Substances (PFAS) Exposure Assessment Technical Tools. Retrieved December 26, 2018, from https://www.health.pa.gov/topics/Documents/Environmental%20Health/PFAS%20Exposure%20Assessment%20Technical%20Tools.pdf
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for Perfluoroalkyls. (Draft for Public Comment). In: U.S. Department of Health and Human Services PHS, editor. Atlanta, GA: 2018. Retrieved December 26, 2018, from https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [Google Scholar]
- Averina M, Brox J, Huber S, Furberg AS, Sorensen M. Serum perfluoroalkyl substances (PFAS) and risk of asthma and various allergies in adolescents. The Tromso study Fit Futures in Northern Norway. Environ Res. 2019. February;169:114–21. Epub 2018/11/18. eng. [DOI] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013. Nov-Dec;121(11–12):1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated environmental assessment and management. 2011. October 1;7(4):513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 1999− 2000. Environmental science & technology. 2007. April 1;41(7):2237–42. [DOI] [PubMed] [Google Scholar]
- C8 Science Panel. The Science Panel Website, 2017. Retrieved December 26, 2018, from http://www.c8sciencepanel.org/.
- CDC (Centers for Disease Control). National Report on Human Exposure To Environmental Chemicals, Updated Tables, January 2019. In: Department of Health and Human Services, ed. Atlanta: 2019. Retrieved March 26, 2019, from https://www.cdc.gov/exposurereport/index.html [Google Scholar]
- CDPHE (Colorado Department of Public Health and the Environment). 2018. Perfluorinated compounds (PFCs and PFAS). Retrieved December 27, 2018, from https://www.colorado.gov/pacific/cdphe/pfcs
- Cho CR, Lam NH, Cho BM, Kannan K, Cho HS. Concentration and correlations of perfluoroalkyl substances in whole blood among subjects from three different geographical areas in Korea. Science of the Total Environment. 2015. April 15;512:397–405. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Thompson BA, Anderson HA. Perfluoroalkyl substances in older male anglers in Wisconsin. Environment international. 2016. May 1;91:312–8. [DOI] [PubMed] [Google Scholar]
- Dalsager L, Christensen N, Husby S, et al. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4 years among 359 children in the Odense Child Cohort. Environ Int. 2016;96:58–64. [DOI] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, et al. Per-and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. International journal of hygiene and environmental health. 2018;221(3):569–77. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ Health Perspect. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, et al. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol. 2009;39(1):76–94. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40(2):300–311. [DOI] [PubMed] [Google Scholar]
- Dinno A Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata Journal. 2015. [Google Scholar]
- Eastoe EF, Halsall CJ, Heffernan JE, Hung H. A statistical comparison of survival and replacement analyses for the use of censored data in a contaminant air database: A case study from the Canadian Arctic. Atmospheric Environment. 2006. 2006/11/01/;40(34):6528–40. [Google Scholar]
- El paso County Health Department. “Air Force Pfos/Pfoa Snapshot Peterson Afb.”. Retrieved March 26, 2019, from https://www.elpasocountyhealth.org/sites/default/files/imce/Peterson%20Snapshot_20Jul17%20(IST).pdf.
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2006;48(8):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson I, Gómez M, Nadal M, van Bavel B, Lindström G, Domingo JL. Perfluorinated chemicals in blood of residents in Catalonia (Spain) in relation to age and gender: a pilot study. Environment international. 2007. July 1;33(5):616–23. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, et al. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE. 2013;8(2):e56969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley B Drinking water in three Colorado cities contaminated with toxic chemicals above EPA limits. Denver Post. 2016. September 23 Retrieved December 26, 2018, from https://www.denverpost.com/2016/06/15/colorado-widefield-fountain-security-water-chemicals-toxic-epa/
- Fisher M, Arbuckle TE, Wade M, Haines DA. Do perfluoroalkyl substances affect metabolic function and plasma lipids?-Analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environmental Research. 2013;121:95–103. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, et al. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med. 2010;164(9):860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wang T, Fu Q, Wang P, Lu Y. Associations between serum concentrations of perfluoroalkyl acids and serum lipid levels in a Chinese population. Ecotoxicol Environ Saf. 2014;106C:246–252. [DOI] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, et al. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect. 2012;120(5):655–660.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res. 2015;136:8–14. [DOI] [PubMed] [Google Scholar]
- Góralczyk K, Pachocki KA, Hernik A, Struciński P, Czaja K, Lindh CH, Jönsson BA, Lenters V, Korcz W, Minorczyk M, Matuszak M. Perfluorinated chemicals in blood serum of inhabitants in central Poland in relation to gender and age. Science of the Total Environment. 2015. November 1;532:548–55. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ Health Perspect. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenhammar I, Berger U, Sundström M, McCleaf P, Eurén K, Eriksson S, Ahlgren S, Lignell S, Aune M, Kotova N, Glynn A. Influence of contaminated drinking water on perfluoroalkyl acid levels in human serum - A case study from Uppsala, Sweden. Environ Res. 2015;140:673–83. doi: 10.1016/j.envres.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol. 2002;36(8):1681–1685. [DOI] [PubMed] [Google Scholar]
- Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, Kamiyama S, Koizumi A. The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years. Journal of occupational health. 2004;46(2):141–7. [DOI] [PubMed] [Google Scholar]
- Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, Oken E, Sagiv SK. Predictors of per-and polyfluoroalkyl substance (PFAS) plasma concentrations in 6–10 year old American children. Environmental science & technology. 2017. April 11;51(9):5193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009. April 1;42(2):377–81.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environmental science & technology. 2009. February 6;43(6):2131–6. [DOI] [PubMed] [Google Scholar]
- Helsel DR. More than obvious: better methods for interpreting nondetect data. ACS Publications; 2005; 419A–423A. [DOI] [PubMed] [Google Scholar]
- Herrick RL, Buckholz J, Biro FM, et al. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991–2012. Environ Pollut. 2017;228:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics. 1979:65–70. [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett. 2016;3(10):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstate Technology Regulatory Council (ITRC). PFAS-Per -and Polyfluoroalkyl Substances 2019. Retrieved January 28, 2019, from https://pfas-1.itrcweb.org/fact-sheets/.
- Jain RB. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: data from National Health and Nutrition Examination Survey 2003–2008. Journal of Toxicology and Environmental Health, Part A. 2013. April 1;76(7):409–21. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Wouwe NV, Yang JH, Aldous KM. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environmental science & technology. 2004. September 1;38(17):4489–95. [DOI] [PubMed] [Google Scholar]
- Karnes C, Winquist A, Steenland K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environmental research. 2014;128:78–83. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, et al. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environmental science & technology. 2014. August 19;48(16):9600–8. Epub 2014/07/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Kalathil AA, Patel AM, Ye X, Calafat AM. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere. 2018. October;209:338–45. Epub 2018/06/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and Environmental Medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lin LY, Chiang CK, et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol. 2010;105(6):1354–1363. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, Wu Y. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environment international. 2011. October 1;37(7):1206–12. [DOI] [PubMed] [Google Scholar]
- Lorber M, Eaglesham GE, Hobson P, Toms LM, Mueller JF, Thompson JS. The effect of ongoing blood loss on human serum concentrations of perfluorinated acids. Chemosphere. 2015. January 1;118:170–7. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson K, Rignell-Hydbom A, Holmberg S, Thelin A, Jönsson BAG, Lindh CH, et al. Levels of perfluoroalkyl substances and risk of coronary heart disease: Findings from a population-based longitudinal study. Environmental Research. 2015. 2015/10/01/;142:148–54. [DOI] [PubMed] [Google Scholar]
- Minnesota Pollution Control Agency (MPCA). PFC Investigation and Clean Up. Retrieved July 9, 2019, from https://www.pca.state.mn.us/waste/pfc-investigation-and-clean.
- New Hampshire Department of Environmental Servies. In-Home Water Filtration Options for PFCs in Household Drinking Water 2016. Retrieved January 28, 2019, from https://www.des.nh.gov/organization/commissioner/documents/pfoa-inhome-treatment-20160518.pdf.
- Olsen GW, Church TR, Larson EB, Van Belle G, Lundberg JK, Hansen KJ, Burris JM, Mandel JH, Zobel LR. Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere. 2004. March 1;54(11):1599–611. [DOI] [PubMed] [Google Scholar]
- Place BJ, Field JA. Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ Sci Technol. 2012;46(13):7120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner S Perfluorinated compounds: occurrence and uses in products In Polyfluorinated chemicals and transformation products 2012. (pp. 25–39). Springer, Berlin, Heidelberg. [Google Scholar]
- Rotander A, Toms LM, Aylward L, Kay M, Mueller JF. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environment international. 2015. September 1;82:28–34. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, et al. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ Sci Technol. 2015;49(19):11849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenaler R, Cameron R, Butt CM, Hoffman K, Higgins CP, Stapleton HM. Serum perfluoroalkyl acids (PFAAs) and associations with behavioral attributes. Chemosphere. 2017. October 1;184:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009;170(10):1268–1278. [DOI] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Winquist A, Parks C. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environ Health Perspect. 2013. August;121(8):900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: a prospective investigation among US women. Environmental health perspectives. 2018;126(3):037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019. March;29(2):131–47. Epub 2018/11/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (United Sates Environmental Protection Agency). Drinking Water Health Advisories for PFOA and PFOS. 2016. Retrieved January 28, 2019, from https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos.
- Vassiliadou I, Costopoulou D, Ferderigou A, Leondiadis L, 2010. Levels of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) in blood samples from different groups of adults living in Greece. Chemosphere. 2010; 80(10):1199–206. [DOI] [PubMed] [Google Scholar]
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013. March;121(3):318–23. Epub 2013/01/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss O, Wiesmuller GA, Bunte A, et al. Perfluorinated compounds in the vicinity of a fire training area--human biomonitoring among 10 persons drinking water from contaminated private wells in Cologne, Germany. Int J Hyg Environ Health. 2012;215(2):212–215. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology. 2014. March;25(2):255–64. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K. Modeled PFOA Exposure and Coronary Artery Disease, Hypertension, and High Cholesterol in Community and Worker Cohorts. Environmental Health Perspectives. 2014. 2014/12/01;122(12):1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environmental science & technology. 2014. July 8;48(15):8807–14. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang Z, Shi Y, Li J, Wang Y, Zhao Y, Wu Y, Cai Z. Human placental transfer of perfluoroalkyl acid precursors: Levels and profiles in paired maternal and cord serum. Chemosphere. 2016. February 1;144:1631–8. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu Q, Sun HW, Zhang XZ, Yun SH, Kannan K. Perfluorinated compounds in whole blood samples from infants, children, and adults in China. Environmental science & technology. 2010. May 4;44(11):4341–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.