Abstract
Fertilization requires the physical combination of gametes, and terrestrial mammals necessitated the evolution of genitalia capable of successfully completing the fertilization process in a non-aqueous environment. Thus, the male mammalian external genitalia evolved as an outgrowth from the body, an appendage sufficient to fertilize eggs housed deep inside the female. In this way, sexual dimorphism of mammalian genitalia became highly pronounced. This highly complex evolutionary divergence both from aqueous fertilization, as well as divergence between the sexes of terrestrial mammals, required exquisitely coordinated, novel patterns of gene expression to regulate the spatial and temporal events governing external genitalia development. Recent studies delineating the genetic regulation of external genitalia development, largely focusing on development of the murine genital tubercle, have vastly enlightened the field of reproductive developmental biology. Murine homologs of human genes have been selectively deleted in the mouse, either in the whole body or using tissue-specific and temporally-specific genetic drivers. The defects in outgrowth and urethral tubularization subsequent to the deletion of specific genes in the developing murine external genitalia delineates which genes are required in which compartments and at what times. This review details how these murine genetic models have created a somewhat modest but rapidly growing library of knowledge detailing the spatial-temporal genetic regulation of external genitalia development.
Keywords: Genitalia, Differentiation, Morphogen
ARGUABLY A THREE-STEP PROCESS
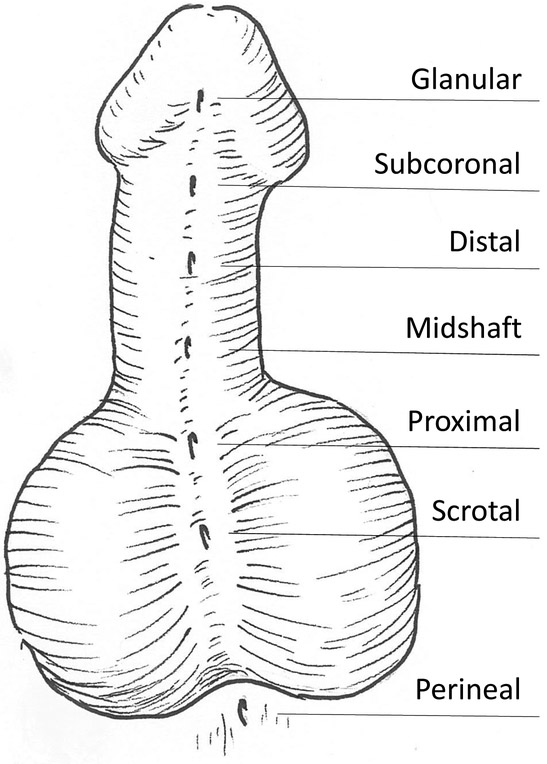
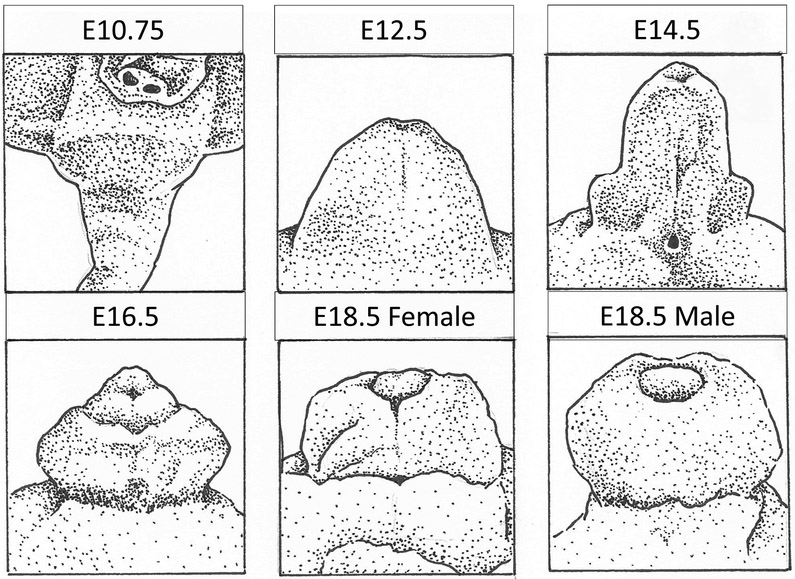
Temporally the development of the genital tubercle can be subdivided roughly into a three-step process. In the mouse, this takes place over the span of embryonic days 10.75 through 18 (Figure 1). The first phase is the pre-androgen phase during which the lateral genital swellings emerge bilaterally adjacent to a primitive genital structure called the cloacal membrane, and grow until the point of sexually dimorphic divergence. In the mouse, the bilateral lateral genital swellings are readily apparent by embryonic day 11.5 (Yamada et al. 2006) and more subtly apparent as early as embryonic day 10.75 (Perriton et al. 2002). The second phase is governed by the presence of masculinizing genetic regulation, specifically the presence of androgen signaling. In the mouse, this phase is evident beginning around embryonic day 15.5 or roughly two thirds of the way through gestation (Suzuki et al. 2017). Prior to this point, although sex-typing (testing for the presence of the Y chromosome) and sometimes gonadotyping (dissecting the embryo to account for either ovaries or testes) can successfully differentiate the female and male murine embryos, the external genitalia are indistinguishably similar at the morphological and histological levels. In humans, defects in these two initial phases can result in congenital anomalies such as ambiguous genitalia and micropenis. A well described syndrome known as 5-alpha-reductase deficiency results in 46XY males presenting as either feminized males or females at birth due to the inability of testosterone to convert to the more potent androgen, dihydrotestosterone (DHT) (Avendaño et al. 2018). Conversely, congenital adrenal hyperplasia (CAH) in 46XX females causes aberrant masculinization of the clitoris via overproduction of testosterone by the adrenal glands (El-Maouche, Arlt and Merke 2017). The third phase of external genitalia development, which is more genetically complex and relevant in the male than in the female due to the increased distal outgrowth, is the successful tubularization of the urethra throughout the length of the penis. If the male urethra does not tubularize completely, the result is a common congenital defect termed hypospadias wherein the meatus (tubal opening) of the urethra is located somewhere along the ventral aspect (underside) of the penile shaft rather than at its distal-most tip. In humans, the incidence of hypospadias is approximately 1 in every 250 live male births and is typically corrected with a complex surgical procedure. Hypospadias, like most other congenital anomalies, can be either isolated or present as part of a genetic syndrome and accompanied by other congenital defects, and can vary in severity according to how far away the meatus is (how proximal it is) from its intended distal position (Baskin 2017) (Figure 2).
Figure 1 -. Murine Genital Tubercle Development.
By E10.75 bilateral lateral genital swellings adjacent to the cloacal membrane are subtly apparent. From this point until E16.5, outgrowth is androgen independent and not significantly different between the sexes. Androgen dependent sexual divergence leads to differences in outgrowth and urethral formation readily apparent by E18.5.
Figure 2 -. Hypospadias.
In humans, hypospadias is defined as an abnormal proximal placement of the urethral opening (meatus) somewhere along the ventral aspect of the penile shaft. Hypospadias is graded mild (glanular) to severe (perineal) based on how proximal the meatus location is.
Although phase two and phase three of external genitalia development can be separated theoretically by their component physiological programs, continued phallic outgrowth versus male urethral formation, the two phases are inherently linked in both their regulation and timing. As soon as sexual divergence begins as measured by differences in tubercle size, so too begins the initial phases of urethral tubularization, which is also androgen dependent. The main reason to differentiate the two phases is due to the independent isolated appearances of outgrowth defects and urethral formation defects, suggesting that although intimately linked and sometimes cooccurring, these two physiological programs are not entirely co-dependent. A patient with micropenis may not have hypospadias, and certainly most patients with hypospadias do not also have micropenis (Springer, van den Heijkant and Baumann 2016).
THE IMPORTANCE OF MORPHOGENS
While endocrine signaling of sex hormones, particularly estrogens and androgens, have long been at the forefront of the study of sexual dimorphisms (Quigley et al. 1995), these are certainly not the only modulators of sexual development. Morphogens are molecular signals whose uneven distribution throughout a tissue during a developmental process is the driving force of differential tissue patterning, including axial patterning in all directions: anterior versus posterior, ventral versus dorsal, left versus right, and proximal versus distal (Sagner and Briscoe 2017). They comprise some of the earliest and most influential molecular signals, differentiating the three primary tissue layers during gastrulation (endoderm, ectoderm, and mesoderm) and establishing the body axes via signaling from a specialized group of cells called the Spemann organizing center (Anderson and Stern 2016). Throughout each developmental patterning process, specialized groups of cells comprising similar organizing centers secrete morphogens in order to communicate their spatial location to surrounding cells via the concentration gradient of the morphogen produced. As surrounding cells are exposed to various levels of the given morphogen, they experience differentiation events dependent on the level and type of morphogen they encounter (Sureda-Gomez and Adell 2019).
The major morphogen signaling pathways controlling body patterning are grouped into, but not limited to, the following pathways: WNT, Hedgehog, FGF, PDGF, Notch (juxtacrine), Retinoic Acid, TGF-beta, GDNF, Activin/Inhibin, BMP, and GDF. Deregulation of any upstream regulators of morphogen expression, any extra- or intracellular receptors or transducers of morphogens, and any transcription factors activated or repressed by the level of morphogen to which a cell is exposed can cause defects in the developmental program of a particular tissue (Durston 2019). These defects typically manifest as congenital structural anomalies; in the case of external genitalia development this includes defects such as micropenis, ambiguous genitalia, and hypospadias (Baetens et al. 2019).
The key to dissecting the molecular mechanisms of morphogen signaling in tissues such as the external genitalia has therefore been to selectively delete members of each morphogen pathway, usually in the mouse, and observe the divergence from normal tissue patterning (the type and extent of congenital defect) that results. Since all of the morphogens are fundamental regulators of development of multiple tissues, typically including vital organs, the study of their roles in external genitalia development has historically been largely occluded by embryonic death in full-body knockout models (Chisaka and Capecchi 1991). It was therefore necessary that the field develop tissue-specific “conditional” mouse models (Skarnes et al. 2011, Sauer and Henderson 1990) as well as temporally inducible (Feil et al. 1996) models in order to dissect the molecular mechanisms of external genitalia development. With the advent of these murine conditional and inducible murine knockout technologies, the study of the genetic regulation of external genitalia development has only recently found its footing. Over the last thirty years, enormous strides have been achieved using conditional and inducible murine knockout models to delineate the genetic regulation of outgrowth, sexual divergence / masculinization, and urethra formation of the external genitalia. The essential genes for external genitalia development discovered using these tools, and their respective roles are described in the sections below.
SPATIAL GENETIC REGULATION DURING PHASE ONE: INITIAL OUTGROWTH
Sonic hedgehog (SHH) is a major morphogenic regulator of the initial outgrowth of the genital tubercle. Identification of SHH as a member of the morphogen signaling center in the distal urethral epithelium (DUE) was first discovered using chick models where transplantation of this signaling center to the limb bud resulted in digit duplication indicating that SHH expression confers polarizing activity. In the same study it was discovered that the mesenchyme of the genital tubercle does not have the same polarizing activity. SHH is among the most heavily expressed proteins in the DUE signaling center located at the ventral midline of the developing genital tubercle. Murine deletion of SHH in a full-body manner resulted in embryonic lethality but only after the GT begins to develop, and showed that embryos lacking SHH exhibited complete agenesis of the genital tubercle, with only the faintest observable bilateral lateral genital swellings at embryonic day eleven, indicating that SHH is secondary to initiation but only to a very small degree. One well-documented mechanism by which SHH controls GT outgrowth is via regulation of cell cycle progression. Inactivation of SHH during murine GT development causes an increase in the length of the cell cycle from 8.5 to 14.4 hours and shows a concomitant growth reduction by 75 percent. This is due to an increase in the length of G1 phase at the expense of entry into S-phase via control of the transcriptional levels of G1 regulating genes. SHH is therefore a master regulator of both the patterning and proliferation programs during the initial phases of external genitalia development (Seifert et al. 2010).
SHH deleted mice also exhibit a persistent cloaca, indicating that septation (the morphological division into separate parts) of the cloaca into the urogenital sinus and rectum fails. This is an interesting finding since the cloaca (a single exterior orifice of the urinary, digestive, and reproductive tracts) is the evolutionary and embryonic precursor to the septated system present in placental mammals (Kluth 2010). Although persistent cloaca is formally considered an anorectal malformation rather than a disorder of sex development (Bischoff et al. 2019), failure of septation by default affects the urinary and reproductive tracts just as much as the gastrointestinal system (Gupta et al. 2014). Recent high resolution episcopic microscopy of normal murine cloacal development versus SHH knockout and WNT-antagonist DKK1 knockout revealed that both hypoplastic (SHH) and hyperplastic (DKK1) defects of the dorsal peri-cloacal mesenchyme (dPCM) result in cloacal anomalies from disorganized patterning of the tissues that grow around the normally stationary dPCM (Huang, Chen and Li 2016). The SHH knockout has thus elucidated at least one conserved genetic pathway required for both cloacal septation and initial external genitalia outgrowth. Similarly, deletion of the transcription factors SIX1 and EYA1 alone or in combination results in variable penetrance of persistent cloaca, restricted GT outgrowth, and hypospadias (Wang et al. 2011).
Downstream of SHH was found to be several additional morphogens including FGF8, FGF10, BMP2, BMP4, and WNT5A (Perriton et al. 2002). In order to further delineate the role of SHH in GT outgrowth, it was necessary to combine temporally inducible (tamoxifen) SHH-cre with conditional floxed SHH alleles. Murine deletion of SHH at embryonic day nine completely abolishes GT outgrowth, whereas deletion at later timepoints shows progressively less severe outgrowth phenotypes. This phenotype was at least partially due to increased apoptosis in the urethral epithelium and decreased proliferation in the mesenchyme. The temporally inducible SHH deletion model showed an obligatory and maintained requirement for SHH in both the initiation and continuance of GT outgrowth. At least part of the function of SHH is to maintain the DUE signaling center, and the key receptor of SHH in the GT was determined to be mesenchymal-expressed smoothened (Lin et al. 2009). Additional mouse studies have linked SHH and canonical WNT signaling in a dose-dependent manner. SHH knockouts show decreased expression of WNT ligands and diminished WNT activity. Constitutive expression of beta-catenin rescues outgrowth defects and FGF8 expression in SHH knockout embryos. This data essentially shows that WNT signaling is downstream of SHH in the DUE (Miyagawa et al. 2009a).
Canonical WNT signaling is indispensible to normal murine GT outgrowth, as evidenced by the complete agenesis of the genital tubercle in knockouts of the essential WNT mediator, beta-catenin. Beta-catenin expression localizes first to the cloacal endoderm, as well as globally with highest expression at the proximolateral sides of the mouse GT during later developmental stages. Conditional knockout or overexpression of beta-catenin was performed using the following cre drivers: SHH-cre in the cloacal endoderm, MSX2-cre in the ectodermal epithelium, and DERMO1-cre in the mesodermal mesenchyme. Using the SHH-cre driver, deletion of beta-catenin resulted in complete lack of genital outgrowth in both males and females, with the generation of a crater-like structure in its place likely the result of decreased proliferation and increased apoptosis. By contrast, expression of constitutively active beta-catenin using the same SHH-cre driver resulted in larger GTs and increased expression of FGF8. By using a tamoxifen inducible version of the SHH-cre driver, it was also shown that the earlier beta-catenin signaling is abrogated, the more severe the outgrowth defect is, and these defects correspond perfectly with concomitant loss of FGF8 expression (Lin et al. 2008). Several WNT ligands are expressed in the developing mouse GT, including WNT3, WNT4, WNT5A, WNT7A, WNT9B. The early (embryonic day 10) and distal centric patterning of WNT5A expression (Yamaguchi et al. 1999) combined with the downregulation of WNT5A in the genital tubercle in response to SHH knockout (Miyagawa et al. 2009a) suggests that WNT5A is one of the main WNT ligands regulating murine GT outgrowth. This role was solidified by the observation that WNT5A full-body knockout mice exhibit severely hypoplastic genital tubercles, among a variety of other severe developmental defects (Tai et al. 2009). WNT5A is a particularly interesting WNT ligand as it can act through both the canonical and non-canonical pathways. Human autosomal dominant mutations in WNT5A are a cause of Robinow Syndrome. One of the many congenital phenotypes of Robinow Syndrome is micropenis and hypoplastic scrotum in males, and hypoplasia of the analogous structures, the clitoris and labia, in females (Person et al. 2010).
Fibroblast growth factor (FGF) signaling has been delineated by the murine conditional deletion of the genitally expressed FGF receptors (FGFR1 and FGFR2) in different tissues: mesenchyme, endoderm, and ectoderm. Mesenchymal FGF signaling is the key regulator during initial outgrowth, as shown by severely hypoplastic outgrowth as early as embryonic day twelve. Given that individual knockout of FGFR1 or FGFR2 does not affect outgrowth significantly when utilizing the same mesenchymal cre (ISL1-cre), these two FGF receptors are functionally redundant at this stage. Additionally, deletion of FGFR1 and FGFR2 specifically in the endoderm or ectoderm has no discernible effects on outgrowth, indicating their importance specifically in the mesenchyme for the initial outgrowth phase (Harada et al. 2015).
Given that the receptors of FGF signaling are integral to initial outgrowth, this begs the question: which FGF ligands are likewise required? Two major candidates were identified based on their expression profiles: FGF8 expressed at the distal most tip of the urethral epithelium and FGF10 localized mostly in the mesenchyme directly adjacent to the DUE. Initial studies indicated that addition of exogenous FGF8 promoted GT outgrowth and neutralizing FGF8 using antibodies caused defects in outgrowth, while murine deletion of FGF10 had minimal effects during the outgrowth phase (Haraguchi et al. 2000). However, more recent studies have shown that although the FGF8 transcript is present at the distal most aspect of the murine DUE, the protein is not detectable by immunohistochemistry, despite the finding the WNT5A mutant mice still exhibiting outgrowth were the same mice that maintained transcription level expression of FGF8. Interestingly, before lateral genital swellings develop, FGF8 is expressed in endodermal cells that are in contact with the cloacal ectoderm. A study using conditional FGF8 deletion with a SHH driver showed that although FGF8 is a clear marker of genital induction, it is not apparently required for the outgrowth phase of genital development. The longstanding assumption that FGF8 is integral to genital tubercle outgrowth based on its unique transcript-level expression profile was therefore brought into question in favor of the conclusion that its transcription is the result of, rather than the signal for outgrowth (Seifert, Yamaguchi and Cohn 2009b). However, the newest studies using higher-level murine genetic models challenge this finding. In mice overexpressing FGF8 using a temporally controlled induction, the genital tubercle is markedly larger. Additionally, overexpression of FGF8 in the absence of canonical beta-catenin signaling can partially rescue the GT agenesis phenotype, indicating that FGF8 functions downstream of canonical WNT signaling to promote genital outgrowth. The same study indicated a delicately balanced autoregulatory feedback loop wherein excess FGF8 causes downregulation of its own transcription, and that in the signaling cascade for outgrowth, the transcription factor SP8 is indispensible for integrating WNT and FGF8. Collectively these findings show that FGF8 is almost certainly the key FGF ligand in the developing genital tubercle and is simply expressed at the protein level at levels too low, and in regions too restricted, to be detected by current histological methods (Lin et al. 2013). Still, these findings beg the question: why does deletion of FGF8 have no effect on mouse GT development? Yamada’s group provided a rational explanation of functional redundancy. They found that FGF4 is upregulated in the DUE in the absence of FGF8, and FGF9 is upregulated in the same tissue in the absence of both (Miyagawa et al. 2009a). Importantly, the findings regarding the WNT-SP8-FGF8 cascade were hypothesized based on the developmental parallels between paired distal-growing appendages (the limbs) and the single medial distal growing appendage of the GT (Lin et al. 2013). Without such innovative comparisons, the FGF cascade of genital tubercle outgrowth would remain largely a mystery.
Most recently, it has been shown that BMP4, a ligand for the TGF-beta superfamily of receptors, is required for initiation of genital tubercle outgrowth, with murine conditional knockout of BMP4 using the HOXA3-cre driver showing genital hypoplasia and reduced expression of genes known to promote outgrowth, including the additional morphogens WNT5A and FGF8, as well as the transcription factors HOXD13 and P63. Interestingly, in this model of genital hypoplasia the SHH signaling pathway appears entirely unaffected, indicating the independent requirements of initial outgrowth on both the BMP and SHH pathways (Kajioka et al. 2019). Additionally, the murine knockout of an upstream regulator of BMP4, ISL1 shows abrogated genital outgrowth (Ching et al. 2018).
The PDGF pathway was until recently entirely neglected in the murine studies of GT development due to the knockout mouse model of the main receptor PDGFRA exhibiting early embryonic lethality. This year however, a fascinating study was published showing that temporal specific deletion of PDGFRA at embryonic day ten or eleven causes cloacal septation defects, defective urogenital fold development, and abnormal urethra tubularization in both males and females. PDGFRA was shown to be required for the maintenance of the urorectal mesenchyme, with deletion causing significant aberrant apoptosis via the caspase pathway (Qian et al. 2019). Alongside SHH, PDGF is another example of a pathway integral to proper proliferation in the developing GT, in this case by inhibiting apoptosis.
SPATIAL GENETIC REGULATION DURING PHASE TWO: SEXUAL DIVERGENCE
Because so many of the genes required for initial outgrowth of the external genitalia are likewise required for the development of vital organs, embryos conceived with mutations in these imperative genes rarely survive to term. Many of the cases of clinical micropenis are therefore caused instead by genetic defects in the pathways regulating the second phase of external genitalia development, sexual divergence, which includes continued distal outgrowth in males.
Micropenis is typically diagnosed in human newborns, affects approximately 0.015% of American newborn males, and is defined as a stretched penile length at least 2.5 standard deviations smaller than the age-matched average (Hatipoğlu and Kurtoğlu 2013). Masculinization of the human external genitalia is dependent largely on androgen signaling. In human females, in the environment of low level androgens, the genital tubercle develops into the clitoris rather than the penis, and the urogenital folds remain unfused to develop into the labia rather than the scrotum The urethra in human and mouse females does not need to traverse the extended length of a masculinized distal outgrowth and therefore need not completely fuse, instead remaining largely as a ventral groove. (Overland et al. 2016).
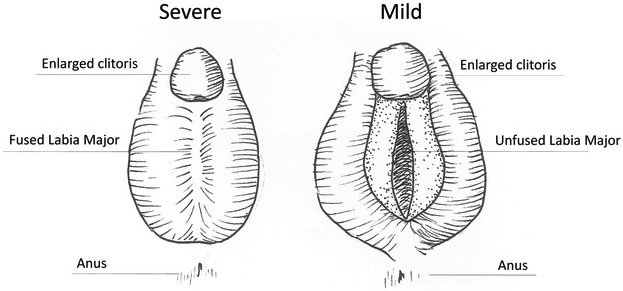
The androgen receptor (AR) gene in humans is located on the X-chromosome and encodes a steroid nuclear receptor activated by androgenic hormones including testosterone (Lubahn et al. 1988) as well as the more potent androgen, dihydrotestosterone (DHT) in the external genitalia. Production of DHT is regulated by the enzyme 5-alpha-reductase, which catalyzes the production of DHT from testosterone. Once activated, AR functions as a transcription factor capable of regulating the expression of a vast network of masculinizing genes. Congenital syndromes affecting both human male and female external genitalia development are frequently the result of significant imbalances in the androgen pathway. Examples of this include 1) androgen insensitivity syndrome caused by mutations in the AR gene (Chauhan et al. 2018) 2) hypogonadotropic hypogonadism in which perturbed hypothalamic or pituitary signaling causes reduced production of androgens (Topaloglu and Kotan 2016) 3) 5-alpha-reductase deficiency in which absence of the catalyzing enzyme results in the inability to generate DHT from testosterone (Okeigwe and Kuohung 2014) and 4) congenital adrenal hyperplasia in females wherein overproduction of testosterone results in an enlarged clitoris (Marei et al. 2016) (Figure 3).
Figure 3 -.
External Genitalia Defects of Congenital Adrenal Hyperplasia are caused by overproduction of androgens in females. In mild cases of CAH, the clitoris is enlarged but the labia major remain unfused. In more severe cases the enlarged clitoris is comorbid with medially fused labia majora. Severe CAH external genitalia can be clinically mistaken for micropenis if chromosomal sex is not tested.
Murine models have vastly improved our understanding of this phase of external genitalia development. AR expression in the murine genital tubercle is located in the mesenchyme bilaterally adjacent to the urethral plate epithelium. AR knockout mice have a feminized external appearance with an anogenital distance comparable to their female littermates, and even develop a clitoris (Weiss et al. 2012). Together these findings closely mimic the human phenotype of androgen insensitivity syndrome. In addition to androgens, local masculinization factors such as the hedgehog and WNT signaling pathways work in concert with AR signaling during this phase to continue regulating outgrowth. Both knockout of the SHH activator GLI2 and overexpression of the SHH repressor GLI3 result in abnormal murine masculinization including limited GT outgrowth (Miyagawa et al. 2011, He et al. 2016). As for the WNT pathway, evidence suggests direct interaction between beta-catenin and the ligand-bound AR, although this has not been tested specifically in genital tissues (Pawlowski et al. 2002), and overexpression of beta-catenin in the mesenchyme of developing mouse female genital tubercles results in a masculinized phenotype, suggesting interplay between WNT and AR (Miyagawa et al. 2009b).
One set of ligands of the TGF-beta superfamily of receptors with roles in androgen regulation during GT development are the activins INHBA and INHBB. While no noticeable GT abnormalities are observed in the knockout mice for these genes, when INHBB is inserted aberrantly into the INHBA locus, both males and females exhibit enlarged external genitalia. In a somewhat similar situation to CAH human females, this phenotype appears to be secondary to an overproduction of androgens (Brown et al. 2000). It is important to note that prenatal exposure to certain endocrine disruptors can cause imbalances in androgen signaling with equally significant phenotypes to patients with inherited mutations (Kalfa et al. 2011).
SPATIAL GENETIC REGULATION DURING PHASE THREE: URETHRAL FORMATION
Formation of the penile urethra is dependent on androgen signaling, and treatment of mice with the AR antagonist flutamide results in hypospadias (Zheng, Armfield and Cohn 2015). One of the many genes downstream of AR in the genital tubercle is FGFR2, specifically FGFR2-IIIB. In FGFR2-IIIB knockout mice, the urethral epithelium does not properly mature due to defects in cell proliferation, resulting in severe hypospadias despite preservation in SHH and FGF8 expression. The probable ligand during this phase is FGF10, as it is likewise downregulated in the presence of AR antagonists (Petiot et al. 2005), and FGF10 mutant mice exhibit failure of the urethral plate to ventrally fuse (Yucel et al. 2004).
Genes regulating the intracellular trafficking of sex steroids also affect urethral formation. Human microduplication of VAMP7, a vesicle trafficking protein that colocalizes with ligand bound estrogen receptor ESR1, is associated with congenital genitourinary defects. Transgenic murine overexpression of VAMP7 causes overt feminization of the male external genitalia including abrogated outgrowth (measured at adult stage), reduced anogenital distance, and partial penetrance of distal hypospadias while aberrantly inducing several estrogen responsive genes (Tannour-Louet et al. 2014). This finding elucidates that androgens and estrogens must be balanced during sexual divergence of the external genitalia, as well as during penile urethral formation (Zheng et al. 2015).
Just as canonical WNT signaling is required for the outgrowth phase, so too is it required for proper urethral formation. When the conditional beta-catenin allele is knocked out at different murine embryonic stages using a tamoxifen-inducible SHH-cre, earlier treatments result in the widest and most proximal hypospadic urethral openings, with a graded less severe phenotype when the knockout is induced at later and later timepoints. By contrast, overexpression of beta-catenin has a normal distal urethral opening similar to wildtype littermates. The urethral defects in the beta-catenin knockout were determined to be the result of failure of distal urethral plate formation and an open proximal urethra secondary to proliferation defects resultant from decreased FGF8 and SHH expression (Lin et al. 2008, Miyagawa et al. 2009a, Seifert et al. 2009a).
Another family of signaling molecules involved in urethral formation are the ephrins. Knockout of the gene EFNB2 in mice results in perineal hypospadias with concurrent anorectal malformations, feminized anogenital distance, and females exhibit persistent cloaca. The ephrin ligands bind to ephrin family receptor tyrosine kinases to regulate signal transduction controlling cellular migration and adhesion during development, and it is speculated that EFNB2 is downstream of AR in the genital tubercle (Yucel et al. 2007). Because the formation of the tubular urethra requires both canalization and fusion events (Liu et al. 2018), it makes sense that genes controlling cell migration, proliferation, and adhesion play important roles. Another such example is LAMA5, a laminin gene expressed in both the ectodermal and endodermal epithelia of the cloaca. LAMA5 knockout mice exhibit wide proximal hypospadias or an entirely exposed urethra that fails to tubularize. LAMA5 appears to be required to maintain the normal basement membrane structure in the ventral ectoderm that is necessary for tubularization (Lin et al. 2016).
Downstream of AR in the process of urethral formation are several other powerful transcription factors. One such example is MAFB, a leucine zipper transcription factor with previously described roles in hematopoiesis (Gemelli et al. 2006). MAFB is dimorphically expressed in the bilateral mesenchyme of the male GT, and knockout mice exhibit normal GT length with defective urethral formation (Suzuki et al. 2014). Later studies from the same group showed definitively that MAFB is downstream of AR, identifying two androgen response elements in the 3’ untranslated region of the MAFB gene. MAFB is also likely regulated by the WNT signal transducer beta-catenin during urethral formation (Matsushita et al. 2016), and it is important to remember that murine beta-catenin knockout results not only in outgrowth defects but also severe hypospadias in both sexes (Lin et al. 2008).
Homeodomain transcription factors are also major players in development, including development of the tubular urethra. HOXA13 deletion in mice causes decreased expression of FGF8 and BMP7, and a distal hypospadic phenotype. HOXA13 mutants show downregulation of AR, which suggests that HOXA13 is either upstream of AR or a participant in a feedback loop (Morgan et al. 2003). Human mutations in HOXA13 are the cause of autosomal dominant hand-foot-genital syndrome, causing limb and Müllerian malformations in females with partially penetrant, variably severe hypospadias in males (Mortlock and Innis 1997). Similarly, human males homozygous for mutations in another HOX family transcription factor, HOXD13, uniformly exhibit hypospadias (Tüzel et al. 2007). Both HOXA13 and HOXD13 are expressed primarily in the outgrowth structures of the limb and genitals. Although HOXD13 mutant mice exhibit fertility defects and absent preputial glands, there is no description in the literature as to whether the genitalia are developmentally normal (Johnson et al. 1998).
Another interesting modulator of urethral formation is retinoic acid signaling. Retinoic acid signaling uses a metabolite of vitamin A (retinol) as a ligand for various nuclear receptors that can then act as transcription factors to regulate expression of downstream genes. Retinoic acid signaling is imperative to the proper development of the body axes, limbs, spinal chord, heart, and most notoriously the eyes (Ghyselinck and Duester 2019). Studies in human stem cells revealed that retinoic acid signaling works in conjunction with BMP4 and p63 to modify chromatin structure in order to promote epithelial differentiation, a system likely at play in many developing tissues (Pattison et al. 2018). Several members of the retinoic acid pathway are dynamically expressed during GT development, including RAR and RXR genes. If pregnant female mice are given a sufficiently high dose of exogenous retinoic acid at gestational day 9, embryos exhibit dramatic malformations in the urethral plate but no concurrent issues with GT outgrowth (Ogino et al. 2001). This is just one example of the differential regulation between continued male-specific distal outgrowth of the GT and the development of the penile urethra.
THE MOUSE AS AN IMPERFECT BUT NECESSARY MODEL
There are undeniable differences between the external genitalia of the mouse versus the human. Despite this, the mouse remains the best animal model for the study of genetic mechanisms in GT development because of the balance it represents between 1) being a relatively rapidly reproducing species 2) possessing a relatively high genetic homology to the human 3) possessing a genome for which gene editing tools are readily available, and perhaps most importantly 4) eliminating the ethical conundrum of prospective human experimentation.
Morphologically, the mouse penis has additional structures that the human does not, including external spines and internal baculum (bone) that over evolutionary time became vestigial to certain primates including humans (Brindle and Opie 2016). One of the most notable differences is the lack of requirement for DHT signaling in mouse GT development, despite it being quite obviously required in the human. Double knockout of the genes encoding 5-alpha-reductase 1 and 2 have no effect on murine external genitalia formation (Mahendroo et al. 2001), although it does affect the other male-specific organs including the seminal vesicles and prostate. This finding implies that testosterone alone is the sufficient androgen in murine GT masculinization, and that the evolutionary distance between mouse and human results in added genetic complexity in the virilization requirements of humans.
The similarities and differences in male urethral formation in mice versus humans, and precisely how applicable and translatable murine studies are to the human condition, is a highly controversial topic among researchers. Dramatic differences between the patterns with which the mouse and human penile urethra tubularize are apparent simply from optical projection tomography images (Li et al. 2015). In the mouse, the proximal portion of the urethra forms from direct canalization of the endodermal urethral plate and the distal portion forms from fusion events at preputial urethral groove. In practically the opposite scenario in humans, the direct canalization events occur in the urethral plate of the distal glans and fusion events occur at the proximal urethra (Liu et al. 2018). We must therefore be careful to avoid aligning too closely murine urethral anomalies with human hypospadias, and consider each murine study of urethral malformation only in the context of how it may or may not translate to humans. Speciation is by definition the evolutionary divergence of one group into two, to the point at which they can no longer procreate, either by incompatibility of chromosomes or incompatibility at copulation (Via 2009). Studies on sexual organs in any species other than human can therefore only shed so much light on the human condition. In spite of this, mouse models have been (and continue to be) truly indispensible tools in illuminating the various upstream signaling pathways conserved across external genitalia development in both species.
FINAL REMARKS
The clinical spectrum of external genitalia development is undeniably wide in range. Researchers worldwide are interested in identifying the genetic underpinnings of various deviations from what is considered anatomically typical, largely in the hopes of creating treatment options for patients who are not happy with either the appearance or function of their genitalia. Historically both researchers and clinicians have referred to variations from the population median as disorders of sexual development (DSD), and in the not so distant past, drastic surgical procedures were regularly performed on DSD infants so that they could more easily conform to the social dichotomies of gender identity and biological sex. Sometimes these drastic surgeries even prioritized aesthetics over sexual function. More recently however, shifts in social awareness have instead put at the forefront each patient’s individual experience, considering the possibility that they do not necessarily want to align perfectly with either pole of these dichotomies, and instead may be most comfortable remaining somewhere along the wide interim spectrum. In the absence of issues in urinary function (which does typically require infant surgery), clinicians and parents have more recently taken to preserving the decisional autonomy of DSD infants, waiting until patients are of an appropriate age to weigh their own treatment options. In the same vein, DSD no longer stands formally for “disorders of sexual development” but instead the literature now largely defines it as “differences of sexual development”. The focus of the field of reproductive developmental genetics continues to be discovering why exactly deviations from the median occur, and developing the widest possible array of options for patients who opt for treatment. This focus simultaneously respects the decisional autonomy of DSD patients, cultivates knowledge surrounding the reason DSDs occur, and provides the best possible care for patients seeking treatment. There continues to be enormous controversy over surgical procedures performed on infant genitalia even when the surgical goal is to improve functionality. We must assume the same controversy will arise when inevitably in utero pharmacological treatments become available to treat DSD. After all, a fetus is no more capable of making decisions for itself than an infant. As researchers, we largely avoid these moral conundrums – our focus is to provide tools and widen options for treatment. It is society that will ultimately decide whether or not the tools are useful, and whether or not the treatment options are appropriate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson C & Stern CD (2016) Organizers in Development. Curr Top Dev Biol, 117, 435–54. [DOI] [PubMed] [Google Scholar]
- Avendaño A, Paradisi I, Cammarata-Scalisi F & Callea M (2018) 5-α-Reductase type 2 deficiency: is there a genotype-phenotype correlation? A review. Hormones (Athens), 17, 197–204. [DOI] [PubMed] [Google Scholar]
- Baetens D, Verdin H, De Baere E & Cools M (2019) Update on the genetics of differences of sex development (DSD). Best Pract Res Clin Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- Baskin L (2017) What Is Hypospadias? Clin Pediatr (Phila), 56, 409–418. [DOI] [PubMed] [Google Scholar]
- Bischoff A, Trecartin A, Alaniz V, Hecht S, Wilcox DT & Peña A (2019) A cloacal anomaly is not a disorder of sex development. Pediatr Surg Int. [DOI] [PubMed] [Google Scholar]
- Brindle M & Opie C (2016) Postcopulatory sexual selection influences baculum evolution in primates and carnivores. Proc Biol Sci, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Houston-Hawkins DE, Woodruff TK & Matzuk MM (2000) Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet, 25, 453–7. [DOI] [PubMed] [Google Scholar]
- Chauhan P, Rani A, Singh SK & Rai AK (2018) Complete Androgen Insensitivity Syndrome due to Mutations in the DNA-Binding Domain of the Human Androgen Receptor Gene. Sex Dev. [DOI] [PubMed] [Google Scholar]
- Ching ST, Infante CR, Du W, Sharir A, Park S, Menke DB & Klein OD (2018) Isl1 mediates mesenchymal expansion in the developing external genitalia via regulation of Bmp4, Fgf10 and Wnt5a. Hum Mol Genet, 27, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O & Capecchi MR (1991) Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature, 350, 473–9. [DOI] [PubMed] [Google Scholar]
- Durston AJ (2019) What are the roles of retinoids, other morphogens, and Hox genes in setting up the vertebrate body axis? Genesis, e23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maouche D, Arlt W & Merke DP (2017) Congenital adrenal hyperplasia. Lancet, 390, 2194–2210. [DOI] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D & Chambon P (1996) Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A, 93, 10887–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemelli C, Montanari M, Tenedini E, Zanocco Marani T, Vignudelli T, Siena M, Zini R, Salati S, Tagliafico E, Manfredini R, Grande A & Ferrari S (2006) Virally mediated MafB transduction induces the monocyte commitment of human CD34+ hematopoietic stem/progenitor cells. Cell Death Differ, 13, 1686–96. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB & Duester G (2019) Retinoic acid signaling pathways. Development, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Bischoff A, Peña A, Runck LA & Guasch G (2014) The great divide: septation and malformation of the cloaca, and its implications for surgeons. Pediatr Surg Int, 30, 1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Omori A, Nakahara C, Nakagata N, Akita K & Yamada G (2015) Tissue-specific roles of FGF signaling in external genitalia development. Dev Dyn, 244, 759–73. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N & Yamada G (2000) Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development, 127, 2471–9. [DOI] [PubMed] [Google Scholar]
- Hatipoğlu N & Kurtoğlu S (2013) Micropenis: etiology, diagnosis and treatment approaches. J Clin Res Pediatr Endocrinol, 5, 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Akbari P, Mo R, Zhang JJ, Hui CC, Kim PC & Farhat WA (2016) Adult Gli2+/−;Gli3Δ699/+ Male and Female Mice Display a Spectrum of Genital Malformation. PLoS One, 11, e0165958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Chen F & Li X (2016) Clarification of mammalian cloacal morphogenesis using high-resolution episcopic microscopy. Dev Biol, 409, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Sweet HO, Donahue LR, Ward-Bailey P, Bronson RT & Davisson MT (1998) A new spontaneous mouse mutation of Hoxd13 with a polyalanine expansion and phenotype similar to human synpolydactyly. Hum Mol Genet, 7, 1033–8. [DOI] [PubMed] [Google Scholar]
- Kajioka D, Suzuki K, Nakada S, Matsushita S, Miyagawa S, Takeo T, Nakagata N & Yamada G (2019) Bmp4 is an essential growth factor for the initiation of genital tubercle (GT) outgrowth. Congenit Anom (Kyoto). [DOI] [PubMed] [Google Scholar]
- Kalfa N, Philibert P, Baskin LS & Sultan C (2011) Hypospadias: interactions between environment and genetics. Mol Cell Endocrinol, 335, 89–95. [DOI] [PubMed] [Google Scholar]
- Kluth D (2010) Embryology of anorectal malformations. Semin Pediatr Surg, 19, 201–8. [DOI] [PubMed] [Google Scholar]
- Li Y, Sinclair A, Cao M, Shen J, Choudhry S, Botta S, Cunha G & Baskin L (2015) Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J Urol, 193, 1353–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Werner R, Ma L & Miner JH (2016) Requirement for basement membrane laminin α5 during urethral and external genital development. Mech Dev, 141, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yin Y, Bell SM, Veith GM, Chen H, Huh SH, Ornitz DM & Ma L (2013) Delineating a conserved genetic cassette promoting outgrowth of body appendages. PLoS Genet, 9, e1003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yin Y, Long F & Ma L (2008) Tissue-specific requirements of beta-catenin in external genitalia development. Development, 135, 2815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yin Y, Veith GM, Fisher AV, Long F & Ma L (2009) Temporal and spatial dissection of Shh signaling in genital tubercle development. Development, 136, 3959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Liu X, Shen J, Sinclair A, Baskin L & Cunha GR (2018) Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation, 101, 46–64. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS & Wilson EM (1988) Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science, 240, 327–30. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Hess DL & Russell DW (2001) Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology, 142, 4652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei MM, Fares AE, Abdelsattar AH, Abdullateef KS, Seif H, Hassan MM, Elkotby M, Eltagy G & Elbarbary MM (2016) Anatomical measurements of the urogenital sinus in virilized female children due to congenital adrenal hyperplasia. J Pediatr Urol, 12, 282.e1–282.e8. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Suzuki K, Ogino Y, Hino S, Sato T, Suyama M, Matsumoto T, Omori A, Inoue S & Yamada G (2016) Androgen Regulates Mafb Expression Through its 3'UTR During Mouse Urethral Masculinization. Endocrinology, 157, 844–57. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, Motoyama J, Iguchi T, Nakagata N, Hui CC & Yamada G (2011) The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology, 152, 2894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM & Yamada G (2009a) Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development, 136, 3969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S & Yamada G (2009b) Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol, 23, 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V & Stadler HS (2003) Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development, 130, 3095–109. [DOI] [PubMed] [Google Scholar]
- Mortlock DP & Innis JW (1997) Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet, 15, 179–80. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Suzuki K, Haraguchi R, Satoh Y, Dolle P & Yamada G (2001) External genitalia formation: role of fibroblast growth factor, retinoic acid signaling, and distal urethral epithelium. Ann N Y Acad Sci, 948, 13–31. [PubMed] [Google Scholar]
- Okeigwe I & Kuohung W (2014) 5-Alpha reductase deficiency: a 40-year retrospective review. Curr Opin Endocrinol Diabetes Obes, 21, 483–7. [DOI] [PubMed] [Google Scholar]
- Overland M, Li Y, Cao M, Shen J, Yue X, Botta S, Sinclair A, Cunha G & Baskin L (2016) Canalization of the Vestibular Plate in the Absence of Urethral Fusion Characterizes Development of the Human Clitoris: The Single Zipper Hypothesis. J Urol, 195, 1275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison JM, Melo SP, Piekos SN, Torkelson JL, Bashkirova E, Mumbach MR, Rajasingh C, Zhen HH, Li L, Liaw E, Alber D, Rubin AJ, Shankar G, Bao X, Chang HY, Khavari PA & Oro AE (2018) Retinoic acid and BMP4 cooperate with p63 to alter chromatin dynamics during surface epithelial commitment. Nat Genet, 50, 1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM & Wierman ME (2002) Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. J Biol Chem, 277, 20702–10. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK & Cohn MJ (2002) Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol, 247, 26–46. [DOI] [PubMed] [Google Scholar]
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ, van Bokhoven H, Hoogeboom JM, Mazzeu JF, Petryk A, Schimmenti LA, Brunner HG, Ekker SC & Lohr JL (2010) WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn, 239, 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C & Cohn MJ (2005) Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development, 132, 2441–50. [DOI] [PubMed] [Google Scholar]
- Qian C, Wu Z, Ng RC, Garcia-Barceló MM, Yuan ZW, Wong KKY, Tam PKH & Lui VCH (2019) Conditional deletion of platelet derived growth factor receptor alpha (Pdgfra) in urorectal mesenchyme causes mesenchyme apoptosis and urorectal developmental anomalies in mice. Cell Death Differ, 26, 1396–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM & French FS (1995) Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev, 16, 271–321. [DOI] [PubMed] [Google Scholar]
- Sagner A & Briscoe J (2017) Morphogen interpretation: concentration, time, competence, and signaling dynamics. Wiley Interdiscip Rev Dev Biol, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B & Henderson N (1990) Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol, 2, 441–9. [PubMed] [Google Scholar]
- Seifert AW, Bouldin CM, Choi KS, Harfe BD & Cohn MJ (2009a) Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development, 136, 3949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Yamaguchi T & Cohn MJ (2009b) Functional and phylogenetic analysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development. Development, 136, 2643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Zheng Z, Ormerod BK & Cohn MJ (2010) Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun, 1, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF & Bradley A (2011) A conditional knockout resource for the genome-wide study of mouse gene function. Nature, 474, 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer A, van den Heijkant M & Baumann S (2016) Worldwide prevalence of hypospadias. J Pediatr Urol, 12, 152.e1–7. [DOI] [PubMed] [Google Scholar]
- Sureda-Gomez M & Adell T (2019) Planarian organizers. Semin Cell Dev Biol, 87, 95–104. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Matsushita S, Suzuki K & Yamada G (2017) 5α-Dihydrotestosterone negatively regulates cell proliferation of the periurethral ventral mesenchyme during urethral tube formation in the murine male genital tubercle. Andrology, 5, 146–152. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Numata T, Suzuki H, Raga DD, Ipulan LA, Yokoyama C, Matsushita S, Hamada M, Nakagata N, Nishinakamura R, Kume S, Takahashi S & Yamada G (2014) Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation. Proc Natl Acad Sci U S A, 111, 16407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CC, Sala FG, Ford HR, Wang KS, Li C, Minoo P, Grikscheit TC & Bellusci S (2009) Wnt5a knock-out mouse as a new model of anorectal malformation. J Surg Res, 156, 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannour-Louet M, Han S, Louet JF, Zhang B, Romero K, Addai J, Sahin A, Cheung SW & Lamb DJ (2014) Increased gene copy number of VAMP7 disrupts human male urogenital development through altered estrogen action. Nat Med, 20, 715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK & Kotan LD (2016) Genetics of Hypogonadotropic Hypogonadism. Endocr Dev, 29, 36–49. [DOI] [PubMed] [Google Scholar]
- Tüzel E, Samli H, Kuru I, Türkmen S, Demir Y, Maralcan G & Güler C (2007) Association of hypospadias with hypoplastic synpolydactyly and role of HOXD13 gene mutations. Urology, 70, 161–4. [DOI] [PubMed] [Google Scholar]
- Via S (2009) Natural selection in action during speciation. Proc Natl Acad Sci U S A, 106 Suppl 1, 9939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gargollo P, Guo C, Tang T, Mingin G, Sun Y & Li X (2011) Six1 and Eya1 are critical regulators of peri-cloacal mesenchymal progenitors during genitourinary tract development. Dev Biol, 360, 186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DA, Rodriguez E, Cunha T, Menshenina J, Barcellos D, Chan LY, Risbridger G, Baskin L & Cunha G (2012) Morphology of the external genitalia of the adult male and female mice as an endpoint of sex differentiation. Mol Cell Endocrinol, 354, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A & Chen Y (2006) Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn, 235, 1738–52. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP & Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development, 126, 1211–23. [DOI] [PubMed] [Google Scholar]
- Yucel S, Dravis C, Garcia N, Henkemeyer M & Baker LA (2007) Hypospadias and anorectal malformations mediated by Eph/ephrin signaling. J Pediatr Urol, 3, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel S, Liu W, Cordero D, Donjacour A, Cunha G & Baskin LS (2004) Anatomical studies of the fibroblast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Adv Exp Med Biol, 545, 123–48. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Armfield BA & Cohn MJ (2015) Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc Natl Acad Sci U S A, 112, E7194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]