Abstract
Introduction
Participant retention is important to maintaining statistical power, minimizing bias, and preventing scientific error in Alzheimer’s disease (AD) and related dementias research.
Methods
We surveyed representative investigators from NIH-funded Alzheimer’s Disease Research Centers (ADRC), querying their use of retention tactics across twelve strategies. We compared survey results to data from the National Alzheimer’s Coordinating Center for each center. We used a generalized estimating equation with independent working covariance model and empirical standard errors to assess relationships between survey results and rates of retention, controlling for participant characteristics.
Results
Twenty-five (83%) responding ADRCs employed an average 42 (standard deviation=7) retention tactics. In a multivariable model that accounted for participant characteristics, the number of retention tactics used by a center was associated with participant retention (OR=1.68, 95% CI 1.42, 1.98; p<0.001 for the middle compared to the lowest tertile survey scores; OR=1.59, 95% CI 1.30, 1.94; p<0.001 for the highest compared to the lowest tertile survey scores) at the first follow-up visit. Participant characteristics such as normal cognition diagnosis, older age, higher education, and Caucasian race were also associated with higher retention.
Conclusions
Retention in clinical research is more likely to be achieved by employing a variety of tactics.
Keywords: missing, missingness, dropout, retention, attrition
Introduction
Achieving adequate recruitment and retention of participants poses a significant barrier to clinical research.1,2 Challenges in accruing a full sample and retaining participants to study completion in Alzheimer’s disease (AD) and Related Dementias (ADRD) research are pronounced, due to the need to recruit older participants who may have multiple co-morbidities as well as cognitive impairment and may lack capacity to provide informed consent.3 Recent commentaries address a crisis in ADRD research recruitment4–6 and national efforts are underway to develop evidence-based methods for improving participant accrual.7 Equally important to the research goals in ADRD will be improved methods to retain participants. Studies with greater than expected loss to follow-up are underpowered, may have limited impact, and provide questionable validity due to the potential for non-ignorable dropout that may bias analytic results.8–10 Thus, a National Academies of Sciences panel on the prevention and treatment of missing data in clinical trials recommends to “design clinical trials consistent with the goal of maximizing the number of participants who are maintained on the protocol-specified intervention until the outcome data are collected.”11 Yet, due to the challenges associated with ADRD research, one review found that 19 of 29 AD clinical trials failed to achieve 80% completion (a common assumption for study power calculations).3
The AD Research Center (ADRC) system is a network of academic centers funded by the National Institute on Aging to perform longitudinal research across the spectrum of ADRD. This includes collecting protocol-derived clinical and neuropsychological assessments.12,13 A critical aspect of this research enterprise is to retain participants longitudinally, ideally across clinical diagnostic transitions and even to death.14
Recent meta-analyses identified more than 900 retention tactics and showed that the number of tactics employed was positively correlated with higher study completion rates.15,16 The use and effectiveness of these retention tactics in ADRD research have been minimally investigated. To examine retention practices among the ADRCs, we developed and administered an on-line survey. To assess whether these practices are associated with retention rates,11 we compared survey results to data each center provided to a common national database, the Uniform Data Set (UDS), housed by the National Alzheimer’s Coordinating Center (NACC). We hypothesized that greater use of retention practices is associated with greater retention rates and that specific populations (e.g., those with cognitive impairment and those lacking a spouse) are more difficult to retain in longitudinal research.
Methods
Survey instrument
Based on recent publications,15,16 we developed a survey that assessed the use of retention tactics categorized into 12 strategies. Strategies included community involvement (involve community in study design, recruitment, and retention); study identity (create a study identity for participants); study personnel (characteristics, training, and management of study personnel); study description (explain study requirements and details, including potential benefits and risks to participants); reminders (provide reminders about appointments and study participation); contact and scheduling methods (use systematic methods for patient contact, appointment scheduling, and cohort retention monitoring); visit characteristics (minimize participant burden through characteristics and procedures of follow-up study visits); benefits of study (provide benefits to participants and families that are directly related to the nature of the study); financial incentives (provide financial incentives or payments); reimbursements or cost coverage (provide reimbursement for research-related expenses or tangible support to facilitate participation); non-financial incentives (provide non-financial incentives or tokens of appreciation); and special tracking methods (methods of tracking or dealing with hard-to-find or difficult participants). In addition to the retention themes and tactics, we asked specific questions related to ADRC practices including the average lengths of study visits and the neuropsychological test battery, and whether centers required some or all subjects to participate in blood draw, neuroimaging, lumbar puncture, and autopsy studies. While clinical and cognitive assessments are standardized parts of the UDS visits performed at every ADRC, the use of optional modules and biomarker assessments, as well as ADRC-specific research studies, result in variability across centers in visit lengths.17,18
The survey was developed by investigators at the UC Irvine ADRC and then reviewed for completion and understandability sequentially by investigators at two geographically dispersed centers (University of Kansas and Oregon Health & Science University). Questions assessed whether centers engaged in up to twelve specific tactics for each retention strategy (Appendix). Additionally, for each strategy, up to 25 additional examples could be provided by respondents. The final survey was distributed on September 29, 2017 via online data collection and a Research Electronic Data Capture (REDCap) database.19 Survey links were sent to leaders of the Outreach, Recruitment and Engagement Cores of the thirty funded ADRCs. These Cores serve as a bridge between the ADRC and the community at large, and are responsible for implementing center recruitment and retention strategies. We requested that only one survey be completed per ADRC and that the survey be completed in collaboration with the ADRC Clinical Core.
Data analyses
We examined the frequencies with which retention tactics were used across ADRCs. In the absence of a priori data to suggest differential importance of tactics, we calculated a total retention score by summing the number of endorsed tactics across the twelve strategies for each center.
To assess whether retention practices were associated with longitudinal research retention rates, we used data from the NACC database for the specific centers that responded to the survey. Data from one center that had recently halted depositing clinical data into NACC were excluded in models of retention rates.
We assessed NACC UDS data from a period of 2012 through 2017. We restricted our analyses to participants newly enrolled between 2012 and 2015 to eliminate potential cohort effects for subjects who had been retained for multiple years prior to data collection. We defined a participant as retained for their first follow-up visit (F1) if the subject returned for a visit within a two-year period. In NACC, participants are anticipated to have annual follow-up visits, based on the original date of initial visit, within a 6-month window (±3-months from the target date, which is the calendar date of the baseline visit). Thus, the 2-year period of assessing retention vs. drop-out was deemed a conservative approach of estimating retention. Participants enrolling at their respective center less than two years prior to the data freeze were removed from the analysis as they were not observed for the full duration of their scheduled visit window. Similarly, participants who died prior to the completion of their first scheduled visit window were also removed from analysis. Retention to the second and third follow-up visits (F2 and F3) was assessed identically, restricted to those participants who had an F1 and F2 visit, respectively, during the study period.
In addition to total retention score (examined as lower, middle and upper tertiles), we examined variables chosen a priori including participant sex, race (Caucasian, African American, Asian, other), ethnicity (Hispanic or non-Hispanic), age (as 5-year increments), study partner type (spousal, adult child, other), comorbidities (the presence or absence of a history of stroke or cardiovascular disease) and diagnostic status. For diagnostic status, we categorized participants as normal, Mild Cognitive Impairment (MCI), or dementia at the baseline visit. In addition to covariates derived from NACC, we included two covariates collected by the survey that were not components of a specific retention strategy: ADRC visit length and neuropsychological test battery length. We also chose, a priori, to include an interaction between gender and marital status. We examined the relative contributions of these associations through a multivariable generalized estimating equation (GEE) logistic regression model. An independence working correlation structure was assumed and empirical sandwich variance estimates were used for all inference.20
To assess whether associations between retention tactics and participant retention were consistent across the duration of participation (e.g., F2 compared to F1 and F3 compared to F2), we considered an interaction term between retention tactics and follow-up periods. We report follow-up visit-specific estimates for the association between retention tactics and the probability of retention. We used multivariate Wald tests to test for effect modification of retention tactics by visit number.
Results
Survey results
Twenty-five centers completed the survey (83% response rate). Table 1 outlines the proportion of ADRCs using specific retention tactics. Most centers reported performing retention tactics within each of the themes.
Table 1.
ADRC Retention Survey Responses
| Strategy/tactic | ADRCs, n (%)* | ADRCs, n (%)# |
|---|---|---|
| Community involvement | 25 (100) | 24 (100) |
| • Involve participants in retention (e.g., testimonials) | 21 (84) | 20 (83) |
| • Maintain community advisory boards to assist with understanding participant groups | 19 (76) | 19 (79) |
| • Involve advocates in review of ongoing practices | 14 (56) | 14 (58) |
| • Involve advocates in study design | 5 (20) | 5 (21) |
| • Other ○ Ethics committee that includes participants ○ Focus groups ○ Participant satisfaction surveys |
9 (36) | 9 (38) |
| Study identity | 25 (100) | 24 (100) |
| • Website | 25 (100) | 24 (100) |
| • Center logo | 23 (92) | 22 (92) |
| • Electronic or print newsletters | 22 (88) | 21 (88) |
| 17 (68) | 17 (71) | |
| 11 (44) | 11 (46) | |
| • YouTube | 7 (28) | 7 (29) |
| • Blog | 4 (16) | 4 (17) |
| • Other ○ Live webinars ○ ‘Ask the Expert’ section of center website and participant feedback link |
6 (24) | 6 (25) |
| Study personnel | 24 (96) | 23 (96) |
| • Diverse staff | 23 (92) | 22 (92) |
| • Identify specific staff members who are responsible for retention of particular participants | 20 (80) | 19 (79) |
| • Ensure that specific staff (study clinician) see participants consistently over time | 18 (72) | 17 (71) |
| • New staff training on retention | 14 (56) | 14 (58) |
| • Annual staff training on retention | 9 (36) | 9 (38) |
| • Other ○ Twice annual staff retreat with focus on retention |
1 (4) | 1 (4) |
| Study description | 25 (100) | 24 (100) |
| • Emphasize importance of long-term participation in recruitment materials and consents | 25 (100) | 24 (100) |
| • Provide opportunity to ask questions of qualified researchers at each visit | 25 (100) | 24 (100) |
| • Offer other opportunities for communication/interaction with faculty/staff | 24 (96) | 23 (96) |
| • Engage in family conferences during participation (e.g., for change in diagnosis) | 20 (80) | 19 (79) |
| • Engage in family conferences prior to enrollment | 10 (40) | 9 (38) |
| • Video description | 4 (16) | 4 (17) |
| • Utilize decision aids during informed consent | 4 (16) | 4 (17) |
| • Other ○ Spanish translation ○ Video for lumbar puncture |
2 (8) | 2 (8) |
| Reminders | 23 (92) | 22 (92) |
| • Between-visit telephone calls | 17 (68) | 16 (67) |
| • Send pre-visit materials for completion | 17 (68) | 16 (67) |
| • Mailed appointment cards | 15 (60) | 15 (63) |
| • Appointment cards at each visit | 8 (32) | 8 (33) |
| • Regular PI letters or emails between visits | 6 (24) | 5 (21) |
| • Text message reminders | 2 (8) | 1 (4) |
| • Other | 0 (0) | 0 (0) |
| Contact and scheduling | 25 (100) | 24 (100) |
| • Use data capture system (e.g., REDCap) to monitor visit windows | 23 (92) | 22 (92) |
| • Use alerts to study staff to send visit reminders | 17 (68) | 16 (67) |
| • Use automated email systems to remind participants about visits | 3 (12) | 3 (13) |
| • Use automated telephone systems to remind participants about visits | 4 (16) | 4 (17) |
| • Use automated text messaging systems to remind participants about visits | 1 (4) | 1 (4) |
| • Other ○ ‘personal’ touch calls or emails from familiar staff |
1 (4) | 1 (4) |
| Visit characteristics | 25 (100) | 24 (100) |
| • Offer breaks during visits | 25 (100) | 24 (100) |
| • Permit telephone completion of study partner activities | 25 (100) | 24 (100) |
| • Follow participants by phone if it becomes impossible for them to attend visits due to disease severity | 25 (100) | 24 (100) |
| • Offer water and snacks during visits | 23 (92) | 22 (92) |
| • Schedule visits around participants’ needs/energy level | 22 (88) | 21 (88) |
| • Permit splitting of visits into 2 or more sessions over 2 or more days | 21 (84) | 20 (83) |
| • Permit splitting of visits into 2 sessions in a single day | 20 (80) | 19 (79) |
| • Hold visits at convenient (off site) locations | 10 (40) | 9 (38) |
| • Perform home visits | 9 (36) | 8 (33) |
| • Hold visits during non-traditional hours (evenings) | 5 (20) | 4 (17) |
| • Hold visits during non-traditional days (weekends) | 4 (16) | 3 (13) |
| • Other ○ Nursing home visits |
1 (4) | 1 (4) |
| Benefits of study | 25 (100) | 24 (100) |
| • Provide feedback on annual UDS evaluation to PCP or other provider if requested by participant | 22 (88) | 21 (88) |
| • Provide diagnostic results to participants | 20 (80) | 19 (79) |
| • Provide feedback on non-UDS evaluations (lab values, evaluations, MMSE, etc) to PCP or other provider if requested by participant | 19 (76) | 18 (75) |
| • Provide neuropsychological test results to participants | 17 (68) | 16 (67) |
| • Provide laboratory test results to participants | 17 (68) | 16 (67) |
| • Offer support groups | 17 (68) | 17 (71) |
| • Provide MRI biomarker results to participants | 12 (48) | 11 (46) |
| • Provide amyloid PET results to participants | 8 (32) | 8 (33) |
| • Provide CSF biomarker results to participants | 7 (28) | 7 (29) |
| • Provide FDG PET biomarker results to participants | 7 (28) | 7 (29) |
| • Provide genetic test results to participants | 3 (12) | 3 (13) |
| • Provide free clinical care (e.g., medication prescriptions) to participants | 3 (12) | 3 (13) |
| • Other ○ Provide access to a social worker |
1 (4) | 1 (4) |
| Financial incentives | 19 (76) | 18 (75) |
| • Provide annual cash payment for completing optional study procedures (e.g., lumbar puncture) | 11 (44) | 11 (46) |
| • Provide annual cash payment for completing study visits | 9 (36) | 9 (38) |
| • Provide annual non-cash payment (e.g., gift card) for completing optional study procedures (e.g., lumbar puncture) | 5 (20) | 5 (21) |
| • Provide annual non-cash payment (e.g., gift card) for completing study visits | 5 (20) | 4 (17) |
| • Other | 0 (0) | 0 (0) |
| Reimbursements or cost coverage | 24 (96) | 23 (96) |
| • Cover parking costs | 23 (92) | 22 (92) |
| • Reimburse for taxis or public transportation | 17 (68) | 16 (67) |
| • Provide meal cards or reimburse for meals during visits | 13 (52) | 12 (50) |
| • Provide gas cards or reimburse for mileage | 7 (28) | 7 (29) |
| • Offer lodging for long visits for participants who travel great distances | 5 (20) | 5 (21) |
| • Offer childcare during visits | 0 (0) | 0 (0) |
| • Other | 0 (0) | 0 (0) |
| Non-financial incentives | 22 (88) | 21 (88) |
| • Hold participant gratitude events | 21 (84) | 20 (83) |
| • Send seasonal holiday cards | 15 (60) | 14 (58) |
| • Offer participants value nominal gifts such as pens, magnets, etc. | 13 (52) | 12 (50) |
| • Provide participants awards or certificates of appreciation for milestone visits (e.g., 5- or 10-year interval) | 11 (44) | 10 (42) |
| • Send birthday cards | 9 (36) | 8 (33) |
| • Hold participant holiday celebrations | 5 (20) | 4 (17) |
| • Other ○ ADRC-stamped chocolate bars ○ Bereavement cards ○ Birthday telephone calls |
4 (16) | 4 (17) |
| Special tracking methods | 18 (72) | 17 (71) |
| • Maintain phone tree of individuals to contact if the participant cannot be reached | 16 (64) | 15 (63) |
| • Follow participants on social media and send private messages to remind of appointments or reach out if difficult to reach through traditional modes of communication | 0 (0) | 0 (0) |
| • Attend patient clinical visits as a means to contact them and request completion of study visits | 10 (40) | 10 (42) |
| • Other ○ No shows triaged for more intense calls and follow-up ○ Letters asking for contact if phone number has changed or cannot be reached |
2 (8) | 2 (8) |
Proportion of responses based on all ADRCs responding (n=25).
Proportion of responses based on ADRCs responses used in multivariable models (n=24).
Nearly all centers reported employing diverse staffs and coordinating visits in a manner such that specific staff members are responsible for seeing and retaining specific participants. Twenty-one centers indicated that they involve participants in retention, for example, through testimonials and advisory boards. Fewer reported involving participants in study design or review of ongoing study practices. While all centers reported having a website, fewer indicated using social media through Facebook, Twitter, YouTube, or blogs.
Every center responded that some form of between-visit communication was used, most frequently telephone calls and sending pre-visit materials. Nearly every center responded that they use an automated system for monitoring visit windows, including alert systems to remind staff to send visit invitations. Fewer acknowledged use of automated communication systems (email and phone) to actually contact participants. Though some centers reported maintaining a phone tree of individuals who could be contacted in the event of inability to reach a participant, few other special tracking methods were reported.
Every center reported flexibility around visit requirements, such as offering breaks and snacks, splitting visits, and performing at least some study aspects by phone. Few centers indicated that they perform visits at convenient locations, days of the week, or times of the day. Nearly all centers indicated that they reimburse for parking and/or public transportation and about half indicated that they provide meals. Most centers reported providing some form of financial incentives for participation, including cash or non-cash incentives. Some centers indicated that they only pay a subset of participants who undergo specific optional procedures such as lumbar puncture.
Nearly every center indicated that they provide diagnostic feedback from the annual visit, either directly to participants or to their treating physicians. This included laboratory and neuropsychological data at two-thirds of the responding centers. The frequency with which centers reported sharing biomarker research data with participants was lower. Twelve centers reported providing participants with structural neuroimaging results while eight reported returning results from molecular neuroimaging and seven reported returning cerebrospinal fluid results. Three centers reported returning genetic testing results to participants.
Five centers reported a total visit length under 3 hours. The most common total visit length reported was 3–4 hours (n=7). Five centers reported a visit length of 4–5 hours, five reported 5–6 hours, and 1 reported >6 hours. Most centers (n=15) reported a neuropsychological test battery below 2 hours. Five centers reported a battery length of 2–3 hours and one reported a battery of 3–4 hours.
Table 2 describes the proportion of ADRCs that require participants to undergo specific research procedures. Most centers indicated that they require blood draw. Fewer indicated requiring biomarker tests or autopsy. For blood draw, MRI, and lumbar puncture, at least some centers reported making exceptions to requirements for unique populations (e.g., underrepresented racial groups).
Table 2.
ADRC requirements of participation to undergo research procedures
| Procedures | Centers requiring procedure, n (%) | Centers requiring procedure with some exceptions (e.g., for underrepresented racial groups), n (%) |
|---|---|---|
| Blood draw | 13 (52) | 15 (60) |
| MRI | 8 (32) | 10 (40) |
| PET | 3 (12) | 3 (12) |
| Lumbar puncture | 2 (8) | 5 (20) |
| Autopsy | 5 (20) | 4 (16) |
Associations with participant retention
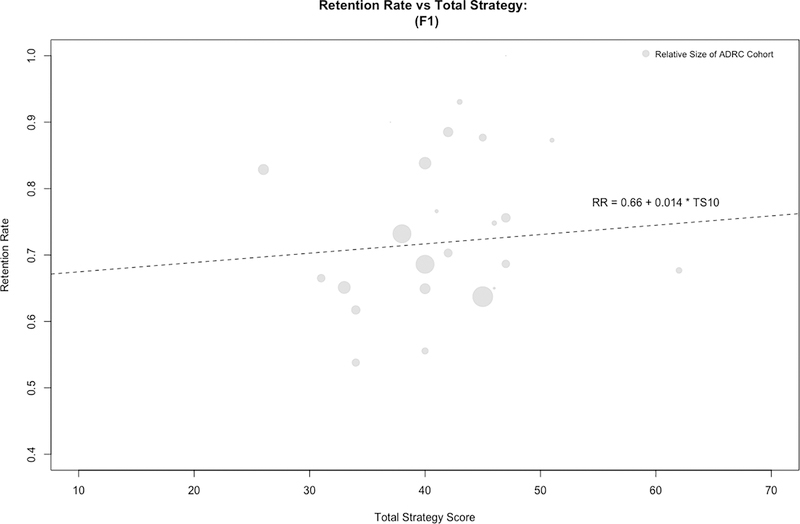
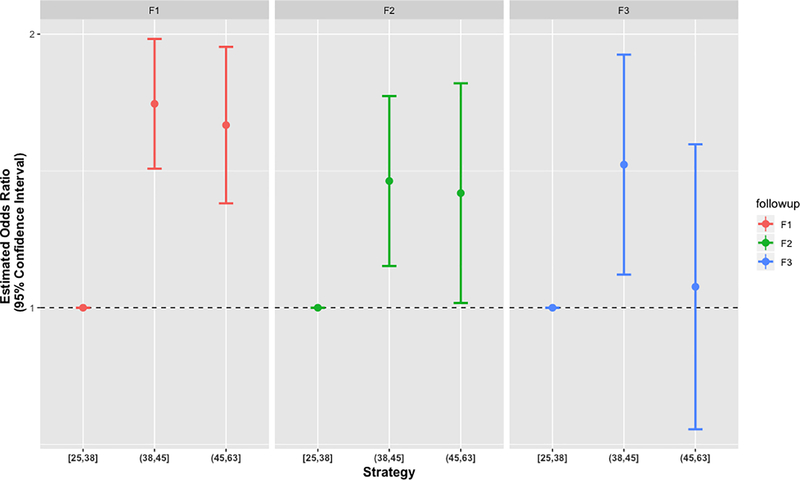
Overall, centers reported employing a mean 42 (SD=7) specific retention tactics, with a range of 25–62. Data were included for 5568 newly enrolled participants at 24 centers during the study period (Supplementary Table). Figure 1 illustrates the relationship between ADRC retention survey scores and first follow-up visit (F1) rates. Data were available for 2994 participants eligible for F2, and 1563 participants eligible for F3. In a GEE regression model, greater retention survey scores were associated with higher retention rates at F1 (Table 3; OR=1.67, 95% CI 1.42, 1.97; p<0.0001 for the middle [38–45] compared to the lowest tertile [25–38] survey scores; OR=1.58, 95% CI 1.30, 1.94; p<0.0001 for the highest [45–62] compared to the lowest tertile [25–38] survey scores). Thus, participants at centers employing at least 38 retention tactics were at least 58% more likely to return for their first follow-up visit, controlling for other covariates. The forest plots in Figure 2 illustrate the consistency of the effect of retention practices across F1, F2, and F3. While no significant interaction between the number of tactics and visit number was observed (p=0.192), the estimated association between retention tactics attenuated over the length of participation. Despite this attenuation, the estimates continued to indicate a positive association between the number of tactics used and the odds of retention at the F2 (Table 3; OR=1.38, 95% CI 1.11, 1.71; p=0.0035 for the middle compared to the lowest tertile survey scores; OR=1.34, 95% CI 1.01, 1.77; p=0.0408 for the highest compared to the lowest tertile survey scores) and F3 visits (Table 3; OR=1.44, 95% CI 1.09, 1.90; p=0.01 for the middle compared to the lowest tertile survey scores; OR=1.05, 95% CI 0.73, 1.51; p=0.77 for the highest compared to the lowest tertile survey scores).
Figure 1.
Relationship between retention strategies and retention rates. ADRC survey responses were used to calculate a total retention score (x-axis) and plotted vs. individual ADRC’s retention rates at F1 (y-axis). A positive correlation was observed, whereby retention rate increased 0.14% for every one unit increase on the survey score. RR=retention rate; TS=total retention survey score.
Table 3.
Multivariable model results
| Covariate | OR | 95% CI | P-value |
|---|---|---|---|
| Total strategy score • F1 (First Follow-up) [Range] ○ Lower [25,38] ○ Middle (38,45] ○ Upper (45,63] • F2 (Second Follow-up) [Range] ○ Lower [25,38] ○ Middle (38,45] ○ Upper (45,63] • F3 (Third Follow-up) [Range] ○ Lower [25,38] ○ Middle (38,45] ○ Upper (45,63] |
1.00 1.68 1.59 1.00 1.38 1.34 1.00 1.44 1.05 |
- (1.42, 1.98) (1.30, 1.94) - (1.11, 1.71) (1.01, 1.77) - (1.09, 1.90) (0.73, 1.51) |
<0.0001 - <0.0001 <0.0001 - 0.0035 0.0408 - 0.01 0.77 |
| Diagnosis • Normal control • MCI • Dementia |
1.00 0.79 0.62 |
- (0.69, 0.89) (0.55, 0.69) |
<0.0001 - 0.0003 <0.0001 |
| Race • White • Asian • African American • Other |
1.00 0.88 0.66 0.93 |
- (0.69, 1.12) (0.57, 0.76) (0.65, 1. 53) |
<0.0001 - 0.31 <0.0001 0.72 |
| Ethnicity • Non-Hispanic • Hispanic |
1.00 0.90 |
- (0.72, 1.12) |
- 0.36 |
| Study partner type • Spouse/partner • Adult child • Other |
1.00 0.86 0.89 |
- (0.73, 1.01) (0.76, 1.06) |
0.1783 - 0.06 0.19 |
| Age (x5 years) | 1.06 | (1.03, 1.09) | <0.0001 |
| Education (years) • Some high school • High school • Some college • College • Graduate |
1.00 1.29 1.47 1.70 1.81 |
- (1.01, 1.66) (1.14, 1.88) (1.32, 2.19) (1.4, 2.31) |
<0.0001 - 0.04 0.002 <0.0001 <0.0001 |
| Visit length* • 0–4 hours • 4–5 hours • >5 hours |
1.00 0.90 0.65 |
- (0.78, 1.03) (0.54, 0.78) |
<0.0001 - 0.14 <0.0001 |
| Battery length* • 0–2 hours • >2 hours |
1.00 1.10 |
- (0.95, 1.27) |
- 0.20 |
| Unmarried participants • Female vs. male |
1.24 |
(1.02, 1.50) |
0.023 |
| Married participants • Female vs. male |
0.83 |
(0.74, 0.93) |
0.002 |
Based on distribution of results and observed regression coefficients, total ADRC visit length and neuropsychological battery length were incorporated as binary covariates in regression models.
Test for interaction between number of tactics and follow-up visit resulted in a p-value of 0.192
Test for interaction between sex and marital status resulted in a p-value of 0.0002599.
Figure 2.
Forest plots of associations between retention scores and retention performance at F1, F2, and F3. The estimates for F2 and F3 (for each survey score tertile) are presented with estimated 95% CIs. All estimates are relative to the rates for the lowest survey score tertile.
Several other factors were associated with participant retention. Compared to participants with a normal control diagnosis, participants with MCI and dementia were estimated to have 21% (OR=0.79, 95% CI 0.69, 0.89; p=0.0003) and 38% (OR=0.62, 95% CI 0.55, 0.69; p<0.0001) lower odds of being retained, respectively. Compared to Caucasians, African Americans were estimated to have 24% lower odds of being retained. Higher education, older age, and having fewer comorbidities were all associated with greater odds of being retained. Among unmarried participants, female participants were estimated to have 24% higher odds of being retained than male participants (OR=1.24, 95% CI 1.02, 1.50; p=0.02). For married participants, female participants were estimated to have a 17% lower odds than male participants (OR=0.83, 95% CI 0.74, 0.93; p=0.002) of being retained (Table 3).
Although the length of the neuropsychological battery had no effect on participant retention, overall visit lengths that were greater than 5 hours, compared to those less than 4 hours, were associated with poorer retention.
Discussion
Retaining participants is critical to minimizing bias and error and ensuring that research advances knowledge.21 Predictors of loss-to-follow-up and means to reduce data missingness remain areas in need of research. We examined participant retention practices at NIH-funded ADRCs. We found that centers consistently engage in a large number of retention activities and that at most centers these activities include most or all of 12 recently outlined retention strategies.15,16 The universal or near-universal practice of some retention tactics by ADRCs (e.g., providing diagnostic feedback and reimbursing for parking, see Table 1), suggests that these tactics are consistently deemed important by investigators. Differences were apparent, however, among the centers in specific tactics employed and there was a sizeable range in the total number of tactics used (25–62). The number of tactics used by a center was associated with rates of participant retention. Centers in the middle and upper tertiles of survey scores, equating to employing at least 38 retention tactics, outperformed the lowest tertile (<38 tactics) in participant retention. This suggests that inadequate retention effort may risk greater than expected dropout and that increasing the number of retention tactics for a given center or study could meaningfully increase participant retention or completion rates.
The relationship between a center’s number of retention tactics and the retention rate was strongest for the first follow-up visit. An initial period of preventing dropout may therefore be most critical, after which participant retention may require less intervention. This conclusion is supported by the observation that, for the centers that reported the lowest numbers of retention tactics, retention rates were higher at F2 and F3, compared to at F1, controlling for other covariates.
In addition to the number of tactics employed at a center, the structure of study visits was also associated with retention. Participants at centers where visits took more than 5 hours were 35% less likely to be retained. In contrast, we observed a trend toward longer cognitive testing batteries being associated with slightly higher retention. This may suggest that the composition of study visits, as well as their total length, is important to optimize participant retention. Across centers, there was consistent use of tactics to ease the burden of participation, such as offering breaks, performing some study aspects by telephone, and breaking visits into multiple days. Opportunities to participate at non-traditional locations (e.g., in the home) and times (e.g., evenings or weekends), however, were less frequently offered. Notable absences from our analyses were whether participants resided in urban or rural settings, whether they used personal or public transportation to attend study visits, the distance they lived from the ADRC, and the time required for participants to commute to study visits.
We did identify a number of demographic characteristics that were associated with participant retention. In most cases, these observations were consistent with our hypotheses and previous observations in the literature. Individuals with MCI and dementia had lower retention rates than those with normal cognition.22 Those with lower education and younger age at enrollment were at greater risk to be lost to follow-up.23 Racial and ethnic minorities had lower retention rates than did non-Hispanic Caucasians.23–25 The identification of risk factors for loss-to-follow-up may be important for instructing elements of study design, recruitment and enrollment practices, and focused retention efforts for particular studies or participants.
One observation that contrasted previous findings was a lack of difference in retention rates among dyad types, based on the relationship between the participant and their study partner.24,26,27 Nonspousal dyads were not significantly more likely to be lost, compared to participants with a spousal study partner. We did, however, observe an interesting dichotomy of dropout risk based on participant sex, depending upon whether the participant was married. Among unmarried participants, females were more likely to be retained, whereas married female participants were less likely than married male participants to be retained. This suggests that study partner sex (most non-spousal study partners are female), may be more important than the relationship to the participant (or the participant’s sex).
Limitations
Several limitations of this study should be noted. We aimed to assess, on a broad level, the general retention practices of ADRCs. We attempted to limit the length of the survey to maximize response rate and there was no opportunity for follow-up questions. Our survey did not permit respondents to provide results specific to diagnostic or other subpopulations for most items. For example, centers may provide amyloid positron emission tomography (PET) results28 to participants deemed appropriate but not to those deemed inappropriate by expert panels.29 The survey items examining center practices related to financial incentives and study procedures (such as amyloid imaging, blood draws, and lumbar puncture), in fact, indicate that at least some centers have different practices for different participants. We focused on the first three follow-up visits because we deemed retention of newly enrolled participants during the study period most pertinent to our research question. Doing so, however, limited data analyses to less than half of the total number of NACC UDS participants and most centers attempt to retain participants for very long (>3 years) periods of follow-up. Though potential interactions could be of interest (e.g., do retention tactics work differently in specific diagnostic populations), we chose to model associations without such interactions to ensure clarity of results. Similarly, systematically examining the impact of specific tactics is beyond the scope of the current study.
Conclusions
These results demonstrate that centers consistently engage in a large number of retention strategies, but that the specific tactics employed vary among centers. The total number of tactics used by a center is associated with the probability that participants will be retained. Specific participants may be more challenging to retain. Future research should examine whether specific tactics are most effective, whether tactics differ in their effectiveness for specific subpopulations, and how they may need to change over time.
Supplementary Material
Acknowledgements
JDG, DLG, and BN were supported by NIA AG016573, 1R21AG056931, and AG059407. JDG is currently supported by UL1 TR000153.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
References
- 1.Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311(10):1045–1051. [DOI] [PubMed] [Google Scholar]
- 2.Amstutz A, Schandelmaier S, Frei R, et al. Discontinuation and non-publication of randomised clinical trials supported by the main public funding body in Switzerland: a retrospective cohort study. BMJ open. 2017;7(7):e016216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers Res Ther. 2010;2(6):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimers Dement. 2016;12(11):1113–1115. [DOI] [PubMed] [Google Scholar]
- 5.Recruitment Vellas B., retention and other methodological issues related to clinical trials for Alzheimer’s disease. The journal of nutrition, health & aging. 2012;16(4):330. [DOI] [PubMed] [Google Scholar]
- 6.Vellas B, Hampel H, Rouge-Bugat ME, et al. Alzheimer’s disease therapeutic trials: EU/US Task Force report on recruitment, retention, and methodology. The journal of nutrition, health & aging. 2012;16(4):339–345. [DOI] [PubMed] [Google Scholar]
- 7.National Institute on Aging. Together we make the difference: National strategy for recruitment and participation in Alzheimer’s and related dementias clinical research. 2018. [DOI] [PMC free article] [PubMed]
- 8.Gross RA, Johnston KC. Levels of evidence: Taking Neurology to the next level. Neurology. 2009;72(1):8–10. [DOI] [PubMed] [Google Scholar]
- 9.French J, Gronseth G. Lost in a jungle of evidence: we need a compass. Neurology. 2008;71(20):1634–1638. [DOI] [PubMed] [Google Scholar]
- 10.Weuve J, Sagiv SK, Fox MP. Quantitative Bias Analysis for Collaborative Science. Epidemiology. 2018;29(5):627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council (US) Panel on Handling Missing Data in Clinical Trials. The prevention and treatment of missing data in clinical trials. Washington, DC: The National Academies Press;2010. [PubMed] [Google Scholar]
- 12.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. [DOI] [PubMed] [Google Scholar]
- 14.Boise L, Hinton L, Rosen HJ, et al. Willingness to Be a Brain Donor: A Survey of Research Volunteers From 4 Racial/Ethnic Groups. Alzheimer Dis Assoc Disord. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identifies number of strategies important for retaining study participants. Journal of clinical epidemiology. 2007;60(8):757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. Journal of clinical epidemiology. 2015;68(12):1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 21.Halpern SD, Karlawish JH, Berlin JA. The continuing unethical conduct of underpowered clinical trials. JAMA. 2002;288(3):358–362. [DOI] [PubMed] [Google Scholar]
- 22.Lo RY, Jagust WJ. Predicting missing biomarker data in a longitudinal study of Alzheimer disease. Neurology. 2012;78(18):1376–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edland SD, Emond JA, Aisen PS, Petersen RC. NIA-funded Alzheimer centers are more efficient than commercial clinical recruitment sites for conducting secondary prevention trials of dementia. Alzheimer Dis Assoc Disord. 2010;24(2):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koss E, Peterson B, Fillenbaum GG. Determinants of attrition in a natural history study of Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13(4):209–215. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy RE, Cutter GR, Wang G, Schneider LS. Challenging Assumptions About African American Participation in Alzheimer Disease Trials. Am J Geriatr Psychiatry. 2017;25(10):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grill JD, Raman R, Ernstrom K, Aisen P, Karlawish J. Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology. 2013;80(3):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coley N, Gardette V, Toulza O, et al. Predictive factors of attrition in a cohort of Alzheimer disease patients. The REAL.FR study. Neuroepidemiology. 2008;31(2):69–79. [DOI] [PubMed] [Google Scholar]
- 28.Lingler JH, Klunk WE. Disclosure of amyloid imaging results to research participants: has the time come? Alzheimers Dement. 2013;9(6):741–744 e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med. 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.