Abstract
Introduction:
The 2010 Deepwater Horizon (DWH) disaster exposed tens of thousands of oil spill response and cleanup (OSRC) workers to hydrocarbons and other hazardous chemicals. Some hydrocarbons, such as toluene and hexane, have been found to have acute adverse effects on the central nervous system in occupational settings. However, no studies have examined the association between oil spill exposures and neurobehavioral function.
Methods:
We used data from the Gulf Long-term Follow-up Study, a cohort of adults who worked on the DWH response and cleanup. Total hydrocarbon (THC) exposure attributed to oil spill cleanup work was estimated from a job-exposure matrix linking air measurement data to detailed cleanup work histories. Participants were also categorized into 6 job categories, or OSRC classes, based on their activity with the highest exposure. Neurobehavioral performance was assessed at a clinical exam 4–6 years after the spill. We used multivariable linear regression to evaluate relationships of ordinal THC levels and OSRC classes with 16 neurobehavioral outcomes.
Results:
We found limited evidence of associations between THC levels or OSRC classes and decreased neurobehavioral function, including attention, memory, and executive function. Workers exposed to ≥3 ppm THC scored significantly worse (difference1.0–2.9ppm =−0.39, 95% confidence interval (CI)=−0.74, −0.04) than workers exposed to <0.30 ppm THC for the digit span forward count test. There was also a possible threshold effect above 1 ppm THC for symbol digit test total errors (difference1.0–2.9ppm =−0.56 (95% CI=−1.13, −0.003), difference≥3.0ppm =−0.55 (95% CI=−1.20, 0.10)). Associations appeared to be stronger in men than in women. A summary latency measure suggested an association between more highly exposed jobs (especially support of operations workers) and decreased neurobehavioral function.
Conclusion:
OSRC-related exposures were associated with modest decreases in neurobehavioral function, especially attention, memory, and executive function.
Keywords: Hydrocarbons, occupational epidemiology, neurobehavioral function, oil spills, disasters
INTRODUCTION:
The 2010 Deepwater Horizon (DWH) disaster was the largest marine oil spill by volume in history. The spill released nearly 5 million barrels of crude oil into the Gulf of Mexico and exposed tens of thousands of oil spill response and cleanup (OSRC) workers and residents to hydrocarbons, particulate matter, dispersants, and other potentially harmful chemicals(1). Several components of crude oil are classified as known or potential neurotoxins, but the exposure threshold for their neurotoxicity is unclear(2,3). Crude oil is a mixture of various hydrocarbons. Even if exposure to each constituent is below its occupational exposure limit, exposure may still cause harm to oil spill workers via additive or synergistic interactions among the chemicals(4).
During past oil spills, cleanup workers reported symptoms such as headaches and visual, cognitive, and memory disturbances(5). Crude oil contains hydrocarbons, which include benzene, toluene, ethylbenzene, xylene (known collectively as BTEX), and hexane, volatile chemicals that can be inhaled by OSRC workers. Inhalation of these chemicals is a concern because they are very lipophilic and readily enter the central nervous system, which can cause acute neurological effects at high airborne concentrations(6–13). Toluene has been the best characterized of these, and chronic toluene exposure has been linked to inattention, memory dysfunction, visuospatial impairment, cognitive impairment, decreased information processing speed, and dementia(8). Elevated blood benzene levels have also been associated with lower neurobehavioral performance(6). Hexane is a known peripheral neurotoxin in humans and has been found to cause toxicity in the peripheral and central nervous systems of rats(14).
Ambient hexane concentrations were higher than those of BTEX during the response and cleanup effort following the DWH disaster because hexane makes up about the same percentage of crude oil (by weight) as the BTEX chemicals combined, but it is more volatile than the BTEX chemicals(15,16). The total hydrocarbon (THC) levels to which most Deepwater Horizon OSRC workers were exposed (including hexane), however, were at the lower end of levels typically encountered in occupational settings, but above levels typically experienced by the general population(17). Understanding oil spill exposures and their relationship to neurotoxic outcomes in humans is important to prevent central nervous system damage to workers and to people who live near future oil disasters, especially for sensitive populations in the community.
Previous studies have reported a variety of health symptoms following exposure from oil spills(18–23), but few studies have examined neurological symptoms or used neurobehavioral tests to assess cognitive function. The Gulf Long-term Follow-up Study (GuLF Study) enrolled Deepwater Horizon OSRC workers and comparison subjects to investigate the health consequences of exposures associated with this oil spill. In this analysis, we examine THC estimates as a marker of oil spill chemical exposure, as well as OSRC job classes(17). The THC estimate is a composite measurement of all oil-related volatile hydrocarbons, including BTEX, hexane, cyclohexane, heptane, and trimethylbenzenes(17). This study examines the association of THC concentrations and OSRC classes with neurobehavioral function among oil spill workers.
METHODS:
To examine the relationship between oil spill exposures and neurobehavioral function, we used data from the GuLF Study, a prospective cohort study of 32,608 adults, ≥21 years old at enrollment, who worked on the Deepwater Horizon OSRC or who received worker safety training but did not work on the spill(1). Phone interviews were conducted from March 2011 to May 2013 to enroll participants and to obtain detailed demographic, health, and OSRC work-related information. Four to six years after the oil spill, cohort members who lived within 60 miles of research clinics in Mobile, AL or New Orleans, LA were invited to complete a clinical exam. A total of 3,401 persons (62% of those eligible) completed the exam (Figure A.1). The clinical exam included standardized neurobehavioral tests, which were completed by 3,291 participants.
Exposure assessment:
GuLF Study industrial hygienists linked detailed, participant-reported work information from the enrollment interview with exposure estimates derived from approximately 28,000 personal air samples to estimate THC exposure by work activity, location, and dates(17). Many participants reported multiple activities, so an individual’s exposure was based on his or her highest exposed activity, expressed as an arithmetic mean. Ordinal maximum THC categories (<0.30 ppm, 0.30–0.99 ppm, 1.0–2.99 ppm, ≥3.00 ppm) were created using a pseudo-log scale based on the empirical exposure range of the means in the study population to allow for uncertainty in the estimates for individual workers(17).
Six broad hierarchical OSRC classes were also created, based on the likelihood of exposure to oil spill-related chemicals, from the work activities in each class to assess the health risk for different types of work. A single class was assigned to each participant, based on the participant’s highest exposure job. From the highest to lowest expected exposure, the job classes were: response workers, who worked on rigs and other large vessels at the wellhead; support of operations workers, who worked mostly on land, handling oily waste; cleanup/water workers, who worked on water away from the wellhead; decontamination workers, who removed oil from vessels, equipment, wildlife, and personnel; cleanup/land workers, who generally worked on beaches and marshes; and administrative support workers, who supported the overall response with a wide range of functions including food service, security, onsite and offsite transportation, and office work (Table A.1). The administrative support workers served as the reference group. All analyses were restricted to OSRC workers to ensure comparability within the study population, as participants who completed the worker safety training but did not work tended to have different demographics than the workers.
Outcome assessment:
Neurobehavioral function was evaluated using the Behavioral Assessment and Research System (BARS), a series of computerized tests with simple verbal instructions and graphics which uses a response keyboard with nine large buttons for ease of use(24). BARS is designed to measure neurobehavioral function in people with limited formal education, literacy, or computer skills. Tests used in the current study included a continuous performance test (CPT) that measures sustained visual attention and short-term memory, a digit span test (DST) that measures attention and memory, a match-to-sample test (MTS) that measures visual memory, a symbol-digit test (SDT) that measures executive function and coding, a simple reaction time test (SRT) that measures response speed, a finger tapping test (TAP) that measures response speed and coordination, and a progressive ratio test (PRT) that measures effort-related motivation (Table 1). Each test has one or more outcome measures, which assess factors such as response latency, error, and correct responses. For example, the continuous performance test measures correct response fraction (number of correctly identified targets over total number of targets), false alarm fraction (number of stimuli identified as targets incorrectly over total number of nontarget stimuli), false latency (number of milliseconds between stimuli and an incorrect response), and d prime (rate of correct hits minus rate of incorrect hits)(25,26).
Table 1.
Individual test descriptions and measured function of Behavioral Assessment and Research Systems.
| Neurobehavioral Test | Function Measured | Brief Test Description | Outcome Variables |
|---|---|---|---|
| Continuous Performance Test (CPT) | Sustained visual attention | Press key when a certain target is presented, but do not press key when other stimuli are presented. | Correct response fraction, d prime, false alarm fraction, false latency, hit fraction |
| Digit Span (DST) | Attention, memory | Press the numbers shown on the screen in the same order or reverse order. Number of digits increases until failure criterion is met. | Forward count, reverse count |
| Match-to-Sample (MTS) | Visual memory | Matrix of blocks is displayed with 3 matrices below Participant matrix that matches the above matrix. | Average correct latency, number of correct responses |
| Symbol Digit (SDT) | Executive function, coding | Digits are paired with symbols in a matrix. Matrix at bottom has symbols but no digits. Participant types correct numbers that correspond with the symbols. | Average correct latency, total errors |
| Simple Reaction Time (SRT) | Response speed | Press button as quickly as possible after seeing a stimulus. | Average correct latency, total number of errors |
| Tapping (TAP) | Response speed, coordination | Press button(s) as rapidly as possible using index finger of one or both hands on 1 or 2 buttons | Alternative hands average number of taps, average number of taps with preferred hand |
| Progressive Ratio Test (PRT) | Effort-related motivation | Tap 10 times to receive a reward (smiling face). Then tap 20 times to get the reward, and then 30 times, etc. | Total taps |
Statistical analysis:
We examined separate multivariable linear regression models to assess the relationship between each of the 16 neurobehavioral outcome measures and OSRC exposures. We assessed neurobehavioral outcome associated with maximum THC exposure by comparing participants in the three higher exposure groups (0.30–0.99 ppm, 1.00–2.99 ppm, ≥3.00 ppm) to those in the lowest exposure group (<0.30 ppm). We also analyzed OSRC classes, where each OSRC class was compared to administrative support workers, who had the lowest chemical exposure. We identified confounders (sex, age, race, education, tobacco use status, as described below), based on a directed acyclic graph and included covariates strongly associated with the outcome if they changed more than half of the 16 estimates by more than 10%(27,28). We examined the associations in the entire analysis cohort as well as stratified by sex, as men and women perform differently on neurobehavioral tests, on average, and metabolism differences may modify the magnitude or nature of chemical effects(29–32). We also examined the relationship between oil spill-related exposures and neurobehavioral function stratified by tobacco use status because of the link between long-term tobacco use and impaired neurobehavioral function and because oil spill exposure includes some of the same neurotoxic components contained in cigarette smoke(33). Additionally, we examined the effect of follow-up time on associations by creating an indicator variable for follow-up time based on the median split (≤1880 days, >1880 days) and including in the models this variable and a multiplicative interaction term of this variable and the exposure categories.
Recent (within 2 hours before the neurobehavioral exam) caffeine use (n=835), recent alcohol consumption (n=8), or recent tobacco use (n=786) did not significantly change estimates, so these were not included as covariates or considered as exclusion factors. Binge drinking (self-reported consumption of ≥5 drinks on one occasion ≥12 times in the year before the clinical exam, n=287) and passive smoking (living with regular smokers at enrollment, n=545) significantly changed estimates and were included in our final models. We also evaluated other thresholds for binge drinking and they yielded minimal changes in effect estimates. The final model controlled for age (continuous), sex (male, female), race (white, black, other), education (less than high school, high school graduate or equivalent, some college, college graduate), binge drinking (yes, no), smoking/tobacco use status (current daily tobacco user, former daily tobacco user, never daily tobacco user, as reported in the clinical exam interview), and passive smoking (living with a smoker, not living with a smoker). We changed the sign of neurobehavioral outcome measures where a higher value indicates worse neurobehavioral function (CPT false alarm fraction, CPT false latency, MTS average correct latency, SDT average correct latency, SDT total errors, SRT average correct latency, SRT total errors) so that all inverse associations between THC or OSRC class and neurobehavioral function measure signify adverse neurobehavioral effects.
We also created a summary measure for response latency (time between stimulus and reaction) to capture, in one overall outcome measure, how long a participant took to complete each of the tasks and to complete them correctly. This measure incorporated latency and tap rate measures from seven tests: SRT average correct latency, SDT average correct latency, MTS average correct latency, CPT false latency, tap rate with preferred hand, tap rate of alternating hands, and PRT tap rate. These measures were converted into z-scores and were summed to create an overall response latency measure. The response latency measures from CPT, MTS, SDT, and SRT were negated so a lower number would indicate worse performance to match the tapping rates (lower tapping rates indicate worse performance). Multivariable linear regression was used to estimate associations of THC level and OSRC class with the response latency summary measure. To contextualize the results, we also age-standardized the risk estimates by dividing the adjusted difference in the summary latency measure between administrative support workers and other OSRC workers (betas for risk factors) by the adjusted association of this outcome measure with a one-year difference in age (betas for age) from the model. This helps make the risk estimates more interpretable by comparing the decrease in neurobehavioral function from OSRC exposure to the impact of aging on neurobehavioral function in this population.
RESULTS:
The final analysis sample of OSRC workers with neurobehavioral outcomes, exposure data, and covariates consisted of 2,819 participants (472 non-workers were excluded from analyses), with an average age of 45 years at enrollment, and over 98% completing the entire BARS exam. Most participants were men (78%) and male workers tended to be exposed to higher levels of THC. Workers exposed to higher levels of THC tended to have lower educational attainment and were more likely to smoke compared to those exposed to lower levels (Table 2a). People who worked in generally more highly exposed OSRC classes (e.g., response, operations) tended to have appreciably higher maximum THC levels than did people in the less exposed OSRC classes (e.g., administrative support, land cleanup) (Table 2b). Participants who did not complete the entire BARS exam (n=44) generally had worse performance on individual tests compared to those who completed the entire exam (data not shown); however, completion of all tests in the exam was not associated with oil spill exposures, and excluding participants who completed only some of the BARS tests did not meaningfully change observed associations.
Table 2a.
Characteristics of overall analysis cohort and sub-groups by maximum THC group.
| Characteristics | Total (n=2,816) | <0.30 ppm (n=406) | 0.30–0.99 ppm (n=935) | 1.0–2.99 ppm (n=1090) | ≥3 ppm (n=388) |
|---|---|---|---|---|---|
| Age, average years±SD | 44.91±12.97 | 45.73±12.90 | 44.11±12.75 | 46.23±13.22 | 42.29+12.32 |
| Sex, female(%) | 631 (22.4) | 171 (42.1) | 295 (31.6) | 122 (11.2) | 43 (11.1) |
| Race, n(%) | |||||
| Black | 1125 (40.0) | 179 (44.4) | 454 (48.6) | 297 (27.3) | 195 (50.4) |
| Other | 234 (8.3) | 25 (6.2) | 69 (7.4) | 110 (10.1) | 30 (7.8) |
| White | 1454 (51.7) | 199 (49.4) | 412 (44.1) | 681 (62.6) | 162 (41.9) |
| Education, n(%) | |||||
| Less than high school | 608 (21.6) | 55 (13.5) | 176 (18.8) | 270 (24.8) | 107 (27.6) |
| High school diploma | 978 (34.7) | 126 (31.0) | 343 (36.7) | 362 (33.2) | 147 (37.9) |
| Some college | 849 (30.1) | 123 (30.3) | 300 (32.1) | 328 (30.1) | 98 (25.3) |
| College grad | 383 (13.6) | 102 (25.1) | 116 (12.4) | 129 (11.8) | 36 (9.3) |
| Smoking status, n(%) | |||||
| Never daily smoker | 934 (33.1) | 180 (44.3) | 305 (32.6) | 355 (32.6) | 94 (24.2) |
| Former daily smoker | 770 (27.3) | 105 (25.9) | 247 (26.4) | 316 (29.0) | 102 (26.3) |
| Current daily smoker | 1115 (39.6) | 121 (29.8) | 383 (41.0) | 419 (38.4) | 192 (49.5) |
| Tobacco use within 2 hours before exam, n(%) | 786 (41.7) | 89 (39.4) | 272 (43.2) | 303 (41.2) | 122 (41.6) |
| Reported passive smoke exposure, n(%) | 545 (28.6) | 70 (24.5) | 190 (29.8) | 210 (29.6) | 75 (27.3) |
| Binge drinkers, n(%)* | 285 (13.9) | 30 (9.8) | 77 (11.6) | 132 (17.2) | 46 (14.8) |
| Consumed alcohol within 2 hours before exam, n(%) | 7 (0.3) | 0 (0.0) | 3 (0.4) | 4 (0.4) | 0 (0.0) |
| Consumed caffeine within 2 hours before exam, n(%) | 835 (29.9) | 117 (29.1) | 291 (31.6) | 341 (31.5) | 86 (22.4) |
| Sleep the night before exam, mean hours±SD | 6.32±1.91 | 6.33±1.89 | 6.36±1.85 | 6.33±1.95 | 6.15±1.97 |
| Proportion of tasks wearing respirators, mean±SD | 0.09±0.20 | 0.14±0.34 | 0.10±0.20 | 0.08±0.18 | 0.08±0.19 |
| Oil spill response and cleanup class, n(%)† | |||||
| Response | 506 (17.9) | 1 (0.2) | 20 (2.1) | 199 (18.3) | 286 (73.7) |
| Operations support | 658 (23.3) | 1 (0.2) | 75 (8.0) | 498 (45.7) | 84 (21.6) |
| Water cleanup | 469 (16.6) | 1 (0.2) | 116 (12.4) | 348 (31.9) | 4 (1.0) |
| Decontamination | 558 (19.8) | 20 (4.9) | 484 (51.8) | 40 (3.7) | 14 (3.6) |
| Land cleanup | 405 (14.4) | 189 (46.6) | 213 (22.8) | 3 (0.3) | 0 (0.0) |
| Administrative support | 223 (7.9) | 194 (47.8) | 27 (2.9) | 2 (0.2) | 0 (0.0) |
| Completed entire BARS exam, n(%) | 2776 (98.5) | 403 (99.3) | 922 (98.6) | 1069 (98.1) | 382 (98.5) |
Reported ≥5 drinks on one occasion ≥12 times during the year before the clinical exam
Response: Stopped the oil release, flared oil/gas, applied dispersant; Support of operations: Handled oily waste; Cleanup on water: Cleanup of oil on water; Decontamination: Decontaminated vessels, equipment, personnel, wildlife; Cleanup on land: Beach cleanup; Administrative: Cooking, security, administrative, various other support jobs. See Supplementary Table 1 for more details.
Table 2b.
Characteristics of overall analysis cohort and sub-groups by hierarchical OSRC classes.
| Characteristics | Administrative Support (n=223)† | Cleanup on land (n=405)† | Decontamination Work (n=558)† | Cleanup on water (n=469)† | Operations (n=658)† | Response work (n=506)† |
|---|---|---|---|---|---|---|
| Age, average years±SD | 48.02±12.35 | 44.29±12.69 | 42.22±12.63 | 49.66±13.39 | 43.81±12.26 | 44.06±13.00 |
| Sex, female(%) | 83 (37.2) | 156 (38.5) | 193 (34.6) | 75 (16.0) | 75 (11.4) | 49 (9.7) |
| Race, n(%) | ||||||
| Black | 47 (21.3) | 224 (55.4) | 341 (61.1) | 65 (13.9) | 257 (39.1) | 191 (37.8) |
| Other | 15 (6.8) | 26 (6.4) | 35 (6.3) | 61 (13.0) | 60 (9.1) | 37 (7.3) |
| White | 159 (71.9) | 154 (38.1) | 182 (32.6) | 342 (73.1) | 340 (51.8) | 277 (54.9) |
| Education, n(%) | ||||||
| Less than high school | 16 (7.2) | 73 (18.0) | 112 (20.1) | 128 (27.3) | 155 (23.6) | 124 (24.6) |
| High school diploma | 49 (22.0) | 157 (38.8) | 213 (38.2) | 150 (32.0) | 233 (35.4) | 176 (34.9) |
| Some college | 76 (34.1) | 120 (29.6) | 180 (32.3) | 130 (27.7) | 209 (31.8) | 134 (26.5) |
| College grad | 82 (36.8) | 55 (13.6) | 53 (9.5) | 61 (13.0) | 61 (9.3) | 71 (14.1) |
| Smoking status, n(%) | ||||||
| Never daily smoker | 106 (47.5) | 143 (35.3) | 188 (33.7) | 172 (36.7) | 176 (26.7) | 149 (29.4) |
| Former daily smoker | 57 (25.6) | 113 (27.9) | 131 (23.5) | 144 (30.7) | 183 (27.8) | 142 (28.1) |
| Current daily smoker | 60 (26.9) | 149 (36.8) | 239 (42.8) | 153 (32.6) | 299 (45.4) | 215 (42.5) |
| Tobacco use within 2 hours before exam, n(%) | 44 (37.6) | 104 (39.7) | 162 (43.9) | 114 (38.4) | 215 (44.7) | 147 (41.2) |
| Reported passive smoke exposure, n(%) | 43 (24.3) | 72 (27.9) | 128 (33.5) | 81 (29.9) | 136 (31.7) | 85 (21.7) |
| Binge drinkers, n(%)* | 16 (9.4) | 30 (10.3) | 41 (10.4) | 52 (16.5) | 93 (19.1) | 53 (13.7) |
| Consumed alcohol within 2 hours before exam, n(%) | 0 (0.0) | 1 (0.3) | 1 (0.2) | 2 (0.5) | 3 (0.5) | 0 (0.0) |
| Consumed caffeine within 2 hours before exam, n(%) | 71 (32.1) | 110 (27.5) | 155 (28.3) | 159 (34.0) | 206 (31.6) | 134 (26.6) |
| Sleep the night before exam, mean hours±SD | 6.41±1.76 | 6.28±1.82 | 6.31±1.96 | 6.42±1.80 | 6.17±2.03 | 6.41±1.91 |
| Proportion of tasks wearing respirators, mean±SD | 0.00±0.00 | 0.10±0.29 | 0.09±0.18 | 0.07±0.08 | 0.11±0.23 | 0.07±0.15 |
| Maximum Total Hydrocarbon (THC) level, n(%) | ||||||
| <0.30 ppm | 194 (87.0) | 189 (46.7) | 20 (3.6) | 1 (0.2) | 1 (0.2) | 1 (0.2) |
| 0.30–0.99 ppm | 27 (12.1) | 213 (52.6) | 484 (86.7) | 116 (24.7) | 75 (11.4) | 20 (4.0) |
| 1.0–2.99 ppm | 2 (0.9) | 3 (0.7) | 40 (7.2) | 348 (74.2) | 498 (75.7) | 199 (39.3) |
| ≥3 ppm | 0 (0.0) | 0 (0.0) | 14 (2.5) | 4 (0.9) | 84 (12.8) | 286 (56.5) |
| Completed entire BARS exam, n(%) | 222 (99.6) | 401 (99.0) | 549 (98.4) | 462 (98.5) | 649 (98.6) | 493 (97.4) |
Reported ≥5 drinks on one occasion ≥12 times during the year before the clinical exam
Response: Stopped the oil release, flared oil/gas, applied dispersant; Support of operations: Handled oily waste; Cleanup on water: Cleanup of oil on water; Decontamination: Decontaminated vessels, equipment, personnel, wildlife; Cleanup on land: Beach cleanup; Administrative: Cooking, security, administrative, various other support jobs. See Supplementary Table 1 for more details.
Overall, we found only limited evidence of associations between THC exposure or OSRC classes with neurobehavioral function. We observed significant associations between increased THC exposure and worse neurobehavioral function, as measured by DST forward count (adjusted difference in score between participants in the highest versus lowest THC exposure group=−0.39, 95% confidence interval (CI)=−0.74, −0.04). We also found a borderline significant association for SDT total errors for the 1.0–2.99 ppm THC group (difference1.0–2.99 ppm THC vs. <0.30 ppm THC =−0.56, 95% CI=−1.13, −0.003) and a non-significant but suggestive, similar magnitude association in the ≥3 ppm group (difference≥3 ppm THC vs. <0.30 ppm THC =−0.55, 95% CI=−1.2, 0.1), indicating a possible threshold effect. We also observed suggestive exposure-response trends or threshold effects (albeit statistically non-significant) for DST reverse count, SDT average correct latency, and CPT d prime (Table 3).
Table 3.
The relationship between maximum THC categories and neurobehavioral outcomes, with each THC category compared to THC <0.30 ppm (n=406).
| Neurobehavioral Outcomes† | THC 0.30–0.99 ppm (n=935)‡ | THC 1.0–2.99 ppm (n=1090)‡ | THC ≥3 ppm (n=388)‡ |
|---|---|---|---|
| CPT correct response fraction | 0.01 (0, 0.02) | 0 (−0.01, 0.01) | 0 (−0.01, 0.02) |
| CPT d prime | 0.01 (−0.15, 0.17) | −0.08 (−0.24, 0.08) | −0.08 (−0.27, 0.10) |
| CPT false alarm fraction§ | 0.01 (0, 0.02) | 0 (−0.01, 0.01) | 0 (−0.01, 0.02) |
| CPT false latency (msec)§ | −26.83 (−58.58, 4.93) | −12.12 (−44.45, 20.21) | −20.59 (−58.23, 17.04) |
| CPT hit fraction | 0 (−0.02, 0.02) | −0.01 (−0.03, 0.01) | −0.01 (−0.03, 0.01) |
| DST forward count | −0.27 (−0.56, 0.03) | −0.19 (−0.49, 0.11) | −0.39 (−0.74, −0.04)* |
| DST reverse count | −0.06 (−0.33, 0.21) | −0.10 (−0.38, 0.18) | −0.21 (−0.53, 0.11) |
| MTS ave correct latency (msec)§ | 23.14 (−81.77, 128.06) | 3.76 (−103.09, 110.60) | −62.76 (−187.05, 61.53) |
| MTS no. of correct responses | 0.25 (−0.12, 0.61) | 0.15 (−0.22, 0.52) | 0.12 (−0.30, 0.55) |
| SDT ave correct latency (msec)§ | −34.16 (−160.96, 92.65) | −96.56 (−225.71, 32.60) | −77.14 (−227.38, 73.11) |
| SDT total errors§ | −0.14 (−0.69, 0.41) | −0.56 (−1.13, −0.003)* | −0.55 (−1.20, 0.10) |
| SRT ave correct latency (msec)§ | 1.60 (−14.19, 17.38) | 1.52 (−14.56, 17.6) | 14.89 (−3.81, 33.59) |
| SRT total no. of errors§ | −0.05 (−0.80, 0.70) | −0.33 (−1.09, 0.43) | 0.13 (−0.76, 1.02) |
| Ave # taps, alternating hands | −0.94 (−4.14, 2.26) | 0.50 (−2.76, 3.76) | 1.70 (−2.09, 5.48) |
| Ave # taps, preferred hand | −0.21 (−2.84, 2.43) | 0.94 (−1.74, 3.63) | −0.26 (−3.38, 2.86) |
| PRT total taps | 1.21 (−12.40, 14.81) | 4.12 (−9.73, 17.97) | −2.01 (−18.13, 14.11) |
| All BARS tests completed^ | 0 (−0.02, 0.02) | 0 (−0.02, 0.02) | 0 (−0.02, 0.02) |
| % of BARS tests completed^ | 0 (−0.01, 0.01) | 0 (−0.01, 0.01) | 0 (−0.01, 0.01) |
Indicates statistical significance of adverse effect (p<0.05).
CPT=continuous performance test (measures sustained visual attention); DST=digit span test (measured attention and memory); MTS=match-to-sample test (measures visual memory); SDT=symbol-digit test (measures executive function and coding); SRT=simple reaction time test (measures response speed); PRT=progressive ratio test (measures effort-related motivation); taps measure response speed and coordination; ave=average; no.=number (see Table 1 for more test details).
Models are adjusted for age, sex, race (white, black, other), education (less than high school, high school diploma or equivalent, some college, college graduate), tobacco use (current, former, never), binge drinking (reported ≥5 drinks on one occasion ≥12 times during the year before the clinical exam; yes/no), and passive smoking (lived with smoker, yes/no). Confidence intervals are shown in parentheses.
These neurobehavioral test measures were negated so that negative results always indicate an adverse effect of THC exposure compared to <0.30 ppm THC.
These measures assess the proportion of BARS tests completed by each participant and whether all of the BARS tests were completed. These results indicate that there was no relationship between participants’ ability to complete the BARS tests and the THC exposure.
In analyses of OSRC classes compared to administrative support workers, all OSRC classes were associated with significant deficits in DST forward count, ranging from −0.40 for operations workers to −0.68 for response workers (Table 4). Operations workers also showed significant deficits for SDT average correct latency (differenceoperations vs. admin = −189.38, 95% CI=−355.05, −23.72) and PRT total taps (differenceoperations vs. admin =−21.66, 95% CI=−39.37, −3.94). In addition to DST forward count, land cleanup workers showed significant deficits in two other measures. This included the average number of taps with alternating hands (differenceland cleanup vs. admin =−5.07, 95% CI=−9.49, −0.64) and PRT total taps (differenceland cleanup vs. admin = −20.38, 95% CI=−39.15, −1.60). While there was no clear pattern of association by OSRC class, it is important to note that the OSRC classes differed not only by type of work and THC exposure, but also by other exposures, heat, education, and health insurance status.
Table 4.
The relationship between OSRC classes and neurobehavioral outcome scores. Each oil spill and response (OSRC) class is compared to administrative workers, n=223. Confidence intervals are shown in parentheses.
| Neurobehavioral Outcomes† | Cleanup on land (n=405)‡ | Decontamination Work (n=558)‡ | Cleanup on water (n=469)‡ | Operations (n=658)‡ | Response work (n=506)‡ |
|---|---|---|---|---|---|
| CPT correct response fraction | 0 (−0.02, 0.01) | 0 (−0.01, 0.02) | −0.01 (−0.02, 0.01) | 0 (−0.02, 0.01) | 0 (−0.02, 0.01) |
| CPT d prime | −0.08 (−0.29, 0.14) | −0.07 (−0.28, 0.14) | −0.11 (−0.34, 0.11) | −0.16 (−0.36, 0.04) | −0.12 (−0.32, 0.08) |
| CPT false alarm fraction§ | 0 (−0.02, 0.01) | 0 (−0.01, 0.02) | −0.01 (−0.02, 0.01) | −0.002 (−0.02, 0.01) | 0 (−0.02, 0.01) |
| CPT false latency (msec)§ | −14.68 (−58.67, 29.3) | −23.06 (−65.16, 19.04) | −4.11 (−49.08, 40.86) | −12.31 (−53.79, 29.18) | −11.70 (−53.04, 29.64) |
| CPT hit fraction | −0.01 (−0.04, 0.01) | −0.02 (−0.04, 0.01) | −0.01 (−0.04, 0.02) | −0.02 (−0.05, 0) | −0.02 (−0.05, 0) |
| DST forward count | −0.58 (−0.98, −0.17)* | −0.51 (−0.90, −0.12)* | −0.6 (−1.01, −0.18)* | −0.40 (−0.78, −0.01)* | −0.68 (−1.06, −0.30)* |
| DST reverse count | −0.17 (−0.54, 0.21) | −0.17 (−0.53, 0.19) | 0.04 (−0.35, 0.42) | −0.33 (−0.69, 0.02) | −0.28 (−0.63, 0.07) |
| MTS ave correct latency (msec)§ | −97.24 (−242.63, 48.15) | −22.84 (−161.94, 116.25) | −42.95 (−191.48, 105.57) | −100.66 (−237.76, 36.45) | −107.95 (−244.61, 28.7) |
| MTS no. of correct responses | 0.03 (−0.47, 0.53) | 0.24 (−0.24, 0.72) | 0.18 (−0.33, 0.69) | 0.10 (−0.38, 0.57) | −0.08 (−0.55, 0.39) |
| SDT ave correct latency (msec)§ | −159.92 (−335.6, 15.76) | −95.77 (−263.79, 72.25) | −94.06 (−273.53, 85.41) | −189.38 (−355.05, −23.72)* | −149.06 (−314.18, 16.06) |
| SDT total errors§ | −0.02 (−0.79, 0.74) | −0.37 (−1.10, 0.36) | −0.54 (−1.32, 0.24) | −0.40 (−1.12, 0.32) | −0.58 (−1.30, 0.14) |
| SRT ave correct latency (msec)§ | −18.12 (−39.99, 3.74) | −14.46 (−35.37, 6.46) | −0.22 (−22.56, 22.12) | −20.41 (−41.03, 0.21) | −13.10 (−33.66, 7.45) |
| SRT total no. of errors§ | −0.78 (−1.82, 0.26) | −0.26 (−1.26, 0.73) | 0.01 (−1.05, 1.07) | −0.68 (−1.66, 0.3) | −0.80 (−1.77, 0.18) |
| Ave # taps, alternating hands | −5.07 (−9.49, −0.64)* | −2.73 (−6.96, 1.50) | −3.96 (−8.47, 0.56) | −2.66 (−6.84, 1.51) | −0.33 (−4.49, 3.83) |
| Ave # taps, preferred hand | −3.58 (−7.23, 0.07) | −1.98 (−5.47, 1.51) | −2.87 (−6.60, 0.85) | −2.66 (−6.10, 0.78) | −0.50 (−3.93, 2.93) |
| PRT total taps | −20.38 (−39.15, −1.60)* | −5.81 (−23.78, 12.17) | −10.30 (−29.50, 8.89) | −21.66 (−39.37, −3.94)* | −6.09 (−23.78, 11.60) |
| All BARS tests completed^ | 0 (−0.03, 0.03) | 0 (−0.02, 0.03) | 0 (−0.03, 0.02) | 0 (−0.02, 0.03) | −0.01 (−0.04, 0.01) |
| % of BARS tests completed^ | 0 (−0.01, 0.01) | 0 (−0.01, 0.01) | 0 (−0.01, 0.01) | 0 (−0.01, 0.01) | 0 (−0.01, 0.01) |
Indicates statistical significance of adverse effect (p<0.05).
CPT=continuous performance test (measures sustained visual attention); DST=digit span test (measured attention and memory); MTS=match-to-sample test (measures visual memory); SDT=symbol-digit test (measures executive function and coding); SRT=simple reaction time test (measures response speed); PRT=progressive ratio test (measures effort-related motivation); taps measure response speed and coordination; ave=average; no.=number (see Table 1 for more test details).
Models are adjusted for age, sex, race (white, black, other), education (less than high school, high school diploma or equivalent, some college, college graduate), tobacco use (current, former, never), binge drinking (reported ≥5 drinks on one occasion ≥12 times during the year before the clinical exam; yes/no), and passive smoking (lived with smoker, yes/no). Confidence intervals are shown in parentheses.
These neurobehavioral test measures were negated so that negative results always indicate an adverse effect of THC exposure compared to <0.30 ppm THC.
These measures assess the proportion of BARS tests completed by each participant and whether all of the BARS tests were completed. These results indicate that there was no relationship between participants’ ability to complete the BARS tests and their OSRC class.
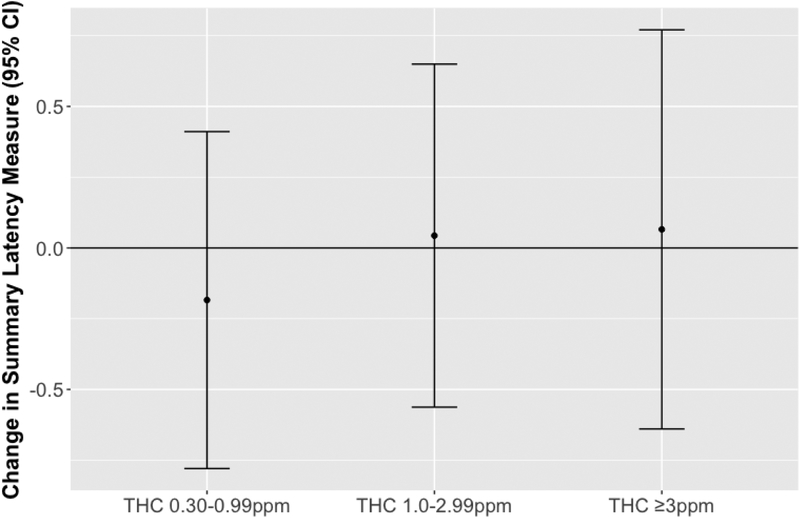
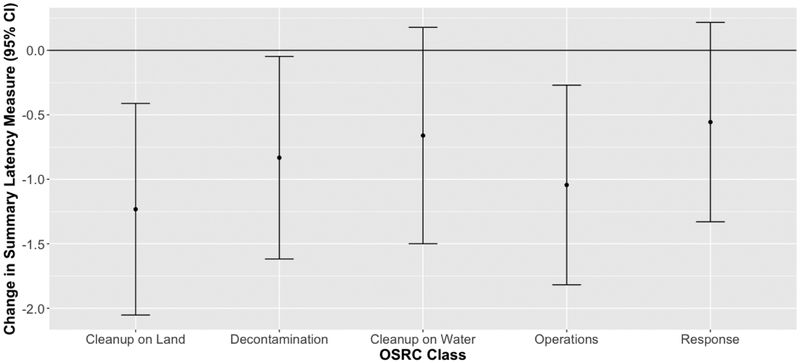
We found no association between higher THC levels and the summary response latency measure (Figure 1), but we observed significant inverse relationships between this summary measure and land cleanup, decontamination, and operations work compared to administrative support work, and suggestive inverse associations for water cleanup and response work (Figure 2). The magnitude of deficit in the summary response latency measure for these OSRC classes compared to administrative support workers is comparable to aging 4 to 9 years (9 years for land cleanup workers (95% CI=3, 15); 6 years for decontamination workers (95% CI=0, 12); 5 years for water cleanup workers (95% CI=−1, 11); 8 years for operations workers (95% CI=2, 13); 4 years for response workers (95% CI=−2, 10).
Figure 1.
Summary latency measures and 95% confidence intervals (CI) in relation to maximum total hydrocarbons (THC) levels. The summary latency measure incorporates latency and tap rate measures from 7 neurobehavioral function tests. To create this summary measure, some component measures were negated so that an inverse relationship between exposure and the summary measure indicates an adverse effect of exposure. Each THC category is compared to workers exposed to THC <0.30 ppm (THC <0.30 ppm n=406; THC 0.03–0.99 ppm n=935; THC 1.0–2.99 ppm n=1090; THC ≥3 ppm n=388). Models are adjusted for age, sex, race, education, smoking status, passive smoking, and binge drinking.
Figure 2.
Summary latency measures and 95% confidence intervals (CI) in relation to oil spill and response (OSRC) classes. The summary latency measure incorporates latency or tap rate measures from 7 neurobehavioral function tests. To create this summary measure, some component measures were negated so that a negative relationship between class and the summary measure indicates an adverse effect of exposure. Each OSRC class is compared to administrative workers (administrative support n=223; cleanup on land n=405; decontamination work n=558; cleanup on water n=469; operations n=658; response work n=506). Models are adjusted for age, sex, race, education, smoking status, passive smoking, and binge drinking. See Table A.1 for OSRC class details.
In sex-stratified analyses, we found generally stronger associations between oil spill exposures (measured both by THC and OSRC classes) and neurobehavioral function in men versus women (Tables A.3 and A.4). We observed apparent exposure-response trends and significant relationships among male workers for several of the neurobehavioral outcomes. For many of these neurobehavioral outcomes (e.g., CPT d prime, DST forward count, DST reverse count, SDT total errors), we had also observed relationships in the entire cohort, but we also observed some additional neurobehavioral outcomes that appeared to be associated with oil spill exposure only in males (e.g., CPT false latency, CPT hit fraction, MTS average correct latency). We saw a small, significant effect among female response workers for CPT correct response fraction and CPT false alarm fraction as well as female operations workers for PRT total taps, compared to administrative support workers. We observed no relationship between THC exposure and the summary latency measure in male or female workers (Figure A.2). Significant associations for the summary latency measure were seen for land cleanup workers and operations workers among both males and females, but an association with decontamination workers was observed only in males (Figure A.3). We observed no differences in effect when stratified by tobacco use.
DISCUSSION:
In this analysis, we found modest evidence of associations between THC levels and OSRC classes with neurobehavioral function. Compared to OSRC workers exposed to maximum THC <0.30 ppm, more highly exposed (THC ≥1 ppm) workers performed worse in multiple domains, with deficits observed in the digit span, symbol digit, and continuous performance tests. Compared to the generally least exposed workers—administrative support workers—land cleanup workers and operations workers showed deficits in multiple tests, including the digit span, progressive ratio, finger tapping, and/or symbol-digit tests. Other OSRC classes (decontamination, water cleanup, and response) showed more limited evidence of association, with deficits observed only in the digit span test. These results suggest possible adverse effects of oil spill-related exposures on attention, memory, executive function, coding, response speed, coordination, and motivation. The evidence is strongest for attention, memory, executive function, and coding, as both higher THC levels and more exposed OSRC classes were associated with decreased function in the digit span and symbol digit tests.
OSRC classes with higher overall oil spill-related exposures tended to have a worse summary latency measure compared to the least exposed OSRC class, i.e. administrative workers, but THC was not associated with this measure. The strongest associations for the summary latency measure and most consistent significant results were found for the land cleanup workers and support of operations workers. The significant results for the land cleanup workers is somewhat surprising as land workers were exposed to lower THC levels than other OSRC workers (aside from administrative support workers). Preliminary analysis of jobs prior to the spill did not indicate that a disproportionate number of land workers had previous jobs that might have resulted in reduced neurobehavioral function. Land workers were also no more likely than other OSRC classes to report stopping work due to heat. Land cleanup workers cleaned oil and tar from beaches and marshes. Support of operations workers refueled vehicles, moved hazardous materials (e.g., oily boom), and operated heavy equipment. The primary exposures the latter class was likely to have were gasoline and diesel fuel, oil and tar. In general, cleanup on land workers had some of the lowest neurobehavioral test performance before controlling for confounders and administrative support workers typically had the highest neurobehavioral test performance (Table A.6). Some associations may be due, in part, to baseline characteristics, as the administrative support workers had, on average, higher educational attainment and were less likely to smoke compared to other OSRC classes, although our models adjusted for the major differences between the OSRC classes, including age, sex, race, education, tobacco use, passive smoking, and binge drinking. We consider the administrative support workers (versus participants who were trained but not called to work) to be the most appropriate comparison group for these analyses because they worked on the oil spill, which reduces the likelihood of healthy worker bias, but were the workers least exposed to oil-related chemicals. We examined effects associated with both OSRC classes and maximum THC levels because OSRC classes can reflect spill-related exposures/experiences not captured by THC level. Thus, associations may be more apparent for OSRC classes than for THC levels due to possible non-THC chemical or non-chemical exposures experienced by some OSRC classes during the spill.
Our results also suggest that these oil spill chemical exposures may have had a stronger effect on men than women, especially for executive function, coding, and sustained visual attention. Animal research suggests that responses to chemicals, such as toluene, often differ by sex(30). Such differences, reported in the literature and found in our study, indicate that physiological differences between the sexes may cause males and females to respond differently to chemical exposures. Additionally, men and women perform differently on neurobehavioral tests, even in the absence of neuroactive chemical exposure(32,34–38). However, it is possible that our observed sex differences in neurobehavioral effects are due to different actual tasks performed (and thus exposures experienced) during the same activity or to patterns of personal protective equipment (PPE) use. For example, in our analysis cohort, male workers reported wearing respirators for an average of 8% of their tasks while female workers reported wearing respirators for 18% of their tasks (Table A.2). Additionally, this study cohort contained over three times as many male workers as female workers; the increased power in the male worker subgroup from the larger sample may explain, in part, the larger number of significant effects among men than women.
To the best of our knowledge, no other studies have examined the association between oil spill exposures and neurobehavioral function in adults, but other studies have found associations between BTEX chemicals and attention, response speed, and memory. A recent occupational study of men in an automotive industry found that painters—exposed to BTEX chemicals from paint—had worse cognitive function than assembly workers, who were not exposed.(13) The association between exposure to higher levels of BTEX and worse memory and reaction time is similar to our results, and the BTEX levels among workers in that study are similar to the levels in our study. They conclude that BTEX affects cognitive functions even when levels are below occupational exposure limits.(13) Another study found an association of occupational exposure to petroleum-derived hydrocarbons with neurotoxicity and excessive sleepiness, based on self-reported symptoms(39). Kicinski et al. found that increased traffic exposure, measured with a benzene biomarker, was associated with decreased sustained attention(40). A study of the association between occupational solvent exposure and cognitive performance found that people with higher exposure to benzene had poorer cognitive performance than those with lower benzene exposure on the digit symbol substitution test, indicating poorer response speed, sustained attention, memory, and visual spatial skills(41). Our results that suggest an association, albeit modest, between OSRC exposure and sustained attention, response speed, and memory are consistent with literature that shows these aspects of neurobehavioral function are affected by benzene and toluene exposure(13,40,41); however, most other studies examined higher exposures to BTEX chemicals than those experienced by most GuLF Study OSRC workers.
Some studies have reported cognitive impairment, including reduced memory, concentration, and visual spatial function, associated with chronic occupational exposure to moderate-to-low airborne concentrations of hydrocarbons(7,42). One study examining occupational exposure to low toluene concentrations found cognitive deficits after >12 years toluene exposure of <20 ppm and ≥4 years of >100 ppm toluene exposure (43). However, another study observed no neurobehavioral effects associated with long-term (mean: 21 years) toluene exposure below 50 ppm(44). Neurologic dysfunction and difficulty concentrating on tasks has been observed among workers with chronic occupational xylene exposure as low as 14 ppm(7,45). THC exposure guidelines depend on the type of total hydrocarbon being measured. The National Institute for Occupational Safety and Health (NIOSH) has a recommended level for petroleum distillates, the closest equivalent of THC measured as total petroleum hydrocarbons, of 500 ppm(46). This level is substantially higher than what most OSCR workers, even those in the >3 ppm THC category, were likely to have experienced. BTEX and hexane levels were also well below current regulations and guidelines.
A limitation of this study is that our measure of THC exposure only captures maximum exposure, as data on cumulative or time-weighted average exposure were not available. There may also be nondifferential exposure misclassification from using ordinal, instead of continuous, exposure estimates(47), but the ordinal estimates were used because of uncertainty in the individual exposure estimates, and maximum THC has been linked to other health outcomes in the GuLF Study(48). The THC estimates used for this study reflect a range of volatile oil-related chemicals and should generally reflect an individual’s cleanup experience(17), but they do not allow assessment of or inference related to individual chemicals, which could be important because THC is a mixture of chemicals of varying toxicity. The THC concentrations found here are relatively low by occupational standards and are below regulatory limits for many of the chemical components. We estimate that approximately 30% of the THC air concentration measured during the oil spill comprised the more highly neurotoxic BTEX and hexane chemicals. The remaining 70% of THC concentrations were comprised of hundreds of other unmeasured volatile oil components, most of which have limited toxicity data. Additionally, tobacco smoke and oil spill-related exposures contain several of the same neurotoxic compounds, and although we adjusted for tobacco use, incomplete adjustment for this factor—possibly due in part to underreported smoking—could obscure a relationship between OSCR exposure and neurobehavioral function(49). Another limitation of this study is the range of follow-up times (4–6 years) between exposure and outcome assessment. In a sensitivity analysis that included a follow-up term based on median split (1880 days) and a multiplicative interaction term of the follow-up term and exposure categories, we found similar patterns and associations as in our original analysis (Tables A.7 and A.8).
This study may also be susceptible to selection bias if participants with greater neurological symptoms were more motivated to participate in the exam or, alternatively, were less able to travel to the clinics for neurobehavioral testing. However, we believe there to be little selection bias based on neurobehavioral function because we found a similar prevalence of neurological symptoms reported during the enrollment interview (e.g., dizziness/lightheadedness, numbness in extremities, tingling/numbness in extremities, frequently stumbling while walking) among cohort members eligible for the neurobehavioral exam (based on residential proximity to a clinic) who did not participate (N=2,165) and cohort members who completed the neurobehavioral exam (our analysis cohort, N=2,819)(Table A.5). We also found no relationship between participants’ OSRC class and their ability to complete the entire BARS exam. Finally, some of the observed associations may be due to chance, as we examined a relatively large number of neurobehavioral outcome measures.
Strengths of this study include the large sample size, detailed data on important potential confounding factors, and comprehensive and objective neurobehavioral test data. By using estimated THC levels derived from personal monitoring data collected on OSRC workers during the oil spill cleanup, this study improves upon studies of previous oil spills, which used only nominal, cruder measures of cleanup-related exposure, such as days worked on the cleanup or distance of residence from the oil spill. Moreover, ours is the first oil spill study to include validated, objective neurobehavioral testing. Additionally, BARS, the neurobehavioral function test system used in this study, was specifically designed for a diverse population with limited computer skills and formal education. Furthermore, participants in this study engaged in a wide range of OSRC tasks at different times, and consequently had a wide range of exposures, increasing our ability to detect any adverse effects. Our study’s neurobehavioral exam also captures multiple domains of neurobehavior.
Conclusions:
We found modest associations between OSRC exposure (both OSRC classes and THC levels) and neurobehavioral function. We observed a moderate association between more highly exposed OSRC classes and attention, memory, and executive function, compared to administrative support workers. This association appeared to be somewhat stronger in men than in women. Additional follow-up time and neurobehavioral testing of this cohort would help determine whether the observed associations persist beyond 4–6 years. Such research will improve our understanding of the long-term health risks associated with oil spills.
Supplementary Material
HIGHLIGHTS:
Attention, memory, and executive function may be impaired by oil spill cleanup exposures
Adverse association between job and executive function was strongest in operations workers
Adverse associations tended to be slightly stronger in men than in women
FUNDING SOURCES:
This research was funded by National Institute of Environmental Health Sciences (NIEHS) training grant T32ES007018, the NIH Common Fund and the Intramural Research Program of the NIH, and National Institute of Environmental Health Sciences (ZO1 ES 102945).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
HUMAN SUBJECTS:
This study was approved by the Institutional Review Boards of both the National Institute of Environmental Health Sciences and the University of North Carolina.
Conflict of Interests:
Oregon Health & Science University (OHSU) and Dr. Diane Rohlman have a significant financial interest in Northwest Education Training and Assessment, LLC, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest was reviewed and a management plan approved by the University of Iowa and the OHSU Conflict of Interest in Research Committee was implemented.
All other authors declare no conflicts of interest.
REFERENCES:
- 1.Kwok RK, Engel LS, Miller AK, et al. The GuLF STUDY: A Prospective Study of Persons Involved in the Deepwater Horizon Oil Spill Response and Clean-Up. Environ. Health Perspect. 2017;125(4):570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrichon P, Le Menach K, Akcha F, et al. Toxicity assessment of water-accommodated fractions from two different oils using a zebrafish (Danio rerio) embryo-larval bioassay with a multilevel approach. Sci. Total Environ. 2016;568:952–966. [DOI] [PubMed] [Google Scholar]
- 3.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet (London, England). 2006;368(9553):2167–2178. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie GD, Still KR, Alexander WK, et al. A review of the neurotoxicity risk of selected hydrocarbon fuels. J. Toxicol. Environ. Health. B. Crit. Rev 2001;4(3):223–312. [DOI] [PubMed] [Google Scholar]
- 5.Ha M, Kwon H, Cheong H-K, et al. Urinary metabolites before and after cleanup and subjective symptoms in volunteer participants in cleanup of the Hebei Spirit oil spill. Sci. Total Environ. 2012;429:167–173. [DOI] [PubMed] [Google Scholar]
- 6.Wu T, Bhanegaonkar AJ, Flowers JW. Blood concentrations of selected volatile organic compounds and neurobehavioral performance in a population-based sample. Arch. Environ. Occup. Health 2006;61(1):17–25. [DOI] [PubMed] [Google Scholar]
- 7.Webb E, Moon J, Dyrszka L, et al. Neurodevelopmental and neurological effects of chemicals associated with unconventional oil and natural gas operations and their potential effects on infants and children. Rev. Environ. Health 2018;33(1):3–29. [DOI] [PubMed] [Google Scholar]
- 8.Filley CM, Halliday W, Kleinschmidt-DeMasters BK. The effects of toluene on the central nervous system. J. Neuropathol. Exp. Neurol 2004;63(1):1–12. [DOI] [PubMed] [Google Scholar]
- 9.McKee RH, Lammers JHCM, Muijser H, et al. Neurobehavioral effects of acute exposure to aromatic hydrocarbons. Int. J. Toxicol 2010;29(3):277–290. [DOI] [PubMed] [Google Scholar]
- 10.Toxicological Profile for Xylene. U.S. Dep. Heal. Hum. Serv. Agency Toxic Subst. Dis. Regist. [electronic article]. 2007;( 10.1155/2013/286524) [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Liang X, Wang X, et al. [Short-term central nervous system symptoms and changes in blood indicators after benzene poisoning in rats]. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(11):1935–1937. [PubMed] [Google Scholar]
- 12.Tegeris JS, Balster RL. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam. Appl. Toxicol 1994;22(2):240–250. [DOI] [PubMed] [Google Scholar]
- 13.Golbabaei F, Dehghani F, Saatchi M, et al. Evaluation of occupational exposure to different levels of mixed organic solvents and cognitive function in the painting unit of an automotive industry. Heal. Promot. Perspect 2018;8(4):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toxicological Profile for n-Hexane. U.S. Dep. Heal. Hum. Serv. Agency Toxic Subst. Dis. Regist. 1999;(July). [PubMed] [Google Scholar]
- 15.Kim S, Chen J, Cheng T, et al. Hexane. PubChem 2019 Updat. Improv. access to Chem. data 2019;(47(D1)):D1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toxicological Profile for Total Petroleum Hydrocarbons (TPH). 1999. [PubMed]
- 17.Stewart PA, Stenzel MR, Ramachandran G, et al. Development of a total hydrocarbon ordinal job-exposure matrix for workers responding to the Deepwater Horizon disaster: The GuLF STUDY. J. Expo. Sci. Environ. Epidemiol 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zock JP, Rodríguez-Trigo G, Pozo-Rodríguez F, et al. Prolonged respiratory symptoms in clean-up workers of the Prestige oil spill. Am. J. Respir. Crit. Care Med. 2007;176(6):610–616. [DOI] [PubMed] [Google Scholar]
- 19.Gorma RW, Berardinelli SP, Bender TR. Health Hazard Evaluation Report: HETA 89-0200 and 89-273-211, Exxon/Valdez Alaska Oil Spill. Cincinnati, OH: 1991. [Google Scholar]
- 20.Cheong H-K, Ha M, Lee JS, et al. Hebei spirit oil spill exposure and subjective symptoms in residents participating in clean-up activities. Environ. Health Toxicol. 2011;26:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meo S, Al-Drees A, Rasheed S, et al. Health complaints among subjects involved in oil cleanup operations during oil spillage from a Greek tanker “tasman Spirit”. Int. J. Occup. Med. Environ. Health 2009;22(2):143–148. [DOI] [PubMed] [Google Scholar]
- 22.Janjua NZ, Kasi PM, Nawaz H, et al. Acute health effects of the Tasman Spirit oil spill on residents of Karachi, Pakistan. BMC Public Health. 2006;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Na JU, Sim MS, Jo IJ, et al. The duration of acute health problems in people involved with the cleanup operation of the Hebei Spirit oil spill. Mar. Pollut. Bull 2012;64(6):1246–1251. [DOI] [PubMed] [Google Scholar]
- 24.Rohlman DS, Gimenes LS, Eckerman DA, et al. Development of the Behavioral Assessment and Research System (BARS) to detect and characterize neurotoxicity in humans. Neurotoxicology. 2003;24(4–5):523–531. [DOI] [PubMed] [Google Scholar]
- 25.Metz CE. Basic principles of ROC analysis. Semin. Nucl. Med 1978;8(4):283–298. [DOI] [PubMed] [Google Scholar]
- 26.Macmillan N, Creelman C. Detection Theory: A User’s Guide. 2nd ed. Taylor & Francis; 2004. [Google Scholar]
- 27.Rothman K, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. 183–209 p. [Google Scholar]
- 28.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 29.Fallon JH, Keator DB, Mbogori J, et al. Gender: a major determinant of brain response to nicotine. Int. J. Neuropsychopharmacol 2005;8(1):17–26. [DOI] [PubMed] [Google Scholar]
- 30.Rogers WR, Miller CS, Bunegin L. A rat model of neurobehavioral sensitization to toluene. Toxicol. Ind. Health 1999;15(3–4):356–369. [DOI] [PubMed] [Google Scholar]
- 31.Brandt M, Brown C, Burkhart J, et al. Mold prevention strategies and possible health effects in the aftermath of hurricanes and major floods. MMWR. Recomm. reports Morb. Mortal. Wkly. report. Recomm. reports 2006;55(RR-8):1–27. [PubMed] [Google Scholar]
- 32.Mann VA, Sasanuma S, Sakuma N, et al. Sex differences in cognitive abilities: a cross-cultural perspective. Neuropsychologia. 1990;28(10):1063–1077. [DOI] [PubMed] [Google Scholar]
- 33.Valentine G, Sofuoglu M. Cognitive Effects of Nicotine: Recent Progress. Curr. Neuropharmacol 2018;16(4):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton L, Pfaff D, Bolt N, et al. Effects of gender and personality on the Conners Continuous Performance Test. J. Clin. Exp. Neuropsychol 2010;32(1):66–70. [DOI] [PubMed] [Google Scholar]
- 35.Arango-Lasprilla JC, Rivera D, Rodriguez G, et al. Symbol Digit Modalities Test: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. 2015;37(4):625–638. [DOI] [PubMed] [Google Scholar]
- 36.Kelly TP, Britton PG. Sex differences on an adaptation of the Digit Symbol subtest of the Wechsler Intelligence Scale for Children-III. Percept. Mot. Skills 1996;83(3 Pt 1):843–847. [DOI] [PubMed] [Google Scholar]
- 37.Rothlein J, Rohlman D, Lasarev M, et al. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and non-agricultural Hispanic workers. Environ. Health Perspect. 2006;114(5):691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi HJ, Lee DY, Seo EH, et al. A normative study of the digit span in an educationally diverse elderly population. Psychiatry Investig. 2014;11(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekkal S, Casas L, Haddam N, et al. Sleep disturbances and neurotoxicity in workers exposed to hydrocarbons. An observational study from Algeria. Am. J. Ind. Med 2016;59(2):129–136. [DOI] [PubMed] [Google Scholar]
- 40.Kicinski M, Vermeir G, Van Larebeke N, et al. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ. Int 2015;75:136–143. [DOI] [PubMed] [Google Scholar]
- 41.Berr C, Vercambre MN, Bonenfant S, et al. Occupational exposure to solvents and cognitive performance in the GAZEL cohort: preliminary results. Dement. Geriatr. Cogn. Disord 2010;30(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker BJ. Neurotoxicity in human beings. J. Lab. Clin. Med 2000;136(3):168–180. [DOI] [PubMed] [Google Scholar]
- 43.Eller N, Netterstrom B, Laursen P. Risk of chronic effects on the central nervous system at low toluene exposure. Occup. Med. (Lond) 1999;49(6):389–395. [DOI] [PubMed] [Google Scholar]
- 44.Seeber A, Demes P, Kiesswetter E, et al. Changes of neurobehavioral and sensory functions due to toluene exposure below 50ppm? Environ. Toxicol. Pharmacol 2005;19(3):635–643. [DOI] [PubMed] [Google Scholar]
- 45.Toxicological Profile for Xylene. Agency Toxic Subst. Dis. Regist. U.S. Public Heal. Serv. U.S. Dep. Heal. Hum. Serv. [electronic article]. 2007;(https://www.atsdr.cdc.gov/toxprofiles/tp71.pdf) [PubMed] [Google Scholar]
- 46.Pocket Guide to Chemical Hazards. NIOSH (National Inst. Occup. Saf. Heal. 2016;(http://www.cdc.gov/niosh/docs/2003-154/%0Dmethod-2000.html). (Accessed February 20, 2018) [Google Scholar]
- 47.Blair A, Stewart PA. Do quantitative exposure assessments improve risk estimates in occupational studies of cancer? Am. J. Ind. Med 1992;21(1):53–63. [DOI] [PubMed] [Google Scholar]
- 48.Strelitz J, Sandler DP, Keil AP, et al. Exposure to Total Hydrocarbons During Clean-up of the Deepwater Horizon Oil Spill and Risk of Heart Attack Across Five Years of Follow-up. Am. J. Epidemiol 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor Gorber S, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob. Res 2009;11(1):12–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.