Abstract
DEGS1‐MH is part of the first wave of the German Health Interview and Examination Survey (DEGS1) covering all relevant health issues. Aims of DEGS1‐MH are to supplement DEGS1 by describing (1) the distribution and frequency, the severity and the impairments of a wide range of mental disorders, (2) risk factors as well as patterns of help‐seeking and health care utilization, and (3) associations between mental and somatic disorders, (4) and by comparisons with a similar survey in the late 1990s (GHS‐MHS), longitudinal trends and changes in morbidity over time. Out of all eligible DEGS1 respondents (nationally representative sample aged 18–79), N = 5318 subjects (conditional response rate 88%) were examined at their place of residence by clinically trained interviewers with a modified version of the standardized, computer‐assisted Composite International Diagnostic Interview (DEGS‐CIDI). Innovative additions were: a comprehensive neuropsychological examination, a broader assessment of psychosis‐like experiences, disorder‐specific disabilities, help‐seeking and health care utilization. The mental health module and its combination with the assessment of somatic and other health issues in DEGS1 allow for internationally unique, detailed and comprehensive analyses about mental disorders and the association of mental and somatic health issues in the community, constituting an improved basis for regular future surveys of this sort. Copyright © 2013 John Wiley & Sons, Ltd.
Keywords: mental disorders, cognitive functioning, prevalence, disability, health service research
Introduction
Recently, the Robert Koch Institute (RKI) presented the background, the aims and the design and methods of the German Health Interview and Examination Survey (DEGS, Scheidt‐Nave et al., 2012). DEGS is part of the continuous national health monitoring system established in Germany and provides nationally representative data on the health status of the adult general population. Against the background of several health interview and examination surveys for different subsets of the population carried out since 1984 (see Scheidt‐Nave et al., 2012), the German Ministry of Health commissioned the RKI to implement a system of health studies for continuous monitoring of the non‐institutionalized population. The first wave of DEGS (DEGS1) is the cornerstone of this ambitious program focusing on the adult, non‐institutionalized general population aged 18–79. Because of the increasing recognition of the societal burden of mental disorders (Wittchen et al., 2011; Wittchen, 2012), it was decided to deal with mental health issues in a separate supplementary module (DEGS1‐MH), to accommodate for the need of differential diagnostic clinical assessments for mental disorders and the time burden associated with such clinically more detailed assessments.
Accordingly two associated surveys were conducted in a coordinated way based on the same nationally representative sample of the German general population. First, the main survey DEGS1, covering the general health status, risk factors, health related behaviours, a wide range of diagnoses of somatic disorders and conditions, along with laboratory tests, assessments of quality of life, a core set of mental health indicators and utilization of health care resources (Scheidt‐Nave et al., 2012). Second, contingent on the completion of the DEGS1, subjects participated additionally in the additional mental health module (DEGS1‐MH) for the assessment of prevalence, severity, and comorbidity of mental disorders and a range of other relevant mental health domains.
The DEGS1 and the DEGS1‐MH are essentially the successor of a previous comprehensive study conducted in the year 1998, namely the “German National Health Interview and Examination Survey 1998” (GNHIES98; Bellach et al., 1998) that was also coupled with a separate mental health module (GHS‐MHS; Jacobi et al., 2002; Wittchen et al., 1999). This mental health module was the first nationally representative adult community study of this sort in Germany, providing comprehensive data about the lifetime and 12‐month prevalence of mental disorders (Jacobi et al., 2004b; Wittchen et al., 2000; Wittchen & Jacobi, 2001), along with a broad range of associated topics of relevance such as associations and interactions between mental and somatic health (Goodwin et al., 2003; Härter et al., 2007; Ratcliffe et al., 2009; Sareen et al., 2006) or associated impairments and help‐seeking (Wittchen and Jacobi, 2004; Jacobi et al., 2004a). This earlier study revealed that the prevalence of mental disorders had been widely underestimated, that most disorders evidently remain undiagnosed and untreated, and that they are associated with high disability and cost burden for the society (Gustavsson et al., 2011; Wittchen, 2002, 2004; Wittchen and Jacobi, 2005, Wittchen et al., 2011). The GHS‐MHS findings also served as input for major pan‐European Union (EU) re‐analyzes on the size and burden of mental disorders in Europe (Wittchen et al., 2011).
When designing the new DEGS1 survey, the availability of the previous GNHIES98 and GHS‐MHS prompted the development of a complex sampling scheme with the goal to define a national representative sample of the adult general population, enriched by participants of the previous 1998 survey. This should allow for both, a nationally representative, general population sample to provide an up to date description (e.g., in terms of prevalence and risk factors) as well as cohort and trend analyses, and prospective risk factors examinations. This complex sampling scheme and the respective methodological aspects of the overall DEGS approach have been presented already in detail in a methods publication (Scheidt‐Nave et al., 2012).
Aims
This paper provides information about the aims, design and methods of the mental health component (DEGS1‐MH) supplementing the recent overall DEGS1 presentation (Scheidt‐Nave et al., 2012). Overall aims of the DEGS1‐MH module are to describe:
the distribution and frequency, the severity and the impairments of a wide range of mental disorders by gender and age groups, including the elderly (65−79 years);
the comorbidity patterns and the interactions between mental disorders and physical conditions, for example with regard to course and outcome, quality of life, role functioning;
risk factors as well as patterns of help‐seeking and health care utilization;
further, we provide a more comprehensive assessment and description of mental disorders according to DSM‐IV‐TR (American Psychiatric Association, 2000), for example by including a neuropsychological module that allows to compare cognitive factors, such as attention, memory and executive functions over the lifespan and across different morbidity patterns, and a broader assessment of psychosis‐like experiences;
DEGS1‐MH will also allow updated descriptions of met and unmet needs in the mental health field in light of recent changes in the health care sector by describing help‐seeking patterns and changes in the morbidity spectrum since 1998 (i.e. incidence, remission, predictors of healthy aging, etc.).
Methods
Study components
The overall DEGS study design with its main survey and the mental health module is presented in Figure 1. The DEGS1‐MH was designed as an independent assessment wave, administered by an independent research group of clinical experts (see authors and acknowledgments), subsequent to completion of the main survey. The study proposal, field procedures, and information for respondents of DEGS1 were approved by the Medical Ethics Review Committees responsible for the RKI main survey (Charité, Berlin) and for DEGS1‐MH by the Ethics Board of the Technische Universität Dresden, respectively.
Figure 1.
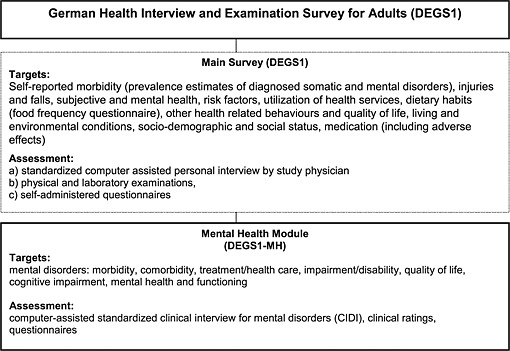
The German Health Interview and Examination Survey (DEGS1).
Sample
Sampling of DEGS1 participants
In order to perform both cross‐sectional and longitudinal analyses, DEGS adopted a mixed study design (see details in Scheidt‐Nave et al., 2012; Kamtsiuris et al., in press) that is only briefly summarized here. A nationally representative sample of persons aged 18‐79 was randomly chosen from local population registries and then supplemented by former participants of the predecessor GNHIES98 study. The random sample was drawn by the RKI in two steps (two‐layered cluster sample). First, among all German political communities, 180 study sample points were determined. In doing so, the 120 former sample points from GNHIES98 were retained and supplemented by 60 newly chosen sample points. Second, subjects were randomly selected from local population registries covering the 180 sample points. Again invited were those GNHIES98 participants who had neither died nor moved abroad and agreed to renewed contact. These people were now 28 to 91 years old. The total number of DEGS1 participants was N = 8152, of which N = 164 subjects were older than 79 and N = 872 were only interviewed, but not assessed by clinical and laboratory examinations. A total of N=7116 DEGS1 participants aged 18–79 years had complete assessments with interview and examination (Scheidt‐Nave et al., 2012). The response rate among previous GNHIES98 participants was consistently higher (64%) than the one for the newly sampled (42%).
Eligibility for the DEGS1‐MH assessment
For the DEGS1‐MH, only DEGS1 participants were eligible who met the following criteria: (a) age 18–79, (b) complete DEGS1 assessment consisting of the medical interview and examination, laboratory tests and self‐report scales, (c) informed consent to be re‐contacted by the independent DEGS1‐MH team for the mental health supplement and (d) meeting general inclusion criteria; that is subjects had to have sufficient language skills to complete the mental health assessment and had to be available during the assessment period (see later and Figure 2). Based on these criteria a total of N = 6028 (100%) of the DEGS1 participants were defined as being eligible for the mental health supplement.
Figure 2.
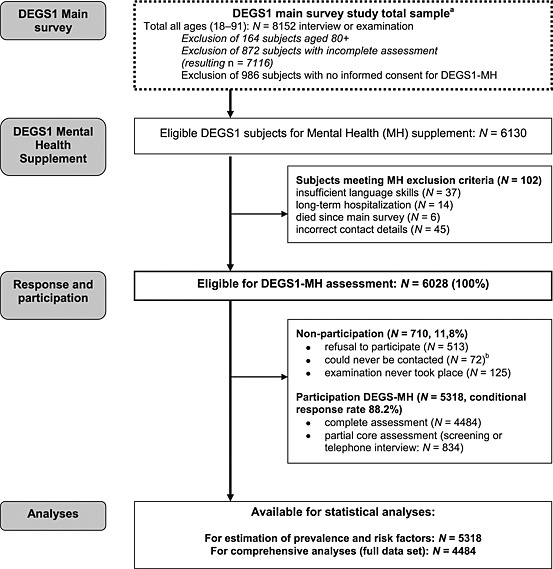
Response and non‐response in DEGS1‐MH. aSee Scheidt‐Nave et al. (2012) and Kamtsiuris et al., in press. bAfter a minimum of 10 contact attempts by telephone or letter (different days and times).
Recruitment procedure, response and non‐response in DEGS1‐MH
During the physical examination, DEGS1 participants were asked to provide informed consent to be re‐contacted for a home visit appointment for additional mental health examinations. However, N = 986 subjects did not wish to be re‐contacted, thus the RKI only transmitted the contact data of N = 6130 subjects who were aged 18–79, had a complete DEGS1 assessment and who agreed to be re‐contacted by the mental health team. A further N = 102 (1.7%) did not meet inclusion criteria because of wrong contact data (N = 45), insufficient language skills (N = 37), long‐term hospitalization (N = 14), or because they were deceased in the time interval between DEGS1 and DEGS1‐MH contact (N = 6). Thus N = 6028 remained for the DEGS1‐MH assessment and were re‐contacted for participation for the additional examinations (Figure 2).
Of the contacted subjects, 513/6028 (8.5%) refused participation, 125/6028 (2.1%) were willing to participate, but the interview never took place, and 72/6028 (1.2%) could never be contacted. The remaining, namely N = 5318/6028, form the final DEGS1‐MH sample (conditional response rate of 88.2%). Of the 5318 DEGS1‐MH participants, N = 2245 subjects (42%) already participated the 1998 predecessor study (GNHIES98), among them N = 1611 subjects (30%) also examined in the GNHIES98 mental health supplement (GHS‐MHS).
Thus, N = 4484/5318 completed the full DEGS1‐MH assessment, while N = 834 completed only the core part of the assessment package, by only completing a screening interview, based on the Composite International Diagnostic Interview (CIDI) stem questions (CID‐S; Wittchen et al., 1999). Reasons for administering the screening interview were: time constraints (N = 450), health problems (N = 49), and other reasons (N = 335, not further explored). Thus, for the statistical analyses, in particular for estimations of prevalence and risk factors N = 5318 subjects were available, while more comprehensive data analyses are restricted to N = 4484 subjects who completed the full assessment. It should be noted, that screening interviews and missing diagnostic information in parts of the interview were imputed to derive probabilities for diagnosis (see later).
Weighting
The complex sampling strategy of DEGS required multiple weighting steps. Adopting the DEGS1 design, sample and attrition weights of the RKI for the N = 7116 DEGS1 respondents aged 18–79 with complete data (Scheidt‐Nave et al., 2012), post stratification weights were calculated for DEGS1‐MH respondents (separate weights for both N=5318 and N=4484). First, marginal (and partially multivariate) distributions of age, sex, federal state of residence, community size, education and German citizenship according to the official records of the local population registries were iteratively adjusted to the German population in the age of 18‐79 (Kamtsiuris et al., in press). Then logistic regression, using the weights provided by the RKI, were applied to predict participation in the mental health module. Eleven demographical, socio‐economical and geographical covariates were considered in the model. In the multiple model which determined the final weight, four variables remained statistically significant (in N = 4484: linear and squared terms of age, German citizenship and total socio‐economical level (dimensional; Lampert et al., in press), in N = 5318 the cubic term of age was included as well). The RKI weight was multiplied with the inverse model‐based predicted probability to be a participant in the mental health module. Finally, the weight was rescaled in both samples so that the average weight equaled one. Weights for longitudinal analysis are yet to be developed.
Age varied between 18 and 79 years (at the time of the physical examination in the main survey). In exceptional cases, however, the time‐lag between core survey and mental health examination was more than one year resulting in the fact that in 15 cases, participants were older than 79 years at the time of the mental health examination. Tables 1 and 2 show the distribution of selected socio‐demographic variables in the DEGS1 main survey and DEGS1‐MH.
Table 1.
Demographic distribution of the German population, the respondents of the main survey (DEGS1) and the respondents of the mental health supplement (DEGS1‐MH) unweighted (N, %) and weighted (%w)
| German population 31 December 2010 (in thousand) | DEGS1 N = 7116a | DEGS1‐MH N = 5318 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | %w RKI | N | % | %w RKI | %w TUD | |
| Men | |||||||||
| 18–24 | 3 444.2 | 10.8 | 318 | 9.3 | 10.8 | 213 | 8.4 | 10.0 | 10.8 |
| 25–34 | 4965.4 | 15.6 | 399 | 11.7 | 15.6 | 283 | 11.1 | 14.6 | 15.2 |
| 35–44 | 5901.3 | 18.5 | 485 | 14.2 | 18.6 | 378 | 14.9 | 19.6 | 19.2 |
| 45–54 | 6766.5 | 21.2 | 673 | 19.7 | 21.3 | 501 | 19.7 | 21.5 | 21.1 |
| 55–64 | 4990.1 | 15.7 | 612 | 17.9 | 15.6 | 456 | 18.0 | 15.7 | 15.2 |
| 65–69 | 2106.1 | 6.6 | 370 | 10.8 | 6.6 | 290 | 11.4 | 7.2 | 7.1 |
| 70–79 | 3 676.1 | 11.5 | 554 | 16.2 | 11.6 | 419 | 16.5 | 11.4 | 11.4 |
| 31 849.7 | |||||||||
| Women | |||||||||
| 18–24 | 3 292.5 | 10.2 | 312 | 8.4 | 10.2 | 213 | 7.7 | 9.4 | 10.4 |
| 25–34 | 4827.8 | 15.0 | 428 | 11.6 | 15.0 | 309 | 11.1 | 14.4 | 14.6 |
| 35–44 | 5693.1 | 17.7 | 562 | 15.2 | 17.7 | 423 | 15.2 | 18.1 | 17.9 |
| 45–54 | 6562.2 | 20.3 | 794 | 21.4 | 20.3 | 625 | 22.5 | 21.1 | 20.3 |
| 55–64 | 5123.4 | 15.9 | 680 | 18.4 | 15.9 | 513 | 18.5 | 16.0 | 15.5 |
| 65–69 | 2275.8 | 7.1 | 373 | 10.1 | 7.1 | 299 | 10.8 | 7.6 | 7.6 |
| 70–79 | 4 479.6 | 13.9 | 556 | 15.0 | 13.9 | 396 | 14.3 | 13.4 | 13.7 |
| 32 254.4 | |||||||||
| Total | |||||||||
| 18–24 | 6 736.7 | 10.5 | 630 | 8.9 | 10.5 | 426 | 8.0 | 9.7 | 10.6 |
| 25–34 | 9793.2 | 15.3 | 827 | 11.6 | 15.3 | 592 | 11.1 | 14.5 | 14.9 |
| 35–44 | 11594.4 | 18.1 | 1047 | 14.7 | 18.1 | 801 | 15.1 | 18.8 | 18.5 |
| 45–54 | 13328.7 | 20.8 | 1467 | 20.6 | 20.8 | 1126 | 21.2 | 21.3 | 20.7 |
| 55–64 | 10113.5 | 15.8 | 1292 | 18.2 | 15.7 | 969 | 18.2 | 15.8 | 15.4 |
| 65–69 | 4381.9 | 6.8 | 743 | 10.4 | 6.8 | 589 | 11.1 | 7.4 | 7.3 |
| 70–79 | 8 155.7 | 12.7 | 1110 | 15.6 | 12.7 | 815 | 15.3 | 12.4 | 12.5 |
| 64 104.1 | |||||||||
DEGS1 respondents aged 18–79 years with both interview and examination data
Note: %wRKI, sample weight provided by RKI, accounting for selection probabilities of sampling points and age groups, regional distribution, German citizenship, education and re‐participation probability of former GNHIES98 participants; %wTUD, post‐stratification weight provided by TUD to additionally account for non‐participation in DEGS1‐MH
Table 2.
Socio‐demographic characteristics of DEGS1 and DEGS1‐MH sample (unweighted and weighted)
| DEGS1 (N = 7116) | DEGS1‐MH (N = 5318) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N a | M | SD | Mw | SDw | N a | M | SD | Mw | SDw | |
| Age | 7116 | 50.6 | 16.7 | 47.4 | 16.7 | 5318 | 51.0 | 16.4 | 47.4 | 16.7 |
| N | % | %w | N | % | %w | |||||
| 18–19 | 217 | 3.0 | 3.9 | 146 | 2.7 | 4.0 | ||||
| 20–29 | 854 | 12.0 | 14.9 | 602 | 11.3 | 15.0 | ||||
| 30–39 | 842 | 11.8 | 14.7 | 609 | 11.5 | 14.3 | ||||
| 40–49 | 1296 | 18.2 | 21.4 | 991 | 18.6 | 21.3 | ||||
| 50–59 | 1399 | 19.7 | 18.2 | 1064 | 20.0 | 18.0 | ||||
| 60–69 | 1398 | 19.6 | 14.1 | 1091 | 20.5 | 14.8 | ||||
| 70–79 | 1110 | 15.6 | 12.7 | 815 | 15.3 | 12.5 | ||||
| Sex | ||||||||||
| male | 7116 | 3411 | 47.9 | 49.7 | 5318 | 2540 | 47.8 | 49.6 | ||
| female | 3705 | 52.1 | 50.3 | 2778 | 52.2 | 50.4 | ||||
| M | SD | Mw | SDw | M | SD | Mw | SDw | |||
| SES 2 – job position (range 1–7) | 6941 | 3.4 | 1.3 | 3.3 | 1.2 | 5242 | 3.5 | 1.3 | 3.3 | 1.2 |
| SES – education (range 1–7) | 7048 | 4.0 | 1.5 | 3.7 | 1.5 | 5280 | 4.0 | 1.5 | 3.7 | 1.4 |
| SES – income (range 1–7) | 7116 | 4.1 | 1.8 | 4.0 | 1.9 | 5318 | 4.2 | 1.8 | 4.0 | 1.8 |
| SES – aggregated (range 1–21) | 7048 | 11.5 | 3.6 | 11.0 | 3.6 | 5280 | 11.7 | 3.6 | 11.1 | 3.5 |
| SES – distribution total | N | % | %w | N | % | %w | ||||
| low SES | 7048 | 1160 | 16.5 | 19.8 | 5280 | 766 | 14.5 | 19.3 | ||
| medium SES | 4209 | 59.7 | 60.1 | 3168 | 60.0 | 60.7 | ||||
| high SES | 1679 | 23.8 | 20.1 | 1346 | 25.5 | 20.0 | ||||
| German citizenship | ||||||||||
| yes | 7116 | 6802 | 95.6 | 90.5 | 5318 | 5146 | 96.8 | 90.9 | ||
| no | 314 | 4.4 | 9.5 | 172 | 3.2 | 9.1 | ||||
| Municipality: Number of inhabitants | ||||||||||
| <2000 | 7116 | 612 | 8.6 | 7.1 | 5318 | 482 | 9.1 | 7.4 | ||
| 2000– < 5000 | 691 | 9.7 | 8.1 | 503 | 9.5 | 8.0 | ||||
| 5000– < 20000 | 1714 | 24.1 | 23.9 | 1277 | 24.0 | 24.2 | ||||
| 20000– < 50000 | 1479 | 20.8 | 19.6 | 1106 | 20.8 | 19.5 | ||||
| 50000– < 100000 | 601 | 8.4 | 9.8 | 452 | 8.5 | 10.1 | ||||
| 100000– < 500000 | 1023 | 14.4 | 15.9 | 749 | 14.1 | 15.1 | ||||
| > = 500000 | 996 | 14.0 | 15.5 | 749 | 14.1 | 15.7 | ||||
| Marital status | ||||||||||
| married (living together) | 7042 | 4477 | 63.6 | 61.0 | 5278 | 3424 | 64.9 | 61.7 | ||
| married (separated) | 121 | 1.7 | 1.8 | 97 | 1.8 | 1.9 | ||||
| never married | 1587 | 22.5 | 26.1 | 1118 | 21.2 | 25.5 | ||||
| divorced | 453 | 6.4 | 6.0 | 347 | 6.6 | 5.9 | ||||
| widowed | 404 | 5.7 | 5.1 | 292 | 5.5 | 5.0 | ||||
Available N; differences to total N due to missing data.
Note: SES, measures of socio‐economic status Lampert et al., in press
Fieldwork DEGS1‐MH
The fieldwork and assessment domains of the main DEGS1 survey have been presented elsewhere (Scheidt‐Nave et al., 2012). The DEGS1‐MH computer assisted personal interviews (CAPI) were performed from September 2009 to March 2012 at the respondent's place of residence either at home (N = 1020), at local study centers that had been already used in the main survey assessment (N = 2715), or at another place of the participant's choice if neither home or study center were suitable (e.g. café, workplace; N = 187). However, N = 562 (12.5%) interviews were conducted via telephone (CATI). Interviews were usually performed 2–8 weeks after the main survey examination (time lag: median = 6 weeks; 55% < 6 weeks, 12% 6–12 weeks, 33% > 12 weeks). The relatively long field period optimized the opportunity to re‐contact respondents when no interview was possible at the first regular tour of the interviewer team to their residence. Appointments were made six to eight weeks before examination.
Interview duration [mean = 66.3 minutes; standard deviation (SD) = 27.7] varied depending on age and diagnostic status and could go up to several hours. Interviews were conducted strictly confidential face‐to‐face interviews involving only interviewer and participant. In 9% of the interviews, however, at least one more person (usually family member or partner) was present at least for some time in the examination room; but post hoc comparisons of these subjects revealed no differences in the reporting behavior of these subjects, thus it is unlikely that this protocol deviation has a significant effect on findings.
Interviewers
The fieldwork was conducted by a total of 49 clinically trained and experienced interviewers (94% clinical psychologists or advanced clinical psychology students, 6% other occupational background, e.g. medical students). Most of the interviewers were highly experienced due to their inclusion in previous studies with the instrument. All interviewers had to complete at least one mandatory DEGS‐CIDI training (two days plus supervision of at least two training interviews). Additionally training components dealt with ethically sensitive issues and issues concerning data protection, and skills in the management of potentially difficult interview situations. For the neuropsychological assessment, additional specialized trainings of one day were conducted.
The average interviewer worked in 21.3 sample points (SD = 13.0; range = 1–45) and conducted 193.4 (SD = 128.6; range = 6–415) interviews. Payment for interviewers varied according to interviewer status (student/postgraduate, service contract/employed) and effort related to length of interview trip.
Monitoring and quality assurance
Fieldwork was monitored closely over the entire data collection period using a monitor protocol. Interviewers were monitored by four supervisors (one regularly present in every interviewer team) according to three standard protocols: (a) the overall DEGS1 fieldwork protocol (see Scheidt‐Nave et al., 2012) defined contact behavior, informed consent and the general procedures during fieldwork (appearance, clothing, etc.); (b) the standard CIDI protocol defined all rules and guidelines to be followed while administration the standardized assessment with the CAPI platform the CIDI; (c) the third protocol defined handling of data after assessment, transmission of data to the study center and data quality control and assurance as well as plausibility checks as defined by the CIDI platform (see Wittchen and Pfister, 1997).
Refreshment training of half‐day duration took place every six months for all current interviewers. Regular external random quality control by RKI group leaders did not reveal any significant violations of the study protocols. Data entry of each participant (including questionnaire data) was independently double checked by two editors. During fieldwork, “critical events” (e.g. aggression, alcohol or drug intoxication of a participant, acute suicidality) were extremely rare (N < 20) and were immediately reported to supervisors who supported the interviewers and contacted the participants by telephone if needed. In no case a negative or harmful outcome related to the examination could be identified.
In order to check acceptance and satisfaction with the examination procedures, 20% of the participants with complete interviews (N = 849) were asked to rate the following items on a four‐point rating scale (see Table 3): initial contact before interview, material and study information, competence of interviewer, atmosphere during interview, effort to participate in study, meaningfulness/relevance of examined topics, computer‐assisted interview tool, and detail of examination. Further they were asked if they would participate again in such a study in the future. Items correlated highly amongst each other and loaded only on one factor (eigenvalue 2.01, all other factors < 0.21) that can be interpreted as overall satisfaction with examination. On average participants were highly satisfied (mean = 1.15, SD = 0.23) on the scale ranging from one (very satisfied) to four (not satisfied at all), and 98% stated that they would participate again. There were some indications of a higher satisfaction in older and females respondents. No associations were found with regard to psychopathology (as measured with the number of positive stem questions of the CIDI and the PHQ9, see later).
Table 3.
Participant satisfaction (%) with DEGS1‐MH assessment procedure (N = 849)
| Satisfaction with …a | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Initial contact before interview | 95.6 | 3.7 | 0.7 | 0.0 |
| Material and study information | 88.0 | 10.4 | 1.3 | 0.3 |
| Competence of interviewer | 97.3 | 2.7 | 0.0 | 0.0 |
| Atmosphere during interview | 95.3 | 4.3 | 0.4 | 0.1 |
| Effort to participate in study | 86.3 | 12.2 | 1.3 | 0.2 |
| Meaningfulness/relevance of examined topics | 77.7 | 20.7 | 1.3 | 0.4 |
| Computer‐assisted interview tool | 78.8 | 11.4 | 1.9 | 7.9 |
| Detail of examination | 83.8 | 15.0 | 0.8 | 0.4 |
| Willingness to participate again | 98% |
Items were rated on a four‐point rating scale (1 = very satisfied, 4 = not satisfied at all).
Diagnostic instruments and domains
Table 4 provides an overview on the diagnostic and non‐diagnostic assessment domains. All components were assessed within one computer‐assisted standardized procedure implying interview questions and ratings by the respondents or the interviewer, the instruction and administration of tests and self‐report scales, and the coding rules. Within this assessment platform, the DEGS‐CIDI (DIA‐X/M‐CIDI) was the main component.
Table 4.
DSM‐IV‐TR diagnoses (A), and other diagnostic and non‐diagnostic (B) domains covered in DEGS1‐MH
| (A) DSM‐IV‐TR Diagnoses (F‐Codes according to ICD‐10) | (B) Other diagnostic and non‐diagnostic domains |
|---|---|
| 1. Mental disorders due to general medical condition | 1. Neuropsychological section |
| General medical condition (GMC; F06.x) | Subjective memory impairment (Jessen, 2007) |
| Substance‐induced diagnoses (F1x.x) | Prospective memory (Kliegel et al., 2007) |
| 2. Substance‐related disorders | Episodic memory (Morris et al., 1989, Luck et al., 2009) |
| Nicotine dependence (F17.2x) | Verbal working memory (von Aster et al., 2006) |
| Alcohol abuse (F10.1x) | Visual search, attention, task‐switching (Reitan and Wolfson, 1993) |
| Alcohol dependence (F10.2x) | Mental speed (Jolles et al., 1995; van der Elst et al., 2006) |
| Any alcohol‐related disorder (F10.1/2) | Verbal fluency (Morris et al., 1989; Jolles et al., 1995; Luck et al., 2009) |
| Medication abuse (F11/F13/F15.1x) | Verbal intelligence (Lehrl, 2005) |
| Medication dependence (F11/F13/F15.2x) | |
| Any medication‐related disorder (F11/F13/F15.1/2) | 2. Impairments and disabilities |
| 3. Schizophrenia and other psychotic disorders (F2x.x) | Impairment days (within past four weeks) due to psychological/psychosomatic and somatic problems, alcohol/drug intake or medication |
| 4. Anxiety disorders | 3. Help‐seeking behavior due to psychological problems |
| Panic‐disorder with and without agoraphobia (F40.01) | ‐ Inpatient: seven types of institutions |
| Agoraphobia without history of panic disorder (F40.00) | ‐ Outpatient: psychiatrist, psychotherapist (four types), general practitioner, counselor (eight types), other institutions (seven types) |
| Generalized anxiety disorder (F41.1) | ‐ Kind of treatment (medication, behavior therapy, other psychotherapy, none of these) |
| Social phobia (40.1) | ‐ Barriers of utilization of health services |
| Specific‐phobia (F40.2) | ‐ Discontinuation of therapy (remitted, partly remitted, dissatisfaction with therapy/therapist, problems with health insurance, stigmatization, change of residence, etc.) |
| ‐ Animal‐type (F40.21) | 4. Subjective generic quality of life (EQ‐5D) (Hinz et al., 2006; Greiner et al., 2003) |
| ‐ Blood‐injection‐injury‐type (F40.23) | 5. (short) BIG Five Inventory (BFI‐10)(Rammstedt, 2007) |
| ‐ Natural‐environmental‐type (F40.22) | 6. Life Orientation Test (LOT) (Scheier et al., 1994) |
| ‐ Situational‐type (F40.24) | 7. Cross‐sectional depressive symptoms (PHQ‐9) (Kroenke et al., 2001) |
| ‐ Other type (F40.25) | |
| 5. Obsessive‐compulsive disorder (F42.x) | 8. Suicidal tendency (five items) (Wittchen and Pfister, 1997) |
| 6. Post‐traumatic stress disorder (F43.1) | |
| 7. Affective (Mood) disorders | 9. Fatigue scale for motor and cognitive functions (FSMC) (Penner et al., 2009) |
| Major depression | |
| Single episode (F32.x) | 10. Psychotic experiences: |
| Recurrent episode (F33.x) | Launay–Slade Hallucinations Scale (LSHS) (Launay and Slade, 1981; Laroi et al., 2004) |
| Non‐remitted recurrent (F32/33.1/2/3) | |
| Specifier for MDD (mild, F32/33.0; moderate, F32/33.1; severe, F32/33.2/3) | Peters et al. Delusions Inventory (PDI) (Peters, 2004) |
| ‐ mild (F32.0) | |
| ‐ moderate (F32.1) | 11. Effort‐Reward‐Imbalance (ERI) for working and non‐working subjects (Siegrist et al., 2004; Siegrist and Jacobi, 2009) |
| ‐ severe (F32.2/3) | |
| ‐ with melancholic features (F3x.x1) | |
| ‐ with postpartum onset | |
| Dysthymic disorder (with hierarchy) (34.1) | |
| Bipolar I affective disorder (F30.1/2, F31.1‐9) | |
| Bipolar II affective disorder (F30/31.0) | |
| 8. Eating disorders | |
| Anorexia nervosa (F50.0) | |
| Bulimia nervosa (F50.2) | |
| Binge eating (F50.9) | |
| 9. Somatoform disorders | |
| SSI4/6 (F45.0) | |
| Pain disorder (F45.4) | |
| 10. Clinical and other interviewer observations | |
| ‐ Brief psychiatric rating scale (BPRS) | |
| ‐ Interview setting, participant's behavior and features |
The DEGS‐CIDI is a modified version of the World Health Organization CIDI (Kessler and Üstün, 2004), available in more than 16 languages, also used in the World Mental Health series and internationally in hundreds of comparable surveys (ESEMeD/MHEDEA 2000 Investigators, 2004; Haro et al., 2006; Kessler and Üstün, 2008; Kessler et al., 2004). The fully structured algorithm‐ and computer‐based DIA‐X/M‐CIDI (Lachner et al., 1998; Reed et al., 1998; Wittchen et al., 1991; Wittchen, 1994; Wittchen and Pfister, 1997) allows for reliable assessments of symptoms and syndromes according to the criteria of DSM‐IV‐TR [with its compatible International Classification of Disease, 10th Revision (ICD‐10; WHO, 1993) codes] for different time frames (four‐week, 12‐month, and lifetime), along with information about onset, duration, and severity of threshold and subthreshold conditions. Moreover, in this study, for all “key syndromes”, the medical and non‐medical help‐seeking behavior as well as medication use were assessed and coded.
Additional standard CIDI probe questions allow the description of physical factors and diseases as well as substances that might be causally associated with the symptoms described by the subjects. Diagnoses are derived in a highly objective manner by using exclusively the standardized CIDI diagnostic program to ensure that the diagnostic criteria are strictly applied on the basis of the symptom information without the interviewer playing any role in making diagnostic statements.
The DEGS‐CIDI maintained the overall structure and rules and protected the integrity of the diagnostic program. However, to allow addressing the research questions in an optimal and efficient way, several adaptations were made:
The initial psychosocial CIDI section (i.e. section A: socio‐demographics) was shortened to avoid overlap with main DEGS1 survey, where this information was already assessed in greater detail.
The CIDI section L was limited to medication use and abuse. Illegal drug use disorders were not assessed because of the associated time burden and previous evidence of low base rates, insufficient for detailed analyses. Further, several regular and specialized drug use surveys already exist in Germany (Kraus et al., 2010).
Standard CIDI questions on impairment and disability were supplemented by a more detailed assessment of disorder‐specific functional impairments.
The use of CIDI skip‐rules in almost all diagnostic sections were minimized, to allow for the assessment of subthreshold conditions (conditions falling short of mandatory DSM‐IV‐TR diagnostic criteria), to improve the dimensional description within the diagnostic status description.
Section Q on help‐seeking, service use and treatment was extended by CIDI questions regarding (a) diagnosis‐specific help‐seeking behavior of general health care providers, specialized psychiatric and psychological institutions, and complementary, informal providers, (b) frequency and type of received treatment/intervention, (c) person‐ and system‐based barriers, e.g. stigmatization, attitude, knowledge about illness and symptoms.
The DEGS‐CIDI covers the following groups of mental disorders: mood disorders (major depression, dysthymia, bipolar disorder I and II, lifetime and past 12 months), anxiety disorders (panic disorder, agoraphobia, generalized anxiety disorder, social phobia, specific phobias in the past 12 months, except for panic (lifetime and 12‐month), obsessive‐compulsive disorder (past 12 month), post‐traumatic stress disorder (lifetime and past 12 month), substance use disorders (nicotine dependence, alcohol and medication abuse and dependence; lifetime and 12‐month), somatoform disorders (pain disorder and undifferentiated somatoform disorder as measured by the Somatic Symptom Index, SSI4,6; lifetime and past 12 month), eating disorders (anorexia nervosa, bulimia nervosa, binge eating disorder; lifetime and past 12 month), psychotic disorders (lifetime) and cognitive impairment (see later).
Due to the diagnostic criteria and the conventions for reporting, only the 12‐month frame should be considered as an appropriate diagnostic reporting standard for all diagnoses, except for two groups of disorders, namely mood disorders that could be interpreted for lifetime and 12‐month, and psychotic disorders for which only lifetime estimates are meaningful. For the disorders only assessed for the past 12 months, additional “prior 12 months” information is only available when symptoms were present within last year.
Neuropsychological section
The DEGS1‐MH includes a cognitive and neuropsychological assessment module for all respondents to map cognitive function over the whole adult life span. The test battery can be used to examine cognitive function scores in relation to a range of other aspects of mental and somatic health, health behaviors and psychosocial characteristics. It can also serve to identify persons with reduced levels of cognitive functioning by applying normative data for individual tests, e.g. from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) test battery (Luck et al., 2009). The neuropsychology section included the following measures: subjective memory impairment (SMI) and related concerns were assessed by the questions: “Do you feel like your memory is becoming worse?” and if the answer was yes, “Does that worry you?” and “Does that worry you a lot?” (Jessen, 2007) and for a subsample (N = 1427) further questions on memory impairment (comparison with peers, impairment rating, health care utilization due to memory problems). Then the following test battery was administered (Table 4). It consisted of 10 cognitive performance tests (administration time: mean = 21.4, SD = 4.3 minutes) and was developed to assess six domains of cognitive functioning with reasonable efficiency: memory: episodic memory (immediate and delayed recall of word lists from CERAD; Morris et al., 1989; Luck et al., 2009), prospective memory (two tasks that could be administered by telephone, analogous to CogTel; Kliegel et al., 2007), and verbal working memory (digit span backwards analogous to Wechsler Intelligence Scale for Adults; Von Aster et al., 2006); executive function and mental speed (verbal fluency task analogous to CERAD; Morris et al., 1989; Luck et al., 2009; letter digit substitution test (LDST); Jolles et al., 1995; van der Elst et al., 2006; Trail making tests (TMT) A and B analogous to CERAD; Morris et al., 1989); and verbal IQ (multiple choice vocabulary test, MWT‐B; Lehrl, 2005).
In addition to analyzing individual test scores, a composite measure of global cognitive function will be created by converting each cognitive performance test into a z‐score and averaging z‐scores of all tests (stratified by age, education, verbal intelligence where appropriate). Composite measures of specific cognitive domains can similarly be created (e.g. memory, mental speed, and executive function).
Additional instruments
The following non‐diagnostic modules were added to the diagnostic interview.
Personality: Inclusion of resource‐oriented variables and personality dimensions as moderators of health‐related behavior (Big Five Inventory short form, BFI‐10; Rammstedt, 2007; Life Orientation Test, LOT‐R, depicting optimism, Scheier et al., 1994).
Subjective generic quality of life: the short questionnaire EQ‐5D was used (Greiner et al., 2003), since the main survey covers further comprehensive assessments.
Suicidal tendency: assessment of suicidal thoughts, plans, attempts, self‐destructive behavior, also in subjects who were not interviewed in the CIDI depression section (this was a limitation of previous studies).
Cross‐sectional depressive symptoms and fatigue were assessed with the PHQ‐9 (Kroenke et al., 2001) and the Fatigue Scale for Motor and Cognitive Functions (FSMC; Penner et al., 2009) for cognitive and physical fatigue.
Effort‐Reward Imbalance: This psychological construct refers to “gratification crisis models”. In collaboration with the author of the original instrument (ERI; Siegrist et al., 2004), a variant for non‐working participants was developed, since a relevant proportion of the sample (N = 2489; 55.5%) was not, not yet, or no longer employed.
Psychotic experiences: We modified and supplemented the CIDI psychosis section by using psychosis‐screenings regarding delusional events along with their frequency, subjective disturbance and conviction of thoughts (PDI; Peters, 2004) and hallucinations in all sensory modalities (LSHS; Launay and Slade, 1981; Bentall and Slade, 1985). Both are validated for surveys in the general population.
Imputation of missing data and screening interviews
In exceptional cases only parts of the interview and the CIDI stem questions were administered. The questionnaire instruments, usually embedded in the interview, were given to complete and send back later. In N = 834 cases, when it was not possible to arrange an interview appointment the screening interview (CIDI stem questions) was conducted. Given that the sensitivity and specificity of the CIDI stem questions for diagnostic status is well established (CID‐S; Wittchen et al., 1999), we calculated for these incomplete data rows, model based estimates of the probability of every diagnosis, to enhance the power of prevalence estimates. Covariates for predicting these probabilities were age, gender and the 11 stem questions. We forced the number of items endorsed (represented by five dummy variables) in the logistic regression models as well as the main effects of age and sex and their interaction. On top of that, specific items as well as the quadratic and cubic terms of age were selected with combined backward and forward selection (exclusion probability = 0.05, inclusion probability = 0.01; when the cubic term of age was in the model, the quadratic term was left in, too). In case of rare diagnoses where numerical problems in prediction occurred, model selection was simplified: Only age and the dimensional count of endorsed stem questions were forced into the model, stem questions with empty cells in combination with a diagnosis were disregarded. All regressions were weighted. This imputation method was also conducted for cases, which did not complete the respective CIDI section for a specific diagnosis. In the final DEGS1‐MH dataset, prevalence estimation will always be reported separately for the N = 4484 respondents with a complete diagnostic data set and the total sample examined of N = 5318 using the model‐based probabilities for the 834 with partial assessment.
Discussion and conclusion
In this paper we presented essential information on design, sampling (response rate, weighting), socio‐demographic sample characteristics, fieldwork and assessment methods of the DEGS1‐MH. The overall aim of DEGS1 is to describe the health status and the morbidity patterns of the adult German population covering both, somatic health and crude indicators of mental health within the DEGS1 main survey and mental disorders and more detailed measures of mental health in the separate mental health module (DEGS1‐MH).
In comparison to previous mental health studies, DEGS1‐MH is expected to provide more detailed and more comprehensive information about the mental health status, mental disorder as well as the health care utilization and the functional limitations in the German general population, aged 18–79. In terms of power considerations for diagnostic issues, the study could be regarded as well powered for all disorders with a prevalence of 1.5% or above, given a total of 5318 respondents. With regard to longitudinal analyses (possible for N = 1611 participants), certain power restrictions will apply, requiring to collapse single diagnoses for some specific diagnoses into larger diagnostic groups. The more comprehensive coverage of diagnostically relevant mental health issues in DEGS1 and DEGS1‐MH will allow for addressing a large range of research questions.
The high acceptance and satisfaction with the study procedures corresponds to other studies using similar demanding interviews for mental disorders (Hoyer et al., 2006). Together with the results of internal and external quality control, this suggests that the fieldwork was designed and conducted properly. Further strengths are the greater emphasis on dimensional measures, improved information about severity, course and disability, the neuropsychological assessment and the opportunity to link data from the somatic and general health component (DEGS1) with the more detailed assessment of mental disorders (DEGS1‐MH) cross‐sectionally and longitudinally.
Limitations of the design
An important limitation in DEGS1 might be the fact that certain high risk groups might not be appropriately covered, such as residents that are long‐term or permanently institutionalized, immigrants not speaking fluent German, and the homeless. Another potential limitation might be that both surveys, DEGS1 and DEGS1‐MH, were in some cases significantly apart in time (out of the target range 2–6 weeks). Thus, some cross‐sectional analyses linking the two survey waves might need caution. However this potential limitation might not be critical, because more than 90% of participants reported during the DEGS1‐MH interview to have the same overall health state as during the preceding DEGS1 examination.
Undoubtedly the sampling design of this ambitious program is quite complex, raising the question of how representative findings will be for the German adult population overall. This question is critical, given that the design tries to accommodate for various goals at a time. First, sample selection was based on an established (Bellach et al., 1998; Kurth et al., 2008) complex two‐stage stratified cluster random sampling, using communities as the primary sample points and population registry data for the non‐institutionalized adult population aged 18–79 with permanent residence in Germany. This ensured, within acceptable limits, the representativeness of the target population (Scheidt‐Nave et al., 2012; Kamstiuris et al., in press). Additionally however the study should be powered for longitudinally analyses by enriching the sample with respondents from the previous 1998 national survey. This additional goal required complex additional strategies and considerations (i.e. replacement, power) to add appropriately the past respondents into the overall sample of the newly sampled. And finally the additional incorporation of the separate mental health module, associated with loss due to lacking informed consent and other reasons for attrition needs to be accommodated for. Thus, due to the complex nature of the overall sampling and design, there was the need of substantial design and post‐stratification weighting to adjust for potential deviations in representativeness, particularly so because of substantial differences in response rates among newly sampled (42%) and past participants (64%, see Scheidt‐Nave et al., 2012, for details). Additionally, the clustering of participants within sample points has to be accounted for in statistical analyses by using procedures designed to analyze data derived from a complex sample survey (Siller, Tompkins, 2006).
Comparisons of the sampled distribution of DEGS1‐MH participants with the true distribution in the population regarding a series of variables (like age, gender, education level) suggest that our findings in the mental health supplement reflect well the true distribution and could be regarded as representative for the German adult population in the age range 18–79. The highly satisfying conditional response rate of 88% at least for the DEGS1‐MH and the fact that the weighting procedures do not change the distribution substantially add further confirmation.
It is also noteworthy that declining response rates in population based health surveys have internationally and consistently been reported over the past decade (Galea and Tracy, 2007; Tolonen et al., 2006). Selection bias resulting from selective participation of healthier persons is a concern in any population‐based survey (Criqui et al., 1978). Survey results may therefore underestimate the overall prevalence of chronic diseases and disability. But, as Galea and Tracy (2007) point out, most studies have found little evidence for substantial bias as a result of non‐participation, and that extreme efforts to increase participation rates may introduce even more bias into the study if the added respondents are not representative for all non‐respondents, or if they are less conscientious in the survey participation.
Coverage of cognitive abilities
Given the current scientific interest in a better characterization of cognitive factors associated with disorders of the brain, the availability of our neuropsychological data, is a particularly exciting strength of our study. This is to our knowledge the first general population study ever that provides such data, focusing on a broad spectrum of cognitive domains over the whole life span. This will allow to determine the distribution of cognitive impairments and to analyze cognitive function in relation to many other aspects of health. However, no information about functional impairment was obtained from informants (e.g. from relatives), and no information on changes to cognitive function over time is available and other clinical causes of reduced cognitive function cannot be reliably excluded. Thus, it will not be possible to identify clinical diagnoses such as a mild cognitive impairment (MCI) and dementia. In general, the study design of DEGS1 was not suitable to estimate prevalences of dementia since it included a random sample of people from the general population who were able to come to the study center, give informed consent and be interviewed and examined for on average two hours without significant language problems. People living in institutions or having functional or cognitive impairments are therefore most likely to be underrepresented in the study (Scheidt‐Nave et al., 2012). Despite this limitation, the data on cognitive function collected in DGES1‐MH is a valuable resource for psychiatric epidemiology and research on public mental health. It can be used to examine cognitive function in relation to a range of other aspects of mental and somatic health, to health behaviors and to psychosocial characteristics both in cross‐sectional and longitudinal analysis. The results of such analyses can contribute importantly to the development of strategies for the prevention of dementia and cognitive decline in Germany and of actions at the population level to enable people to preserve their cognitive function as a key component of healthy aging. In future study waves, for which the current wave can serve as a baseline assessment, it may be possible to identify persons with cognitive decline or new dementia or MCI in later longitudinal analyses. One particular advantage of DEGS1‐MH is that cognitive function has been assessed in participants of all age groups. Thus, it will be possible to examine the long‐term effects of predictors of cognitive decline starting in early or middle adult age.
Coverage of psychosis‐like experiences
Another innovative component is the section dealing with psychosis‐like experiences. The main rational to include the assessment of psychotic and psychosis‐like experiences is that previous studies (Cougnard et al., 2007; Dominguez et al., 2010; Dominguez et al., 2011; Kaymaz et al., 2012; Wigmann et al., 2012) showed highly variable prevalences of such experiences in unselected samples of the general population, and that the prospective significance of such experiences for the presence or the future development of mental disorders is still unclear. Psychosis‐like experiences were reported in up to 30% of the general population (reviewed by Nuevo et al., 2012). However, in most cases, such experiences are not “psychotic” (i.e. in a strict sense of the term referring to delusions or hallucinations), but comprise near‐normal experiences like magical thinking (reviewed by Nelson et al., 2012). Assessing the symptoms with psychometric scales usually results in much higher prevalence rates compared to using the CIDI psychosis scale (around 10%) or operationalized diagnostic assessments for ICD‐10/DSM‐IV diagnoses (around 1%) (Nuevo et al., 2012). Also, such symptoms and experiences are usually of a fluctuating or transient nature. Obviously, the point prevalence of such experiences is much higher than the point prevalence of psychotic disorders (around 1%), which indicates that there are different patterns or that additional factors must play a role in determining the progression from transient experiences to a psychotic disorder. Factors like the type of symptom experiences (for example, truly paranoid ideas like persecution versus “near‐normal” experiences like magical thinking), environmental, neurocognitive and genetic factors may play a role (Nelson et al., 2012). There is a need to explore the interplay between these experiences and such associated factors with a view to establish risk factors for the development of mental disorders following the occurrence of psychosis‐like experiences in otherwise healthy persons. Such knowledge would be essential for assessing the individual risk of progression from psychosis‐like experiences to frank mental disorders. This could lead to effective prevention and early detection due to intensified medical follow‐up of those persons with high risks of progression. In addition to the prognostic significance of psychosis‐like experiences, Nuevo et al. (2012) showed that the occurrence of any such symptom is associated with distress and functional impairments irrespective of the development of a mental disorder. This indicates that the health‐outcome of persons with such symptoms needs to be monitored and long‐term studies are needed. DEGS1‐MH provides a unique opportunity to assess the mental health outcome of persons with such findings.
Conclusion and further perspectives
To conclude, the DEGS1‐MH provides up‐to‐date and internationally uniquely detailed and comprehensive data on the distribution of mental and somatic health symptoms and diagnoses. The standardized methodology used in this study program allows cross‐national comparisons with similar surveys such as the National Comorbidity Survey Replication in the United States (NCS‐R; Kessler et al., 2004, 2005) or the Netherlands Mental Health Survey and Incidence Study (NEMESIS‐2; de Graaf et al., 2010, 2012). In line with the study of Kessler et al. (2012), such more coherent prevalence and morbid risk estimates resulting from our study will inform policy‐makers and the public and will also provide science and research with more solid evidence about determinants, severity, course, and associated psychological and social disabilities of mental disorders, as well as their association with somatic disorders.
DEGS1‐MH provides a sustainable basis for future research. Currently several research projects are underway using DEGS1‐MH data for in depth analysis, among them: (1) methodological and statistical support of the DEGS1‐MH research consortium; (2) affective disorders and their correlates; (3) associations of mental disorders with chronic stress and the so called “burnout syndrome”, (4) estimation of changes of mental disorders in the general population during the last ten years; (5) cognitive performance and its association with mental and somatic disorders; (6) processes and circumstances of remissions and chronic courses of mental disorders; (7) help seeking behavior and the use of the health care system; (8) investigation of the comorbidity of somatic and mental disorders, and (9) development of short research instruments for the monitoring of mental disorders in the general population.
Declaration of interest statement
The authors have no competing interest to declare.
Acknowledgments
DEGS1 and DEGS1‐MH are commissioned by the German Ministry of Health (BMG), steered and coordinated by the Robert Koch Institute (Berlin, Germany, Department of Epidemiology and Health Monitoring: Dr. Ulfert Hapke and Dr. Bärbel‐Maria Kurth, Former Head of the Department).
Within this program, DEGS1‐MH was designed and conducted by the Technische Universität Dresden (Institute of Clinical Psychology and Psychotherapy & Center of Clinical Epidemiology and Longitudinal Studies, CELOS; Director: Prof. Dr. Hans‐Ulrich Wittchen). Principal Investigators of DEGS1‐MH were H.‐U. Wittchen (PI) and Frank Jacobi (Co‐PI), supported by the Stiftung Seelische Gesundheit inaugurated by the German Association for Psychiatry and Psychotherapy (DGPPN, Prof. Wolfgang Gaebel and Prof. Wolfgang Maier) with regard to the modules for psychosis and neurocognitive assessment. Funding was primarily provided by the BMG/RKI, supplemented by internal resources of the TU Dresden and the Foundation of the German Association for Psychiatry and Psychotherapy.
We would like to acknowledge the support of all RKI staff in the field work of the project, in particular Dr. Heike Hölling, and Dr. Stefan Dahm (for statistical support regarding sample weights), and the responsible RKI project officers for DEGS1‐MH, namely Michael Lange and Ulfert Hapke. We also thank the many staff members and interviewers of DEGS1‐MH at Technische Universität Dresden and the Institute, in particular Lisa Beyer, Marlen Gehrke, Gerry Gwozdz, Esther Lochmann, Doreen Opitz, Kristina Schäfer, Yuliya Stankevich and Anke Vogel for their exceptional contributions during the field phase of DEGS1‐MH. Above all, we wish to thank all the DEGS1‐MH study participants.
References
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision, Washington, DC, American Psychiatric Association, 6(4). [Google Scholar]
- Bellach B.M., Knopf H., Thefeld W. (1998) The German Health Survey 1997/98. Gesundheitswesen, 60(2), 59–68 (in German). [PubMed] [Google Scholar]
- Bentall R.P., Slade P.D. (1985) Reliability of a scale measuring disposition towards hallucination: a brief report. Personality and Individual Differences, 6(4), 527–529. [Google Scholar]
- Cougnard A., Marcelis M., Myin‐Germeys I., De Graaf R., Vollebergh W.A.M., Krabbendam L., Lieb R., Wittchen H.‐U., Henquet C.E.C., Spauwen J., van Os J. (2007) Does normal developmental expression of psychosis combine with environmental risk to cause persistence of psychosis? A psychosis proneness – persistence model. Psychological Medicine, 37(4), 513–527. [DOI] [PubMed] [Google Scholar]
- Criqui M., Barrett‐Connor E., Austin M. (1978) Differences between respondents and nonrespondents in a population‐based cardiovascular disease study. American Journal of Epidemiology, 108(5), 367–372. [DOI] [PubMed] [Google Scholar]
- Dominguez M.d.G., Can Saka M., Lieb R., Wittchen H.‐U., van Os J. (2010) Early expression of negative/disorganized symptoms predicts psychotic experiences and subsequent clinical psychosis; a 10‐year study. The American Journal of Psychiatry, 167(9), 1075–1082. [DOI] [PubMed] [Google Scholar]
- Dominguez M.G., Wichers M., Lieb R., Wittchen H.‐U., van Os J. (2011) Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8‐year cohort study. Schizophrenia Bulletin, 37(1), 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Elst W., van Boxtel M.P.J., van Breukelen G.J.P., Jolles J. (2006) The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. Journal of Clinical and Experimental Neuropsychology, 28(6), 998–1009. [DOI] [PubMed] [Google Scholar]
- ESEMeD/MHEDEA 2000 Investigators (2004) Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica, 109, 21–27. [DOI] [PubMed] [Google Scholar]
- Goodwin R., Jacobi F., Thefeld W. (2003) Mental disorders and asthma in the community. Archives of General Psychiatry, 60(11), 1125–1130. [DOI] [PubMed] [Google Scholar]
- de Graaf R., Ten Have M., Van Dorsselaer S. (2010) The Netherlands Mental Health Survey and Incidence Study‐2 (NEMESIS): design and methods. International Journal of Methods in Psychiatric Research, 19(3), 125–141, DOI: 10.1002/mpr.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R., Ten Have M., van Gool C., van Dorsselaer S. (2012) Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study‐2. Social Psychiatry and Psychiatric Epidemiology, DOI: 10.1007/s00127-010-0334-8 [DOI] [PubMed] [Google Scholar]
- Greiner W., Weijnen T., Neuwenhuizen M., Oppe S., Badia X., Busschbach J., Buxton M., Dolan P., Kind P., Krabbe P., Ohinmaa A., Parkin D., Roset M., Sintonen H., Tsuchiya A., de Charro F. (2003) A single European currency for EQ‐5D health states. The European Journal of Health Economics, 4(3), 222–231. [DOI] [PubMed] [Google Scholar]
- Gustavsson A., Svensson M., Jacobi F., Allgulander C., Alonso J., Beghi E., Dodel R., Ekman M., Faravelli C., Fratiglioni L., Gannon B., Jones D.H., Jennum P., Jordanova A., Jönsson L., Karampampa K., Knapp M., Kobelt G., Kurth T., Lieb R., Linde M., Ljungcrantz C., Maercker A., Melin B., Moscarelli M., Musayev A., Norwood F., Preisig M., Pugliatti M., Rehm J., Salvador‐Carulla L., Schlehofer B., Simon R., Steinhausen H.C., Stovner L.J., Vallat J.M., Van den Bergh P., van Os J., Vos P., Xu W., Wittchen H.‐U., Jönsson B., Olesen J. (2011) Cost of disorders of the brain in Europe European Neuropsychopharmacology, 21(10), 718–779. [DOI] [PubMed] [Google Scholar]
- Haro J.M., Arbabzadeh‐Bouchez S., Brugha T.S., De Girolamo G., Guyer M.E., Jin R., Lepine J.P., Mazzi F., Reneses B., Vilagut G., Sampson N.A., Kessler R.C. (2006) Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health Surveys. International Journal of Methods in Psychiatric Research, 15(4), 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härter M., Baumeister H., Reuter K., Jacobi F., Höfler M., Bengel J., Wittchen H.‐U. (2007) Increased 12‐month prevalence rates of mental disorders in patients with chronic somatic diseases. Psychotherapy and Psychosomatics, 76(6), 354–360. [DOI] [PubMed] [Google Scholar]
- Hinz A., Klaiberg A., Brähler E., König H. (2006) Der Lebensqualitätsfragebogen EQ − 5D: Modelle und Normwerte für die Allgemeinbevölkerung. Psychotherapie Psychosomatik Medizinische Psychologie, 56(2), 42–48. [DOI] [PubMed] [Google Scholar]
- Hoyer J., Ruhl U., Scholz D., Wittchen H.‐U. (2006) Patients' feedback after computer‐assisted diagnostic interviews for mental disorders. Psychotherapy Research, 16(3), 357–363. [Google Scholar]
- Jacobi F., Wittchen H.‐U., Müller N., Hölting C., Sommer S., Lieb R., Höfler M., Pfister H. (2002) Estimating the prevalence of mental and somatic disorders in the community: aims and methods of the German National Health Interview and Examination Survey. International Journal of Methods in Psychiatric Research, 11(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi F., Klose K., Wittchen H.‐U. (2004a) Psychische Störungen in der deutschen Allgemeinbevölkerung: Inanspruchnahme von Gesundheitsleistungen und Ausfalltage. Bundesgesundheitsblatt, 47, 736–744. [DOI] [PubMed] [Google Scholar]
- Jacobi F., Wittchen H.‐U., Hölting C., Höfler M., Pfister H., Müller N., Lieb R. (2004b) Prevalence, co‐morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS). Psychological Medicine, 34, 1–15, DOI: 10.1017/S0033291703001399 [DOI] [PubMed] [Google Scholar]
- Jessen F. (2007) Patterns of subjective memory impairment in the elderly: association with memory perfomance. Psychological Medicine, 37(12), 1753–1762. [DOI] [PubMed] [Google Scholar]
- Jolles J., Houx P.J.., Van Boxtel M.P.J., Ponds R.W.H.M. (1995) Maastricht Aging Study: Determinants of Cognitive Aging, Maastricht, Neuropsych Publishers. [Google Scholar]
- Kamtsiuris P., Lange M.., Hoffmann R., Schaffrath Rosario A., Dahm S., Kurth B.‐M. (in press) Die erste Welle der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1): Stichprobendesign, Response, Gewichtung und Repräsentativität. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz, 56(5/6), 620–630. [DOI] [PubMed] [Google Scholar]
- Kaymaz N., Drukker M., Lieb R., Wittchen H.‐U., Werbeloff N., Weiser M., Lataster T., van Os J. (2012) Do subthreshold psychotic experiences predict clinical outcomes in unselected non‐help‐seeking population‐based samples? A systematic review and meta‐analysis, enriched with new results. Psychological Medicine, 42(11), 2239–2253. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Üstün T.B. (2004) The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13(2), 93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Üstün T.B. (2008) The World Health Organization Composite International Diagnostic Interview In Kessler R.C., Üstün T.B. (eds) The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders, pp. 58–90, Cambridge, Cambridge University Press. [Google Scholar]
- Kessler R.C., Berglund P., Chiu W.T., Demler O., Heeringa S., Hiripi E., Jin R., Pennell B.E., Walters E.E., Zaslavsky A., Zheng H. (2004) The US National Comorbidity Survey Replication (NCS‐R): design and field procedures. International Journal of Methods in Psychiatric Research, 13(2), 69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Walters E.E. (2005) Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R., Petukhova M., Sampson N., Zaslavsky A., Wittchen H.‐U. (2012) Prevalence and morbid risk of anxiety and mood disorders in the US. International Journal of Methods in Psychiatric Research, 21(3), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M., Martin M., Jäger T. (2007) Development and validation of the Cognitive Telephone Screening Instrument (COGTEL) for the assessment of cognitive function across adulthood. The Journal of Psychology, 141(2), 147–170. [DOI] [PubMed] [Google Scholar]
- Kraus L., Pabst A., Piontek D., Müller S. (2010) Trends des Substanzkonsums und substanzbezogener Störungen. Ergebnisse des Epidemiologischen Suchtsurveys 1995–2009. Sucht, 56(5), 337–348. [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J. (2001) Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth B.M., Kamtsiuris P., Hölling H., Schlaud M., Dölle R., Ellert U., Kahl H., Knopf H., Lange M., Mensink G.B., Neuhauser H., Rosario A.S., Scheidt‐Nave C., Schenk L., Schlack R., Stolzenberg H., Thamm M., Thierfelder W., Wolf U. (2008) The challenge of comprehensively mapping children's health in a nation‐wide health survey: design of the German KiGGS‐Study. BMC Public Health, 8, 196, DOI: 10.1186/1471-2458-8-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner G., Wittchen H.‐U., Perkonigg A., Holly A., Schuster P., Wunderlich U., Türk D., Garczynski E., Pfister H. (1998) Structure, content and reliability of the Munich‐Composite International Diagnostic Interview (M‐ CIDI). Substance use sections. European Addiction Research, 4(1‐2), 28–41. [DOI] [PubMed] [Google Scholar]
- Lampert T., Kroll L., Müters S., Stolzenberg H. (in press) Messung des sozioökonomischen Status Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz, 56(5/6), 631–636. [DOI] [PubMed] [Google Scholar]
- Laroi F., Marczewski P., Van der Linden M. (2004) Further evidence of the multi‐dimensionality of hallucinatory predisposition: factor structure of a modified version of the Launay–Slade Hallucinations Scale in a normal sample. European Psychiatry, 19(1), 15–20. [DOI] [PubMed] [Google Scholar]
- Launay G., Slade P. (1981) The measurement of hallucinatory predisposition in male and female prisoners. Personality and Individual Differences, 2(3), 221–234. [Google Scholar]
- Lehrl S. (2005) Mehrfachwahl‐Wortschatz‐Intelligenztest (MWT‐B), Göttingen, Hogrefe. [Google Scholar]
- Luck T., Riedel‐Heller S.G., Wiese B., et al (2009) CERAD‐NP battery: age‐, gender‐ and education‐specific reference values for selected subtests – results of the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe). Zeitschrift für Gerontologie und Geriatrie, 42(5), 372–384, DOI: 10.1007/s00391-009-0031-y [DOI] [PubMed] [Google Scholar]
- Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G., Mellits E.D., Clark C. (1989) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's Disease. Neurology, 39(9), 1159–1165. [DOI] [PubMed] [Google Scholar]
- Nelson B., Fusar‐Poli P., Yung A.R. (2012) Can we detect psychotic‐like experiences in the general population? Current Pharmaceutical Design, 18(4), 376–385. [DOI] [PubMed] [Google Scholar]
- Nuevo R., Chatterji S., Verdes E., Naidoo N., Arango C., Ayuso‐Mateos J.L. (2012) The continuum of psychotic symptoms in the general population: a cross‐national study. Schizophrenia Bulletin, 38(3), 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner I.K., et al (2009) The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis‐related fatigue. Multiple Sclerosis, 15(12), 1509–1517. [DOI] [PubMed] [Google Scholar]
- Peters E. (2004) Measuring delusional ideation: the 21‐item Peters et al. Delusions inventory. Schizophrenia Bulletin, 30(4), 1005–1022. [DOI] [PubMed] [Google Scholar]
- Rammstedt B. (2007) The 10‐item big five inventory. Norm values and investigation of sociodemographic effects based on a German population representative sample. European Journal of Psychological Assessment, 23(3), 193–201. [Google Scholar]
- Ratcliffe G.E., Enns M.W., Jacobi F., Belik S.‐L., Sareen J. (2009) The relationship between migraine and mental disorders in a population‐based sample. General Hospital Psychiatry, 31(1), 14–19. [DOI] [PubMed] [Google Scholar]
- Reed V., Gander F., Pfister H., Steiger A., Sonntag H., Trenkwalder C., Hundt W., Wittchen H.‐U. (1998) To what degree the Composite International Diagnostic Interview (CIDI) correctly identifies DSM‐IV disorders? Testing validity issues in a clinical sample. International Journal of Methods in Psychiatric Research, 7(3), 142–155. [Google Scholar]
- Reitan R.M., Wolfson D. (1993) The Halstead–Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation (2nd edition), Tucson, AZ, Neuropsychology Press. [Google Scholar]
- Sareen J., Jacobi F., Cox B., Belik S.‐L., Clara I., Stein M.B. (2006) Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Archives of Internal Medicine, 166(19), 2109–2116. [DOI] [PubMed] [Google Scholar]
- Scheidt‐Nave C., Kamtiuris P., Goesswald H. et al (2012) German health interview and examination survey for adults (DEGS) – design, objectives and implementation of the first data collection wave. BMC Public Health, 12, 730, DOI: 10.1186/1471-2458-12-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier M.F., Carver C.S., Bridges M.W. (1994) Distinguishing optimism form neuroticism (and trait anxiety, self‐mastery, and self‐esteem): a re‐evaluation of the Life Orientation Test. Journal of Personality and Social Psychology, 67(6), 1063–1078. [DOI] [PubMed] [Google Scholar]
- Siegrist J., Jacobi F. (2009) Adoption of the Effort–Reward Imbalance Questionnaire for Non‐working Adults. DEGS‐CIDI (unpublished), TU Dresden.
- Siegrist J., Starke D., Chandola T., Godin I., Marmot M., Niedhammer I., Peter R. (2004) The measurement of effort‐reward imbalance at work: European comparisons. Social Science & Medicine, 58(8), 1483–1500. [DOI] [PubMed] [Google Scholar]
- Siller A.B., Tompkins L. (2006) The Big Four: Analyzing Complex Sample Survey Data Using SAS, SPSS, STATA, and SUDAAN Thirty‐First SAS Users Group International conference (SUGI); March 27, 2006; San Francisco, CA: http://www2.sas.com/proceedings/sugi31/172-31.pdf [Google Scholar]
- Tolonen H., Helakorpi S., Talala K., Helasoja V., Martelin T., Prättälä R. (2006) 25‐year trends and socio‐demographic differences in response rates: Finnish adult health behaviour survey. European Journal of Epidemiology, 21(6), 409–415. [DOI] [PubMed] [Google Scholar]
- Von Aster M., Neubauer A., Horn R. (2006) Wechsler‐Intelligenztest für Erwachsene. WIE – Manual, Frankfurt/M, Harcourt Test Services. [Google Scholar]
- Wigmann J.T.W., Nierop V., Vollebergh W.A.M., Lieb R., Beesdo‐Baum K., Wittchen H.‐U., Van Os J. (2012) Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity‐implications for diagnosis and ultra‐high risk research. Schizophrenia Bulletin, 38(2), 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.‐U. (1994) Reliability and validity studies of the WHO Composite International Diagnostic Interview (CIDI): a critical review. Journal of Psychiatric Research, 28(1), 57–84. [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U. (2002) Generalized anxiety disorder: prevalence, burden and cost to society. Depression and Anxiety, 16(4), 162–171. [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U. (2004) Continued needs for epidemiological studies of mental disorders in the community. Psychotherapy and Psychosomatics, 73(4), 197–206. [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U. (2012) The burden of mood disorders. Science, 338, 15, DOI: 10.1126/science.1230817 [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U., Jacobi F. (2004) Angststörungen. Gesundheitsberichterstattung des Bundes, Berlin, Robert‐Koch‐Institut. [Google Scholar]
- Wittchen H.‐U., Jacobi F. (2001) Die Versorgungssituation psychischer Störungen in Deutschland. Eine klinisch‐epidemiologische Abschätzung anhand des Bundes‐Gesundheitssurveys 1998. Bundesgesundheitsblatt‐Gesundheitsforschung‐Gesundheitsschutz, 44(10), 993–1000. [Google Scholar]
- Wittchen H.‐U., Jacobi F. (2005) Size and burden of mental disorders in Europe – a critical review and appraisal of 27 studies. European Neuropsychopharmacology, 15(4), 357–376. [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U., Pfister H. (1997) DIA‐X‐Interviews: Manual für Screening‐Verfahren und Interview; Interviewheft Längsschnittuntersuchung (DIA‐X‐Lifetime); Ergänzungsheft (DIA‐X‐Lifetime); Interviewheft Querschnittuntersuchung (DIA‐X‐12 Monate); Ergänzungsheft (DIA‐X‐12Monate); PC‐Programm zur Durchführung des Interviews (Längs‐ und Querschnittuntersuchung); Auswertungsprogramm, Frankfurt, Swets & Zeitlinger. [Google Scholar]
- Wittchen H.‐U., Robins L.N., Cottler L., Sartorius N., Burke J., Regier D. (1991) Cross‐cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI) – Results of the multicenter WHO/ADAMHA Field Trials (wave I). The British Journal of Psychiatry, 159(5), 645–653. [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U., Höfler M., Gander F., Pfister H., Storz S., Üstün T.B., Müller N., Kessler R.C. (1999) Screening for mental disorders: performance of the Composite International Diagnostic‐Screener (CID‐S). International Journal of Methods in Psychiatric Research, 8(2), 59–70. [Google Scholar]
- Wittchen H.‐U., Müller N., Pfister H., Winter S., Schmidtkunz B. (1999) Affektive, somatoforme und Angststörungen in Deutschland‐Erste Ergebnisse des bundesweiten Zusatzsurveys. Psychische Störungen, Das Gesundheitswesen, 61(Sonderheft 2), 216–222. [PubMed] [Google Scholar]
- Wittchen H.‐U., Müller N., Schmidtkunz B., Winter S., Pfister H. (2000) Erscheinungsformen, Häufigkeit und Versorgung von Depressionen. Ergebnisse des bundesweiten Gesundheitssurveys. Psychische Störungen, MMW Fortschritte der Medizin, 118(Sonderheft 1), 4–10. [Google Scholar]
- Wittchen H.‐U., Jacobi F., Rehm J., Gustavsson A., Svensson M., Jönsson B., Olesen J., Allgulander C., Alonso J., Faravelli C., Fratiglioni L., Jennum P, Lieb R, Maercker A., van Os J., Preisig M., Salvador‐Carulla L., Simon R., Steinhausen H.‐C. (2011) The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21(9), 655–679. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (1993) Tenth Revision of the International Classification of Diseases, Chapter V (F): Mental and Behavioral Disorders. Clinical Descriptions and Diagnostic Guidelines, Geneva, WHO. [Google Scholar]


