Abstract
The objective of this study is to present the rationale, methods, design and preliminary results from the High Risk Cohort Study for the Development of Childhood Psychiatric Disorders. We describe the sample selection and the components of each phases of the study, its instruments, tasks and procedures. Preliminary results are limited to the baseline phase and encompass: (i) the efficacy of the oversampling procedure used to increase the frequency of both child and family psychopathology; (ii) interrater reliability and (iii) the role of differential participation rate. A total of 9937 children from 57 schools participated in the screening procedures. From those 2512 (random =958; high risk =1554) were further evaluated with diagnostic instruments. The prevalence of any child mental disorder in the random strata and high‐risk strata was 19.9% and 29.7%. The oversampling procedure was successful in selecting a sample with higher family rates of any mental disorders according to diagnostic instruments. Interrater reliability (kappa) for the main diagnostic instrument range from 0.72 (hyperkinetic disorders) to 0.84 (emotional disorders). The screening instrument was successful in selecting a sub‐sample with “high risk” for developing mental disorders. This study may help advance the field of child psychiatry and ultimately provide useful clinical information. Copyright © 2014 John Wiley & Sons, Ltd.
Keywords: longitudinal, development, genetics, psychiatry, cohort
Introduction
In this study we describe the methods and present some preliminary results from the High Risk Cohort Study for the Development of Childhood Psychiatric Disorders. This cohort was designed for the purpose of understanding developmental trajectories of psychopathology and mental disorders. Using a two‐stage design we first assessed child symptoms and family history of psychiatric disorders in a screening interview. In the second stage a random sub‐sample (aimed to be representative from the community) and a high‐risk sub‐sample (a sample with children with increased risk for mental disorders) were selected for further evaluation. The evaluation of the sub‐samples includes an extensive assessment of environmental and genetic risk factors, brain structure and function and peripheral biomarkers combined with a clinical evaluation of psychiatric disorders and symptoms.
This higher risk sampling was based on the presence of early psychiatric symptoms and high family loading of psychopathology assessed by the screening interview. Using this strategy we hoped to find a higher incidence of mental disorders in this sub‐sample, and therefore, to enhance the power to identify developmental trajectories and causal pathways for five main disorders: (1) attention deficit hyperactivity disorder (ADHD); (2) anxiety disorders; (3) obsessive compulsive disorder (OCD); (4) psychosis; (5) learning disorders.
The aim of this report is to provide a detailed description of the study methodology, and to present some preliminary results from the baseline (wave‐1) of this ongoing cohort project. Preliminary results are limited to the baseline phase and encompass: (i) the efficacy of the oversampling procedure used to increase the frequency of both child and family psychopathology; (ii) interrater reliability; (iii) the role of differential participation rate.
Methods
Baseline assessment (wave‐1) comprised six major steps: (1) screening of the families at schools at the registry day (n =9937); (2) high risk and random selection sampling (n =2512); (3) a household parent interview conducted by a lay interviewer (n =2512); (4) child evaluation at schools conducted by a psychologist and a hearing and speech pathologist (n =2401); (5) collection of saliva for genetic studies (simultaneously during household interviews for parents and school interviews for children); (6) acquisition of neuroimaging and collection of blood samples for analyses of biomarkers.
Screening of the families at the registry day
A total of 57 schools (22 in Porto Alegre and 35 in São Paulo) were included in the study (Figure 1). Porto Alegre and São Paulo are capitals of two Brazilian states. São Paulo is the capital of one of the southeast states of Brazil and the most populated city of the country, with 11,316,149 inhabitants. Porto Alegre is the capital of the southernmost state of Brazil and has 1,409,939 inhabitants. For logistic reasons (specially for the neuroimaging study), only public schools close to the research centers with more than 1000 students in the age of interest were selected to participate.
Figure 1.
Subjects and schools geographic distribution in Porto Alegre and São Paulo.
The enrollment for the screening phase was conducted at public schools during the early registry days. Attendance to schools is compulsory in Brazil for all children 7–14 years old and by law at least one carer is needed to register the child. Eligible subjects were those: (1) being registered by a biological parent that was a primary carer and could provide sufficient information about the children's behavior; and (2) 6–12 years old at enrollment. All parents present at the selected schools on registry days were invited to participate and those who agreed were interviewed in loco or later, by telephone, with a modified version of the Family History Screen (FHS) administered by a lay interviewer (Weissman et al., 2000).
Family History Screen (FHS)
The FHS is an interview used to screen all members of a family for DSM‐IV mental disorder symptoms based on the information provided by one family member. In this study, this screening instrument was adapted to allow the collection of information about the index child, his/her biological parents, biological siblings and half‐siblings of the index child, instead of asking about family members of the informant.
The FHS is completely structured, with a mean time of administration of approximately 40–60 minutes, assessed by trained interviewers using an electronic data collection system. In Porto Alegre (site 1) 19% of the interviews were performed by telephone and in São Paulo (site 2) all interviews were performed by telephone. At the beginning of the interview, the interviewer asked the informant to make a complete list of all biological first‐degree family members of each eligible child. Subsequently, the informant was asked about the presence of DSM‐IV screening symptoms for each diagnosis (e.g, “Did anyone on the list feel sad, blue, or depressed for most of the time for two days or more? If yes, who was that?”). The screening question serves as gate to ask about impairment, duration/frequency, and/or exclusion questions, asked only for those individuals who screened‐positively (conditional questions). The instrument was adapted for the purposes of this study. Briefly, the version we have used has 48 items, 29 main questions accompanied by 19 conditional questions. It has questions about the main psychiatric syndromes: depression, mania, specific phobia, social phobia, generalized anxiety disorder, panic disorder, agoraphobia, OCD, psychotic experiences, alcohol use and problems due to alcohol use, drug use and problems due to drug use, ADHD, separation anxiety, oppositional defiant disorder, conduct disorder and inhibited behavior towards unfamiliar people.
In total, we were able to interview 9937 children (from 8012 families) and collect information about 45,394 family members. In this phase we obtained information primarily from the biological mother (in 87.6% of the cases) or biological father. The total number of parents approached in the registry day in both states with children in the age range of the project was approximately 12,500.
Training procedures for the screening phase
The research team trained lay interviewers to perform the FHS assessment. The training procedure consisted of two sessions lasting two hours each with lectures about main psychiatric syndromes and symptoms, familiarization with the instrument and ethical/confidentiality issues. We further conducted five simulations using videotaped recorded interviews of real patients and control subjects. Researchers answered interviewers' doubts about rating and instrument procedures (including the digital platform used).
High risk and random selection sampling
Based on the information collected with the FHS, we computed an index of family load for each of the 9937 potential eligible children. This index expresses the percentage of members in the family that screened positively for each of the disorders assessed, adjusted for relatedness.
In which: BM, the biological mother is positive for the presence of symptoms; BF, the biological father is positive for the presence of symptoms; BS, the biological sibling is positive for the presence of symptoms; HS, the half‐sibling are positive for the presence of symptoms. All variables were coded as zero for absence and one for presence. The relatedness adjustment (0.5) takes into account that half siblings contribute to half of genetic information if compared to biological siblings. This index was constructed based on previous work using this instrument (Milne et al., 2008).
Among the 9937 eligible children, 1500 were randomly selected to compose the random study sample (without replacement). Among the remaining children, those who had screened positively for any of the five disorders of interest for this study were ranked according to percentage of members in their families presenting symptoms of the same disorder. We further invited subjects to be enrolled in the second phase of the selection process with replacement until a fixed number of 2512 individuals was achieved (our budget limit), prioritizing those with higher FLI.
Only one child per family was enrolled. In families with more than one child eligible, one of them was chosen based on a simple randomization procedure. In summary: high‐risk selection aimed at those screening positively for any of the five main psychiatric diagnoses of interest (ADHD, anxiety disorders, OCD, psychotic experiences, and learning disorders). Among those, children with a higher number of family members affected were prioritized. A schematic representation of the selection procedure is depicted in the Supplementary Material (Figures S1 and S2).
A total of 2512 subjects selected through the earlier mentioned procedures were comprehensively studied during the parent interview (household interview) and child interview and testing (school evaluation). The flowchart describes the selection procedures (Figure 2).
Figure 2.
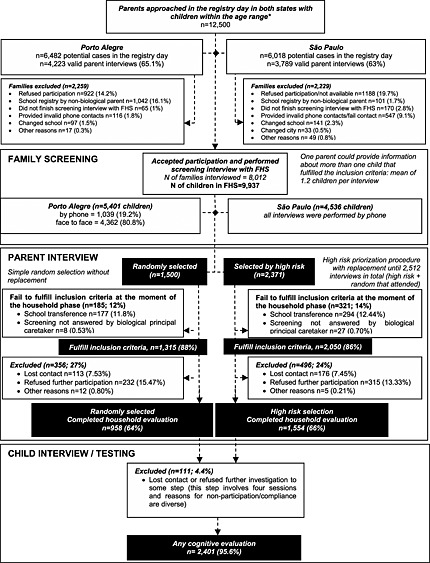
Flowchart of participants in the study.
Household parent interview
The parent interview consisted of: (1) a detailed evaluation of general risk factors for mental disorders; (2) an assessment of childhood psychiatric diagnosis; (3) an assessment of parental diagnosis; (4) questions about child's treatment history and service use.
General risk factors
Questions about risk factors were drawn based on a careful literature review about known major risk factors for mental disorders. The risk factors can be divided in to demographical, prenatal and perinatal, and early life stressors and are presented in Table 1.
Demographics: (i) age; (ii) sex; (iii) socio‐economic status (ABEP, 2010); (iv) socio‐economic progression since birth; (v) parental marital status; (vi) parental age at birth;
Prenatal and perinatal: (i) gestational age at birth; (ii) stress during pregnancy; (iii) prematurity, (iv) low birth weight; (v) low birth length; (vi) exposure to tobacco intra‐utero and during childhood (quantified); (vii) exposure to alcohol intra‐utero and during childhood (quantified); (viii) gestational infections and clinical conditions (diabetes, hypertension); (ix) delivery conditions; (x) perinatal complications; (xi) psychiatric medications use during pregnancy; (xii) breastfeeding and exclusive breastfeeding time; (xiii) prenatal care;
Early life stressors: (i) time and quality of contact with caregivers since birth; (ii) bullying perpetration and victimization; (iii) head trauma; (iv) brain injury; (v) parental warmth and protection [selected questions from Parental Bonding Instrument (Parker, 1990)]; (vi) childhood maltreatment [selected questions from Childhood Trauma Questionnaire (Bernstein et al., 1997)]; (vii) family cohesion, conflict and control [dimensions from the Family Environment Scale (Moos, 1990)].
Table 1.
Neuropsychological tasks used in the study
| Domain | Tasks | Main function |
|---|---|---|
| Computerized tasks | ||
| Basic information processing | Two‐choice reaction time (Hogan et al., 2005) | Baseline speed, accuracy, intra‐subject reaction time variability. Processing efficiency, cautiousness, motor response/encoding (diffusion model) |
| Inhibitory‐based executive function | Conflict control task (Hogan et al., 2005) | Inhibition of a pre‐potent response and initiation of an alternative more appropriate response |
| Go/No‐Go (Bitsakou et al., 2008). | Inhibition of a pre‐potent response | |
| Attention | Attention network task (Fan et al., 2002) | Aspects of alerting, orienting and executive attention |
| Attention orienting towards threat faces and happy faces | Dot‐probe task (500 ms exposure time; 80 trials) (Mogg et al., 1997) | Attention bias towards threat faces and attention bias towards happy faces at initial stages of attention orienting |
| Dot‐probe task (500 ms and 1250 ms exposure time; 160 trials) (Mogg et al., 1997) | Attention bias towards threat faces and attention bias towards happy faces at initial and late stages of attention orienting | |
| Temporal processing | Time anticipation 400 ms and 2000 ms (Toplak and Tannock, 2005) | Store a time interval in the memory and reproduce it several times without the cue stimuli |
| Duration discrimination task (Toplak et al., 2003) | Discriminate the relative length of time intervals through visual stimuli | |
| Delay aversion | Delay reaction task (Sonuga‐Barke and Taylor, 1992) | Estimate the relative performance in speed, variability and accuracy in tasks with long time delays if compared to baseline levels |
| Choice delay task (Sonuga‐Barke and Taylor, 1992) | Preference of immediate less advantageous rewards over delayed more advantageous rewards. | |
| Non‐computerized tasks | ||
| General intelligence (estimated) | Wechsler Intelligence Scale for Children (Wechsler, 2002) – subtests vocabulary and block design | General intelligence |
| Working memory | Digit span forwards and backwards (Wechsler, 2002) | Short‐term memory and manipulation capacity of verbal information |
| Corsi blocks forwards and backwards (Vandierendonck et al., 2004) | Short‐term memory and manipulation capacity of visual‐spatial information | |
| Immediate and late non‐verbal memory | Rey Osterrieth complex figure test (Rey and Osterrieth, 1993) | Visuo‐motor abilities, planning, non‐verbal immediate and late visual memory |
| Visuo‐motor abilities | Bender Gestalt task Bender, 1938) | Visuo‐motor maturity |
| Motor functions | Luria motor tasks (Luria, 1973) | Motor coordination |
| Non‐verbal design fluency | Five‐point task | Non‐verbal design fluency |
Child diagnosis and dimensions of psychopathology
Child psychiatric diagnosis was established using the Development and Well‐being Assessment (DAWBA, Goodman et al., 2000a). The DAWBA is a structured interview administered by lay interviewers, which also contains the Strength and Difficulties Questionnaire (SDQ, Goodman et al., 2000b) (a 25‐item questionnaire providing four risk groups of behavioral and emotional difficulties) and recorded verbatim responses of any reported problems (for further details see http://www.dawba.info). Verbatim responses as well as structured answers are then carefully evaluated by psychiatrists that confirm, refute or alter the initial computerized diagnosis. All questions are closely related to DSM‐IV diagnostic criteria and focus on current problems causing significant distress or social impairment. The DAWBA has been translated to several languages, and for the present study the Brazilian Portuguese version (Fleitlich‐Bilyk and Goodman, 2004) was administered to the biological parents of all children included in the project. Administrations were performed in accordance with previously reported procedures.
A total of nine certified child psychiatrists performed the rating procedures. All of them were trained and supervised closely by a senior child psychiatrist with extensive experience in rating the DAWBA (BFB). In addition, all cases in which raters had doubts about any specific diagnosis were scaled up and discussed between two child psychiatrists until consensus about the diagnosis was achieved.
In order to perform a reliability analysis of the rating procedure, a sub‐sample of 200 subjects received a second rating by a trained child psychiatrist. We selected subjects divided equally into DAWBA bands (Goodman et al., 2011). DAWBA bands represent computer‐generated categories based on answers to the DAWBA questions that inform to the rater the probability of a positive diagnosis (<0.1%, ~3%, ~15%, ~50% and higher than 70%). The second rater was informed that the 200 cases (40 cases from each band) did not represent the population distribution of mental disorders.
In addition to the DAWBA, we also used the Child Behavior Checklist (CBCL) in order to have an assessment of dimensions of psychopathology that have shown to be valid in several cultures (Ivanova et al., 2007).
Parental diagnosis
History of psychiatric disorder in the respondent was assessed using the Mini International Psychiatric Interview (MINI) and the MINI Plus (Amorim et al., 1998; Sheehan et al., 1998). The following modules were used in this research: (1) major depressive episode; (2) manic episode; (3) panic disorder; (4) agoraphobia; (5) social anxiety disorder; (6) alcohol abuse and dependence; (7) drug abuse and dependence; (8) psychotic disorders; (9) generalized anxiety disorder (GAD); (10) ADHD. To assess obsessive‐compulsive symptoms dimensionally, we used the Dimensional Yale–Brown Obsessive‐Compulsive Scale (Rosario‐Campos et al., 2006). At the end of each MINI module we added a four‐point response option question (“None”, “A Little”, “Moderate” and “Severe”) about impairment, an item about age of onset of the symptoms, and an item about treatment‐seeking.
Child medication, psychotherapy and service use
During household interview, parents were asked on whether the child had ever received any type of treatment for behavioral, attention, learning, or emotional problems, such as fear, anxiety and depression. A positive response would lead to other questions aiming to evaluate the detailed history of medication and psychotherapy treatment. A list of the most common psychiatric medications and their commercial names was presented in order to facilitate communication. We asked additional questions with respect to hospitalization due to psychiatric problems and specific interventions for learning disorders.
Training procedures for the parental interviews
In this phase, training procedures lasted two full days. Lay interviewers attended to instructions provided by clinical psychiatrists from the research team covering the following topics: (1) project structure and design; (2) main aspects of psychiatric syndromes, psychopathology, and risk factors; (3) how to deal with difficult situations/interviews; (4) confidentiality and ethical issues of collecting psychiatric information. Researchers reviewed the full protocol together with the interviewers several times, and simulated difficult situations and potential doubts in role‐playing activities. We also asked them to rate videotaped interviews. Given the importance of this procedure, each interviewer was rated by the research team and those not achieving acceptable retained trained information were excluded from participation in the project. Since this was a household phase, interviews were scheduled and confirmed previously by telephone.
Child interview and testing (school interview)
The child interview and testing comprised: (1) a detailed child evaluation regarding psychosis and psychotic experiences, anxiety and temperament; (2) specific neurocognitive tests; (3) an evaluation of literacy and phonological awareness. For logistical purposes, only participants who remained in the same school of original registry were evaluated, that resulted in a sub‐sample of subjects from the screening phase not fulfilling the project entry criteria.
Child evaluation
Trained psychologists read to children the 20 items about positive symptoms and one item about negative symptoms of the Community Assessment of Psychotic Experiences (CAPE, Konings et al., 2006). Two items about difficulty in differentiating reality and fantasy were added. Additionally, psychologists rated an overall clinical judgment using four anchored ratings from the Comprehensive Assessment of At‐risk Mental States (CAARMS, Yung et al., 2003). The rating consisted of four questions, with a score from zero to six, related to the following aspects: unusual mental contents, unusual perceptual experiences, speech organization, and blunted affect. Finally, psychologists adjudicated the presence of psychotic symptoms according to the K‐SADS‐PL (Kiddie–Sads – Present and Lifetime Version) evaluation of delusions and hallucinations (Kaufman et al., 1997).
Since parents tend to underestimate emotional symptoms (Kuhn et al., 2011), we used the child version of the Portuguese Brazilian version of the Screen for Children Anxiety Related Emotional Disorders (SCARED, Isolan et al., 2011) to directly assess anxiety symptoms.
A scale was also used to evaluate a child's temperament, the Early Adolescent Temperament Questionnaire – revised version (EATQ‐R, Ellis and Rothbart, 2001). This scale provides measures of Effortful Control, Negative Affect and Surgency.
Neuropsychological evaluation
The neurocognitive tests used in this study are depicted in Table 1. The tests were chosen based on a priori hypothesis related to specific cognitive domains for each of the main five disorders investigated.
Intelligence quotient was estimated using the vocabulary and block design subtests of the Weschler Intelligence Scale for Children, third edition (WISC‐III, Wechsler, 2002), using the Tellegen and Briggs (1967) method and Brazilian norms (Figueiredo, 2001).
Procedures and computerized tests battery
All computerized tests were administered by mental health professionals previously trained. Tests were performed in 26 notebooks Acer 14 inches Intel Pentium Dual Core T4300 running in Windows 7 Premium software. All computerized neuropsychological tests were programmed using e‐prime 2.0. Professionals were instructed to adjust computer to 70% volume, 100% brightness and to perform the tests with the computer linked to a power font. Children were instructed to be 50 cm from the computer screen, which should be kept at a 90° angle to the base of the notebook supported by a fixed structure. Before each computerized task, professionals received a structured instruction to certify that the children were positioned at the center of the computer screen and with both index fingers positioned at the notebook mouse or spacebar. Professionals were instructed to follow narrowly the standard procedures.
To assign tasks between sessions 1 and 2, we used a randomized block approach that takes into consideration the sum of the estimated time sessions in each block of tasks. For half of the children, Block 1 was administered in the first session, and for the other half, Block 2 was administered in the first session.
Block 1 comprised (1) go/no‐go task*, (2) conflict control task, (3) long dot‐probe task (500/1200 ms version; 160 trials), (4) two‐choice task, (5) delay reaction task* and (6) duration discrimination task*.
Block 2 involved (1) attention network task*, (2) choice‐delay task, (3) short dot‐probe task (500 ms; 80 trials) and (4) 400 and (5) 2000 ms time anticipation task. Note: * Indicates tasks with practice trials.
The order of administration of each task within the two blocks was also randomized in five randomly generated sequences that were attributed to each one of the children randomly. We also recoded quality of control of procedural task‐related variables (followed random sequence, technical/logistic problems, professional's perception about the understanding of the task, collaboration, motivation, level of noise, impairment of test quality by environmental factors).
Literacy evaluation (reading, writing, mathematics)
The School Performance Test (“Teste de Desempenho Escolar”, TDE) (Stein, 1998) assessed literacy. This instrument is composed of three subtests: writing (isolated words in dictation); mathematics (oral problem solving and written calculations of mathematical operations); and reading (recognition of words isolated from context). The instrument was elaborated and validated to Brazilian population (Stein, 1998).
Central auditory processing and phonological awareness
The Simplified Assessment of Auditory Processing (Pereira and Schochat, 1997) screened for problems in auditory function. This is an auditory screening test composed of cochleopalpebral reflex (CPR), sound localization test and a test of memory for non‐verbal sounds in sequence. Performance difficulties in sound localization test or in the test of sequenced sounds indicative of harmed auditory processing.
The CONFIAS (“Consciência fonológica instrumento de avaliação sequencial”, Phonological Awareness Instrument) (Moojen et al., 2003) test was used to evaluate phonologic processing. It consists of a tool that assesses phonologic awareness in sequence and is standardized and validated for use in Brazil. It is composed of two levels (syllable and phoneme), and each level has specific metalinguistic tasks: segmentation, identification, rhyme, and syllable or phoneme exclusion or transposition. The Phonology Test, part of the ABFW Test (“Teste de linguagem infantil nas áreas de fonologia, vocabulário, fluência e pragmática”) (Wertzner, 2000), was also used in order to detect the presence of one or more phonologic processes, characterizing phoneme replacements, omissions and/or distortion in the child's speech.
Training procedures for child interview and testing
Psychologists and hearing and speech pathologist conducted child evaluations in this phase. Training consisted of two sessions with a specialized hearing and speech pathologist experienced in the study protocol. Full explanation about the project, instruments, procedures and standardization for the clinical evaluations was provided.
Molecular genetic evaluation
Saliva was collected from the child and both parents using an Oragene© salivary kit. If saliva from one of the biological parents was not available, the brother with the closest age to the child was chosen to provide a saliva sample. More detail about genetic analyses will be provided in specific studies from this cohort.
Neuroimaging and peripheral biomarkers
A sub‐sample of approximately 750 subjects was selected to participate in a neuroimaging and blood biomarkers study. A detailed description of the neuroimaging/biomarkers sample and design will be provided elsewhere. Brain magnetic resonance imaging (MRI) was acquired in two centers using a 1.5T General Electric Scanner. The sequences acquired were: (a) high‐resolution tridimensional T1‐weighted; (b) diffusion tensor imaging (DTI); (c) intrinsic connectivity fMRI; (d) MT ON/OFF. Total scan time was 28 minutes.
Children were invited to arrive one‐hour earlier to the exam site and were engaged in a recreational and therapeutic technique in order to reduce exam related distress. Dancing technique and theater games, relaxation, music, games, and various recreational and interactive games were used as a desensitization method. A simulation of the brain scan, using a cloth tunnel on a stretcher with simultaneous presentation of the background noise of each MRI sequence to be performed in the research protocol was also used. Since this is a follow‐up study, these procedures were taken to improve the quality of the assessment and to enhance engagement in future assessments. These procedures were highly successful, since 739 (98%) of children that come to the MRI center finished the MRI scan and 606 (81%) provided blood samples. No anesthetic or sedative medications were used.
After the MRI acquisition, they were invited to provide blood samples. A blood sample was collected for plasmatic biomarkers, mRNA and DNA. Blood samples were stored at 4°C at collecting sites and processed the same day within a four‐hour interval for plasma aliquots, which were stored in a freezer at −80°C. EDTA tubes for DNA extraction were stored at 4°C and PAX gene tubes, for RNA extraction, at −20°C after incubating them for at least two hours at room temperature, following manufacturer's instructions.
Ethical considerations
This study was approved by the ethics committee of the University of São Paulo [IORG0004884/National Council of Health Registry number (CONEP): 15.457/Project IRB registration number: 1132/08]. Written consent was obtained from parents of the participants as well as from those participants that were able to read, write and understand the written consent. From others, verbal agreement was obtained. All families were invited for an appointment with a trained psychologist and social worker in case they were interested in receiving the results of the study evaluation and all children identified as being under the need of care were referred for clinical evaluation. Situations involving serious risk of physical or psychological harm received special attention in accordance to competent authorities’ guidelines. No compensation or financial incentive was made available for those taking part in the household and school interviews. Compensation for transport expenses was provided for those attending the neuroimaging study.
Statistical analysis
Data is presented as absolute and relative frequencies, and variables with a non‐Gaussian distribution are presented with percentiles (50th and 75th; 95th in some cases due to extreme skewness). We compared proportions between samples using Chi‐square test (with Yates correction for df =1). For continuous variables with non‐Gaussian distribution differences between mean ranks are compared using the independent samples Mann–Whitney U test. Interrater reliability was calculated using Kappa. All tests were two‐tailed and we adopted a significance level of 5%.
Results
We report data about: (1) the efficacy of the oversampling procedure; (2) diagnostic reliability; (3) study participation and differences between those individuals who attended household and scholar interviews.
The total sample comprises children with an average age of 9.7 years at the diagnostic phase [standard deviation (SD) =1.92], 53.1% male, included mostly subjects in medium strata of socio‐economic status (64.6% medium; 6% low/very low and 29.4% comfortable/high), with a mean number of 1.44 siblings (SD =1.29). The rate of any mental disorders in the random strata was 19.9% if compared to 29.7% in the high‐risk strata. Rates for parental and child psychopathology as well as demographic data for each study selection strata are depicted in Tables 2, 3, 4, 5, 6.
Table 2.
Diagnoses compared by selection groups (random versus high risk) in those that had information on the Development and Well‐being Assessment (DAWBA)
| Total (n =2512) | Randomly selected (n =958) | Select by high risk (n =1554) | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | OR (CI 95%) | χ 2 Yates (df =1) | p‐Value | |
| Any disorder | 652 | 26.0 | 191 | 19.9 | 461 | 29.7 | 1.694 (1.397–2.053) | 28.68 | <0.001 |
| Any emotional | 335 | 13.3 | 93 | 9.7 | 242 | 15.6 | 1.716 (1.331–2.212) | 17.14 | <0.001 |
| Separation anxiety | 72 | 2.9 | 20 | 2.1 | 52 | 3.3 | 1.624 (0.963–2.737) | 2.96 | 0.087 |
| Specific Phobia | 89 | 3.5 | 28 | 2.9 | 61 | 3.9 | 1.357 (0.861–2.139) | 1.46 | 0.227 |
| Social phobia | 26 | 1.0 | 3 | 0.3 | 23 | 1.5 | 4.782 (1.432–15.971) | 6.78 | 0.009 |
| Panic disorder | 1 | 0.0 | 0 | 0.0 | 1 | 0.1 | — | — | — |
| Agoraphobia | 4 | 0.2 | 0 | 0.0 | 4 | 0.3 | — | — | — |
| PTSD | 23 | 0.9 | 8 | 0.8 | 15 | 1.0 | 1.157 (0.489–2.740) | 0.014 | 0.907 |
| OCD | 7 | 0.3 | 2 | 0.2 | 5 | 0.3 | 1.543 (0.299–7.968) | 0.017 | 0.895 |
| GAD | 47 | 1.9 | 17 | 1.8 | 30 | 1.9 | 1.090 (0.598–1.986) | 0.017 | 0.898 |
| Other anxiety | 44 | 1.8 | 15 | 1.6 | 29 | 1.9 | 1.195 (0.638–2.242) | 0.161 | 0.689 |
| Major depression | 73 | 2.9 | 11 | 1.1 | 62 | 4.0 | 3.578 (1.874–6.828) | 15.967 | <0.001 |
| Other depression | 8 | 0.3 | 1 | 0.1 | 7 | 0.5 | 4.330 (0.532–35.251) | 1.279 | 0.258 |
| Undifferentiated anxiety/depression | 6 | 0.2 | 0 | 0.0 | 6 | 0.4 | — | — | — |
| Mania/bipolar | 5 | 0.2 | 3 | 0.3 | 2 | 0.1 | 0.410 (0.068–2.459) | 0.299 | 0.585 |
| Any hyperkinetic | 274 | 10.9 | 79 | 8.2 | 195 | 12.5 | 1.597 (1.213–2.101) | 10.85 | <0.001 |
| ADHD combined | 105 | 4.2 | 29 | 3.0 | 76 | 4.9 | 1.647 (1.066–2.546) | 4.683 | 0.030 |
| ADHD inattentive | 95 | 3.8 | 24 | 2.5 | 71 | 4.6 | 1.863 (1.165–2.981) | 6.380 | 0.012 |
| ADHD hyp‐imp | 40 | 1.6 | 13 | 1.4 | 27 | 1.7 | 1.285 (0.660–2.503) | 0.332 | 0.565 |
| Other hyperactivity | 34 | 1.4 | 13 | 1.4 | 21 | 1.4 | 0.996 (0.496–1.998) | <0.001 | >0.999 |
| Any CD/ODD | 171 | 6.8 | 53 | 5.5 | 118 | 7.6 | 1.403 (1.004–1.961) | 3.65 | 0.056 |
| ODD | 131 | 5.2 | 39 | 4.1 | 92 | 5.9 | 1.483 (1.011–2.176) | 3.734 | 0.053 |
| CD | 40 | 1.6 | 11 | 1.1 | 29 | 1.9 | 1.637 (0.814–3.293) | 1.518 | 0.218 |
| Other disruptive | 9 | 0.4 | 6 | 0.6 | 3 | 0.2 | 0.307 (0.077–1.230) | 2.021 | 0.155 |
| PDD/Autism | |||||||||
| Autism, NOS | 5 | 0.2 | 1 | 0.1 | 4 | 0.3 | — | 4.123* | 0.248 |
| Asperger | 1 | 0.0 | 0 | 0.0 | 1 | 0.1 | |||
| Autism | 9 | 0.4 | 1 | 0.1 | 8 | 0.5 | |||
| Tic disorder | — | 4.938* | 0.176 | ||||||
| Other tic | 3 | 0.1 | 0 | 0.0 | 3 | 0.2 | |||
| Chronic tic | 7 | 0.3 | 2 | 0.2 | 5 | 0.3 | |||
| Tourette | 9 | 0.4 | 1 | 0.1 | 8 | 0.5 | |||
| Eating disorder | — | 1.588* | 0.452 | ||||||
| Eating, NOS | 9 | 0.4 | 2 | 0.2 | 7 | 0.5 | |||
| Bulimia nervosa | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |||
| Anorexia nervosa | 1 | 0.0 | 0 | 0.0 | 1 | 0.1 | |||
| Psychosis | 1 | 0.0 | 0 | 0.0 | 1 | 0.1 | 0.618 (0.600–0.638) | <0.001 | >0.999 |
Note: ADHD, attention deficit hyperactivity disorder; CD, conduct disorder; ODD, oppositional defiant disorder; PTSD, post‐traumatic stress disorder; OCD, obsessive compulsive disorder; GAD, generalized anxiety disorder; PDD, pervasive developmental disorder; NOS, not otherwise specified.
df =3
Table 3.
Comparison of psychiatric diagnoses according to the Mini International Neuropsychiatric Interview (MINI) from the respondent (94.9% mother) between random and high risk samples that attended diagnostic interview (n =2512)
| Total (n =2512) | Randomly selected (n =958) | Select by high risk (n =1554) | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | N | % | n | % | OR | χ 2 Yates (df =1) | p‐Value | |
| Any | 738 | 29.4 | 240 | 25.1 | 498 | 32.0 | 1.411 (1.177–1.691) | 13.638 | <0.001 |
| Any mood | 491 | 19.5 | 150 | 15.7 | 341 | 21.9 | 1.514 (1.225–1.871) | 14.493 | <0.001 |
| Bipolar | 75 | 3.0 | 22 | 2.3 | 53 | 3.4 | 1.502 (0.908–2.486) | 2.170 | 0.141 |
| Unipolar depression | 360 | 14.3 | 111 | 11.6 | 249 | 16.0 | 1.456 (1.146–1.851) | 9.143 | 0.002 |
| Recurrent Depression | 251 | 10.0 | 76 | 7.9 | 175 | 11.3 | 1.473 (1.111–1.953) | 6.933 | 0.008 |
| Any anxiety | 584 | 23.2 | 191 | 19.9 | 393 | 25.3 | 1.359 (1.118–1.653) | 9.217 | 0.002 |
| Panic | 175 | 7.0 | 49 | 5.1 | 126 | 8.1 | 1.637 (1.165–2.300) | 7.738 | 0.005 |
| Agoraphobia | 288 | 11.5 | 95 | 9.9 | 193 | 12.4 | 1.288 (0.993–1.671) | 3.416 | 0.065 |
| Social anxiety | 129 | 5.1 | 37 | 3.9 | 92 | 5.9 | 1.347 (1.060–1.777) | 4.739 | 0.029 |
| GAD | 420 | 16.7 | 141 | 14.7 | 279 | 18.0 | 1.268 (1.017–1.581) | 4.226 | 0.040 |
| Any substance | 33 | 1.3 | 8 | 0.8 | 25 | 1.6 | 1.942 (0.872–4.322) | 2.172 | 0.141 |
| Alcohol dependence | 15 | 0.6 | 4 | 0.4 | 11 | 0.7 | 1.700 (0.540–5.355) | 0.423 | 0.515 |
| Alcohol abuse | 6 | 0.2 | 1 | 0.1 | 5 | 0.3 | 3.089 (0.360–26.481) | 0.440 | 0.507 |
| Drug dependence | 13 | 0.5 | 3 | 0.3 | 10 | 0.6 | 2.062 (0.566–7.510) | 0.697 | 0.404 |
| Drug abuse | 13 | 0.5 | 3 | 0.3 | 10 | 0.6 | 2.062 (0.566–7.510) | 0.697 | 0.404 |
| Other syndromes | |||||||||
| Psychotic syndrome | 119 | 4.8 | 33 | 3.5 | 86 | 5.6 | 1.638 (1.087–2.468) | 5.224 | 0.022 |
| Child ADHD | 44 | 1.8 | 12 | 1.3 | 32 | 2.1 | 1.676 (0.859–3.270) | 1.885 | 0.170 |
| Adult ADHD | 3 | 0.1 | 1 | 0.1 | 2 | 0.1 | 1.238 (0.112–13.668) | <0.001 | >0.999 |
Note: GAD, generalzed anxiety disorder; ADHD, attention deficit hyperactivity disorder; OR, Odds Ratio.
Table 4.
Inter‐rater agreement for DAWBA clinical ratings (n =200)
| Agreement (%) | Expected agreement (%) | Kappa | Standard error | Z | p‐Value | n Positive (rater 1/2) | |
|---|---|---|---|---|---|---|---|
| Any disorder | 90.95 | 54.62 | 0.8007 | 0.0706 | 11.34 | <0.001 | 65/73 |
| Any hyperkinetic | 92.46 | 72.66 | 0.7243 | 0.0708 | 10.23 | <0.001 | 31/34 |
| Any emotional | 95.48 | 70.65 | 0.8459 | 0.0706 | 11.98 | <0.001 | 33/38 |
| Any conduct | 95.48 | 83.13 | 0.7318 | 0.0709 | 10.33 | <0.001 | 19/18 |
Note: DAWBA, Development and Well‐being Assessment.
Table 5.
Demographics and child psychopathology according to the Family History Screen (FHS) stratified by selection strata, inclusion criteria and participation in the total sample (n =9937) and comparisons between those fulfill versus not fulfill selection criteria and between those evaluated versus non‐evaluated in household phase
| Not selected | Randomly selected | Select by high risk | Total | Randomly selected (n =1500) | Select by high risk (n =2371) | Random with inclusion criteria (n =1315) | High risk with inclusion criteria (n =2050) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n =6066) | Total (n =1500) | Total (n =2371) | (n =9937) | Not fulfill (n =185) | Fulfill (n =1315) | Not fulfill (n =321) | Fulfill (n =2050) | Not Evaluated (n =357) | Evaluated (n =958) | Not Evaluated (n =496) | Evaluated (n =1554) | |||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Respond (father) | 850 | 14.0 | 195 | 13.0 | 196 | 8.3 | 1241 | 12.5 | 20 | 10.8 | 175 | 13.3 | 22 | 6.9 | 174 | 8.5 | 58 | 16.2 | 117 | 12.2 | 55 | 11.1 | 119 | 7.7 |
| Gender (male) | 3036 | 50.0 | 792 | 52.8 | 1351 | 57.0 | 5179 | 52.1 | 100 | 54.1 | 692 | 52.6 | 189 | 58.9 | 1162 | 56.7 | 188 | 52.7 | 504 | 52.6 | 274 | 55.2 | 888 | 57.1 |
| State (Porto Alegre) | 3455 | 57.0 | 750 | 50.0 | 1196 | 50.4 | 5401 | 54.4 | 124 | 67 | 626 | 47.6 | 226 | 70.4 | 970 | 47.3 | 143 | 40.1 | 483 | 50.4 | 198 | 39.9 | 772 | 49.7 |
| Depression | 67 | 1.1 | 23 | 1.5 | 92 | 3.9 | 182 | 1.8 | 2 | 1.1 | 21 | 1.6 | 19 | 5.9 | 73 | 3.6 | 3 | 0.8 | 18 | 1.9 | 14 | 2.8 | 59 | 3.8 |
| Mania | 44 | 0.7 | 22 | 1.5 | 60 | 2.5 | 126 | 1.3 | 2 | 1.1 | 20 | 1.5 | 10 | 3.1 | 50 | 2.4 | 4 | 1.1 | 16 | 1.7 | 7 | 1.4 | 43 | 2.8 |
| Panic | 166 | 2.7 | 69 | 4.6 | 245 | 10.3 | 480 | 4.8 | 11 | 5.9 | 58 | 4.4 | 40 | 12.5 | 205 | 10 | 7 | 2 | 51 | 5.3 | 37 | 7.5 | 168 | 10.8 |
| GAD | 95 | 1.6 | 39 | 2.6 | 157 | 6.6 | 291 | 2.9 | 4 | 2.2 | 35 | 2.7 | 21 | 6.5 | 136 | 6.6 | 8 | 2.2 | 27 | 2.8 | 20 | 4 | 116 | 7.5 |
| Agoraphobia | 207 | 3.4 | 82 | 5.5 | 293 | 12.4 | 582 | 5.9 | 13 | 7 | 69 | 5.2 | 41 | 12.8 | 252 | 12.3 | 17 | 4.8 | 52 | 5.4 | 53 | 10.7 | 199 | 12.8 |
| Specific phobia | 1403 | 23.1 | 408 | 27.2 | 959 | 40.4 | 2770 | 27.9 | 49 | 26.5 | 359 | 27.3 | 138 | 43 | 821 | 40 | 83 | 23.2 | 276 | 28.8 | 184 | 37.1 | 637 | 41 |
| Social anxiety | 559 | 9.2 | 199 | 13.3 | 655 | 27.6 | 1413 | 14.2 | 28 | 15.1 | 171 | 13 | 103 | 32.1 | 552 | 26.9 | 33 | 9.2 | 138 | 14.4 | 117 | 23.6 | 435 | 28 |
| OCD | 395 | 6.5 | 217 | 14.5 | 847 | 35.7 | 1459 | 14.7 | 24 | 13 | 193 | 14.7 | 117 | 36.4 | 730 | 35.6 | 48 | 13.4 | 145 | 15.1 | 181 | 36.5 | 549 | 35.3 |
| Psychosis | 451 | 7.4 | 217 | 14.5 | 850 | 35.8 | 1518 | 15.3 | 27 | 14.6 | 190 | 14.4 | 132 | 41.1 | 718 | 35 | 32 | 9 | 158 | 16.5 | 164 | 33.1 | 554 | 35.6 |
| Alcohol | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drugs | 1 | 0.0 | 1 | 0.0 | 1 | 0.0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Learning | 1020 | 16.8 | 359 | 23.9 | 1032 | 43.5 | 2411 | 24.3 | 45 | 24.3 | 314 | 23.9 | 154 | 48 | 878 | 42.8 | 83 | 23.2 | 231 | 24.1 | 198 | 39.9 | 680 | 43.8 |
| Language | 501 | 8.3 | 214 | 14.3 | 653 | 27.5 | 1368 | 13.8 | 32 | 17.3 | 182 | 13.8 | 111 | 34.6 | 542 | 26.4 | 33 | 9.2 | 149 | 15.6 | 103 | 20.8 | 439 | 28.2 |
| Separation | 453 | 7.5 | 158 | 10.5 | 516 | 21.8 | 1127 | 11.3 | 17 | 9.2 | 141 | 10.7 | 85 | 26.5 | 431 | 21 | 25 | 7 | 116 | 12.1 | 95 | 19.2 | 336 | 21.6 |
| Conduct | 77 | 1.3 | 19 | 1.3 | 86 | 3.6 | 182 | 1.8 | 2 | 1.1 | 17 | 1.3 | 23 | 7.2 | 63 | 3.1 | 2 | 0.6 | 15 | 1.6 | 8 | 1.6 | 55 | 3.5 |
| ODD | 457 | 7.5 | 151 | 10.1 | 486 | 20.5 | 1094 | 11 | 20 | 10.8 | 131 | 10 | 91 | 28.3 | 395 | 19.3 | 19 | 5.3 | 112 | 11.7 | 69 | 13.9 | 326 | 21 |
| ADHD | 1028 | 16.9 | 384 | 25.6 | 1126 | 47.5 | 2538 | 25.5 | 52 | 28.1 | 332 | 25.2 | 165 | 51.4 | 961 | 46.9 | 76 | 21.3 | 256 | 26.7 | 203 | 40.9 | 758 | 48.8 |
Note: GAD, generalized anxiety disorder; OCD, obsessive compulsive disorder; ODD, oppositional defiant disorder; ADHD, attention deficit hyperactivity disorder. All differences between random and high risk group were significant, except for alcohol, drug use and state (compare columns #2 and #3). Italic typeface represents significant differences between proportions in each category of symptoms (p <0.05) – Fulfill versus Not fulfill and Evaluated versus Not Evaluated in each selection stratum. All percentages are presented within columns. Statistics: z‐test
Table 6.
Family demographics and family liability index (FLI) according to the Family History Screen (FHS) stratified by selection strata, inclusion criteria and participation in the total sample (n =9937) and comparisons between those fulfill versus not fulfill selection criteria and between those evaluated versus non‐evaluated in the household phase
| Not selected | Randomly selected | Select by high risk | Total | Randomly selected (n =1500) | Select by high risk (n =2371) | Random with inclusion criteria (n =1315) | High risk with inclusion criteria (n =2050) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n =6066) | Total (n =1500) | Total (n =2371) | (n =9937) | Not fulfill (n =185) | Fulfill (n =1315) | Not fulfill (n =321) | Fulfill (n =2050) | Not Evaluated (n =357) | Evaluated (n =958) | Not Evaluated (n =496) | Evaluated (n =1554) | |||||||||||||
| p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | p75 | p95 | |
| Age respectively (years) | 40 | 48 | 40 | 50 | 40 | 48 | 40 | 48 | 38 | 49 | 40 | 50 | 39 | 49 | 40 | 48 | 40 | 51 | 40 | 50 | 39 | 48 | 40 | 48 |
| Age (years) | 10 | 12 | 10 | 12 | 11 | 12 | 10 | 12 | 10 | 12 | 10 | 12 | 11 | 12 | 10 | 12 | 11 | 12 | 10 | 12 | 11 | 12 | 10 | 12 |
| Biological siblings | 2 | 3 | 1 | 2 | 2 | 3 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 3 | 2 | 3 | 1 | 3 | 1 | 2 | 1 | 3 | 2 | 3 |
| Half siblings | 1 | 3 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 1 | 3 | 1 | 2 | 1 | 2 |
| Total | 0.75 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Depression | 0 | 0.25 | 0 | 0.33 | 0 | 0.4 | 0 | 0.33 | 0 | 0.33 | 0 | 0.33 | 0 | 0.4 | 0 | 0.4 | 0 | 0.33 | 0 | 0.33 | 0 | 0.33 | 0 | 0.43 |
| Mania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Panic | 0 | 0.33 | 0 | 0.37 | 0 | 0.5 | 0 | 0.33 | 0 | 0.33 | 0 | 0.4 | 0.22 | 0.5 | 0 | 0.5 | 0 | 0.33 | 0 | 0.4 | 0 | 0.4 | 0 | 0.5 |
| GAD | 0 | 0.33 | 0 | 0.5 | 0.29 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0.33 | 0.5 | 0.29 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0.25 | 0.5 | 0.29 | 0.5 |
| Agoraphobia | 0 | 0.33 | 0 | 0.4 | 0 | 0.5 | 0 | 0.33 | 0 | 0.4 | 0 | 0.4 | 0.14 | 0.5 | 0 | 0.5 | 0 | 0.33 | 0 | 0.4 | 0 | 0.5 | 0 | 0.5 |
| Specific phobia | 0.33 | 0.6 | 0.33 | 0.6 | 0.4 | 0.75 | 0.33 | 0.67 | 0.33 | 0.5 | 0.33 | 0.6 | .5 | 0.67 | 0.4 | 0.75 | 0.33 | 0.6 | 0.33 | 0.64 | 0.4 | 0.71 | 0.4 | 0.75 |
| Social anxiety | 0.13 | 0.5 | 0.2 | 0.5 | 0.33 | 0.67 | 0.25 | 0.5 | 0.25 | 0.5 | 0.2 | 0.5 | 0.33 | 0.67 | 0.33 | 0.67 | 0 | 0.5 | 0.22 | 0.5 | 0.33 | 0.57 | 0.33 | 0.67 |
| OCD | 0.33 | 0.5 | 0.36 | 0.67 | 0.5 | 1 | 0.33 | 0.67 | 0.4 | 0.75 | 0.33 | 0.67 | 0.5 | 0.8 | 0.5 | 1 | 0.33 | 0.67 | 0.4 | 0.67 | 0.5 | 0.83 | 0.5 | 1 |
| Psychosis | 0 | 0.4 | 0 | 0.5 | 0.33 | 0.67 | 0.14 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0.33 | 0.67 | 0.33 | 0.6 | 0 | 0.5 | 0.13 | 0.5 | 0.25 | 0.6 | 0.33 | 0.6 |
| Alcohol | 0 | 0.33 | 0 | 0.5 | 0.22 | 0.5 | 0 | 0.4 | 0 | 0.5 | 0 | 0.5 | 0.25 | 0.5 | 0.2 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0.15 | 0.5 | 0.2 | 0.5 |
| Drugs | 0 | 0.29 | 0 | 0.33 | 0 | 0.4 | 0 | 0.33 | 0 | 0.5 | 0 | 0.33 | 0.13 | 0.5 | 0 | 0.4 | 0 | 0.33 | 0 | 0.33 | 0 | 0.4 | 0 | 0.4 |
| Learning | 0.33 | 0.5 | 0.33 | 0.63 | 0.5 | 0.75 | 0.33 | 0.67 | 0.33 | 0.67 | 0.33 | 0.6 | 0.5 | 0.71 | 0.5 | 0.75 | 0.33 | 0.57 | 0.33 | 0.67 | 0.5 | 0.67 | 0.5 | 0.75 |
| Language | 0 | 0.33 | 0 | 0.44 | 0.22 | 0.5 | 0 | 0.4 | 0 | 0.5 | 0 | 0.4 | 0.25 | 0.5 | 0.2 | 0.5 | 0 | 0.5 | 0 | 0.4 | 0 | 0.5 | 0.25 | 0.5 |
| Separation | 0 | 0.33 | 0 | 0.5 | 0.13 | 0.5 | 0 | 0.4 | 0 | 0.4 | 0 | 0.5 | 0.25 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 |
| Conduct | 0 | 0.33 | 0 | 0.4 | 0 | 0.5 | 0 | 0.33 | 0 | 0.4 | 0 | 0.4 | 0.25 | 0.5 | 0 | 0.5 | 0 | 0.33 | 0 | 0.45 | 0 | 0.5 | 0 | 0.5 |
| ODD | 0 | 0.33 | 0 | 0.5 | 0.25 | 0.5 | 0 | 0.5 | 0.2 | 0.5 | 0 | 0.5 | 0.29 | 0.5 | 0.25 | 0.5 | 0 | 0.33 | 0 | 0.5 | 0.17 | 0.5 | 0.25 | 0.5 |
| ADHD | 0 | 0.4 | 0.11 | 0.5 | 0.33 | 0.5 | 0.17 | 0.5 | 0.18 | 0.5 | 0.1 | 0.5 | 0.33 | 0.67 | 0.33 | 0.5 | 0 | 0.4 | 0.14 | 0.5 | 0.25 | 0.5 | 0.33 | 0.5 |
Note: GAD, generalized anxiety disorder; OCD, obsessive compulsive disorder; ODD, oppositional defiant disorder; ADHD, attention deficit hyperactivity disorder. Median values returned in a vast majority of zeros and therefore only precentiles 75th (p75) and 95th (p95) are presented. All differences between random and high risk groups were significant, except for respondent age and family liability for mania, (compare columns #2 and #3). Italic typeface represents significant rank differences in each category of symptoms (p <0.05) – Fulfill versus Not fulfill and Evaluated versus Not Evaluated in each selection stratum. Statistics: Independent samples Mann–Whitney test. FLI measures the percentage of family members affected for each psychiatric disorder.
Efficacy of the oversampling procedure
Adopting the described procedures, we tried to oversample children with psychiatric symptoms and families with high percentage of members affected by psychiatric disorders according to the screening instrument. Here, we evaluate the efficacy of the oversampling procedure comparing groups according to the selection procedure: the random procedure and the high‐risk selection procedure.
We found that those in the high‐risk group presented a higher number of childhood psychiatric disorders according to the child diagnostic evaluation with the DAWBA (Table 2). We also found that the high‐risk group (based on FHS) presented a higher number of parents with psychopathology (the respondent of the household interview) in comparison with the random group as assessed by the MINI (Table 3).
Diagnostic reliability
Diagnostic reliability for the rating procedure of DAWBA resulted in acceptable indexes with agreements ranging from 90% to 95% and Kappa from 0.72 to hyperkinetic disorders to 0.84 for emotional disorders (Table 4).
Attendance to household/scholar interviews
Those children who were selected but failed to fulfill inclusion criteria along the study (e.g. school transference before child evaluation) did differ in some aspects from those that met criteria for being evaluated. They were more likely to be from the Porto Alegre site in both random and high‐risk strata. No other differences were found in the random selection group. However, within the high‐risk group, those who failed to fulfill inclusion criteria were also more likely to have depressive, psychotic, language, separation anxiety, conduct and oppositional defiant symptoms (Table 5).
Regarding family liability index (FLI), respondents who did not fulfill the criteria were younger and had higher family loading of drug abuse within randomly selected subjects. A lower family loading of generalized anxiety, alcohol, drugs, separation anxiety and conduct was found for those who fulfilled diagnostic criteria within those selected by high‐risk (Table 6).
Those who were selected and fulfill inclusion criteria but were not evaluated in the household interview (e.g. refused further participation, not found, etc.) also differed from those evaluated in some specific variables. Within the randomly selected subjects, the non‐evaluated were less likely to be from Porto Alegre and to have symptoms of panic, specific phobia, social anxiety, psychosis, language difficulties, separation anxiety, oppositional defiant, and attention deficit hyperactivity symptoms. Within the high‐risk group, the non‐evaluated were less likely to be from Porto Alegre, to be male and to present panic, generalized anxiety, language difficulties, conduct, oppositional defiant and attention deficit hyperactivity symptoms. Moreover, within randomly selected subjects, those who did not fulfill inclusion criteria were older, had lower FLIs for generalized anxiety, social anxiety, psychosis, learning, oppositional defiant, conduct and attention deficit hyperactivity symptoms. Within the high‐risk sample, they had lower FLIs for generalized anxiety, social anxiety, psychosis, language, oppositional defiant and attention deficit hyperactivity symptoms.
Discussion
We described the rationale, methods, design and preliminary results of the High Risk Cohort Study for the Development of Childhood Psychiatric Disorders. Each of the study phases were described in detail, including their instruments, methods and procedures.
The significant efficacy of the oversampling procedure based on the FHS interview evidenced by the higher rate of psychiatric disorders and family psychopathology according to diagnostic instruments (DAWBA and MINI) in the high‐risk sub‐sample was a key point of this study. This positive result encourages us to think our design will provide advantages in the follow‐up phases, since potentially a higher number of subjects, well characterized in terms of clinical and neurobiological measures, will be affected by traits (outcomes) of interest in this study. Despite that, an increase in rates of family and child mental disorders does not necessarily mean higher follow‐up risk. However, a series of studies have reported that individual and family psychiatric symptoms and disorders are reliable predictors of psychopathology later in life (Kim‐Cohen et al., 2003; Milne et al., 2008; Moffitt et al., 2007).
We showed that whereas those who did not fulfill the inclusion criteria for participation in the study (mainly due to school transference) were found to have more child and family psychopathology, those who did not attended the diagnostic evaluation (mainly due to lost contact or refusal) had lower child and family psychopathology as assessed by the FHS. The participation analysis clearly shows that some family and child psychopathological measures were different between those who failed to fulfill inclusion criteria and those that were not evaluated by the household interview. This underscores that we have to be careful in interpreting prevalence rates in this sample, given its lack of general representativeness for the main population. Indeed, our prevalence rates were somewhat higher than previous studies with representative samples in our country from both south and southeast regions using the same instruments (Anselmi et al., 2010; Fleitlich‐Bilyk and Goodman, 2004). Therefore results from our analysis may not be generalized for prevalence purposes. However, this project was designed to be a high‐risk cohort, enriched for individual and family psychopathology, and not to assess population prevalence of psychiatric disorders. Still, the slightly higher prevalence rates of psychiatric disorders found when compared to other Brazilian studies, even in the random stratum of our sample, may be accounted to by differences in demographical aspects, such as urbanicity.
Our design has some limitations. First, we needed to select children that lived closely to the research centers in order to carry out the neuroimaging procedures, limiting the representativeness of the sample. Second, in order to keep the enrolled subjects independent from one another, we selected only one child per family, thus children from large families are underrepresented. Third, although five main areas of interest were covered in this study, other important psychiatric disorders such as depression, autism, eating disorders and substance use disorders were not targeted in the oversampling procedure. However, for those outcomes that were measured we believe our cohort will be informative for mental health problems. Finally, although the oversampling procedure with the screening instrument was able to increase the frequency of psychiatric disorders and number of familial cases in the high‐risk cohort strata according to diagnostic instruments, it is still not clear if the procedure will increase our power to detect incident and persistent cases of psychiatric disorders.
Our study was specifically designed to combine a longitudinal epidemiological approach with a comprehensive study of gene variants, environmental factors and diverse phenotypes – both clinical and those related to brain structure and functions. We believe studies like the High Risk Cohort Study for Childhood Psychiatric Disorder may help advance the field forward and provide useful information to understand the nature, prevent and treat mental disorders.
Financial support
This work is supported by the National Institute of Developmental Psychiatry for Children and Adolescents, a science and technology institute funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; National Council for Scientific and Technological Development; grant number 573974/2008‐0) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; Research Support Foundation of the State of São Paulo; grant number 2008/57896‐8).
The author's scholarships are supported by the following Brazilian government institutions: CNPq, FAPESP, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Brazilian Federal Agency for Support and Evaluation of postgraduate education) and Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS; Research Support Foundation from the State of Rio Grande do Sul). Giovanni Abrahão Salum is in receipt of a CAPES/FAPERGS post‐doctoral scholarship; Ary Gadelha is in receipt of a CAPES PhD scholarship; Pedro Mario Pan is in receipt of a CNPq/CAPES master's degree scholarship; Ana Carina Tamanaha is in receipt of a FAPESP post‐doctoral scholarship; Tais Moriyama is in receipt of a CAPES PhD scholarship; Guilherme V. Polanczyk is in receipt of a senior research CNPq scholarship; Pedro Gomes de Alvarenga is in receipt of a CAPES PhD scholarship; Andrea Jackowski, Helena Brentani, Jair de Jesus Mari, Maria Conceição do Rosário, Gisele Gus Manfro, and Eurípedes Constantino Miguel are in receipt of a senior research CNPq scholarship (302463/2011‐9).
Financial disclosures
Giovanni Abrahão Salum, Ana Carina Tamanaha, Ana Soledade Graeff‐Martins, Pedro Alvarenga, Fernanda Valle Krieger, Andrea Jackowski, João Ricardo Sato, Elisa Brietzke, Helena Brentani, Jair de Jesus Mari, Gisele Gus Manfro and Eurípedes Constantino Miguel declare no potential conflicts of interest.
Ary Gadelha, Pedro Mario Pan, and Tais Silveira Moriyama have received continuous medical education support from Astra Zeneca, Eli‐Lilly and Janssen‐Cilag. Guilherme Vanoni Polanczyk has aided as a speaker and/or consultant to Eli‐Lilly, Novartis, and Shire Pharmaceuticals, developed educational material to Janssen‐Cilag, and receives unrestricted research support from Novartis. Maria Conceição do Rosário has worked for the last five years as a speaker for the companies Novartis and Shire. Rodrigo A. Bressan was on the speakers' bureau and/or acted as consultant for Eli‐Lilly, Janssen‐Cilag, Novartis, Lundbek and Roche in the last three years (received less than US$10,000 per year, which is less than 5% of Lundbek and Roche's gross income per year). RAB has also received travel awards (air tickets and hotel costs) from Janssen‐Cilag for taking part in a psychiatric meeting. The Schizophrenia Program by RAB received unrestricted educational and research support from the following pharmaceutical companies in the last three years: Janssen‐Cilag; Novartis; Lundbek. Luis Augusto Rohde was on the speakers' bureau and/or acted as consultant for Eli‐Lilly, Janssen‐Cilag, Novartis and Shire in the last three years. LAR received travel awards (air tickets and hotel costs) from Novartis and Janssen‐Cilag in 2010 for taking part of two child psychiatric meetings. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by LAR received unrestricted educational and research support from the following pharmaceutical companies in the last three years: Abbott; Eli‐Lilly; Janssen‐Cilag; Novartis; Shire. He also receives authorship royalties from Oxford University Press and Artmed.
Supporting information
Supporting info item
Acknowledgements
The authors would like to thank the children and families for their participation, which made this research possible; the collaborators for the neuropsychological evaluation (Bruno Sini Scarpato, Sandra Lie Ribeiro do Valle and Carolina Araújo); Dr Robert Goodman for his research support regarding the DAWBA instrument procedures and Professor Heinrich Hasenack for the geocoding procedures.
Salum G. A., Gadelha A., Pan P. M., Moriyama T. S., Graeff‐Martins A. S., Tamanaha A. C., Alvarenga P., Krieger F. V., Fleitlich‐Bilyk B., Jackowski A., Sato J. R., Brietzke E., Polanczyk G. V., Brentani H., Mari J. d. J., Do Rosário M. C., Manfro G. G., Bressan R. A., Mercadante M. T., Miguel E. C., and Rohde L. A. (2015) High risk cohort study for psychiatric disorders in childhood: rationale, design, methods and preliminary results, Int. J. Methods Psychiatr. Res., 24, pages 58–73. doi: 10.1002/mpr.1459.
References
- Amorim P., Lecrubier Y., Weiller E., Hergueta T, Sheehan D. (1998) DSM‐IH‐R psychotic disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. European Psychiatry: The Journal of the Association of European Psychiatrists, 13(1), 26–34, DOI: 10.1016/S0924-9338(97)86748-X [DOI] [PubMed] [Google Scholar]
- Anselmi L., Fleitlich‐Bilyk B., Menezes A.M., Araujo C.L., Rohde L.A. (2010) Prevalence of psychiatric disorders in a Brazilian birth cohort of 11‐year‐olds. Social Psychiatry and Psychiatric Epidemiology, 45(1), 135–142, DOI: 10.1007/s00127-009-0052-2 [DOI] [PubMed] [Google Scholar]
- Associação Brasiliera de Empresas de Pesquisa (ABEP) . (2010) Critério de Classificação Econômica Brasil. São Paulo: ABEP. [Google Scholar]
- Bender L. (1938) A Visual‐motor Gestalt Test and its Clinical Use . New York: American Orthopsychiatric Association. [Google Scholar]
- Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. (1997) Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry, 36(3), 340–348, DOI: 10.1097/00004583-199703000-00012 [DOI] [PubMed] [Google Scholar]
- Bitsakou P., Psychogiou L., Thompson M., Sonuga‐Barke E.J. (2008) Inhibitory deficits in attention‐deficit/hyperactivity disorder are independent of basic processing efficiency and IQ. Journal of Neural Transmission, 115(2), 261–268, DOI: 10.1007/s00702-007-0828-z [DOI] [PubMed] [Google Scholar]
- Ellis L.K., Rothbart M.K. (2001) Revision of the Early Adolescent Temperament Questionnaire. Poster presented at the 2001 Biennial Meeting of the Society for Research in Child Development.
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. (2002) Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347, DOI: 10.1162/089892902317361886 [DOI] [PubMed] [Google Scholar]
- Figueiredo V.L.M. (2001) Uma adaptação brasileira do teste de inteligência WISC‐III. Brasília, DF: Curso de Pós‐Graduação em Psicologia, Instituto de Psicologia – Universidade de Brasília. [Google Scholar]
- Fleitlich‐Bilyk B., Goodman R. (2004) Prevalence of child and adolescent psychiatric disorders in southeast Brazil. Journal of the American Academy of Child and Adolescent Psychiatry, 43(6), 727–734, DOI: 10.1097/01.chi.0000120021.14101.ca [DOI] [PubMed] [Google Scholar]
- Goodman A, Heiervang E., Collishaw S., Goodman R. (2011) The ‘DAWBA bands’ as an ordered‐categorical measure of child mental health: description and validation in British and Norwegian samples. Social Psychiatry and Psychiatric Epidemiology, 46(6), 521–532, DOI: 10.1007/s00127-010-0219-x [DOI] [PubMed] [Google Scholar]
- Goodman R., Ford T., Richards H., Gatward R., Meltzer H. (2000a) The Development and Well‐being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry, 41(5), 645–655. [PubMed] [Google Scholar]
- Goodman R., Ford T., Simmons H., Gatward R., Meltzer H. (2000b) Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. British Journal of Psychiatry, 177, 534–539. [DOI] [PubMed] [Google Scholar]
- Hogan A.M., Vargha‐Khadem F., Kirkham F.J., Baldeweg T. (2005) Maturation of action monitoring from adolescence to adulthood: an ERP study. Developmental Science, 8(6), 525–534, DOI: 10.1111/j.1467-7687.2005.00444.x [DOI] [PubMed] [Google Scholar]
- Isolan L., Salum G.A., Osowski A.T., Amaro E., Manfro G.G. (2011) Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in Brazilian children and adolescents. Journal of Anxiety Disorders, 25(5), 741–748, DOI: 10.1016/j.janxdis.2011.03.015 [DOI] [PubMed] [Google Scholar]
- Ivanova M.Y., Dobrean A., Dopfner M., Erol N., Fombonne E., Fonseca A.C., Frigerio A., Grietens H., Hannesdottir H., Kanbayashi Y., Lambert M., Achenbach T.M., Larsson B., Leung P., Liu X., Minaei A., Mulatu M.S., Novik T.S., Oh K.J., Roussos A., Sawyer M., Simsek Z., Dumenci L., Steinhausen H.C., Metzke C.W., Wolanczyk T., Yang H.J., Zilber N., Zukauskiene R., Verhulst F.C., Rescorla L.A., Almqvist F., Weintraub S., Bilenberg N., Bird H., Chen W.J. (2007) Testing the 8‐syndrome structure of the child behavior checklist in 30 societies. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53 36(3), 405–417, DOI: 10.1080/15374410701444363 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. (1997) Schedule for Affective Disorders and Schizophrenia for School‐age Children – present and lifetime version (K‐SADS‐PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988, DOI: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kim‐Cohen J., Caspi A., Moffitt T.E., Harrington H., Milne B.J., Poulton R. (2003) Prior juvenile diagnoses in adults with mental disorder: developmental follow‐back of a prospective‐longitudinal cohort. Archives of General Psychiatry, 60(7), 709–717, DOI: 10.1001/archpsyc.60.7.709 [DOI] [PubMed] [Google Scholar]
- Konings M., Bak M., Hanssen M., van Os J., Krabbendam L. (2006) Validity and reliability of the CAPE: a self‐report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatrica Scandinavica, 114(1), 55–61, DOI: 10.1111/j.1600-0447.2005.00741.x [DOI] [PubMed] [Google Scholar]
- Kuhn S., Schmiedek F., Schott B., Ratcliff R., Heinze H.J., Duzel E., Lindenberger U., Lovden M. (2011) Brain areas consistently linked to individual differences in perceptual decision‐making in younger as well as older adults before and after training. Journal of Cognitive Neuroscience, 23(9), 2147–2158, DOI: 10.1162/jocn.2010.21564 [DOI] [PubMed] [Google Scholar]
- Luria A.R. (1973) The Working Brain: An Introduction to Neuropsychology. New York: Basic Books. [Google Scholar]
- Milne B.J., Moffitt T.E., Crump R., Poulton R., Rutter M., Sears M.R., Taylor A., Caspi A. (2008) How should we construct psychiatric family history scores? A comparison of alternative approaches from the Dunedin Family Health History Study. Psychological Medicine, 38(12), 1793–1802, DOI: 10.1017/S0033291708003115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Harrington H., Caspi A., Kim‐Cohen J., Goldberg D., Gregory A.M., Poulton R. (2007) Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry, 64(6), 651–660, DOI: 10.1001/archpsyc.64.6.651 [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., de Bono J., Painter M. (1997) Time course of attentional bias for threat information in non‐clinical anxiety. Behaviour Research and Therapy, 35(4), 297–303. [DOI] [PubMed] [Google Scholar]
- Moojen S., Lamprecht R., Santos R., Freitas G., Brodacz R., Costa A. (2003) CONFIAS Consciência Fonológica Instrumento de Avaliação Sequencial . São Paulo: Casa do Psicólogo. [Google Scholar]
- Moos R.H. (1990) Conceptual and empirical approaches to developing family‐based assessment procedures: resolving the case of the Family Environment Scale. Family Process, 29(2), 199–208; discussion 209–111. [DOI] [PubMed] [Google Scholar]
- Parker G. (1990) The Parental Bonding Instrument. A decade of research. Social Psychiatry and Psychiatric Epidemiology, 25(6), 281–282. [DOI] [PubMed] [Google Scholar]
- Pereira L.D., Schochat E. (1997) Processamento auditivo central: manual de avaliação. São Paulo: Lovise. [Google Scholar]
- Rey A., Osterrieth P. (1993) Translations of excerpts from Rey's ‘Psychological Examination of Traumatic Encephalopathy’ and Osterrieth's ‘The Complex Figure Test’. The Clinical Neuropsychologist, 7, 2–21. [Google Scholar]
- Rosario‐Campos M.C., Miguel E.C., Quatrano S., Chacon P., Ferrao Y., Findley D., Katsovich L., Scahill L., King R.A., Woody S.R., Tolin D., Hollander E., Kano Y., Leckman J.F. (2006) The Dimensional Yale–Brown Obsessive‐Compulsive Scale (DY‐BOCS): an instrument for assessing obsessive‐compulsive symptom dimensions. Molecular Psychiatry, 11(5), 495–504, DOI: 10.1038/sj.mp.4001798 [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. (1998) The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Sonuga‐Barke E.J., Taylor E. (1992) The effect of delay on hyperactive and non‐hyperactive children's response times: a research note. Journal of Child Psychology and Psychiatry, 33(6), 1091–1096. [DOI] [PubMed] [Google Scholar]
- Stein L.M. (1998) TDE Teste de desempenho escolar. São Paulo: Casa do Psicológo. [Google Scholar]
- Tellegen A., Briggs P.F. (1967) Old wine in new skins: grouping Wechsler subtests into new scales. Journal of Consulting Psychology, 31(5), 499–506. [DOI] [PubMed] [Google Scholar]
- Toplak M.E., Rucklidge J.J., Hetherington R., John S.C., Tannock R. (2003) Time perception deficits in attention‐deficit/ hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(6), 888–903. [DOI] [PubMed] [Google Scholar]
- Toplak M.E., Tannock R. (2005) Tapping and anticipation performance in attention deficit hyperactivity disorder. Perceptual and Motor Skills, 100(3 Pt 1), 659–675. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A., Kemps E., Fastame M.C., Szmalec A. (2004) Working memory components of the Corsi blocks task. British Journal of Psychology, 95(Pt 1), 57–79, DOI: 10.1348/000712604322779460 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2002) WISC‐III: Escala de Inteligência Wechsler para Crianças: Manual. São Paulo: Casa do Psicólogo. [Google Scholar]
- Weissman M.M., Wickramaratne P., Adams P., Wolk S., Verdeli H., Olfson M. (2000) Brief screening for family psychiatric history: the family history screen. Archives of General Psychiatry 57(7), 675–682. [DOI] [PubMed] [Google Scholar]
- Wertzner H.F. (2000) Prova de Fonologia In Andrade C.R., Befi‐Lopes D.M., Fernandes F.D.M., Wertzner H.F. (eds) ABFW Teste de Linguagem infantil. São Paulo: Pró fono. [Google Scholar]
- Yung A.R., Phillips L.J., Yuen H.P., Francey S.M., McFarlane C.A., Hallgren M., McGorry P.D. (2003) Psychosis prediction: 12‐month follow up of a high‐risk (“prodromal”) group. Schizophrenia Research, 60(1), 21–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


