Abstract
Objective
A longitudinal focus on gene–environment vulnerability and resilience in both patients, their unaffected family members and non‐related controls offers the opportunity to elucidate etiological and pathogenetic factors influencing the onset and course of psychotic disorders. The current paper delineates the objectives, sample characteristics, recruitment and assessment procedures of the Genetic Risk and Outcome of Psychoses (GROUP) study.
Methods
A naturalistic longitudinal cohort study with assessments at baseline, after three and six years of follow‐up. The study is conducted by a consortium of four university psychiatric centres, with their affiliated mental health care institutions in the Netherlands covering more than 7.5 million inhabitants. Extensive assessment of genetic factors, environmental factors, (endo)phenotypes, and outcome.
Results
At baseline, 1120 patients, 1057 siblings, 919 parents and 590 healthy controls were included.
Conclusion
The GROUP study will contribute to insight in risk and protective factors in the aetiology of non‐affective psychotic disorders, and in the variation in their course and outcome. Copyright © 2012 John Wiley & Sons, Ltd.
Keywords: schizophrenia, methodology, gene, environment, interaction, aetiology, longitudinal
Introduction
With a lifetime prevalence of nearly 1.5% (Perälä et al., 2007) non‐affective psychotic disorders belong to the most disabling and expensive medical disorders. These disorders have a multifactorial polygenetic aetiology and heterogeneous course (Menezes et al., 2006). For schizophrenia, being most common within the spectrum of non‐affective psychoses, heritability has been estimated at around 80% (EU‐GEI, 2008). It has been suggested that the main factor behind this is the interaction between genes and environment (van Os and Sham, 2003). A better understanding of the complex ways in which nature and nurture factors influence humans can thereby improve our knowledge of schizophrenia and related disorders. Therefore we investigated subjects with schizophrenia, and also with clinically related but less severe other non‐affective psychotic disorders. An approach that focuses on gene–environment interaction differs from the linear gene–phenotype approach by positing a causal role, not only for either genes or environment as isolated factors, but also for their synergistic co‐participation in the cause of non‐affective psychosis where the effect of one factor is conditional on the other (van Os et al., 2010). For example, the effect of cannabis may be stronger in those with a high genetic risk for the development of psychosis (Moore et al., 2007; Arseneault et al., 2002; Verdoux et al., 2003). Moreover, the widespread geographic, temporal, and ethnic variation in incidence of psychosis (McGrath et al., 2004), as well as the marked variability in response to environmental risk factors such as drug abuse, urbanicity or migration (van Os et al., 2008), suggest an interaction between genes and environment.
Research that focuses on the gene–environment interaction is still at an early stage. Studies on genetic risk and variations in expression and course of non‐affective psychosis have been hampered by relatively small sample sizes, short follow‐up periods, limited phenotyping and limited assessment of environmental factors (Gur et al., 2007; El‐Missiry et al., 2011). Studying both genes and environment may prove a fruitful strategy to identify variations that may give rise to the psychotic vulnerability and variability of its course. Recent research indicates that subclinical expressions of psychotic vulnerability have a comparable symptom structure and association with risk and protective factors (Baskak et al., 2008; Fanous et al., 2001; Hanssen et al., 2006; Kendler and Hewitt, 1992; Kendler et al., 1993). There is evidence that the expression of psychotic vulnerability is higher in family members of patients, as compared to the normal population (Vollema and Postma, 2002). Furthermore, cognitive impairments in the realm of verbal learning and memory, attention, and working memory have been found in relatives of patients, albeit to a lesser extent (Gur et al., 2007). Trait markers that are stable over time, such as cognition and psychotic experiences are also referred to as endophenotypes. The inclusion of family members of psychotic probands enables us to establish whether subclinical psychotic symptoms and cognitive impairments in siblings and parents are associated with the same genetic and non‐genetic risk factors as has been found among patients (Krabbendam et al., 2001; Vollema and Postma, 2002; Vollema and Ormel, 2000). The participation of family‐members in research offers a unique possibility to study pathogenetic mechanisms, predictors of functioning and psychiatric disorders in a non‐clinical population with an increased genetic risk, without confounding therapy related factors. Since both genetic and environmental factors could have an impact on endophenotypes (Gervais et al., 2004; Zink et al., 2008), it is necessary for the study of the gene–environment interaction underlying these phenotypes to determine their genetic variants.
Consequently, the Genetic Risk and Outcome of Psychosis (GROUP) study has been designed to study genetic and non‐genetic vulnerability and resilience factors for variation in the expression of non‐affective psychotic disorders and variation in the course of these disorders in a naturalistic cohort study of patients with non‐affective psychotic disorders, their siblings, parents, and non‐related controls. Follow‐up is scheduled three years and six years after the baseline assessment.
The purpose of the current report is to provide a detailed description of GROUP objectives, an overview of the sample characteristics, recruitment and assessment methods in order to guide other, similar efforts and to serve as a definitive point of reference for GROUP publications.
Objectives
The objectives of the GROUP study are two‐fold: (1) investigating the genetic and environmental factors, and their interaction, contributing to the expression of psychosis; (2) investigating factors of vulnerability and protectiveness, and response to medication and clinical outcome. Furthermore, the GROUP study has the goal to build new research infrastructures in routine mental health service settings, by means of collaborations between university medical centres and mental health institutions. These purposes can be achieved by inclusion of a group of incident and prevalent patients with a non‐affective psychosis presenting consecutively at these services either as outpatients or inpatients, representative of the prevalence of treated patients with a non‐affective psychosis. This cohort will be relevant for the improvement of patient care, and serve as a vehicle to systematic evaluation of treatment practices. In order to test hypotheses about the aetiology of non‐affective psychosis, a cohort of family members with resilience for psychosis is being included, consisting of both siblings and parents. In order to test hypotheses about the aetiology of non‐affective psychosis, a cohort of family members with resilience for psychosis is being included, consisting of both siblings and parents. Furthermore, a cohort of non‐related control subjects will participate in the project. This allows for comparisons with a group that represents the variation of subclinical symptoms being present in the normal population. It is known that the majority of the first‐degree relatives and controls will never develop a psychotic disorder despite carrying components of vulnerability.
The mean age of onset of psychosis is approximately 24 years old, after which the following six years have been suggested to be most indicative for outcome. In order to investigate both the transition of patients to other psychiatric diagnoses, as well as possible transitions of non‐affected siblings to psychosis, the project aims at relatively young affected and non‐affected siblings, which will be studied for six years using two follow‐up assessments.
Genetic risk is assessed both indirectly, using a familial liability score for psychosis (Verdoux et al., 1996), and directly using genetic sequence variation. It has been argued that gene environment interactions need a hypothesis‐driven strategy focusing on pathways at which biological synergism may take place between genetic and environmental mechanisms (Van Os et al., 2008). After reviewing the literature, genes were selected that (1) were previously suggested to be associated with schizophrenia; (2) are important for dopaminergic neurotransmission (Kapur, 2003; Hirvonen et al., 2005; Huttunen et al., 2008); (3) have a role in regulating differential sensitivity to broadly defined environmental influences; and (4) may be involved in epigenetic regulation of environmental factors. The GROUP study will initially focus on 250 functional single‐nucleotide polymorphisms (SNPs).
Environmental risk factors that will be included in the study are substance abuse, urbanicity, ethnicity/migration and premorbid adaptation. Evidence for induced psychotic symptoms following substance abuse is especially high for consumption of cannabis. This is also been found to be true of men brought up in, or born in, an urban living environment (Kelly et al., 2010). With respect to ethnicity and migration, there is consistent evidence that psychoses have a higher incidence in many migrants and ethnic minorities (Morgan et al., 2010). Finally, poor premorbid adjustment in terms of peer relationships and scholastic performance has been found to be present in those who later develop a non‐affective psychosis, schizophrenia in particular (Saracco‐Alvarez et al., 2010).
Endophenotypes are quantifiable intermediate factors in the genes‐to‐behaviour pathways which make genetic and biological studies for disease categories more manageable (Gottesman and Gould, 2003). Endophenotypes vary quantitatively among individuals at risk for the disorder, regardless of whether the disorder is expressed phenotypically, making clinically unaffected relatives of disordered patients informative for genetic studies. The GROUP study will focus on expression and longitudinal course of neurocognition, social cognition and schizotypal features as endophenotypes of schizophrenia and other non‐affective psychotic disorders. Neuropsychological assessment focuses on cognitive domains known to play an important role in schizophrenia research (Nuechterlein et al., 2004), such as processing speed and working memory. Factor analyses have shown that these domains represent replicable and separable dimensions of cognitive impairment in schizophrenia. The evidence for cognition as an endophenotypic marker is particularly strong for verbal learning and memory, attention/vigilance, and working memory (Gur et al., 2007). Recent research suggests that potentially significant endophenotypes can also be found in the realm of social cognition (Baas et al., 2008; Gur et al., 2007; Janssen et al., 2003; Versmissen et al., 2008).
The significant variation in response and side effects of antipsychotic medications on patients with a psychotic disorder has been attributed to differences in genetic factors. The individual differences in medication effects are thought to be associated with polymorphisms of genes that are associated with neurotransmitter receptors, metabolic enzymes, and with the permeability of the blood–brain. The GROUP study will focus on the association between genetic variation and effectiveness and side effects of antipsychotic medication. In addition, heterogeneity has been found with respect to outcome in terms of course, functioning, and subjective quality of life. These factors will also be subject of investigation.
The scale of the study with its inclusion of a well‐characterized patient population as well as siblings and parents make this study suitable to investigate the complexity of genomic architecture and genetic regulation underlying psychotic disorders.
Power calculations
In order to establish sufficient power to address the study objectives, a large sample for all cohorts is needed. In order to investigate gene–environment interaction in a case‐only design (Khoury and Flandres, 1996), at least 1000 patients are required in order to establish a power of 89.7% (α = 0.05). A gene–environment study using a case‐sibling design (Ottman, 1996) requires at least 1000 patients and 1000 siblings in order to reach a power of 81% (α = 0.05). To detect statistical differences (α = 0.05) between the cohorts longitudinally, at least 1000 patients, 1000 siblings, and 350 controls are needed to establish a power of 93% in a case–control and a case‐sibling design (standard deviation = 0.15) on a continuous variable (cognitive function, schizotypy). For a sibling‐control design, 1000 siblings and 350 controls would be required for a power of 89% (standard deviation = 0.10).
Methods
Overview of GROUP‐project structure and participating institutes
The GROUP steering committee consists of the scientific leaders of schizophrenia research programmes in four university departments of psychiatry in the Netherlands (Amsterdam, Groningen, Maastricht and Utrecht). In each academic centre one or two senior site‐coordinators are responsible for the clinical and endophenotype assessment training, recruitment, quality control and quality assurance. Each centre has formalized collaborations with several mental health care institutions in their region, both in the Netherlands and Belgium. The mental health care services committed themselves to provide research physicians and nurses to the screening, inclusion and follow‐up during the GROUP study for the duration of 10 years. GROUP steering committee and site coordinators work in close collaboration with representatives of the family association Ypsilon, the client association Anoiksis, and the Dutch Knowledge Centre for Schizophrenia. These associations are the only ones that apply to psychosis and schizophrenia in the Netherlands. Ypsilon is an association founded and run by family members (N = 7000 members) of individuals suffering from psychosis or schizophrenia. Their main goal is to provide support for the family members. Anoiksis is an association founded and run by patients who suffer from psychosis or schizophrenia. Their main goal is to facilitate contact between patients who suffer from psychosis or schizophrenia. In addition they advocate and give advice about psychosis. The Dutch Knowledge Centre for Schizophrenia contributes to the improvement of the quality of care for individuals with schizophrenia.
Thirty‐six mental health care institutes participated in the GROUP study. Together, these mental health care institutions cover about 75% of the population in the Netherlands. They are acknowledged at the end of this article. See Figure 1 for a flow diagram of the GROUP‐project organization structure, referred participants and collected data. The number of participants targeted before onset of the study is unfortunately not available. Of the individuals that did finally participate, data was collected. The current paper is based on the baseline assessment of the longitudinal setup of the GROUP project and therefore drop out rates will become available after the second assessment. However, even within the baseline assessments there were some drop outs that lead to incomplete datasets, as showed in the flow diagram, Figure 1. Reasons for these drop outs were: refusal, physical illness, not available for further assessment or other reasons.
Figure 1.
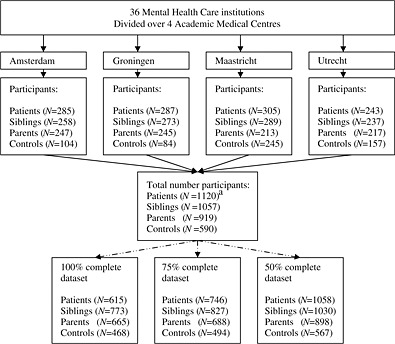
Flow diagram of the GROUP‐project organization structure, referred participants and collected data. aNineteen parents and 24 siblings received a patient status.
Inclusion criteria and recruitment
In selected representative geographical areas of the Netherlands and (Dutch speaking part of) Belgium, patients were identified through clinicians working in regional psychosis departments or academic centres, whose caseload was screened for inclusion criteria. Subsequently, a group of patients presenting consecutively at these services either as outpatients or inpatients were recruited for the study. Persons identified as potentially eligible were given detailed explanation of the study procedures and were asked for informed consent for detailed assessment and for contacting their first degree family members (brothers, sisters, parents). Controls were selected through a system of random mailings to addresses in the catchment areas of the cases. Inclusion criteria for patients were the following: (1) age range of 16 to 50 years (extremes included); (2) a diagnosis of non‐affective psychotic disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria (APA, 2000); (3) good command of the Dutch language; and (4) able and willing to give written informed consent. Inclusion criteria for siblings were the following: (1) age range of 16 to 50 years (extremes included); (2) good command of the Dutch language; (3) able and willing to give written informed consent. Similar criteria, excluding age, applied to the parents. Inclusion criteria for healthy controls were the following: (1) age range of 16 and 50 years (extremes included), (2) no lifetime psychotic disorder; (3) no first degree family member with a lifetime psychotic disorder (4) good command of the Dutch language; and (5) able and willing to give written informed consent. Comorbidity in patients, siblings and parents was not an exclusion criterion. When siblings or parents appeared to have a lifetime psychotic disorder they were included in the patient group. Only a lifetime psychotic disorder or lifetime psychotic disorder of first degree relative was an exclusion criterion in the healthy control group. Respective effects were accounted for by controlling for psychopathology. Moreover, several research questions specifically focused on differences between patients with and without comorbid psychopathology (for instance: comorbid cannabis abuse or obsessive compulsive disorder).
The study protocol was approved centrally by the Ethical Review Board of the University Medical Centre Utrecht and subsequently by local review boards of each participating institute. After full verbal and written information about the study, written informed consent was obtained from all participants before the start of the first assessment. Confidentiality of data is maintained by using a unique research identification (ID) for each respondent. The ID number does not include any data related to the name of the participant or information that could lead to the identification of the person. The personal data linked to the ID are securely stored by each local centre.
Assessment training
GROUP investigators were convinced of the necessity of uniformity in experimental procedures and experimenter behaviour. Over 30 interviewers per site were trained for administering the assessments. The interviewers consisted of research assistants, psychologists, psychiatrists, nurses and PhD students. Before the start of the study all interviewers met for three days of training workshops at one site (Utrecht), to practise the assessments of all measures used in the GROUP project. Issues which arose during training and official assessments were adjusted by mutual agreement with the makers and the interviewers for optimal and practical use. Specific issues that arose during assessment training were: insufficient reliability in assessing movement disorders, differences in assessing severity of schizotypal traits with the Structured Interview for Schizotypy‐revised (SIS‐R) and systematic bias in assessing the Global Assessment of Functioning (GAF)‐scores in part of the researchers. These issues were addressed by adding extra training and repeated training in bimonthly sessions.
All researchers were retrained every two months and ongoing effort was directed at increasing reliability of assessments, in order to minimize the impact a large collaborative research project such as described in the current study could have on the quality of assessment. When new interviewers entered the project, training was supervised by the senior clinical site coordinators who implemented the training protocol. The training procedure consisted of didactic sessions, observation, and supervised practice. Training materials for each assessment included written procedure manuals and instructional videos. The procedure manuals contained scripts for task instructions, which were stated verbatim to each participant by an interviewer. Procedure manuals and instructional videos are web‐based and at all times available for all interviewers. Reliability training was performed four times a year. Formal assessment of reliability of all rating scales was carried out.
An important issue concerns systematic differences between assessment of patients versus siblings and controls due to differences with respect to commitment to collaborate and/or fatigue. Differences due to paranoia or difficulties in working alliance due to symptoms can not be excluded. However, researchers were particularly focussed on establishing an optimal collaboration with the included subjects. To minimize the influence of fatigue on cognitive task performance, sessions started with neuropsychological testing. Subjects could take short rest breaks whenever necessary.
Measurements
Assessments took place at one of the participating regional psychosis departments or academic centres in and around Amsterdam, Utrecht, Groningen and Maastricht. The assessments were administered by research assistants. If participants were unable to visit the institute, in‐home assessments were being offered. Since some of the participating institutions were inpatient and other outpatient clinics, the amount of in‐home assessments differed. The same protocol was being used during in‐home assessments and assessments at the institute. In both instances neuropsychological testing was performed with the use of a laptop computer. In both instances urine was sampled to account for effects of drug of abuse. The assessment lasted on average four hours for patients and three hours for other participants. To account for fatigue effects, the four hours lasting assessment for patients was divided over two separate assessments of two hours each, within a week from each other.
Genetic factors
For genotyping purposes, 20 ml of blood was collected from each subject at the participating mental health institutes and sent to the University Medical Centre Utrecht by mail where DNA was extracted from peripheral blood lymphocytes using established procedures. A contribution to the development of a first non‐affective psychotic episode is likely to be applicable for the following candidate genes: neuroregulin‐1 (NRG‐1), dysbindin (DTNBP1), G72, d‐aminoacid oxidase (DAAO), regulator of G‐protein signalling‐4 (RGS4), catechol‐O‐methyltransferase (COMT), and praline dehydrogenase (PRODH) (Harrison and Weinberger, 2005).
The Family Interview for Genetic Studies (FIGS; Maxwell, 1992) was assessed for systematically gathering diagnostic information from an informant about relatives in the pedigrees being studied. This method is less sensitive than direct assessment of each family member, although it has proven to be useful in situations where reliance on direct information from each family member is not possible. The FIGS is administered to the parents of the proband and if they were not available, to the siblings and to the controls. It is administered in the first interview session, and subjects are invited to collect all missing information prior to the next session. The FIGS starts with the drawing of a pedigree containing all first‐ and second‐degree relatives of the proband. Next, psychiatric information about all relatives in the pedigree is obtained by asking general screening questions. In case of endorsed symptom categories, these are followed up using a checklist asking details of symptoms, number of episodes, duration, age of onset, treatment, and impairment rating. Symptom categories of depression, mania, and psychosis were included. Familial loading was calculated using a method described by Derks et al. (2009).
Environmental factors
To assess the quality and severity of tobacco, alcohol and other drug abuse or dependence and its course, the Composite International Diagnostic Interview (CIDI; WHO, 1990) was used. A special Substance Abuse Module covers tobacco, alcohol, and other drug abuse in considerable detail, allowing the assessment of the quality and severity of dependence and its course. In a field trial the cross‐cultural acceptability and reliability of the questions were found to be high (Cottler et al., 1989).
In addition, urine was screened by means of immunoassays: for cocaine CEDIA® (cutoff 300 ng/ml), for amphetamines CEDIA® amphetamine/XTC (cutoff 1000 ng/ml) with additional analyses added when the first screening result was positive, and for cannabis (cutoff 50 ng/ml). For cannabis, given the relatively high cutoff level of 50 ng/ml we used a detection window of one month (Musshoff and Madea, 2006). In addition, the creatinine level of every sample was measured as an integrity parameter.
Ethnicity was evaluated as follows. If the country of origin of three or more of the subject's grandparents was similar, the subject's ethnicity was equal to this. In all other cases, the ethnicity was mixed. For the assessment of urbanicity and migration, participants were asked to report all the addresses were they had lived before the age of 21. These addresses were then coupled to the national database of Statistics Netherlands to determine the level of urbanicity during childhood.
The Premorbid Adjustment Scale (PAS; Cannon‐Spoor et al., 1982) is designed to evaluate the degree of achievement of developmental goals at each of several periods of a subject's life before the onset of schizophrenia. Interrater reliability and internal consistency has been found to be high (Small et al., 1984). High scores in PAS representing a bad premorbid social adjustment correlated significantly with a low age of onset, high positive and negative syndrome scale (PANSS) scores, an insidious onset and a long period of hospitalization.
Phenotypes
To assess DSM‐IV diagnosis two structured diagnostic instruments were used, in accordance with the standard practice in the local area. Three sites used the Comprehensive Assessment of Symptoms and History (CASH; Andreasen et al., 1992) and one site used the Schedules for Clinical Assessment for Neuropsychiatry (SCAN 2.1; Wing et al., 1990). The CASH includes the Scale for the Assessment of Positive Symptoms [SAPS, with 34 items measured on an ordinal scale ranging from zero (absent) to five (severe); Andreasen, 1984a] and the Scale for the Assessment of Negative Symptoms (SANS, with 21 items; Andreasen, 1984b).
The SCAN is the successor of the Present State Examination, 9th edition (PSE‐9; Wing et al., 1974), which has been created by the World Health Organization (WHO). It is a semi‐structured computer‐based interview to assess psychiatric symptoms, using DSM‐IV and the Internation Classification of Diseases, 10th Revision (ICD‐10) compatible algorithms, constructed by the WHO‐SCAN Advisory Committee (Wing et al., 1990). The SCAN covers practically all of the axis I disorders. For several of these diagnostic groups its reliability and validity has been established (Andrews and Peters, 1998). Brugha et al. (1999) reported good reliability between lay interviewers and trained clinicians for SCAN sections for psychotic disorders. All raters were trained psychologist or psychiatrist, with extensive clinical experience. Diagnostic consensus was achieved in the presence of an independent psychiatrist. Psychiatric diagnosis was established according to criteria of DSM‐IV. The two different diagnostic tools are comparable in that they both assess diagnoses after careful consideration with a clinician. Moreover, all diagnoses were discussed between researchers and involved clinicians and in the case of incongruence the site‐coordinators made a final decision. The CASH or SCAN were administered to all participant cohorts.
The PANSS (Kay et al., 1987) was administered to measure severity of a variety of symptoms in the patient population. The PANSS (Kay et al., 1987) is currently the most widely used scale to assess the severity of a variety of symptoms in patients with schizophrenia. Originally the PANSS consists of three subscales: positive syndrome scale (item P1–P7), a negative syndrome scale (items N1–N7) and general psychopathology scale (item G1–G16). Recently, Van der Gaag et al. (2006a, 2006b) developed a complex model with a substantial number of double loading items and improved stability using confirmatory factor analysis on a large data set. This five‐factor model presumably reflects the complex reality of schizophrenia and comorbid symptoms. Data reduction with the use of this model is thought to produce results that have better face validity and associations with other measures (Van der Gaag et al., 2006a, 2006b). Therefore, in our analyses we will use this latter model next to the widely used original PANSS scale with its three subscales.
The Schedule for the Deficit Syndrome (SDS; Kirkpatrick et al., 1989) was used to categorize patients with schizophrenia into deficit or non‐deficit subgroups. The SDS is a semi‐structured interview designed to assess six enduring (lasting > 1 year) negative symptoms of restricted affect, diminished emotional range, poverty of speech, curbed interests, diminished sense of purpose, and diminished social drive. To meet criteria for the deficit syndrome, an individual must demonstrate a moderate or higher level of severity on at least two of these symptoms.
To measure Parkinsonism, the motor examination part of the Unified Parkinson Disease Rating Scale (UPDRS) was being used (Martinez‐Martin et al., 1994). The UPDRS is a valid and reliable instrument and widely used in research in Parkinson's disease (Martinez‐Martin et al., 1994). Dyskinesia was measured with items 1–7 of the Abnormal Involuntary Movement rating Scale (AIMS; Guy, 1976). Akathisia was measured with the Barnes Akathisia Rating Scale (BARS; Barnes, 1989) with one extra item added to measure acute dystonia with a severity score from zero to four.
The Obsessive Compulsive Drug Use Scale (OCDUS; Schippers et al., 1997) was used in all participants to assess self‐rated cannabis craving over the past seven days. In a study among heroin users, Franken et al. (2002) found a three factor solution: (1) thoughts and interference; (2) desire and control; (3) resistance to thoughts and intention to use.
The semi‐structured Yale–Brown Obsessive Compulsive Scale (YBOCS; Goodman et al., 1989a, 1989b) was used in all participants to measure obsessive compulsive symptoms. Investigations of the psychometric properties of the YBOCS in non‐psychotic OCD patients show high internal consistency, interrater reliability and test–retest reliability (Goodman et al., 1989a, 1989b; Woody et al., 1995, Kim et al., 1990). Internal consistency in patients with schizophrenia and comorbid obsessive‐compulsive symptoms (OCS) were even better than those found in non‐psychotic patients with obsessive‐compulsive disorder (OCD) (Woody et al., 1995; De Haan et al., 2006, Boyette et al., 2011). A three‐factor solution is thought optimal: obsessions, compulsions, and resistance.
Endophenotypes
The neuropsychological assessment focuses on verbal learning and memory, attention/vigilance, working memory, speed of processing, reasoning and problem solving, verbal comprehension and social cognition, thereby covering all domains as included in the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus, with the exception of visual learning and memory (Nuechterlein et al., 2004). Tests for each of these domains are selected on the basis of established reliability and validity, as well as on their feasibility for use in large multisite studies (see Table 1). For a number of the tasks, assessment was computerized using E‐prime 1.3 (Psychology Software Tools, Inc., Pittsburgh, PA, 2001). The tests are administered in a fixed order. Total testing time is approximately two hours. A break is scheduled in case of subject fatigue.
Table 1.
Neuropsychological assessment at baseline
| Task | Domaina | Reference |
|---|---|---|
| Word Learning Taskb | Verbal learning and memory | Brand and Jolles, 1985 |
| Continuous Performance Test‐HQ (CPT‐HQ)b | Attention/vigilance | Nuechterlein and Dawson, 1984; Wohlberg and Kornetsky, 1973 |
| WAIS‐III Digit Symbol Substitution Test | Processing speed | Wechsler, 1997 |
| WAIS‐III Information | World knowledge | Wechsler, 1997 |
| WAIS‐III Arithmetic | Working memory | Wechsler, 1997 |
| WAIS‐III Block Design | Reasoning and problem solving | Wechsler, 1997 |
| Response Set‐shifting Task (RST)b | Reasoning and problem solving/working memory | Bilder et al., 1992 |
| Benton Facial Recognition Test | Visuoperception | Benton et al., 1983 |
| Degraded Affect Recognition Task (DFAR)b | Social cognition | Van 't Wout et al., 2004 |
| Hinting Task | Social cognition | Corcoran et al., 1995 |
The domains which the tasks correspond to are based on factor‐analytic work (Nuechterlein et al., 2004), with the exception of the Response Set‐shifting Task and the Benton Facial Recognition Test.
Computerized assessment using E‐prime 1.3.
To rate self‐reports of lifetime psychotic experiences, the Community Assessment of Psychic Experiences (CAPE; http://www.cape42.homestead.com) was administered. Research with the CAPE has shown (i) a three‐factor structure of positive, negative and depressive dimensions in a large and representative sample of young men (Stefanis et al., 2002) and in a large sample of undergraduate female students (Verdoux et al., 2003), (ii) discriminative validity across groups of individuals with schizophrenia, affective and anxiety disorders and individuals from the general population (Hanssen et al., 2003), (iii) family‐specific variation for positive and negative subclinical psychosis dimensions (Hanssen et al., 2006), and (iv) stability over time and specific and independent associations with the corresponding interview‐based dimensions (Konings et al., 2006).
Siblings and healthy controls were assessed with the SIS‐R (Kendler et al., 1989; Vollema and Ormel, 2000; Vollema et al., 2002) to measure schizotypy. Questions and rating procedures are standardized. Schizotypal symptoms and signs can be assessed reliably using the SIS‐R (Vollema and Ormel, 2000). Item scores were reduced to three‐dimensional scores of schizotypy of positive symptoms (such as referential thinking, delusional mood, magical ideation, illusions, and suspiciousness), of disorganization symptoms (such as goal directedness of thinking, loosening of associations, and oddness) and of negative symptoms (such as social isolation, social anxiety, introversion, restricted affect).
Outcome
Course of psychopathology, needs and care‐consumption were assessed with the Life Chart Schedule (LCS; Susser et al., 2000) by clinical trained interviewers. The LCS was used in patients and other informants, e.g. one or both parents and to collect data from the clinical records. LCS ratings proved reliable in all four key domains The reliability was fair to excellent for ratings of duration of experience [interclass correlation coefficient (ICC) ranged from 0.53 to 0.99], quality of experience (kappa ranged from 0.46 to 0. 92) and long‐term time trends (kappa ranged from 0.66 to 0.94). The LCS can be used to obtain reliable ratings of the long‐term course of schizophrenia and related disorders in multiple domains.
Treatment response, adverse events and compliance were assessed by the treating physician at the start of the study and six months later. The Clinical Global Impression‐Schizophrenia Scale (CGI‐SCH and CGI‐IMP) was used to evaluate treatment response. The CGI‐SCH and the CGI‐IMP have been shown to have good concurrent validity and substantial reliability and sensitivity to change when compared with the PANSS and the GAF scales (Haro et al., 2003; Leucht et al., 2006).
History of currently prescribed anti‐psychotic medication, co‐medication, and reasons for recent medication‐switches were registered. Endocrine side‐effects, subjective experiences and extra‐pyramidal side effects were evaluated as absent, minimal or present. Medication compliance was rated on a seven‐item Likert‐type scale, as described by Janssen et al. (2006). This scale is adapted from a four‐category psychosis insight scale (Amador et al., 1993) and consists of seven categories reflecting an increasing degree of positive compliance and cooperation in taking medication (from “refuses all kinds of medication” to “cooperates actively, is fully responsible for intake”).
The Camberwell Assessment scale of Need Short Appraisal Schedule (CANSAS; Slade et al., 1999) was used to assess need of care. The interrater reliability of the CANSAS was assessed under routine conditions. Agreement on the identification of an area of need was high, higher on patient ratings than on staff ratings (Andresen et al., 2000).
Quality of life was assessed with the 26‐item the World Health Organization Quality of Life‐BREF (WHOQOL‐BREF 1998). The domain scores of the WHOQOL‐BREF have been shown to correlate highly with the WHOQOL‐100, which has demonstrated criterion validity (Orley and Kuyken, 1994). In a Dutch psychiatric outpatient population the WHOQOL‐BREF was demonstrated to be an adequate measure for assessing quality of life with a high construct validity and reliability (Trompenaars et al., 2005).
Procedure
The four sites all used the same measures (with one exception see Phenotypes section), and all measurements were administered in the same set order for standardization. The self report questionnaires were sent to the participants prior to the first assessment meeting in order for them to fill out and bring the questionnaires to the meeting. At the start of the assessment informed consent was signed and the self report questionnaires were checked for missing data. The neuropsychological test battery was first administered to make sure participating individuals were fully concentrated and focused. Order effects are not accounted for. However, by starting with neuropsychological testing we maximized the chance that subjects achieved optimally. Trained researchers applied the tests. Urine screening was administered at the same time to rule drug use effects out and the PANSS interview was also assessed. For the second assessment meeting, or in the case of siblings, parents and controls after the break, the diagnostic tools were administered first, then other questionnaires and it closed with physical examination. Some of the questionnaires had overlapping items and were therefore administered consecutively to avoid repetition.
Overview of data flow, quality control and quality assurance
All co‐workers of the GROUP project manually filled out the interview assessments on pre‐prepared template documents and they asked the participants to fill out the self report questionnaires on similar pre‐prepared template documents. In each one of the four sites, the data was uploaded by scanning these data mask template documents. Cardiff Teleform, the program used to create these template documents, automatically captures data from paper forms and documents (virtually eliminating all human intervention and errors), validate the data and pass it to the local database. A secure (encrypted) channel was installed between each site and the central GROUP data centre in Groningen, over which the local database was auto‐synchronized daily. After all sites got their data flow geared up and initial setup issues were resolved, the central data centre started keeping history of all synced data sets. Clinical assessment data and endophenotype data were processed locally at specific sites and then synchronized with the central data centre using the same secure channels. Before transmission of data from the sites to the central data centre, quality controls were performed on site to eliminate human errors. Before scanning the documents all data was checked for errors by co‐workers of the GROUP project, then after scanning the documents the data was verified again and if necessary, corrected. At the central data centre a set of scripts was run each night to create a daily build of the aggregated central database from the collection of raw data streams from each site. These scripts included sanity checks, ranging from simple range checks to complex consistency checks. Modifications and data‐cleaning at this level was also done by scripts and thus fully reversible. Each (internal) release of the central database contained fresh auto‐generated codebooks and elaborate syntaxes (SPSS and STATA) and for transparency all scripts used to generate the central database were also published alongside. Histories of the aggregated central databases were kept every night, therefore all central data sets are archived. This is a powerful protection and quality policy. The anonymized central database was made available to all site coordinators by means of a secured website. This included auto‐generated reports presenting statistics of processed instruments per assessment per site per subject‐type. Suspect data (data failing sanity checks) was presented by reports and by extra data‐files containing only suspect data to speed up the cleaning process. Using these reports each site would clean or re‐run their data locally which would then show up in the next central database iteration after auto‐synchronizing and rebuilding of the central database. After the triple checks, a group of database managers, at least one from each site, had weekly meetings to discuss the quality checks and find solutions for the remaining errors. Each senior site coordinator was responsible for quality assessment (technical and logical errors in the data) of a part of the assessments instruments. Any data questions that arose during this quality assessment process were reviewed by the research staff at the site where the data were collected. Thereafter a definitive data set was established and changes were only possible with permission of all senior site coordinators.
Results
Demographic and clinical characteristics of participants
In this study 1120 patients, 1057 siblings, 919 parents, 590 healthy controls. Nineteent parents and 24 siblings received a patient status. Demographical data and clinical characteristics of the participant patients, siblings, parents and controls are provided in Table 2. Analysis of variance (ANOVA) and Chi square tests were applied to investigate the group differences in all groups and independent t test and chi to investigate between the groups. There was a main effect of age (F [3, 3682] = 2339.24, p < 0.001). Besides the age difference between parents and the other groups, healthy controls were older than siblings (p < 0.001) and patients (p < 0.001). There was a main effect of the Wechsler Adult Intelligence Scale, third edition (WAIS‐III) estimated intelligence quotient (IQ) (F[3,3548] = 113.250, p < 0.001). Controls had significantly higher IQ's as compared to parents, siblings and patients (p < 0.001). Parents and siblings had significantly higher IQ scores, as compared to patients (p < 0.001). Finally, there was a main effect of Educational degree (F [3,3578] = 74.326, p < 0.001), adapted from Verhage (1964). Controls were significantly higher educated as compared to siblings (p = 0.012), parents (p = 0.007) and patients (p < 0.001). Patients were significantly lower educated as compared to their siblings (p < 0.001) and parents (p < 0.001), although the latter two did not significantly differ. The included groups differed significantly with respect to gender (χ 2(3) = 315.320, p < 0.001). Seventy‐six percent of the patients were male. The proportion of females was greater among siblings (54%), parents (57%) and healthy controls (54%). Groups differed significantly with respect to ethnicity (χ 2(3) = 64.413, p < 0.001). The majority of patients (79%) were Caucasian, which applied even more so to the other participants (>80%). The origins of non‐Caucasians were from a great variety of countries and cultures, including Morocco, Turkey, Surinam and several Asian countries. Most prevalent lifetime psychiatric morbidity among siblings, parents and controls was depressive disorder. Cannabis use/dependence was more frequent in the male population of all the participant groups, but compared to females the differences did not reach significance. There was a significant difference between the groups with respect to current residential status (χ 2(12) = 694.957, p < 0.001) and marital status (χ 2(9) = 765.098, p < 0.001). Patients were more often non‐married, and single or living at their parents home.
Table 2.
Demographic and clinical characteristics of participants in the GROUP study, means (standard deviations in parentheses) and absolute numbers
| Variable | Patients (N = 1120) | Siblings (N = 1057) | Parents (N = 919) | Controls (N = 590) |
|---|---|---|---|---|
| Age (years) at T0 | 27.7 (8.0) | 27.8 (8.3) | 54.7 (6.9) | 30.4 (10.6) |
| Gender, male (%) | 76.2 | 45.6 | 42.7 | 45.8 |
| Education, Verhagea | 4.0 (2.1) | 5.1 (2.1) | 5.1 (2.3) | 5.4 (1.8) |
| WAIS‐III Estimated IQ | 94.9 (16.1) | 102.6 (15.6) | 103.1 (17.0) | 109.6 (15.2) |
| Ethnicity, Caucasian (%) | 79.1 | 83.2 | 88.9 | 92.0 |
| Marital status (%) | ||||
| Not married | 87.9 | 57.4 | 4.7 | 55.0 |
| Married/living together | 9.2 | 40.3 | 70.7 | 41.0 |
| Other | 2.9 | 2.3 | 24.7 | 4.0 |
| Residential status (%) | ||||
| Single | 33.7 | 20.5 | 8.3 | 22.1 |
| With parents(s) | 39.5 | 27.7 | 5.5 | 26.8 |
| With partner/family | 10.3 | 46.3 | 84.8 | 46.9 |
| Sheltered living | 9.7 | 0.1 | 0.0 | 0.0 |
| Other | 6.8 | 5.3 | 1.4 | 4.3 |
| Lifetime psychopathology | ||||
| Depressive disorder | ||||
| Male (%) | 0 | 5.8 | 12.5 | 3.0 |
| Female (%) | 0.4 | 13.6 | 20.9 | 12.5 |
| Bipolar disorder | ||||
| Male (%) | 1.3 | 0.8 | 0.5 | 0 |
| Female (%) | 1.5 | 1.2 | 1.0 | 0.3 |
| Substance abuse | ||||
| Male (%) | 45.6 | 17.6 | 3.8 | 13.7 |
| Female (%) | 17.2 | 8.7 | 2.1 | 3.8 |
| Age onset psychosis (years) | 22.4 (6.8) | |||
| Duration of illness (years) | 4.4 (4.1) | |||
| Psychotic episodes (N) | 1.7 (1.1) | |||
| Recent onset psychosis (%) | 19.3 | |||
| PANSS, | ||||
| Positive | 1.8 (0.8) | |||
| Negative | 2.1 (0.8) | |||
| General | 1.8 (0.5) | |||
| Hospitalizations (N) | 2.6 (2.9) | |||
| Global Assessment of Functioning (GAF) | ||||
| Symptoms | 55.9 (16.0) | |||
| Disabilities | 54.5 (16.2) | |||
| Diagnostic (%) | ||||
| SZb, paranoid type | 53.8 | |||
| Schizo‐affective | 10.7 | |||
| Psychosis NOS | 10.4 | |||
| SZ undifferentiated type | 5.7 | |||
| Schizophreniform | 5.6 | |||
| SZ disorganized type | 3.7 | |||
| Other | 10.1 |
Education (Verhage): range 0 (no education), 3‐5 (school diploma) to 8 (university degree).
SZ, schizophrenia.
At baseline, the mean age of patients was 28, of which a minority had experienced their first psychotic episode during the past year. The spectrum of non‐affective psychotic disorders consisted of various diagnostic groups. The diagnosis of paranoid schizophrenia was most frequent (54%), followed by schizoaffective disorder (11%) and psychotic disorder not otherwised specified (NOS) (10%). Within the patient group the comorbidity rates were as follows: depressive disorder in the female sample (1,4%), bipolar disorder in the male (1.3%) and female (1.5%) sample and substance abuse in the male (45,4%) and female (17.2%) sample. Differential diagnoses in the patient group were divided as follows: schizophrenia subtypes (65.5%), schizoaffective disorder (10.7%), psychotic disorder not otherwise specified (10.5%), schizophreniform disorder (5.6%), Brief psychotic disorder (2.9%), delusional disorder (2.0%) and bipolar disorder (1.4%).
Interrater reliability
We chose to test the interrater reliability of the most important diagnostic instruments and the measures of severity of (sub)clinical psychotic symptoms (respectively CASH, SCAN, SIS‐R and PANSS). In follow‐up assessments interrater reliability of all other measure will be assessed. As described in the assessed measures section, the YBOCS, PAS and CANSAS have shown to have good interrater reliability after extensive training.
Interrater reliability of the PANSS and SIS‐R assessment in the GROUP study was evaluated with ICC by using total and subscale scores of 16 randomly selected researchers (four of each study site) who rated four videotaped interviews: ICC of PANSS positive subscale score was 0.957 (95% confidence interval 0.808 to 0.997); ICC of PANSS negative subscale score 0.911 (95% confidence interval 0.606 to 0.994); ICC of PANSS total score 0.946 (95% confidence interval 0.758 to 0.996); and ICC of SIS‐R total score was 0.986 (95% confidence interval 0.868 to 1.000). Interrater reliability of the diagnostic classification according to DSM‐IV was evaluated by assessing the concordance between the diagnosis assessed by the raters involved in the GROUP project and the diagnosis as assessed by the treating clinician. A randomly selected comparison of 65 subjects with a psychotic disorder revealed a difference in diagnosis in one case: schizoaffective disorder versus schizophrenia undifferentiated type.
Proposed analyses for testing hypotheses
To compare resilience and vulnerability factors between patients, siblings, parents and controls a regression model that takes familial clustering into account will be used. Odds ratios will be calculated to determine the magnitude of the associations. Genetic polymorphisms will be analysed in the same way. Familial clustering will be conveyed as the random effect in multilevel random regression models, and will be reported as the proportion of total variance explained by random effect. To determine the extend of familial clustering, cross‐sib within trait analyses will be used, in which the presence of an endophenotype in sib 1 is used to predict the presence of the same endophenotype in sib 2. Next, a cross‐sib cross trait analyses will be performed to test the relation between endophenotypes within families. Herewith the presence of endophenotype 1 in sib 1 will be used to predict endophenotype 2 in sib 2. If necessary, Bonferroni corrections will be performed to control for multiple testing.
Discussion
This article presents objectives, sample characteristics overview, recruitment and assessment methods of the GROUP study. The objectives with respect to the inclusion of the large cohorts have been met. The descriptive purpose of the current paper sets the stage for future GROUP reports. A main objective of the project was to include at least 1000 patients with non‐affective psychosis, 1000 siblings, 1000 parents, and 350 controls, originating from a large part of the Netherlands and Belgium. This objective has largely been met. Strengths of the GROUP study are the sample size, the longitudinal nature, the comprehensive and detailed assessment of genetic factors, environmental factors, (endo)phenotypes, and clinical outcome. Inclusion of patients presenting consecutively at representative services either as outpatients or inpatients provides a representative sample of treated patients from which it is possible to derive meaningful population attributable fractions. Inclusion of brothers and sisters of these patients offers the unique possibility to study pathogenetic mechanisms and predictors of functioning and psychiatric disorders in a non‐clinical population with an increased genetic risk.
A number of limitations of the GROUP study should be noted. Firstly, selection bias: subjects willing to participate in a demanding study protocol may be different from subjects in other psychiatric studies that are less demanding, or from subjects refusing participation in research. Unfortunately, the response rate of the subjects approached was not evaluated.
In addition, it is possible that subjects who did not complete the whole assessment procedure differ from other subjects. Future studies should take this into account. Secondly, differences between relatives and patients may be difficult to detect when siblings who are not available or willing to participate, share relevant clinical characteristics with their ill family members. However, the availability of FIGS data on non‐participating relatives will enable an estimate of the magnitude of these potential biases. Thirdly, it is possible that adolescent siblings of affected probands subsequently develop a psychotic disorder during the follow‐up period of six years. However, measurable subclinical symptoms are expected to be present in these subjects. Fourthly, although we strived for minimization and constancy of environmental influences on the subjects' behaviour during the assessment, some of the subjects had to be visited in their own living environment due to inability to visit the institute. We can not rule out the possibility that this may have influenced assessments. Finally, despite massive investment, interrater reliability remains a vulnerability of large collaborations. The data quality of interview assessments may have been influenced by the large number of research assistants and participants in the GROUP study. However, an advantage was that for the duration of the full baseline assessment all research assistants remained connected to the research project.
Studying both genes and environment in interaction is thought to be a sound strategy to identify the environmental and genetic variations that may give rise to the psychotic vulnerability and variability in its course. It should be noted that factors beyond this interaction may be part of the pathway to psychosis as well. One such a factor is the gene–environment correlation. The GROUP study may be suitable to investigate these other forms of gene–environment interaction as well (Rutter et al., 2006).
Longitudinal multi‐centre collaborations aimed at detailed assessment of vulnerability and resilience factors (environmental and genetic) in populations with variable expressions of psychotic vulnerability offer a unique opportunity to take forward the study of the aetiology and prognosis of non‐affective psychosis. Such investigations require the involvement of many researchers and clinicians and therefore assessment methods that are precisely defined and uniformly applied. Research projects on such a scale are only possible with the dedication of many patients, family members, control subjects, researchers, clinicians as well as boards of directors of universities, health care institutes and funding agencies.
Better understanding of predictors of clinical and subclinical presentations of psychotic disorders will allow more accurate knowledge of possible preventive measures and thereby ultimately contribute to health improvement of patients with non‐affective psychotic disorders and those at high risk to develop such disorders.
Declaration of interest statement
GROUP investigators designed the study and wrote the protocol.
Authors Nikie Korver, Piotr J. Quee, Heleen Boos, Claudia Simons, René S. Kahn, Don H. Linszen, Jim van Os, Durk Wiersma; Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, Lydia Krabbendam and Inez Myin‐Germeys managed the literature searches and analyses.
Authors Nikie Korver, Piotr J. Quee and L. de Haan wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
All other authors declare that they have no conflicts of interest.
Appendix
GROUP investigators are René S. Kahna, Don H. Linszenb, Jim van Osc, Durk Wiersmad; Richard Bruggemand, Wiepke Cahna, Lieuwe de Haanb, Lydia Krabbendamc, Inez Myin‐Germeysc.
aDepartment of Psychiatry, University Medical Centre Utrecht, Rudolf Magnus Institute of Neuroscience, The Netherlands.bDepartment of Psychiatry, Academic Medical Centre University of Amsterdam, Amsterdam, The Netherlands. cSouth Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, EURON, Maastricht, The Netherlands. dDepartment of Psychiatry & Rob Giel Research Center, University Medical Centre Groningen, University of Groningen, The Netherlands.
Acknowledgements
This work was supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw). The GROUP project was also supported by a grant from ZonMw, within the Mental Health programme (project number: 10.000.1002). ZonMw had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Acknowledgement GROUP investigators
Amsterdam
Academic Medical Centre: S.E. van den Berg, C.L.A. Schroeder, R. van der Valk, N. Dekker, C.J. Meijer, N. Korver, L.N. Boyette, J. Meijer, D. van Dam; GGZ Ingeest: I. de Rijke, S. Huinink, R.J. de Vries, M. Jansen, D. Bos; Arkin: W.P. Hoen, E.M. te West, S.H.J. Groeneveld, E.M. Vergunst, M.Swets, S. Vothknecht, I.Poleacov; Dijk en Duin: D. van Dijk, S. de Metz, M.T. Hasty, G. Geertsa, P.M. de Baaij, A. Metzger; Erasmus University Medical Center: NJM van Beveren, M. Baldini; GGZ INgeest: F.D. Grimbergen, M.A.M. Boerma, C. Agsteribbe; GGZ Noord Holland Noord: H.P. Wisman, M.A.H. Monden, M.M. Bosman, M. van Dijk; Rivierduinen: R.Klaassen, T.van der Tang, B. Luteijn, H. Winkel, H. Weisz, A.C.P. Strater, A. Landman, M. Vorstenbosch.
Maastricht
D. Op 't Eijnde, K. Lenders, S. Loyen, R. Roberts, K. Sweers, H. Gielen, N. Soons, A. Hintzen, V. Habets, I. Riské, C. Vossen, E. Martens, S. Apers, L. Wijnhoven, E. Konings, T. Lataster, M. Lardinois, D. Versmissen, C. Simons, M. van der Werf, P. Habets, S. Pfeiffer, E. de Loore, M. Heins, M. Oorschot, M. Meys, M. Dietvorst, C. van Zelst, I. Crolla, R. Mengelers, F. van Goethem, W. Beuken, D. Byniam, T. Driesen, M. Marcelis, G. Driessen, A. Shazad, R. van Winkel, C. Henquet, G. Kenis, P. Delespaul.
Utrecht
Utrecht Medical Centre: P. Anema, E.M.J. van Baaren, S.C. Bakker, H.B.M. Boos, E. Caspers, E.M. Derks, S. van Hemert, A. Hemkes, R. Hijman, M. van Leeuwen, J.E.H. Machielsen, R.A. Ophoff, M. Rais, M. Salden, H. van Someren, E. Strengman, M. Vleesschouwer; Symfora: M. van Beek, P.N. van Harten, J.P.F. Koning, W. Schep; Meerkanten: M.G. Vollema, P. Prins, T. Viester; Altrecht: A. Akdeniz, K. Verweij, N. Kaymaz; RIAGG Amersfoort: F. v.d. Heuvel, B. v.d. Goot; Delta: J.E. Hovens, A.J.M. Loonen.
Groningen
UMCG Groningen: J. Bous, M. Veenstra, E. van 't Hag, P.J. Quee, F.J. Nienhuis, E. Veermans, A.A. Bartels; Lentis: F. Duijndam, T. Lugtenberg, H. Knegtering; GGZ Friesland: W. Blaauw, A. Wunderink; GGZ Drenthe: K.P. Touw, J. Arends, C.J. Slooff; Dimence: J. Brilman, M. Schomaker, M van Wijk, A. Wessels; Mediant GGZ Twente: E. Vroom, K. Meijerink; GGNet: I. Bogert, W. Janssen, H. de Berk, E.O. Noorthoorn; Parnassia: H. Ising, J.D. Blom, M. van der Gaag; De Grote Rivieren: G. Mensen, R. van der Snoek, R. Smit, G. Faber.
References
- Amador X.F., Strauss D.H., Yale S.A., Flaum M.M., Endicott J., Gorman J.M. (1993) Assessment of insight in psychosis. The American Journal of Psychiatry, 150(6), 873–879. [DOI] [PubMed] [Google Scholar]
- APA (2000) Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM‐IV‐TR), Washington, DC: American Psychiatric Association. [Google Scholar]
- Andreasen N.C. (1984a) Scale for the Assessment of Positive Symptoms (SAPS), Iowa City, IA, University of Iowa. [Google Scholar]
- Andreasen N.C. (1984b) Modified Scale for the Assessment of Negative Symptoms (SANS), Iowa City, IA, University of Iowa. [Google Scholar]
- Andreasen N.C., Flaum M., Arndt S. (1992) The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry, 49(8), 615–623. [DOI] [PubMed] [Google Scholar]
- Andresen R., Caputi P., Oades L.G. (2000) Interrater reliability of the Camberwell assessment of need short appraisal schedule. Austalian and New Zealand Journal of Psychiatry, 34(5), 856–861. [DOI] [PubMed] [Google Scholar]
- Andrews G., Peters L. (1998) The psychometric properties of the Composite International Diagnostic Interview. Social Psychiatry and Psychiatric Epidemiology, 33(2), 80–88. [DOI] [PubMed] [Google Scholar]
- Arseneault L., Cannon M., Poulton R., Murray R., Caspi A., Moffitt T.E. (2002) Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BioMed Central, 325(7374), 1183–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D., van 't Wout M., Aleman A., Kahn R.S. (2008) Social judgement in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychological Medicine, 38, 747–754. [DOI] [PubMed] [Google Scholar]
- Barnes T.R. (1989) A rating scale for drug‐induced akathisia. The British Journal of Psychiatry, 154, 672–676. [DOI] [PubMed] [Google Scholar]
- Baskak B., Ozel E.T., Atbasoglu E.C., Baskak S.C. (2008) Peculiar word use as a possible trait marker in schizophrenia. Schizophrenia Research, 103(1–3), 311–317. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Sivan A.B., de Hamsher K.S., Varney N.R., Spreen O. (1983) Benton's Test of Facial Recognition, New York: Oxford University Press. [Google Scholar]
- Bilder R.M., Turkel E., Lipschutz‐Broch L., Lieberman J.A. (1992) Antipsychotic medication effects on neuropsychological functions. Psychopharmacology Bulletin, 28(4), 353–366. [PubMed] [Google Scholar]
- Boyette L.N.J., Swets M., Meijer C.J., Wouters L., Genetic Risk and Outcome of Psychosis (GROUP) . (2011) Factor structure of the Yale–Brown Obsessive‐Compulsive Scale (Y‐BOCS) in a large sample of patients with schizophrenia or related disorders and comorbid obsessive‐compulsive symptoms. Psychiatry Research, 30(186), 409–413. [DOI] [PubMed] [Google Scholar]
- Brand N., Jolles J. (1985) Learning and retrieval rate of words presented auditorily and visually. The Journal of General Psychology, 112(2), 201–210. [DOI] [PubMed] [Google Scholar]
- Brugha T.S., Nienhuis F.J., Bagchi D., Smith J., Meltzer H. (1999) The survey form of SCAN: the feasibility of using experienced lay survey interviewers to administer a semi‐structured systematic clinical assessment of psychotic and non psychotic disorders. Psychological Medicine, 29(3), 703–712. [DOI] [PubMed] [Google Scholar]
- Cannon‐Spoor H.E., Potkin S.G., Wyatt R.J. (1982) Measurement of premorbid adjustment in chronic schizophrenia. Schizophrenia Bulletin, 8(3): 470–484. [DOI] [PubMed] [Google Scholar]
- Corcoran R., Mercer G., Frith C.D. (1995) Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophrenia Research, 17(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Cottler L.B., Robins L.N., Helzer J.E. (1989) The reliability of the CIDI‐SAM: a comprehensive substance abuse interview. British Journal of Addiction, 84(7), 801–814. [DOI] [PubMed] [Google Scholar]
- De Haan L., Hoogenboom B., Beuk N., Wouters L., Dingemans P.H., Linszen D.Z. (2006) Reliability and validity of the Yale‐Brown Obsessive‐Compulsive Scale in schizophrenia patients. Psychopharmacology Bulletin, 39(1), 25–30. [PubMed] [Google Scholar]
- Derks E.M., Verweij K.H., Kahn R.S., Cahn W.C. (2009) The calculation of familial loading in schizophrenia. Schizophrenia Research, 111(1–3), 198–199. [DOI] [PubMed] [Google Scholar]
- El‐Missiry A., Aboraya A.S., Manseur H., Manchester J., France C., Border K. (2011) An update on the epidemiology of schizophrenia with a special reference to clinically important risk factors. International Journal of Mental Health and Addition, 9(1): 39–59. [Google Scholar]
- European Network of Schizophrenia Networks for the Study of Gene–Environment Interactions (EU‐GEI) . (2008) Schizophrenia aetiology: Do gene–environment interactions hold the key? Schizophrenia Research, 102(1–3), 21–26. [DOI] [PubMed] [Google Scholar]
- Fanous A., Gardner C., Walsh D., Kendler K.S. (2001) Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Archives of General Psychiatry, 58(7): 669–673. [DOI] [PubMed] [Google Scholar]
- Franken I.H., Hendriksa V.M., van den Brink W. (2002) Initial validation of two opiate craving questionnaires the obsessive compulsive drug use scale and the desires for drug questionnaire. Addictive Behaviors, 27(5), 675–685. [DOI] [PubMed] [Google Scholar]
- Gervais H., Belin P., Boddaert N., Leboyer M., Coez A., Sfaello I., Barthelemy C., Brunelle F., Samson Y., Zilbovicius M. (2004) Abnormal cortical voice processing in autism. Nature Neuroscience, 7(8): 801–802. [DOI] [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. (1989a) The Yale–Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry, 46(11): 1006–1011. [DOI] [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. (1989b) The Yale–Brown Obsessive Compulsive Scale II: validity. Archives of General Psychiatry, 46(11): 1012–1016. [DOI] [PubMed] [Google Scholar]
- Gottesman I.I., Gould T.D. (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. The American Journal of Psychiatry, 160(4), 636–645. [DOI] [PubMed] [Google Scholar]
- Gur R.E., Calkins M.E., Gur R.C., Horan P.H., Nuechterlein K.H., Seidman L.J., Stone W.S. (2007) The consortium on the genetics of schizophrenia: neurocognitive endophenotypes. Schizophrenia Bulletin, 33(1), 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W.A. (1976) Abnormal Involuntary Movement Scale (AIMS) In EDCEU Assessment Manual for Psychopharmacology, Guy W.A. (ed), pp. 534–537, Washington, DC: Department of Health Education and Welfare. [Google Scholar]
- Hanssen M., Krabbendam L., Vollema M., Delespaul P., van Os J. (2006) Evidence for instrument and family specific variation of subclinical psychosis dimensions in the general population. Journal of Abnormal Psychology, 115(1): 5–14. [DOI] [PubMed] [Google Scholar]
- Hanssen M., Peeters F., Krabbendam L., Radstake S., Verdoux H., Van Os J. (2003) How psychotic are individuals with non‐psychotic disorders? Social Psychiatry and Psychiatric Epidemiology, 38(3), 149–154. [DOI] [PubMed] [Google Scholar]
- Haro J.M., Kamath S.A., Ochoa D., Rele K., Fargas A., Rodrigues M.J., Rele R., Orta J., Kharbeng A., Araya S., Gervin M., Alonso J., Mavreas V., Lavrentzou E., Liontos N., Gregor K., Jones P.B., SOHO Study Group . (2003) The Clinical Global Impression‐Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatrica Scandinavica, 107(Suppl. 416): 16–23. [DOI] [PubMed] [Google Scholar]
- Harrison P.J., Weinberger D.R. (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular Psychiatry, 10(1), 40–68. [DOI] [PubMed] [Google Scholar]
- Hirvonen J., van Erp T.G., Huttunen J., Aalto S., Nagren K., Huttunen M., Lonnqvist J., Kaprio J., Hietala J., Cannon T.D. (2005) Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Archives of General Psychiatry, 62(4): 371–378. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Braff D.L., Nuechterlein K.H., Sugar C.A., Cadenhead K.S., Calkins M.E., Dobie D.J., Freedman R., Greenwood T.A., Gur R.E., Gur R.C., Light G.A., Mintz J., Olincy A., Radant A.D., Schork N.J., Seidman L.J., Siever L.J., Silverman J.M., Stone W.S., Swerdlow N.R., Tsuang D.W., Tsuang M.T., Turetsky B.I., Green M.F. (2008) Verbal working memory impairments in individuals with schizophrenia and their first‐degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophrenia Research, 103(1–3): 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J., Heinimaa M., Svirskis T., Nyman M., Kajander J., Forsback S., Solin O., Ilonen T., Korkeila J., Ristkari T., McGlashan T., Salokangas R.K., Hietala J. (2008) Striatal dopamine synthesis in first‐degree relatives of patients with schizophrenia. Biological Psychiatry, 63(1): 114–117. [DOI] [PubMed] [Google Scholar]
- Janssen B., Gaebel W., Haerter M., Komaharadi F., Lindel B., Weinmann S. (2006) Evaluation of factors influencing medication compliance in inpatient treatment of psychotic disorders. Psychopharmacology, 187(2), 229–236. [DOI] [PubMed] [Google Scholar]
- Janssen I., Krabbendam L., Jolles J., van Os J. (2003) Alterations in theory of mind in patients with schizophrenia and non‐psychotic relatives. Acta Psychiatrica Scandinavica, 108(2): 110–117. [DOI] [PubMed] [Google Scholar]
- Kapur S. (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American Journal of Psychiatry, 160(1), 13–23. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. [DOI] [PubMed] [Google Scholar]
- Kelly B.D., O'Callaghan E., Waddington J.L., Feeney L., Browne S., Scully P.J., Clarke M., Quinn J.F., McTigue O., Morgan M.G., Kinsella A., Larkin C. (2010) Schizophrenia and the city: a review of literature and perspective study of psychosis and urbanicity in Ireland. Schizophrenia Research, 116(1): 75–89. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Hewitt J. (1992) The structure of self‐report schizotypy in twins. Journal of Personality Disorders, 6(1): 1–17. [Google Scholar]
- Kendler K.S., Lieberman J.A., Walsh D. (1989) The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophrenia Bulletin, 15(4), 559–571. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., McGuire M., Gruenberg A.M., O'Hare A., Spellman M., Walsh D. (1993) The Roscommon Family Study. III. Schizophrenia‐related personality disorders in relatives. Archives of General Psychiatry, 50(10): 781–788. [DOI] [PubMed] [Google Scholar]
- Khoury M.J., Flandres W.D. (1996) Nontraditional epidemiologic approaches in the analysis of gene–environment interaction: case–control studies with no controls! American Journal of Epidemiology, 37(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Dysken M.W., Kuskowski M. (1990) The Yale–Brown Obsessive‐Compulsive Scale: a reliability and validity study. Psychiatry Research, 34(1): 99–106. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B., Buchanan R.W., McKenney P.D., Alphs L.D., Carpenter W.T. Jr (1989) The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Research, 30(2): 119–123. [DOI] [PubMed] [Google Scholar]
- Konings M., Bak M., Hanssen M., van Os J., Krabbendam L. (2006) Validity and reliability of the CAPE: a self‐report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatrica Scandinavica, 114(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Krabbendam L., Marcelis M., Delespaul P., Jolles J., van Os J. (2001) Single or multiple familial cognitive risk factors in schizophrenia? American Journal of Medical Genetics, 105(2), 183–188. [DOI] [PubMed] [Google Scholar]
- Leucht S., Kane J.M., Etschel E., Kissling W., Hamann J., Engel R.R. (2006) Linking the PANSS, BPRS, and CGI: clinical implications. Neuropharmacology, 31(10): 2318–2325. [DOI] [PubMed] [Google Scholar]
- Martinez‐Martin P., Gil‐Nagel A., Gracia L.M., Gomez J.B., Martinez‐Sarries J., Bermejo F. (1994) Unified Parkinson's Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Movement Disorders, 9(1): 76–83. [DOI] [PubMed] [Google Scholar]
- Maxwell M.E. (1992) Manual for the FIGS (Family Interview for Genetic Studies), Bethesda, MD, Clinical Neurogenetics Branch, National Institute of Mental Health, Intramural Research Program. [Google Scholar]
- McGrath J., Saha S., Welham J., El Saadi O., MacCauley C., Chant D. (2004) A systematic review of the incidence of schizophrenia: the distrivution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Medicine, 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes N.M., Arenovich T., Zipursky R.B. (2006) A systematic review of longitudinal outcome studies of first‐episode psychosis. Psychological Medicine, 36(10), 1349–1362. [DOI] [PubMed] [Google Scholar]
- Moore T.H., Zammit S., Lingford‐Hughes A., Barnes T.R., Jones P.B., Burke M., Lewis G. (2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet, 370(9584): 319–328. [DOI] [PubMed] [Google Scholar]
- Morgan C., Charalambides M., Hutchinson G., Murray R.M. (2010) Migration, ethnicity, and psychosis: toward a social developmental model. Schizophrenia Bulletin, 36(4), 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musshoff F., Madea B. (2006) Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Therapeutic Drug Monitoring, 28(2): 155–163. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Barch D.M., Gold J.M., Goldberg T.E., Green M.F., Heaton R.K. (2004) Identification of separable cognitive factors in schizophrenia. Schizophrenia Research, 72(1): 29–39. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H.R., Dawson M.E. (1984) Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin, 10, 160–203. [DOI] [PubMed] [Google Scholar]
- Orley J., Kuyken W. (1994) Quality of Life Assessment, International Perspectives, Heidelberg: Springer Verlag. [Google Scholar]
- Ottman R. (1996) Gene–environment interaction: definitions and study designs. Preventive Medicine, 25(6), 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perälä J., Suvisaari J., Saarni S.I., Kuoppasalmi K., Isometsä E., Pirkola S., Partonen T., Tuulio‐Henriksson A., Hintikka J., Kieseppä T., Härkänen T., Koskinen S., Lönnqvist J. (2007) Lifetime prevalence of psychotic and bipolar I disorders in a general population. Archives of General Psychiatry, 61(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Rutter M., Moffitt T.E., Caspi A. (2006) Gene–environment interaction and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry, 47(3–4): 226–261. [DOI] [PubMed] [Google Scholar]
- Saracco‐Alvarez R., Rodríguez‐Verdugo S., García‐Anaya M., Fresán A. (2010) Premorbid adjustment in schizophrenia and schizoaffective disorder. Psychiatry Research, 165(3), 234–240. [DOI] [PubMed] [Google Scholar]
- Schippers G.M., Dejong C.A.J., Lehert P., Potgieter A., Deckers F., Casselman J., Geerlings P.J. (1997) The Obsessive Compulsive Drinking Scale – translation into Dutch and possible modifications. European Addition Research, 3(3), 116–122. [Google Scholar]
- Slade M., Beck A., Bindman J., Thornicroft G., Wright S. (1999) Routine clinical outcome measures for patients with severe mental illness: CANSAS and HoNOS. The British Journal of Psychiatry, 174(May): 404–408. [DOI] [PubMed] [Google Scholar]
- Small N.E., Mohs R.C., Halperin R., Rosen W.G., Masterson C., Kendler K.S., Horvath T.B., Davis K.L. (1984) A study of the reliability of reported premorbid adjustment in schizophrenic patients. Biological Psychiatry, 19(2), 203–211. [PubMed] [Google Scholar]
- Stefanis N.C., Hanssen M., Smirnis N.K., Avramopoulos D.A., Evdokimidis I.K., Stefanis C.N., Verdoux H., van Os J. (2002) Evidence that three dimensions of psychosis have a distribution in the general population. Psychological Medicine, 32(2), 347–358. [DOI] [PubMed] [Google Scholar]
- Susser E., Finnerty M., Mojtabai R., Yale S., Conover S., Goetz R., Amador X. (2000) Reliability of the life chart schedule for assessment of the long‐term course of schizophrenia. Schizophrenia Research, 42(1): 67–77. [DOI] [PubMed] [Google Scholar]
- Trompenaars F.J., Masthoff E.D., Van Heck G.L., Hodiamont P.P., De Vries J. (2005) Content validity, construct validity, and reliability of the WHOQOL‐Bref in a population of Dutch adult psychiatric outpatients. Quality of Life Research, 14(1), 151–160. [DOI] [PubMed] [Google Scholar]
- Van 't Wout M., Aleman A., Kessels R.P., Laroi F., Kahn R.S. (2004) Emotional processing in a non‐clinical psychosis‐prone sample. Schizophrenia Research, 68(2–3), 271–281. [DOI] [PubMed] [Google Scholar]
- Van der Gaag M., Cuijpers A., Hoffman T., Remijsen M., Hijman R., de Haan L., van Meijel B., Van Harten P.N., Valmaggia L., de Hert M., Wiersma D. (2006a) The five‐factor model of the Positive and Negative Syndrome Scale I: Confirmatory factor analysis fails to confirm 25 published five‐factor solutions. Schizophrenia Research, 85(1–3), 273–279. [DOI] [PubMed] [Google Scholar]
- Van der Gaag M., Cuijpers A., Hoffman T., Remijsen M., Hijman R., de Haan L., van Meijel B., Van Harten P.N., Valmaggia L., de Hert M., Wiersma D. (2006b) The five‐factor model of the Positive and Negative Syndrome Scale II: A ten‐fold cross‐validation of a revised model. Schizophrenia Research, 85(1–3), 280–287. [DOI] [PubMed] [Google Scholar]
- Van Os J., Sham P. (2003) Gene–environment interactions In The Epidemiology of Schizophrenia Murray R.M., Jones P.B., Susser E. (eds), pp. 235–254, Cambridge: Cambridge University Press. [Google Scholar]
- Van Os J., Rutten B.P.F., Paulton R. (2008) Gene‐environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophrenia Bulletin, 34(6), 1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J., Rutten B.P.F., Paulton R. (2010) Gene–environment interactions for searchers: collaborations between epidemiology and molecular genetics In Advances in Schizophrenia Research Gattaz W.F., Busatto G. (eds), pp. 19–50, New York: Springer Science ;+ ;Business Media. [Google Scholar]
- Verdoux H., Sorbara F., Gindre C., Swendsen J.D., van Os J. (2003) Cannabis use and dimensions of psychosis in a nonclinical population of female subjects. Schizophrenia Research, 59(1), 77–84. [DOI] [PubMed] [Google Scholar]
- Verdoux H., van Os J., Sham P., Jones P., Gilvarry K., Murray R.. (1996) Does familiality predispose to both emergence and persistence of psychosis? A follow‐up study. The British Journal of Psychiatry, 168(5), 620–626. [DOI] [PubMed] [Google Scholar]
- Verhage F. (1964) Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar, Assen, Van Gorcum; (in Dutch). [Google Scholar]
- Versmissen D., Janssen I., Myin‐Germeys I., Mengelers R., Campo J.A., van Os J., Krabbendam L. (2008) Evidence for a relationship between mentalising deficits and paranoia over the psychosis continuum. Schizophrenia Research, 99(1–3), 103–110. [DOI] [PubMed] [Google Scholar]
- Vollema M.G., Ormel J. (2000) The reliability of the structured interview for schizotypy‐revised. Schizophrenia Bulletin, 26(3), 619–629. [DOI] [PubMed] [Google Scholar]
- Vollema M.G., Postma B. (2002) Neurocognitive correlates of schizotypy in first degree relatives of schizophrenia patients. Schizophrenia Bulletin, 28(3), 367–377. [DOI] [PubMed] [Google Scholar]
- Vollema M.G., Sitskoorn M.M., Appels M.C., Kahn R.S. (2002) Does the Schizotypal Personality Questionnaire reflect the biological‐genetic vulnerability to schizophrenia? Schizophrenia Research, 54(1–2), 39–45. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997) WAIS‐III: Wechsler Adult Intelligence Scale (3rd edition) Administration and Scoring Manual, San Antonio, TX, Psychological Corporation. [Google Scholar]
- Wing J.K., Babor T., Brugha T., Burke J., Cooper J.E., Giel R., Jablenski A., Regier D., Sartorius N. (1990) SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry, 47(6): 589–593. [DOI] [PubMed] [Google Scholar]
- Wing J.K., Cooper J.E., Sartorius N. (1974) The Measurement and Classification of Psychiatric Symptoms, London: Cambridge University Press. [Google Scholar]
- Wohlberg G.W., Kornetsky C. (1973) Substained attention in remitted schizophrenics. Archives of General Psychiatry, 28(4), 533–537. [DOI] [PubMed] [Google Scholar]
- Woody S.R., Steketee G., Chambless D.L. (1995) Reliability and validity of the Yale–Brown Obsessive‐Compulsive Scale. Behaviour Research and Therapy, 33(5): 597–605. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (1990) Composite International Diagnostic Interview (CIDI): a) CIDI‐interview (version 1.0), b) CIDI‐user manual, c) CIDI‐training manual, d) CIDI‐computer programs, Geneva, WHO. [Google Scholar]
- Zink C.F., Tong Y., Chen Q., Bassett D.S., Stein J.L. Meyer‐Lindenberg A. (2008) Know your place: neural processing of social hierarchy in humans. Neuron, 58(2) 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


