Abstract
Depression, alcohol use disorders and post‐traumatic stress disorder (PTSD) are serious issues among military personnel due to their impact on operational capability and individual well‐being. Several military forces screen for these disorders using scales including the Kessler Psychological Distress Scale (K10), Alcohol Use Disorders Identification Test (AUDIT), and Post‐traumatic Stress Disorder Checklist (PCL). However, it is unknown whether established cutoffs apply to military populations. This study is the first to test the diagnostic accuracy of these three scales in a population‐based military cohort.
A large sample of currently‐serving Australian Defence Force (ADF) Navy, Army and Air Force personnel (n = 24,481) completed the K10, AUDIT and PCL‐C (civilian version). Then, a stratified sub‐sample (n = 1798) completed a structured diagnostic interview detecting 30‐day disorder. Data were weighted to represent the ADF population (n = 50,049).
Receiver operating characteristic (ROC) analyses suggested all three scales had acceptable sensitivity and specificity, with areas under the curve from 0.75 to 0.93. AUDIT and K10 screening cutoffs closely paralleled established cutoffs, whereas the PCL‐C screening cutoff resembled that recommended for US military personnel.
These self‐report scales represent a cost‐effective and clinically‐useful means of screening personnel for disorder. Military populations may need lower cutoffs than civilians to screen for PTSD. Copyright © 2014 John Wiley & Sons, Ltd.
Keywords: Military, sensitivity and specificity, K10, PCL, AUDIT
Introduction
Experiencing deployment‐related trauma like direct combat and witnessing atrocities (rather than simply having been deployed) is significantly associated with subsequent disorder in military personnel, including post‐traumatic stress disorder (PTSD), depression and alcohol use disorder (Fear et al., 2010; Hoge et al., 2006; Hoge et al., 2004; Iversen et al., 2008; Sareen et al., 2007). Although mental disorders have negative repercussions for military productivity and personal well‐being (e.g. Erbes et al., 2011; Hoge et al., 2006; Hoge et al., 2002; Hoge et al., 2005; Rona et al., 2009), they often go untreated, seen in the relatively low service use rates among affected personnel (Hoge et al., 2004; Kim et al., 2010; Sareen et al., 2007). Thus, it is imperative that military forces are better able to identify mental disorders. To achieve this, more information is needed regarding the accuracy of military screening instruments; particularly, whether they can sensitively detect mental disorders among personnel, at which cutoffs they function optimally, and where further identification resources should be directed if needed. This study examines the diagnostic accuracy of several commonly‐used scales to screen for mental disorders in a large military sample.
Screening for mental disorders in the military
Several nations (e.g. Canada, the United States, New Zealand, and Australia) conduct mental health screening for personnel returning from deployment, to identify those most likely to benefit from an intensive diagnostic interview, target those at‐risk for education and prevention, and refer disordered personnel to services (Rona et al., 2005; Steele and Twomey, 2008). Various self‐completed questionnaires assess depressive and post‐traumatic stress symptoms and alcohol use; these are often accompanied by brief semi‐structured interviews to contextualize questionnaire responses and provide brief intervention (Steele and Twomey, 2008).
However, the diagnostic accuracy of these screens has not been clearly established in serving personnel; thus, the effectiveness of military screening programmes remains unknown (Dunt, 2009). It is vital that military forces can be confident their screening measures are identifying the correct people, and that benefits of screening outweigh the costs (Rona et al., 2005). Thus, careful implementation must occur alongside thorough testing (Bliese et al., 2008; Dunt, 2009; French et al., 2004; Rona et al., 2004a; Rona et al., 2005; Wright et al., 2005a).
In particular, it cannot be assumed that scale cutoffs derived from civilian samples apply in military settings: personnel may require different cutoffs for reasons like regular scale completion (which may lead to habitual responding, or learning the response pattern needed to avoid follow‐up) and an ethos of ‘fighting through’ distress. Additionally, a significant proportion of personnel perceive that seeking help for problems will result in social stigma, including experiencing career harm, and being stopped from deployment (French et al., 2004; Gould et al., 2010; Hoge et al., 2004; Iversen et al., 2011; McFarlane et al., 2011; Sareen et al., 2007). Relatedly, a greater proportion of personnel screen positive for disorders within de‐identified research than when results are identifiable within post‐deployment screening, with many personnel reporting feeling reluctant to disclose problems during post‐deployment screening (Warner et al., 2011).
Suboptimal cutoffs may result in poor sensitivity (the proportion of disordered personnel who are correctly identified) and/or specificity (the proportion of non‐disordered personnel who are correctly identified), which both have negative repercussions for personnel. Specifically, if cutoffs are too high and sensitivity too low, a significant proportion of psychologically vulnerable personnel may go undetected and sent into combat. Alternatively, low cutoffs accompanied by low specificity may subject disorder‐free personnel to stigmatizing attitudes, and increase the workload of mental health service providers to unmanageable levels. Thus, it is critical to determine optimal cutoffs for military personnel.
The Australian Defence Force (ADF) mental health screen
The Australian Defence Force (ADF) uses the Return to Australia Psychological Screen (RtAPS) upon departing for the area of operations, and the Post‐operational Psychological Screen (POPS) three to six months after returning (Department of Defence, 2008; Dunt, 2009; Steele and Goodman, 2006). These instruments guide a brief universal semi‐structured interview conducted by a mental health professional. Three self‐report scales are used: the Alcohol Use Disorders Identification Test (AUDIT) assesses alcohol consumption (Babor et al., 2001); the Post‐traumatic Stress Disorder Checklist – civilian version (PCL‐C) assesses post‐traumatic stress symptoms (Weathers et al., 1993); and the Kessler Psychological Distress Scale (K10) assesses general psychological distress, meaning it can detect symptoms shared between several common mental disorders, like anxiety and depressive symptoms (Kessler et al., 2002; Department of Defence, 2009; Dunt, 2009). These scales are also used within Defence primary health care and periodic health examinations. The AUDIT and PCL have also been used to screen military personnel internationally (Rona et al., 2004b; Steele and Twomey, 2008; Wright et al., 2005b).
The ADF selected these scales given their validation and use in international military studies and Australian community/veteran populations (Department of Defence, 2009; Nicholson, 2006). However, their diagnostic accuracy – including optimal screening cutoffs – has not been adequately confirmed within currently‐serving military samples.
Diagnostic accuracy of ADF screening scales
The PCL (Weathers et al., 1993) was developed in Vietnam combat veterans. Although it has shown good overall diagnostic accuracy in primary care and veteran samples (see McDonald and Calhoun, 2010), there is evidence that the established screening cutoff score of 50 is not optimal in all populations/settings, with the optimal1 cutoff varying from 30 to 60 (McDonald and Calhoun, 2010). This between‐sample variability highlights the need to obtain validation evidence for the population to be screened. In the only study of currently‐serving military personnel (Bliese et al., 2008), the cutoff of 50 resulted in near‐perfect specificity (0.98) but poor sensitivity (0.24), suggesting that this cutoff would be best used for estimating true population disorder prevalence within epidemiological research. Alternatively, a cutoff of 30 produced high specificity (0.88) and sensitivity (0.78). As data came from mandatory post‐deployment screening, this lower cutoff may have reflected under‐reporting due to fear of social stigma. While Bliese et al.’s (2008) results highlight that military personnel may require different cutoffs, it is unclear whether their results, developed from a relatively small sample (n = 352) of US Army personnel recently‐returned from combat deployment in Iraq, apply to broader military populations, including personnel returned from any operational deployment, as well as personnel who have never been deployed.
The AUDIT (Babor et al., 2001) shows high sensitivity (around the high 80s) in primary care and epidemiological samples using the recommended screening cutoff of eight, with slightly lower though acceptable specificity (Degenhardt et al., 2001; Reinert and Allen, 2002). However, in seemingly the only diagnostic accuracy study in currently‐serving military personnel, a slightly higher optimal cutoff of 10 was found for detecting 12‐month DSM‐IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) alcohol disorder in male Australian Navy veterans (sensitivity = 0.85, specificity = 0.77) (McKenzie et al., 2006).
The K10 (Kessler et al., 2002) shows high levels of overall diagnostic accuracy in numerous population‐level studies, with areas under the curve from 0.80 to 0.96 (Andrews and Slade, 2001; Furukawa et al., 2003; Kessler et al., 2002; Kessler et al., 2003; Oakley Browne et al., 2010). However, its diagnostic accuracy has not been examined in military populations. The slightly shorter K6 demonstrated high specificity in US military personnel, but sensitivity was too low for a military screen (Wright et al., 2007). Importantly, no information regarding sensitivity and specificity of particular cutoffs is available. Two sets of cutoffs are used in Australia: (1) national surveys use scores ≥16 and ≥21 to indicate moderate and high distress, respectively, and (2) primary care settings use scores ≥20 and ≥25 to indicate mild and moderate disorder, respectively (Australian Bureau of Statistics, 2003).
In sum, due to the few military diagnostic accuracy studies (among small and specific samples), and the possibility that military personnel may need distinct cutoffs, there is a great need to establish optimal screening cutoffs in actively‐serving military populations.
The nature of epidemiological cutoffs
These screening scales are also widely used to estimate population prevalence in military epidemiological studies, given it is impractical to administer diagnostic interviews to large samples (e.g. Fear et al., 2010; Riddle et al., 2007). However, optimal screening cutoffs (identified through sensitivity and specificity indices) are often quite different from optimal epidemiological cutoffs, with the distinction between them often misunderstood (McDonald and Calhoun, 2010). For screening, a relatively low cutoff is preferable as it is generally desirable for few true positives to be missed. However, for epidemiological purposes, it is important that the number of false classifications is minimal and those screening positive actually have the disorder, meaning a relatively high cutoff is preferable (McDonald and Calhoun, 2010). Furthermore, the number of incorrect classifications (and thus the optimal epidemiological cutoff) is greatly impacted by population disorder prevalence, so that when prevalence is relatively low (as often seen in military populations e.g. Sareen et al., 2007), screening cutoffs will tend to overestimate prevalence even when sensitivity and specificity are high (McDonald and Calhoun, 2010; Terhakopian et al., 2008). In these cases, higher epidemiological cutoffs are needed, even though they result in lower sensitivity. As these two types of cutoffs are distinct and not interchangeable, it is important to establish both within the same population.
The aim of this study was to examine the diagnostic accuracy of these three screening scales (the AUDIT, PCL‐C, and K10) against ‘gold standard’ structured diagnostic interviews. This is the first study to assess these scales in a large and representative sample of actively‐serving military personnel. While our primary aim within the context of military screening was to establish optimal screening cutoffs, a secondary aim was to identify optimal epidemiological cutoffs, after establishing the prevalence of disorder in this military population.
Method
Participants
Participants came from the 2010 ADF Mental Health Prevalence and Well‐being Study (MHPWS: McFarlane et al., 2011; Van Hooff et al; 2014), which measured the prevalence of mental disorders in a representative sample of currently‐serving ADF personnel. Detailed methodology is described elsewhere (McFarlane et al., 2011; Van Hooff et al., 2014).
A two‐phase assessment was used. First, all currently‐serving ADF personnel in the Navy, Army, and Air Force (as at 11 December 2009) but excluding trainees and reservists were considered eligible and contacted for Phase 1 participation: this was 50,049 personnel. Of these, 24,481 (49% of the ADF population) agreed to participate and completed self‐report questionnaires. Among the remaining 25,568 eligible personnel who did not participate, 76% never responded (and some may not have even read our correspondence); 17% actively declined; and 7% consented, but never completed a questionnaire. Second, a stratified sub‐sample of 3688 (15% of the Phase 1 sample) were sought for Phase 2, of which 1798 (49% response rate) completed a telephone interview. The interview sub‐sample pool was stratified by Service, sex (oversampling for females, to ensure sufficient numbers in each Service), and the combination of members’ Phase 1 screening scores (oversampling for high scorers, to reduce the possibility of error in prevalence estimates by limiting the number without mental disorders). A detailed participant flow chart can be found in the online supplementary material.
Table 1 provides sample demographic characteristics. The sample was predominantly male (76%), and aged, on average, 38.3 years [standard deviation (SD) = 9.4]. The sample comprised members from all Services (40% Army, 39% Air Force, and 21% Navy) and ranks (49% commissioned officers, 36% non‐commissioned officers, and 14% other ranks). Compared with the total ADF population, respondents were slightly older and had served for longer, comprised a greater proportion of personnel who were married and in the Army, and a smaller proportion of males, deployed personnel, and personnel in other ranks. This sample was not intended to resemble the ADF population, as females and those with higher screening questionnaire scores were oversampled. Moreover, these observed differences were subsequently used in the population weighting process, so that the estimates generated effectively represented the entire ADF population.
Table 1.
Demographic characteristics of study respondents and the total ADF population
| Variable | Phase 2 study sample (n = 1798) | Total ADF population (n = 50,049) |
|---|---|---|
| Age | 38.3 (9.4) | 33.2 (9.2) |
| Male (%) | 75.6 | 86.4 |
| Service | ||
| Army (%) | 39.8 | 50.7 |
| Navy (%) | 21.4 | 23.2 |
| Air Force (%) | 38.8 | 26.1 |
| Rank | ||
| Commissioned officer (%) | 36.4 | 24.0 |
| Non‐commissioned officer (%) | 49.4 | 44.6 |
| Other ranks (%) | 14.1 | 31.4 |
| Time in ADF (years) | 16.2 (9.8) | 11.6 (8.8) |
| Been deployed (%) | 61.8 | 65.4 |
| Married (%) | 77.2 | 62.9 |
| Highest educational qualifications | ||
| High school or less (%) | 13.1 | —a |
| Certificate/diploma (%) | 36.9 | —a |
| University degree (%) | 50.0 | —a |
| MEC status | ||
| MEC 1 (%) | 50.4 | 65.6 |
| MEC 2 (%) | 34.0 | 23.4 |
| MEC 3 (%) | 12.5 | 8.9 |
| MEC 4 (%) | 3.2 | 2.1 |
Note: ADF, Australian Defence Force; MEC, medical employment classification (MEC 1/2 = fit to deploy, MEC 3/4 = unfit to deploy).
Unable to determine education of non‐responders.
Measures
Screening scales (the index tests)
Alcohol Use Disorders Identification Test (AUDIT)
The AUDIT comprises 10 questions on alcohol consumption, dependence and problems, typically or in the last 12 months. Total scores range from zero to 40, with higher scores indicating more problematic alcohol consumption. The AUDIT demonstrates high internal consistency, factorial convergent and criterion validity (Allen et al., 1997; Degenhardt et al., 2001; Reinert and Allen, 2002). Internal consistency was good in our sample (alpha = 0.75). The ADF uses a screening cutoff of eight (warranting simple advice), with scores above 20 resulting in comprehensive assessment and referral to drug/alcohol services (Department of Defence, 2009).
Post‐traumatic Stress Disorder Checklist – civilian version (PCL‐C)
The ADF uses the PCL‐C (Weathers et al., 1993) as it allows members’ ratings to be based on any trauma, not just trauma experienced during military service (Nicholson, 2006). The 17 questions correspond with the DSM‐IV PTSD symptomatic criteria. Respondents rate these symptoms in the past month which, once summed, give a total score ranging from 17 to 85, with higher scores indicating higher levels of PTSD symptoms. Overall, the PCL shows high validity and reliability (McDonald and Calhoun, 2010; Wilkins et al., 2011). Internal consistency was excellent in our sample (alpha = 0.95). The ADF uses a screening cutoff (indicating the need for psychologist follow‐up) of 30, with scores above 50 triggering automatic referral (Department of Defence, 2009; Nicholson, 2006).
Kessler Psychological Distress Scale (K10)
As a measure of general psychological distress, the K10 (Kessler et al., 2002) detects symptoms found in several common disorders, including depressive and anxiety symptomatology. Participants rate the 10 questions in reference to the last four weeks. Total scores range from 10 to 50,2 with higher scores indicating higher psychological distress. The K10 is widely used in clinical screening and epidemiological research, shows high factorial validity and internal consistency, and performs as well as/better than other relevant questionnaires (Andrews and Slade, 2001; Baillie, 2005; Furukawa et al., 2003; Hides et al., 2007; Kessler et al., 2002; Kessler and Üstün, 2004). Internal consistency was excellent in our sample (alpha = 0.91). The ADF uses a screening cutoff of 20 (indicating the need for follow‐up, and potential referral) (Department of Defence, 2009; McFarlane et al., 2011).
Structured diagnostic interview (the reference standard)
Selected sections of the computerized Composite International Diagnostic Interview 3.0 (CIDI: Kessler and Üstün, 2004) were administered by trained Psychology (Honours) graduates via telephone. The modules administered were depression, mania, panic disorder, specific and social phobia, agoraphobia, generalized anxiety disorder, obsessive‐compulsive disorder, PTSD, alcohol use, tobacco, and separation anxiety (although the last two modules were not used here); all other CIDI modules (e.g. psychosis, personality) were not administered. The World Health Organization's International Classification of Diseases system (ICD‐10: World Health Organization, 1992) was used to diagnose 30‐day anxiety disorder, affective disorder, PTSD, alcohol harmful use,3 and alcohol dependence. The CIDI is widely used in epidemiological surveys, and shows high convergent and predictive validity (Haro et al., 2006).
Procedure
Data collection spanned April 2010 and January 2011. In Phase 1, personnel were contacted by email and mail, to seek participation and distribute study materials. Emails, letters, defence base visits and telephone calls followed‐up non‐respondents. For Phase 2, the Phase 1 participants who were selected as eligible for the CIDI interview sample (through the abovementioned stratified sampling process) were telephoned and invited to complete a telephone interview. Only those who could be interviewed within 60 days of completing their questionnaire were eligible. At most, 10 phone call attempts were made (as well as two recorded telephone messages). Informed consent was digitally recorded via telephone. Interviewers were blind to participants’ screening scores. On average, 42 days (SD = 25.3) elapsed between survey and interview completion. Interviews took, on average, 30 minutes for non‐symptomatic and 60 minutes for symptomatic personnel.
This study was approved by the Australian Defence Human Research Ethics Committee, the University of Queensland Behavioural and Social Sciences Ethical Review Committee, the Department of Veterans’ Affairs Human Research Ethics Committee and the University of Adelaide Human Research Ethics Committee.
Statistical analyses
Statistical analyses were conducted in SAS version 9.2 and Stata version 11.2. Data were weighted to correct for differential non‐response, and obtain prevalence estimates for the entire ADF population. Questionnaire results were weighted by sex, Service, rank and medical employment classification (MEC) status. CIDI results were weighted using the interview selection strata (Service, sex and Phase 1 screening scores). Within each stratum the weight was calculated as the population size divided by the number of stratum respondents. A finite population correction was also applied to adjust variance estimates for the reasonably large sampling fraction within each stratum.
Receiver operating characteristic (ROC) analysis evaluated screening scale cutoffs for detecting 30‐day ICD‐10 disorders (the criterion variables). Diagnostic accuracy was evaluated with respect to: (1) the area under the ROC curve (or AUROC, representing the probability that a randomly selected participant with the specified disorder scores higher than a randomly selected member without the disorder); (2) sensitivity (the probability of accurately detecting those with a specified disorder using the specified cutoff); (3) specificity (the probability of correctly identifying those who do not have the specified disorder using the specified cutoff); (4) overall diagnostic efficiency (the proportion of the total sample that has been correctly identified); (5) positive predictive value (the proportion of those screening positive who have the disorder); (6) negative predictive value (the proportion of those screening negative who do not have the disorder). Weighted estimates of proportions were used to estimate these indices. Jackknife sampling was used for the estimation of AUROC and standard error.
Using ROC analysis, we identified two optimal cutoffs for each scale, corresponding with our primary and secondary aims:
The screening cutoff maximized the sum of the sensitivity and specificity (the proportion of those with and without the disorder that are correctly classified), and is suited to identify personnel who might need care.
The epidemiological cutoff brought the number of false positives (incorrect disorder identifications) and false negatives (missed disorder identifications) closest together, counterbalancing these sources of error most accurately. Therefore, this cutoff would give the closest estimate to the true prevalence of 30‐day disorder, and is suited to monitor trends.
Epidemiological cutoffs are always higher than screening cutoffs as they aim to identify only those with clinical disorders, whereas screening cutoffs are designed to be more inclusive, given that any false positives may be ruled out following diagnostic interview.
Results
Descriptive statistics are provided in Tables 2 and 3. Military personnel showed low symptomatology according to the screening scales, with mean values towards lower scale limits. The prevalence of 30‐day disorder ranged from 9.2% for any anxiety/affective disorder, to 0.2% for alcohol harmful use.
Table 2.
Mental health screening scale scores (n = 50,049)
| Variable | Mean (M) or % (95% CI) |
|---|---|
| Levels of mental health problems | |
| AUDIT | 6.0 (5.9, 6.0) |
| Zone II or above (8+) % | 26.4% (25.5, 27.2) |
| Zone IV or above (20+) % | 1.4% (1.2, 1.5) |
| PCL | 22.7 (22.6, 22.8) |
| At least moderate (30+) % | 15.4% (14.7, 16.0) |
| Very high (50+) % | 3.0% (2.8, 3.1) |
| K10 | 15.4 (15.3, 15.5) |
| At least ‘moderate’ (16+) % | 35.4% (34.3, 36.3) |
| At least ‘high’ (22+) % | 12.9% (12.3, 13.4) |
Table 3.
Prevalence of CIDI ICD‐10 30‐day disorder (n = 50,049)
| 30‐day disorder variable | % (95% CI) |
|---|---|
| Any anxiety or affective disorder | 9.1% (6.8, 11.4) |
| Any anxiety disorder | 7.5% (5.4, 9.7) |
| Any affective disorder | 2.6% (1.9, 3.4) |
| Post‐traumatic stress disorder (PTSD) | 3.4% (2.6, 4.2) |
| Any alcohol disorder | 1.0% (0.5, 1.4) |
| Alcohol harmful use | 0.2% (0.0, 0.5) |
| Alcohol dependence | 0.8% (0.3, 1.2) |
We present abridged diagnostic accuracy tables, which include the optimal screening and epidemiological scores, and one score above and below each cutoff (full tables available upon request).
Alcohol Use Disorders Identification Test (AUDIT)
Table 4 presents optimal AUDIT scores for detecting 30‐day ICD‐10 alcohol harmful use/dependence.
Table 4.
Properties of the AUDIT for predicting 30‐day ICD‐10 alcohol disorders
| Sensitivity | Specificity | PPV | NPV | Overall efficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff | Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI |
| Alcohol harmful use | ||||||||||
| 7 | 1.00 | 1.00–1.00 | 0.69 | 0.66–0.72 | 0.01 | 0.00–0.01 | 1.00 | 1.00–1.00 | 0.69 | 0.66 – 0.72 |
| 8a | 1.00 | 1.00–1.00 | 0.75 | 0.73–0.78 | 0.01 | 0.00–0.02 | 1.00 | 1.00–1.00 | 0.75 | 0.73 – 0.78 |
| 9 | 0.57 | 0.07–1.00 | 0.82 | 0.80–0.84 | 0.01 | 0.00–0.01 | 1.00 | 1.00–1.00 | 0.82 | 0.80 – 0.84 |
| … | ||||||||||
| 25 | 0.00 | 0.00–0.00 | 1.00 | 0.99–1.00 | 0.00 | 0.00–0.00 | 1.00 | 1.00–1.00 | 0.99 | 0.99 – 1.00 |
| 26b | 0.00 | 0.00–0.00 | 1.00 | 1.00–1.00 | 0.00 | 0.00–0.00 | 1.00 | 1.00–1.00 | 0.99 | 0.99 – 1.00 |
| 27 | 0.00 | 0.00–0.00 | 1.00 | 1.00–1.00 | 0.00 | 0.00–0.00 | 1.00 | 1.00–1.00 | 0.99 | 0.99 – 1.00 |
| Alcohol dependence | ||||||||||
| 8 | 0.94 | 0.85–1.00 | 0.76 | 0.73–0.78 | 0.03 | 0.01–0.04 | 1.00 | 1.00–1.00 | 0.76 | 0.73–0.78 |
| 9a | 0.91 | 0.81–1.00 | 0.83 | 0.81–0.85 | 0.04 | 0.02–0.06 | 1.00 | 1.00–1.00 | 0.83 | 0.81–0.85 |
| 10 | 0.80 | 0.59–1.00 | 0.86 | 0.84–0.88 | 0.04 | 0.02–0.07 | 1.00 | 1.00–1.00 | 0.86 | 0.84–0.87 |
| … | ||||||||||
| 20 | 0.23 | 0.01–0.45 | 0.99 | 0.99–1.00 | 0.20 | 0.01–0.39 | 0.99 | 0.99–1.00 | 0.99 | 0.98–0.99 |
| 21b | 0.08 | 0.01–0.18 | 0.99 | 0.99–1.00 | 0.09 | 0.02–0.19 | 0.99 | 0.99–1.00 | 0.99 | 0.98–0.99 |
| 22 | 0.08 | 0.01–0.18 | 0.99 | 0.99–1.00 | 0.11 | 0.02–0.23 | 0.99 | 0.99–1.00 | 0.99 | 0.98–0.99 |
| Any alcohol disorder | ||||||||||
| 7 | 1.00 | 1.00–1.00 | 0.70 | 0.66–0.73 | 0.03 | 0.02–0.05 | 1.00 | 1.00–1.00 | 0.70 | 0.67–0.73 |
| 8a | 0.95 | 0.89–1.00 | 0.76 | 0.73–0.78 | 0.04 | 0.02–0.06 | 1.00 | 1.00–1.00 | 0.76 | 0.73–0.79 |
| 9 | 0.83 | 0.64–1.00 | 0.83 | 0.81–0.85 | 0.05 | 0.02–0.07 | 1.00 | 1.00–1.00 | 0.83 | 0.81–0.85 |
| … | ||||||||||
| 19 | 0.21 | 0.03–0.39 | 0.99 | 0.99–0.99 | 0.17 | 0.03–0.32 | 0.99 | 0.99–1.00 | 0.98 | 0.98–0.99 |
| 20b | 0.19 | 0.02–0.37 | 0.99 | 0.99–1.00 | 0.22 | 0.03–0.41 | 0.99 | 0.99–1.00 | 0.99 | 0.98–0.99 |
| 21 | 0.08 | 0.00–0.16 | 0.99 | 0.99–1.00 | 0.12 | 0.00–0.23 | 0.99 | 0.99–1.00 | 0.98 | 0.98–0.99 |
Optimal screening cutoff.
Optimal epidemiological cutoff.
Note: PPV, positive predictive value; NPV, negative predictive value. The scores above and below the optimal screening and epidemiological cutoffs are also displayed.
The AUROC for detecting any 30‐day ICD‐10 alcohol harmful use was 0.87 [95% confidence interval (CI) 0.72–0.98], indicating good discriminating value. The optimal screening cutoff was eight: using this cutoff, the AUDIT had a sensitivity of 1.00 (95% CI 1.00–1.00), indicating this cutoff would detect 100% of those with alcohol harmful use, and a specificity of 0.75 (95% CI 0.73–0.78), indicating that 75% of those without disorder will score below this cutoff. This cutoff resulted in 12,350 false positive and zero false negative diagnoses. In contrast, the optimal epidemiological cutoff was 26, with a specificity of 1.00 (95% CI 1.00–1.00) (but with zero sensitivity). This cutoff resulted in 109 false positive and 118 false negative diagnoses.
The AUROC for detecting any 30‐day ICD‐10 alcohol dependence was 0.93 (95% CI 0.89–0.97), indicating good discriminating value. The optimal screening cutoff was nine: sensitivity was 0.91 (95% CI 0.81–1.00), and specificity was 0.83 (95% CI 0.81–0.85). This cutoff resulted in 8688 false positive and 34 false negative diagnoses. In contrast, the optimal epidemiological cutoff was 21, with a specificity of 0.99 (95% CI 0.99–1.00) [but a sensitivity of 0.08 (95% CI 0.01–0.18)]. This cutoff resulted in 316 false positive and 347 false negative diagnoses.
The AUROC for detecting 30‐day ICD‐10 any alcohol disorder was 0.91 (95% CI 0.87–0.96), indicating good discriminating value (see Figure 1). The optimal screening cutoff was 8: sensitivity was 0.95 (95% CI 0.89–1.00), and specificity was 0.76 (95% CI 0.73–0.78). This cutoff resulted in 11,996 false positive and 23 false negative diagnoses. In contrast, the optimal epidemiological cutoff was 20, with a specificity of 0.99 (95% CI 0.99–1.00) [but a sensitivity of 0.19 (95% CI 0.02–0.37)]. This cutoff resulted in 343 false positive and 400 false negative diagnoses.
Figure 1.
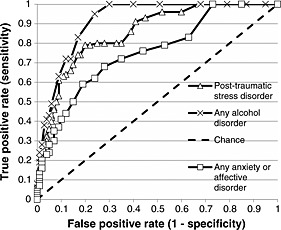
ROC curves predicting 30‐day ICD‐10 disorders from the three screening scales: the AUDIT predicts any alcohol disorder (combining harmful use and dependence); the PCL‐C predicts post‐traumatic stress disorder; and the K10 predicts any anxiety/affective disorder.
Overall, conservative screening and epidemiological cutoff values of eight and 20 (respectively) would identify both alcohol harmful use and dependence.
Post‐traumatic Stress Disorder Checklist – civilian version (PCL‐C)
Table 5 presents optimal PCL‐C scores for detecting 30‐day ICD‐10 PTSD, and Figure 1 presents the ROC curve. The AUROC was 0.85 (CI 95% 0.79–0.91), indicating good discriminating value. The optimal screening cutoff was 29, with a sensitivity of 0.79 (95% CI 0.65–0.92) indicating that 79% of those with PTSD will be detected. The specificity was 0.80 (95% CI 0.77–0.82), indicating that there is an 80% probability that those who do not have PTSD will score below the cutoff. This cutoff resulted in 9897 false positive and 359 false negative diagnoses. In contrast, the optimal epidemiological cutoff was 53, with a specificity of 0.97 (95% CI 0.97–0.98), but a sensitivity of 0.25 (95% CI 0.15–0.35). This cutoff resulted in 1215 false positive and 1247 false negative diagnoses. Using its established cutoff of 50, the PCL‐C showed very low sensitivity (0.30, 95% CI 0.19–0.40) though high specificity (0.97 95% CI 0.96–0.97).
Table 5.
Properties of the PCL‐C for predicting 30‐day ICD‐10 post‐traumatic stress disorder
| Sensitivity | Specificity | PPV | NPV | Overall efficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff | Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI |
| 28 | 0.79 | 0.65–0.92 | 0.78 | 0.75–0.80 | 0.11 | 0.08–0.13 | 0.99 | 0.98–1.00 | 0.78 | 0.75–0.80 |
| 29a | 0.79 | 0.65–0.92 | 0.80 | 0.77–0.82 | 0.12 | 0.09–0.15 | 0.99 | 0.98–1.00 | 0.80 | 0.77–0.82 |
| 30 | 0.74 | 0.60–0.87 | 0.82 | 0.80–0.84 | 0.12 | 0.09–0.15 | 0.99 | 0.98–1.00 | 0.82 | 0.80–0.83 |
| … | ||||||||||
| 50 | 0.30 | 0.19–0.40 | 0.97 | 0.96–0.97 | 0.23 | 0.15–0.31 | 0.98 | 0.97–0.98 | 0.94 | 0.93–0.95 |
| … | ||||||||||
| 52 | 0.28 | 0.18–0.39 | 0.97 | 0.96–0.98 | 0.26 | 0.17–0.35 | 0.98 | 0.97–0.98 | 0.95 | 0.94–0.96 |
| 53b | 0.25 | 0.15–0.35 | 0.97 | 0.97–0.98 | 0.26 | 0.16–0.36 | 0.97 | 0.97–0.98 | 0.95 | 0.94–0.96 |
| 54 | 0.21 | 0.12–0.30 | 0.98 | 0.97–0.98 | 0.24 | 0.14–0.34 | 0.97 | 0.96–0.98 | 0.95 | 0.94–0.96 |
Optimal screening cutoff.
Optimal epidemiological cutoff. PPV = positive predictive value; NPV = negative predictive value. The scores above and below the optimal screening and epidemiological cutoffs are also displayed (as well as the established cutoff of 50).
Kessler Psychological Distress Scale (K10)
Table 6 presents optimal K10 scores for detecting 30‐day ICD‐10 anxiety/affective disorder. The AUROC for detecting any 30‐day anxiety disorder was 0.75 (95% CI 0.60–0.89), indicating fair to good discriminating value. The optimal screening cutoff was 17: sensitivity was 0.68 (95% CI 0.49–0.87), indicating this cutoff would detect 68% of those with anxiety disorder, and specificity was 0.72 (95% CI 0.68–0.75), indicating that 72% of those without anxiety disorder will score below this cutoff. This cutoff resulted in 13,115 false positive and 1210 false negative diagnoses. In contrast, the optimal epidemiological cutoff was 26, with a specificity of 0.95 (95% CI 0.93–0.96) [but a sensitivity of 0.30 (95% CI 0.19–0.40)]. This cutoff resulted in 2470 false positive and 2674 false negative diagnoses.
Table 6.
Properties of the K10 for predicting 30‐day ICD‐10 anxiety and affective disorders
| Cutoff | Sensitivity | Specificity | PPV | NPV | Overall efficiency | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Any anxiety disorder | ||||||||||
| 16 | 0.73 | 0.53–0.93 | 0.64 | 0.60–0.68 | 0.14 | 0.12–0.17 | 0.97 | 0.93–1.00 | 0.65 | 0.61–0.69 |
| 17a | 0.68 | 0.49–0.87 | 0.72 | 0.68–0.75 | 0.16 | 0.13–0.20 | 0.96 | 0.94–0.99 | 0.71 | 0.68–0.75 |
| 18 | 0.61 | 0.44–0.79 | 0.76 | 0.72–0.79 | 0.17 | 0.14–0.21 | 0.96 | 0.93–0.99 | 0.75 | 0.71–0.78 |
| … | ||||||||||
| 20 | 0.49 | 0.35–0.64 | 0.84 | 0.82–0.87 | 0.21 | 0.17–0.25 | 0.95 | 0.93–0.98 | 0.82 | 0.79–0.85 |
| … | ||||||||||
| 25 | 0.31 | 0.21–0.42 | 0.93 | 0.92–0.95 | 0.27 | 0.20–0.34 | 0.94 | 0.92–0.97 | 0.88 | 0.86–0.91 |
| 26b | 0.30 | 0.19–0.40 | 0.95 | 0.93–0.96 | 0.31 | 0.23–0.39 | 0.94 | 0.92–0.97 | 0.90 | 0.87–0.92 |
| 27 | 0.25 | 0.16–0.34 | 0.95 | 0.94–0.96 | 0.30 | 0.21–0.38 | 0.94 | 0.92–0.96 | 0.90 | 0.87–0.92 |
| Any affective disorder | ||||||||||
| 18 | 0.76 | 0.60–0.92 | 0.74 | 0.71–0.77 | 0.07 | 0.05–0.09 | 0.99 | 0.98–1.00 | 0.74 | 0.71–0.77 |
| 19a | 0.75 | 0.59–0.91 | 0.79 | 0.76–0.82 | 0.09 | 0.06–0.11 | 0.99 | 0.98–1.00 | 0.79 | 0.76–0.82 |
| 20 | 0.69 | 0.54–0.85 | 0.83 | 0.81–0.85 | 0.10 | 0.07–0.13 | 0.99 | 0.98–1.00 | 0.83 | 0.81–0.85 |
| … | ||||||||||
| 30 | 0.28 | 0.17–0.40 | 0.97 | 0.97–0.98 | 0.22 | 0.13–0.31 | 0.98 | 0.97–0.99 | 0.95 | 0.94–0.96 |
| 31b | 0.23 | 0.13–0.33 | 0.98 | 0.97–0.98 | 0.21 | 0.13–0.30 | 0.98 | 0.97–0.99 | 0.95 | 0.95–0.97 |
| 32 | 0.17 | 0.10–0.25 | 0.98 | 0.98–0.99 | 0.20 | 0.12–0.28 | 0.98 | 0.97–0.99 | 0.96 | 0.95–0.97 |
| Any anxiety or affective disorder | ||||||||||
| 18 | 0.62 | 0.46–0.77 | 0.76 | 0.73–0.80 | 0.21 | 0.17–0.24 | 0.95 | 0.92–0.98 | 0.75 | 0.71–0.78 |
| 19a | 0.59 | 0.44–0.73 | 0.81 | 0.78–0.84 | 0.24 | 0.19–0.28 | 0.95 | 0.92–0.98 | 0.79 | 0.76–0.82 |
| 20 | 0.50 | 0.37–0.63 | 0.85 | 0.83–0.87 | 0.25 | 0.21–0.29 | 0.94 | 0.92–0.97 | 0.82 | 0.79–0.85 |
| … | ||||||||||
| 24 | 0.35 | 0.25–0.45 | 0.93 | 0.91–0.94 | 0.33 | 0.26–0.40 | 0.93 | 0.91–0.96 | 0.88 | 0.85–0.90 |
| 25b | 0.30 | 0.21–0.39 | 0.93 | 0.92–0.95 | 0.32 | 0.24–0.39 | 0.93 | 0.91–0.96 | 0.88 | 0.85–0.90 |
| 26 | 0.28 | 0.20–0.37 | 0.95 | 0.94–0.96 | 0.36 | 0.27–0.44 | 0.93 | 0.91–0.95 | 0.89 | 0.86–0.91 |
Optimal screening cutoff.
Optimal epidemiological cutoff.
Note: PPV, positive predictive value; NPV, negative predictive value. The scores above and below the optimal screening and epidemiological cutoffs are also displayed (as well as the current ADF cutoff of 20).
The AUROC for detecting any 30‐day ICD‐10 affective disorder was 0.81 (95% CI 0.70–0.91), indicating good discriminating value. The optimal screening cutoff was 19: sensitivity was 0.75 (95% CI 0.59–0.91), and specificity was 0.79 (95% CI 0.76–0.82). This cutoff resulted in 10,207 false positive and 336 false negative diagnoses. In contrast, the optimal epidemiological cutoff was 31 [specificity = 0.98 (95% CI 0.97–0.98), sensitivity = 0.23 (95% CI 0.13–0.33)]. This cutoff resulted in 1117 false positive and 1021 false negative diagnoses. Both of these cutoffs were slightly higher than the cutoff scores identified for any anxiety disorder.
Finally, the AUROC for detecting any 30‐day ICD‐10 anxiety or affective disorder was 0.75 (95% CI 0.63–0.86), indicating fair to good discriminating value (see Figure 1). The optimal screening cutoff was 19: sensitivity was 0.59 (95% CI 0.44–0.73), and specificity was 0.81 (95% CI 0.78–0.84). This cutoff resulted in 8530 false positive and 1883 false negative diagnoses. In contrast, the optimal epidemiological cutoff was 25, with a specificity of 0.93 (95% CI 0.92–0.95) [but a sensitivity of 0.30 (95% CI 0.21–0.39)]. This cutoff resulted in 2974 false positive and 3169 false negative diagnoses.
Overall, conservative screening and epidemiological cutoff values of 17 and 25 (respectively) would identify both anxiety and affective disorders within the ADF population.
Discussion
This is the first study to test the diagnostic validity of three routinely‐used mental health screening scales in a large representative military sample. All scales showed good to excellent levels of overall diagnostic validity, and more specifically, their optimal screening cutoffs could sensitively detect disorder whilst maintaining good specificity (although the degree to which each scale did this differed). In most cases, these screening cutoffs paralleled those already established in other populations. In sum, these scales appear to be useful for military personnel.
The AUDIT showed excellent discriminating ability between military personnel with and without alcohol disorders, particularly alcohol dependence. This is consistent with research in various populations, including Australian Navy personnel (Degenhardt et al., 2001; McKenzie et al., 2006; Reinert and Allen, 2002). The optimal screening cutoff of eight is identical to that recommended by the World Health Organization, used in military research (Fear et al., 2010), and used for ADF screening, though it was slightly lower than the optimal cutoff found in Australian Navy personnel (McKenzie et al., 2006). This cutoff showed excellent sensitivity and good specificity: thus, while the AUDIT may detect the majority of personnel with alcohol disorders (having only missed 34 ADF members within our analyses), it will require rigorous follow‐up interview procedures to screen out the significant number of false positives (up to 13,000 members). Our results support the AUDIT's use (and the cutoff of eight) in military personnel.
The PCL‐C also showed good discrimination between personnel with and without PTSD, consistent with previous research (McDonald and Calhoun, 2010). The optimal screening cutoff of 29 showed a balance of good sensitivity and specificity; results were similar to those in actively‐serving personnel and primary care veterans (Bliese et al., 2008; McDonald and Calhoun, 2010). This cutoff was also similar to the current ADF cutoff of 30 (Department of Defence, 2009; Nicholson, 2006). However, the originally‐recommended cutoff of 50 (Weathers et al., 1993) appeared too high, and did not identify most personnel with PTSD. Bliese et al. (2008) speculated that perceived stigma might explain lower optimal cutoffs in primary care samples (like theirs) and post‐deployment settings, compared with treatment‐seeking or anonymous epidemiological samples. However, this reasoning cannot be applied to our study, as participation was voluntary and results confidential. Perhaps because many personnel knew the questionnaire from post‐deployment screening, they completed it in the same manner, as if it was not confidential. Regardless, it appears that the current ADF screening cutoff of 30 performs well, although a slight score reduction may improve the proportion of correct diagnoses.
The K10 was the least effective, despite showing reasonable diagnostic accuracy overall, with good ability for predicting affective disorders and lower though fair ability for predicting anxiety disorders. This relatively poorer performance was perhaps unsurprising given the K10 was designed to measure non‐specific distress rather than any particular disorder (Kessler et al., 2002). However, the K10 has performed excellently in predicting anxiety and mood disorders in community populations (Andrews and Slade, 2001; Furukawa et al., 2003; Kessler et al., 2002; Kessler et al., 2003; Oakley Browne et al., 2010). Perhaps our sample was more likely to suffer from non‐specific pathology not reflected in formal diagnoses. In the only study assessing cutoffs, the range of ‘optimal screening cutoffs’ (using our criterion) was 16–18, spanning our cutoff of 17 (Andrews and Slade, 2001); however, our cutoff demonstrated lower sensitivity. Our optimal cutoff is also similar to the cutoff of 20 used by Australian primary care clinics and the ADF. Although this score difference was only slight, the established cutoff of 20 showed particularly lower sensitivity, only detecting about half of ADF members with anxiety/affective disorders. Thus, the established cutoff could be slightly lowered to improve the proportion of correct classifications.
Compared with the optimal screening cutoffs, the diagnostic properties of the optimal epidemiological cutoffs highlight the different purposes of these cutoffs. While the epidemiological cutoffs minimized the number of incorrect diagnoses, as our sample showed low disorder prevalence favoured specificity, and the resultant poor sensitivity illustrated these cutoffs are not suited for screening as they are poor predictors of disorders for individuals. As case in point, the optimal PCL‐C epidemiological cutoff of 53 was very close to the originally‐recommended screening cutoff of 50, suggesting that the best use for the original cutoff may be for estimating true population disorder prevalence within military epidemiological research, rather than for screening purposes (as also concluded by Bliese et al., 2008). However, given the low population PTSD prevalence (3.4%), slightly increasing the epidemiological cutoff to 53 would be recommended in order to avoid overestimating prevalence in military personnel (see also McDonald and Calhoun, 2010; Terhakopian et al., 2008). The epidemiological cutoff could also be used to develop screening score bandings to triage personnel, and more efficiently target resources. That is, personnel scoring between screening and epidemiological cutoffs may need cautious and detailed follow‐up to determine the presence of disorder. Many of these personnel may experience transient rather than severe problems (see Wright et al., 2005b), especially in the immediate decompression phase, and may be quickly returned to duty, with regular follow‐up rather than extensive treatment indicated. However, personnel scoring above epidemiological cutoffs might be more quickly referred to appropriate services.
Clinical implications
These results have important clinical implications for military mental health screening. While we defined optimal screening cutoffs as those that maximized the proportion of correct diagnoses (i.e. optimal statistically), optimal cutoffs must ultimately be decided according to each user's needs. Thus, rather than being prescriptive, our results may guide clinical decision‐making regarding the best use of these scales. For example, although the optimal K10 cutoff resulted in the highest proportion of total correct classifications, it favoured specificity, showing less than ideal sensitivity. Given that sensitivity is generally preferred for screening purposes, as false positives may be identified through follow‐up interviews, a lower cutoff score may be preferred for military personnel. A preference for early intervention and managing potential under‐reporting might also lead military forces to consider lower cutoffs. However, in the ADF, a K10 cutoff with a sensitivity of 0.80 or higher would come at the cost of 16,000 additional false positives (assuming an ADF population of 50,049, and that all ADF personnel were screened). Thus, lowering cutoffs would necessitate rigorous follow‐up procedures to detect these false positives while maintaining high confidentiality to avoid exposure to social stigma, and would require greater follow‐up resources. Alternatively, the potential experience of stigma among disorder‐free personnel may be considered too great a cost to increase sensitivity at the expense of specificity, especially if an increased likelihood of screening positive reduced personnel ‘buy‐in’. These and other potential costs and benefits must be considered when selecting cutoffs for military personnel.
Though beyond the scope of our study, scales must also be considered in relation to external criteria to determine their effectiveness within clinical military screening contexts. Importantly, screening will only be optimally effective if it is considered acceptable by personnel. Although ADF post‐deployment screening is, with some exceptions, compulsory regardless of acceptability, personnel may be less inclined to report honestly, and to engage in suggested treatment options if they have low faith in the process. Thus, broader cultural issues like confidentiality, stigma, career repercussions, and availability and efficacy of treatment options should also be assessed for potential improvement. It is possible that by improving personnel's trust in the management of mental disorders, such positive cultural shifts may even slightly reduce reliance on screening, if personnel who recognize they have problems feel free to seek help.
Limitations
Several limitations must be considered. First, participants’ results were not released to the ADF. While confidentiality is essential for maintaining ethical standards, this study does not parallel the circumstances of ADF screening, where members’ results are disclosed to medical officers, and thus can have career implications. It is possible that slightly lower cutoffs may have been found had we conducted this study within standard military screening, as members may under‐report symptoms if they believe that negative consequences will result from screening positive.
Moreover, as our procedures differed from those in the ADF, diagnostic validity may differ somewhat. In our study, interviewers with undergraduate degrees (i.e. not clinically trained) and blind to participants’ screening scores administered structured diagnostic interviews. In contrast, for post‐deployment screening, ADF psychologists and psychological assistants (ADF‐trained, not clinically trained) conduct semi‐structured interviews based on personnel's screening responses, and follow basic guidelines and personal judgement when making decisions. While some aspects of our study may overestimate real‐life validity, others may underestimate it, and determining the net effect is difficult. Future studies could replicate the actual military experience, and assess the scales’ ability to predict referral by ADF psychologists in the context of full disclosure. It is possible that scale validity may be lower in real‐life screening contexts (Rona et al., 2004a).
As the demographic characteristics of the ADF population were known, our use of inverse probability weighting means that results are representative of the entire ADF population. Of course, the weighting process can contain a degree of error as it involves statistically estimating population‐level data from available responders. However, our two‐phase design and stratification strategy reduces the possibility of error and improves prevalence estimate precision by focussing diagnostic interviews on the respondents most likely to have a disorder. Additionally, because the interviewees were drawn from the large proportion of the ADF population who completed the Phase 1 questionnaire, the potential for sampling error was further reduced.
These population‐based results have important implications for military forces internationally. While other forces show different contextual features from the ADF (e.g. US forces have on average younger personnel and fewer officers, and have experienced longer deployments: Fear et al., 2010; Sundin et al., 2013) we would suggest that generalizability of our results would not be substantially impacted given (1) our disorder prevalence rates are not dissimilar to those from other whole‐of‐population military studies (e.g. Sareen et al., 2007; Riddle et al., 2007; Fear et al., 2010) when accounting for the differing assessment methodologies used, and (2) our PCL results resemble those documented by Bliese et al. (2008) in US soldiers. While it is common practice for researchers and clinicians to use cutoffs that were derived in different countries (see Sundin et al., 2013; Terhakopian et al., 2008), we and others recommend that cutoffs always be validated before use in the population of interest to ensure that the scales are working optimally (McDonald and Calhoun, 2010; Terhakopian et al., 2008).
In conclusion, these three scales represent promising options for military screening. However, only long‐term follow‐up within military contexts can determine if their use results in referral to and uptake of needed services, and the reduction of mental disorders.
Declaration of interest statement
HB, NS and SEH received salaries from the Department of Defence in the last five years. Views and opinions expressed within this report are those of the authors, and do not necessarily reflect those of the Department of Defence. While the Department Defence was involved with the study design and data collection, it had no role in the analysis and interpretation of the data, or the decision to submit the manuscript for publication.
Supporting information
Supporting info item
Acknowledgements
The authors thank Dr Alan Verhagen for his contribution to the design of this project, Ms Michelle Lorimer for her statistical assistance, and the many research officers and interviewers who assisted with data collection. This research was funded by the Department of Defence.
Searle A. K., Van Hooff M., McFarlane A. C., Davies C. E., Fairweather‐Schmidt A. K., Hodson S. E., Benassi H., and Steele N. (2015) The validity of military screening for mental health problems: diagnostic accuracy of the PCL, K10 and AUDIT scales in an entire military population, Int. J. Methods Psychiatr. Res., 24, pages 32–45. doi: 10.1002/mpr.1460.
Endnotes:
As there is no one indicator of optimal performance, optimal cutoffs have been defined in various ways, including those that (1) maximize overall efficiency, (2) maximize the sum of sensitivity and specificity, and (3) balance sensitivity and specificity (see McDonald and Calhoun, 2010).
The Australian scoring system is different from the US system, where each response is scored from zero to four, and total scores range from zero to 40 (see http://www.hcp.med.harvard.edu/ncs/k6_scales.php)
Although the specific alcohol use disorder ‘alcohol harmful use’ has also been referred to as ‘alcohol abuse’ (including within the DSM‐IV classification system), we refer to ‘alcohol harmful use’ herein, to be consistent with the World Health Organization ICD‐10 classification used in this paper.
References
- Allen J.P., Litten R.Z., Fertig J.B., Babor T. (1997) A review of research on the Alcohol Use Disorders Identification Checklist (AUDIT). Alcoholism: Clinical and Experimental Research, 21, 613–619. [PubMed] [Google Scholar]
- Andrews G., Slade T. (2001) Interpreting scores on the Kessler Psychological Distress Scale (K10). Australian and New Zealand Journal of Public Health, 25, 494–497. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics . (2003) Information paper: use of the Kessler Psychological Distress Scale in ABS health surveys, Australia 2001. http://www.abs.gov.au/ausstats/abs@.nsf/mf/4817.0.55.001
- Babor T., Higgins‐Biddle J.C., Saunders J., Monteiro M.G. (2001) AUDIT – The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care (2nd edition), Department of Mental Health and Substance Dependence, World Health Organization, Geneva. [Google Scholar]
- Baillie A.J. (2005) Predictive gender and education bias in Kessler's psychological distress scale (K10). Social Psychiatry and Psychiatric Epidemiology, 40, 743–748. [DOI] [PubMed] [Google Scholar]
- Bliese P.D., Wright K.M., Adler A.B., Cabrera O., Castro C.A., Hoge C.W. (2008) Validating the Primary Care Posttraumatic Stress Disorder Screen and the Posttraumatic Stress Disorder Checklist with soldiers returning from combat. Journal of Consulting and Clinical Psychology, 76, 272–281. [DOI] [PubMed] [Google Scholar]
- Degenhardt L., Conigrave K., Wutzke S., Saunders J. (2001) The validity of an Australian modification of the AUDIT questionnaire. Drug and Alcohol Review, 20, 143–154. [Google Scholar]
- Department of Defence . (2008) Defence Instruction (General) Personnel 16–28. Operational Mental Health Screening, Department of Defence, Canberra. [Google Scholar]
- Department of Defence . (2009) Health Bulletin No 11/2009. Mental Health Screen for Casework, Department of Defence, Canberra. [Google Scholar]
- Dunt D. (2009) Review of mental health care in the ADF and transition through discharge. http://www.defence.gov.au/health/DMH/Review.htm.
- Erbes C.R., Meis L.A., Polusny M.A., Compton J.S. (2011) Couple adjustment and posttraumatic stress disorder symptoms in National Guard veterans of the Iraq war. Journal of Family Psychology, 25, 479–487. [DOI] [PubMed] [Google Scholar]
- Fear N.T., Jones M., Murphy D., Hull L., Iversen A.C., Coker B., Machell L., Sundin J., Woodhead C., Jones N., Greenberg N., Landau S., Dandeker C., Rona R.J., Hotopf M., Wessely S. (2010) What are the consequences of deployment to Iraq and Afghanistan on the mental health of the UK armed forces? A cohort study. Lancet, 375, 1783–1797. [DOI] [PubMed] [Google Scholar]
- French C., Rona R., Jones D., Wessely S. (2004) Screening for physical and psychological illness in the British Armed Forces: II. Barriers to screening – learning from the opinions of Service personnel. Journal of Medical Screening, 11, 153–157. [DOI] [PubMed] [Google Scholar]
- Furukawa T.A., Kessler R.C., Slade T., Andrews G. (2003) The performance of the K6 and K10 screening scales for psychological distress in the Australian National Survey of Mental Health and Well‐Being. Psychological Medicine, 33, 357–362. [DOI] [PubMed] [Google Scholar]
- Gould M., Adler A., Zamorski M., Castro C., Hanily N., Steele N., Kearney S., Greenberg N. (2010) Do stigma and other perceived barriers to mental health care differ across Armed Forces? Journal of the Royal Society of Medicine, 103, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro J.M., Saena A.‐B., Brugha T.S., De Girolamo D., Guyer M.E., Jin R., Lepine J.P., Mazzi F., Reneses B., Vilagut G., Sampson N.A., Kessler R.C. (2006) Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health Surveys. International Journal of Methods in Psychiatric Research, 15, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hides L., Lubman D.I., Devlin H., Cotton S., Aitken C., Gibbie T., Hellard M. (2007) Reliability and validity of the Kessler 10 and Patient Health Questionnaire among injecting drug users. Australian and New Zealand Journal of Psychiatry, 41, 166–168. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Auchterlonie J.L., Milliken C.S. (2006) Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA – Journal of the American Medical Association, 295, 1023–1032. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Castro C.A., Messer S.C., McGurk D., Cotting D.I., Koffman R.L. (2004) Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine, 351, 13–22. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Lesikar S.E., Guevara R., Lange J., Brundage J.F., Engel C.C., Messer S.C., Orman D.T. (2002) Mental disorders among U.S. military personnel in the 1990s: Association with high levels of health care utilization and early military attrition. American Journal of Psychiatry, 159, 1576–1583. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Toboni H.E., Messer S.C., Bell N., Amoroso P., Orman D.T. (2005) The occupational burden of mental disorders in the U.S. military: psychiatric hospitalizations, involuntary separations, and disability. American Journal of Psychiatry, 162, 585–591. [DOI] [PubMed] [Google Scholar]
- Iversen A., van Staden L., Hughes J.H., Greenberg N., Hotopf M., Rona R.J., Thomicroft G., Wessely S., Fear N.T. (2011) The stigma of mental health problems and other barriers to care in the UK Armed Forces. BMC Health Services Research, 11, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen A.C., Fear N.T., Ehlers A., Hughes J.H., Hull L., Earnshaw M., Greenberg N., Rona R., Wessely S., Hotopf M. (2008) Risk factors for post‐traumatic stress disorder among UK Armed Forces personnel. Psychological Medicine, 38, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Andrews G., Colpe L.J., Hiripi E., Mroczek D.K., Normand S.L.T., Walters E.E., Zaslavsky A.M. (2002) Short screening scales to monitor population prevalences and trends in non‐specific psychological distress. Psychological Medicine, 32, 959–976. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Barker P.R., Colpe L.J., Epstein J.F., Gfroerer J.C., Hiripi E., Howes M.J., Normand S.L.T., Manderscheid R.W., Walters E.E., Zaslavsky A.M. (2003) Screening for serious mental illness in the general population. Archives of General Psychiatry, 60, 184–189. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Üstün T.B. (2004) The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13, 93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P.Y., Thomas J.L., Wilk J.E., Castro C.A., Hoge C.W. (2010) Stigma, barriers to care, and use of mental health services among active duty and Naitonal Guard soliders after ocmbat. Psychaitric Services, 61, 572–588. [DOI] [PubMed] [Google Scholar]
- McDonald S.D., Calhoun P.S. (2010) The diagnostic accuracy of the PTSD checklist: a review. Clinical Psychology Review, 30, 976–987. [DOI] [PubMed] [Google Scholar]
- McFarlane A.C., Hodson S.E., Van Hooff M., Davies C. (2011) Mental Health in the Australian Defence Force: 2010 ADF Mental Health and Wellbeing Study: Full Report, Department of Defence, Canberra. [Google Scholar]
- McKenzie D.P., McFarlane A.C., Creamer M., Ikin J.F., Forbes A.B., Kelsall H.L., Clarke D.M., Glass D.C., Ittak P., Sim M.R. (2006) Hazardous or harmful alcohol use in Royal Australian Navy veterans of the 1991 Gulf War: identification of high risk subgroups. Addictive Behaviors, 31, 1683–1694. [DOI] [PubMed] [Google Scholar]
- Nicholson C. (2006) A Review of the PTSD Checklist. Research Report 08/2006, Department of Defence, Canberra. [Google Scholar]
- Oakley Browne M.A., Wells J.E., Scott K.M., McGee M.A., for the New Zealand Mental Health Survey Research Team . (2010) The Kessler Psychological Distress Scale in Te Rau Hinengaro: the New Zealand Mental Health Survey. Australian and New Zealand Journal of Psychiatry, 44, 314–322. [DOI] [PubMed] [Google Scholar]
- Reinert D.F., Allen J.P. (2002) The Alcohol Disorders Use Identification Test (AUDIT): a review of recent research. Alcoholism: Clinical and Experimental Research, 26, 272–279. [PubMed] [Google Scholar]
- Riddle J.R., Smith T.C., Smith B., Corbeil T.E., Engel C.C., Wells T.S., Hoge C.W., Adkins J., Zamorski M., Blazer D., for the Millenium Cohort Study Team . (2007) Millennium Cohort: The 2001–2003 baseline prevalence of mental disorders in the U.S. military. Journal of Clinical Epidemiology, 60, 192–201. [DOI] [PubMed] [Google Scholar]
- Rona R., Hooper R., Jones D., French C., Wessely S. (2004a) Screening for physical and psychological illness in the British Armed Forces: III. The value of a questionnaire to assist a Medical Officer to decide who needs help. Journal of Medical Screening, 11, 158–161. [DOI] [PubMed] [Google Scholar]
- Rona R., Hyams K.C., Wessely S. (2005) Screening for psychological illness in military personnel. JAMA – Journal of the American Medical Association, 293, 1257–1260. [DOI] [PubMed] [Google Scholar]
- Rona R., Jones M., Iversen A., Hull L., Greenberg N., Fear N.T., Hotopf M., Wessely S. (2009) The impact of posttraumatic stress disorder on impairment in the UK military at the time of the Iraq war. Journal of Psychiatric Research, 43, 649–655. [DOI] [PubMed] [Google Scholar]
- Rona R.J., Jones M., French C., Hooper R., Wessely S. (2004b) Screening for physical and psychological illness in the British Armed Forces: I. The acceptability of the programme. Journal of Medical Screening, 11, 148–153. [DOI] [PubMed] [Google Scholar]
- Sareen J., Cox B.J., Afifi T.O., Stein M.B., Belik S.‐L., Meadows G., Asmundson G.J.G. (2007) Combat and peacekeeping operations in relation to prevalence of mental disorders and perceived need for mental health care: findings from a large representative sample of military personnel. Archives of General Psychiatry, 64, 843–852. [DOI] [PubMed] [Google Scholar]
- Steele N., Goodman M. (2006) History of ADF Mental Health Screening 1999–2005. Psychology Research and Technology Group Technical Brief 4/2006, Department of Defence, Canberra. [Google Scholar]
- Steele N., Twomey A. (2008) Post‐deployment mental health screning, surveillance and research in TTCP countries, Report for the Technical Cooperation Program Human Resources and Performance Group.
- Sundin J., Herrell R.K., Hoge C.W., Fear N.T., Adler A., Greenberg N., Riviere L., Thomas J., Wessely A., Bliese P. (2013) Mental health outcomes in US and UK military personnel returnin from Iraq. The British Journal of Psychiatry, 203, 1–8. [DOI] [PubMed] [Google Scholar]
- Terhakopian A., Sinaii N., Engel C.C., Schnurr P.P., Hoge C.W. (2008) Estimating population prevalence of posttraumatic stress disorder: an example using the PTSD checklist. Journal of Traumatic Stress, 21(3), 290–300. [DOI] [PubMed] [Google Scholar]
- Van Hooff M., McFarlane A.C., Davies C.E., Searle A.K., Fairweather‐Schmidt A.K., Verhagen A., Benassi H., Hodson S. (2014) The Australian Defence Force Mental Health Prevalence and Wellbeing Study: Design and methods. European Journal of Psychotraumatology, 5, 23950 10.3402/ejpt.v5.23950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C.H., Appenzeller G.N., Grieger T., Belenkiy S., Breitbach J., Parker J., Warner C.M., Hoge C. (2011) Importance of anonymity to encourage honest reporting in mental health screening after combat deployment. Archives of General Psychiatry, 68, 1065–1071. [DOI] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Herman D.S., Huska J.A., Keane T.M. (1993) The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility, Annual meeting of the International Society for Traumatic Stress Studies, San Antonio, Texas.
- Wilkins K.C., Lang A.J., Norman S.B. (2011) Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depression and Anxiety, 28, 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (1992) The International Classification of Diseases: Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. 10, World Health Organization, Geneva. [Google Scholar]
- Wright K.M., Bliese P.D., Adler A.B., Hoge C., Castro C.A., Thomas J.L. (2005a) Letter to the editor: screening for psychological illness in the military. JAMA – Journal of the American Medical Association, 294, 41–42. [DOI] [PubMed] [Google Scholar]
- Wright K.M., Bliese P.D., Thomas J.L., Adler A.B., Eckford R.D., Hoge C. (2007) Contrasting approaches to psychological screening with U.S. combat soldiers. Journal of Traumatic Stress, 20, 965–975. [DOI] [PubMed] [Google Scholar]
- Wright K.M., Thomas J.L., Adler A.B., Ness J.W., Hoge C.W., Castro C.A. (2005b) Psychological screening procedures for deploying US forces. Military Medicine, 170, 555–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


