Abstract
The National Vietnam Veterans Longitudinal Study (NVVLS) is the second assessment of a representative cohort of US veterans who served during the Vietnam War era, either in Vietnam or elsewhere. The cohort was initially surveyed in the National Vietnam Veterans Readjustment Study (NVVRS) from 1984 to 1988 to assess the prevalence, incidence, and effects of post‐traumatic stress disorder (PTSD) and other post‐war problems. The NVVLS sought to re‐interview the cohort to assess the long‐term course of PTSD.
NVVLS data collection began July 3, 2012 and ended May 17, 2013, comprising three components: a mailed health questionnaire, a telephone health survey interview, and, for a probability sample of theater Veterans, a clinical diagnostic telephone interview administered by licensed psychologists. Excluding decedents, 78.8% completed the questionnaire and/or telephone survey, and 55.0% of selected living veterans participated in the clinical interview.
This report provides a description of the NVVLS design and methods. Together, the NVVRS and NVVLS constitute a nationally representative longitudinal study of Vietnam veterans, and extend the NVVRS as a critical resource for scientific and policy analyses for Vietnam veterans, with policy relevance for Iraq and Afghanistan veterans. Copyright © 2015 John Wiley & Sons, Ltd.
Keywords: Vietnam veterans, epidemiology, trauma, PTSD, methodology
Introduction
Background: the national Vietnam veterans readjustment study (NVVRS)
In the decade following the May 17, 1975 proclamation by President Gerald R. Ford that the “Vietnam Era” was over, controversy arose concerning the well‐being of the men and women who served in the war and returned to civilian life. One faction believed that Vietnam veterans had answered the Nation's call, served honorably, and returned successfully to civilian life; another believed that for an important minority of Vietnam veterans “the war was not yet over.” Because both beliefs were based largely on anecdotal evidence, in 1983 Congress passed Public Law 98‐160, which directed the US Department of Veterans Affairs (VA) to contract for an independent national study of “the prevalence, incidence, and effects of post‐traumatic stress disorder (PTSD) and related post‐war psychological problems” among Vietnam veterans (Veterans' Health Care Amendments, 1983).
The National Vietnam Veterans Readjustment Study (NVVRS) was implemented in 1984–1988 as a comprehensive, methodologically rigorous study that included probability samples; a quasi‐experimental design, with two comparison groups (era veterans, who served in the military during the years of the Vietnam War between 1964 and 1975 but not in the war, and civilian counterparts, who did not serve in the military, matched for demographics with the theater veterans; in‐person survey and clinical interviews with veterans; and in‐person interviews with veterans’ spouses. The NVVRS was the only national probability survey of Vietnam veterans and their peers that represented all veterans from this cohort – including men and women from all service branches.
The initial findings from the NVVRS indicated that: (1) although the majority of Vietnam veterans had readjusted to civilian life successfully, 15.2% of the men and 8.5% of the women who served in the war had current PTSD 15 or more years after their service; (2) nearly 30% of men and women met criteria for PTSD at some time after returning from the warzone; (3) those with higher levels of exposure to combat and other warzone stressors were four times more likely to have PTSD than those with lesser exposure; and (4) PTSD from warzone trauma was frequently comorbid with major depression, alcohol use disorders, and many other post‐war readjustment problems. For both males and females, rates of current PTSD for veterans serving in the Vietnam War theater of operations were dramatically higher than rates for Vietnam era veterans (2.5% male, 1.1% female) or civilian counterparts (1.2% males, 0.3% females). Among men who served in the Vietnam theater, substantial differences in current PTSD prevalence were found by minority status, with Hispanics and Blacks more likely to have current PTSD than Whites (27.9% Hispanics, 20.6% Blacks, 13.7% Whites). After controlling for warzone exposure Black–White differences were no longer significant but Hispanics still had higher PTSD rates than the other groups. Vietnam veterans with post‐war psychological problems were more likely to have sought mental health care provided by the VA than those without such problems; however, a substantial proportion (37.5% male; 25% female) of Vietnam theater veterans with PTSD had never used the VA or any other source for their mental health problems (Kulka et al., 1990a, 1990b).
Because of its comprehensive, multidimensional assessment of warzone stress exposure, its broader, more representative and inclusive sample, and its use of multiple methods to assess a wide range of potential post‐war readjustment problems, the NVVRS findings quickly became an important component of the empirical underpinning of federal policies and programs for America's veterans (e.g. Congressional renewal of the Vet Center program in 1988, acceleration of VA's nationwide implementation of PTSD Clinical Teams, a new, comprehensive approach to PTSD care designed by VA's Special Committee on PTSD several years before the NVVRS findings were made public). In literature reviews published in the 1990s, the NVVRS was described as “the most methodologically rigorous epidemiological study of psychological problems ever conducted in the United States” (Keane, 1990, p. 2), and “the classic epidemiological study in the field” (Yehuda and McFarlane, 1995, p. 1707). Additionally, because of its multiple scientific strengths (e.g. probability sampling from military records, high response rates, clinical assessment of key outcomes, multiple quasi‐experimental comparison groups), the findings were embraced by researchers, clinicians, and policy‐makers nationwide.
The release of the NVVRS Public Use Analysis Files (Hunt et al., 1994) further spurred the use of these data to address a broad range of important issues, permitting investigators from a variety of fields, including many VA researchers, to conduct and publish studies of important scientific and clinical issues related to the consequences of warzone stress exposure. More than 60 peer‐reviewed scientific papers have been published based on analyses of the NVVRS Public Use data (listed in a separate file as Supporting Information online).
The NVVRS has also been subject to criticism, some based on misperception of study aims and methods, and others on analysis of other data sources as well as re‐analysis of the public use data set. In a high profile example, Dohrenwend et al. (2006) used data from the NVVRS and other archival sources to validate self‐report measures of warzone stress exposure collected in the NVVRS, and presented alternative “adjusted” PTSD prevalence estimates – based on different definitions, criteria and assumptions – that were somewhat lower than those presented in the original report. Thus, the comprehensive use of multiple measures, modes, methods and criteria to assess PTSD, warzone stress exposure and post‐war readjustment problems in the NVVRS enables researchers to explore empirical questions in a way that no other data source available on Vietnam veterans does. It is reasonable to anticipate that these data will continue to be used for such purposes in the years ahead.
Need for a longitudinal follow‐Up: the national Vietnam veterans longitudinal study (NVVLS)
Although findings from the NVVRS have helped to inform science and policy, the passage of time revealed important questions that NVVRS could not address, due to the fact that the cohorts were not reassessed. These include questions about the course of disorder for warzone‐related PTSD, the relationship of PTSD with physical illnesses, many of which have clinical onset later in life than psychiatric disorders, risk factors for chronic and delayed onset PTSD, and the impact of treatment on the course of PTSD.
Consequently, Congress enacted Public Law 106‐419, directing the VA to contract for an independent follow‐up of the NVVRS cohorts (Veterans Benefits and Health Care Improvement Act, 2000). The mandate for the National Vietnam Veterans Longitudinal Study (NVVLS) focused more narrowly on the course of warzone‐related PTSD; the relationship of PTSD and its frequent psychiatric comorbidities with physical health problems; whether particular Veteran subgroups are at greater risk for chronicity or severity of PTSD; and the effect of mental health and other services on the course of their PTSD.
The purpose of this article is to disseminate a description of the design and methods of the NVVLS as a follow‐up longitudinal study of the cohort to facilitate the continued use of the NVVRS and accelerated use of these new data. Together, these two data sets and their documentation constitute a 25 year representative national longitudinal study of Vietnam veterans, and extend use of the NVVRS as a critical source of data for scientific and policy analyses for this important cohort of the nation's veterans, as well as new cohorts of veterans emerging from Iraq and Afghanistan.
Design and methods of the NVVLS
Overview
The NVVLS is the second wave assessment of a probability sample cohort of US veterans who served in the Vietnam War theater of operations (stationed in Vietnam, Laos, Cambodia, waters in or around these countries, or who flew missions over these areas), and a comparison sample of US veterans who served during the war era but not in the Vietnam theater. As with the NVVRS, the NVVLS was designed to be a “community” (non‐institutionalized) epidemiologic study using probability sampling, survey and clinical assessments, and a non‐equivalent comparison group quasi‐experimental design.
The NVVRS used a two‐stage design developed by psychiatric epidemiologists (e.g. Dohrenwend, 1989; Schlenger et al., 2004) to increase the validity of prevalence estimates obtained in community epidemiologic studies for conditions that have low prevalence in the population, and thus are challenging to detect. In the first stage, all study participants underwent survey interviews that included screening assessments for the primary outcome(s), along with socio‐demographic characteristics and other hypothesized risk and/or resilience factors. In the second stage, those who screened positive for PTSD, and a stratified probability subsample of those who screened negative, were asked to participate in a clinician administered, semi‐structured diagnostic interview.
Sample
The NVVLS sample includes all veterans who participated in the NVVRS who were located and consented to being contacted in the future for research purposes. The original NVVRS sample was constructed by using military records to draw stratified random samples of the 8.3 million veterans who served in the active military during the Vietnam era and were still alive in 1987. At the time of the NVVRS, the research team succeeded in enrolling 2348 Vietnam veterans in the NVVRS including 1632 (83.4% response rate) theater veterans and a comparison group of 716 (76.7% response rate) era veterans. In both groups, specific subgroups were purposefully oversampled (e.g. Black and Hispanic males, females, and wounded Veterans) so that they could be studied with greater precision, and analysis weights were developed to remove bias introduced by the complex sampling procedures and non‐response.
For the NVVLS clinical assessment, we created a clinical subsample – by combining (a) living theater veterans who were eligible to participate in the NVVRS Clinical Interview (n = 346); and (b) a new probability sample drawn from the 58% (n = 421) of NVVRS theater veterans who were not eligible for the Time 1 clinical subsample due to geographic constraints. The addition of this new subsample made the NVVLS clinical sample a probability sample of Vietnam theater veterans, supporting direct estimation of the clinically‐identified prevalence of PTSD and other psychiatric conditions as well as psychometric validation of survey measures. Ultimately, 767 of the 1632 participants from the NVVRS theater sample were selected for the NVVLS clinical subsample and were alive at the time of the feasibility study.
Cohort mortality and locatability between assessments
To address concerns regarding the likelihood of locating NVVRS participants after a 25 year interval with no interim contact, a two‐pronged feasibility study was conducted. First, the team conducted an address search using identifying information from the NVVRS and the secure consolidated credit information from the LexisNexis Credit Information Bureau. Second, we conducted a search of two national death registries to identify deceased cohort members. We obtained medium to high probability location information for 99.7% (n = 2340) of the NVVRS theater veterans, including the participants who were reported as deceased prior to data collection. Hence, we were confident that the NVVLS could successfully be implemented.
Fieldwork
The collection of survey and clinical interview data began on July 3, 2012 and ended on May 17, 2013. The study included the following sequential components.
Phase 1: health questionnaire
A package was mailed to all eligible veterans (n = 1920) containing (a) an introduction letter and (b) a self‐report survey booklet with a pre‐addressed, postage paid envelope for return when completed.
Phase 2: health interview
After returning the health questionnaire, participants were invited to complete a computer assisted self‐report telephone interview, which was conducted by experienced survey interviewers.
Phase 3: clinical interview
After completion of the health interview, the subset of theater veterans who were pre‐selected for the stratified random clinical subsample were contacted to schedule an appointment for the clinical diagnostic interview, conducted over the telephone by an experienced licensed psychologist.
Study recruitment
The Phase 1 enrollment plan included several steps, including sending a personalized introduction letter; maintaining and supplementing a comprehensive contact database; maintenance of a toll‐free number; employing the Dillman method of five contacts (three mailings plus reminder/thank‐you cards) for the health questionnaire (Dillman, 1999); and a telephone follow‐up. Non‐contact non‐response to surveys can be reduced substantially by sending a Field Representative to the home to “knock on the door” and ask the sample member to participate and this method has been employed to enhance response rate in large surveys such as the Decennial Census and the American Community Survey (Heimel, 2011; US Census Bureau, 2009, 2010). Therefore, field representatives were used for location, contact and non‐response follow‐up to boost location and response rates to the first two study phases. An incentive payment of $75 was provided to participants after completion of each of the study phases. During the last four months of data collection, incentive payments were raised to $100 for all eligible participants who had not yet initiated or completed study participation and for those reached by Field Representatives.
Figure 1 describes the sequenced NVVLS data collection process. Although the standard sequence of interviews was a self‐administered health questionnaire followed by the health interviews, this sequence was altered if the subject did not return the mail questionnaire, and was reached first by telephone and was willing to conduct the health interview at that time. In those cases, the health questionnaire was sent again to the study subject. Of the total 1450 participants who completed either the Phase 1 mail or Phase 2 telephone survey, 152 (10.5%) were recruited by Field Representatives, increasing the overall response rate from 70.6% to 78.8%.
Figure 1.
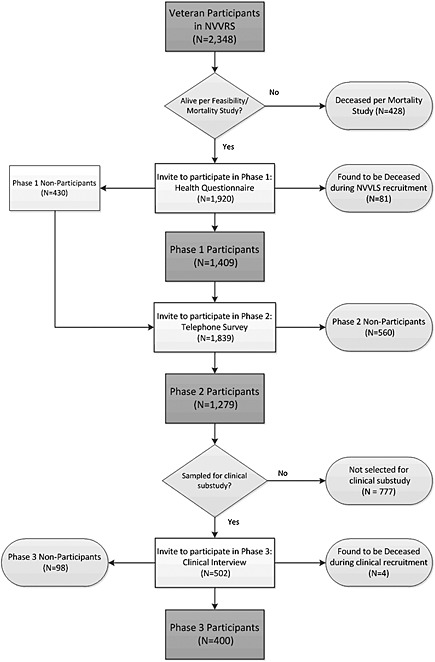
Flowchart of data collection.
Training and assessment calibration
In addition to the specific training provided for the survey and clinical interviewers, all interviewers were trained in protocols for dealing with participant distress, and adverse events were documented and reported to the appropriate oversight entities. In addition, a study clinical psychologist was on call 24 hours a day for the duration of data collection.
Quality control activities were incorporated across study phases, to assure consistent implementation of the study protocol and ascertainment of complete data. The Phase 2 computer assisted telephone interview included a review of study‐specific reports (e.g. call history reports, interviewer comments), embedded quality checks such as verification by interviewer of each key entry before progressing through the interview, as well as supervisor verification of the accuracy of interviewer data recording and live monitoring of 15% of the interviews.
During data collection for the Phase 3 clinical interviews, all sessions were audio recorded and 15% (N = 60) were randomly selected and rescored by independent clinical raters who were blind to the study team diagnoses. The inter‐rater reliabilities were excellent: Kappa = 0.93 for CAPS derived current PTSD diagnosis and Kappa = 1.0 for SCID derived current major depressive disorder. During data collection, the clinical supervisor also conducted weekly calibration meetings with the clinical interviewers to establish diagnostic consensus. Because the psychologists on the team were highly experienced in administering the SCID (structured clinical interview for DSM‐IV), the focus of calibration meetings was on calibration of the new CAPS‐5 (clinician‐administered PTSD scale for DSM‐5). During the first three months of Phase 3, all audio recordings of the CAPS‐5 assessment were reviewed, scored and discussed for adherence by the clinical supervisor and the psychologists conducting the diagnostic assessments. Thereafter, a random sample of the audio recordings was reviewed during calibration meetings and all assessments were reviewed during individual supervision with the clinical supervisor on the study.
Discrepancies that resulted from the evaluation of participants were resolved by group consensus and/or consultation with Dr Frank Weathers, co‐developer of the CAPS‐5, as well as quality control checks and verification of data entry. Dr Weathers joined our calibration meetings by telephone as needed to clarify scoring guidelines and establish scoring rules for the CAPS‐5. We also corresponded with Dr Weathers and his colleagues who were conducting the field studies for the CAPS‐5 to corroborate our scoring with their guidelines.
Measures
Maximum inferential power is attained in longitudinal studies when measures are identical across time, effectively ruling out measurement differences as an alternative explanation of any observed outcome differences. Therefore, assessments for constructs that were germane to the NVVLS research questions and included in the NVVRS were assessed using the same scale(s). Table 1 provides a summary of the constructs and corresponding scales included in both the NVVRS and NVVLS.
Table 1.
National Vietnam Veterans Longitudinal Study (NVVLS) measures administered in both the baseline and follow‐up studies (repeated from National Vietnam Veterans Readjustment Study [NVVRS])
| Construct | Instrument/items (Source)a | NVVLS study phase |
|---|---|---|
| Demographics | ||
| Sex | 1: Health Questionnaire | |
| Date of Birth | 1: Health Questionnaire | |
| Household and Personal Income | 1: Health Questionnaire | |
| Household Composition | 1: Health Questionnaire | |
| Highest Educational Achievement | 2: Health Interview | |
| Marital Satisfaction and Social Support | ||
| Current Marital Status and History | 2: Health Interview | |
| Marital Satisfaction | 2: Health Interview | |
| Social Support (Structural, Socio‐emotional, Instrumental) | 1: Health Questionnaire | |
| Occupational Status | ||
| Health Condition Interfere with Work | 1: Health Questionnaire | |
| Employment Status | 1: Health Questionnaire | |
| Psychological Well‐being | ||
| General Life Satisfaction (Veroff et al., 1981) | 1: Health Questionnaire | |
| Stressful and Traumatic Life Events | ||
| Exposure to and Effects of Stressful Life Events and Traumatic Event Probes (Weiss et al., 1984) | 2: Health Interview | |
| Post‐traumatic Stress Disorder (PTSD) | ||
| Mississippi Scale for Combat‐related PTSD (M‐PTSD; Keane et al., 1988) | 1: Health Questionnaire | |
| Psychiatric Comorbidities b | ||
| Structured Clinical Interview for DSM‐IV (SCID; First et al., 2002)c Overview | 3: Clinical Interview | |
| SCID Screener: Current and Lifetime Diagnoses | 3: Clinical Interview | |
|
SCID Module A. Mood Disorders: Major Depression; Bipolar I/II; Dysthymia; Depressive Disorder not otherwise specified (NOS) |
3: Clinical Interview | |
|
SCID Module F. Anxiety Disorders: Panic Disorder; Social Phobia |
3: Clinical Interview | |
| Substance Use | ||
|
SCID Module E. Substance Use Disorders: Alcohol Use and Dependence Drug Abuse and Dependence |
3: Clinical Interview | |
| Physical Health Status and Health Behaviors | ||
| Perceived Health Status | 1: Health Questionnaire | |
| NHIS Chronic Health Conditions List (Ward et al., 2013) | 2: Health Interview | |
| Disability Days (Bed, Work Loss, Cut‐Down Days) | 2: Health Interview | |
| Functional Limitations | 2: Health Interview | |
| Mental and Physical Health Service Utilization | ||
| Inpatient and Outpatient Health/Mental Health Service Utilization and Reasons for Not Seeking Care (Veroff et al., 1981) | 2: Health Interview | |
For a detailed description of the construction of NVVRS variables derived from these instruments and items, refer to the NVVRS Public Use Analysis File Documentation: Analysis Variables from the National Vietnam Veterans Readjustment Study (Hunt et al., 1994).
Psychiatric comorbidities in the era veterans were assessed with noted screening measures in the mail survey.
Assessed both current and lifetime prevalence. For participants who completed the NVVRS interview, restricted history to period following NVVRS. The NVVRS included the SCID for the DSM‐III‐R.
Additionally, the research protocol included measures that were not in the NVVRS to reflect changes including 25 years of participant aging, changes in diagnostic criteria, and improvements in instrumentation. For example, the NVVRS PTSD assessment was based on the DSM‐III‐R (American Psychiatric Association, APA, 1987) diagnostic criteria, whereas the NVVLS PTSD measures incorporated the most up to date diagnostic criteria from DSM‐5 (American Psychiatric Association, APA, 2013). The measures administered only in the NVVLS are summarized in Table 2, and were used only in cross‐sectional analyses. Altogether, the Phases 1 and 2 each required an average of one hour to complete and the Phase 3 clinical interview averaged three hours.
Table 2.
National Vietnam Veterans Longitudinal Study (NVVLS) measures administered only in the follow‐up study (not repeated from National Vietnam Veterans Readjustment Study [NVVRS])
| Construct | Instrument/items | NVVLS study phase |
|---|---|---|
| Occupational Status | ||
| Concerns About Retirement Item Subset (King et al., 2007) | 1: Health Questionnaire | |
| Cognitive Impairment | ||
| Self‐reported Memory Problems | 2: Health Interview | |
| Telephone Interview for Cognitive Status (TICS; Brandt et al., 1988)a | 2: Health Interview | |
| Military Experience Evaluation | ||
| Positive Appraisal of Military Experiences Scale (PAMES; King et al., 2007) | 1: Health Questionnaire | |
| Late Onset Stress Symptomatology (LOSS)‐11 item short form (Davison et al., 2006; King et al., 2007) | 1: Health Questionnaire | |
| Psychological Well‐being | ||
| Patient Health Questionnaire (PHQ‐9; Kroenke et al., 2001) | 1: Health Questionnaire | |
| Kessler‐6 Plus (K‐6+; Kessler et al., 2003) | 1: Health Questionnaire | |
| Stressful and Traumatic Life Events | ||
| Military Warzone Traumatic Events (Refer to Stressful Events Survey) (Goodman et al., 1998) | 3: Clinical Interview | |
| Post‐traumatic Stress Disorder (PTSD) | ||
| PTSD Checklist – Military Version (PCL‐M; Weathers et al., 1993; Weathers et al., 2013a) | 2: Health Interview | |
| PTSD Checklist – Specific Version (PCL‐S; Weathers et al., 1993; Weathers et al., 2013a) | 2: Health Interview | |
| Post‐traumatic Disorders Scale Items (PDS; Foa, 1996) | 2: Health Interview | |
| Clinician Administered PTSD Scale (CAPS‐5; Weathers et al., 2013b)b | 3: Clinical Interview | |
| Peri‐traumatic Dissociation Experiences Questionnaire (PDEQ‐RV; Marmar et al., 1997) | 3: Clinical Interview | |
| Peri‐traumatic Distress Inventory (PDI‐RV) (Brunet et al., 2001) | 3: Clinical Interview | |
| Self‐reported treatment history for PTSD | 3: Clinical Interview | |
| Substance Use | ||
| Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993) | 1: Health Questionnaire | |
| The Drug Abuse Screening Test (DAST; Skinner, 1982) | 1: Health Questionnaire | |
| Tobacco Use (Substance Abuse and Mental Health Services Administration, 2011) | 2: Health Interview | |
| Physical Health Status and Health Behaviors | ||
| Height | 1: Health Questionnaire | |
| Weight | 1: Health Questionnaire | |
| Recent weight loss/gain | 1: Health Questionnaire | |
| Vision (EPESE) (Cornoni‐Huntley et al., 1993) | 2: Health Interview | |
| Hearing (EPESE) (Cornoni‐Huntley et al., 1993) | 2: Health Interview | |
| Wisconsin Brief Pain Inventory (BPI) (Cleeland, 1989) | 1: Health Questionnaire | |
| Pittsburgh Sleep Quality Index (Buyesse et al., 1989) | 1: Health Questionnaire | |
| Insomnia Severity Index (Morin, 1993) | 1: Health Questionnaire | |
| Mental and Physical Health Service Utilization | ||
| Review of Treatment History | 3: Clinical Interview | |
If respondent endorsed memory problems or screener responses indicated significant impairment, survey interviewer administered full TICS.
Assessed current PTSD only; if respondent did not endorse current PTSD, assessed lifetime PTSD.
Note: EPESE, Established Populations for Epidemiologic Studies of the Elderly.
Phase 1 and 2: PTSD survey measures
Identifying true cases of PTSD is a challenge in the context of a community longitudinal, epidemiologic study. PTSD is a relatively new disorder in the psychiatric lexicon, having first appeared in the 1980 DSM‐III. Substantive changes have been made to PTSD's definition and/or criteria in each of the three DSM revisions that have been made since 1980. Dozens of diverse tools and approaches for identifying PTSD have been developed since 1980, and changes in assessment protocols reduce the internal validity of longitudinal studies. Given these and other challenges, including resource limitations, the NVVLS team implemented a broad, multi‐instrument assessment that included both survey‐based and clinical components, similar but not identical to the NVVRS protocol. The rationale for the assessment strategy was (1) to assess all survey participants with two established self‐report survey measures described in this section, and (2) to create a probability subsample that was also assessed via clinical assessment, administered by an experienced licensed psychologist.
The M‐PTSD symptom‐based scale (Keane et al., 1988) was administered in the NVVLS Phase 1 self‐report Health Questionnaire and was the only PTSD assessment that was also included in the NVVRS (Kulka et al., 1990a, 1990b). The M‐PTSD items were scored on a five‐point Likert scale, with higher scores corresponding to greater symptom severity. The measure can be scored to produce a continuous index of level of PTSD symptomatology and distress, as well as a dichotomous score using empirically established cutoff(s) (Schlenger et al., 1992).
The PTSD Checklist for DSM‐5 (PCL‐5), a self‐report measure of 20 DSM‐5 PTSD symptoms (Weathers et al., 2013a) 16 was administered by survey interviewers to theater veterans with reference to the most distressing warzone incident and for theater and era veterans to the most distressing non‐warzone military and civilian incidents. Given the early stage of development of the PCL‐5 at the time of study initiation, we administered the full PCL‐4 supplemented with new, PCL‐5 items. Specifically, during the Phase 2 survey interview we administered all 17 items of the PCL‐4, plus the additional four PCL‐5 items capturing negative alterations in cognition, behavior, and mood. This allowed us to create both PCL‐4 and PCL‐5 scores for each survey participant The administered PCL is identical to the current PCL‐5 version for 13 of the 20 items, with slight but not substantive wording differences in the other 7 items (all consistent with PCL‐4 assessment of the respective PTSD criteria).
The response categories for the PCL‐5 items were on a five‐point Likert scale (0–4), with higher values indicating more distress. The measure may be scored in several ways, including summing the responses of the 20 items to produce a continuous sum of symptom ratings or counting the number of symptoms after converting the Likert scale rating into symptomatic (e.g. ratings of two to four) versus non‐symptomatic (e.g. ratings of zero or one) to produce a continuous symptom count. The DSM dichotomous scoring methods requires conversion of item responses from a five‐point Likert rating scale into symptomatic versus non‐symptomatic, and scoring the items for each of the DSM Criterion. Because the PCL‐5 does not directly assess DSM‐5 Criterion F (Duration of Symptoms) and Criterion G (Functional Significance), 10 items from the Post‐traumatic Diagnostic Scale (PDS; Foa, 1996) were used to assess symptom duration and functional impairment.
Phase 3: clinical interview diagnostic measures
The NVVLS Phase 3 telephone clinical interview included the following instruments.
Warzone traumatic events list
The Warzone Traumatic Events List assesses the participant's self‐identified “most distressing event” in military warzone experience in the Vietnam War.
Clinician‐administered PTSD scale (CAPS‐5)
The CAPS provides symptom severity assessment and diagnosis as defined by the DSM‐5 (Blake et al., 1995; Weathers et al., 2013b). The CAPS was administered with reference to the cumulative impact of warzone trauma. Resource limitations and concerns about participant burden precluded administering the CAPS to assess PTSD symptoms related to non‐warzone or civilian traumatic incidents.
Structured clinical interview for DSM‐IV, non‐patient version (SCID‐NP)
The SCID‐non‐patient version (SCID‐NP; First et al., 2002) is a structured diagnostic clinical interview for DSM‐IV disorders. The study included the SCID overview screener, Module A: Mood Disorders, Module F: Anxiety Disorders (Panic and Social Phobia only), and Module E: Substance Use Disorders.
Development of weights
Weighting for the NVVRS
Because the NNVLS survey weights are based on weights originally developed for the NVVRS, a brief summary of that process follows. A more detailed description is given in Appendix A of the Contractual Report of Findings from the National Vietnam Veteran Readjustment Study (Kulka et al., 1988). The sample design was developed to permit comparisons among subgroups of the theater veteran population and between the theater veteran population and either the era veterans or the civilian counterparts. The NVVRS response rates were 83.3% for theater veterans, 76.3% for era veterans and over 80% for the clinical examination sample.
The initial sampling weight for a case was computed from the inverse of that case's selection probability: a product of the probability of being selected for the screening sample and the conditional probability of being selected within the sampling strata defined by (a) theater status, (b) sex, (c) race/ethnicity (for male Veterans only, because 94% of female theater veterans were White), (d) occupation (for female veterans only, because 85% were nurses), and (e) age. Post‐stratification adjustments were made to the sampling weights to account for several sources of non‐response, including missing information in personnel records, inability to locate participants, and refusal to participate. The final NVVRS sampling weight for a case accounts for both probability of selection and non‐response.
Weighting for the NVVLS
Phases 1 and 2: mail and telephone surveys
The NVVLS attempted to interview all living veterans from the NVVRS cohort; hence, the basic sample stratification for the NVVLS is the same as for NVVRS veteran sample, and the original response‐adjusted post‐stratified veteran weights developed for NVVRS is the appropriate base weight. Three sets of response‐adjusted weights were created for the NVVLS: (1) a “health questionnaire weight” for the 1409 veterans completing the Phase 1 Health Questionnaire (whether or not they completed the Phase 2 telephone interview); (2) a “health interview weight” for the 1279 veterans completing the Phase 2 Health Interview (whether or not they completed the mail survey); and (3) a “health questionnaire and interview weight” for the 1238 veterans who completed both Phases 1 and 2.
Each of these weights was estimated by multiplying the original NVVRS veteran weight by the ratio of the sum of the weights for the living veterans to the sum of the original weights for those completing Phase 1, 2, or both, with the ratio calculated separately for each of 10 sampling strata. The 10 strata included six levels for men (three race/ethnicity groups by theater and era status) and four for women (nurses and non‐nurses by theater and era status). Thus constructed, the resulting weights sum to the estimated population of living Veterans in each of the sampling strata.
Phase 3: clinical diagnostic telephone interview sample
Estimation of weights for the NVVLS Phase 3 Clinical Subsample also used the original response‐adjusted post‐stratified veteran weights developed for NVVRS as the base weight. Similar to the survey weights, after eliminating those who were deceased at the time of the NVVLS, the weights were adjusted to account for differential sampling across strata defined by risk of PTSD and for non‐response to the NVVLS clinical interview. Only theater veterans were sampled for the NVVLS clinical interview, but, in contrast to the NVVRS, no restrictions were made based on geography. Of the 1632 theater veterans participating in the NVVRS, 743 (45.5%) were geographically eligible for the NVVRS clinical sample, of which 403 (54.2%) were selected for the clinical interview and 344 (85.4%) were interviewed. Of the 889 theater veterans (55.5%) participating in the NVVRS but who were not geographically eligible for the NVVRS clinical interview, 524 (58.9%) were selected for the NVVLS clinical interview.
In order to adjust for non‐response and account for both the NVVRS sampling strata and the clinical sampling strata, a raking ratio estimation technique was used (Battaglia et al., 2009); after raking, the resulting weights summed to the estimated population size of living theater veterans within each stratum (i.e. raking margin).
Data collection results and analysis of non‐response
Disposition results for NVVLS phases 1 and 2
Of the 2348 Vietnam veterans completing the NVVRS survey interviews, 428 (18.2%) had died prior to initiation of the NVVLS, and an additional 81 were identified as deceased during NVVLS data collection. While the proportions of those deceased were approximately the same for theater and era veterans (18.6% and 17.3%, respectively), there were some notable differences by gender, race/ethnicity and warzone stress exposure, and several indicators of high levels of psychological distress. Table 3 shows the final field dispositions for theater and era veterans. Of the 1839 living, eligible veterans, a total of 1450 (78.8%) participated in at least one of the first two study phases: 1238 (67.3%) completed both Phase 1 and Phase 2; 171 (9.3%) completed Phase 1 only; and 41 (2.2%) completed Phase 2 only.
Table 3.
Final National Vietnam Veterans Longitudinal Study (NVVLS) field disposition results for Phases 1 and 2 for those still alive as of the feasibility/mortality search (N = 1920)
|
Theater veterans (N = 1328) |
Era veterans (N = 592) |
All veterans (N = 1920) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Percent excluding Deceased | N | % | Percent excluding Deceased | N | % | Percent excluding Deceased | |
| Completes (Phase l and/or Phase 2) | 1007 | 75.8 | 78.9 | 443 | 74.8 | 78.7 | 1450 | 75.5 | 78.8 |
| Completed Phase 1 and 2 | 863 | 65.0 | 67.6 | 375 | 63.3 | 66.6 | 1,238 | 64.5 | 67.3 |
| Completed Phase 1 only | 117 | 8.8 | 9.2 | 54 | 9.1 | 9.6 | 171 | 8.9 | 9.3 |
| Completed Phase 2 only | 27 | 2.0 | 2.1 | 14 | 2.4 | 2.5 | 41 | 2.1 | 2.2 |
| Not Completed | 321 | 24.2 | 21.1 | 149 | 25.2 | 21.3 | 470 | 24.5 | 21.2 |
| Refused/located & not completed | 116 | 8.7 | 9.1 | 46 | 7.8 | 8.2 | 162 | 8.4 | 8.8 |
| Sick | 27 | 2.0 | 2.1 | 5 | 0.8 | 0.9 | 32 | 1.7 | 1.7 |
| Away/out of country/incarcerated | 6 | 0.5 | 0.5 | 7 | 1.2 | 1.2 | 13 | 0.7 | 0.7 |
| Spanish speaking only | 2 | 0.2 | 0.2 | 2 | 0.3 | 0.4 | 4 | 0.2 | 0.2 |
| Could not be located/no contact | 118 | 8.9 | 9.2 | 60 | 10.1 | 10.7 | 178 | 9.3 | 9.7 |
| Deceased | 52 | 3.9 | 29 | 4.9 | 81 | 4.2 | |||
| Total | 1328 | 100.0 | 592 | 100.0 | 1920 | 100.0 | |||
Disposition results for NVVLS phase 3
For the clinical interview (Phase 3), out of 767 originally selected for the clinical subsample and not identified as deceased during the feasibility study, 36 were found to be deceased during recruitment for the first two study phases, and an additional four were found to be dead when contacted for the clinical interview. Of the remaining 727, 400 (55.0%) began the clinical interview, and 390 completed the full interview. Participation in Phase 3 was contingent on completing Phase 2. Of the 45% who did not participate in the clinical interview, the majority (70%) were non‐respondents prior to even being passed to the clinical interview phase (i.e. in prior phases of data collection). Of the 13.5% who completed Phase 2 but did not participate in the clinical interview, the most prominent reasons for non‐participation were lack of re‐contact (n = 46) and refusals (n = 35).
Unanticipated and adverse events
During data collection, there were 33 “incidents” for which a case report form was completed. The vast majority involved subjects experiencing emotional distress during the Phase 2 Health Interview, typically when describing their worst traumatic event. The research team followed the safety protocol to offer participants the Veterans Helpline, and in very few cases, telephone follow‐up by the on call clinician. All incidents and follow‐up steps were documented and resolved satisfactorily and none of the incidents rose to the level of a serious or reportable adverse event.
Comparison of respondents and non‐respondents to NVVLS phases 1 and 2 for those still living
Table 4 summarizes differences between (a) respondents to both Phases 1 and 2 of the NVVLS (i.e. those who completed both the self‐report Health Questionnaire and survey telephone Health Interview and (b) all non‐respondents (i.e. all sources of non‐response combined). Differences between respondents and non‐respondents were not statistically significant for: theater versus era Veterans; between those selected or not selected for the clinical subsample; warzone stress exposure; demoralization; and current major depression. Differences by race/ethnicity and a high probability of PTSD at the time of the NVVRS were both statistically significant. Statistical differences by sex, age and high PTSD symptomatology with a higher cutoff score were of marginal significance (p‐values ranging from 0.076 to 0.125).
Table 4.
Comparison of respondents and non‐respondents to Phase 1 mail and Phase 2 telephone of the National Vietnam Veterans Longitudinal Study (NVVLS) for those still living (N = 1839)
| Characteristic |
Respondents to Phase 1 and Phase 2 (N = 1238) |
Non‐respondents to Phase 1 and/or Phase 2 (N = 601) |
Chi‐square (df) | p‐Value | ||
|---|---|---|---|---|---|---|
| N | % (SE) | N | % (SE) | |||
| Theater versus Era | ||||||
| Theater | 863 | 67.6 (1.3) | 413 | 32.4 (1.3) | 0.19 (1) | 0.664 |
| Era | 375 | 66.6 (2.0) | 188 | 33.4 (2.0) | ||
| Sex | ||||||
| Men | 816 | 66.0 (1.3) | 421 | 34.0 (1.3) | 3.14 (1) | 0.076 |
| Women | 422 | 70.1 (1.9) | 180 | 29.9 (1.9) | ||
| Age at NVVRS | ||||||
| <40 | 464 | 65.7 (1.8) | 242 | 34.3 (1.8) | 4.22 (2) | 0.121 |
| 40–44 | 554 | 69.9 (1.6) | 239 | 30.1 (1.6) | ||
| 45+ | 220 | 64.7 (2.6) | 120 | 35.3 (2.6) | ||
| Race/ethnicity | ||||||
| Whitea | 838 | 70.1 (1.3) | 358 | 29.9 (1.3) | 15.84 (2) | <.001 |
| Black | 205 | 66.1 (2.7) | 105 | 33.9 (2.7) | ||
| Hispanic | 195 | 58.6 (2.7) | 138 | 41.4 (2.7) | ||
| Selected for clinical subsample b | ||||||
| Yes | 488 | 66.8 (1.7) | 243 | 33.2 (1.7) | 0.60 (1) | 0.440 |
| No | 375 | 68.8 (2.0) | 170 | 31.2 (2.0) | ||
| Warzone stressor exposure b | ||||||
| Low/moderate | 568 | 67.5 (1.6) | 273 | 32.5 (1.6) | 0.03 (1) | 0.870 |
| High | 291 | 68.0 (2.2) | 137 | 32.0 (2.2) | ||
| M‐PTSD with cutoff at 89 c | ||||||
| Positive (M‐PTSD 89+) | 171 | 61.7 (2.9) | 106 | 38.3 (2.9) | 4.60 (1) | 0.032 |
| Negative (M‐PTSD < 89) | 1054 | 68.3 (1.2) | 489 | 31.7 (1.2) | ||
| M‐PTSD with cut‐off at 94 d | ||||||
| Positive (M‐PTSD 94+) | 125 | 62.5 (3.4) | 75 | 37.5 (3.4) | 2.35 (1) | 0.125 |
| Negative (M‐PTSD < 94) | 1100 | 67.9 (1.2) | 520 | 32.1 (1.2) | ||
| Demoralization | ||||||
| High | 197 | 65.2 (2.7) | 105 | 34.8 (2.7) | 0.80 (1) | 0.372 |
| Not high | 1041 | 67.9 (1.2) | 493 | 32.1 (1.2) | ||
| Major depressive episode in last six months | ||||||
| Yes | 39 | 68.4 (6.2) | 18 | 31.6 (6.2) | 0.03 (1) | 0.864 |
| No | 1198 | 67.3 (1.1) | 581 | 32.7 (1.1) | ||
Includes a small number of individuals of other races (N = 28).
Among theater veterans.
Cutoff of 89 was based on findings from the NVVRS Preliminary Validation Study (n = 210), and was used as the screen‐positive level for the clinical subsample (Kulka et al., 1988).
Cutoff of 94 was based on clinical subsample analyses aimed at balancing false positives and false negatives (Kulka et al., 1991).
Comparison of Vietnam theater veteran respondents and non‐respondents to NVVLS phase 3 for those still living
Large, statistically significant differences in participation were apparent by both gender and race/ethnicity for the clinical interview. Among veterans living at the time of the NVVLS, 63.9% of women eligible for the clinical interview completed the assessment compared to only 50.3% of men; and 59.0% of White completed the clinical interview compared with 46.6% and 44.1% of Blacks and Hispanics, respectively. The other variables show substantially smaller differences in clinical study response rates and none are statistically significant. Thus, in spite of a smaller sample size, these comparisons also suggest a potential for non‐response bias in the clinical interview component as well for at least two of these characteristics.
Comparisons of weighted distributions on selected characteristics of respondents to the NVVRS (still living) and respondents to NVVLS phases 1–2 and phase 3
Although these unweighted comparisons of both clinical and survey non‐respondents to those who responded to each of these phases show some significant differences on selected characteristics, a key purpose for using respondent weights and weighted estimates is to adjust for potential non‐response bias (while also adjusting for different probabilities of selection). Thus, to further assess the potential for non‐response bias, it is important to compare weighted estimates for these same characteristics.
The first set of comparisons of these two sets of weighted estimates for the population is shown in Table 5 for the survey Phases 1 and 2. Tests of statistical significance were not computed for these estimates (as one sample is a subset of the other); however, confidence intervals are shown for all estimates and, as these confidence intervals overlap for all estimates, it is reasonable to conclude that for the Phase 1 and 2 survey interviews, the weighting adjustments essentially eliminated these variables as potential sources of non‐response bias.
Table 5.
Comparison of weighted distributions of NVVRS respondents (still living) (N = 1839) to respondents to Phases 1 and 2 of the NVVLS (N = 1238] on selected characteristics
| Characteristic | Alive at NVVLS (N = 1839) | Respondents to Phase 1 and 2 (N = 1238) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unweighted N | Weighted N a | Col %b (95% CI) | Unweighted N | Weighted N c | Col %b (95% CI) | |||
| Theater versus Era | ||||||||
| Theater | 1276 | 2,531,802 | 37.9 | (36.7, 39.0) | 863 | 2,531,802 | 37.9 | (36.6, 39.1) |
| Era | 563 | 4,156,693 | 62.1 | (61.0, 63.3) | 375 | 4,156,693 | 62.1 | (60.9, 63.4) |
| Sex | ||||||||
| Men | 1237 | 6,457,639 | 96.5 | (95.8, 97.3) | 816 | 6,457,639 | 96.5 | (95.7, 97.4) |
| Women | 602 | 230,856 | 3.5 | (2.7, 4.2) | 422 | 230,856 | 3.5 | (2.6, 4.3) |
| Age at NVVRS | ||||||||
| <40 | 706 | 3,451,485 | 51.6 | (49.0, 54.2) | 464 | 3,186,727 | 47.6 | (44.5, 50.8) |
| 40–44 | 793 | 2,380,085 | 35.6 | (32.5, 38.7) | 554 | 2,514,573 | 37.6 | (33.5, 41.7) |
| 45+ | 340 | 856,925 | 12.8 | (10.5, 15.2) | 220 | 987,196 | 14.8 | (11.6, 17.9) |
| Race/ethnicity | ||||||||
| Whited | 1196 | 5,766,155 | 86.2 | (85.4, 87.0) | 838 | 5,761,737 | 86.1 | (84.9, 87.4) |
| Black | 310 | 597,852 | 8.9 | (8.4, 9.4) | 205 | 599,768 | 9.0 | (8.6, 9.4) |
| Hispanic | 333 | 324,489 | 4.9 | (4.0, 5.7) | 195 | 326,990 | 4.9 | (3.7, 6.1) |
| Selected for clinical subsample e | ||||||||
| Yes | 731 | 1,449,495 | 21.7 | (19.9, 23.4) | 488 | 1,418,471 | 21.2 | (19.3, 23.1) |
| No | 545 | 1,082,307 | 16.2 | (14.5, 17.9) | 375 | 1,113,331 | 16.6 | (14.6, 18.7) |
| Warzone stressor exposure e | ||||||||
| Low/moderate | 841 | 1,910,028 | 76.4 | (73.0, 79.9) | 568 | 1,968,102 | 78.4 | (74.5, 82.3) |
| High | 428 | 588,475 | 23.6 | (20.1, 27.0) | 291 | 540,713 | 21.6 | (17.7, 25.5) |
| M‐PTSD with cutoff at 89 f | ||||||||
| Positive (M‐PTSD 89+) | 277 | 899,663 | 13.6 | (10.0, 17.1) | 171 | 912,761 | 13.7 | (9.1, 18.3) |
| Negative (M‐PTSD < 89) | 1543 | 5,738,753 | 86.4 | (82.9, 90.0) | 1,054 | 5,731,835 | 86.3 | (81.7, 90.9) |
| M‐PTSD with cutoff at 94 g | ||||||||
| Positive (M‐PTSD 94+) | 200 | 646,026 | 9.7 | (6.6, 12.9) | 125 | 631,647 | 9.5 | (5.5, 13.5) |
| Negative (M‐PTSD < 94) | 1620 | 5,992,391 | 90.3 | (87.1, 93.4) | 1,100 | 6,012,949 | 90.5 | (86.5, 94.5) |
| Demoralization | ||||||||
| High | 302 | 1,034,080 | 15.5 | (11.6, 19.4) | 197 | 1,025,443 | 15.3 | (10.3, 20.4) |
| Not high | 1534 | 5,643,733 | 84.5 | (80.6, 88.4) | 1,041 | 5,663,053 | 84.7 | (79.6, 89.7) |
| Major depressive episode in last six months | ||||||||
| Yes | 57 | 89,146 | 1.3 | (0.7, 2.0) | 39 | 102,505 | 1.5 | (0.7, 2.4) |
| No | 1779 | 6,590,288 | 98.7 | (98.0, 99.3) | 1,198 | 6,578,599 | 98.5 | (97.6, 99.3) |
Weighted using response‐adjusted post‐stratification weight for veterans from National Vietnam Veterans Readjustment Survey (NVVRS).
Column percentage.
Using the National Vietnam Veterans Longitudinal Survey (NVVLS) weight for respondents to mail and telephone interview.
Includes a small number of individuals of other races (N = 28).
Among theater veterans.
Cutoff of 89 was based on findings from the NVVRS Preliminary Validation Study (n = 210), and was used as the screen‐positive level for the clinical subsample (Kulka et al., 1988).
Cutoff of 94 was based on clinical subsample analyses aimed at balancing false positives and false negatives (Kulka et al., 1991).
However, while overlapping confidence intervals are reasonable proxies for statistical significance, they do not address the issue of practical or substantive significance with regard to the likely importance of the differences observed and their potential biasing effects on estimates. The observed differences in percentages for NVVLS in Table 5 range from a low of 0.0 to a maximum of 4.0 percentage points (most at 0.3 or less). Given that the conventional estimate of bias due to non‐response equals the non‐response rate (32%) times the difference in sample means between respondents and non‐respondents (Groves and Couper, 1998), the maximum observed differences (2.0 and 4.0) imply biases of about 0.64 to 1.28%. While these are important to note, the impact of these biases would likely be minimal on analyses of these data.
Although based on smaller samples, the results for the Phase 3 clinical interview showed a very similar pattern (Table 6). In all cases, after development of weighting adjustments for sample selection probabilities and non‐response, estimates for these characteristics were nearly identical between the two groups, and their confidence intervals overlap substantially, including for the PTSD measures. Although based on smaller samples, the results for the Phase 3 clinical interview showed essentially the same pattern. In all cases, after development of weighting adjustments for sample selection probabilities and non‐response, estimates for these characteristics were nearly identical between the two groups, and their confidence intervals overlap substantially. Thus, after weighting adjustments none of these variables (or those strongly related to them) appears to be important potential sources of non‐response bias. Once again, looking beyond statistical significance, the differences in percentages in Table 6 range from a low of 0.0 to a maximum of 1.8 percentage points (most at 0.3 or less). Based on the conventional estimate of non‐response bias applied in Table 5, with the maximum observed differences ranging from 0.8 to 1.8, and with a non‐response rate of 45% imply biases of about 0.36 to 0.81% suggesting that these differences as well are likely of minimal substantive importance. While these are important to note, the impact of these biases would likely be minimal on analyses of these data.
Table 6.
Comparison of weighted distributions of theater veterans respondents to NVVRS (still living) (N = 1276), to respondents Phase 3 of the NVVLS with complete CAPS (N = 390) on selected characteristics
| Characteristic | Theater veterans still living at NVVLS (N = 1276) | Respondents to clinical interview with complete CAPS (N = 390) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unweighted N | Weighted N a | Percent of living (95% CI) | Unweighted N | Weighted N b | Percent of respondents (95% CI) | |||
| Sex | ||||||||
| Men | 921 | 2,525,720 | 99.8 | (99.7, 99.8) | 275 | 2,502,346 | 99.8 | (99.7, 99.8) |
| Women | 355 | 6,082 | 0.2 | (0.2, 0.3) | 115 | 6,054 | 0.2 | (0.2, 0.3) |
| Age at NVVRS | ||||||||
| <40 | 474 | 1,169,227 | 46.2 | (42.6, 49.8) | 149 | 1,204,200 | 48.0 | (41.2, 54.8) |
| 40–44 | 561 | 1,031,077 | 40.7 | (37.0, 44.4) | 174 | 951,416 | 37.9 | (31.3, 44.5) |
| 45+ | 241 | 331,498 | 13.1 | (11.3, 14.9) | 67 | 352,785 | 14.1 | (10.2, 17.9) |
| Race/ethnicity | ||||||||
| Whitec | 817 | 2,151,473 | 85.0 | (83.6, 86.3) | 261 | 2,129,082 | 84.9 | (82.5, 87.2) |
| Black | 221 | 245,135 | 9.7 | (8.7, 10.7) | 62 | 244,070 | 9.7 | (8.7, 10.7) |
| Hispanic | 238 | 135,194 | 5.3 | (4.4, 6.2) | 67 | 135,248 | 5.4 | (3.3, 7.5) |
| Warzone stressor exposure | ||||||||
| Low/moderate | 841 | 1,910,028 | 76.4 | (73.0, 79.9) | 230 | 1,903,185 | 76.7 | (71.0, 82.5) |
| High | 428 | 588,475 | 23.6 | (20.1, 27.0) | 157 | 576,765 | 23.3 | (17.5, 29.0) |
| M‐PTSD with cutoff at 89 d | ||||||||
| Positive (M‐PTSD 89+) | 247 | 499,775 | 19.8 | (16.4, 23.3) | 124 | 498,667 | 19.9 | (14.9, 24.9) |
| Negative (M‐PTSD < 89) | 1019 | 2,018,502 | 80.2 | (76.7, 83.6) | 264 | 2,004,492 | 80.1 | (75.1, 85.1) |
| M‐PTSD with cutoff at 94 e | ||||||||
| Positive (M‐PTSD 94+) | 179 | 351,721 | 14.0 | (10.9, 17.0) | 87 | 329,318 | 13.2 | (9.1, 17.2) |
| Negative (M‐PTSD <94) | 1087 | 2,166,557 | 86.0 | (83.0, 89.1) | 301 | 2,173,841 | 86.8 | (82.8, 90.9) |
| Demoralization | ||||||||
| High | 229 | 387,364 | 15.4 | (12.4, 18.3) | 91 | 375,654 | 15.0 | (10.0, 20.0) |
| Not high | 1,044 | 2,133,756 | 84.6 | (81.7, 87.6) | 299 | 2,132,747 | 85.0 | (80.0, 90.0) |
| Major depressive episode in last six months | ||||||||
| Yes | 49 | 60,602 | 2.4 | (1.3, 3.6) | 24 | 60,602 | 2.4 | (0.8, 4.0) |
| No | 1224 | 2,462,139 | 97.6 | (96.4, 98.7) | 366 | 2,447,798 | 97.6 | (96.0, 99.2) |
Weighted using response‐adjusted post‐stratification weight for veterans from National Vietnam Veterans Readjustment Survey (NVVRS).
Weighted using National Vietnam Veterans Longitudinal Survey (NVVLS) clinical weight, which accounts for non‐response (including the 10 cases who did not complete the CAPS [clinician‐administered PTSD scale]), based on six raking variables (NVVRS sampling strata, clinical sampling strata, warzone stress exposure, M‐PTSD 89+, high demoralization, and major depressive episode in last six months).
Includes a small number of individuals of other races (N = 28).
Cutoff of 89 was based on findings from the NVVRS Preliminary Validation Study (n = 210), and was used as the screen‐positive level for the clinical subsample (Kulka et al., 1988).
Cutoff of 94 was based on clinical subsample analyses aimed at balancing false positives and false negatives.(Kulka et al., 1991).
Conclusions
Answering questions about the heterogeneous, long‐term course of warzone‐related PTSD and related mental and physical health disorders has become critically important, not only to Vietnam veterans, who currently comprise the largest number of living veterans, but also to a new generation of US veterans of the conflicts in Iraq and Afghanistan. The NVVRS and NVVLS data have a unique capacity to address such questions.
Strengths and limitations
The important strengths of the NVVLS include a relatively high response rate among the cohort and probability sampling, which provides strong external validity (i.e. generalizability of the findings). Excluding those identified as deceased, 67.3% of all eligible veterans completed both the Phase 1 and Phase 2 survey components, and 78.8% completed the mail and/or telephone surveys. This is a remarkable retention rate for a longitudinal follow‐up survey conducted over 25 years after the NVVRS, with no contact with the participants between assessments. Moreover, the participation profiles for theater and era veterans were essentially the same. The NVVLS quasi‐experimental study design with comparison groups also supports the ruling out of important competing hypotheses, and the longitudinal design allows for comparisons in the same subject at two points in time. The team employed comprehensive assessment of behavioral health outcomes with empirically validated measures, including self‐report questionnaires, survey interviews, and clinical interviews.
However, the lengthy interval between the two data collection points is not ideal, diluting our ability to establish the course of illness in detail. Although participation levels were remarkably high given the long interval between the NVVRS and NVVLS, nearly 20% of the cases in the original cohort had died, and others could not be interviewed in the NVVLS for the reasons enumerated earlier, thereby reducing the follow‐up sample sizes and precision of estimates, as well as potentially subjecting results to both known and unknown biases. The 25‐year window between assessments also limits the ability to evaluate the course of illness and to address many questions and hypotheses that have emerged as important issues in the military and among veterans during this period. Relying heavily on participant self‐reports may also raise questions of validity, as does the lack of biomarkers, medical records, and other external sources of information.
Final conclusions
To date, the NVVRS is the most rigorous and comprehensive study of the prevalence of PTSD and other psychological problems in readjusting to civilian life among Vietnam veterans. The publicly available study data has been the basis for a large number of scientific papers. Because the second wave assessment, the NVVLS, employs assessment at two points in time and a registry that records the deaths in the only nationally representative sample of Vietnam veteran cohorts, the study data are more valuable than ever. This paper describes the design and methods of the NVVLS to facilitate the use of these data in future research endeavors and to inform the interpretation of studies based on these data. Several manuscripts are currently in development to address the core questions raised in the congressional mandate (PL 106‐419), including assessing the prevalence and long‐term course of PTSD and psychiatric and physical health comorbidities, examining risk factors for mortality, examining risk factors for PTSD, and assessing service utilization within and outside of the VA among the Vietnam veteran generation.
Declaration of interest statement
The authors have no competing interests.
Supporting information
Supporting info item
Acknowledgements
The National Vietnam Veterans Longitudinal Study (NVVLS) was funded by the US Department of Veterans Affairs (VA), Office of Research and Development, under Contract No. GS‐10 F‐0086 K, Task Order No. VA101‐DO7008. Although the contract was awarded to Abt Associates, the research team comprised multiple partners from collaborating organizations, including colleagues in the Department of Psychiatry at New York University Langone School of Medicine, Abt SRBI, Health Research and Analysis, and HMS Technologies Inc. Together, the team thanks the following VA staff for their support and guidance in conducting the NVVLS: Timothy O'Leary, MD, PhD, F. Alex Chiu, PhD, Theresa Gleason, PhD, and C. Karen Jeans, PhD, CCRN, CIP. Additionally, the authors thank the following individuals for their contributions to the design and methods of the study: Michael Battaglia, PhD (Battaglia Consulting, LLC); Dan G. Blazer II, MD, MPH, PhD (Psychiatry and Behavioral Sciences, Duke University Medical Center); James R. Chromy (RTI International); Sandro Galea, MD, MPH, DrPH (Mailman School of Public Health, Columbia University); Daniel W. King, PhD (Psychological and Brain Sciences, Boston University); Lynda A. King, PhD (Psychological and Brain Sciences, Boston University); Lisa LaVange, PhD (Office of Biostatics, Center for Drug Evaluation and Research, US Food and Drug Administration); and Daniel S. Weiss, PhD (Department of Psychiatry, University of California, San Francisco).
Schlenger, WILLIAM. E. , Corry, NIDA. H. , Kulka, RICHARD. A. , Williams, CHRISTIANNA. S. , Henn‐Haase, C. , and Marmar, CHARLES. R. (2015) Design and methods of the national Vietnam veterans longitudinal study. Int. J. Methods Psychiatr. Res., 24: 186–203. doi: 10.1002/mpr.1469.
Footnotes
For a full account of the NVVRS design and methods, refer to: (1) the final Contractual Report of Findings from the National Vietnam Veterans Readjustment Study (Kulka et al., 1988) available from the US Department of Veterans Affairs); (2) “Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study” (Kulka et al., 1990a); (3) The National Vietnam Veterans Readjustment Study: Tables of Findings and Technical Appendices (Kulka et al., 1990b); and (4) NVVRS Public Use Analysis File Documentation: Analysis Variables from the National Vietnam Veterans Readjustment Study (Hunt et al., 1994).
References
- American Psychiatric Association (APA) . (1987) Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised, Washington, DC, APA. [Google Scholar]
- American Psychiatric Association (APA) . (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edition, Washington, DC, APA. [Google Scholar]
- Battaglia M.P., Izrael D., Hoaglin D.C., Frankel M.R. (2009) Practical considerations in raking survey data. Survey Practice, 2(5). [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. (1995) The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Brandt J., Spencer M., Folstein M. (1988) The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1, 111–117. [Google Scholar]
- Brunet A., Weiss D.S., Metzler T.J., Best S.R., Neylan T.C., Rogers C., Fagan J., Marmar C.R. (2001) The peritraumatic distress inventory: a proposed measure of PTSD criterion a2. American Journal of Psychiatry, 158(9), 1480–1485. [DOI] [PubMed] [Google Scholar]
- Buyesse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Cleeland C.S. (1989) Measurement of pain by subjective report In Chapman C.R., Loeser J.D. (eds) Issues in Pain Measurement, pp. 391–403, New York, Raven Press. [Google Scholar]
- Cornoni‐Huntley J., Ostfeld A.M., Taylor J.O., Wallace R.B., Blazer D., Berkman L.F., Evans D.A., Kohout F.J. (1993) Established populations for epidemiologic studies of the elderly: study design and methodology. Aging (Milano), 5(1), 27–37. [DOI] [PubMed] [Google Scholar]
- Davison E.H., Pless A.P., Gugliucci M.R., King L.A., King D.W., Salgado D.M., Spiro A. III, Bachrach P. (2006) Late‐life emergence of early‐life trauma the phenomenon of late‐onset stress symptomatology among aging combat veterans. Research on Aging, 28(1), 84–114. [Google Scholar]
- Dillman D.A. (1999) Mail and other self‐administered surveys in the 21st Century: The beginning of a New Era. The Gallup Research Journal, 2(1), 121–140. [Google Scholar]
- Dohrenwend B.P. (1989) The problem of validity in field studies of psychological disorders revisited In Robins L.N. (ed.) Validity of Psychiatric Diagnosis, New York, Raven Press. [Google Scholar]
- Dohrenwend B.P., Turner J.B., Turse N.A., Adams B.G., Koenen K.C., Marshall R. (2006) The psychological risks of Vietnam for US veterans: a revisit with new data and methods. Science, 313(5789), 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. (2002) Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐patient Edition (SCID‐I/NP), New York, Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Foa E.B. (1996) PDS Scale Manual/User’s Guide. Minneapolis, MN, National Computer Systems. [Google Scholar]
- Goodman L., Corcoran C., Turner K., Yuan N., Green B. (1998) Assessing traumatic event exposure: general issues and preliminary findings for the Stressful Life Events Screening Questionnaire. Journal of Traumatic Stress, 11(3), 521–542. [DOI] [PubMed] [Google Scholar]
- Groves R.M., Couper M.P. (1998) Nonresponse in Household Interview Surveys, New York, John Wiley & Sons. [Google Scholar]
- Heimel S.K. (2011) Characteristics of the 2010 Census Nonresponse Followup Operation. Paper presented to the Joint Statistical Meetings, Survey Methods Section. See Table 1 http://www.amstat.org/sections/srms/proceedings/y2011/Files/302821_69162.pdf
- Hunt P.N., Schlenger W.E., Jordan B.K., Fairbank J.A., LaVange L.M., Potter F.J. (1994) NVVRS Public Use Analysis File Documentation: Analysis Variables from the National Vietnam Veterans Readjustment Study, Appendix E, Research Triangle Park, NC, Research Triangle Institute. [Google Scholar]
- Keane T.M. (1990) The epidemiology of post‐traumatic stress disorder: some comments and concerns. PTSD Research Quarterly, 1, 1–7. [Google Scholar]
- Keane T.M., Caddell J.M., Taylor K.L. (1988) Mississippi Scale for combat‐related posttraumatic stress disorder: three studies in reliability and validity. Journal of Consulting and Clinical Psychology, 56, 85–90. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Barker P.R., Colpe L.J., Epstein J.F., Gfroerer J.C., Hiripi E., Howes M.J, Normand S.‐L.T., Manderscheid R.W., Walters E.E., Zaslavsky A.M. (2003) Screening for serious mental illness in the general population. Archives of General Psychiatry, 60(2), 184–189. [DOI] [PubMed] [Google Scholar]
- King L.A., King D.W., Vickers K., Davison E.H., Spiro A. III (2007) Assessing late‐onset stress symptomatology among aging male combat veterans. Aging & Mental Health, 11(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Kulka R., Schlenger W., Fairbank J., Hough R., Jordan B., Marmar C., Weiss D. (1988) Contractual Report of Findings from the National Vietnam Veterans Readjustment Study. RTI Project 3035, Research Triangle Park, NC, Research Triangle Institute. [Google Scholar]
- Kulka R., Schlenger W., Fairbank J., Jordan B., Hough R., Marmar C., Weiss D. (1990a) Trauma and the Vietnam War generation: report of findings from the National Vietnam Veterans Readjustment Study, New York, Brunner/Mazel Publishers. [Google Scholar]
- Kulka R., Schlenger W., Fairbank J., Jordan B., Hough R., Marmar C., Weiss D. (1990b) The National Vietnam Veterans Readjustment Study: tables of findings and technical appendices, New York, Brunner/Mazel Publishers. [Google Scholar]
- Kulka R.A., Schlenger W.E., Fairbank J.A., Jordan B.K., Hough R.L., Marmar C., Weiss D.S. (1991) Assessment of PTSD in the community: prospects and pitfalls from recent studies of Vietnam veterans. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 3, 547–560. [Google Scholar]
- Marmar C.R., Weiss D.S., Metzler T.J. (1997) The peritraumatic dissociative experiences questionnaire In Wilson J.P., Keane T.M. (eds) Assessing Psychological Trauma and PTSD, pp. 412–442, New York, Guilford Press. [Google Scholar]
- Morin C.M. (1993) Insomnia: Psychological Assessment and Management, New York, Guilford Press. [Google Scholar]
- Saunders S.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. (1993) Development of the alcohol use disorders identification test (audit): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schlenger W.E., Jordan B.K., Caddell J.M., Ebert L., Fairbank J.A. (2004) Epidemiological methods for assessing trauma and PTSD In Wilson J.P., Keane T.M. (eds) Assessing Psychological Trauma and PTSD, 2nd edition, New York, Guilford Press. [Google Scholar]
- Schlenger W.E., Kulka R.A., Fairbank J.A., Hough R.L., Jordan B.K., Marmar C.R., Weiss D.S. (1992) The prevalence of post‐traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. Journal of Traumatic Stress, 5(3), 333–363, DOI: 10.1002/jts.2490050303 [DOI] [Google Scholar]
- Skinner H.A. (1982) The drug abuse screening test. Addictive Behaviors, 7(4), 363–371. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . (2011) Substance Abuse and Mental Health Services Administration, Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings , NSDUH series H‐41, HHS publication No. (SMA) 11‐4658, Rockville, MD, US Department of Health and Human Services.
- US Census Bureau . (2009) Design and Methodology: American Community Survey , Chapter 7, US Government Printing Office, Washington, DC. https://www.census.gov/acs/www/Downloads/survey_methodology/acs_design_methodology.pdf
- US Census Bureau . (2010) 2010 Census: Cooperation with Enumerators is Critical to a Successful Headcount GAO‐10‐665 T : April 30, 2010. http://www.gao.gov/products/GAO-10-665T.
- Veroff J., Kulka R.A., Douvan E. (1981) Mental Health in America: Patterns of Help‐seeking from 1957 to 1976, New York, Basic Books. [Google Scholar]
- Benefits Veterans, Health Care Improvement Act (2000) Pub. L. No. 106‐419, §212, 114 Stat. 1843 (2000), Washington, DC, Department of Veteran Affairs. [Google Scholar]
- Veterans' Health Care Amendments . (1983) Pub. L. No. 98‐160, §102, 97 Stat. 994 (1983), Washington, DC, Department of Veteran Affairs. [Google Scholar]
- Ward B.W., Schiller J.S. (2013) Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Preventing Chronic Disease, 10, 1202–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Herman D.S., Huska J.A., Keane T.M. (1993) The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility . Paper presented at the 9th Annual Conference of the ISTSS, San Antonio, TX.
- Weathers F.W., Litz B.T., Keane T.M., Palmieri P.A., Marx B.P., Schnurr P.P. (2013a) The PTSD Checklist for DSM‐5 (PCL‐5), Scale available from the National Center for PTSD at http://www.ptsd.va.gov
- Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., Marx B.P., Keane T.M. (2013b) The Clinician‐Administered PTSD Scale for DSM‐5 (CAPS‐5), Interview available from the National Center for PTSD at http://www.ptsd.va.gov
- Weiss D., Horowitz M., Wilner N. (1984) The Stress Response Rating Scale: a clinician's measure for rating the response to serious life events. British Journal of Clinical Psychology, 23, 202–215. [PubMed] [Google Scholar]
- Yehuda R., McFarlane A. (1995) Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. American Journal of Psychiatry, d152, 1702–1713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


