Abstract
Few studies have investigated the natural history of post‐traumatic stress disorder (PTSD). Project VALOR (Veterans' After‐discharge Longitudinal Registry) was designed as a longitudinal patient registry assessing the course of combat‐related PTSD among 1600 male and female Veterans who served in Operation Enduring Freedom (OEF) in Afghanistan or Operation Iraqi Freedom (OIF). Aims of the study include investigating patterns and predictors of progression or remission of PTSD and treatment utilization. The study design was based on recommendations from the Agency for Healthcare Quality and Research for longitudinal disease registries and used a pre‐specified theoretical model to select the measurement domains for data collection and interpretation of forthcoming results. The registry will include 1200 male and female Veterans with a recent diagnosis of PTSD in the Department of Veteran Affairs (VA) electronic medical record and a comparison group of 400 Veterans without a medical record‐based PTSD diagnosis, to also allow for case‐control analyses. Data are collected from administrative databases, electronic medical records, a self‐administered questionnaire, and a semi‐structured diagnostic telephone interview. Project VALOR is a unique and timely registry study that will evaluate the clinical course of PTSD, psychosocial correlates, and health outcomes in a carefully selected cohort of returning OEF/OIF Veterans. Copyright © 2011 John Wiley & Sons, Ltd.
Keywords: post‐traumatic stress disorder (PTSD), study design, disease registry, combat‐exposed Veterans, psychosocial outcomes
Introduction
Post‐traumatic stress disorder (PTSD) is a common and potentially disabling psychiatric disorder that affects a large number of active duty military personnel and Veterans. The prevalence of PTSD in service men and women returning from overseas operations in Afghanistan and Iraq is estimated to be at least 10% immediately post‐deployment, with an approximate doubling of the prevalence within five years after deployment (Hoge et al., 2006; Hoge et al., 2004; Tanielian and Jaycox, 2008). Similar or higher rates are reported in Veterans of previous military conflicts (Richardson et al., 2010; Seal et al., 2009). Despite the prevalence and potential impact of PTSD on multiple areas of patient function (Hoge et al., 2007; Kubzansky et al., 2007; Marx et al., 2009a; Schnurr and Jankowski, 1999; Vasterling et al., 2008), surprisingly little is known about the natural history, course of outcomes, and treatment utilization patterns in returning service members (Wolfe et al., 1999). As a result, diagnostic and treatment services for combat‐exposed Veterans with PTSD may not be adequately allocated, and long‐term health policy and planning in this regard may not be adequately informed.
Epidemiologic studies have identified pre‐ and post‐trauma factors that influence the development of PTSD (Brewin et al., 2000; Ozer et al., 2003). However, the course of PTSD differs across individuals, with some patients recovering quickly and others experiencing symptoms for years or even decades, and the natural history and predictors of remission or progression are not well understood. Knowledge about the efficacy and safety of treatments for PTSD has been obtained from a limited number of randomized controlled trials (RCTs), most of which were performed in non‐Veteran populations (Friedman et al., 2007; Monson et al., 2006; Schnurr et al., 2007; Schnurr et al., 2003). While randomized trials are the “gold standard” for assessing efficacy and/or safety of one treatment over another or a suitable control, observational longitudinal studies, such as patient registries, have some unique advantages, such as their ability to evaluate treatment utilization patterns, outcomes, and factors influencing treatment utilization in a real‐world setting (Conway and Clancy, 2009). Differences in the course of disease for subgroups of individuals, including men versus women, those with multiple versus few exposures, and those who are actively being treated versus those who are not, can also be assessed in a well‐designed patient registry. Moreover, the manner in which treatments for PTSD are applied in everyday practice, including who receives which treatments and for how long, the use of concomitant medical or psychiatric services, and the likely or common psychosocial sequelae, are key areas for assessment, particularly in the context of Veterans with PTSD.
Project VALOR (Veterans' After‐discharge Longitudinal Registry) is an observational patient registry study of PTSD among combat‐exposed Veterans who served in the recent military operations in Iraq and Afghanistan. The objective of the VALOR registry is to provide data on the natural history and outcomes associated with PTSD in Veterans who have utilized the Department of Veteran Affairs (VA) health care system. An additional goal of this project is to determine predictors of a PTSD diagnosis by comparing diagnosed cases to combat‐exposed Veterans without a diagnosis of PTSD.
In this paper, we describe the design and methods of Project VALOR, as well as the strengths, limitations, and challenges of establishing a large patient registry of Veterans with or without a recent medical record‐based diagnosis of PTSD.
Methods
Overview
To guide the selection of study measures, we developed a conceptual model (Figure 1) based on current evidence regarding psychosocial predictors of recovery and outcome in PTSD (Brewin et al., 2000; King et al., 1998; Ozer et al., 2003). Although this model does not consider all possible factors that may influence PTSD, it served to guide the selection of conceptual domains and measurement methods in the study, in addition to providing a general conceptual framework for statistical analyses and theoretical interpretation of the final results. Accordingly, the registry is designed to provide relevant data and to allow an evaluation of current theoretical models of symptom development in a large sample of service men and women who utilize the VA medical system. Diagnostic, demographic, and service‐related data are being collected from existing medical and military records as well as detailed information on symptoms of PTSD and potential risk factors and outcomes from a diagnostic telephone interview and a self‐administered questionnaire. In addition, we plan to collect one‐year follow‐up data through medical record abstraction; further follow‐up, including recontacting study participants, is planned for future years based on continued funding.
Figure 1.
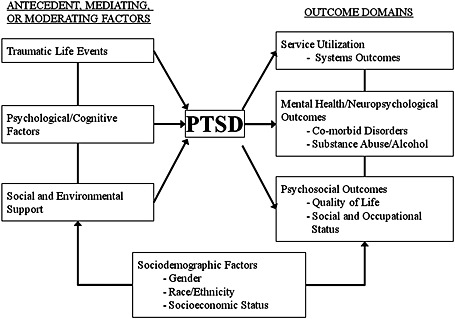
Project VALOR conceptual model.
This project is the result of a joint effort of researchers at the National Center for PTSD at the VA Boston Healthcare System (clinical center), Washington, DC VA Medical Center, and New England Research Institutes, Inc. (NERI) (data and statistical center). The study has received approval from the Institutional Review Boards (IRBs) of all participating institutions as well as the US Army Medical Research and Materiel Command Office of Research Protections.
Study population
The source population for study participants is US Army or Marine Veterans who were deployed to combat in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF) and are in the VA health care system inpatient or outpatient databases. To be eligible for this study, participants must have either: (a) separated from active duty after serving in OEF/OIF or (b) completed at least one Reserve/Guard deployment in support of OEF/OIF. In addition, they must have undergone a mental health evaluation at a VA facility, as indicated by a diagnostic interview or psychotherapy procedure code, between July 2008 and December 2009, and must not currently be participating in a clinical (intervention) trial. From this source population, we will enroll in the study 1200 men and women with a recent diagnosis of PTSD in the VA electronic medical record and 400 men and women without a PTSD diagnosis in the medical record. For the purposes of study enrollment, we defined a diagnosis of PTSD as at least two PTSD diagnoses (primary or secondary ICD‐9‐CM code 309.81) associated with two separate VA visits that occurred on or after the date of the mental health evaluation but before December 2009, and we defined the absence of PTSD as no ICD‐9‐CM code 309.81 in the VA electronic medical record since the beginning of OEF/OIF (2002 health care records). We exclude individuals with a single PTSD diagnosis during the time period of interest to avoid including those with an unconfirmed referral for PTSD or a diagnostic or coding error. Individuals with major depressive disorder, traumatic brain injury (TBI), or other concomitant psychiatric disorders are included in the registry, so that the registry is inclusive of a broad range of comorbidities associated with PTSD. To evaluate predictors of PTSD risk and recovery separately for men and women, we plan to oversample females to provide adequate power for analyses stratified by gender. Approximately equal numbers of male and female Veterans will be included in each diagnostic group, by recruiting eligible participants until gender‐specific enrollment targets are achieved.
Study recruitment
The procedures for recruiting participants are shown in Figure 2. Project VALOR team members from the Washington, DC VA created a roster of potential participants for the PTSD registry and the non‐PTSD comparison group using the inclusion criteria described previously. The initial roster included 3000 men and women with a mental health evaluation (defined earlier) completed between July 2008 and July 2009. The target sample for this roster was 2250 individuals with a diagnosis of PTSD between July 2008 and July 2009 and 750 individuals without a PTSD diagnosis in the VA medical record, with equal numbers of men and women within each group. However, due to a limited number of women who met the inclusion criteria for the non‐PTSD group, the non‐PTSD sample consisted of 527 men and 223 women, while the PTSD sample included equal numbers of men and women. For the second roster of potential participants, we extended the timeframe for selection to December 2009, to increase the number of women without a diagnosis of PTSD who were eligible for the study.
Figure 2.
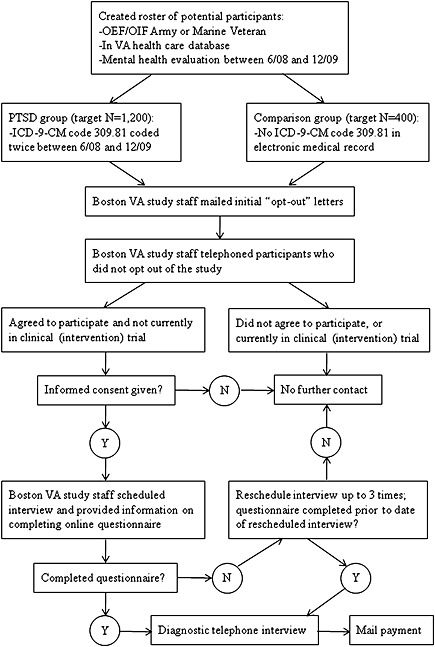
Flowchart of data collection procedures.
VA Boston study staff mail initial “opt‐out” letters to all individuals in the roster, to introduce the study and ask the Veteran whether or not he/she would like further contact about the study. Veterans who request no further contact regarding the study are omitted from the list of potential participants; those who agree to future contact are retained as potential participants. Veterans who do not respond to the initial letter within 30 days are sent a second letter, and those who do not respond to the second letter within 14 days are included in the list of potential participants. Demographic data and service‐related characteristics of Veterans who decline participation are collected through medical record abstraction, for comparison with registry participants.
Participants who do not opt out of the study are telephoned by VA Boston study staff, who provide additional details about the study, assess the exclusion criteria of no current participation in a clinical (intervention) research trial, and formally invite the Veteran to participate in the study. Those who agree to continue are then provided with an opportunity to give their informed consent for participation in the study. Given the online and telephone nature of the study, a Waiver of Documentation of Informed Consent was granted by the local IRB and consent is obtained verbally, to avoid sending personally identifiable information through the mail. Trained research assistants administer standardized, IRB‐approved informed consent and Health Insurance Portability and Accountability Act (HIPAA) release scripts while on the telephone. After informed consent is obtained, the research assistant schedules a date and time for the telephone interview and reminds the participant to complete the self‐administered questionnaire prior to the interview. Participants are compensated $50.00 for their participation in the registry.
Data collection and measures
Medical history data were abstracted from the electronic medical records at baseline and will be abstracted again at year one of follow up. The VA health care system database contains information on all inpatient and outpatient visits, including the ICD‐9 (International Classification of Diseases) and procedure codes to describe the condition being treated and the nature of the visit, respectively. However, the database is structured chronologically by visit, rather than unique patient identifiers or diagnoses, and it offers no readily accessible way to assess the longitudinal outcomes of patients with PTSD or their utilization of VA health care. Therefore, a fundamental objective of this project is to establish a database of patients with and without PTSD from the existing VA utilization database. However, to do this the data must be extracted, transformed into records indexed by patient, and loaded into a new database, together with data imported from other sources (Gliklich and Dreyer, 2010). Other sources of data for the PTSD registry include the OEF/OIF Veteran roster (particularly for military specific data, such as branch, rank, and deployment dates), a self‐administered questionnaire, and a diagnostic interview conducted by telephone (described earlier).
The questionnaire and interview are used to fill key gaps in information obtained from the electronic medical record by assessing factors such as exposure to traumatic events, comorbid symptoms of anxiety or depression, substance abuse, social and occupational status, and overall quality of life. Recognizing the limitations of self‐report data, each of these domains are assessed by means of brief, validated scales which measure current symptoms and outcomes (Table 1). The specific measures were selected based on psychometric criteria (reliability, sensitivity, and specificity), public health and policy relevance, and level of burden for the respondent. Details of the validation and utilization of these measures are provided in the references noted in Table 1. For some measures, we selected items from instruments in development or constructed questions based on empirically‐demonstrated construct relevance.
Table 1.
Project VALOR data collection domains, measures and assessment methods
| Domain | Assessment measure(s) | Project VALOR administration | References |
|---|---|---|---|
| Suicidal ideation | Mini‐International Neuropsychiatric Interview (MINI) suicide module | Telephone interview | (Sheehan et al., 1998) |
| PTSD | Structured Clinical Interview for DSM‐IV (SCID‐IV) PTSD module | Telephone interview | (Spitzer et al., 1992; Williams et al., 1992) |
| Self‐assessed effects of military experiences on post‐discharge life | Two open‐ended questions (qualitative data): “How have your military experiences affected your [ability to do work] or [personal relationships] after you came home?” | Telephone interview | — |
| Traumatic brain injury (TBI) | Defense and Veterans Brain Injury Center TBI questionnaire, modified for brief administration | Telephone interview | (Brown et al., 2005; Levin, 1995; Wilde et al., 2006) |
| Combat exposure | DRRI Section I (Combat Experiences) and Section J (Post‐Battle Experiences) | Self‐administered questionnaire | (King et al., 2006) |
| Quality of life | SF‐12v2 | Self‐administered questionnaire | (Ware et al., 1996) |
| Sleep | Sleep Problem Scale | Self‐administered questionnaire | (Jenkins et al., 1988) |
| Absenteeism | World Health Organization Health and Work Performance Questionnaire (HPQ) | Self‐administered questionnaire | (Kessler et al., 2003) |
| Life stressors/trauma | Life Events Checklist (LEC) | Self‐administered questionnaire | (Gray et al., 2004) |
| Social support | Deployment Risk and Resilience Inventory (DRRI) Section L (Post‐deployment support) | Self‐administered questionnaire | (King et al., 2006) |
| Mental health disorders (including depression, anxiety, panic, somatoform disorder) | Prime‐MD Patient Health Questionnaire (PHQ) | Self‐administered questionnaire | (Spitzer et al., 1999) |
| Alcohol/drug use | Alcohol Use Disorder Identification Test (AUDIT)
Two‐Item Conjoint Screen (TICS) |
Self‐administered questionnaire | AUDIT (Reid et al., 1999; Saunders et al., 1993)
TICS (Brown et al., 2001) |
| Anger/hostility | Dimensions of Anger Reactions, revised short form (DAR‐5) | Self‐administered questionnaire | (Forbes et al., 2004; Hawthorne et al., 2006) |
| Treatment utilization | New questions | Self‐administered questionnaire | — |
| Functional impairment | Psychosocial Functioning Inventory (PFI) | Self‐administered questionnaire | (Marx et al., 2009b) |
Self‐administered questionnaire
Participants are required to complete a self‐administered questionnaire prior to the interview. The questionnaire, which is a compilation of specific scales (Table 1), is accessed via a secure website hosted by an online survey vendor specializing in psychiatric and social science research (PsychData, LLC, State College, PA, USA). All stored data are encrypted, and no identifying information (including IP address) is collected in the online survey. Participants without access to the internet or who are uncomfortable using the internet are given the option of completing a paper‐and‐pencil version of the survey through the postal mail. In addition to the measures noted in Table 1, the questionnaire collects background/demographic data and information on current health status and health‐related impairment of activities of daily living. This measures broader aspects of daily living than the more psychosocially‐focused Psychosocial Functioning Inventory (Marx et al., 2009b).
The process of questionnaire administration is shown in Figure 2. Consented individuals are given approximately two weeks to complete the questionnaire prior to their scheduled telephone interview. If the questionnaire is not completed by the interview date, a research staff member contacts the participant to reschedule the phone interview. Once the participant completes the online questionnaire, the data are transferred to a database at VA Boston and the participant's record is permanently deleted from the PsychData web server, in order to maintain participant privacy and data security. In addition, a paper copy of the questionnaire is printed and securely stored at VA Boston, to provide a backup in the event of hardware or software failure.
Diagnostic telephone interview
The diagnostic telephone interviews are administered by doctoral‐level clinicians with specialized training in PTSD assessment who are blinded with respect to the participant's PTSD status in the VA medical record. Study interviewers are specifically trained in the administration of the PTSD module of the Structured Clinical Interview for DSM‐IV (SCID‐IV) (Spitzer et al., 1992; Williams et al., 1992). Assessment measures were selected based on prior use and validation in both clinical and epidemiological studies, and ease of standardization across interviewers (see Table 1). Each interview is digitally recorded, and 5% (i.e. 80 interviews) will be randomly selected and assessed for reliability of the SCID diagnosis by two independent raters.
Prior to administration of the SCID‐IV PTSD module, participant suicidality is assessed using the Mini‐International Neuropsychiatric Interview (MINI) suicide module. Interviewers follow a standardized protocol for proceeding with the interview based on the participant's score on the MINI suicide module or expression of suicidal ideation in other components of the interview. In the event that a participant is thought to be at imminent risk of suicide, the interviewer discontinues the study protocol, further assesses the participant's suicidal intent, and contacts the participant's local VA or Department of Defense (DoD) health care facility and notifies the mental health provider on call or suicide prevention coordinator, as appropriate.
Participants who complete the questionnaire but do not complete the diagnostic interview as scheduled are telephoned up to four times to reschedule the interview. Those who cannot be reached or who refuse to complete the interview are compensated $25 rather than $50 for their participation in a portion of the study.
Outcomes assessment
An important goal of the interview is to assess the concordance between the PTSD diagnosis found in the electronic medical record and the SCID‐based diagnosis from the diagnostic telephone interview. As noted previously, participants' PTSD status at study entry is based on whether or not their VA medical records contain a current or recent (past 18 months) diagnosis of PTSD. Blinded, doctoral‐level clinicians administer the SCID‐IV PTSD module during the telephone interview to determine each participant's current PTSD status.
Several comorbidities of interest, including TBI, substance use disorders, and depression, are assessed through the online questionnaire or diagnostic interview. In addition, due to the common overlap between PTSD and TBI, the interview includes a retrospective lifetime assessment of history of head injuries that may have led to TBI. The interviewer obtains details regarding the timing, associated events, and injury characteristics of the five most serious head injuries or close exposures to explosive blasts in the participant's life that caused altered consciousness, seizures, and/or required brain surgery. The TBI interview was derived in part from questions used by the Defense and Brain Injury Center, to assure comparability with other recent studies of OEF/OIF Veterans (Schwab et al., 2007). The interview questions also reflect empirically‐derived indicators (e.g. duration of post‐traumatic amnesia) of brain injury severity (Brown et al., 2005; Wilde et al., 2006) and capture information pertinent to current classification standards (Kay et al., 1993).
Data analysis
The final registry database will include merged data from the self‐administered questionnaire and telephone interview, along with select baseline and follow‐up data from the Veterans Health Administration (VHA) electronic medical record. We will conduct descriptive analyses to characterize the PTSD and non‐PTSD samples in terms of demographics, diagnosis, symptomatology, quality of life, current therapies used, and clinical trajectories. In addition, we will assess the prevalence of PTSD based on both the medical records and the standardized SCID interview assessment, and will examine the concordance between medical record‐based and SCID‐based diagnoses. We will conduct analyses comparing the 1200 PTSD group participants with the 400 non‐PTSD participants. These analyses will include the “false positive” PTSD cases (individuals with a medical record‐based diagnosis of PTSD who do not meet the research‐based SCID diagnostic criteria for PTSD) and/or the “false negative” cases (individuals in the non‐PTSD comparison group who nevertheless meet the SCID diagnostic criteria for PTSD) as appropriate, based on the goal of the analysis. For individuals in the control group who meet SCID criteria for a positive diagnosis but do not have a medical record diagnosis, we will examine potential risk factors and determinants. Conversely, for those individuals who do not meet SCID criteria but have a medical record diagnosis, we will examine likely factors (e.g. treatment or deployment status) that might account for the discrepancy. A major strength of the registry design is that it permits examination of potential reasons for and correlates of these discordant diagnostic assessments.
The operational definitions for selected outcomes of interest are listed in Table 2. Covariates (and interaction terms) will be retained in the models if they are found to be significant predictors of the outcome (at the 0.05 level of significance) or if they confound the effects of significant predictors, defined as changing the effect size estimate by at least 20%. Analyses will be conducted in SAS 9.2 (SAS Institute, Cary, NC) and SUDAAN 10.0.1 (Research Triangle Institute International, Research Triangle Park, NC). Because the target population will be oversampled to achieve equal proportions of male and female participants, weighted estimation methods will be used to achieve unbiased overall prevalence estimates of comorbidities and other conditions that reflect the status of the target PTSD population.
Table 2.
Operational definitions of selected outcomes of interest
| Outcome | Definition | Anticipated variable form |
|---|---|---|
| PTSD – clinical diagnosis | Diagnosis of PTSD in electronic medical record (ICD‐9 code 309.81) | Dichotomous |
| PTSD – research‐based diagnosis | Meets criteria for PTSD based on SCID assessment | Dichotomous |
| Psychiatric comorbidities | Diagnoses of depression, anxiety, substance abuse or other DSM‐IV diagnoses | Dichotomous |
| Treatment utilization | Number of health care visits, pharmaceutical usage | Continuous |
| Productivity loss | Self‐reported absentee days, decreased accomplishments, decreased diligence | Continuous |
| Quality of life | SF‐12 scores | Continuous |
Statistical power
The primary aim of the registry is to describe the natural history of PTSD, characterized by the long‐term psychosocial, medical, and quality of life outcomes associated with the disorder, and to assess whether these outcomes differ by subgroups defined by socio‐demographic, military, and post‐deployment factors. The size of each study group and subgroup was determined based on statistical power as well as feasibility, given the duration and resources of the research funding for the project. As noted earlier, we oversampled women to allow for analyses stratified by gender. We plan to continue enrolling participants until we achieve the target number of individuals in each subgroup with a completed questionnaire and diagnostic interview. Our power calculations therefore are based on the goal of 1200 participants with and 400 participants without a PTSD diagnosis. With 1200 participants with a diagnosis of PTSD based on the VA electronic records, there is high precision to estimate comorbidity rates. If the comorbidity rate is as high as 40%, the relative precision (half‐width) of a 90% confidence interval is less than 6% (0.023/0.40). If the rate is only 10%, then the relative precision of the confidence interval is 14% (0.014/0.10).
Power is also sufficient for potential subgroup comparisons where moderate to large effect sizes are expected. For example, it is of interest to assess whether comorbidity rates differ by subgroup in the PTSD cohort (e.g. by socio‐economic status or service branch). In the case of a subgroup factor that divides the sample according to a 30/70 split (360 versus 840 subjects), if the comorbidity rate of the lesser affected subgroup is relatively rare (10%), there is over 84% power to detect an odds ratio of 1.71 (0.10 versus 0.16 rates). If the comorbidity rate of the lesser affected subgroup is 20%, there is over 89% power to detect an odds ratio of 1.56 (0.20 versus 0.28 rates). If the comorbidity is quite prevalent (40%), there is 87% power to detect a smaller effect size (odds ratio of 1.44). If the subgroup sizes are more evenly split amongst the 1200 PTSD cases, power will be even greater.
Secondary analyses of case‐control data from the 1200 Veterans in the PTSD group and 400 Veterans in the comparison group will have sufficient power in numerous scenarios, even for conservative assumptions. For example, if the outcome is low social support as a dichotomous indicator (defined by a pre‐specified cutoff), and if the control rate of low social support is 20%, there is approximately 80% power to detect an odds ratio of 1.5 for low social support in cases versus controls. For analyses treating outcomes as continuous variables, only 503 cases (versus 167 controls) are required to detect an effect size (defined as the sample standard deviation divided by the mean difference) of 0.25 standard deviations with 80% power, which is a minimum clinically significant difference. With 1200 cases and 400 controls, there is >99% power to detect a 0.25 effect size. Therefore, continuous analyses of case‐control differences in functioning can support multivariate modeling as well as subgroup analyses defined by gender and race/ethnicity.
Results
To test the procedures for study recruitment and data collection, we conducted a pilot study over a three‐month period prior to the launch of full study recruitment. Twenty‐seven participants (13 men and 14 women) were enrolled during the pilot phase.
Characteristics of the pilot study participants are shown in Table 3. On average, participants were 42 years of age (range: 28 to 58 years), and 74% of participants had a diagnosis of PTSD based on the VA medical records. Concordance between the PTSD diagnosis from the medical records and the SCID was 82%; 18 participants (67%) had a positive diagnosis on both the medical records and the SCID, three (11%) were positive on the SCID but not the medical records, two (7%) were positive based on the medical records but not the SCID, and four (15%) were negative for both.
Table 3.
Baseline characteristics of pilot phase participants in Project VALOR
| Covariate | All pilot study participants |
|---|---|
| N | 27 |
| Age (mean and standard deviation [SD]) | 41.9 (8.6) |
| Female (%) | 52 |
| Race (%) | |
| Black | 15 |
| White | 82 |
| Other | 3 |
| Hispanic ethnicity (%) | 11 |
| Branch of military service (%) | |
| Army | 96 |
| Marines | 4 |
| Location of deployment (%) | |
| Afghanistan only | 19 |
| Iraq only | 70 |
| Multiple deployments/other | 11 |
| PTSD based on SCID (%) | 78 |
| Interview duration (mean and SD) | 31.9 (12.3) |
The pilot phase demonstrated the feasibility of the recruitment and data collection procedures. All participants completed both the self‐administered questionnaire and diagnostic telephone interview, and no difficulties related to comprehension or completion of the questionnaire or interview were reported by the participant or the interviewer. The average length of the diagnostic interview was 32 minutes (range 10 to 59 minutes).
Several changes to the study protocol were implemented as a result of the pilot phase. The number of times an interview can be rescheduled was increased to three, to give participants additional time to complete the self‐administered questionnaire. In addition, revisions were made to the TBI scale and the Life Events Checklist was added to the interview to collect detailed information on the participant's trauma history.
The pilot phase was completed in January 2010, and full study recruitment began in May 2010. As of March 4, 2011, 419 study interviews have been completed. With recruitment of additional interviewers for the project, we anticipate completing study enrollment in early 2012.
Discussion
Project VALOR is the first longitudinal patient registry study of PTSD in returning service men and women who have utilized the VA health care system. This study is designed to provide longitudinal data over time to characterize the course of PTSD and associated outcomes in combat‐exposed Veterans, as diagnosed by a SCID‐based interview and electronic medical record information. The registry will provide a unique opportunity to characterize the diagnostic and treatment services received by a well‐defined cohort of Veterans with or without a diagnosis of PTSD, and to describe associated patient characteristics, including demographic, medical and psychosocial information. Our observational registry design will include assessment of the number, type and duration of treatments received, presence or absence of other medical or psychiatric disorders, and the use of conjoint treatments for these disorders. We will use a broad array of functional and psychosocial assessments at baseline, as well as medical record abstraction at baseline and year one of follow‐up, to obtain detailed data on potential risk factors for and outcomes of PTSD. Our use of a mixed mode data collection system, including a combination of medical record abstraction, a self‐administered questionnaire, and a structured telephone interview, is a unique feature and potential strength of the study design. We will use brief, validated measures to assess PTSD and other outcomes and exposures of interest, with data obtained in a format specifically suited to the topic or domain under investigation. This convergent validity approach is intended to provide internal validity checks on the accuracy and completeness of diagnostic data obtained for each participant. An additional strength of the study design is the large number of female participants, as this will allow us to examine differences in the predictors of risk and recovery for PTSD by gender.
The registry will create an opportunity, most importantly, to assess the longitudinal trajectory of psychosocial outcomes over time. Funding has been obtained for the first wave (12 months) of follow up, and we plan to seek funding for additional follow‐up cycles. The longitudinal component of the registry will provide data not only on progression and remission of PTSD symptoms, but also trajectories of other psychiatric comorbidities (e.g. depression, substance abuse, suicide risk), resource and treatment utilization, and social and occupational outcomes in participants with or without a diagnosis of PTSD. A comparison group of male and female Veterans without a medical record‐based diagnosis of PTSD, but with similar service history and combat experience, is included in our design for case‐control comparisons. An important aspect of the registry will be assessment of longitudinal data obtained from medical record sources within the VA, compared to the structured, telephone interview and self‐administered questionnaire specifically designed for the study. A registry database of potentially eligible participants for future ancillary studies will also be developed.
Although there are several strengths of the study design, limitations include the potential for loss to follow up over time and the possibility that our sample will not be representative of the underlying population of Veterans with PTSD, as our study population will only include those who seek care for PTSD or other mental health conditions at VA health care facilities. Although some loss to follow up is inevitable, we will use proven strategies for maximizing retention in order to retain as many participants as possible at each stage of follow up (Kessler et al., 2008; Scott et al., 2006). Further, by using medical record abstraction we will be able to obtain follow‐up data for some participants who are unavailable for recontact but continue to receive care at VA facilities. Due to the sampling strategy, our population will not be broadly representative of the prevalence of PTSD or patterns of treatment utilization in combat‐exposed Veterans; however, the influence of known predictors of PTSD risk and recovery, such as social support and exposure to additional life stressors, would not be expected to differ among individuals who seek versus those who do not seek care at VA facilities. In addition, our sample will include some Veterans with a history of PTSD prior to their OEF/OIF deployment. However, the inclusion of these prevalent or historical cases would not be expected to influence our primary aims, as participants will be classified based on their current or recent PTSD status. Other limitations include the possibility of recall bias in assessing trauma history and other exposures, and the inability to definitively determine whether differences in PTSD status based on the medical records versus the study interview are due to true changes in status or diagnostic errors. In addition, we chose not to include a genetic or neurobiological component in our baseline assessment due to the methodological challenges and costs involved; however, we are currently evaluating extensions to the registry that will incorporate these and other objectives of interest.
Overall, we anticipate that findings from Project VALOR will inform public health policy and decision‐making in the years to come. Specifically, we expect that the study will point to potentially underserved subgroups or populations, such as individuals who have a diagnosis of PTSD or meet criteria for the diagnosis but are not receiving appropriate care for their condition. We expect to learn how specific treatments – medical or psychosocial – are being applied in everyday practice, and how male and female Veterans with PTSD respond to treatment outside the setting of clinical trials. As noted in one recent commentary: “Registries are being used to fill important gaps in evidence and contribute to understanding how trial results can be applied in practice” (Dreyer and Garner, 2009, p. 790). Project VALOR is intended to serve this important function and to provide a complementary viewpoint and means for long‐term assessment of outcomes associated with PTSD in an observational setting.
Declaration of interest statement
The authors have no competing interests.
Acknowledgements
This work was funded by the US Department of Defense Awards W81XWH‐08‐2‐0102 and W81XWH‐08‐2‐0100.
The authors would like to thank the members of the Scientific Advisory Panel for contributions to the design and methods of the study: David H. Barlow, PhD, Director, Center for Anxiety and Related Disorders, Boston University, Boston, Massachusetts; Evelyn J. Bromet, PhD, Professor of Psychiatry and Preventive Medicine, State University of New York at Stony Brook, Department of Psychiatry, Stony Brook, New York; Dennis S. Charney, MD, Professor and Dean, Mt Sinai School of Medicine, New York; Bruce P. Dohrenwend, PhD, Chief, Division of Social Psychiatry, New York State Psychiatric Institute, New York; Javier I. Escobar, PhD, Associate Dean of Global Health and Professor of Psychiatry and Family Medicine, Robert Wood Johnson Medical School – UMDNJ, New Brunswick, New Jersey; Matthew J. Friedman, MD, PhD, Director, VA Medical Center, National Center for PTSD, White River Junction, Vermont; Stanislav V. Kasl, PhD, Head, Division of Chronic Disease Epidemiology, Social and Science Behavior and Professor of Epidemiology, Yale University School of Medicine, Department of Epidemiology and Public Health, New Haven, Connecticut; Harvey S. Levin, PhD, Professor, Cognitive Neuroscience Laboratory, Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas.
References
- Brewin C.R., Andrews B., Valentine J.D. (2000) Meta‐analysis of risk factors for posttraumatic stress disorder in trauma exposed adults. Journal of Consulting and Clinical Psychology, 68, 748–766. [DOI] [PubMed] [Google Scholar]
- Brown R.L., Leonard T., Saunders L.A., Papasouliotis O. (2001) A two‐item conjoint screen for alcohol and other drug problems. Journal of the American Board of Family Practice, 14, 95–106. [PubMed] [Google Scholar]
- Brown A.W., Malec J.F., McClelland R.L., Diehl N.N., Englander J., Cifu D.X. (2005) Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision‐tree) analysis. Journal of Neurotrauma, 22, 1040–1051. [DOI] [PubMed] [Google Scholar]
- Conway P.H., Clancy C. (2009) Comparative‐effectiveness research – implications of the Federal Coordinating Council's report. New England Journal of Medicine, 361, 328–330. [DOI] [PubMed] [Google Scholar]
- Dreyer N.A., Garner S. (2009) Registries for robust evidence. Journal of the American Medical Association, 302, 790–791. [DOI] [PubMed] [Google Scholar]
- Forbes D., Hawthorne G., Elliott P., McHugh T., Biddle D., Creamer M., Novaco R.W. (2004) A concise measure of anger in combat‐related posttraumatic stress disorder. Journal of Traumatic Stress, 17, 249–256. [DOI] [PubMed] [Google Scholar]
- Friedman M.J., Marmar C.R., Baker D.G., Sikes C.R., Farfel G.M. (2007) Randomized, double‐blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. Journal of Clinical Psychiatry, 68, 711–720. [DOI] [PubMed] [Google Scholar]
- Gliklich R.E., Dreyer N.A. (eds). (2010) Registries for Evaluating Patient Outcomes: A User's Guide, 2nd ed, AHRQ Publication No. 10‐EHC049, Rockville, MD, Agency for Healthcare Research and Quality; [PubMed] [Google Scholar]
- Gray M.J., Litz B.T., Hsu J.L., Lombardo T.W. (2004) Psychometric properties of the Life Events Checklist. Assessment, 11, 330–341. [DOI] [PubMed] [Google Scholar]
- Hawthorne G., Mouthaan J., Forbes D., Novaco R.W. (2006) Response categories and anger measurement: do fewer categories result in poorer measurement? Development of the DAR5. Social Psychiatry and Psychiatric Epidemiology, 41, 164–172. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Auchterlonie J.L., Milliken C.S. (2006) Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. Journal of the American Medical Association, 295, 1023–1032. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Castro C.A., Messer S.C., McGurk D., Cotting D.I., Koffman R.L. (2004) Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine, 351, 13–22. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Terhakopian A., Castro C.A., Messer S.C., Engel C.C. (2007) Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. American Journal of Psychiatry, 164, 150–153. [DOI] [PubMed] [Google Scholar]
- Jenkins C.D., Stanton B.A., Niemcryk S.J., Rose R.M. (1988) A scale for the estimation of sleep problems in clinical research. Journal of Clinical Epidemiology, 41, 313–321. [DOI] [PubMed] [Google Scholar]
- Kay T., Harrington D.E., Adams R., Anderson T., Berrol S., Cicerone K., Dahlberg C., Gerber D., Goka R., Harley P., Hilt J., Horn L., Lehmkuhl D., Malec J., Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine . (1993) Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 8, 86–87. [Google Scholar]
- Kessler R.C., Barber C., Beck A., Berglund P., Cleary P.D., McKenas D., Pronk N., Simon G., Stang P., Ustun T.B., Wang P. (2003) The World Health Organization Health and Work Performance Questionnaire (HPQ). Journal of Occupational and Environmental Medicine, 45, 156–174. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Keane T.M., Ursano R.J., Mokdad A., Zaslavsky A.M. (2008) Sample and design considerations in post‐disaster mental health needs assessment tracking surveys. International Journal of Methods in Psychiatric Research, 17(Suppl. 2), S6–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.A., King S.W., Fairbank J.A., Keane T.M., Adams G.A. (1998) Resilience‐recovery factors in post‐traumatic stress disorder among female and male Vietnam veterans: hardiness, postwar social support and additional stressful life events. Journal of Personality and Social Psychology, 74, 420–434. [DOI] [PubMed] [Google Scholar]
- King L.A., King D.W., Vogt D.S., Knight J., Samper R. (2006) Deployment risk and resilience inventory: a collection of measures for studying deployment‐related experiences of military personnel and veterans. Military Psychology, 18, 89–120. [Google Scholar]
- Kubzansky L.D., Koenen K.C., Spiro A., 3rd , Vokonas P.S., Sparrow D. (2007) Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of General Psychiatry, 64, 109–116. [DOI] [PubMed] [Google Scholar]
- Levin H.S. (1995) Prediction of Recovery from Traumatic Brain Injury. Journal of Neurotrauma, 12, 913–922. [DOI] [PubMed] [Google Scholar]
- Marx B.P., Brailey K., Proctor S.P., Macdonald H.Z., Graefe A.C., Amoroso P., Heeren T., Vasterling J.J. (2009a) Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment. Archives of General Psychiatry, 66, 996–1004. [DOI] [PubMed] [Google Scholar]
- Marx B.P., Schnurr P.P., Rodriguez P., Holowka D.W., Lunney C., Weathers F., Sloan D.M., Keane T.M. (2009b) Development of a Functional Impairment Scale for Active Duty Service Members and Veterans, Symposium presented at the Annual Meeting of the International Society for Traumatic Stress Studies, Atlanta, GA.
- Monson C.M., Schnurr P.P., Resick P.A., Friedman M.J., Young‐Xu Y., Stevens S.P. (2006) Cognitive processing therapy for veterans with military‐related posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 74, 898–907. [DOI] [PubMed] [Google Scholar]
- Ozer E.J., Best S.R., Lipsey T.L., Weiss D.S. (2003) Predictors of posttraumatic stress disorder and symptoms in adults: a meta‐analysis. Psychology Bulletin, 129, 52–73. [DOI] [PubMed] [Google Scholar]
- Reid M.C., Fiellin D.A., O'Connor P.G. (1999) Hazardous and harmful alcohol consumption in primary care. Archives of Internal Medicine, 159, 1681–1689. [DOI] [PubMed] [Google Scholar]
- Richardson L.K., Frueh B.C., Acierno R. (2010) Prevalence estimates of combat‐related post‐traumatic stress disorder: critical review. Australian and New Zealand Journal of Psychiatry, 44, 4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption – II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Schnurr P.P., Jankowski M.K. (1999) Physical health and post‐traumatic stress disorder: review and synthesis. Seminars in Clinical Neuropsychiatry, 4, 295–304. [DOI] [PubMed] [Google Scholar]
- Schnurr P.P., Friedman M.J., Engel C.C., Foa E.B., Shea M.T., Chow B.K., Resick P.A., Thurston V., Orsillo S.M., Haug R., Turner C., Bernardy N. (2007) Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. Journal of the American Medical Association, 297, 820–830. [DOI] [PubMed] [Google Scholar]
- Schnurr P.P., Friedman M.J., Foy D.W., Shea M.T., Hsieh F.Y., Lavori P.W., Glynn S.M., Wattenberg M., Bernardy N.C. (2003) Randomized trial of trauma‐focused group therapy for posttraumatic stress disorder: results from a Department of Veterans Affairs cooperative study. Archives of General Psychiatry, 60, 481–489. [DOI] [PubMed] [Google Scholar]
- Schwab K.A., Ivins B., Cramer G., Johnson W., Sluss‐Tiller M.M., Kiley K., Lux W., Warden D. (2007) Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: initial investigation of the usefulness of a short screening tool for traumatic brain injury. Journal of Head Trauma Rehabilitation, 22, 377–389. [DOI] [PubMed] [Google Scholar]
- Scott C.K., Sonis J., Creamer M., Dennis M.L. (2006) Maximizing follow‐up in longitudinal studies of traumatized populations. Journal of Traumatic Stress, 19, 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal K.H., Metzler T.J., Gima K.S., Bertenthal D., Maguen S., Marmar C.R. (2009) Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. American Journal of Public Health, 99, 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. (1998) The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry, 59(Suppl. 20), 22–33; quiz 4–57. [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B. (1999) Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. Journal of the American Medical Association, 282, 1737–1744. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Gibbon M., First M.B. (1992) The Structured Clinical Interview for DSM‐III‐R (SCID). I: History, rationale, and description. Archives of General Psychiatry, 49, 624–629. [DOI] [PubMed] [Google Scholar]
- Tanielian T., Jaycox L.H. (eds). (2008) Invisible Wounds of War: Psychological and cognitive injuries, their consequences, and services to assist recovery, Santa Monica, CA, RAND Corporation. [Google Scholar]
- Vasterling J.J., Schumm J., Proctor S.P., Gentry E., King D.W., King L.A. (2008) Posttraumatic stress disorder and health functioning in a non‐treatment‐seeking sample of Iraq war veterans: a prospective analysis. Journal of Rehabilitation Research and Development, Clinical Supplement, 45, 347–358. [DOI] [PubMed] [Google Scholar]
- Ware J.J., Kosinski M., Keller S.D. (1996) A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Bigler E.D., Pedroza C., Ryser D.K. (2006) Post‐traumatic amnesia predicts long‐term cerebral atrophy in traumatic brain injury. Brain Injury, 20, 695–699. [DOI] [PubMed] [Google Scholar]
- Williams J.B., Gibbon M., First M.B., Spitzer R.L., Davies M., Borus J., Howes M.J., Kane J., Pope H.G., Jr , Rounsaville B., et al. (1992) The Structured Clinical Interview for DSM‐III‐R (SCID). II. Multisite test–retest reliability. Archives of General Psychiatry, 49, 630–636. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Erickson D.J., Sharkansky E.J., King D.W., King L.A. (1999) Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. Journal of Consulting and Clinical Psychology, 67, 520–528. [DOI] [PubMed] [Google Scholar]


