Abstract
Background/aims: Smoking cessation has been shown to be effective in randomized controlled trials. It is unclear though, whether interventions also work in routine primary care.
Methods: In 167 primary care settings we conducted a randomized four‐armed smoking cessation trial to examine the efficacy of a minimal intervention (MI; n = 81), cognitive‐behavioral therapy (CBT; n = 175), bupropion (BUP; n = 108) and nicotine replacements (NRT; n = 103). Overall, 467 current smokers were enrolled. Abstinence rates at the end of treatment (12 weeks) were 32.8% for MI patients, 34.8% for CBT, 35.3% for NRT, and 46.5% for BUP patients (ITT, intention to treat) (no differential effects). Retention rates were highest in the BUP group (59.3%) and lowest in the NRT group (50.5%). Completer findings were: MI, 56.4%; CBT, 64%; BUP, 79.3%; NRT, 69.2% (LOCF, lost to follow‐up). No serious adverse events occurred during or after the medication phase. At 12‐month follow‐up continuous abstinence rates were: BUP, 29.0%; CBT, 20.9%; NRT, 29.6%; MI, 29.6%.
Conclusion: Our findings suggest that established smoking cessation treatments are effective when applied by non‐specialist primary care physicians. Our data supports a structured, multimodal treatment structure as core ingredient of successful smoking cessation in primary care. Copyright © 2011 John Wiley & Sons, Ltd.
Keywords: Smoking cessation, bupropion, nicotine replacement, CBT, efficacy, primary care
Introduction
Over the last three decades, evidence has accumulated for the efficacy of a variety of drug and non‐drug smoking cessation treatments including bupropion (BUP), nicotine replacement therapy (NRT), and psychological interventions. Meta‐analysis has shown that 31% of smokers who received bupropion SR for smoking cessation maintained abstinence for 12 months (Fiore et al., 2000). Additionally, meta‐analysis of 123 clinical trials has demonstrated that all commercially available forms of NRT (gum, transdermal patch, nasal spray, inhaler, and sublingual tablets/lozenges) are effective in promoting smoking cessation (Silagy et al., 2004). NRT increases the odds of quitting approximately 1.5‐ to two‐fold regardless of treatment setting. Most clinical trials of BUP and NRT provide low intensity psychosocial interventions in addition to the pharmacological agent, such as physician advice, brief counseling or follow‐up telephone support (Gold et al., 2002; Hughes et al., 1999). Behavioral therapy and counseling have also shown efficacy when administered alone, with estimated abstinence rates of 12 to 20% (Fiore et al., 2000). Despite growing evidence for the efficacy of each of these smoking cessation treatments, trials comparing pharmacological and psychological interventions are notably absent (Gold et al., 2002).
There is a considerable number of randomized, double‐blind and placebo‐controlled study designs (Fagerström et al., 1993; Fiore et al., 1994; Gonzales et al., 2001; Hays et al., 1999, 2001; Hurt et al., 1997; Jorenby et al., 1999; Richmond and Zwar, 2003; Swan et al., 2003; Tonnesen et al., 1999, 2003; Tonstad et al., 2002; Yudkin et al., 1996). Characteristics of these efficacy studies include the following: (1) patient samples are highly selected with regard to motivation and treatment compliance; (2) treatment providers are well‐informed and rigorously trained in patient recruitment, motivational enhancement, counseling and more complex smoking cessation interventions; (3) structured diagnostic and evaluative measures are used intensively; (4) patients frequently obtain behavioral counseling; (5) treatment is provided at no cost to patients.
These “artificial” conditions imposed by randomized controlled trials are difficult, if not impossible, to transfer to real‐life clinical settings. Research in routine health care shows that physicians face multiple barriers when treating smokers including time constraints, sub‐optimal financial reimbursement, lack of educational training or knowledge in smoking cessation treatments, and low motivation and treatment compliance on the part of patients (Hoch et al., 2004a, 2004b) A growing number of psychotherapy and drug therapy researchers have recognized the need for additional studies conducted under naturalistic conditions (Glasgow et al., 2004, 2003; Roy‐Byrne et al., 2003). Such “effectiveness” studies aim to determine “whether treatments are feasible and have measurable beneficial effects across broad populations … in real‐world settings” with particular emphasis on primary care settings (Nathan et al., 2000; p. 965).
To date, few studies have investigated the advantages and disadvantages of BUP in applied settings (Johnstone et al., 2004; Kohlenberg et al., 2004; Tonstad et al., 2002). Moreover, existing studies have not compared “first‐line” smoking cessation treatments such as BUP, nicotine replacement and psychological interventions (e.g. self‐help manuals) in a manner that would help translate research results into practice (Glasgow et al., 2003).
The current trial was conducted within the nationwide epidemiological research program “Smoking and Nicotine Dependence Awareness and Screening Study” (SNICAS), with a nationwide cross‐sectional target day assessment of a nationally representative sample of 897 primary care doctors and 28,707 patients (Phase I) (Muehlig et al., 2003; Hoch et al., 2004a, 2004b). The presented trial (Phase II) is based on a regional sub‐sample in Greater Munich and Dresden area, Germany.
We hypothesized that (1) BUP, NRT, and cognitive‐behavioral therapy (CBT) are each superior to a minimal intervention (MI) involving physician advice to quit, and (2) BUP – due to its effects on reinforcement and withdrawal via the dopaminergic and noradrenergic systems (Hurt et al., 1997) – will be associated with superior results compared to NRT and CBT. More generally, we also expected that the success rate in the BUP, NRT and CBT conditions will be slightly lower than those reported for controlled clinical trials.
Methods
To test our hypotheses, we conducted a four‐arm smoking cessation intervention study. Primary outcome measures were complete abstinence at the end of treatment (three months) and continuous abstinence during a subsequent 12‐month follow‐up assessment period. Secondary outcome measures included retention rates, reasons for treatment discontinuation, and safety of interventions. In absence of a control group without any systematic intervention, we defined MI (structured quit advice by the doctor) as the control group condition, against which the effects of other groups were tested.
Protocol
Patients were recruited from a total of 167 primary care physicians in two catchment areas of the SNICAS Phase 1 program. The two areas were the greater Dresden and Munich areas, where the two study centers were located. The target population for the study was originally the total of all smoking patients in each participating setting on the Phase 1 SNICAS target day. All patients with regular smoking behavior, participating on that target day, were eligible for the study, provided that inclusion and exclusion criteria (specified later) were met. Figure 1 depicts this recruitment process and reveals that it yielded only 51 patients for the study. We added additional screening days to recruit further patients in these settings. To avoid any systematic selection, participating doctors were required to approach all regular smokers, irrespective of their presenting complaint and reason for visit. Each patient was read a standardized study invitation for a smoking cessation treatment to be offered by his or her doctor. If patients were willing to consider treatment immediately (within seven days), they were screened for inclusion and exclusion criteria. If eligible, patients received an explanation of study procedures and provided informed consent. All doctors willing to participate were required to sign the study enrolment form, and they were offered a compensation of 85€ for each patient documented and treated in the main study (MI 55€). The first subject was enrolled in May 2002 and the last patient in July 2003. The follow‐up of the last patient was completed in October 2004.
Figure 1.

Flow diagram of the progress through the phases of the trial.
The experiment was undertaken with the understanding and written consent of each subject, and that the study conforms with “The Code of Ethics” of the World Medical Association (Declaration of Helsinki). It was reviewed and approved by the ethics committee of Technische Universität Dresden, Germany.
Elegibility criteria
To be eligible for the study, subjects needed to complete a standardized colored patient questionnaire (which appeared in four colors that were used for randomization purposes). They had to be current regular smokers (i.e. smoking in the past four weeks), at least 18 years of age, weigh at least 100 pounds [or body mass index (BMI) > 16], speak and understand the German instructions and manuals, and sign the informed consent form. Only one member per household was allowed to enroll in the study. Exclusion criteria (as assessed by the study‐site primary care physician) were: serious or unstable renal, cardiac, hypertensive, pulmonary, endocrine, or neurologic disorders; ulcerous, seizure or dermatologic disorders; a current diagnosis of major depressive episode or a history of panic disorder, psychosis, bipolar disorder, or eating disorder; use of NRT within six months before study enrollment; abuse of alcohol or a non‐nicotine containing drug within the preceding year; current use of BUP; or concurrent use of tricyclics, selective serotonin re‐uptake inhibitors (SSRIs), serotonin‐norepinephrine re‐uptake inhibitors (SNRIs), neuroleptics or monoamine oxidase (MAO) inhibitors.
Treatment assignment and participant flow
On SNICAS target day n = 720 subjects were eligible for participation, n = 395 (55%) subjects did not meet the criteria, n = 256 (36%) were not willing to participate in the study (Figure 1).
In the second screening wave n = 467 subjects were enrolled in the trial, n = 51 were excluded because they did not meet the criteria. A total of n = 467 subjects enrolled in the trial were allocated to interventions. For each enrolled subject, end of treatment status was determined. Twelve‐month follow‐up information was available for 402 subjects (86.1%).
Assignment to treatment conditions was randomized through use of the patient questionnaire, which was available in four different colors presented in a randomized order (generated by the study center). These questionnaires were distributed consecutively to all attending patients on the target days by nurses. Thus, the assignment of patients was entirely dependent on the consecutive attendance of patients and the random assignment of a color. Doctors were not allowed to interfere with this study procedure. Monitors provided random checks of this procedure in 15% of all settings and performed other quality assurance tests.
Pharmacological and psychological interventions
Baseline session
All subjects in all four treatment arms received the same standardized face‐to‐face oral brief motivational intervention to quit smoking (<3 minutes) plus a motivational information sheet (“Reasons to Quit Smoking Immediately”). Subsequently though, the four treatment arms differed with regard to number of treatment components and their intensity (see Figure 2).
Figure 2.
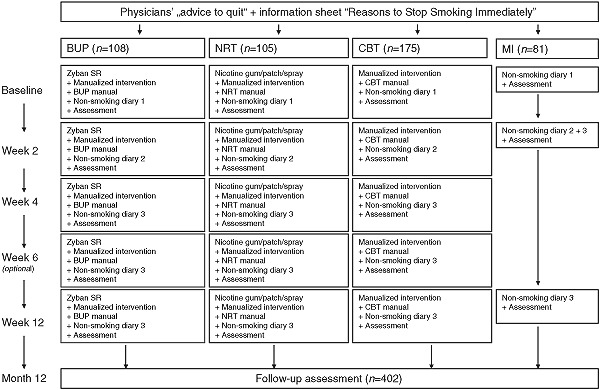
Intervention and assessment schedule.
Subjects in the MI group were given additionally only a “non‐smoking diary”. At week 2 and 12 subjects received a total of two brief (5–10 minutes) feedback sessions, in which the non‐smoking diary for the last weeks was reviewed, along with a repeat of the motivational intervention and the motivational sheet information. Subjects who had stopped were reinforced, those who had not yet stopped smoking were encouraged to do so.
In the CBT condition, the following further treatment components were added to the three MI elements, namely the structured quit advice, the “non‐smoking diaries”, and the review of the diary: First patients in the CBT condition received a total of four (optional five) counseling sessions. Secondly, using a standardized therapist CBT manual, the doctor reviewed and discussed in each of theses sessions the diary information and provided structured guidance for various domains, such as the quit day preparation, relapse prevention, managing withdrawal, etc. using a standardized set of simple cognitive‐behavioral techniques. Thirdly, the patient received a cognitive‐behavioral self‐help manual, that was matched to the version used by the doctor. Finally homework exercises were assigned. At each counseling session, smoking cessation outcomes and homework assignments were assessed by the physicians.
Subjects in the BUP group received the same treatment as the CBT group plus 150‐mg tablets of sustained‐release BUP (Zyban®, GlaxoSmithKline). The dose schedule was 150 mg q.d. (quaque die, each day) for the first six days, and 150 mg b.i.d. (bis in die, twice a day) thereafter. Subjects in the NRT condition received the same treatment as the CBT group plus standard NRT. Physicians were allowed to prescribe a nicotine‐replacement product in accordance with the subject's choice (patches: NiQuitin 21‐mg, 14‐mg, 7‐mg; Nicotinell 52.5‐mg, 35‐mg, 17.5‐mg; Nicorette 24.9‐mg, 16.6‐mg, 8.3‐mg; gum: Nicotinell 2‐mg, 4‐mg; Nicorette: 2‐mg, 4‐mg; nasal spray 10‐mg/10 ml). Patients in the BUP and NRT groups had to cover all expenses for the pharmacological treatment.
Number of sessions, intensity and structure of counseling were identical for each of the three active intervention group. Four (optional five) individual counseling sessions were scheduled. The total amount of contact time was 20–30 minutes at each session. Counseling topics included: motivational enhancement, identification of smoking triggers, social support, coping with side effects of medication and withdrawal, positive reinforcement, and relapse prevention. Core elements of the adjunctive cognitive‐behavioral self‐help manual were: motivational enhancement, “quit day” procedures, smoking trigger recognition, problem solving, social support, coping with withdrawal, weight management, problem solving skills, positive reinforcement and relapse prevention. In the self‐help manuals given to the BUP and NRT groups, pharmacotherapy‐specific information was added. To ensure adherence to treatment content and structure, primary care physicians were asked to use a standardized treatment manual developed by our work group. Moreover, fidelity to treatment was regularly evaluated by the study monitors who supervised the physicians.
Treatment period
In all active treatment conditions, the intervention period was nine to 12 weeks, depending on holidays and other logistics. Target quitting dates were set for the second week, usually at day 8. Individual counseling was scheduled at baseline and at weeks 2, 4, 6 (optional) and 12. Prior to each session, physicians administered measures of the primary and secondary outcomes of interest. The MI group was assessed at the two follow‐up sessions (week 2 and week 12).
Assessments and follow‐up
A pre‐study questionnaire was administered to collect data on personal characteristics and office settings of participating doctors (Muehlig et al., 2003). A patient questionnaire was used in the baseline session to survey the patients' demographic characteristics, physical and mental health, smoking status and history. Assessment of nicotine dependence syndrome was based on the items of the Munich‐Composite International Diagnostic Interview tobacco section (DIA‐X/M‐CIDI) according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) (APA, 1994; Wittchen and Pfister, 1997). The CIDI's reliability and validity has been established (Lachner et al., 1998; Wittchen, 1994). We also used the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) to obtain a quantitative measure of nicotine dependence. Motivation to change was assessed by a categorical staging algorithm of Jaekle et al. (1999) adopted from DiClemente et al. (1991). The pre‐treatment doctors' clinical appraisal form included among others, ratings of the patients' current smoking status, somatic and mental disorders, health risks, and the motivation for smoking cessation. Presence of mental disorders and use of drugs was screened by questionnaire using the M‐CIDI as a model in the baseline examination questionnaire during the first treatment session. For the use of alcohol (frequency, quantity) items of the Alcohol Use Disorders Identification Test (AUDIT) were administrated (Saunders et al., 1993). To structure and standardize treatment sessions the physicians were provided with standardized treatment manuals. Manuals included guidance for motivating and counseling smokers and structured assessment forms (e.g. patient smoking status and progress, motivation to quit, medication use, adverse treatment effects, and reasons for drop‐out). Subjects were asked to keep a daily non‐smoking diary that included information on smoking status, craving, withdrawal symptoms, positive and negative changes, and a semi‐structured relapse protocol. At post‐treatment (three months) and at 12‐month follow‐up, self‐reported smoking status, number and reasons for relapses, stability of abstinence, and positive and negative changes were assessed.
Measures of outcome
The primary outcome variable was the point‐prevalence of abstinence at the end of treatment. Data were available on this variable for 467 patients. Subjects were considered abstinent if they reported no smoking during seven days prior to the assessment. At 12‐month follow‐up, the primary outcome measure was continuous abstinence (i.e. no smoking since the end of treatment). Data were available on this variable for 402 patients. Secondary outcome variables included rates and reasons for treatment discontinuation as well as safety of treatment.
Statistical analysis
Baseline demographic, clinical, and smoking‐related characteristics of subjects were compared by analysis of variance for continuous variables and chi‐square analysis for categorical variables. Following the recommendations of Peto et al. (1976), data were subjected to an intention‐to‐treat (ITT) analysis. In other words, case ascertainment and follow‐up continued regardless of whether patients maintained participation in the trial. Last observation of abstinence or non‐abstinence was carried forward if subjects discontinued treatment or were lost to follow‐up (LOCF analysis). All participants who received at least one dose of study medication were included in the safety analysis. Logistic regression analyses were used to determine pairwise differences among groups in post‐treatment and follow‐up abstinence rates, and rates of treatment discontinuation. Statistical analyses were performed with the Stata software package, version 8.0. (Stata Corporation, 2003).
Results
Baseline characteristics and retention rates
The baseline characteristics of study participants are shown in Table 1. There were no significant differences between groups. The retention rate among the 467 subjects in the complete observation period was 54% (Table 2). A total of 215 participants (46%) did not complete all mandatory treatment and assessment sessions. Retention rates were highest in the BUP group (59.3%) and lowest in the NRT group (50.5%); there were no significant between‐group differences in terms of treatment discontinuation. Most frequent reasons for treatment discontinuation were, that the patients reconsidered their initial commitment to stop after starting the program (“mistimed treatment”, 27%) and insufficient motivation to continue (“no interest anymore”, 22%; “no motivation to stop smoking”, 21%). Symptoms of withdrawal, price of medication and weight gain were mentioned rarely as reasons to discontinue the interventions (all <3%; full analysis available upon request).
Table 1.
Baseline characteristics of the subjectsa
| Characteristics | BUP (n = 108) | CBT (n = 175) | NRT (n = 103) | MI (n = 81) |
|---|---|---|---|---|
| Age (yr) | 45.0 ± 13.5 | 42.4 ± 13.9 | 41.9 ± 13.3 | 41.5 ± 15.1 |
| Male gender (%) | 49.1 | 49.1 | 42.2 | 53.8 |
| Body mass index (BMI)b | 24.6 ± 3.9 | 24.4 ± 4.2 | 24.3 ± 4.1 | 24.3 ± 3.6 |
| Number of cigarettes smoked/day in past four weeks | 21.8 ± 9.0 | 18.7 ± 9.0 | 20.1 ± 9.5 | 18.1 ± 8.9 |
| Fagerström scorec | 3.7 ± 2.4 | 4.0 ± 2.5 | 3.2 ± 2.4 | 3.2 ± 2.4 |
| DSM‐IV nicotine dependenced (%) | 79.3 | 71.2 | 70.5 | 64.1 |
| Age of onset (years) | 16.4 ± 3.5 | 16.5 ± 4.8 | 16.2 ± 2.7 | 16.3 ± 3.4 |
| Number of previous serious quit attempts | 5.1 ± 4.2 | 6.7 ± 14.4 | 4.4 ± 3.9 | 6.1 ± 13.8 |
| Previous use of nicotine patch (%) | 18.5 | 20.3 | 21.3 | 11.7 |
| Previous use of nicotine gum (%) | 19.8 | 11.5 | 18.8 | 8.3 |
| Previous use of nicotine spray (%) | — | — | 1.3 | — |
| Previous use of BUP (%) | 7.4 | 4.7 | 6.3 | 1.7 |
| Marital status (%) | ||||
| Single | 29.1 | 35.3 | 37.9 | 47.6 |
| Married | 41.9 | 44.9 | 44.8 | 36.5 |
| Divorced, widowed or separated | 29.1 | 19.9 | 17.2 | 15.9 |
| Occupational situation (%) | ||||
| Employed | 71.8 | 54.7 | 61.6 | 57.1 |
| Unemployed | 7.1 | 18.0 | 11.6 | 11.1 |
| Homemaker | 3.5 | 4.0 | 5.8 | — |
| Retired | 9.4 | 14.0 | 12.8 | 17.5 |
| Other | 8.2 | 9.3 | 8.1 | 14.3 |
| Stages of changee (%) | ||||
| Precontemplation | 3.8 | 6.8 | 5.2 | 5.2 |
| Contemplation | 53.2 | 58.1 | 61.0 | 61.0 |
| Preparation | 43.0 | 35.1 | 33.8 | 33.8 |
Plus‐minus values are means ± standard deviation. Because of rounding, not all percentages total 100.
BMI is measure of body fat based on height and weight that applies to both adult men and women (BMI in kg/m2).
Fagerström Test for Nicotine Dependence by Heatherton et al. (1991).
DSM‐IV nicotine dependence syndrome criteria were assessed by use of items taken from the Composite International Diagnostic Interview – Module Nicotine Dependence (Cottler et al., 1990, 1991) and CIDI/DIA‐X (Wittchen and Pfister, 1997) as a model.
Categorical staging algorithm of Jaekle et al. (1999).
Table 2.
Retention rates and most frequent reasons for treatment discontinuation
| Total (n = 467) | BUP (n = 108) | CBT (n = 175) | NRT (n = 105) | MI (n = 81) | |
|---|---|---|---|---|---|
| Retention rate (%, n) | 54.0 (252) | 59.3 (64) | 50.9 (89) | 50.5 (52) | 58.0 (47) |
| Reasons for treatment discontinuation a | |||||
| Number assessed | 208 | 44 | 86 | 51 | 27 |
| Mistimed treatment (%, n) | 26.9 (56) | 27.3 (12) | 31.4 (27) | 23.5 (12) | 18.5 (5) |
| Not interested (%, n) | 22.1 (46) | 13.6 (6) | 20.9 (18) | 15.7 (8) | 51.8 (14) |
| No motivation to stop (%, n) | 20.7 (43) | 9.1 (4) | 19.8 (17) | 27.5 (14) | 29.6 (8) |
| Hassles or life events (%, n) | 18.8 (39) | 22.7 (10) | 17.4 (15) | 19.6 (10) | 14.8 (4) |
| Relapse (%, n) | 16.8 (35) | 11.4 (5) | 19.5 (17) | 17.7 (9) | 14.8 (4) |
| No time (%, n) | 15.4 (32) | 11.4 (5) | 15.1 (13) | 13.7 (7) | 26.0 (7) |
| Intervention is too time consuming (%, n) | 13.5 (28) | 6.8 (3) | 12.6 (11) | 13.7 (7) | 25.9 (7) |
Reasons for treatment discontinuation stated by at least 10% of all subjects are listed in decreasing order.
Abstinence rates: baseline to post‐treatment (three months)
Point‐prevalence of abstinence was assessed at weeks 2, 4, and at post‐treatment (three months) using ITT and a completer analysis. As compared to baseline, ITT 12 weeks abstinence rates (Figure 3) were lowest in the MI group (32.8%), slightly higher in CBT (34.8%) and NRT (35.3%) and highest the BUP group (46.5%). Group comparisons revealed no significant difference amongst each other. All groups revealed the highest abstinence rates in week 2 (32.8% to 52.9%), except for the BUP group, that revealed highest abstinence rates at week 4 (50.5%). Moreover, all groups revealed a reduction in abstinence rates from week 4 to 12, that was particularly pronounced in the NRT group, mainly due to discontinuation of treatment. Rates of abstinence were considerably higher in those patients that completed the treatment (completer analysis, Figure 3). Here, the MI performed significantly worse than the BUP group. It is noteworthy that in contrast to the ITT analysis, the completer analysis did not show a reduction in abstinence rates at week 12. Also, there is a continued increase in abstinence rates in the CBT group.
Figure 3.
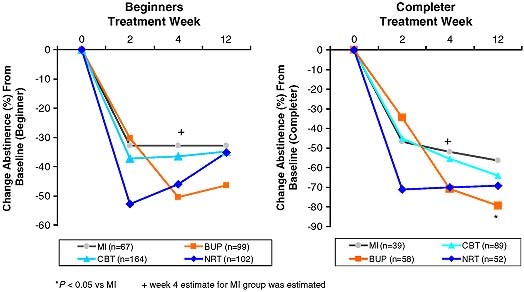
Change in abstinence rates (%) in the intervention groups from baseline to 12‐weeks post‐assessment.
Table 3 reveals the size of the treatment effects (baseline versus 12 weeks) by group. Although abstinence rates for BUP appear to be higher, differences in the point‐prevalence of abstinence between the BUP group and the MI group were not statistically significant [odds ratio (OR) 1.8, 95% confidence interval (CI) 0.9–3.4). Nor were NRT or CBT more effective than MI (NRT versus MI: OR 1.1, 95% CI 0.6–2.1; CBT versus MI: OR 1.1, 95% CI 0.6–1.9). The three intervention groups did also not differ significantly from each other in terms of the primary outcome measure. Among those who completed the assigned intervention (n = 238) 68% were abstinent at the end of treatment (BUP, 79.3%; CBT, 64%; NRT, 69.2%; MI, 6.4%) (Figure 3). Between group differences in the point‐prevalence of abstinence were only significant for BUP versus MI (OR 3.0, 95% CI 1.2–7.3) (Table 3).
Table 3.
Abstinence rates at three and 12 months: comparison between groups (odds ratiosd and 95% confidence intervals)
| Total | MI | BUP | CBT | NRT | |
|---|---|---|---|---|---|
| Beginner | (n = 432)a | (n = 67) | (n = 99) | (n = 164) | (n = 102) |
| Abstinence at three monthsb, c (n, %) | 37.3 (161) | 32.8 (22) | 46.5 (46) | 34.8 (57) | 35.3 (36) |
| Comparison with the MI group | 1.8 (0.9–3.4) | 1.1 (0.6–1.9) | 1.11 (0.6–2.1) | ||
| Comparison with the BUP group | 0.6 (0.4–1.0) | 0.6 (0.4–1.1) | |||
| Comparison with the CBT group | 1.0 (0.6–1.7) | ||||
| Completer | (n = 238)a | (n = 39) | (n = 58) | (n = 89) | (n = 52) |
| Abstinence at three monthsb, c (n, %) | 67.7 (161) | 56.4 (22) | 79.3 (46) | 64.0 (57) | 69.2 (36) |
| Comparison with the MI group | 3.0 (1.2–7.3) | 1.4 (0.6–2.9) | 1.7 (0.7–4.1) | ||
| Comparison with the BUP group | 0.5 (0.2–1.0) | 0.6 (0.3–1.4) | |||
| Comparison with the CBT group | 1.3 (0.6–2.6) | ||||
| 12‐Month follow‐up e | (n = 325) | (n = 44) | (n = 76) | (n = 129) | (n = 76) |
| Abstinence at 12 months (%, n) | 25.9 (84) | 29.6 (13) | 29.0 (22) | 20.9 (27) | 29.6 (22) |
| Comparison with the MI group | 1.0 (0.4–2.2) | 0.6 (0.3–1.4) | 1.0 (0.4–2.2) | ||
| Comparison with the BUP group | 0.6 (0.3–1.2) | 1.0 (0.5–2.0) | |||
| Comparison with the CBT group | 1.5 (0.8–2.9) |
Thirty‐five patients excluded from analysis due to abstinence before first intervention.
LOCF analysis: last observation of abstinence/non‐abstinence is carried forward if subject dropped‐out or were lost.
Point‐prevalence of abstinence at end of treatment: self‐report of abstinence at the final visit and seven days prior to the assessments, no biochemical confirmation, the treatment period was nine to 12 weeks.
Odds ratios were computed by logistic regression analysis, which was used to determine pairwise differences in abstinence rates.
Continuous abstinence since end of treatment: self‐reported abstinence in follow‐up interview assessment or by mail (questionnaire), no biochemical confirmation.
Continuous abstinence (Table 3, lower part) during the 12‐month follow‐up period showed that 26% of subjects assessed were completely abstinent. Continuous abstinence rates for the active treatment groups were neither significantly different from each other, (BUP, 29%; CBT, 20.9%; NRT, 29.6%; MI, 29.6%) nor from the MI condition.
Safety
Among all patients who discontinued treatment (n = 215), 18 patients stopped due to adverse effects (11 in the NRT group who used the patch; seven in the BUP group). The most common adverse effects were: headache (29%), application‐site reactions (24%), and dry mouth (8%) in the NRT group (patch); nausea (13%) and disturbing taste (12%) in the NRT group (gum); and insomnia (7%) and dry mouth (7%) in the BUP group (full analysis available upon request). No serious adverse events occurred during or after the medication phase.
Comment
This study examined in a fairly unselected sample of non‐specialized primary care physicians the efficacy of four established smoking cessation interventions in primary care patients under routine conditions. Notable special features of our study are: (a) patients were sampled randomly from primary care settings, (b) random assignment of patients to four different treatment conditions, (c) treatments were administered in a structured way according to the recommended optimal format for both the drug and the non‐drug intervention components, (d) the administration of the structured intervention packages by doctors that were previously neither specialized nor specifically trained in the use of the four interventions, (e) the use of standardized intervention and assessment packages under routine case conditions to ensure treatment integrity and (d) standardized symptom and diagnostic assessments.
Focusing on the abstinence as the primary outcome, the core study finding is that BUP, NRT and CBT as established smoking cessation treatments result in quit rates that are similar or even better than those reported in controlled clinical trials. This suggest overall that established smoking cessation treatments – if supplied in an attractive manual form with simple instructions – are feasible and similarly effective as compared to standard clinical trials, even in non‐specialized primary care settings, with minimal training even with doctors that have no or little previous expertise in this field.
Abstinence rates
More than one‐third of those who started treatment and two‐thirds of those completing treatment were abstinent three months after the interventions began. Contrary to our initial hypothesis “more intensive” treatments such as BUP, CBT, and NRT were not significantly more efficacious in promoting initial and continued abstinence from smoking than MI. It is noteworthy though that the three intensive treatments revealed partly remarkably different patterns. NRT patients showed some strong initial effect on abstinence as early as week 2, with some considerable decline in efficacy at week 4 and 12. BUP patients revealed highest abstinence rates later, not before week 4. There were no consistent effects to support the initial hypothesis that BUP was superior to other active treatment conditions. In contrast to both medication groups, CBT reveals a more modest increase in abstinence initially, but in contrast to the two drug groups they did not relapse that frequently up to week 12. It should be noted that the study was not designed and powered to detect differences between all intensive treatment conditions.
The abstinence rates achieved in the MI group with 33% in the ITT and 56% in the completer analysis is remarkable. Even more so, if one takes into account that in the MI group, a noteworthy 18% became abstinent without any additional formal intervention and that a substantial proportion remained abstinent at 12‐months follow‐up. This effect seems to be in agreement with the fairly consistent finding, that even simple advice to quit by the doctor is a powerful and effective intervention in itself (Fiore et al., 2000). Yet, rates of prolonged abstinence for our MI condition exceed by far the pooled abstinence rates for low intensity counseling (3–10 minutes) we know from the literature (Fiore et al., 2000). This finding might suggest that the initial baseline motivation intervention to stop was more effective in the MI group than in the other groups. MI patients were aware that there is not much to follow in terms of more intensive treatment components. The consistently and substantially lower immediate quit rates in the more intensive treatment conditions could be related to the patients' attitude that they can wait to quit smoking until the “real” treatment starts. Another explanation for the substantial and stable MI abstinence rates might be that we applied in all groups a personalized monitoring strategy. Following the baseline advice to quit patients were asked to fill‐out the attractive diary booklets and to bring them back at each follow‐up session for feedback and support. Moreover, the arrangement of two brief follow‐up sessions – also given to the MI group – may have contributed to the positive result by producing additional treatment effects such as social support and positive reinforcement. In comparison to other trials where only the physicians' advice was given, our format of “minimal intervention” thus could be regarded as more complex. We suggest, that these findings indicate, that in routine primary care MI can be quite effective, if it is combined with at least two structured and continuous brief follow‐up consultations based on smoking diaries. The stability of the MI abstinence rates at four and 12 weeks and follow‐up, suggests that there is obviously a considerable proportion of primary care patients, that profits in a stable way from such MI. We are unable to dissect to what degree the additional assessments and indirect doctor attention might have lead to this sustained response. This finding prompts the need to identify variables that inform the doctor upfront reliably which types of patients are most likely to profit from such short intervention, without the effort of more intensive treatment.
Analyses of prolonged abstinence show that over a quarter of participants were completely and continuously abstinent 12 months after treatment termination, underlining that sustained abstinence at 12 weeks predicts a high probability of 12 month abstinence. The magnitude of abstinence rates resembles findings from previous clinical trials in specialty research clinics. Fiore et al.'s (2000) meta‐analytic study found abstinence rates of 31% for BUP (95% CI 23.2–37.8), 18% for nicotine patch (95% CI 16.2–19.5), 24% for nicotine gum (95% CI 20.6–26.7), and 31% for nicotine nasal spray (95% CI 21.8–29.2). Moreover, our CBT intervention was associated with an abstinence rate similar to the 12–20% found for various types of counseling and behavioral therapies. In the context of these positive findings it should be noted that involvement of primary care doctors in the treatment of tobacco dependence in Germany is not ensured by reimbursement of treatment provision.
Despite the failure to demonstrate significant differences between the three intensive treatments we caution the interpretation, that there are no differences and that choice of treatment does not matter. At this stage of the analyses we only focused on the categorical measure abstinence and did not consider yet, which type of patients profits the most from which type of intervention, by using other secondary outcome measures, such as dimensional measures of use and more flexible pattern of relative abstinence. Future analyses of secondary categorical and dimensional outcome variables (e.g. partial abstinence, number of smoked cigarettes, positive and negative changes, severity of dependence, etc.) may provide evidence of more subtle differences between the different treatment conditions.
Retention rates
The study also impressively revealed that retention in treatment is the core challenge in routine care and primary care settings in particular. The retention rate of 54% in our study appears to be considerably lower than those reported in efficacy studies in specialized clinics (e.g. 80.2% in a BUP trial) (Jorenby et al., 1999). As evidenced in the comparison of ITT and completer analyses, the decline in initial abstinence rates as early as four weeks, 12 weeks and in follow‐up is mainly driven by non‐retention. The low retention thus seems to be typical for routine and primary care settings. Our study purposely examined patients in routine primary care and not patients in specialized clinics, which might have considerably more expertise and techniques to keep patients in treatment. Smokers in our study had to simply agree to immediate study participation and had to indicate their willingness to quit in the next two weeks. We did not exclude the substantial proportion of about 50% of study patients whose motivational stage was low (pre‐contemplater or contemplater). This is reflected by the rank order of the most frequent reasons for treatment discontinuation, where loss or reduction in motivation was by far the most frequent reason. In future analyses, we hope to provide more information on the predictive power of readiness to change as well as a wide range of patient and doctor characteristics with regard to positive primary and secondary treatment outcomes.
Safety
In December 2001, six months prior to the start of this intervention study, four cases of death among 330,000 patients treated with BUP were registered at the German Federal Institute for Pharmaceutics and Medical Devices. Although a careful analysis could not attribute cause of death to use of BUP, concerns about safe prescription of BUP for smoking cessation arose among health experts and members of the media. In our study no serious adverse events occurred with administration of BUP. Unpleasant side effects were similar to those reported in other studies (Gonzales et al., 2001; Hays et al., 1999, 2001; Hurt et al., 1997; Jorenby et al., 1999).
Limitations
This study was conducted under routine primary care conditions. We attempted to combine high internal validity (e.g. maximization of treatment effects by use of standardized treatment manuals and materials; control checks by study monitors) and high external validity (e.g. representativeness of treatment seekers and providers; employment of smoking cessation products as currently licensed, marketed, and implemented for use in routine primary care; no extensive doctor training). However, we cannot entirely exclude the possibility that internal validity and the validity of statistical results were compromised by departures from our randomization scheme. Random checks of this procedure and quality assurance tests by study monitors revealed that in some cases in the latter part of the study treatment was based on patient and physician preferences. Another limitation is that our results rely on self‐report measures of abstinence at post‐treatment and 12‐month follow‐up. We were neither financially nor logistically able to implement biochemically‐confirming CO (carbon monoxide)‐assays in all the participating primary care settings.
Conclusion
The present study demonstrates that smoking cessation treatments applied by non‐specialist physicians are similarly effective in achieving abstinence as shown under controlled clinical trial conditions. BUP, NRTs and CBT for smoking cessation are feasible, effective, and safe when applied in routine care, even without formal training for the physicians. Such complex interventions with multiple treatment components and structured counseling sessions are clearly more effective than a “one shot” minimal intervention consisting of some short motivational intervention with the physicians' advice to quit! In comparison to specialist clinics, the retainment of patients in the treatment appears to be a major obstacle, reducing the efficacy of treatments.
Perspectives
Further research is needed to find appropriate methods for enhancing patients' motivation to quit smoking as well as treatment retention for use in routine care. In order to optimally allocate limited resources in primary health care, further studies should determine which patient or doctor characteristics are associated with favorable treatment outcomes in primary care and particularly which characteristics are reliably and easily accessible markers for sustained response.
Declaration of interest statement
All authors declare that they do not have any competing interests.
Acknowledgments
We acknowledge the commitment of participating primary care physicians, their staff, patients, and members of the SNCIAS research team (Sabine Apelt, Kerstin Baunack, Annett Franke, Anne Geißler, Katja Hagenau, Silke Haebold, Dr Eva Hoch, Birgit Jahn, Sylke Jupe, Lars‐Uwe Korn, Jens Klotsche, Antje Kullowatz, Marion Kramer‐Rosenzweig, Christian Langer, Katja Laemmerhirt, Jasmin Lorenz, Katrin Poettrich, Hristo Tzvetkov, Sandy Moll, Dr Stephan Muehlig, Dr Ulla Nagel, Bernd Schneider, Andrea Schultheiß, Angela Settele, Holger Sonntag, Barbara Spiegel, Juergen von Consbruch, Dr Hans‐Ulrich Wittchen, Sonja Zimmermann) without whom the present study would not have been possible. We further acknowledge the helpful advice and suggestions of Dr Juergen Rehm, Toronto.
This paper has been prepared in the context of the project F4 “Smoking Cessation in Primary Care” (PI: Professor Dr Hans‐Ulrich Wittchen) of the Addiction Research Network ASAT (Allocating Substance Abuse Treatments to Patient Heterogeneity). Contact information: e‐mail: asatkoordination@mpipsykl.mpg.de (http://www.asat-verbund.de). ASAT is sponsored by a federal grant of the Federal Ministry of Education and Research (01EB 0440 ‐ 0441, 01EB 0142). During the preparatory phase of the study we received further support of GlaxoSmithKline GmbH & Co. KG (Munich), GlaxoSmithKline Consumer Health Care GmbH & Co KG (Buehl) and Pharmacia GmbH (Erlangen). They provided the mandatory treatment manuals for use of their products.
References
- American Psychiatric Association (APA) (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Washington DC, APA. [Google Scholar]
- Cottler L.B. (1990) The CIDI and CIDI‐substance abuse module (SAM): cross‐cultural instruments for assessing DSM‐III, DSM‐III‐R and ICD‐10 criteria. NIDA Research Monograph, 105, 220–226. [PubMed] [Google Scholar]
- Cottler L.B., Robins L.N., Grant B.F., Blaine J., Towle L.H., Wittchen H.U., Sartorius N. (1991) The CIDI‐Core Substance ‐abuse and dependence questions ‐ cross‐cultural and nosological issues. British Journal of Psychiatry, 159, 653–658. [DOI] [PubMed] [Google Scholar]
- DiClemente C.C., Prochaska J.O., Fairhurst S.K., Velicer W.F., Velasquez M.M., Rossi J.S. (1991) The process of smoking cessation: an analysis of precontemplation, contemplation and preparation stages of change. Journal of Consulting and Clinical Psychology, 59, 295–304. [DOI] [PubMed] [Google Scholar]
- Fagerström K.O., Schneider N.G., Lunell E. (1993) Effectiveness of nicotine patch and nicotine gum as individual versus combined treatment for tobacco withdrawal symptoms. Psychopharmacology, 111(3), 271–277. [DOI] [PubMed] [Google Scholar]
- Fiore M.C., Bailey W.C., Cohen S.J., et al. (2000) Treating Tobacco Use and Dependence. Clinical Practice Guideline, Rockville, MD, US Department of Health and Human Services. [Google Scholar]
- Fiore M.C., Smith S.S., Jorenby D.E., Baker T.B. (1994) The effectiveness of the nicotine patch for smoking cessation – a metaanalyses. JAMA, 271(24), 1940–1947. [PubMed] [Google Scholar]
- Glasgow R.E., Klesges L.M., Dzewaltowski D.A., Bull S.S., Estabrooks P. (2004) The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Annals of Behavioral Medicine, 27(1), 3–12. [DOI] [PubMed] [Google Scholar]
- Glasgow R.E., Lichtenstein E., Marcus A.C. (2003) Why don't we see more translation of health promotion research to practice? Rethinking the efficacy‐to‐effectiveness transition. American Journal of Public Health, 93(8), 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D.H., Nides M.A., Ferry L.H., Kustra R.P., Jamerson B.D., Segall N., Herrero L.A., Krishen A., Sweeney A., Buaron K., Metz A. (2001) Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomised placebo‐controlled study. Clinical Pharmacology & Therapeutics, 69(6), 438–444. [DOI] [PubMed] [Google Scholar]
- Gold P.B., Rubey M.D., Harvey R.T. (2002) Naturalistic, self‐assignment comparative trial of BupropionSR, a nicotine patch, or both for smoking cessation treatment in primary care. American Journal on Addiction, 11, 315–331. [DOI] [PubMed] [Google Scholar]
- Hays J.T., Croghan I.T., Schroeder D.R., Offord K.P., Hurt R.D., Wolter T.D., Nides M.A., Davidson M. (1999) Over‐the‐Counter nicotine patch therapy for smoking cessation: results from randomised, double‐blind, placebo‐controlled, and open label trials. American Journal of Public Health, 89, 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J.T., Hurt R.D., Rigotti N.A., Niaura R., Gonzales D., Durcan M.J., Sachs D.P.L., Wolter T.D., Buist A.S., Johnston J.A., White J.D. (2001) Sustained‐release bupropion for pharmacologic relapse prevention after smoking cessation – a randomized, controlled trial. Annals of Internal Medicine, 135(6), 423–433. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerström K.O. (1991) The Fagerstroem‐Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hoch E., Franke A., Sonntag H., Jahn B., Mühlig S., Wittchen H.‐U. (2004a) Raucherentwöhnung in der primärärztlichen Versorgung – Chance oder Fiktion? Ergebnisse der SNICAS‐Studie. Suchtmedizin, 6, 92–96. [Google Scholar]
- Hoch E., Muehlig S., Höfler M., Lieb R., Wittchen H.‐U. (2004b) How prevalent is smoking and nicotine dependence in primary care? Addiction, 99, 1586–1598. [DOI] [PubMed] [Google Scholar]
- Hughes J.R., Goldstein M.G., Hurt R.D., Shiffman S. (1999) Recent advances in the pharmacotherapy of smoking cessation. JAMA, 281, 72–76. [DOI] [PubMed] [Google Scholar]
- Hurt R.D., Sachs D.P.L., Glower E.D., Offord K.P., Johnston J.A., Dale L.C., Khayrallah M.A., Schroeder D.R., Glover P.N., Sullivan C.R., Croghan I.T., Sullivan P.M. (1997) A comparison of sustained‐release bupropion and placebo for smoking cessation. New England Journal of Medicine, 337, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Jaekle C., Keller S., Baum E., Basler H.‐D. (1999) Skalen zur Selbstwirksamkeit und Entscheidungsbalance im Prozess der Verhaltensänderung von Rauchern. [Scales of self‐efficacy and decisional balance in the process of smoking behavior change]. Diagnostica, 45(3), 138–146. [Google Scholar]
- Johnstone E., Hey K., Drury M., Roberts S., Welch S., Walton R., Murphy M. (2004) Zyban for smoking cessation in a general practice setting: the response to an invitation to make a quit attempt. Addiction Biology, 9(3–4), 227–232. [DOI] [PubMed] [Google Scholar]
- Jorenby D.E., Leischow S.J., Nidel M.A., Rennard S.I., Johnston J.A., Hughes A.R., Smith S.S., Muramoto M.L., Daughton D.M., Doan K., Fiore M.C., Baker T.B. (1999) A controlled trial of sustained‐release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine, 340, 685–691. [DOI] [PubMed] [Google Scholar]
- Kohlenberg B.S., Antonuccio D.O., Hayes S.C., Gifford E.V., Piasecki M.P. (2004) Suitability of bupropion SR for nicotine‐dependent smokers: problems in a practice setting. Psychotherapy and Psychosomatics, 73(4), 252–254. [DOI] [PubMed] [Google Scholar]
- Lachner G., Wittchen H.‐U., Perkonigg A., Holly A., Schuster P., Wunderlich U., Tuerk D., Garczynski E., Pfister H. (1998) Structure, content and reliability of the Munich‐Composite International Diagnostic Interview (M‐CIDI). Substance use sections. European Addiction Research, 4(1–2), 28–41. [DOI] [PubMed] [Google Scholar]
- Muehlig S., Hoch E., Höfler M., Pittrow D., Wittchen H.‐U. (2003) Aims, design and methods of the Smoking and Nicotine Dependence Awareness and Screening (SNICAS) study. IJMPR, 12(4), 208–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan P.E., Stuart S.P., Dolan S.L. (2000) Research on psychotherapy efficacy and effectiveness: between scylla and charybdis? Psychological Bulletin, 26(6), 964–981. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M., Armitage P., Breslow N.E., Cox D.R., Howard S.V., Mantel N., McPherson K., Peto J., Smith P.G. (1976). Design and analysis of randomised clinical trials requiring prolonged observation of each patient. I. Introduction and design. British Journal of Cancer, 34(6), 585–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R., Zwar N. (2003) Review of bupropion for smoking cessation. Drug and Alcohol Review, 22(2), 203–220. [DOI] [PubMed] [Google Scholar]
- Roy‐Byrne P., Sherbourne C.D., Craske M.G., Stein M.B., Wyne K., Greer S., Adrienne M.C., Bystritsky A. (2003) Moving treatment research from clinical trials to the real world. Psychiatric Services, 54(3), 327–332. [DOI] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Bebor T.F., De La Fuente J.R., Grant M. (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Silagy C., Lancaster T., Stead L., Mant D., Fowler G. (2004) Nicotine replacement therapy for smoking cessation (Cochrane Review) In The Cochrane Library (Issue 4), Chichester, John Wiley & Sons. [DOI] [PubMed] [Google Scholar]
- Stata Corporation (2003) Stata Statistical Software: Release 8.0, Stata Corporation, College Station, TX. [Google Scholar]
- Swan G.E., McAfee T., Curry S.J., Jack L.M., Javitz H., Dacey S., Bergman K. (2003) Effectiveness of bupropion sustained release for smoking cessation in a health care setting – a randomised trial. Archives of Internal Medicine, 163(19), 2337–2344. [DOI] [PubMed] [Google Scholar]
- Tonnesen P., Paoletti P., Gustavsson G., Russell M.A., Saracci R., Gulsvik A., Rijcken B. (1999) Higher dosage nicotine patches increase one‐year smoking cessation rates: results from the European CEASE trial. European Respiratory Journal, 13(2), 238–246. [DOI] [PubMed] [Google Scholar]
- Tonnesen P., Tonstad S., Hjalmarson A., Lebargy F., van Spiegel P.I., Hider A., Sweet R., Townsend J. (2003) A multicentre, randomised, double‐blind, placebo‐controlled, 1‐year study of bupropion SR for smoking cessation. Journal of Internal Medicine, 254(2), 184–192. [DOI] [PubMed] [Google Scholar]
- Tonstad S., Murphy M., Astbury C., Hider A., Sweet R. (2002) Zyban (TM) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease – 12 month follow‐up data. European Heart Journal, 23(Supplement S), 362–362. [Google Scholar]
- Wittchen H.‐U. (1994) Reliability and validity studies of the WHO‐Composite International Diagnostic Interview (CIDI): a critical review. Journal of Psychiatric Research, 28(1), 57–84. [DOI] [PubMed] [Google Scholar]
- Wittchen H.‐U., Pfister H. (1997) DIA‐X‐Interviews: Manual für Screening‐Verfahren und Interview; Interviewheft Längsschnittuntersuchung (DIA‐X‐Lifetime); Ergänzungsheft (DIA‐X‐Lifetime); Interviewheft Querschnittuntersuchung (DIA‐X‐12‐Monate); PC‐Programm zur Durchführung der Interviews (Längs‐ und Querschnittuntersuchung), Auswertungsprogramm. Frankfurt, Swets & Zeitlinger. [Google Scholar]
- Yudkin P.L., Jones L., Lancaster T., Fowler G.H. (1996) Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomised, double‐blind, placebo‐controlled trial. British Journal of General Practice, 46(404), 145–148. [PMC free article] [PubMed] [Google Scholar]


