Abstract
Brain derived neurotrophic factor (BDNF) is a molecular trophic factor that plays a key role in neuronal survival and plasticity. Single nucleotide polymorphisms (SNPs) of the BDNF gene have been associated with specific phenotypic traits in a large number of neuropsychiatric disorders and the response to psychotherapeutic medications in patient populations. Nevertheless, due to study differences and occasionally contrasting findings, substantial further research is required to understand in better detail the association between specific BDNF SNPs and these psychiatric disorders. While considerable progress has been made recently in developing advanced genotyping platforms of SNPs, many high‐throughput probe‐ or array‐based detection methods currently available are limited by high costs, slow processing times or access to advanced instrumentation. The polymerase chain reaction (PCR)‐based, tetra‐primer amplification refractory mutation system (T‐ARMS) method is a potential alternative technique for detecting SNP genotypes efficiently, quickly, easily, and cheaply. As a tool in psychopathology research, T‐ARMS was shown to be capable of detecting five common SNPs in the BDNF gene (rs6265, rs988748, rs11030104, 11757G/C and rs7103411), which are all SNPs with previously demonstrated clinical relevance to schizophrenia and depression. The present technique therefore represents a suitable protocol for many research laboratories to study the genetic correlates of BDNF in psychiatric disorders. Copyright Copyright © 2015 John Wiley & Sons, Ltd.
Keywords: SNP genotyping, T‐ARMS, brain‐derived neurotrophic factor, psychiatric disorders, schizophrenia
Introduction
The human brain‐derived neurotrophic factor (BDNF) gene is approximately 70 kb long and is located on chromosome 11p13 (Maisonpierre et al., 1991; Pruunsild et al., 2007). BDNF stimulates the tyrosine kinase receptor B, which promotes neuronal survival, coordinates synaptic remodeling, and regulates neurotransmitter biochemistry (Ashe et al., 2001; Binder et al., 2001). As BDNF is posited to play a key role in neuronal function (Agartz et al., 2006; Jonsson et al., 2006; Lencz et al., 2009; Pruunsild et al., 2007), a considerable body of research has been undertaken to understand the role of BDNF polymorphisms in the context of psychiatric disorders, including schizophrenia (Buckley et al., 2011), bipolar disorder (Rakofsky et al., 2012), depression (Bulgin et al., 2008; Jiang and Salton, 2013; Schumacher et al., 2005; Strauss et al., 2004), attention deficit hyperactivity disorder (ADHD, Lee et al., 2007) as well as many others (Hong et al., 2011).
In particular, the literature indicates that multiple individual BDNF single nucleotide polymorphisms (SNPs) have demonstrated associations with the phenotype of schizophrenia or the response to antipsychotic drugs. These include the specific SNPs: rs6265 (G to A mutation), rs988748 (C to G mutation), rs11030104 (T to C mutation), 11757 G/C (G to C mutation) and rs7103411 (C to T mutation) (Agartz et al., 2006; Chao et al., 2008; Hong et al., 2003; Jonsson et al., 2006; Lencz et al., 2009; Neves‐Pereira et al., 2005; Novak et al., 2010; Qian et al., 2007; Schumacher et al., 2005; Skibinska et al., 2008; Varnas et al., 2008; Zhang et al., 2013). The rs6265 polymorphism is notable as an exonal SNP that causes a Val66Met amino acid change in the precursor protein, altering secretion and intracellular trafficking of BDNF (Egan et al., 2003). The polymorphisms rs988748, rs11030104, 11757 G/C and rs7104311 in contrast are intronal SNPs suspected of contributing to haplotypes that affect BDNF function (Beuten et al., 2005; Novak et al., 2010; Schumacher et al., 2005). As a large number of BDNF SNPs are found to be relevant to schizophrenia, a simple and efficient genotyping method is necessary to concurrently detect these multiple SNPs in the same samples to facilitate genetic association studies.
Genetic research with SNPs can be achieved through sensitive, high‐throughput genotyping assays (Kim and Misra, 2007). However, a significant number of clinical laboratories may not have the resources or specialized equipment to utilize these sophisticated and highly specialized techniques. To date, restriction fragment length polymorphism (RFLP) has been a common and well‐established low‐throughput choice for BDNF SNP detection (Bueller et al., 2006; Jonsson et al., 2006; Neves‐Pereira et al., 2005; Sears et al., 2011). While RFLP is simple and cost‐effective, it is also time‐consuming and dependent on post‐amplification modifications (Kim and Misra, 2007; Twyman and Primrose, 2003). Such concerns motivate the need for a more streamlined genotyping alternative.
Tetra‐primer amplification refractory mutation system (T‐ARMS) is an established genotyping strategy that involves a single polymerase chain reaction (PCR) amplification followed by gel electrophoresis (Hamajima et al., 2000; Wirz et al., 2004). The amplification is a multiplex reaction of two primer pairs, which amplify the two defining alleles (mutant or wild type) of a SNP, respectively (Liu et al., 2012; Ye et al., 2001). Several T‐ARMS assays have been reported to reliably genotype commonly investigated mutations in genes such as methylenetetrahydrofolate reductase (Lajin et al., 2012), coagulation Factor V, and α1‐antitrypsin (Etlik et al., 2011). Of interest, Sheikh et al. (2010) previously applied the T‐ARMS method to detect BDNF rs6265. The aim of the present work was to demonstrate the versatility and suitability of T‐ARMS to genotype BDNF by extending the assay to four additional BDNF SNPs. This study evaluated the capacity of T‐ARMS to provide an easily optimized yet practical technique that can be performed in any standard molecular biology laboratory for cheap, rapid and simple genotyping of BDNF.
Methods
DNA extraction
Control genomic DNA for this study was obtained by venous blood draws from healthy adult volunteers; DNA from 96 subjects was used for the validation of the technique. DNA was extracted using QIAamp DNA Blood Maxi Kit (Qiagen) and quantified using the Nanodrop‐1000 Spectrophotometer (Thermo Scientific). The study protocol was approved by the University of British Columbia Research Ethics Board. The study was conducted in accordance with the principles of Good Clinical Practices and the Declaration of Helsinki.
Primer design
T‐ARMS primer pairs were designed using Primer1 software (http://primer1.soton.ac.uk/primer1.html). The inner T‐ARMS primer pair was designed with a deliberate mismatch −2 bp from the 3′ terminus to increase allele‐specificity (Ye et al., 2001). T‐ARMS primer lengths were adjusted to ensure the melting temperatures of the primer set were within the annealing temperature range of the touchdown PCR protocol. RFLP primers were designed to flank the restriction enzyme cut sequence. When possible, primers were selected with GC content percentage between 40 and 60% while possessing fewer than four sequential repeats of a same base at any location. Both T‐ARMS and RFLP primers were verified for non‐specificity using the Primer‐BLAST online application.
T‐ARMS PCR genotyping
T‐ARMS reactions were conducted on the Arktik thermocycler (Thermo Scientific) by two thermocyling protocols using touchdown PCR. Each PCR reaction was carried out in a total volume of 20 µl containing 100 ng DNA, 200 nM dNTP mix (Invitrogen), 1.5 μM MgCl2, SNP‐specific tetra‐primer mix (0.1–1 μM; Table 1), 1X PCR Buffer and 1 U Platinum Taq DNA Polymerase (Invitrogen). T‐ARMS PCR reactions were performed as follows: two minute initial denaturation (94°C), 30 cycles of 30 seconds denaturation (94°C), 30 seconds annealing (variable temperature), and 30 seconds elongation (72°C), with final five minute extension (72°C). For rs6265, rs988748, and rs7103411, annealing temperature decreased from 63 to 57°C (−0.3°C/cycle) for the first 20 cycles followed by 10 cycles of steady 57°C annealing. For rs11030104 and 11757 G/C, annealing temperature decreased from 61 to 54°C (−0.5°C/cycle) for the first 14 cycles followed by 16 cycles of steady 54°C annealing.
Table 1.
Primers used in PCR for T‐ARMS genotyping and sequencing reactions
| Primer | Primer sequence (5′–3′) | PCR [Primer] (μM) |
|---|---|---|
| rs6265_TARMS_OF | CCT ACA GTT CCA CCA GGT GAG AAG | 0.4 |
| rs6265_TARMS_OR | GAC CCT CAT GGA CAT GTT TGC | 0.4 |
| rs6265_TARMS_IF | GGC TGA CAC TTT CGA ACc CA | 0.8 |
| rs6265_TARMS_IR | GGT CCT CAT CCA ACA GCT CTT CTA TaA C | 0.8 |
| rs988748_TARMS_OF | CTA AGT GAG CAG CCT TCA GAA TGC | 0.25 |
| rs988748_TARMS_OR | CGA GTG TCA CAC ATT TAG CTT TGC | 0.25 |
| rs988748_TARMS_IF | GGA ACC AAC GCA GAG GGT aTG | 0.75 |
| rs988748_TARMS_IR_II | AGC TGG ATA CCG CTA CCC aAG | 0.75 |
| rs11030104_TARMS_OF | TGT GAT AAA GAG TCA TCC GAA GGT | 0.25 |
| rs11030104_TARMS_OR | AGT AGC AGG GTG GAT GGT | 0.25 |
| rs11030104_TARMS_IF | CAG GAA ATT GTA GGA CAG TTA GgA C | 0.75 |
| rs11030104_TARMS_IR | AAT AAT TAA AAA GCA GAT AAC ACT ACC tCA | 0.75 |
| 11757 G/C_TARMS_OF | GCT TCA ACT TAG TAA AGC CCT TTG G | 0.1 |
| 11757 G/C_TARMS_OR | GGA TTC TAA TGA CTG GGA ATT AAC AAG AC | 0.1 |
| 11757 G/C_TARMS_IF | TCA CAG GGA ATC TAA AGA AAT TTA AGc AG | 0.2 |
| 11757 G/C_TARMS_IR | GAG CTC CTG AAC GAc GG | 1.0 |
| rs7103411_TARMS_OF | ACA TCA AGA GCG GGC TGA CTT G | 0.25 |
| rs7103411_TARMS_OR | TGA TAC AGC TTT GAC CAT CCG TTT AAT GG | 0.25 |
| rs7103411_TARMS_IF | GGA GCG CAC TGT AAA GAT ACT GAg AC | 0.75 |
| rs7103411_TARMS_IR | CAA CAT TGA TCT CAC ATT CGT GTg CA | 0.75 |
Note: Lower case bases denote deliberately substituted mismatch (Ye et al., 2001) to increase primer specificity. Abbreviations: OF, outer forward; OR, outer reverse; IF, inner forward; IR, inner reverse.
Validation by RFLP or Sanger sequencing
RFLP PCR reactions were conducted on the Arktik thermocycler (Thermo Scientific) by two separate thermocyling protocols. Each PCR reaction was carried out in a total volume of 20 µl, containing 100 ng DNA, 200 nM dNTP mix (Invitrogen), 1 μM each of forward and reverse primers (Table 2), 1X Phire Green Reaction Buffer and Phire Green Hot Start II DNA Polymerase (Thermo Scientific). rs6265 and rs988748 used the following cycling protocol: 30 seconds initial denaturation at 98°C, followed by 35 cycles of five seconds denaturation (98°C), five seconds annealing (68.2°C), three seconds extension (72°C) with final one minute extension (72°C). rs11030104 and 11757 G/C had an annealing temperature at 64.3°C with otherwise identical conditions to the first RFLP cycling protocol. PCR reactions were cleaned using QIAquick PCR Purification Kit (Qiagen), and quantified on the Nanodrop‐1000 Spectrophotometer (Thermo Scientific). Amplicons were digested with restriction enzyme as follows: Hin1II for rs6265, Alw26I for rs988748, RsaI for rs11030104, Eco47I for 11757 G/C. Each digestion was carried out in 30 µl, containing 1 µg DNA, 0.1 mg/ml BSA (only rs11030104), 0.7X Buffer, and 5–10 U enzyme (Thermo Scientific, Promega). Digestion reactions were incubated at 37°C for two hours, followed by 20 minutes inactivation at 65°C.
Table 2.
PCR primers for amplification prior to genotyping by RFLP
| Primer name | Primer sequence (5’ – 3’) |
|---|---|
| rs6265_RFLP_F | AAA GAA GCA AAC ATC CGA GGA CAA G |
| rs6265_RFLP_R | ATT CCT CCA GCA GAA AGA GAA GAG G |
| rs988748_RFLP_F | TTG GAG TAG GGT TCC TCC AGT |
| rs988748_RFLP_R | AGA GGG CAT GAA GCT GGA TA |
| rs11030104_RFLP_F | ACA TCC TGA TGG TAA AAG CT |
| rs11030104_RFLP_R | GGT AAA TAT AGG CTG TGC TAA C |
| 11757 G/C_RFLP_F | AAT ACA AGT AGG ACC CTA GC |
| 11757 G/C_RFLP_R | CCT CCT GCA GCC ATT AGT |
Note: F, forward; R, reverse.
Due to unavailability of RFLP enzyme, alleles of rs7103411 suggested by T‐ARMS were confirmed by DNA Sanger Sequencing in collaboration with the CMMT DNA Sequencing Core Facility (Vancouver, Canada). Each 15 µl sequencing reaction contained 10 pmol of “rs7103411_TARMS_OF” primer with 55 ng of cleaned PCR product.
Gel electrophoresis
PCR amplicons or restriction digestions were mixed with 6X Track‐it Cyan‐Yellow Dye (Invitrogen) prior to loading on 2% (w/v) agarose gel (4% for 11757 G/C RFLP genotyping) pre‐stained with 1:10000 Sybr‐Safe DNA Stain (Invitrogen). Gels were run at 100 V for one hour and visualized by blue light fluorescence (595 nm wavelength) on LAS‐3000 imager (Fujifilm).
Results
The T‐ARMS assay unambiguously detected all three genotypes (homozygous wild type, heterozygous, and homozygous mutant) for five BDNF SNPs: rs6265, rs988748, rs11030104, 11757 G/C and rs7103411 in the DNA of the 96 subjects; the call rate was 100% for all SNPs. These genotypes were concordant when validated by PCR‐RFLP or Sanger Sequencing (Figures 1, 2, 3, 4, 5).
Figure 1.
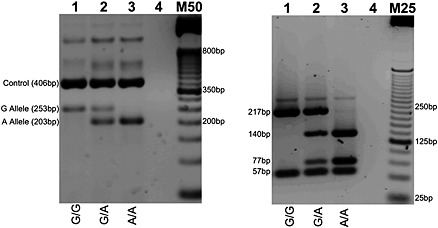
rs6265 (G to A reverse strand) genotyping by T‐ARMS PCR (left) with cross‐validation by PCR‐RFLP (right). RFLP genotyping by Hin1II digestion: G Allele (57 bp and 217 bp); A Allele (57 bp, 77 bp, and 140 bp). Homozygous wild type, heterozygous, and homozygous mutant genotypes presented in lanes 1, 2, and 3, respectively. Lane 4 contains no genomic DNA. M50 designates a 50 bp DNA molecular weight marker; M25 is a 25 bp molecular weight marker. PCR products were resolved on 2% agarose gel.
Figure 2.
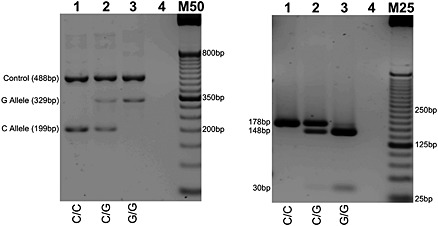
rs988748 (C to G reverse strand) genotyping by T‐ARMS PCR (left) with cross‐validation by PCR‐RFLP (right). RFLP genotyping by Alw26I digestion: C Allele (178 bp); G Allele (148 bp and 30 bp). Homozygous wild type, heterozygous, and homozygous mutant genotypes presented in lanes 1, 2, and 3, respectively. Lane 4 contains no genomic DNA. M50 designates a 50 bp DNA molecular weight marker; M25 is a 25 bp molecular weight marker. PCR products were resolved on 2% agarose gel.
Figure 3.
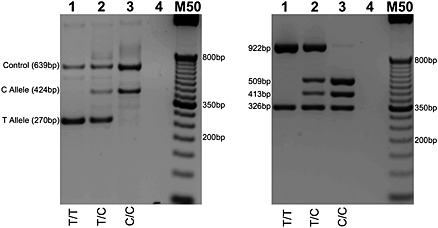
rs11030104 (T to C reverse strand) genotyping by T‐ARMS PCR (left) with cross‐validation by PCR‐RFLP (right). RFLP genotyping by RsaI digestion: T Allele (326 bp and 922 bp); C Allele (326 bp, 413 bp, and 509 bp). Homozygous wild type, heterozygous, and homozygous mutant genotypes presented in lanes 1, 2, and 3, respectively. Lane 4 contains no genomic DNA. M50 designates a 50 bp DNA molecular weight marker. PCR products were resolved on 2% agarose gel.
Figure 4.
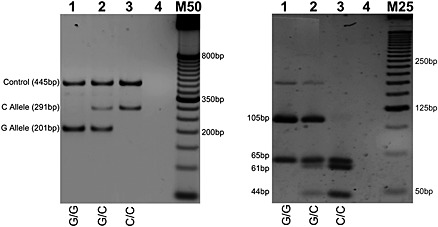
11757G/C (G to C forward strand) genotyping by T‐ARMS PCR (left) with cross‐validation by PCR‐RFLP (right). RFLP genotyping by Eco47I digestion: G Allele (65 bp and 105 bp); C Allele (65 bp, 61 bp, and 44 bp). Homozygous wild type, heterozygous, and homozygous mutant genotypes presented in lanes 1, 2, and 3, respectively. Lane 4 contains no genomic DNA. M50 designates a 50 bp DNA molecular weight marker; M25 is a 25 bp molecular weight marker. T‐ARMS PCR products were resolved on 2% agarose gel; PCR‐RFLP products were resolved on 4% agarose gel.
Figure 5.
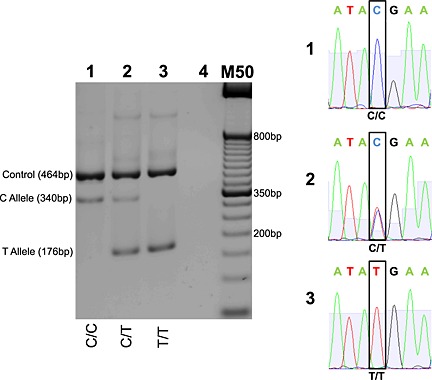
rs7103411 (C to T forward strand) genotyping by T‐ARMS PCR (left) with cross‐validation by Sanger Sequencing (right). Homozygous wild type, heterozygous, and homozygous mutant genotypes presented in lanes 1, 2, and 3, respectively. Lane 4 contains no genomic DNA. M50 designates a 50 bp DNA molecular weight marker. T‐ARMS PCR products were resolved on 2% agarose gel. Sequence output for homozygous wildtype, heterozygous, and homozygous mutant samples presented on chromatograms 1, 2, and 3, respectively. Sequence flanking SNP: 5′‐ATA[C/T]GAA‐3′.
Indeed, the T‐ARMS results appeared to be less ambiguous than the RFLP results, which were sometimes liable to incomplete enzymatic digestion leading to false heterozygote results. PCR products produced by T‐ARMS were well separated and more distinguishable than the digested PCR products from RFLP. With rs7103411, T‐ARMS was still capable of genotyping whereas RFLP could not due to the lack of an enzyme restriction site. This affirms the utility of the T‐ARMS as a genotyping technique, as its amplified product sizes can be easily customized, whereas the digested product sizes from RFLP is dictated by the enzyme cut sites flanking the SNP.
It should be noted that non‐specific bands were present in a few T‐ARMS assays (Figures 1, 3, and 5). However, the sizes of the non‐specific products were not within the range of the desired PCR products and do not appear to interfere with the interpretation of the results.
Discussion
Polymorphisms of the BDNF gene have been linked to numerous psychiatric disorders, including schizophrenia (Agartz et al., 2006; Ashe et al., 2001; Jonsson et al., 2006), and affect variables ranging from clinical response to antipsychotic drugs through to brain morphology (Smith et al., 2012). Given the complex phenotype of the psychiatric disorders such as schizophrenia, there is an unmet need for simplified SNP genotyping. The T‐ARMS protocol presently described addresses this issue by providing a cheap, fast and efficient yet robust method of detecting BDNF SNPs. T‐ARMS successfully detected five BDNF SNPs: rs6265, rs988748, rs11030104, 11757 G/C, and rs7103411. Comparatively, T‐ARMS improves upon conventional genotyping because it relies on a single PCR without additional post‐amplification manipulation (Kim and Misra, 2007; Twyman and Primrose, 2003). This distinction represents a significant saving in both cost and time.
Although T‐ARMS is a relatively straightforward procedure, certain parameters should be considered. The biggest hurdle to performing a successful T‐ARMS assay is selection of compatible primers. The allele specificity T‐ARMS derives from the inner primer pair where one primer is complementary to the wild type allele and the other complementary to the mutant allele. As reported previously (Liu et al., 2012; Ye et al., 2001), allele specificity is further increased by introducing deliberate mismatches at the −2 location of the 3′ primer end. Besides allele specificity, care also needs to be taken regarding the primer concentrations used in the assay. Following the observations of Ye et al. (2001), the current T‐ARMS protocol used a primer mixture with preferentially higher concentration of inner primers versus outer primers to promote amplification of allele specific products. However, primer concentrations were not constant across all five SNPs (Table 1). Prospective users of T‐ARMS would be advised to optimize working primer concentrations on a case‐by‐case basis prior to applying the method to larger sample sizes.
Beyond primers, the choice of polymerase is also important. Notably, absence of exonuclease activity by the polymerase enzyme is an important requirement for the T‐ARMS assay. Proof‐reading enzymes (such as Pfu) are incompatible with T‐ARMS as their 3′ exonuclease activity would eliminate the 3′ mismatches critical to primer‐allele specificity. Another assay parameter to consider is the thermal cycling temperatures. The present study relied on a touchdown PCR where annealing temperatures gradually decreased with each amplification cycle (Ye et al., 2001). Touchdown PCR theoretically increases detection sensitivity because initial amplification at high temperatures allows primer‐specific products to preferentially outcompete against primer‐non‐specific amplicons with each cycle. The optimal touchdown range would encompass the melting temperature of all four primers. It would begin a few degrees higher than the highest primer melting temperature and end a few degrees below the lowest primer melting temperature.
As with any assay, T‐ARMS also possess certain limitations that need to be recognized. In particular, any multiplex PCR is prone to generate non‐specific PCR products. Although primer non‐specific binding is greatly reduced through touchdown cycling, the formation of non‐specific products cannot be eliminated. Thus, the possibility of non‐specific product interference in genotype interpretation is a concern. Difficulty of designing appropriate primers is another factor hindering this technique's applicability. Since allele‐specific primers bind to the SNP, the primer melting temperature is constrained by the sequence immediately flanking the SNP. This poses a concern when suitable outer primers with similar melting temperatures cannot be found. Lajin et al. (2012) proposed the use of betaine additive to circumvent this problem. As betaine decreases base pair composition dependency, the addition of betaine may facilitate primers of the same length but different compositions to have similar melting temperatures.
Nevertheless, the benefits of T‐ARMS are numerous. For one, the T‐ARMS technique requires minimal instrumentation. Only a PCR thermocycler and gel electrophoresis apparatus are needed, making the assay accessible to any standard molecular biology laboratory. Execution of the assay is also fast with typical results turnaround within three hours or less, which is in strong contrast to most “next‐gen” sequencing where results have a significantly longer turnaround, not least due to the much greater amount of information that is obtained and must be processed. Additionally, this method can be applied not only to genotype a single gene, but also to examine haplotypes of SNPs in parallel across different genes. The adaptable nature of T‐ARMS opens the possibility of conducting low‐throughput genotyping studies previously deemed impermissible due to restraints, such as a lack of an enzyme cut site for RFLP. The T‐ARMS protocol might also potentially be envisioned as a practical technique to validate results obtained from more high‐throughput means, although further validation with other genes is required. We have recently confirmed that the technique can work reliably when detecting SNPs for the catecholamine degrading enzyme catechol‐O‐methyltransferase (COMT) (manuscript in preparation), but whether the technique works in more complex regions, such as the MHC (major histocompatibility complex) region where risk of non‐specific amplification is much higher, needs to be addressed in future studies.
Considering the active interest in SNP detection and the role of SNPs as markers in complex diseases (Kim and Misra, 2007), much progress has been made in developing robust and versatile genotyping assays. Despite their greater throughput, advanced genotyping technologies (e.g. melting curve analysis or SNP microarrays) are often inaccessible to laboratories without the specialized resources to carry out such analyses (Kim and Misra, 2007; Kwok and Chen, 2003; Twyman and Primrose, 2003). Few assays are available to laboratories looking for greater efficiency without incurring the costs of specialized instrumentation. T‐ARMS is one such method where SNPs are detected quickly, easily, and cheaply. When applied to BDNF as presently done, T‐ARMS detected five select SNPs believed to have clinical importance in schizophrenia (Agartz et al., 2006; Ashe et al., 2001; Jonsson et al., 2006; Lencz et al., 2009; Pruunsild et al., 2007). In light of this work, interested investigators in psychiatric disorders such as schizophrenia, depression or other BDNF‐related pathologies may choose to adopt T‐ARMS as a suitable tool to study the genetic correlates of neuropsychiatric conditions and drug‐associated effects (Barr et al., 2008; Boyda et al., 2012; Boyda et al., 2010; Honer et al., 2007; Leung et al., 2012).
Declaration of interest statement
Dr Procyshyn is a paid consultant for and is on the speaker's bureau for AstraZeneca, Bristol‐Myers Squibb, Janssen, Otsuka, Pfizer, and Sunovion. He also has funding from the Canadian Institute of Health Research.
Dr Honer has received consulting fees or sat on paid advisory boards for: MDH Consulting, In Silico (no honorarium), Novartis, Roche, Otsuka, and Lundbeck; received honoraria from Rush University, the Korean Society for Schizophrenia Research, the Center for Addiction and Mental Health (Toronto), the BC Schizophrenia Society, the Fraser, Vancouver Coastal and the Providence Health Authorities, and the Canadian Agency for Drugs and Technology in Health; provided expert testimony for Fasken Martineau DuMoulin, Cave and Company, and Hartshorne & Mehl; and received grants from the Canadian Institute of Health Research.
Dr Barr has received grants from Bristol‐Myers Squibb.
All other authors declare no potential competing interests.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Kevin Tsai in PCR set‐up and optimization. Financial support for the current project was provided by the British Columbia Provincial Health Services Authority and NSERC grant 356069‐09 to AMB.
Wang, C. K. , Xu, M. S. , Ross, C. J. , Lo, R. , Procyshyn, R. M. , Vila‐Rodriguez, F. , White, R. F. , Honer, W. G. , and Barr, A. M. (2015) Development of a cost‐efficient novel method for rapid, concurrent genotyping of five common single nucleotide polymorphisms of the brain derived neurotrophic factor (BDNF) gene by tetra‐primer amplification refractory mutation system. Int. J. Methods Psychiatr. Res., 24: 235–244. doi: 10.1002/mpr.1475.
References
- Agartz I., Sedvall G.C., Terenius L., Kulle B., Frigessi A., Hall H., Jonsson E.G. (2006) BDNF gene variants and brain morphology in schizophrenia. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 141B(5), 513–523. [DOI] [PubMed] [Google Scholar]
- Ashe P.C., Berry M.D., Boulton A.A. (2001) Schizophrenia, a neurodegenerative disorder with neurodevelopmental antecedents. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 25(4), 691–707. [DOI] [PubMed] [Google Scholar]
- Barr A.M., Procyshyn R.M., Hui P., Johnson J.L., Honer W.G. (2008) Self‐reported motivation to smoke in schizophrenia is related to antipsychotic drug treatment. Schizophrenia Research, 100(1–3), 252–260. [DOI] [PubMed] [Google Scholar]
- Beuten J., Ma J.Z., Payne T.J., Dupont R.T., Quezada P., Huang W., Crews K.M., Li M.D. (2005) Significant association of BDNF haplotypes in European‐American male smokers but not in European‐American female or African‐American smokers. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 139B(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Binder D.K., Croll S.D., Gall C.M., Scharfman H.E. (2001) BDNF and epilepsy: too much of a good thing? Trends in Neurosciences, 24(1), 47–53. [DOI] [PubMed] [Google Scholar]
- Boyda H.N., Procyshyn R.M., Tse L., Wong D., Pang C.C., Honer W.G., Barr A.M. (2012) Intermittent treatment with olanzapine causes sensitization of the metabolic side‐effects in rats. Neuropharmacology, 62(3), 1391–1400. [DOI] [PubMed] [Google Scholar]
- Boyda H.N., Tse L., Procyshyn R.M., Wong D., Wu T.K., Pang C.C., Barr A.M. (2010) A parametric study of the acute effects of antipsychotic drugs on glucose sensitivity in an animal model. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 34(6), 945–954. [DOI] [PubMed] [Google Scholar]
- Buckley P.F., Pillai A., Howell K.R. (2011) Brain‐derived neurotrophic factor: findings in schizophrenia. Current Opinion in Psychiatry, 24(2), 122–127. [DOI] [PubMed] [Google Scholar]
- Bueller J.A., Aftab M., Sen S., Gomez‐Hassan D., Burmeister M., Zubieta J.K. (2006) BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry, 59(9), 812–815. [DOI] [PubMed] [Google Scholar]
- Bulgin N.L., Strauss J.S., King N.A., Shaikh S.A., George C.J., Fox N.A., Barr C.L., Kovacs M., Kennedy J.L. (2008) Association study of theta EEG asymmetry and brain‐derived neurotrophic factor gene variants in childhood‐onset mood disorder. NeuroMolecular Medicine, 10(4), 343–355. [DOI] [PubMed] [Google Scholar]
- Chao H.M., Kao H.T., Porton B. (2008) BDNF Val66Met variant and age of onset in schizophrenia. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 147B(4), 505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D.R. (2003) The BDNF val66met polymorphism affects activity‐dependent secretion of BDNF and human memory and hippocampal function. Cell, 112(2), 257–269. [DOI] [PubMed] [Google Scholar]
- Etlik O., Koksal V., Arican‐Baris S.T., Baris I. (2011) Development and validation of a cost‐effective in‐house method, tetra‐primer ARMS PCR assay, in genotyping of seven clinically important point mutations. Molecular and Cellular Probes, 25(4), 177–181. [DOI] [PubMed] [Google Scholar]
- Hamajima N., Saito T., Matsuo K., Kozaki K., Takahashi T., Tajima K. (2000) Polymerase chain reaction with confronting two‐pair primers for polymorphism genotyping. Japanese Journal of Cancer Research, 91(9), 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honer W.G., Thornton A.E., Sherwood M., MacEwan G.W., Ehmann T.S., Williams R., Kopala L.C., Procyshyn R., Barr A.M. (2007) Conceptual and methodological issues in the design of clinical trials of antipsychotics for the treatment of schizophrenia. CNS Drugs, 21(9), 699–714. [DOI] [PubMed] [Google Scholar]
- Hong C.J., Huo S.J., Yen F.C., Tung C.L., Pan G.M., Tsai S.J. (2003) Association study of a brain‐derived neurotrophic‐factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. Neuropsychobiology, 48(4), 186–189. [DOI] [PubMed] [Google Scholar]
- Hong C.J., Liou Y.J., Tsai S.J. (2011) Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Research Bulletin, 86(5–6), 287–297. [DOI] [PubMed] [Google Scholar]
- Jiang C., Salton S.R. (2013) The role of neurotrophins in major depressive disorder. Translational Neuroscience, 4(1), 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson E.G., Edman‐Ahlbom B., Sillen A., Gunnar A., Kulle B., Frigessi A., Vares M., Ekholm B., Wode‐Helgodt B., Schumacher J., Cichon S., Agartz I., Sedvall G.C., Hall H., Terenius L. (2006) Brain‐derived neurotrophic factor gene (BDNF) variants and schizophrenia: an association study. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 30(5), 924–933. [DOI] [PubMed] [Google Scholar]
- Kim S., Misra A. (2007) SNP genotyping: technologies and biomedical applications. Annual Review of Biomedical Engineering, 9, 289–320. [DOI] [PubMed] [Google Scholar]
- Kwok P.Y., Chen X. (2003) Detection of single nucleotide polymorphisms. Current Issues in Molecular Biology, 5(2), 43–60. [PubMed] [Google Scholar]
- Lajin B., Alachkar A., Sakur A.A. (2012) Triplex tetra‐primer ARMS‐PCR method for the simultaneous detection of MTHFR c.677C>T and c.1298A>C, and MTRR c.66A>G polymorphisms of the folate‐homocysteine metabolic pathway. Molecular and Cellular Probes, 26(1), 16–20. [DOI] [PubMed] [Google Scholar]
- Lee J., Laurin N., Crosbie J., Ickowicz A., Pathare T., Malone M., Tannock R., Kennedy J.L., Schachar R., Barr C.L. (2007) Association study of the brain‐derived neurotropic factor (BDNF) gene in attention deficit hyperactivity disorder. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 144B(8), 976–981. [DOI] [PubMed] [Google Scholar]
- Lencz T., Lipsky R.H., DeRosse P., Burdick K.E., Kane J.M., Malhotra A.K. (2009) Molecular differentiation of schizoaffective disorder from schizophrenia using BDNF haplotypes. British Journal of Psychiatry, 194(4), 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J.Y., Barr A.M., Procyshyn R.M., Honer W.G., Pang C.C. (2012) Cardiovascular side‐effects of antipsychotic drugs: the role of the autonomic nervous system. Pharmacology & Therapeutics, 135(2), 113–122. [DOI] [PubMed] [Google Scholar]
- Liu J., Huang S., Sun M., Liu S., Liu Y., Wang W., Zhang X., Wang H., Hua W. (2012) An improved allele‐specific PCR primer design method for SNP marker analysis and its application. Plant Methods, 8(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P.C., Le Beau M.M., Espinosa R. 3rd, Ip N.Y., Belluscio L., de la Monte S.M., Squinto S., Furth M.E., Yancopoulos G.D. (1991) Human and rat brain‐derived neurotrophic factor and neurotrophin‐3: gene structures, distributions, and chromosomal localizations. Genomics, 10(3), 558–568. [DOI] [PubMed] [Google Scholar]
- Neves‐Pereira M., Cheung J.K., Pasdar A., Zhang F., Breen G., Yates P., Sinclair M., Crombie C., Walker N., St Clair D.M. (2005) BDNF gene is a risk factor for schizophrenia in a Scottish population. Molecular Psychiatry, 10(2), 208–212. [DOI] [PubMed] [Google Scholar]
- Novak G., LeBlanc M., Zai C., Shaikh S., Renou J., DeLuca V., Bulgin N., Kennedy J.L., Le Foll B. (2010) Association of polymorphisms in the BDNF, DRD1 and DRD3 genes with tobacco smoking in schizophrenia. Annals of Human Genetics, 74(4), 291–298. [DOI] [PubMed] [Google Scholar]
- Pruunsild P., Kazantseva A., Aid T., Palm K., Timmusk T. (2007) Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics, 90(3), 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Zhao J., Shi Y., Zhao X., Feng G., Xu F., Zhu S., He L. (2007) Brain‐derived neurotrophic factor and risk of schizophrenia: an association study and meta‐analysis. Biochemical and Biophysical Research Communications, 353(3), 738–743. [DOI] [PubMed] [Google Scholar]
- Rakofsky J.J., Ressler K.J., Dunlop B.W. (2012) BDNF function as a potential mediator of bipolar disorder and post‐traumatic stress disorder comorbidity. Molecular Psychiatry, 17(1), 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Jamra R.A., Becker T., Ohlraun S., Klopp N., Binder E.B., Schulze T.G., Deschner M., Schmal C., Hofels S., Zobel A., Illig T., Propping P., Holsboer F., Rietschel M., Nothen M.M., Cichon S. (2005) Evidence for a relationship between genetic variants at the brain‐derived neurotrophic factor (BDNF) locus and major depression. Biological Psychiatry, 58(4), 307–314. [DOI] [PubMed] [Google Scholar]
- Sears C., Markie D., Olds R., Fitches A. (2011) Evidence of associations between bipolar disorder and the brain‐derived neurotrophic factor (BDNF) gene. Bipolar Disorders, 13(7–8), 630–637. [DOI] [PubMed] [Google Scholar]
- Sheikh H.I., Hayden E.P., Kryski K.R., Smith H.J., Singh S.M. (2010) Genotyping the BDNF rs6265 (val66met) polymorphism by one‐step amplified refractory mutation system PCR. Psychiatric Genetics, 20(3), 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinska M., Kapelski P., Slopien A., Leszczynska‐Rodziewicz A., Godlewska B., Wilkosc M., Tomaszewska M., Dmitrzak‐Weglarz M., Czerski P. (2008) Association study of four polymorphisms of brain‐derived neurotrophic factor (BDNF) gene with schizophrenia. European Neuropsychopharmacology, 18: S215. [Google Scholar]
- Smith G.N., Thornton A.E., Lang D.J., Macewan G.W., Ehmann T.S., Kopala L.C., Tee K., Shiau G., Voineskos A.N., Kennedy J.L., Honer W.G. (2012) Hippocampal volume and the brain‐derived neurotrophic factor Val66Met polymorphism in first episode psychosis. Schizophrenia Research, 134(2–3), 253–259. [DOI] [PubMed] [Google Scholar]
- Strauss J., Barr C.L., George C.J., King N., Shaikh S., Devlin B., Kovacs M., Kennedy J.L. (2004) Association study of brain‐derived neurotrophic factor in adults with a history of childhood onset mood disorder. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 131B(1), 16–19. [DOI] [PubMed] [Google Scholar]
- Twyman R.M., Primrose S.B. (2003) Techniques patents for SNP genotyping. Pharmacogenomics, 4(1), 67–79. [DOI] [PubMed] [Google Scholar]
- Varnas K., Lawyer G., Jonsson E.G., Kulle B., Nesvag R., Hall H., Terenius L., Agartz I. (2008) Brain‐derived neurotrophic factor polymorphisms and frontal cortex morphology in schizophrenia. Psychiatric Genetics, 18(4), 177–183. [DOI] [PubMed] [Google Scholar]
- Wirz S.A., Morale M.C., Marchetti B., Barr A.M., Sotgiu S., Rosati G., Pugliatti M., Sanna M.V., Giliberto O., Bartfai T., Conti B. (2004) High frequency of TNF alleles –238A and –376A in individuals from northern Sardinia. Cytokine, 26(4), 149–154. [DOI] [PubMed] [Google Scholar]
- Ye S., Dhillon S., Ke X., Collins A.R., Day I.N. (2001) An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Research, 29(17), E88–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.P., Lencz T., Geisler S., DeRosse P., Bromet E.J., Malhotra A.K. (2013) Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophrenia Research, 146(1–3), 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]


