Abstract
There is substantial evidence that patient compliance or rather adherence to medical measures and recommendations for lifestyle changes can pivotally influence the prognosis of the patients or disease progression. However, the scientific evaluation and the statistical analysis of “patient adherence” are extremely difficult due to the fact that the construct of “adherence” is complex and comprised of many layers, and varies greatly in different disease groups. With this paper, we describe the development and structure of this novel assessment tool that takes past and prospective information on different facets of drug and behavioural adherence into account, expected to result in considerably improved prediction of future cardiovascular risk. We suggest a simple scoring scheme and explore the psychometric properties and the higher order factorial structure. In this exploratory study the “Diabetes Cardiovascular Risk Evaluation Targets and Essential Data for Commitment of Treatment” (DETECT) adherence score revealed good psychometric properties in terms of internal consistency and factorial structure, suggesting that its further exploration in terms of external validity is promising. Findings also underline that it is useful and informative to cover within one score both, pharmacologic and non‐pharmacologic interventions in primary care. Our combination in this respect is unique, as most studies conducted on this subject so far aimed at assessing solely drug adherence or behavioural adherence. Copyright © 2011 John Wiley & Sons, Ltd.
Keywords: DETECT adherence score, drug adherence, behavioural adherence, primary care, cardiovascular disease
Background
There is substantial evidence that patient compliance or rather adherence to medical measures and recommendations for lifestyle changes can pivotally influence the prognosis of the patients or disease progression. Recent reviews and meta‐analytic studies suggest fairly consistently that on average 26% of all patients across various disease groups experience a good outcome by adhering than by not adhering to medical recommendations (DiMatteo et al., 2002; McDermott et al., 1997). Non‐adherence is further known to increase physician and patient frustration and is associated with increased risk of incorrect diagnoses and unnecessary treatment (Joshi and Milfred, 1995). However, the scientific evaluation and the statistical analysis of “patient adherence” are extremely difficult for several reasons due to the fact that the construct of “adherence” is complex and comprised of many layers, and varies greatly in different disease groups. Apart from drug adherence as a measure of reliable and regular medication intake, there remains – often unnoted – the adherence to medically recommended behavioural modifications.
Therefore we developed a novel multidimensional adherence measure, intended for use in the primary care setting. The focus of this measure is on the primary prevention of cardiovascular disease in patients with metabolic and cardiovascular risk constellations as routinely treated by primary care physicians. The rationale for developing the scale refers to our observation that primary care physicians typically know their patients for longer time periods and see their patients on a more or less regular basis. In our multidimensional approach to adherence, the doctor first assesses the patients at baseline with regard to clinical, diagnostic and behavioural characteristics, supplemented by a self‐report of the patient. Secondly, the doctor defines individual target problems that he would like to address in therapy. Finally, after a certain time period, the doctor evaluates the success of recommended therapeutic interventions.
Aims
With this paper, we describe the development and structure of this novel assessment tool that takes into account past and prospective information on different facets of adherence and is expected to result in considerably improved prediction of future cardiovascular risk. We suggest a simple scoring scheme and explore the psychometric properties and the higher order factorial structure.
Methods
Study methods and study sample
The development of our adherence score took place in the context of the “Diabetes Cardiovascular Risk Evaluation Targets and Essential Data for Commitment of Treatment” (DETECT) study (Wittchen et al., 2005). DETECT is a large three‐stage prospective‐longitudinal clinical epidemiological study programme (Boehler et al., 2004; Pieper et al., 2005; Wittchen et al., 2005), based on a nationwide representative sample of initially over 3000 German primary care physicians and their patients. The primary aim of this study was to describe the real‐life situation of patients with diabetes mellitus, hyperlipidemia, arterial hypertension and other cardiovascular risk factors and predictors for outcome in primary care over a course of five years, focusing on: (i) prevalence and comorbidity rates of these disease and risk constellations, (ii) their progression, (iii) physician treatment patterns as well as (iv) met and unmet needs of patients (Boehler et al., 2007; Pieper et al., 2005; Pittrow et al., 2006; Wittchen et al., 2005). The adherence score development was triggered as part of the risk factor and predictor modelling.
The methods, design and procedures have been published elsewhere in greater detail (Bischoff et al., 2006; Pieper et al., 2005; Wittchen et al., 2005). Briefly, at baseline 55,518 unselected consecutive patients were enrolled of which a random subset of 7519 patients was chosen for a comprehensive laboratory test programme within a multi‐wave prospective longitudinal follow‐up study, with two additional assessments after one year and five years (Figure 1). All patients completed a standardized clinical assessment by their physician as well as a laboratory assessment, signed an informed consent form and completed a self‐report questionnaire before being assessed by their physician within a structured clinical interview. Physicians also documented symptoms, diagnoses, treatments and health behaviour of all patients at each visit.
Figure 1.
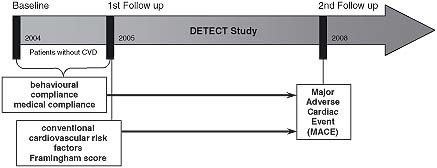
Study design for the adherence score.
The DETECT survey received the approval of the Ethics Committee of the Carl Gustav Carus Medical Faculty at the Technische Universitaet Dresden (AZ: EK149092003; 16 September 2003) and was registered at http://clinicaltrials.gov (NCT01076608).
For the present analysis, we used the subset of those DETECT study participants with complete information on baseline data and outcome on 2nd Follow up (n = 6826 patients). We further excluded all patients who had a cardiovascular disease at baseline, because we ultimately aimed to predict cardiovascular outcomes in primary prevention. Thus, 5645 patients were chosen for the present analysis. The sample characteristics are rleported in Table 1. In this sample of 5465 patients, the mean age was 55.8 years [standard deviation (SD) = 13.8; range = 18–95] and 62.2% of participants were female. Consistent with the age and gender distribution in German primary care, the point prevalence in this sample was 12.4% for diabetes mellitus, 34.5% for hypertension and 28.0% for hyperlipidemia. The majority of patients was married and employed.
Table 1.
Baseline characteristics of the study population (n = 5645)
| Characteristics | Totala | Femalea | Malea |
|---|---|---|---|
| No. of subjects | 5645 | 3512 | 2133 |
| Age, mean (SD), years | 55.8 (13.8) | 55.3 (14.2) | 56.8 (13.0) |
| Female, No. | 3512 (62.2%) | ||
| Family status, No. | |||
| Single | 543 (9.8%) | 333 (9.6%) | 210 (10.0%) |
| Married | 4001 (71.9%) | 2310 (66.8%) | 1691 (80.3%) |
| Divorced/ widowed | 1022 (18.4%) | 817 (23.6%) | 205 (9.7%) |
| Professional status, No. | |||
| Employed | 2539 (45.7%) | 1518 (43.8%) | 1021 (48.9%) |
| Homemaker | 328 (5.9%) | 199 (5.7%) | 129 (6.2%) |
| Unemployed | 458 (8.2%) | 454 (13.1%) | 4 (0.2%) |
| Retired | 2165 (39.0%) | 1264 (36.4%) | 901 (43.2%) |
| Other | 67 (1.2%) | 34 (1.0%) | 33 (1.6%) |
| Current smoker, No. | 1105 (21.2%) | 668 (20.8%) | 437 (21.9%) |
| Ex‐smoker, No. | 1272 (24.4%) | 553 (17.2%) | 719 (36.1%) |
| Waist to height ratio, mean (SD) | 0.56 (0.09) | 0.55 (0.09) | 0.57 (0.07) |
| Waist circumference; mean (SD), cm | 93.8 (14.7) | 89.4 (14.3) | 101.2 (12.2) |
| Body mass index, mean (SD), kg/m2 | 26.9 (4.8) | 26.5 (5.2) | 27.6 (4.0) |
| Hypertension, No. | 1948 (34.5%) | 1136 (32.4%) | 812 (38.1%) |
| Systolic blood pressure, mean (SD), mmHg | 131.6 (18.1) | 129.7 (18.6) | 134.8 (16.9) |
| Diastolic blood pressure, mean (SD), mmHg | 80.1 (9.8) | 79.3 (10.1) | 81.4 (9.2) |
| Antihypertensive treatment, No. | 1707 (31.9%) | 1006 (30.3%) | 701 (34.5%) |
| Diabetes mellitus, No. | 702 (12.4%) | 359 (10.2%) | 343 (16.1%) |
| Fasting plasma glucose, mean (SD), mg/dl | 99.5 (32.9) | 96.2 (29.9) | 105.0 (36.7) |
| HbA1c, mean (SD), % | 5.5 (0.8) | 5.4 (0.7) | 5.6 (0.9) |
| Oral drug treatment, No. | 448 (8.4%) | 215 (6.5%) | 233 (11.5%) |
| Insulin treatment, No. | 191 (3.6%) | 111 (3.3%) | 80 (3.9%) |
| Hyperlipidemia, No. | 1578 (28.0%) | 914 (26.0%) | 664 (31.1%) |
| Total cholesterol, mean (SD), mg/dl | 225.8 (42.2) | 226.9 (41.8) | 223.9 (42.7) |
| HDL cholesterol, mean (SD), mg/dl | 55.8 (18.6) | 60.7 (18.7) | 47.7 (15.1) |
| LDL cholesterol, mean (SD), mg/dl | 129.3 (33.3) | 128.8 (33.7) | 130.2 (32.5) |
| Depression,b No. | 298 (5.5%) | 219 (6.5%) | 79 (3.9%) |
| Depression score,b mean (SD) | 3.4 (3.3) | 3.7 (3.4) | 3.0 (3.1) |
All percentages refer to number of subjects with existing data.
Depression screening questionnaire (DSQ); at least three screening items “at most days” and a total score above eight.
Procedure for the scale and score construction
Based on theoretical considerations and a series of data explorations, we first selected seven domains, believed to be of particular interest. We used the laboratory, clinician and self‐reported patient data from the DETECT baseline and follow‐up assessment that appropriately matched these domains. Emphasis in this selection process was placed on choosing those measures that are typically routinely assessed by a primary care physician anyhow, and are easy to assess under routine conditions without major time investment.
Table 2 shows measures of baseline ratings, target areas and goal achievement for each of the seven domains of the adherence score. Most of the indicators tap into established risk factors for cardiovascular disease (e.g. physical activity, waist circumference, blood pressure, cholesterol levels, HbA1c and smoking), and clinicians' diagnoses of diabetes mellitus, hyperlipidemia and arterial hypertension. Additionally, we selected core patient factors, such as patient awareness of diagnosis and patient behavioural health salience ratings for risk factor modification as well as doctors' experiences with past patient behavioural interventions and drug interventions, supplemented by an overall compliance rating.
Table 2.
Measures of baseline ratings, target areas and goal attainment for the adherence score
| Baseline ratings and measures | One‐year outcome | |||
|---|---|---|---|---|
| I. Current and past Indicators assessment domains by doctor | II. Future target area chosen for intervention by doctor in prospective observation period | III. Degree of goal attainment (measure) | Source | |
| A. Measured risk factors | a. Physical activity (low) | Increase physical activity | ≥ two hours physical activity per week | P |
| b. Waist circumference [> 88 cm (female); 102 cm (male)] | Weight reduction | Decrease in waist circumference by ≥5 % | LAB | |
| c. Smoking | Quit or reduce smoking | Non‐smoker or ≥30% reduction of daily cigarette smoking | MD | |
| B. Diagnostic risk factors | a. Diabetes (Hba1c) | Improve diabetes (reduce HbA1c) | HbA1c reduction ≥7% or HbA1c at baseline or at target level | LAB |
| b. Hyperlipidemia (LDL/HDL) | Improve cholesterol levels | Reduction of LDL‐ and HDL‐cholesterol ratio by ≥10% or LDL‐cholesterol at baseline ≤160 mg/dl or total cholesterol at baseline ≤200 mg/dl | LAB | |
| c. Arterial hypertension (blood pressure) | Improve blood pressure | Reduction of MAP by ≥10% or normotensive at baseline | MD, LAB | |
| C. Patients awareness of his diagnosis | a. Diabetes mellitus | Diagnostic awareness | Patient knows his diagnosis of diabetes mellitus | P |
| b. Hyperlipidemia | Diagnostic awareness | Patient knows his diagnosis of hyperlipidemia | P | |
| c. Hypertension | Diagnostic awareness | Patient knows his diagnosis of hypertension | P | |
| D. Patients behavioural health salience ratings | a. Physical activity habits | Increase salience/motivation | Patient never or seldom has difficulty in making physical activity a priority | P |
| b. Dietary habits | Increase salience/motivation | Patient never or seldom has difficulty with improved dietary habits | P | |
| c. Waist circumference above 88 cm (female); 102 cm (male) | Increase salience/motivation | Patient never or seldom has difficulty with weight reduction | P | |
| d. Smoking habits | Increase salience/motivation | Patient never or seldom has difficulty with smoking cessation | P | |
| E. Doctors past behavioural interventions | a. Past lifestyle intervention, nutrition | Nutrition counselling | Patient participated in nutrition counselling | P |
| b. Past lifestyle intervention, physical activity | Physical education classes | Patient participated in physical education classes | P | |
| c. Past lifestyle intervention, stress | Stress management classes | Patient participated in stress management classes | P | |
| d. Past smoking cessation | Smoking cessation programme | Patient participated in a smoking cessation programme | P | |
| F. Doctors drug interventions | a. Past medication treatment | Improve/ensure medication adherence | Never or rarely problems with prescribed medication | MD |
| G. Overall compliance rating (drug & behavioural) | a. MDs overall adherence rating | Ensure drug & behavioural adherence | Doctor perceives total adherence as high | MD |
Abbreviations: P: patient self report; MD: doctors clinical interview; LAB: measured clinical parameter; MAP: mean arterial pressure (diastolic blood pressure + 1/3(difference between systolic and diastolic blood pressure)).
Acknowledging that primary care physicians typically choose primary targets for future intervention and do not address all risk factor constellations simultaneously, we asked each physician to attach a weighting to the indicators in order to highlight to which degree the targets will be emphasized in future interventions.
After a period of 12‐months – each patient was reappraised with regard to the degree in which treatment goals were achieved by use of a predefined goal attainment rating – using the same measuring tools as in the initial examination.
To take the complexity of drug and behavioural adherence into account, the scale consists of various categorical and dimensional measures. These measures were taken from the existing baseline and 12‐month follow‐up data for the current analysis. The baseline indicators were: (i) low physical activity as rated by patients (less than two hours of physical activity per week); (ii) waist circumference (WC) as measured at baseline assessment by the treating physician (WC was measured with a measuring tape midway between the lowest rib and the pelvis; elevated WC was defined as >88 cm in woman and >102 cm in men); (iii) diabetes mellitus, hyperlipidemia and arterial hypertension were defined according to physician diagnosis. In the domain behavioural health salience ratings patients were considered dependent on the individual risk profile. It includes in the categories patients with (a) low physical activity, (b) a diagnosis of diabetes mellitus, hyperlipidemia, hypertension, (c) with increased WC and (d) former or current smokers at baseline.
Further, physicians made concrete non‐pharmacologic therapeutic recommendations, such as dietary consultation, participation in a physical education class, stress management class or smoking cessation programme. Past pharmacologic treatment was defined by any drug prescription at baseline assessment. The medical appraisal of individual patient adherence was assessed with one single item in the physicians diagnostic interview.
As future target areas for interventions in the domain of cardiovascular risk factors, we defined the physician advice to increase physical activity, to lose weight and to reduce or quit smoking. Targets for the course of disease were the aim to reduce HbA1c in diabetic patients, to improve cholesterol levels in hyperlipidemic patients and to reduce systolic and diastolic blood pressure in hypertensive patients. Patients who were unaware of their diagnoses were advised to obtain information themselves of their disease.
To evaluate the degree of goal attainment in each of the seven domains we used data from the 1st Follow up assessment after 12 month. In the domain of cardiovascular risk factors, we examined if the patients increased their physical activity to more than two hours per week, or if they decreased their WC by 5% or more. In case of smoking reduction goal, we checked if patients had stopped smoking at the one‐year follow‐up or if they reduced their daily cigarette smoking by ≥30%. In diabetic patients, goal attainment was defined as a reduction of HbA1c to a specific physician target level or a level <7%Hb. In hyperlipidemic patients, the goal was a reduction of the LDL/HDL‐cholesterol ratio by ≥10%, LDL‐cholesterol level ≤160 mg/dl or total cholesterol level ≤200 mg/dl; in hypertensive patients the goal was a reduction of mean arterial pressure (MAP) by ≥10%. Patient awareness was achieved if the patients knew their diagnoses at follow‐up. Goal attainment in patient behavioural salience ratings was defined as never or seldom having difficulties in following the physician advice to change health behaviours. Targets were reached in the domain of behavioural intervention if the patients participated in the prescribed interventions and programmes. The goal of drug adherence was for patients to indicate that they never or rarely had problems with intake of prescribed medication. Overall adherence was achieved, if the physician perceived, that total adherence was high.
Scoring scheme
We applied two different strategies in the development of the adherence score. In the crude scoring scheme we rated if each of the 19 indicators (allocated to the seven domains) is relevant for the patient. If the indicator was not relevant or not target for intervention, a score of “zero” was assigned. If the indicator is relevant/targeted for the patient and the intervention goal is attained, then a score of “one” was given. In case of target area not chosen for intervention the indicator variable was scored “two” and if the target area was chosen for intervention and the treatment goal was not attained, “three”. In the scoring scheme for the adherence score corrected by risk factors, we scored “one” if the treatment goal was attained or the indicator was not relevant for the patient. The adherence indicator was coded by “zero” in case of not attaining the treatment goal or the relevant indicator was not targeted by the physician.
Statistical analyses
The baseline variables were processed towards a simplified categorical coding scheme and combined with the physicians target area as described earlier and reported in Table 2. Confirmatory factor analysis was applied to identify the contribution of each indicator for the latent total behavioural and drug adherence scores to be derived. All indicator variables were categorical, resulting in polychoric correlation matrix as the basis for the confirmatory factor analyses. The behavioural adherence score was estimated by the indicator variables from the domains A to E and the drug adherence score by the domains F and G as described in Table 2. The two scores are the base for the second‐order factor model to estimate a total adherence measure. In order to assess the goodness‐of‐fit of the factor analysis model, we calculated the chi‐squared goodness‐of‐fit test, the standardized root mean square residual (RMSR), Comparative Fit Index (CFI) and Tucker–Lewis Index (TLI). A RMS of lower than 0.08 and CFI/ TLI above 0.9 indicate acceptable fit of the factor model (Hu and Bentler, 1998). The weighted least squares estimator based on a diagonal weight matrix was applied for estimating the model parameters. The adherence scores were estimated as linear combination of all indicators weighted by the factor loadings based on the confirmatory factor analyses by maximum a posteriori method (Shi and Lee, 1997).
The confirmatory factor analyses and estimation of factor scores were conducted in MPLUS 6.0 (Muthén and Muthén, 1998–2010).
Results
Sample characterization by indicator and domain
Table 3 reports the baseline risk factor profile of patients (A, B), describes proportion of patients aware of having a particular diagnosis (C) and salient behavioural health ratings (D) along with information on patient's past adherence with behavioural interventions, drug interventions and the overall compliance rating (E–G). Most frequent risk factors were elevated WC (43.5%), arterial hypertension (34.5%) and low physical activity (29.1%). A total of 1275 patients (80.8% of all hyperlipidemic patients) were aware of the diagnosis of hyperlipidemia. The proportion was higher for arterial hypertension (83.8% of 1632) and diabetes mellitus (90.9% of 633).
Table 3.
Patients baseline characterization by domain and scores (I), proportion of patients for which the respective target areas was chosen for intervention (II) and proportion of patients among those targeted (see II) reaching the intervention goal (see III)
| I. Baseline characterization by domain and scores/number (%) of patients | II. Proportion of patients for which the respective target area (A) was chosen for intervention | III. Proportion of patients among those targeted (B) reaching the intervention goal | ||||
|---|---|---|---|---|---|---|
| N | Percentage | N | Percentage | N | Percentage | |
| A. Measured risk factors | ||||||
| a. Physical activity (low) | 1645 | 29.1 | 687 | 41.8 | 251 | 36.5 |
| b. Waist circumference [> 88 cm (female); 102 cm (male)] | 2455 | 43.5 | 1418 | 57.8 | 288 | 20.3 |
| c. Smoking | 1200 | 21.3 | 730 | 60.8 | 288 | 39.5 |
| B. Diagnostic risk factors | ||||||
| a. Diabetes (Hba1c) | 702 | 12.4 | 702 | 100.0 | 394 | 56.1 |
| b. Hyperlipidemia (LDL/HDL) | 1578 | 28.0 | 1578 | 100.0 | 896 | 56.8 |
| c. Arterial hypertension (blood pressure) | 1948 | 34.5 | 1948 | 100.0 | 731 | 37.5 |
| C. Patients awareness of his diagnosis | ||||||
| a. Diabetes mellitus | 633 | 11.2 | 633 | 100.0 | 227 | 35.9 |
| b. Hyperlipidemia | 1275 | 22.6 | 1275 | 100.0 | 966 | 75.8 |
| c. Arterial hypertension | 1632 | 28.9 | 1632 | 100.0 | 613 | 37.6 |
| D. Patients behavioural health salience ratings | ||||||
| a. Physical activity habits | 1645 | 29.1 | 687 | 41.8 | 280 | 40.8 |
| b. Dietary habits | 3548 | 62.9 | 1707 | 48.1 | 941 | 55.1 |
| b. Waist circumference [> 88 cm (female); 102 cm (male)] | 2455 | 43.5 | 1418 | 57.8 | 509 | 35.9 |
| d. Smoking habits | 580 | 10.3 | 310 | 53.5 | 35 | 11.3 |
| E. Doctors past behavioural interventions | ||||||
| a. Past lifestyle intervention, nutrition | 5265 | 93.3 | 2240 | 42.6 | 485 | 21.7 |
| b. Past lifestyle intervention, physical activity | 5265 | 93.3 | 1170 | 22.2 | 295 | 25.2 |
| c. Past lifestyle intervention, stress | 5265 | 93.3 | 460 | 8.7 | 59 | 12.8 |
| d. Past smoking cessation | 2379 | 42.1 | 326 | 13.7 | 18 | 5.5 |
| F. Doctors drug interventions | ||||||
| a. Past medication treatment | 3292 | 58.3 | 3292 | 100.0 | 2996 | 91.0 |
| G. Overall compliance rating (drug & behavioural) | ||||||
| a. MDs overall adherence rating | 3724 | 66.0 | 3724 | 100.0 | 3615 | 97.1 |
Category B, C, F, and G in column I and II are in italics to highlight that this indicators is invariably associated with doctors target decision.
It is noteworthy, that despite of the fact that many more patients had critically elevated risk constellations, not all of these were targeted. The most frequent intervention targets of physicians were quitting or reducing smoking (60.8%), weight reduction (57.8%) and the recommendation of a smoking cessation programme (53.5%). Less frequently targeted were physical education classes (22.2%) and stress management classes (8.7%).
At the one‐year follow‐up the majority of the patients (91.0%) rarely or never had problems with the intake of prescribed medication, more than 50% of the patients improved their cardiovascular risk profile by lowering the elevated HbA1c level (56.1%) and lipid level (56.8%), whereas only 37.5% improved their blood pressure level. The patient awareness with respect to risk factors was low: only 11.3% of the current smokers rated the importance of a smoking cessation as middle or high; the rate was slightly higher for perception of importance of weight reduction (35.9%), increasing physical activity (40.8%) and improving dietary habits (55.1%).
Adherence score characterization
If these data were entered into a “second‐order factor model” for adherence, an acceptable fit (CFI = 0.91, TLI = 0.91; RMSR = 0.078) was found, based on the guidelines for fit indices suggested by Hu and Bentler (1998). A common measure for homogeneity of an estimated score is the correlation based internal consistency ranging between zero and one. Internal consistency is the ability of the several adherence indicators whether they measure the same general construct. The internal consistency for the crude total adherence score is 0.83, for the behavioural adherence score 0.83 and for the drug adherence score 0.74. An internal consistency of above 0.70 is considered to be adequate and values above 0.80 are good (Cronbach, 1951).
Basic characteristics of the crude and risk factor corrected adherence scores are reported in Table 4 and Figure 2. Female patients had higher adherence than male patients. The crude adherence score decreased with age, whereas the association of age and the risk factor corrected score was smaller; this was due to the construction of the scores. The indictor variables for the crude score differ between relevance for the patients and goal attainment, whereas the two categories are collapsed in the risk factor corrected score. The mean crude adherence was 85.3 (SD = 13.7) for patients not targeted by the physician. If the physician targeted at least one domain, the mean adherence score was 43.1 (9.8).
Table 4.
Adherence score
| Crude | Corrected by risk factors | ||||||
|---|---|---|---|---|---|---|---|
| Total | Behavioural | Drug | Total | Behavioural | Drug | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Total | 58.3 (21.6) | 52.1 (26.9) | 54.5 (21.0) | 73.2 (16.4) | 85.1 (15.1) | 67.5 (17.4) | |
| Gender | |||||||
| Female | 59.9 (21.9) | 53.4 (26.9) | 56.0 (21.3) | 73.7 (16.4) | 86.0 (14.9) | 67.6 (17.5) | |
| Male | 55.8 (21.0) | 49.9 (26.9) | 51.9 (20.3) | 72.3 (16.4) | 83.7 (15.3) | 67.5 (17.2) | |
| Age group | |||||||
| 18–45 | 70.4 (21.9) | 69.4 (25.0) | 66.1 (21.7) | 81.1 (12.0) | 92.3 (9.8) | 72.7 (14.6) | |
| 46–65 | 56.7 (21.0) | 50.8 (26.4) | 52.9 (20.3) | 76.0 (15.9) | 87.2 (14.5) | 68.2 (17.1) | |
| 66+ | 50.7 (17.8) | 39.4 (20.9) | 47.2 (17.1) | 69.8 (15.8) | 79.1 (15.4) | 63.1 (17.2) | |
| Number of targeted behaviour | |||||||
| 0 | 85.3 (13.7) | 84.6 (15.1) | 81.4 (14.4) | 89.7 (4.7) | 100.0 (0.0) | 82.2 (8.8) | |
| 1 | 74.4 (17.5) | 78.3 (14.9) | 67.8 (16.0) | 89.5 (4.5) | 100.0 (0.0) | 81.5 (8.2) | |
| 2–5 | 62.0 (15.4) | 53.9 (21.2) | 57.8 (14.6) | 78.7 (9.2) | 88.8 (9.8) | 74.0 (11.6) | |
| 6–10 | 44.7 (10.6) | 36.4 (18.1) | 41.3 (10.0) | 63.7 (11.7) | 77.1 (12.9) | 58.7 (13.0) | |
| 11+ | 32.4 (9.4) | 24.4 (13.1) | 29.5 (8.9) | 48.5 (12.5) | 65.4 (14.1) | 42.2 (13.5) | |
| Number of not reached intervention goals | |||||||
| 0 | 78.3 (16.5) | 71.1 (24.0) | 74.4 (16.7) | 86.7 (6.9) | 96.1 (6.3) | 81.2 (9.1) | |
| 1 | 60.6 (16.2) | 51.2 (23.5) | 56.2 (14.9) | 77.7 (9.7) | 87.4 (9.7) | 73.8 (10.8) | |
| 2–5 | 48.3 (15.5) | 42.7 (23.2) | 44.5 (14.3) | 67.0 (13.6) | 80.3 (14.1) | 61.2 (14.1) | |
| 6–10 | 34.3 (11.5) | 33.0 (20.8) | 31.1 (10.5) | 48.9 (15.0) | 67.4 (18.5) | 40.6 (13.6) | |
| 11+ | 22.0 (9.6) | 30.1 (21.6) | 19.1 (9.0) | 28.9 (17.4) | 46.3 (26.0) | 24.5 (13.0) | |
Figure 2.
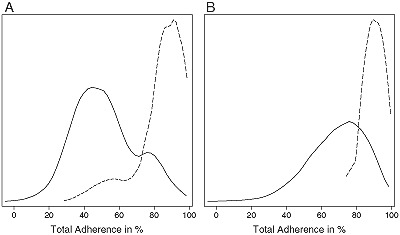
Distribution of the total adherence score: (A) crude adherence score; (B) corrected by risk factor adherence score (dashed line: distribution of total adherence score for patients with no targeted area for intervention; solid line: distribution of total adherence score for patients with at least one targeted area for intervention).
Discussion
In this exploratory study the DETECT adherence score revealed good psychometric properties in terms of internal consistency and factorial structure, suggesting that its further exploration in terms of external validity is promising. Findings also underlie that it is useful and informative to cover within one score both, pharmacologic and non‐pharmacologic interventions in primary care. Our combination in this respect is unique, as most studies conducted on this subject so far aimed at assessing solely drug adherence (Glynn et al., 1994; Horwitz et al., 1990) or behavioural adherence (Brownell and Cohen, 1995a, 1995b; Ettinger et al., 1997).
Based on sophisticated moderator analyses, DiMatteo et al. (2002), highlighted four aspects of measurement that are essential for an effective measurement of adherence: (i) the scaling (continuous rather than dichotomous is preferred, because of increased reliability and power), (ii) the number of adherence indicator measures, (iii) the frequency of their assessment over time and (iv) the incorporation of self‐report measures. Most existing measures (Burke and Dunbar‐Jacob, 1995; Evangelista and Dracup, 2000) have not observed all of these issues equally. Further, most have an exclusive and or primary focus on drug adherence and only few cover behavioural aspects. Our novel DETECT adherence score considers (see Table 2) various factors of patients and physicians that influence the complex construct of “incompliance” (DiMatteo et al., 2002) in seven domains and thus meets these requirement to a larger degree than any previous score. Its feasibility and reliance on routinely assessed laboratory, clinical and behavioural domains further adds to the utility of the DETECT adherence score in routine care, particularly in health care and primary care systems like in Germany, that are characterized typically by a long‐standing patient–doctor relationship in most cases. Also, cardiovascular disease is an ideal entity to decipher adherence in primary prevention, as many medications in the armamentarium against cardiovascular disease have a preventive mechanism that does not demonstrate noticeable symptom relief to the subjects.
There are several limitations to this study. The DETECT study was designed to examine the prevalence and comorbidity of diabetes mellitus, hypertension, hyperlipidemia, coronary heart disease, associated medical conditions and frequency of behavioural and clinical risk factors. Therefore, the adherence score indicators are not based on specific adherence measures, rather the adherence score is based on exploratory ad hoc measures. Further studies should test the concurrent validity of this instrument by using objective measurements (e.g. biomarkers, electronically monitoring systems, behavioural monitoring) and investigate whether the score can be shortened for a more time and cost effective instrument for the use in primary care offices.
Declaration of interest statement
The authors have no competing interests. The first three authors contributed equally to this work.
Acknowledgements
DETECT (Diabetes Cardiovascular Risk‐Evaluation: Targets and Essential Data for Commitment of Treatment) is a cross‐sectional and prospective‐longitudinal, nationwide clinical epidemiological study. DETECT is supported by an unrestricted educational grant of Pfizer GmbH, Karlsruhe, Germany.
Members of the DETECT study group include: Principal investigator: Professor Dr H.‐U. Wittchen; Staff members: Dipl.‐Psych. L. Pieper, Dipl.‐Math. J. Klotsche, Dr T. Eichler, Dr H. Glaesmer, E. Katze. Steering Committee: Professor Dr H. Lehnert (Lübeck), Professor Dr G. K. Stalla (München), Professor Dr A. M. Zeiher (Frankfurt); Advisory Board: Professor Dr W. März (Heidelberg/Graz), Professor Dr S. Silber (München), Professor Dr U. Koch (Hamburg), Professor Dr D. Pittrow (München/Dresden), Professor Dr M. Wehling (Mannheim), Dr D. Leistner (Frankfurt), Dr H. J. Schneider (München), Dr C. Sievers (München).
References
- Bischoff B., Silber S., Richartz B.M., Pieper L., Klotsche J., Wittchen H.U. (2006) Inadequate medical treatment of patients with coronary artery disease by primary care physicians in Germany. Clinical Research in Cardiology, 95(8), 405–412. [DOI] [PubMed] [Google Scholar]
- Boehler S., Glaesmer H., Pittrow D., Lehnert H., Stalla G.K., Zeiher A.M., Maerz W., Silber S., Wehling M., Ruf G., Reinecke A., Wittchen H.U. (2004) Diabetes and cardiovascular risk evaluation and management in primary care: progress and unresolved issues – rationale for a nationwide primary care project in Germany. Experimental and Clinical Endocrinology & Diabetes, 112(4), 157–170. [DOI] [PubMed] [Google Scholar]
- Boehler S., Scharnagl H., Freisinger F., Stojakovic T., Glaesmer H., Klotsche J., Pieper L., Pittrow D., Kirch W., Schneider H., Stalla G.K., Lehnert H., Zeiher A.M., Silber S., Koch U., Ruf G., Maerz W., Wittchen H.U. (2007) Unmet needs in the diagnosis and treatment of dyslipidemia in the primary care setting in Germany. Atherosclerosis, 190(2), 397–407. [DOI] [PubMed] [Google Scholar]
- Brownell K.D., Cohen L.R. (1995a) Adherence to dietary regimes. 1. An overview of research. Behavioral Medicine, 20(4), 149–154. [DOI] [PubMed] [Google Scholar]
- Brownell K.D., Cohen L.R. (1995b) Adherence to dietary regimes. 2. Components of effective interventions. Behavioral Medicine, 20(4), 155– 164. [DOI] [PubMed] [Google Scholar]
- Burke L.E., Dunbar‐Jacob J. (1995) Adherence to medication, diet, and activity recommendations: From assessment to maintenance. Journal of Cardiovascular Nursing, 9(2), 62–79. [DOI] [PubMed] [Google Scholar]
- Cronbach L.J. (1951) Coefficient alpha and the internal structure of tests. Psychometrika, 16(3), 297–334. [Google Scholar]
- DiMatteo M.R., Giordani P.J., Lepper H.S., Croghan T.W. (2002) Patient adherence and medical treatment outcomes – a meta‐analysis. Medical Care, 40(9), 794–811. [DOI] [PubMed] [Google Scholar]
- Ettinger W.H., Burns R., Messier S.P., Applegate W., Rejeski W.J., Morgan T., Shumaker S., Berry M.J., Otoole M., Monu J., Craven T. (1997) A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis – the Fitness Arthritis and Seniors Trial (FAST). Journal of the American Medical Association, 277(1), 25–31. [PubMed] [Google Scholar]
- Evangelista L.S., Dracup K. (2000) A closer look at compliance research in heart failure patients in the last decade. Progress in Cardiovascular Nursing, 15(3), 97–103. [DOI] [PubMed] [Google Scholar]
- Glynn R.J., Buring J.E., Manson J.E., Lamotte F., Hennekens C.H. (1994) Adherence to aspirin in the prevention of myocardial‐infarction – the physicians health study. Archives of Internal Medicine, 154(23), 2649–2657. [DOI] [PubMed] [Google Scholar]
- Horwitz R.I., Viscoli C.M., Berkman L., Donaldson R.M., Horwitz S.M., Murray C.J., Ransohoff D.F., Sindelar J. (1990) Treatment adherence and risk of death after myocardial‐infarction. Lancet, 336(8714), 542–545. [DOI] [PubMed] [Google Scholar]
- Hu L.T., Bentler P.M. (1998) Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods, 3(4), 424–453. [Google Scholar]
- Joshi N., Milfred D. (1995) The use and misuse of new antibiotics – a perspective. Archives of Internal Medicine, 155(6), 569–577. [PubMed] [Google Scholar]
- McDermott M.M., Schmitt B., Wallner E. (1997) Impact of medication nonadherence on coronary heart disease outcomes – a critical review. Archives of Internal Medicine, 157(17), 1921–1929. [PubMed] [Google Scholar]
- Muthén B., Muthén L. (1998–2010) Mplus Version 6 Software Users Guide. Los Angeles, CA, Muthén & Muthén. [Google Scholar]
- Pieper L., Wittchen H.U., Glaesmer H., Klotsche J., Marz W., Stalla G., Lehnert H., Zeiher A.M., Silber S., Koch U., Bohler S., Pittrow D., Ruf G. (2005) Cardiovascular high‐risk constellations in primary care. DETECT Study 2003. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz, 48(12), 1374–1382. [DOI] [PubMed] [Google Scholar]
- Pittrow D., Stalla G.K., Zeiher A.M., Silber S., Marz W., Pieper L., Klotsche J., Glaesmer H., Ruf G., Schneider H.J., Lehnert H., Bohler S., Koch U., Wittchen H.U. (2006) Prevalence, drug treatment and metabolic control of diabetes mellitus in primary care. Medizinische Klinik, 101(8), 635–644. [DOI] [PubMed] [Google Scholar]
- Shi J.Q., Lee S.Y. (1997) Estimation of factor scores with polytomous data by the EM algorithm. British Journal of Mathematical & Statistical Psychology, 50, 215–226. [Google Scholar]
- Wittchen H.U., Glaesmer H., Marz W., Stalla G., Lehnert H., Zeiher A.M., Silber S., Koch U., Bohler S., Pittrow D., Ruf G., Grp D.E.‐S. (2005) Cardiovascular risk factors in primary care: Methods and baseline prevalence rates – the DETECT program. Current Medical Research and Opinion, 21(4), 619–629. [DOI] [PubMed] [Google Scholar]


