Abstract
We have examined the predictive utility of motor activity in infancy towards diagnosis of attention deficit hyperactivity disorder (ADHD) in later childhood. We conducted a nested case‐control study using videos of infants in the Avon Longitudinal Study of Parents and Children (ALSPAC). Sixty videos of children who received any Development and Well‐being Assessment (DAWBA) psychiatric diagnosis at age 91 months (including 16 with ADHD) plus two controls per case were selected for data analysis. Body movements were measured at age one year: associations between motor activity‐derived variables using factor analysis, and later ADHD diagnoses were sought. No significant association was found between infant motor activity and later ADHD. A positive association between motor activity and inattentive ADHD was found in males. Motor activity at age one year did not predict ADHD at age seven years. The positive association with inattentive ADHD in males requires further investigation. Copyright © 2014 John Wiley & Sons, Ltd.
Keywords: attention deficit hyperactivity disorder, motor development, infant mental health, early identification
Introduction
The development of targeted interventions to reduce the population prevalence of child psychopathology depends upon accurate early identification. While early signs of autism have received a great deal of attention (Yirmiya and Ozonoff, 2007), there is a highly compelling case for early identification of the less intensively investigated disruptive behaviour disorders which are both more prevalent and more amenable to low‐intensity therapeutic interventions (Hutchings et al., 2007; Rappaport et al., 1998). There is an increasingly strong case (Wilson et al., 2009) for screening for lifecourse‐persistent conduct disorder (Moffitt and Caspi, 2001) but similar arguments could be applied to screening for attention deficit hyperactivity disorder (ADHD), which is equally persistent and shares its poor prognosis (Billstedt et al., 2005). In this paper we examine the predictive utility of one of the core features of ADHD – abnormal levels of motor activity – assessed in infancy.
Predictive value of generalized motor activity
Generalized movements of the body involve the head, trunk, arms and legs (Hadders‐Algra and Groothuis, 1999). They occur antenatally and until four postnatal months, when more direct, purposeful, goal‐orientated movements begin to develop (Hadders‐Algra et al., 2009). A study of generalized movements of 52 infants with a range of risk factors (low risk preterm and hypoxic‐ischaemic term babies) for neurodevelopmental problems found that while “definitely abnormal” movement was associated with a diagnosis of cerebral palsy between 4–9 years of age, “mildly abnormal” movement was associated with questionnaire‐reported ADHD and aggressive behaviour (Hadders‐Algra and Groothuis, 1999). In this study, “normal” movements showed complexity, variation and fluency, while “abnormal” movements lacked some or all of these qualities. A similar study of low and high risk infants under four months in age found abnormal general movements were related to ADHD with psychiatric comorbidity, but not to ADHD alone, at 9–12 years of age (Hadders‐Algra et al., 2009). In contrast, Yuge et al. (2011) found association between the quality of general movements of high risk infants at age 3–5 months and later cerebral palsy only, with no correlation to other neurological or neuropsychiatric outcome. Finally, Friedman et al. (2005) found that increased body movement in normal infants at three months of age is associated with parent‐reported attention problems in their child at age eight years but not hyperactivity.
Auerbach et al. (2004) studied infants at high genetic risk of ADHD at seven months of age, and found them to be significantly less interested than controls in block play, as well as having higher levels of anger and higher general levels of activity. Laboratory observations of vigorous movements in the infants in this study were also related to maternal reports of more anger. Parent‐completed questionnaires at age 7‐, 12‐ and 25‐months for infants at‐risk due to paternal ADHD symptoms also highlight consistently higher levels of activity, as well as higher levels of anger, and lower levels of attention and inhibitory control compared to the control group (Auerbach et al., 2008): the authors suggest that activity level in the infant is a strongly predictive behavioural marker of ADHD risk.
Gross motor functioning of children with Autism spectrum disorders (ASDs) and ADHD has been shown to be significantly impaired compared with controls, with ASDs children generally performing more poorly (Dewey et al., 2007; van Waelvelde et al., 2010; Pan et al., 2009); while children with emotional disorders perform better than those with behavioural or pervasive developmental disorders, they still have poorer skills compared to the population norm (Emck et al., 2011). Motor problems are most prevalent in children with comorbid psychiatric disorders; ADHD alone was not found to be associated with motor problems unless other disorders were present (Martin et al., 2010). It is worth noting that ADHD is commonly comorbid with other diagnoses: up to 50% of children with ADHD also have developmental coordination disorder (DCD) (Flapper and Schoemaker, 2006), and there is growing opinion that in early childhood impairing neurodevelopmental symptoms can lead to overlapping clinical syndromes which include ADHD (Gillberg, 2010). Children with, or at risk of ASD, ADHD and/or DCD were found to have poor motor coordination, though those with or at risk of ASD show little improvement in these symptoms over time while slightly more improvement is seen in the ADHD group (van Waelvelde et al., 2010). Locomotor difficulties associated with ADHD are, however, not improved with medication (Verret et al., 2010).
There is therefore limited evidence that motor activity in infancy predicts later ADHD diagnosis, but none of the studies is based on a large population sample and it is therefore not possible to assess positive and negative predictive values for the types of assessment used. Here we report findings based on a community‐based large sample of infants who were later assessed for psychopathology, testing the hypothesis that activity levels will be predictive of later ADHD diagnosis.
Methods
The sample
The 16 ADHD cases and 120 controls analysed in this study were selected from a larger study of 60 psychiatric cases and 120 controls. This larger study was itself nested within the Avon Longitudinal Study of Parents and Children (ALSPAC) (Golding, 1990), a community‐based longitudinal study of 14,541 pregnancies with an expected date of delivery in 1991–1992. Cases and controls were selected as follows (Figure 1).
Figure 1.
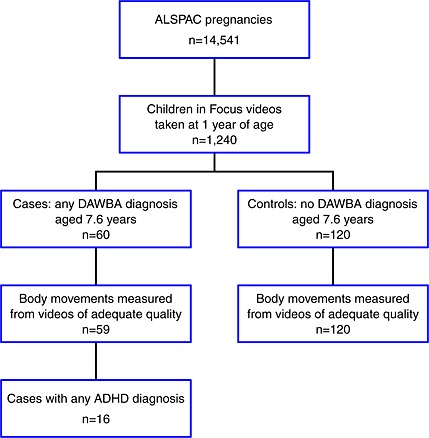
Flow diagram showing the selection of ADHD cases and controls.
A representative randomly selected subcohort of 10% of ALSPAC participants was recruited to the Children in Focus study, which involved children being brought to a study centre for assessment. This sample was selected from the last six months of ALSPAC births, occurring from 6 June to 11 December 1992. In 1993, when the children had a mean age of 54 weeks [standard deviation (SD) = 1.1 weeks], 1240 parents and infants in the Children in Focus study took part in a video recorded interaction – the Thorpe Interactive Measure (TIM) (Thorpe et al., 2003). The videos ranged in duration from one to 14 minutes and involved parents and children in a “naturalistic” environment (on a living room sofa) looking at a picture book. Parents (90% mothers) were asked to engage their child in this activity as they would at home, for as long as the child was interested.
At age 91 months, all ALSPAC parent participants were asked to complete the Development and Well‐being Assessment (DAWBA, http://www.dawba.com) (Goodman et al., 2000) for the index child. This structured assessment based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) diagnoses uses closed questions and branching rules to elicit details of psychiatric disorder. Parent reports were elicited by a lay researcher and review of the responses was made by a child psychiatrist. Data are available for 8240 (58.9%) of the 13,988 children who had survived to age one year. One hundred and seventy‐five children (2.1%) had any ADHD diagnosis. DAWBA assessments are available for 845 (68%) of the Children in Focus sample with video data.
Video assessments for all 60 children with any psychiatric diagnosis at age 91 months, together with two randomly selected sex‐matched controls per case, were selected by one of the authors (JH) and these videos were transferred to the study team, who remained blind to the case or control status of the children (and to all other data) until the video data collection process was complete. One of the videos was of such poor quality that it could not be coded.
DAWBA diagnoses in the 179 (59 cases, 120 control) videos which could be analysed are presented in Table 1.
Table 1.
Sample description. Thirteen children had more than one diagnosis including eight children with at least one ADHD diagnosis
| Controls | Any disorder | Any ADHD disorder | Combined ADHD | Inattentive ADHD | Hyperactive‐impulsive ADHD | Any oppositional‐conduct disorder | Pervasive development disorder | Any anxiety disorder | Any depressive disorder | |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 120 | 59 | 16 | 8 | 5 | 3 | 27 | 6 | 25 | 5 |
| Male | 82 | 41 | 14 | 8 | 3 | 3 | 20 | 5 | 13 | 3 |
| Female | 38 | 18 | 2 | 0 | 2 | 0 | 7 | 1 | 12 | 2 |
We excluded the children who had non‐ADHD diagnoses from the comparison group, leaving 16 ADHD cases and 120 controls. These numbers of cases and controls gave 80% power to reject a null hypothesis of no association [odds ratio (OR) = 1] against a two‐sided alternative hypothesis at 5% significance, assuming an OR of 2.2 associated with a one SD difference in a normally distributed motion summary.
Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and Local Research Ethics Committees.
Video analysis
SkillSpector Motion Tracking software (v1.2, freeware published by Video4Coach) was used to track, frame‐by‐frame, eight body markers [nose (N), right (RH) and left hand (LH), right elbow (RE) and left elbow (LE), right shoulder (RS) and left shoulder (LS) and pelvis (P)]. Legs and feet were not tracked as these were obscured for the greater part of most videos. The x‐y coordinates (in pixels) were captured for each point (an animation created using the x‐y coordinates from one video is available as supporting information). Mean head length was estimated every 1500 frames (100 seconds), allowing correction for distance of the child from the camera.
Thirteen motion summaries were created with a view to determining robust indices of general motor activity, summarizing speed, acceleration, variability of speed and acceleration, periodicity and restlessness. These summaries are described fully in the Appendix.
One hundred and four (eight body markers × 13 motion summaries) variables were therefore available.
Fourteen of the 104 motion variables were selected for further investigation on the basis of length of time visible in the video (≥ 32 s, or 480 frames, in total), data completeness (≥ 90% data points present), inter‐ and intra‐rater reliability [inter‐ and intra‐rater intraclass correlation coefficient (ICC) and inter‐rater rank correlation all > 0.5] and non‐redundancy (absolute pairwise Pearson correlation coefficient < 0.9).
The 14 variables selected were:
Speed of five markers (N, RH, LH, LE, LS): average speed in pixels/s over the duration of the video;
Variability of speed of five markers (N, RH, LH, LE, LS): the standard deviation of speed, where speed was averaged over 8‐second blocks;
Restlessness of three markers (N, LH, LE): the percentage of the video where the body part was moving;
Rhythmic motion of one marker (RH): the percentage of non‐overlapping 8‐second blocks within which speed was judged to have displayed significant (p < 0.001) periodicity at frequencies of < 1.2 Hz, according to Fisher's exact g test (Fisher, 1929).
Statistical methods
Factor analysis was used to cluster the 14 variables into a smaller number of underlying factors. The number of non‐trivial factors to retain was determined by parallel analysis (Horn and Engstrom, 1979). Maximum likelihood factor analysis with a promax rotation was used to fit the factor model and calculate factor loadings. The factor loadings were used to derive “factor scores”. Each factor score was calculated as the sum of the z‐scores (standardized to have mean = 0 and SD = 1) of the variables with loadings > 0.6. Finally, the summed z‐scores were themselves converted to z‐scores.
The derived factor scores were then tested for association with control and case status in relation to ADHD at age seven years (any ADHD disorder, combined ADHD, inattentive ADHD and hyperactive‐impulsive ADHD). Sex‐adjusted ORs with 95% confidence intervals (CIs) and p‐values were estimated by exact logistic regression (see Appendix for further details). Adjusting for sex accounted for the sex imbalance between cases and controls caused by selecting the 16 ADHD cases from the 60 psychiatric cases. We used exact logistic regression because it is unbiased at small sample sizes, unlike standard logistic regression. Associations between factor scores and case‐control status were also explored within male subjects. Associations within females were not assessed due to low numbers.
All statistical analyses were conducted using R (R Development Core Team, 2008). Exact logistic regression was performed with the “elrm” package for R (Zamar et al., 2007).
Results
A three‐factor model provided the best fit to the data (Table 2).
Table 2.
Factor analysis, assuming three factors, of the 14 motion variables (numerical summaries of infant motor activity – see methods for details) that passed filters for missingness, reliability and redundancy
| Item | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Speed, N | 0.90 | 0.08 | 0.03 |
| Speed, RH | 0.81 | –0.05 | –0.06 |
| SD of 8 s block speed, N | 0.72 | –0.25 | 0.35 |
| SD of 8 s block speed, RH | 0.69 | –0.18 | 0.16 |
| Percentage time active, N | 0.67 | 0.48 | –0.42 |
| Speed, LS | 0.54 | 0.37 | 0.27 |
| Speed periodicity (% p < 0.001, frequency < 1.2), RH | 0.53 | 0.05 | –0.06 |
| Percentage time active, LH | –0.10 | 0.97 | –0.07 |
| Percentage time active, LE | 0.03 | 0.94 | 0.02 |
| Speed, LH | –0.06 | 0.72 | 0.38 |
| Speed, LE | 0.06 | 0.66 | 0.50 |
| SD of 8 s block speed, LE | 0.11 | 0.04 | 0.88 |
| SD of 8 s block speed, LH | –0.06 | 0.13 | 0.80 |
| SD of 8 s block speed, LS | 0.55 | –0.16 | 0.57 |
Factor loadings were estimated using the promax rotation from pairwise Pearson correlations between 179 subjects; 3% of data were missing; loadings exceeding ± 0.6 are shown in italic type
Factor 1 could represent central body movement variables, where speed, activity and variability of speed of the nose (N) are presented. It is difficult to interpret the loading of nose (N) and right hand (RH) together but it may in part reflect the fact that the right hand of the infant was often obscured due to the camera position, particularly when holding the book, and thus relatively inactive. During periods of greater activity right hand movement may have correlated more with central body (N) movement than left hand movement. Factor 2 shows left arm (LH, LE) movement, and Factor 3 indicates the variability of speed of the left arm. The left arm is preferentially represented in our data because it was the most visible limb in relation to the camera position.
Table 3 summarizes the relationship of the three derived factors to the diagnostic categories. None of the three factor scores was significantly associated with any of the diagnostic categories (Figure 2). In the exploratory males‐only analysis, inattentive ADHD was positively associated with Factor 1 [OR = 5.2 (95% CI: 1.1, 40.5); p = 0.021] and Factor 3 [OR = 11.4 (95% CI: 1.5, 570.5); p = 0.006] (Figure 3).
Table 3.
Mean (SD) factor scores within ADHD diagnostic groups
| Factor | Controls | Case definition | ||||
|---|---|---|---|---|---|---|
| Any ADHD disorder | Combined ADHD | Inattentive ADHD | Hyperactive‐impulsive ADHD | |||
| 1 | Mean (SD) | 0.0 (0.9) | 0.2 (1.3) | 0.2 (0.7) | 0.3 (2.3) | 0.0 (0.7) |
| N | n = 120 | n = 16 | n = 8 | n = 5 | n = 3 | |
| OR (95% CI) | 1.1 (0.6, 2.0) | 1.0 (0.4, 2.4) | 1.4 (0.5, 4.1) | 1.0 (0.3, 4.3) | ||
| p | p = 0.892 | p = 1.000 | p = 0.647 | p = 1.000 | ||
| 2 | Mean (SD) | −0.1 (1.1) | −0.1 (1.0) | 0.2 (0.7) | −0.4 (1.5) | −0.3 (1.3) |
| N | n = 118 | n = 16 | n = 8 | n = 5 | n = 3 | |
| OR (95% CI) | 0.9 (0.6, 1.6) | 1.2 (0.6, 3.1) | 0.8 (0.4, 2.0) | 0.8 (0.3, 2.9) | ||
| p | p = 0.702 | p = 0.859 | p = 0.525 | p = 0.553 | ||
| 3 | Mean (SD) | −0.1 (1.0) | −0.1 (1.2) | −0.2 (0.5) | 0.3 (1.9) | −0.3 (1.4) |
| N | n = 118 | n = 16 | n = 8 | n = 5 | n = 3 | |
| OR (95% CI) | 1.0 (0.6, 1.8) | 0.9 (0.4, 2.0) | 1.6 (0.6, 4.8) | 0.8 (0.2, 2.7) | ||
| p | p = 1.000 | p = 0.707 | p = 0.387 | p = 0.561 | ||
For each diagnosis, sex‐adjusted odds ratios (ORs) associated with a one SD difference in factor score are presented, with 95% confidence intervals (CIs) and p‐values estimated by exact logistic regression. Each factor score was calculated as the sum of the z‐scores (standardized to have mean = 0 and SD = 1) of the variables with factor loadings > 0.6 (Table 2).
Figure 2.
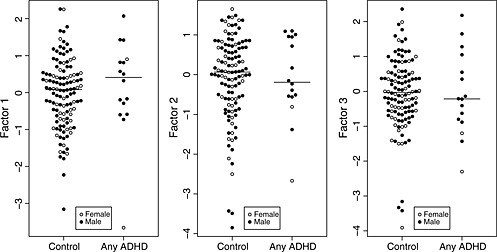
Motion factor scores in controls and infants who developed ADHD. Medians are indicated by horizontal lines.
Figure 3.
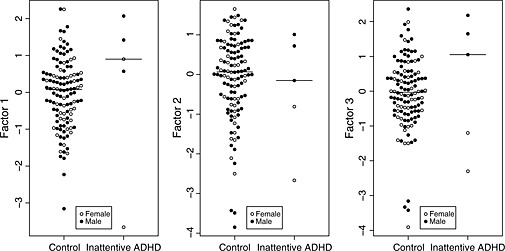
Motion factor scores in controls and infants who developed inattentive ADHD. Medians are indicated by horizontal lines.
Discussion
Our findings
We found no significant association between the motion variables measured at age 12 months and diagnosis of ADHD at age seven years. This negative result has two possible explanations: either the null hypothesis of no association between ADHD and our motion factor scores is true, or the association is too weak for a study of 16 ADHD cases and 120 controls to detect. The CIs around the OR estimates in Table 3 suggest that, broadly speaking, ORs outside the range 0.5–2 are implausible, while ORs in the range 0.7–1.5 are still plausible. An example might serve to give these numbers some context: an OR of 2 implies that the risk of being diagnosed with ADHD aged seven years for a one‐year‐old infant with a factor score of two, which is on the upper limit of normal (2 SDs above the mean), is four times the risk of an infant with a factor score of zero, which is in the middle of the normal range (since ADHD is a relatively rare disorder in the population, ORs are approximately equal to relative risks). A more powerful study would be required to detect smaller (thus less useful to the clinician) effects in the OR range 0.7–1.5. This could be achieved by increasing the sample size substantially (e.g. 109 ADHD cases and 218 controls would give 80% power to detect an OR of 1.4) and by decreasing the variability of the factor scores, perhaps via repeated video assessments.
The detection of a positive association between two of the motion factors and risk of inattentive ADHD in male subjects is intriguing. However, these associations were detected in an exploratory retrospective subgroup analysis where only three cases were available, and should therefore be viewed with scepticism until replicated in a larger study.
Weaknesses
The major weakness of this study is the low number of ADHD cases available which limited the power of the study to detecting only very strong associations. Furthermore, in common with most cohort studies, ALSPAC suffered some selective attrition of children with disruptive behaviour disorders (Wolke et al., 2009).
The camera angle in the videos made it inevitable that some body parts could not be seen at all times (e.g. legs, right arm) and it is possible that some relevant motion data may have been lost. There is also the possibility that passive movement of the child by the parent, rather than self‐initiated movement may have obscured some inter‐individual differences. The motion variables we used were derived from plotted coordinates which were observed directly from the videos. Our data reduction attempted to create summary measures to simplify analysis but it is possible that some important component of infant movement may have been lost.
Previous reports have described direct observations of general movements before children have developed purposeful motion (Hadders‐Algra and Groothuis, 1999; Hadders‐Algra et al., 2009; Yuge et al., 2011). It is possible that by 12 months of age, more goal‐orientated movements would overshadow more generalized movements: the latter could be more significant in terms of prognostic utility.
The DAWBA has been shown to be sufficiently accurate at diagnosing ADHD without direct clinical assessment of the child, using multiple informants including the parents, teachers and the child themselves if aged 11 years or above, compared to face‐to‐face clinical assessment (Foreman et al., 2009). The method used in the ALSPAC study used only the parent interview – it may be that using multiple sources of information about the child could add to diagnostic accuracy.
Strengths
Our sample was derived from a large population‐based longitudinal study, and therefore provided an excellent opportunity to explore early indicators of disorders in childhood in a sample representative of the normal population. Longitudinal studies of this type can provide otherwise unobtainable quantitative data on the utility of measures in the prediction of psychiatric disorders in children (Thompson et al., 2010). We are not aware of any cohort study which could yield comparable data.
We used direct, largely objective, measures of several types of motion of a range of body parts and we believe that our summary data represent a good measure of overall physical activity.
Implications for policy and further work
We were not able to confirm earlier literature suggesting a relationship between early movement patterns and later childhood ADHD diagnoses. It appears unlikely that assessment of levels of motor activity at one year will be very strongly predictive of ADHD in primary care pediatric practice or in early intervention clinics. Our results could inform the design of a larger study powered to detect weaker associations between motor activity and ADHD, and sex differences in the strength of these associations. Further longitudinal studies with regular collection of video material could yield useful data on normal and abnormal development of motor activity. Further insights from such studies might include precursors of other psychiatric diagnoses as well as, for example, poor physical fitness and obesity.
Declaration of interest statement
None of the authors have any financial or other conflicts of interest to declare.
Supporting information
supporting info item
Acknowledgements
The authors are grateful to all the families who took part in this study, the midwives for help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The United Kingdom Medical Research Council, the Wellcome Trust, and the University of Bristol provide core support for ALSPAC. The project reported here was supported by small grants from the Yorkhill Children's Foundation, the Gillberg Neuropsychiatry Centre and the Waterloo Foundation. This article is the work of the authors, and Philip Wilson will serve as guarantor for the contents of this article.
Derivation of motion factors
Sixteen motion summaries were created with a view to determining robust indices of general motor activity over the duration of the video, summarizing speed, acceleration, variability of speed and acceleration, periodicity and restlessness.
Mean speed in units per second across the duration of the video, k∑d i/(n − 1), where k is the number of frames per second (15), n is the number of frames and d is the vector of length n − 1 of distances travelled between n frames, √(Δxi 2 + Δyi 2), and where Δxi and Δyi are the differences in x and y coordinates between frames i and i + 1.
Mean scalar acceleration, k∑Δdi/(n − 2), where Δd is the vector of length n − 2 of differences between distances travelled between consecutive frames.
Mean vector acceleration is calculated as mean speed, except that differences in x and y coordinates between frames are replaced by second‐order differences (the differences between the differences).
Standard deviation of mean speed in 8‐second blocks. Mean speed is calculated as mentioned earlier for each consecutive 8‐second block of frames, and the standard deviation of speed across the blocks is calculated.
Standard deviation of mean scalar acceleration in 8‐second blocks. Calculated as for the standard deviation of mean speed in 8‐second blocks.
Standard deviation of mean vector acceleration in 8‐second blocks. Calculated as for the standard deviation of mean speed in 8‐second blocks.
Periodicity of speed, low frequency: the percentage of non‐overlapping 8‐second blocks within which speed was judged to have displayed significant (p < 0.001) periodicity at frequencies of < 1.2 Hz, according to Fisher's exact g test (Fisher, 1929).
Periodicity of scalar acceleration, low frequency. Calculated as for periodicity of speed, low frequency.
Periodicity of vector acceleration, low frequency. Calculated as for periodicity of speed, low frequency.
Periodicity of speed, high frequency. Calculated as for periodicity of speed, low frequency, except that periodicities in the range 1.2–3.1 were tested for.
Periodicity of scalar acceleration, high frequency. Calculated as for periodicity of speed, high frequency.
Periodicity of vector acceleration, high frequency. Calculated as for periodicity of speed, high frequency.
Percentage time active. The proportion of frames at which speed was greater than zero.
Some of these proved unreliable and were rejected. Summary data were generated as follows:
Reliability estimates from two classes of variable (scalar acceleration and high frequency periodicity) were consistently low. Removal of these variables left 72 motion variables.
Data points derived from less than 32 second video time (480 frames in total) were set to missing and variables with more than 10% missing data were excluded, leaving 45 variables.
All speed and acceleration variables (that is, all variables except those gauging activity and periodicity) were adjusted for distance from the camera by standardizing to mean head length.
All speed and acceleration variables were log‐transformed to give approximately normal distributions.
Three reliability statistics (inter‐ and intra‐rater ICC and inter‐rater rank correlation) were calculated from videos rated by two raters. Thirty‐three variables scoring > 0.5 on all three reliability statistics were retained.
Groups of closely correlated variables were identified by calculating pairwise Pearson correlation coefficients. Particular sets of motion summaries were consistently highly correlated (absolute correlation > 0.9) across body markers. “Accel‐v”, “Mean 8 s block accel‐v”, “Mean 8 s block speed” and “Speed” variables tended to be closely correlated. Of these, only “Speed” was retained. Likewise, “SD 8 s block accel‐v” and “SD 8 s block speed” variables were closely correlated and only “SD 8 s block speed” was retained. This step reduced the number of motion variables to 14.
| N variables excluded | N variables retained | |
|---|---|---|
| Summary variables originally considered (eight body markers × 13 motion summaries) | — | 104 |
| Exclude all summaries of scalar acceleration and high frequency periodicity due to low reliability | 48 | 56 |
| Exclude variables with > 10% missing data, after setting to missing data points derived from < 32 second video time | 21 | 35 |
| Exclude variables with any of inter‐ and intra‐rater ICC and inter‐rater rank correlation < 0.5 | 12 | 23 |
| Exclude variables with Pearson correlation of > 0.9 with any other variable | 9 | 14 |
Logistic regression
Odds ratios and associated 95% confidence intervals and p‐values were estimated by exact logistic regression using a Markov chain Monte Carlo (MCMC) algorithm implemented in the “elrm” package for R (Zamar et al., 2007). To prevent degeneracy in the distribution explored by the Markov chain, factor scores were rounded to the nearest integer for exact logistic regression, reducing the number of distinct values to seven for each factor. The Markov chain mixing parameter, r, was set to two after the mixing properties of r values of two, four and six had been explored. Following 5000 initial iterations which were discarded as burn‐in, the chain was run until the effective number of MCMC samples generated (that is, the number of iterations adjusted for autocorrelation) had reached 10,000.
References
- Auerbach J.G., Atzaba‐Poria N., Berger A., Landau R. (2004) Emerging developmental pathways to ADHD: possible path markers in early infancy. Neural Plasticity, 11(1‐2), 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach J.G., Berger A., Atzaba‐Poria N., Arbelle S., Cypin N., Friedman A., Landau R. (2008) Temperament at 7, 12 and 25 months in children at familial risk for ADHD. Infant and Child Development, 17(4), 321–338. [Google Scholar]
- Billstedt E., Gillberg C., Gillberg C. (2005) Autism after adolescence: population‐based 13‐ to 22‐year follow‐up study of 120 individuals with autism diagnosed in childhood. Journal of Autism & Developmental Disorders, 35(3), 351–360. [DOI] [PubMed] [Google Scholar]
- Dewey D., Cantell M., Crawford S.G. (2007) Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 13(2), 246–256. [DOI] [PubMed] [Google Scholar]
- Emck C., Bosscher R.J., Van Wieringen P.C.W., Doreleijers T., Beek P.J. (2011) Gross motor performance and physical fitness in children with psychiatric disorders. Developmental Medicine & Child Neurology, 53(2), 150–155. [DOI] [PubMed] [Google Scholar]
- Fisher R.A. (1929) Tests of significance in harmonic analysis. Proceedings of the Royal Society, A, 125(796), 54–59. [Google Scholar]
- Flapper B.C.T. Schoemaker M.M. (2006) Fine motor skills and effects of methylphenidate in children with attention‐deficit‐hyperactivity disorder and developmental coordination disorder. Developmental Medicine & Child Neurology, 48(3), 165–169. [DOI] [PubMed] [Google Scholar]
- Foreman D., Morton S., Ford T. (2009) Exploring the clinical utility of the Development and Well‐being Assessment (DAWBA) in the detection of hyperkinetic disorders and associated diagnoses in clinical practice. Journal of Child Psychology and Psychiatry, 50(4), 460–470. [DOI] [PubMed] [Google Scholar]
- Friedman A.H., Watamura S.E., Robertson S.S. (2005) Movement‐attention coupling in infancy and attention problems in childhood. Developmental Medicine & Child Neurology, 47(10), 660–665. [DOI] [PubMed] [Google Scholar]
- Gillberg C. (2010) The ESSENCE in child psychiatry: Early Symptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations. Research in Developmental Disabilities, 31(6), 1543–1551. [DOI] [PubMed] [Google Scholar]
- Golding J. (1990) Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC). West of England Medical Journal, 105(3), 80–82. [PMC free article] [PubMed] [Google Scholar]
- Goodman R., Ford T., Richards H., Gatward R., Meltzer H. (2000) The Development and Well‐being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry, 41(5), 645–655. [PubMed] [Google Scholar]
- Hadders‐Algra M., Bouwstra H., Groen S.E. (2009) Quality of general movements and psychiatric morbidity at 9–12 years. Early Human Development, 85(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Hadders‐Algra M., Groothuis A. (1999) Quality of general movements in infancy is related to neurological dysfunction, ADHD, and aggressive behaviour. Developmental Medicine & Child Neurology, 41(6), 381–391. [DOI] [PubMed] [Google Scholar]
- Horn J.L., Engstrom R. (1979) Cattell's scree test in relation to Bartlett's chi‐square test and other observations on the number of factors problem. Multivariate Behavioral Research, 14(3), 283–300. [DOI] [PubMed] [Google Scholar]
- Hutchings J., Gardner F., Bywater T., Daley D., Whitaker C., Jones K., Eames C., Edwards R.T. (2007) Parenting intervention in Sure Start services for children at risk of developing conduct disorder: pragmatic randomised controlled trial. British Medical Journal, 334(7595), 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N.C., Piek J., Baynam G., Levy F., Hay D. (2010) An examination of the relationship between movement problems and four common developmental disorders. Human Movement Science, 29(5), 799–808. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Caspi A. (2001) Childhood predictors differentiate life‐course persistent and adolescence‐limited antisocial pathways among males and females. Development & Psychopathology, 13(2), 355–375. [DOI] [PubMed] [Google Scholar]
- Pan C.Y., Tsai C.L., Chu C.H. (2009) Fundamental movement skills in children diagnosed with autism spectrum disorder and attention deficit hyperactivity disorder. Journal of Autism and Developmental Disorders, 39(12), 1694–1705. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . (2008) R: A Language and Environment for Statistical Computing, Vienna, R Foundation for Statistical Computing. http://www.rproject.org
- Rappaport G.C., Ornoy A., Tenenbaum A. (1998) Is early intervention effective in preventing ADHD? Israel Journal of Psychiatry & Related Sciences, 35(4), 271–279. [PubMed] [Google Scholar]
- Thompson L., Kemp J., Wilson P., Pritchett R., Minnis H., Toms‐Whittle L., Puckering C., Law J., Gillberg C. (2010) What have birth cohort studies asked about genetic, pre‐ and peri‐natal exposures and child and adolescent onset mental health outcomes? A systematic review. European Child & Adolescent Psychiatry, 19(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Thorpe K., Rutter M., Greenwood R. (2003) Twins as a natural experiment to study the causes of mild language delay: II: Family interaction risk factors. Journal of Child Psychology and Psychiatry, 44(3), 342–355. [DOI] [PubMed] [Google Scholar]
- van Waelvelde H., Oostra A., Dewitte G., van den Broeck C., Jongmans M.J. (2010) Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder. Developmental Medicine & Child Neurology, 52(8), e174–e178. [DOI] [PubMed] [Google Scholar]
- Verret C., Gardiner P., Beliveau L. (2010) Fitness level and gross motor performance of children with attention‐deficit hyperactivity disorder. Adapted Physical Activity Quarterly, 27(4), 337–351. [DOI] [PubMed] [Google Scholar]
- Wilson P., Minnis H., Puckering C., Gillberg C. (2009) Should we aspire to screen preschool children for conduct disorder? Archives of Disease in Childhood, 94(10), 812–816. [DOI] [PubMed] [Google Scholar]
- Wolke D., Waylen A., Samara M., Steer C., Goodman R., Ford T., Lamberts K. (2009) Selective drop‐out in longitudinal studies and non‐biased prediction of behaviour disorders. British Journal of Psychiatry, 195(3), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N., Ozonoff S. (2007) The very early autism phenotype. Journal of Autism and Developmental Disorders, 37(1), 1–11. [Google Scholar]
- Yuge M., Marschik P.B., Nakajima Y., Yamori Y., Kanda T., Hirota H., Yoshida N., Einspieler C. (2011) Movements and postures of infants aged 3 to 5 months: to what enxtent is their optimality related to perinatal events and to the neurological outcome? Early Human Development, 87(3), 231–237. [DOI] [PubMed] [Google Scholar]
- Zamar D., McNeney B., Graham J. (2007) elrm: software implementing exact‐like inference for logistic regression models. Journal of Statistical Software, 21(3), 1–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supporting info item


